Abstract
Common features of neurodegenerative diseases (NDDs) include progressive dysfunctions and neuronal injuries leading to deterioration in normal brain functions. At present, ginseng is one of the most frequently used natural products. Its use has a long history as a cure for various diseases because its extracts and active compounds exhibit several pharmacological properties against several disorders. However, the pathophysiology of NDDs is not fully clear, but researchers have found that various ion channels and specific signaling pathways might have contributed to the disease pathogenesis. Apart from the different pharmacological potentials, ginseng and its active compounds modulate various ion channels and specific molecular signaling pathways related to the nervous system. Here, we discuss the signal modulating potential of ginseng and its active compounds mainly focusing on those relevant to NDDs.
Keywords: Active compounds, Ginseng, Ion channels, Neurodegenerative diseases, Signaling pathway
1. Introduction
In the nervous system, neurodegenerative diseases (NDDs) are multifactorial debilitating disorders that are characterized by progressive dysfunction and neuronal injury leading to a slow but irreversible deterioration of brain functions which affects around 30 million individuals worldwide [1], [2], [3], [4]. Although some symptomatic treatments are available, specific treatments have not yet been discovered [5]. Ginseng is considered to be one of the widely used traditional herbal medicines [6]. Ginseng and its constituents have been documented to produce aphrodisiac, adaptogenic, immunomodulatory, antiinflammatory, antioxidant, antiaging, anticancer, antifatigue, antidiabetic, pulmonary protective, hepatoprotective, cardioprotective, hypolipidemic, and renoprotective effects in various studies [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21].
Furthermore, ginseng extracts and its active compounds have exhibited properties including antistress, antidepressive, and neuroprotective in various studies of neurological disease models [22], [23], [24], [25], [26], [27], [28], [29], [30]. They also enhanced cognitive performance and help maintain brain health [30], [31], [32], [33], [34]. Recently, various studies have reported on the potential role of ginseng and its active compounds in treating neurological disorders. Here, focusing on potential therapies for NDDs, we present the signal modulating potential of ginseng and its active compounds.
2. Role of ginseng and its active compounds in modulating ion channel signaling pathways
2.1. Modulation of voltage-gated ion channel
Various sources of evidence have suggested that ginsenosides regulate the neuronal Na+ channels. In Xenopus oocytes, ginsenoside Rg3 carbohydrate component inhibited the inward Na+ peak current [35]. Also, in Xenopus oocytes, Rg3 inhibited the Na+ channel by acting on the resting and open states of the Na+ channel via contact with the S4 voltage sensor segment of domain II [36]. Moreover, ginsenoside Rh2 inhibited the Na+ channel function by inhibiting veratridine-dependent depolarization of mouse synaptoneurosomes following the inhibition of l-glutamate and γ-amino butyric acid releases [37].
By activating the K+ channel, some ginsenosides regulated the electrical state of excitable neurons. In Xenopus oocytes, ginsenoside Rg3 enhanced outward large-conductance Ca2+ and voltage-gated big K+ (BKCa) channel currents in a concentration-dependent, voltage-dependent and reversible manner [38]. Moreover, in rat's brain, ginsenoside Rf–activated G protein-gated inwardly rectifying potassium (GIRK) channels through an unidentified G protein-coupled receptor [39].
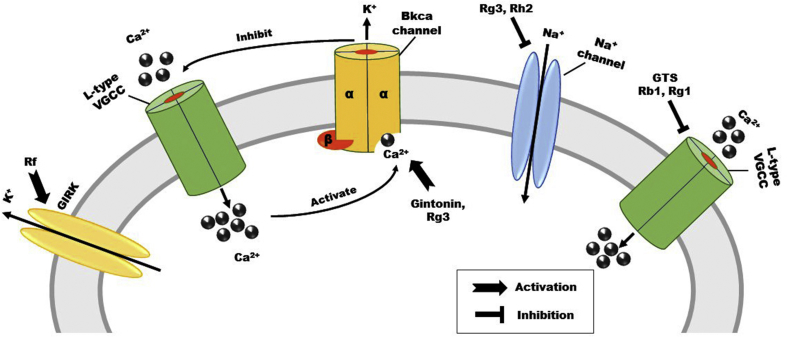
In neuronal cells, ginsenosides are capable of inhibiting the Ca2+ channels. Ginseng total saponins are the main bioactive ingredients of P. ginseng. By inhibiting L-type Ca2+ channels, the ginseng total saponins decreased the KCl-induced neuronal loss in primary cortical neurons [40]. In Xenopus oocytes, BKCa channels are heterologously expressed. Gintonin treatment activated the BKCa channel and expressed the α-subunit of the BKCa channel in a concentration-dependent manner [41]. In primary hippocampal neurons, through L-type Ca2+ channels, ginsenoside Rg1 inhibited Ca2+ influx [42]. Furthermore, in the amyloid beta (Aβ)25–35 model, ginsenoside Rg1 inhibits high-voltage–activated calcium channel currents in hippocampal neurons [43]. In addition, ginsenoside Rb1 inhibited voltage-gated calcium channel currents in a concentration-dependent manner, and upon washout, the current was mostly recovered. In hippocampal neurons, Rb1 selectively inhibits the action of L-type voltage-gated calcium channels without affecting the N-type or P/Q-type Ca2+ channels [44]. The signal modulating effects of active ginseng compounds on voltage-gated ion channels are shown in Fig. 1.
Fig. 1.
Effects of active ginseng compounds on voltage-gated ion channels. Active ginseng compounds modulate the channels activities. GTS, ginseng total saponins; GIRK, G protein-gated inwardly rectifying potassium; L-type VGCC, L-type voltage-gated calcium channel; BKca channel, large-conductance Ca2+ and voltage-gated big K+ channel.
2.2. Modulation of the ligand-gated ion channel
2.2.1. Nicotinic acetylcholine receptors
Active ginseng compounds modulate nicotinic acetylcholine receptors (nAChRs). In oocytes expressing nAChR subunits (α1β1δε or α3β4), ginsenosides inhibited the acetylcholine (ACh)-induced inward currents (IACh). This potential indicates that ginsenosides directly control the nAChR channel activities. In the case of IACh inhibition, protopanaxatriol (PPT) ginsenosides (Re, Rf, Rg1, or Rg2) were more powerful than protopanaxadiol (PPD) ginsenosides including Rb1, Rb2, Rc, and Rd [45]. Conversely, by the desensitization of ACh induced in oocytes expression, ginsenoside Rg2 reduced the peak current and elevated the inward currents in human nAChR subunits α3β4, α3β2, α4β4, and α4β2 [46]. Moreover, through nAChR channel activity, ginsenosides show the inhibitory effects on IACh reduction of catecholamine release. Regarding the heterologous expression of the α9α10 subunits of nAChR in Xenopus oocytes, IACh is blocked by ginsenosides with a potency order of Rg3 > Rb2 > CK > Re = Rg2 > Rf > Rc > Rb1 > Rg1 in a rescindable means. Ginsenoside Rg3's blocking effects on IACh were the same after preapplication linked to ginsenoside Rg3 co-application. Furthermore, α10 subunit of α9α10 nAChR might play an important role in Rg3-induced regulation of the α9α10 nAChR [47]. Besides, a recent study described the ameliorative effect of Rg1 on lipopolysaccharide (LPS)-induced cognitive deficits. Rg1 treatment inhibited LPS-induced reduction of ACh levels and an increase in acetylcholinesterase activity. LPS treatment reduced the α7 nAChR protein expression in the prefrontal cortex and hippocampus, but Rg1 treatment reverted the changes [48]. Furthermore, choline acetyltransferase (ChAT) and vesicular acetylcholine transporter (VAChT) are essential for cholinergic neurotransmission. Ginsenosides Rd and Re regulated both ChAT and VAChT. In Neuro-2a cells, the Rd and Re effectively induced the ChAT/VAChT genes expression and ACh promotion [49].
2.2.2. γ-amino butyric acid receptors
Ginsenosides interact with the γ-amino butyric acid (GABAA) receptor and might regulate the ligand binding with the GABAA receptor. In a rat brain membrane fraction, ginsenosides differentially regulate the binding of [3H]-flunitrazepam or [3H]-muscimol to the GABAA receptor [50]. Conversely, longer infusion of ginsenoside Rc raises [3H]-muscimol binding to the GABAA receptor in a region-specific way in the rat brain [51]. Another study showed that ginsenosides enhance the GABA-mediated channel activity and thus control the GABAA receptor channel activity [52]. Therefore, in studies using Xenopus oocytes, ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, and Rg2 affected the activity of GABAA receptor channel in human recombinant GABAA receptor expression. Ginsenoside Rc utmost potently improved the GABA-induced inward peak current (IGABA). A GABAA receptor antagonist (bicuculline) and a GABAA channel blocker (picrotoxin) blocked the ginsenoside Rc stimulatory effect on IGABA. Regarding the Cl− channel blockers, niflumic acid and, on the IGABA, 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid both attenuated the ginsenoside Rc effect. Therefore, by affecting the binding affinities of the GABAA receptor ligands, ginsenosides may regulate the GABAA receptor [52]. Besides, the regulatory effects of ginsenoside metabolites differ from those of ginsenosides. The human recombinant GABAA receptor (α1β1γ2s) channel activity expressed in Xenopus oocytes, M4, a metabolite of PPT ginsenosides, more potently inhibited the IGABA than PPD. The effect of M4 and PPD on IGABA was both concentration-dependent and reversible. The half-inhibitory concentration (IC50) values of M4 and PPD were 17.1 ± 2.2 and 23.1 ± 8.6 μM, respectively. The inhibition of IGABA by M4 and PPD was voltage-independent and noncompetitive [53]. Using a conventional whole-cell patch-clamp technique in acutely isolated rat hippocampal CA3 pyramidal neurons, compound K produced a potential effect on GABAergic spontaneous miniature inhibitory postsynaptic currents. Compound K increases spontaneous GABA release by increasing intraterminal Ca2+ concentration through Ca2+ release from presynaptic Ca2+ stores [54]. A recent report indicated that GABAA receptor agonist pretreatment significantly potentiated the Panax quinquefolius neuroprotective effect. Regarding the sleep deprivation, GABAergic mechanism induced anxiety-like behavior, mitochondrial dysfunction, oxidative stress, hypothalamic–pituitary–adrenal axis activation and neuroinflammation that could be involved in the P. quinquefolius neuroprotective outcome [55].
2.2.3. Glutamate receptors channel activity
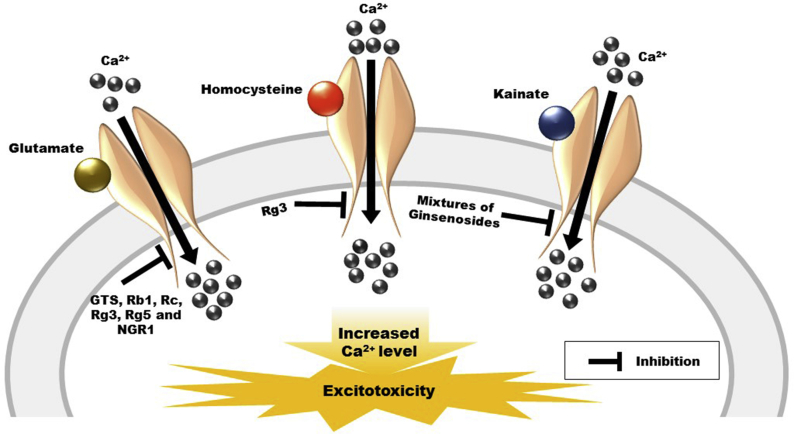
In different neurotoxic agents–induced models, active ginseng compounds produced effects that confirmed the potential effects of ginseng active compounds on glutamate receptors. In rat hippocampal cultures, Rg3 reduced the high K+-, glutamate-, and N-methyl-D-aspartate (NMDA)-induced Ca2+ influx [56]. Ginseng total saponins decreased glutamate-induced cultured rat astrocytes swelling [57]. Ginsenosides Rb1 and Rg3 produced a protective effect against glutamate-induced neurotoxicity. In this study, Rb1 and Rg3 prevent the nitric oxide (NO) overproduction, malondialdehyde formation. The treatments also preserved the superoxidase dismutase level and diminished the Ca2+ influx in rat cortical cultures [58]. In the Huntington's disease model, the neuroprotective effects of ginsenosides Rb1, Rc, and Rg5 might be due to the inhibition of glutamate-induced Ca2+ responses in cultured medium spiny striatal neuronal culture [59]. In cultured mouse cortical neurons, notoginsenoside R1 (NGR1) also prevented glutamate-mediated neurotoxicity [60]. Pretreatment with ginsenosides attenuated NMDA- or substance P-induced nociceptive behavior through the intrathecal route [61], [62]. With respect to the ginsenosides, the pretreatment attenuates the kainate-induced cellular death in hippocampal neurons [63]. Ginsenoside Rb3 could exert a neuroprotective role on hippocampal neurons, a role which was partly mediated by the facilitating Ca2+-dependent deactivation of NMDA receptors and the resulting reduction of intracellular free Ca2+ level [64]. Ginsenosides Rh2 and Rg3 prevent the NMDA receptor channel currents in cultured rat hippocampal neurons [65]. Moreover, in in vitro and in vivo studies of homocysteine (HC)-induced hippocampal excitotoxicity, ginsenoside Rg3 produced neuroprotective activity. Furthermore, in vivo experiments showed that intracerebroventricular Rg3 preadministration significantly and dose-dependently decreased the HC-induced hippocampal damage in rats. Treatment with Rg3 has been found to dose-dependently inhibit the HC-induced elevation of intracellular Ca2+ levels. In addition, in Xenopus oocytes expressing the NMDA receptor, Rg3 treatment dose-dependently repressed HC-induced currents [66]. Regarding the effects of ginsenoside metabolites on NMDA receptor channel activity, PPT contrasting compound K and PPD were reversibly repressed the NMDA-mediated inward currents (INMDA) in a dose-dependent manner. INMDA inhibition by PPT was noncompetitive with NMDA and was self-regulating the membrane holding potential, although ginsenoside Rh2, Rg3, and PPT interrelate with the NMDA receptor [67]. Ginsenoside Rb1 produced the anti-fatigue effect through the inflammatory cytokine-mediated NMDA receptor pathway [68]. The protective effects of active ginseng compounds on toxins-induced excitotoxicity through the glutamate receptors are shown in Fig. 2.
Fig. 2.
Potential activities of active ginseng compounds in toxin-induced neurotoxicity models. GTS, ginseng total saponins; NGR1, notoginsenoside R1. Glutamate, homocysteine and kainate cause an increase in intracellular calcium level leading to excitotoxicity. Active ginseng compounds produce protective activities against toxins-induced excitotoxicity.
3. Various specific molecular signaling and their modulating effect by ginseng and its active compounds
3.1. Toll-like receptor involving pathways
In an Alzheimer's disease (AD) cellular model, ginsenoside Rg1 produced antineuroinflammatory activity through a toll-like receptor (TLR) pathway. In NG108-15 cells, Aβ25–35 markedly increased the expressions of TLR3 and TLR4. In a concentration-dependent manner, Rg1 significantly reduced the expressions of both proteins as well as mRNA of TLR3 and TLR4 [69].
3.1.1. Mitogen-activated protein kinase pathway
Red ginseng marc oil (RMO) reduced the p38 mitogen-activated protein kinase (MAPK) and its upstream kinases including MAPK kinases 3/6 (MKK3/6) phosphorylation. By blocking the p38 MAPK pathway, RMO produced an antiinflammatory effect in LPS-induced RAW 264.7 macrophages [70]. Ginseng pectin pretreatment enhanced the phosphorylation of both the extracellular signal-regulated kinases 1 and 2 (ERK1/2) in cortical neuron cells and in U87 cells, it also increased the ERK1/2 phosphorylation. Owing to the activation of the phosphorylation of ERK1/2, ginseng pectin produced this neuroprotective activity against H2O2-induced apoptosis [71]. In LPS-induced RAW 264.7 cells, synthesized gold nanoparticles (AuNPs) using Panax ginseng exert antiinflammatory effects through p38 MAPK [72]. Likewise, gintonin produced antiinflammatory activity via the signal transduction through MAPK, and it potently suppressed the NO production and also efficiently suppressed the proinflammatory cytokines levels in RAW 264.7 cells [73].
Different doses of ginsenoside Rb1 pretreatment noticeably attenuated tau protein hyperphosphorylation and the expression of c-Jun N-terminal kinase (JNK)/p38 MAPK in Aβ25-35-induced model [74]. In 1-methyl-4-phenylpyridinium (MPP+)-treated PC12 cells, Rb1 improved ERK1/2 phosphorylation and reduced phosphorylated p38 or stress activated protein kinase/JNK. Rb1 increased ERK1/2 phosphorylation, and it was abrogated by estrogen receptor siRNA [75]. By upregulating the growth-associated protein 43 (GAP-43) expression through ERK-dependent signaling pathways, ginsenoside Rd may help PC12 cells neurite outgrowth [76]. In addition, Rd efficiently inhibited the activation of the MAPK signaling pathway induced by spinal cord injury in the rat model, which might be involved in the neuroprotective activity of Rd against spinal cord injury [77].
In various studies, ginsenosides Rg1 and Rg5 have shown potential effects through this pathway. To promote cell proliferation, ginseng and Rg1 increase the MAPK signaling pathways. In RSC96 cells, ginseng and Rg1 were recognized to have proliferative effects that are MAPK signaling-dependent [78]. In addition, Rg1 produced antiinflammatory activity in BV-2 microglial cells. In this study, phosphoinositide phospholipase C-γ1 inhibition was moderately abolished, and Rg1 produced an inhibitory effect on ERK1/2, JNK, and p38 MAPK phosphorylation. Therefore, by suppressing the neurotoxic proinflammatory mediators and cytokines expression, Rg1 expressively attenuates the overactivation of microglial cells through phospholipase C-γ1 signaling pathway activation [79]. Besides, Rg1 improves the neurite outgrowth and defends against Aβ25–35-induced damage, and this mechanism may be involved in the activation of ERK1/2 signaling [80]. Additionally, Rg1 activated the ERK/MAPK pathway in another study [81]. Ginsenoside Rg5 inhibited the phosphorylation of MAPKs and the DNA binding activities in LPS-stimulated BV-2 microglial cells and primary rat microglia [82].
NGR1 and compound K show potential activity through the modulation of this pathway. NGR1 produces a sufficient effect by inducing an estrogen receptor–dependent ERK1/2 pathway [83]. Compound K significantly suppressed the Phorbol-12-myristate-13-acetate-mediated p38 MAPK, ERK, and JNK activation, which are upstream modulators of activator protein-1. In addition, compound K also inhibited the invasiveness of in vitro glioma cells [84].
3.1.2. Nuclear factor kappa-light-chain-enhancer of activated B cells pathway
RMO and AuNPs obtained from the leaf extract of P. ginseng and gintonin produced antiinflammatory activities in the LPS-induced RAW 264.7 macrophage cell line [70], [72], [73]. RMO treatment reduced the inducible nitric oxide species and cyclooxygenase-2 at both the mRNA and protein levels, with a blockade of the nuclear translocation of the p65 subunit. Hence, due to the inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) transcriptional activity, RMO might produce this antiinflammatory effect [70]. Moreover, AuNPs decreased inflammatory mediators, including NO, prostaglandin E2, interleukin-6, and tumor necrosis factor-α, expression. In RAW 264.7 cells, AuNPs suppressed the LPS-induced activation of NF-κB signaling [72]. At the given doses, gintonin effectively suppressed the NO production without any cytotoxicity and also proficiently suppressed the proinflammatory cytokines levels. Furthermore, it facilitates signal transduction through NF-κB pathways and recovers the mir-34a and mir-93 levels [73].
In the AD model, P. ginseng ginsenosides Rg1, Rg3, Rd, and Rg3 enriched the extract, producing potential activities [85], [86], [87], [88]. In an advanced glycation end product–induced AD model, ginseng showed the neuroprotective effects through the significant decrease in the expression of the receptor for advanced glycation end-products and NF-κB in a rat [86]. In transgenic mice, Rd might improve learning and memory ability in used Aβ precursor by inhibiting the transcription activity of NF-κB. Moreover, by suppressing the activated NF-κB pathway, the proinflammatory cytokines were further reduced, and the protective factors were generated [87]. In a scopolamine-induced model, the oral administration of ginsenoside Rg3-enriched ginseng extract (Rg3GE) suppressed the increase in acetylcholinesterase activity and stimulation of the NF-κB pathway (i.e., phosphorylation of p65) in the hippocampus [88]. In the LPS-induced BV-2 microglia cell line, ginsenosides Re and Rh1 produced antiinflammatory activity [89], [90]. Re produced neuroprotective events through phospho-p38, iNOS, and COX-2 signaling pathways [89]. Without affecting NF-κB DNA binding, ginsenoside Rh1 inhibited LPS-induced NF-κB–mediated transcription. In addition, an increase in cAMP responsive element-binding protein was identified to result in the suppression of NF-κB–mediated transcription [90]. Moreover, in a study of oxidative stress, ginsenoside Rg1 normalized the oxidative stress–induced nonmuscle myosin heavy chain IIA (NMMCH IIA) overexpression in PC12 cells. The collected data showed that the NMMCH IIA-NF-kappa B/p65 pathway was involved in oxidative stress–induced cell death [91]. The effects of active ginseng compounds through the MAPK and NF-κB pathways are summarized in Table 1.
Table 1.
Potential effects of ginseng and its active compounds through the MAPK and NF-κB pathways.
| Ginseng/active compounds | Models | Major effects | References |
|---|---|---|---|
| Rb1 | Aβ25-35-induced embryo rat cortical neurons | Different doses attenuate tau protein hyperphosphorylation and the expression of JNK/p38 MAPK in the process. | [74] |
| MPP+-treated PC12 cells | Improves ERK1/2 phosphorylation levels and reduced phosphorylated p38 or SAPK/JNK. | [75] | |
| Rd | PC12 cells | Helps the neurite outgrowth through upregulating the growth associated protein 43 expression through ERK-dependent signaling pathways. | [76] |
| Spinal cord injury in rat | Produces neuroprotective activity by efficiently inhibiting the activation of the MAPK signaling pathway. | [77] | |
| APP transgenic mice | By inhibiting the transcription activity of NF-κB, might improve learning and memory when APP is used. Moreover, by suppressing the activated NF-κB pathway, further reduces the pro-inflammatory cytokines and generates protective factors. | [87] | |
| Rg1 | Aβ25–35-induced NG108-15 cells | Reduces the increased expressions of both TLR3 and TLR4. | [69] |
| RSC96 cells | Produces the proliferative effects through the MAPK signaling–dependent pathway. | [78] | |
| BV-2 microglial cells | Attenuates the overactivation of phosphoinositide phospholipase C-γ1 and produces the inhibitory effect on the ERK1/2, JNK and p38 MAPK phosphorylation. | [79] | |
| Aβ25–35-induced cultured hippocampal neurons | Improves neurite outgrowth and defends against damage, and the mechanism may comprise the activation of ERK1/2 signaling. | [80] | |
| PC12 cells | By CaMKIIα, it activates the ERK/MAPK pathway. | [81] | |
| H2O2-induced PC12 cells | Normalizes the oxidative stress-induced NMMCH IIA overexpression. Regarding the collected data, NMMHC IIA-NF-kappa B/p65 pathway involved in oxidative stress-induced cell death. | [91] | |
| Rg3GE | Scopolamine-induced mice | Suppresses the increase in acetylcholinesterase activity and stimulation of the NF-κB pathway (i.e., phosphorylation of p65) in the hippocampus. | [88] |
| Rg5 | LPS-stimulated BV-2 microglial cells and rat primary microglia | Inhibits the phosphorylation of MAPKs and the DNA binding activities. | [82] |
| Rh1 | LPS-induced microglia | Without affecting NF-κB DNA binding, it inhibits NF-κB–mediated transcription. In addition, due to the NF-κB–mediated transcription, an increase of cAMP responsive element-binding protein might have been identified. | [90] |
| Re | LPS-induced BV-2 microglia | Produces the neuroprotective events through the phospho-p38, iNOS and COX-2 signaling pathways. | [89] |
| RMO | LPS-induced RAW 264.7 macrophages | Reduces the p38 MAPK and its upstream kinases including MAPK kinases 3/6 (MKK3/6) phosphorylation. | [70] |
| LPS-induced RAW 264.7 macrophages | Reduces the iNOS and COX-2 at both mRNA and protein levels, blockade of nuclear translocation of the p65 subunit. Henceforth, due to the inhibition of NF-κB transcriptional activity, it might have produced this anti-inflammatory effect. | [70] | |
| Ginseng Pectin | H2O2-induced apoptosis in cortical neuron cells and U87 cells | Pretreatment enhances the phosphorylation of both the extracellular signal-regulated kinases 1 and 2 (ERK1/2). | [71] |
| AuNPs | LPS-induced RAW 264.7 cells | Exerts anti-inflammatory effects through the suppression of NF-κB signaling pathway activation through p38 MAPK. | [72] |
| LPS-induced RAW 264.7 cells | Decreases inflammatory mediators such as NO, prostaglandin E2, interleukin-6 and tumor necrosis factor-α, expression. | [72] | |
| Gintonin | LPS-induced RAW 264.7 cells | Produces anti-inflammatory activity via the signal transduction through MAPK, and potently suppresses the nitric oxide production and also efficiently suppressed the proinflammatory cytokines levels. | [73] |
| LPS-induced RAW 264.7 | Effectively suppresses the NO production without any cytotoxicity and also proficiently suppresses the proinflammatory cytokines levels. Furthermore, facilitates signal transduction through NF-κB pathways and recovers the mir-34a and mir-93 levels. | [73] | |
| NGR1 | H2O2-induced PC12 cells | Produces neuroprotective activity the effect by inducing an estrogen receptor-dependent ERK1/2 pathway. | [83] |
| Compound K | Phorbol myristate acetate–mediated human astroglioma cells | Significantly suppresses the p38 MAPK, ERK, and JNK activation, which are upstream modulators of activator protein-1. | [84] |
| Ginseng | Advanced glycation end product– induced AD in rat | Shows neuroprotective effects through the significant decreasing the expression of receptor for advanced glycation end-products and NF-κB. | [86] |
AD, Alzheimer's disease; APP, amyloid β-protein precursor; AuNPs, synthesized gold nanoparticles; CaMKIIα, calcium/calmodulin-dependent protein kinase type II alpha chain; JNK, c-Jun N-terminal kinase; LPS, lypopolysaccharide; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated; NGR1: notoginsenoside R1; NMMCH IIA, nonmuscle myosin heavy chain IIA; NO, nitric oxide; Rg3GE, Rg3-enriched ginseng extract; RMO, Red ginseng marc oil; SAPK, stress activated protein kinase; TLR, toll-like receptor.
3.2. Caspase-3 and Bcl-2-like protein 4-mediated pathways
Ginsenosides showed potential activity through these pathways. NGR1 produced neuroprotective activity by suppressing caspase-3 activation [83]. In PC12 cells, ginsenoside Rb1 prevented MPP+-induced caspase-3 activation and DNA fragmentation. Besides, Rb1 also activated B-cell lymphoma-extra-large (Bcl-xL) and reduced apoptosis [75]. Ginsenoside Rg1 inhibited the caspase-3 signaling pathway and myosin IIA-actin interaction, and through these inhibitions, it produced neuroprotective activity [92]. In an MPP+-induced apoptosis in human neuroblastoma (SH-SY5Y) cells model, Rg1 can effectively decrease the expression of MPP+-induced upregulation of Bax and decrease the B-cell lymphoma 2 (Bcl-2) expressions. In addition, Rg1 can effectively decrease the MPP+-induced toxicity by inhibiting the activation of caspase-3 [93]. A recent study revealed that ginsenoside Rd prevented trimethyltin-induced cell apoptosis via regulation of caspase-3, Bcl-2, and Bcl-2–like protein 4 [94]. With respect to the ginsenoside Rb1, it suppressed the activation of ER stress-associated proteins including protein kinase RNA–like endoplasmic reticulum kinase and C/EBP homology protein and high glucose induced Bcl-2 downregulation in hippocampal neurons [95].
3.3. Phosphoinositide 3-kinase/protein kinase B pathway
Ginseng protein produced neuroprotective activity in D-galactose/AlCl3-induced AD model, and protective effect is connected to the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) signaling pathway [96]. Ginseng protein decreased the Aβ1-42 content and phosphor-tau and enhanced the mRNA and PI3K and phospho-Akt/Akt protein expression in the hippocampus [96]. Ginsenoside Rd promotes the glutamate clearance, which was achieved by upregulating the glutamate transporter 1 expression via the PI3K/Akt pathway [97]. Neurite outgrowth is a crucial process associated with neuronal repair. In addition, Rd produced an effect on neurite outgrowth, and the upregulation of GAP-43 expressions through PI3K/Akt-dependent pathways might be responsible for this activity [76]. Furthermore, an experimental Parkinson's disease model induced by MPP+ in SH-SY5Y showed that different concentrations of Rd had a neuroprotective effect. This protective effect might be due to the PI3K/Akt survival-signaling pathway [98].
Various studies have shown that ginsenosides Rg1 and Rg5 show beneficial effects through this pathway. Ginsenoside Rg1 enhances neurite outgrowth and protects against Aβ25–35-induced damage, and its mechanism may involve the activation of Akt signaling [71]. In LPS-stimulated BV-2 microglial cells and rat primary microglia, Rg5 produced an antiinflammatory activity. The studies indicated that Rg5 inhibited the phosphorylation of PI3K which are upstream molecules controlling the inflammatory reactions [82]. Moreover, pseudoginsenoside-F11 produced antineuroinflammatory activity on activated microglia. Pseudoginsenoside-F11 inhibited neuroinflammation LPS-induced in N9 microglia by inhibiting the activation of TLR4 mediated PI3K/Akt pathways [99]. NGR1 produced a neuroprotective activity by suppressing the H2O2-induced intracellular reactive oxygen species accumulation and increasing the product of lipid peroxidation (malondialdehyde), protein oxidation (protein carbonyl), DNA fragmentation (8-OHdG), mitochondrial membrane depolarization as well as caspase-3 activation. This neuroprotective activity is occurred by inducing estrogen receptor-dependent crosstalk between Akt pathways [83].
3.4. Insulin-like growth factor-I receptor signaling
A study of MPP+-induced apoptosis model in PC12 cells showed that ginsenoside Rb1 had neuroprotective effects. In caspase-3-dependent apoptosis pathway, Rb1 protects the PC12 cells through the estrogen receptor [75]. In a study on RSC96 Schwann cells, ginseng and ginsenoside Rg1 exhibited proliferation and migration-enhancing properties. Ginseng and ginsenoside Rg1 improve the insulin-like growth factor-I receptor (IGF-IR) pathway regulators' protein expression. Moreover, Rg1 with biomedical materials would be a possible method to improve neuron regeneration [78]. Ginsenoside Rg1 produced neuroprotective effects against 6-hydroxydopamine (6-OHDA)–induced neurotoxicity [100], [101]. In the 6-OHDA–induced model of nigrostriatal injury, Rg1 showed a neuroprotective activity, and this effect might involve the activation of the IGF-IR signaling pathway. Rg1 treatment ameliorated this behavior in apomorphine-induced rotational behavior. In addition, 6-OHDA significantly reduced the striatum dopamine content, and Rg1 reversed the significant effects of 6-OHDA [101]. Besides, in 6-OHDA–induced neuronal damages in SK-N-SH cells, Rg1 produced neuroprotective effects through the activation of the IGF-IR, ER signaling pathways, and its antiapoptotic potentials [100].
3.5. Nuclear factor (erythroid-derived 2)-like-2 factor pathway
P. ginseng extract produced antidepressant activity in a chronic restraint stress -induced depression model in mice. In this study, P. ginseng extract showed anti-neuroinflammatory and antioxidant, nuclear factor (erythroid-derived 2)-like-2 factor/heme oxygenase-1 (Nrf2/HO-1 activation) activity by inhibiting the hypothalamo–pituitary–adrenal axis mechanism [102]. Ginsenoside Rb1 activates the Nrf2/HO-1 signaling pathway to display a potent neuroprotective activity against tert-butylhydroperoxide–induced oxidative injury. In cultured neural progenitor cells, Rb1 activates the Nrf2 pathway and led to an elevated HO-1 expression [103], [104]. In iron-induced neurotoxicity, the antioxidative properties that trigger the Akt/Nrf2 pathway and increase the Nrf2-induced HO-1 and Cu/Zn superoxidase dismutase expression allowed ginsenoside Rg1 protect the human neuroblastoma SK-N-SH cells [105]. Similarly, ginsenoside Rh3 improves the Nrf2 DNA-binding activity [106]. PPT ginsenosides improve the Nrf2 inflowing to the nucleus and induced antioxidant response elements (ARE) such as HO-1 and the expression of nicotinamide adenine dinucleotide phosphate (NADPH) quinone oxidase 1. Subsequently, PPT shows a neuroprotective activity against 3-nitropropionic acid–induced injury (oxidative stress) in the rat model of Huntington's disease [107]. Additionally, NGR1 improves Nrf2 nuclear translocation and ARE activity. It also upregulates the expression and effects of HO-1, NADPH quinone oxidase 1, and gamma-glutamylcysteine synthetase. NGR1 provides neuroprotective activity by inducing the estrogen receptor-dependent activating Nrf2/ARE signaling [83].
3.6. Nerve growth factor and brain-derived neurotrophic factor pathways
Various research studies have shown that ginseng extracts, as well as its active compounds, exert potential activity through these pathways. In Neuro-2a cells, ginsenosides Rd and Re treatments meaningfully improved the microtubule-associated protein-2, nerve growth factor receptor (p75), p21, and tropomyosin receptor kinase A genes and proteins expression. Therefore, Re and Rd play a significant role in differentiation of neuron and the nerve growth factor–tropomyosin receptor kinase A signaling pathway [49].
In the rat model, P. ginseng showed neuroprotective activity against LPS-induced brain injury. LPS causes elevated brain NO and serum HC associated with a reduction of brain-derived neurotrophic factor (BDNF) level. On the other hand, in the treated group, P. ginseng significantly attenuated these compared to the LPS group [108]. A study showed that P. notoginseng saponins promote the rat embryonic cortical neural stem cells survival, self-renewal, proliferation, and differentiation through neurotrophic factors in the autocrine or paracrine signaling [109].
Ginsenoside Rg1 also improved the memory performance [110], [111]. In the AD model, Rg1 treatment reduced the Aβ1-42 accumulations and phosphorylated (p)-Tau protein. Rg1 treatment also upregulated the BDNF and phosphorylated tropomyosin receptor kinase B. Similarly, Rg1 treatment also restored the long-term potentiation in the AD mice model. In another study, through the hippocampal BDNF upregulation, Rg1 treatment improved the memory performance in middle-aged mice, changing apical spines and helping hippocampal long-term potentiation [111].
Ginsenoside Rb1 revealed a neuroprotective activity against cerebral ischemia. After Rb1 infusion, the number of nestin-positive cells apparently increased. At different points in time, Rb1-treated rats showed a BDNF level that significantly improved compared to that of ischemic rats. These neuroprotective effects might be because of the promotion of the neurogenesis and expression of BDNF regulation [112]. In addition, Rb1 showed the preventive and therapeutic effects on the neural injury during cerebral infarction in rat's model via middle cerebral artery occlusion. The increases in occlusion duration resulted in a decline in interleukin-1 levels and GAP-43 level and an increase in BDNF levels and neurofilaments. These effects might be due to a decrease in inflammation and assistance in the growth of nerve cells [113].
Ginsenosides Rb3, Rg1, and Rg3 produced antidepressant activity through the BDNF signaling pathway [114], [115], [116], [117]. In a study of the chronic mild stress model, treatment with Rb3 significantly increased the BDNF level in the prefrontal cortex and hippocampus area [114]. Rg1 produced antidepressant activity via BDNF signaling pathway. It upregulates the BDNF expression and hippocampal neurogenesis [115], [116]. Recently, Rg1 treatment prevented chronic social defeat stress–induced depressive-like symptoms in a depression-induced mouse model [116]. Furthermore, Rg3 completely restored the chronic social defeat stress–induced reduction in the hippocampal BDNF signaling pathway [117].
3.7. Mechanistic target of rapamycin signaling, Wnt/β-catein and Rho-associated kinase 1/Myosin light-chain kinase pathways
Compound K is produced potential activity through the mechanistic target of rapamycin pathway. In a study of AD model, compound K promotes Aβ clearance and enhances autophagy in primary astrocytes. It also slows AD pathological progression. In addition, due to the mechanistic target of rapamycin phosphorylation, it might contribute to an enhancement in the autophagy [118]. In in vivo and in vitro Parkinson's disease models, ginsenoside Rg1 showed neuroprotective potentials through the Wnt/β-catenin signaling pathway. In both the in vivo and in vitro studies, Rg1 showed protective effects on the protein and mRNA expression levels of this pathway marker. With respect to the in vitro study, the neuroprotective potentials were blocked by Dickkopf-related protein 1 [119]. In a H2O2-induced model study, treatment with Rg1 eliminated H2O2-induced different morphological fluctuations such as cell rounding, membrane blebbing, neurite retraction, and nuclei condensation. The mechanism of neuroprotection of Rg1 is connected to the inhibition of myosin IIA–actin interaction and the signaling pathway of Rho-associated kinase 1/Myosin light-chain kinase [92].
4. Conclusion
Recently, medicinal plants have shown potent pharmacological roles in various disorders that have been proven in several preclinical and clinical studies. Identifying the proper therapeutic targets is essential to setting up treatment strategies for various neurological disorders. In case of NDDs, no cure has yet been discovered, and this is a major topic of research today. In addition, identifying therapeutic targets and therapeutic molecules are crucial steps for the designing of therapeutic management for NDDs. Considering the importance of having targets, several signals have demonstrated a potential pharmacological role in various neurological disorders. Detecting these targets, allows the possibility to discover novel compounds suitable to treat the NDDs. Ginseng and its active substances have been known as multipotential therapeutics against various acute and chronic diseases. With respect to their multipotential role, researchers have concentrated their research. Various reports have indicated that they may be great therapeutic agents for neurological disorders due to their potential activities that modulate the various molecular signaling pathways.
However, research has not concluded how ginseng active compounds can have specific medicinal effects on NDDs. We focused on NDDs and here present the ion channels and specific molecular signals that are modulated by ginseng and its active substances. The overview shows that ginseng components might emerge as candidates for NDDs due to their versatile potential, specifically, their neuroprotective activities against neuroinflammation and apoptotic cell death. It might be helpful to conduct deeper research and clinical trials regarding NDDs. Also, based on signal modulating potential, molecular modification of ginseng compounds may be useful in obtaining superior pharmaceutical drugs. In addition, understanding of the complex response to ginseng could allow developing synergistic drug therapy.
To examine and validate the potential, several disease models were designed including toxin-induced cellular and animal models as well as transgenic models to conduct for different NDDs. Further studies concerning specific ion channels and molecular signals known in the pathogenesis and as therapeutic targets for NDDs are suggested to confirm the exact pharmacological and therapeutic activities of ginseng and its active compounds. The design and execution of clinical studies for active ginseng compounds to treat NDDs is a major challenge for the clinical scientist. Before clinical studies, more investigations using laboratory-based and informatics-based approaches should be conducted to understand the precise medicinal efficacy, potency, and safety in the NDDs. These approaches might give direction to modify and synthesize new derivatives of active ginseng compounds targeting the NDDs. Finally, the diverse potential role of ginseng active compounds might serve a good role in treatment strategies to cure NDDs. In near future, rigorous studies of active ginseng compounds with their modified forms will produce drugs for therapy of NDDs.
Conflicts of interest
The authors have no conflicts of interest.
Acknowledgment
This research was supported by The Leading Human Resource Training Program of Regional Neo industry through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (grant number: NRF-2016H1D5A1909610).
References
- 1.Sheikh S., Haque E., Mir S.S. Neurodegenerative diseases: multifactorial conformational diseases and their therapeutic interventions. J Neurodegener Dis. 2013;2013:8. doi: 10.1155/2013/563481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Błaszczyk J.W. Parkinson's disease and neurodegeneration: GABA-collapse hypothesis. Front Neurosci. 2016;10 doi: 10.3389/fnins.2016.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrell R.W., Lomas D.A. Conformational disease. Lancet. 1997;350(9071):134–138. doi: 10.1016/S0140-6736(97)02073-4. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs G.G. Molecular pathological classification of neurodegenerative diseases: turning towards precision medicine. Int J Mol Sci. 2016;17(2):189. doi: 10.3390/ijms17020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X., Pan W. The treatment strategies for neurodegenerative diseases by integrative medicine. Integr Med Int. 2014;1(4):223–225. [Google Scholar]
- 6.Xiang Y.Z., Shang H.C., Gao X.M., Zhang B.L. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother Res. 2008;22(7):851–858. doi: 10.1002/ptr.2384. [DOI] [PubMed] [Google Scholar]
- 7.Hwang I.H., Kwon Y.K., Cho C.K., Lee Y.W., Sung J.S., Joo J.C., Lee K.B., Yoo H.S., Jang I.S. Modified Panax ginseng extract inhibits uPAR-mediated α 5 β1-integrin signaling by modulating caveolin-1 to induce early apoptosis in lung cancer cells. Am J Chin Med. 2016;44(05):1081–1097. doi: 10.1142/S0192415X16500609. [DOI] [PubMed] [Google Scholar]
- 8.Leung K.W., Wong A.S. Ginseng and male reproductive function. Spermatogenesis. 2013;3(3):e26391. doi: 10.4161/spmg.26391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nocerino E., Amato M., Izzo A.A. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia. 2000;71:S1–S5. doi: 10.1016/s0367-326x(00)00170-2. [DOI] [PubMed] [Google Scholar]
- 10.Lee J.S., Hwang H.S., Ko E.J., Lee Y.N., Kwon Y.M., Kim M.C., Kang S.M. Immunomodulatory activity of red ginseng against influenza A virus infection. Nutrients. 2014;6(2):517–529. doi: 10.3390/nu6020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong M., Lee Y.H., Kim S., Suk K.T., Bang C.S., Yoon J.H., Baik G.H., Kim D.J., Kim M.J. Anti-inflammatory and antifatigue effect of Korean Red Ginseng in patients with nonalcoholic fatty liver disease. J Ginseng Res. 2016;40(3):203–210. doi: 10.1016/j.jgr.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu T., Rhee M.H., Lee J., Kim S.H., Yang Y., Kim H.G., Kim Y., Kim C., Kwak Y.-S., Kim J.-H. Ginsenoside Rc from Korean Red Ginseng (Panax ginseng CA Meyer) attenuates inflammatory symptoms of gastritis, hepatitis and arthritis. Am J Chin Med. 2016;44(03):595–615. doi: 10.1142/S0192415X16500336. [DOI] [PubMed] [Google Scholar]
- 13.Kim M.H., Lee E.J., Cheon J.M., Nam K.J., Oh T.H., Kim K.S. Antioxidant and hepatoprotective effects of fermented red ginseng against high fat diet-induced hyperlipidemia in rats. Lab Anim Res. 2016;32(4):217–223. doi: 10.5625/lar.2016.32.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H.J., Lee S.G., Chae I.G., Kim M.J., Im N.K., Yu M.H., Lee E.J., Lee I.S. Antioxidant effects of fermented red ginseng extracts in streptozotocin-induced diabetic rats. J Ginseng Res. 2011;35(2):129–137. doi: 10.5142/jgr.2011.35.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang E., Park S.Y., Yin C.S., Kim H.T., Kim Y.M., Yi T.H. Antiaging effects of the mixture of Panax ginseng and Crataegus pinnatifida in human dermal fibroblasts and healthy human skin. J Ginseng Res. 2017;41(1):69–77. doi: 10.1016/j.jgr.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X.J., Zhang X.J., Shui Y.M., Wan J.B., Gao J.L. Anticancer activities of protopanaxadiol-and protopanaxatriol-type ginsenosides and their metabolites. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/5738694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Im K., Kim J., Min H. Ginseng, the natural effectual antiviral: protective effects of Korean Red Ginseng against viral infection. J Ginseng Res. 2016;40(4):309–314. doi: 10.1016/j.jgr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uzkeser M., Karakus E., Albayrak A., Kiki İ., Bayir Y., Cadirci E., Unal D., Halici Z., Karadeniz A. Protective effect of Panax ginseng against N-acetyl-p-aminophenol-induced hepatotoxicity in rats. African J Pharmacy and Pharmacol. 2012;6(36):2634–2642. [Google Scholar]
- 19.Lim K.H., Ko D., Kim J.H. Cardioprotective potential of Korean Red Ginseng extract on isoproterenol-induced cardiac injury in rats. J Ginseng Res. 2013;37(3):273–282. doi: 10.5142/jgr.2013.37.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee L.S., Cho C.W., Hong H.D., Lee Y.C., Choi U.K., Kim Y.C. Hypolipidemic and antioxidant properties of phenolic compound-rich extracts from white ginseng (Panax ginseng) in cholesterol-fed rabbits. Molecules. 2013;18(10):12548–12560. doi: 10.3390/molecules181012548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin H.S., Yu M., Kim M., Choi H.S., Kang D.H. Renoprotective effect of red ginseng in gentamicin-induced acute kidney injury. Lab Investig. 2014;94(10):1147–1160. doi: 10.1038/labinvest.2014.101. [DOI] [PubMed] [Google Scholar]
- 22.Kim E.H., Kim I.H., Ha J.A., Choi K.T., Pyo S., Rhee D.K. Antistress effect of red ginseng in brain cells is mediated by TACE repression via PADI4. J Ginseng Res. 2013;37(3):315–323. doi: 10.5142/jgr.2013.37.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y., Choi E.H., Doo M., Kim J.Y., Kim C.J., Kim C.T., Kim I.H. Anti-stress effects of ginseng via down-regulation of tyrosine hydroxylase (TH) and dopamine β-hydroxylase (DBH) gene expression in immobilization-stressed rats and PC12 cells. Nutr Res Prac. 2010;4(4):270–275. doi: 10.4162/nrp.2010.4.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng L., Liu X.M., Cao F.R., Wang L.S., Chen Y.X., Liao Y.H., Wang Q., Chang Q. Anti-stress effects of ginseng total saponins on hindlimb-unloaded rats assessed by a metabolomics study. J Ethnopharmacol. 2016;188:39–47. doi: 10.1016/j.jep.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 25.Dang H., Chen Y., Liu X., Wang Q., Wang L., Jia W., Wang Y. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1417–1424. doi: 10.1016/j.pnpbp.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 26.He D.F., Ren Y.P., Liu M.Y. Effects of ginseng fruit saponins on serotonin system in Sprague-Dawley rats with myocardial infarction, depression, and myocardial infarction complicated with depression. Chin Med J (Engl) 2016;129(24):2913. doi: 10.4103/0366-6999.195462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao B., Newmark H., Zhou R. Neuroprotective effects of ginseng total saponin and ginsenosides Rb1 and Rg1 on spinal cord neurons in vitro. Exp Neurol. 2002;173(2):224–234. doi: 10.1006/exnr.2001.7841. [DOI] [PubMed] [Google Scholar]
- 28.Van Kampen J., Robertson H., Hagg T., Drobitch R. Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson's disease. Exp Neurol. 2003;184(1):521–529. doi: 10.1016/j.expneurol.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Naval M., Gómez-Serranillos M., Carretero M., Villar A. Neuroprotective effect of a ginseng (Panax ginseng) root extract on astrocytes primary culture. J Ethnopharmacol. 2007;112(2):262–270. doi: 10.1016/j.jep.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Seo J.Y., Ju S.H., Oh J., Lee S.K., Kim J.S. Neuroprotective and cognition-enhancing effects of compound K isolated from red ginseng. J Agric Food Chem. 2016;64(14):2855–2864. doi: 10.1021/acs.jafc.5b05789. [DOI] [PubMed] [Google Scholar]
- 31.Christensen L.P. Ginsenosides: chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2008;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 32.Radad K., Moldzio R., Rausch W.D. Ginsenosides and their CNS targets. CNS Neu Ther. 2011;17(6):761–768. doi: 10.1111/j.1755-5949.2010.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rokot N.T., Kairupan T.S., Cheng K.C., Runtuwene J., Kapantow N.H., Amitani M., Morinaga A., Amitani H., Asakawa A., Inui A. A role of ginseng and its constituents in the treatment of central nervous system disorders. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/2614742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeo H.B., Yoon H.K., Lee H.J., Kang S.G., Jung K.Y., Kim L. Effects of Korean red ginseng on cognitive and motor function: a double-blind, randomized, placebo-controlled trial. J Ginseng Res. 2012;36(2):190–197. doi: 10.5142/jgr.2012.36.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J.H., Hong Y.H., Lee J.H., Kim D.H., Nam G., Jeong S.M., Lee B.H., Lee S.M., Nah S.Y. A role for the carbohydrate portion of ginsenoside Rg 3 in Na+ channel inhibition. Mol Cells. 2005;19(1):137–142. [PubMed] [Google Scholar]
- 36.Lee J.H., Jeong S.M., Kim J.H., Lee B.H., Yoon I.S., Lee J.H., Choi S.H., Kim D.H., Rhim H., Kim S.S. Characteristics of ginsenoside Rg3-mediated brain Na+ current inhibition. Mol Pharmacol. 2005;68(4):1114–1126. doi: 10.1124/mol.105.015115. [DOI] [PubMed] [Google Scholar]
- 37.Duan Y., Nicholson R.A. 20 (S)-protopanaxadiol and the ginsenoside Rh 2 inhibit Na+ channel-activated depolarization and Na+ channel-dependent amino acid neurotransmitter release in synaptic fractions isolated from mammalian brain. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147(3):351–356. doi: 10.1016/j.cbpc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Choi S.H., Shin T.J., Lee B.H., Hwang S.H., Lee S.M., Lee B.C., Park C.S., Ha T.S., Nah S.Y. Ginsenoside Rg3 enhances large conductance Ca2+-activated potassium channel currents: a role of Tyr360 residue. Mol Cells. 2011 Feb;31(2):133–140. doi: 10.1007/s10059-011-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi S., Jung S.Y., Ko Y.S., Koh S.R., Rhim H., Nah S.Y. Functional expression of a novel ginsenoside Rf binding protein from rat brain mRNA in Xenopus laevis oocytes. Mol Pharm. 2002;61(4):928–935. doi: 10.1124/mol.61.4.928. [DOI] [PubMed] [Google Scholar]
- 40.Kim S., Nah S.Y., Rhim H. Neuroprotective effects of ginseng saponins against L-type Ca2+ channel-mediated cell death in rat cortical neurons. Biochem Biophys Res Commun. 2008;365(3):399–405. doi: 10.1016/j.bbrc.2007.10.048. [DOI] [PubMed] [Google Scholar]
- 41.Choi S.H., Lee B.H., Hwang S.H., Kim H.J., Lee S.M., Kim H.C., Rhim H.W., Nah S.Y. Molecular mechanisms of large-conductance Ca2+-activated potassium channel activation by ginseng gintonin. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/323709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y.F., Fan X.J., Li X., Peng L.L., Wang G.H., Ke K.F., Jiang Z.L. Ginsenoside Rg 1 protects neurons from hypoxic–ischemic injury possibly by inhibiting Ca2+ influx through NMDA receptors and L-type voltage-dependent Ca2+ channels. Eur J Pharmacol. 2008;586(1):90–99. doi: 10.1016/j.ejphar.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 43.Quan Q.K., Li X., Yuan H.F., Wang Y., Liu W.L. Ginsenoside Rg1 inhibits high-voltage-activated calcium channel currents in hippocampal neurons of beta-amyloid peptide-exposed rat brain slices. Chinese J Integr Med. 2016:1–6. doi: 10.1007/s11655-015-2301-4. [DOI] [PubMed] [Google Scholar]
- 44.Lin Z.Y., Chen L.M., Zhang J., Pan X.D., Zhu Y.G., Ye Q.Y., Huang H.P., Chen X.C. Ginsenoside Rb1 selectively inhibits the activity of L-type voltage-gated calcium channels in cultured rat hippocampal neurons. Acta Pharmacol Sin. 2012;33(4):438–444. doi: 10.1038/aps.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi S., Jung S.Y., Lee J.H., Sala F., Criado M., Mulet J., Valor L.M., Sala S., Engel A.G., Nah S.Y. Effects of ginsenosides, active components of ginseng, on nicotinic acetylcholine receptors expressed in Xenopus oocytes. Eur J Pharmacol. 2002;442(1):37–45. doi: 10.1016/s0014-2999(02)01508-x. [DOI] [PubMed] [Google Scholar]
- 46.Sala F., Mulet J., Choi S., Jung S.Y., Nah S.Y., Rhim H., Valor L.M., Criado M., Sala S. Effects of ginsenoside Rg2 on human neuronal nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2002;301(3):1052–1059. doi: 10.1124/jpet.301.3.1052. [DOI] [PubMed] [Google Scholar]
- 47.Lee B.H., Choi S.H., Hwang S.H., Kim H.J., Lee S.M., Kim H.C., Rhim H., Nah S.Y. Effects of ginsenoside Rg3 on α9α10 nicotinic acetylcholine receptor-mediated ion currents. Biol Pharm Bull. 2013;36(5):812–818. doi: 10.1248/bpb.b12-01009. [DOI] [PubMed] [Google Scholar]
- 48.Jin Y., Peng J., Wang X., Zhang D., Wang T. Ameliorative effect of ginsenoside Rg1 on lipopolysaccharide-induced cognitive impairment: role of cholinergic system. Neurochem Res. 2017;42(5):1299–1307. doi: 10.1007/s11064-016-2171-y. [DOI] [PubMed] [Google Scholar]
- 49.Kim M.S., Yu J.M., Kim H.J., Kim H.B., Kim S.T., Jang S.K., Choi Y.W., Lee D.I., Joo S.S. Ginsenoside Re and Rd enhance the expression of cholinergic markers and neuronal differentiation in Neuro-2a cells. Biol Pharm Bull. 2014;37(5):826–833. doi: 10.1248/bpb.b14-00011. [DOI] [PubMed] [Google Scholar]
- 50.Kimura T., Saunders P., Kim H., Rheu H., Oh K., Ho I. Interactions of ginsenosides with ligand-bindings of GABAA and GABAB receptors. Gen Pharmacol. 1994;25(1):193–199. doi: 10.1016/0306-3623(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 51.Kim H.S., Hwang S.L., Nah S.Y., Oh S. Changes of [3H] MK-801,[3H] muscimol and [3H] flunitrazepam binding in rat brain by the prolonged ventricular infusion of ginsenoside Rc and Rg1. Pharmacol Res. 2001;43(5):473–479. doi: 10.1006/phrs.2001.0809. [DOI] [PubMed] [Google Scholar]
- 52.Choi S.E., Choi S., Lee J.H., Whiting P.J., Lee S.M., Nah S.Y. Effects of ginsenosides on GABA A receptor channels expressed in Xenopus oocytes. Arch Pharm Res. 2003;26(1):28–33. doi: 10.1007/BF03179927. [DOI] [PubMed] [Google Scholar]
- 53.Lee B.H., Choi S.H., Shin T.J., Hwang S.H., Kang J., Kim H.J., Kim B.J., Nah S.Y. Effects of ginsenoside metabolites on GABAA receptor-mediated ion currents. J Ginseng Res. 2012;36(1):55. doi: 10.5142/jgr.2012.36.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bae M.Y., Cho J.H., Choi I.S., Park H.M., Lee M.G., Kim D.H., Jang I.S. Compound K, a metabolite of ginsenosides, facilitates spontaneous GABA release onto CA3 pyramidal neurons. J Neurochem. 2010;114(4):1085–1096. doi: 10.1111/j.1471-4159.2010.06833.x. [DOI] [PubMed] [Google Scholar]
- 55.Chanana P., Kumar A. GABA-BZD receptor modulating mechanism of panax quinquefolius against 72-h sleep deprivation induced anxiety like behavior: possible roles of oxidative stress, mitochondrial dysfunction and neuroinflammation. Front Neurosci. 2016;10 doi: 10.3389/fnins.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim S., Ahn K., Oh T.H., Nah S.Y., Rhim H. Inhibitory effect of ginsenosides on NMDA receptor-mediated signals in rat hippocampal neurons. Biochem Biophys Res Commun. 2002;296(2):247–254. doi: 10.1016/s0006-291x(02)00870-7. [DOI] [PubMed] [Google Scholar]
- 57.Seong Y., ChangSik S., HackSeang K. Inhibitory effect of ginseng total saponins on glutamate-induced swelling of cultured astrocytes. Biol Pharm Bull. 1995;18(12):1776–1778. doi: 10.1248/bpb.18.1776. [DOI] [PubMed] [Google Scholar]
- 58.Kim Y., Kim S., Markelonis G., Oh T. Ginsenosides Rb1 and Rg3 protect cultured rat cortical cells from glutamate-induced neurodegeneration. J Neurosci Res. 1998;53(4):426–432. doi: 10.1002/(SICI)1097-4547(19980815)53:4<426::AID-JNR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 59.Wu J., Jeong H.K., Bulin S.E., Kwon S.W., Park J.H., Bezprozvanny I. Ginsenosides protect striatal neurons in a cellular model of Huntington's disease. J Neurosci Res. 2009;87(8):1904–1912. doi: 10.1002/jnr.22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu B., Nakamichi N., Zhang W.S., Nakamura Y., Kambe Y., Fukumori R., Takuma K., Yamada K., Takarada T., Taniura H. Possible protection by notoginsenoside R1 against glutamate neurotoxicity mediated by N-methyl-D-aspartate receptors composed of an NR1/NR2B subunit assembly. J Neurosci Res. 2009;87(9):2145–2156. doi: 10.1002/jnr.22021. [DOI] [PubMed] [Google Scholar]
- 61.Yoon S.R., Nah J.J., Shin Y.H., Kim S.K., Nam K.Y., Choi H.S., Nah S.Y. Ginsenosides induce differential antinociception and inhibit substance P induced-nociceptive response in mice. Life Sci. 1998;62(21):319–325. doi: 10.1016/s0024-3205(98)00168-4. [DOI] [PubMed] [Google Scholar]
- 62.Shin Y.H., Jung O.M., Nah J.J., Nam K.Y., Kim C.Y., Nah S.Y. Ginsenosides that produce differential antinociception in mice. Gen Pharmacol. 1999;32(6):653–659. doi: 10.1016/s0306-3623(98)00239-0. [DOI] [PubMed] [Google Scholar]
- 63.Lee J.H., Kim S.R., Bae C.S., Kim D., Hong H.N., Nah S.Y. Protective effect of ginsenosides, active ingredients of Panax ginseng, on kainic acid-induced neurotoxicity in rat hippocampus. Neurosci Lett. 2002;325(2):129–133. doi: 10.1016/s0304-3940(02)00256-2. [DOI] [PubMed] [Google Scholar]
- 64.Peng L.L., Shen H.M., Jiang Z.L., Li X., Wang G.H., Zhang Y.F., Ke K.F. Inhibition of NMDA receptors underlies the neuroprotective effect of ginsenoside Rb3. Am J Chin Med. 2009;37(4):759–770. doi: 10.1142/S0192415X09007223. [DOI] [PubMed] [Google Scholar]
- 65.Lee E., Kim S., Chung K.C., Choo M.K., Kim D.H., Nam G., Rhim H. 20 (S)-ginsenoside Rh 2, a newly identified active ingredient of ginseng, inhibits NMDA receptors in cultured rat hippocampal neurons. Eur J Pharmacol. 2006;536(1):69–77. doi: 10.1016/j.ejphar.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 66.Kim J.H., Cho S.Y., Lee J.H., Jeong S.M., Yoon I.S., Lee B.H., Lee J.H., Pyo M.K., Lee S.-M., Chung J.M. Neuroprotective effects of ginsenoside Rg 3 against homocysteine-induced excitotoxicity in rat hippocampus. Brain Res. 2007;1136:190–199. doi: 10.1016/j.brainres.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 67.Shin T.J., Hwang S.H., Choi S.H., Lee B.H., Kang J., Kim H.J., Zukin R.S., Rhim H., Nah S.Y. Effects of protopanaxatriol-ginsenoside metabolites on rat N-methyl-d-aspartic Acid receptor-mediated ion currents. Korean J Physiol Pharmacol. 2012;16(2):113–118. doi: 10.4196/kjpp.2012.16.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen W.Z., Liu S., Chen F.F., Zhou C.J., Yu J., Zhuang C.L., Shen X., Chen B.C., Yu Z. Prevention of postoperative fatigue syndrome in rat model by ginsenoside Rb1 via down-regulation of inflammation along the NMDA receptor pathway in the hippocampus. Biol Pharm Bull. 2015;38(2):239–247. doi: 10.1248/bpb.b14-00599. [DOI] [PubMed] [Google Scholar]
- 69.Zhao B.S., Liu Y., Gao X.Y., Zhai H.Q., Guo J.Y., Wang X.Y. Effects of ginsenoside Rg1 on the expression of toll-like receptor 3, 4 and their signalling transduction factors in the NG108-15 murine neuroglial cell line. Molecules. 2014;19(10):16925–16936. doi: 10.3390/molecules191016925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bak M.J., Hong S.G., Lee J.W., Jeong W.S. Red ginseng marc oil inhibits iNOS and COX-2 via NFκB and p38 pathways in LPS-stimulated RAW 264.7 macrophages. Molecules. 2012;17(12):13769–13786. doi: 10.3390/molecules171213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fan Y., Sun C., Gao X., Wang F., Li X., Kassim R.M., Tai G., Zhou Y. Neuroprotective effects of ginseng pectin through the activation of ERK/MAPK and Akt survival signaling pathways. Mol Med Rep. 2012;5(5):1185–1190. doi: 10.3892/mmr.2012.811. [DOI] [PubMed] [Google Scholar]
- 72.Ahn S., Singh P., Castro-Aceituno V., Yesmin Simu S., Kim Y.J., Mathiyalagan R., Yang D.C. Gold nanoparticles synthesized using Panax ginseng leaves suppress inflammatory-mediators production via blockade of NF-κB activation in macrophages. Artif Cells Nanomed Biotechnol. 2017;45(2):270–276. doi: 10.1080/21691401.2016.1228661. [DOI] [PubMed] [Google Scholar]
- 73.Saba E., Jeon B.R., Jeong D.H., Lee K., Goo Y.K., Kwak D., Kim S., Roh S.S., Kim S.D., Nah S.Y. A novel Korean Red Ginseng compound gintonin inhibited inflammation by MAPK and NF-κB pathways and recovered the levels of mir-34a and mir-93 in RAW 264.7 cells. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/624132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song J., Chen X., Zhang J., Huang T., Zeng Y., Shen J., Chen L. JNK/p38 MAPK involves in ginsenoside Rb1 attenuating beta-amyloid peptide (25-35)-induced tau protein hyperphosphorylation in embryo rat cortical neurons. Yao Xue Xue Bao. 2008;43(1):29–34. [PubMed] [Google Scholar]
- 75.Hashimoto R., Yu J., Koizumi H., Ouchi Y., Okabe T. Ginsenoside Rb1 prevents MPP+-induced apoptosis in PC12 cells by stimulating estrogen receptors with consequent activation of ERK1/2, Akt and inhibition of SAPK/JNK, p38 MAPK. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/693717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu S.D., Xia F., Lin X.M., Duan K.L., Wang F., Lu Q.L., Cao H., Qian Y.H., Shi M. Ginsenoside-Rd promotes neurite outgrowth of PC12 cells through MAPK/ERK-and PI3K/AKT-dependent pathways. Int J Mol Sci. 2016;17(2):177. doi: 10.3390/ijms17020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cong L., Chen W. Neuroprotective effect of ginsenoside Rd in spinal cord injury rats. Basic Clin Pharmacol Toxicol. 2016;119(2):193–201. doi: 10.1111/bcpt.12562. [DOI] [PubMed] [Google Scholar]
- 78.Lu M.C., Lai T.Y., Hwang J.M., Chen H.T., Chang S.H., Tsai F.J., Wang H.L., Lin C.C., Kuo W.W., Huang C.Y. Proliferation-and migration-enhancing effects of ginseng and ginsenoside Rg1 through IGF-I-and FGF-2-signaling pathways on RSC96 Schwann cells. Cell Biochem Funct. 2009;27(4):186–192. doi: 10.1002/cbf.1554. [DOI] [PubMed] [Google Scholar]
- 79.Zong Y., Ai Q.L., Zhong L.M., Dai J.N., Yang P., He Y., Sun J., Ling E.A., Lu D. Ginsenoside Rg1 attenuates lipopolysaccharide-induced inflammatory responses via the phospholipase C-γ1 signaling pathway in murine BV-2 microglial cells. Curr Med Chem. 2012;19(5):770–779. doi: 10.2174/092986712798992066. [DOI] [PubMed] [Google Scholar]
- 80.Huang L., Liu L.F., Liu J., Dou L., Wang G.Y., Liu X.Q., Yuan Q.L. Ginsenoside Rg1 protects against neurodegeneration by inducing neurite outgrowth in cultured hippocampal neurons. Neural Regen Res. 2016;11(2):319–325. doi: 10.4103/1673-5374.177741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu J.F., Xue W., Ning N., Zhang J.T., Chen N.H. Ginsenoside Rg1 activated CaMKIIα mediated extracellular signal-regulated kinase/mitogen activated protein kinase signaling pathway. Acta Pharmacol Sin. 2008;29(9):1119–1126. doi: 10.1111/j.1745-7254.2008.00867.x. [DOI] [PubMed] [Google Scholar]
- 82.Lee Y.Y., Park J.S., Jung J.S., Kim D.H., Kim H.S. Anti-inflammatory effect of ginsenoside Rg5 in lipopolysaccharide-stimulated BV2 microglial cells. Int J Mol Sci. 2013;14(5):9820–9833. doi: 10.3390/ijms14059820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meng X., Sun G., Ye J., Xu H., Wang H., Sun X. Notoginsenoside R1-mediated neuroprotection involves estrogen receptor-dependent crosstalk between Akt and ERK1/2 pathways: a novel mechanism of Nrf2/ARE signaling activation. Free Radic Res. 2014;48(4):445–460. doi: 10.3109/10715762.2014.885117. [DOI] [PubMed] [Google Scholar]
- 84.Jung S.H., Woo M.S., Kim S.Y., Kim W.K., Hyun J.W., Kim E.J., Kim D.H., Kim H.S. Ginseng saponin metabolite suppresses phorbol ester–induced matrix metalloproteinase-9 expression through inhibition of activator protein-1 and mitogen-activated protein kinase signaling pathways in human astroglioma cells. Int J Cancer. 2006;118(2):490–497. doi: 10.1002/ijc.21356. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y., Lin L., Liu G., Liu J., Li T. Pharmacokinetics and brain distribution of ginsenosides after administration of sailuotong. Zhongguo Zhong Yao Za Zhi. 2014;39(2):316–321. [PubMed] [Google Scholar]
- 86.Tan X., Gu J., Zhao B., Wang S., Yuan J., Wang C., Chen J., Liu J., Feng L., Jia X. Ginseng improves cognitive deficit via the RAGE/NF-κB pathway in advanced glycation end product-induced rats. J Ginseng Res. 2015;39(2):116–124. doi: 10.1016/j.jgr.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu J., Yan X., Li L., Li Y., Zhou L., Zhang X., Hu X., Zhao G. Ginsenoside Rd improves learning and memory ability in APP transgenic mice. J Mol Neurosci. 2015;57(4):522–528. doi: 10.1007/s12031-015-0632-4. [DOI] [PubMed] [Google Scholar]
- 88.Kim J., Shim J., Lee S., Cho W.H., Hong E., Lee J.H., Han J.S., Lee H.J., Lee K.W. Rg3-enriched ginseng extract ameliorates scopolamine-induced learning deficits in mice. BMC Complement Altern Med. 2016;16(1):66. doi: 10.1186/s12906-016-1050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee K.W., Jung S.Y., Choi S.M., Yang E.J. Effects of ginsenoside Re on LPS-induced inflammatory mediators in BV2 microglial cells. BMC Complement Altern Med. 2012;12(1):196. doi: 10.1186/1472-6882-12-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jung J.S., Shin J.A., Park E.M., Lee J.E., Kang Y.S., Min S.W., Kim D.H., Hyun J.W., Shin C.Y., Kim H.S. Anti-inflammatory mechanism of ginsenoside Rh1 in lipopolysaccharide-stimulated microglia: critical role of the protein kinase A pathway and hemeoxygenase-1 expression. J Neurochem. 2010;115(6):1668–1680. doi: 10.1111/j.1471-4159.2010.07075.x. [DOI] [PubMed] [Google Scholar]
- 91.Kou J., Liu Q., Guan T., Wang Y., Lv Y., Huang Y., Cao Z., Yu B. Ginsenoside Rg1 protects hydrogen peroxide-induced PC12 cell death: involvement of NMMHC IIA-NF-κB/p65 pathway (1143.15) The FASEB J. 2014;28(1) 1143–1215. [Google Scholar]
- 92.Wang Y., Liu Q., Xu Y., Zhang Y., Lv Y., Tan Y., Jiang N., Cao G., Ma X., Wang J. Ginsenoside Rg1 protects against oxidative stress-induced neuronal apoptosis through myosin IIA-actin related cytoskeletal reorganization. Int J Biol Sci. 2016;12(11):1341. doi: 10.7150/ijbs.15992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao P., Teng J., Zhu H., Zheng Y., Zhu X. Ginsenoside Rg1 prevents MPP+-induced apoptosis of SHSY5Y cells via the inhibition of a Bax-mediated mitochondrial pathway and by suppressing oxidative stress. Int J Clin Exp Med. 2016;9(6):10811–10819. [Google Scholar]
- 94.Hou J., Xue J., Lee M., Sung C. Ginsenoside Rd as a potential neuroprotective agent prevents trimethyltin injury. Biomed Rep. 1899;6(4):435–440. doi: 10.3892/br.2017.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu D., Zhang H., Gu W., Liu Y., Zhang M. Neuroprotective effects of ginsenoside Rb1 on high glucose-induced neurotoxicity in primary cultured rat hippocampal neurons. PLoS One. 2013;8(11):e79399. doi: 10.1371/journal.pone.0079399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li H., Kang T., Qi B., Kong L., Jiao Y., Cao Y., Zhang J., Yang J. Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of d-galactose/AlCl 3 inducing rats model of Alzheimer's disease. J Ethnopharmacol. 2016;179:162–169. doi: 10.1016/j.jep.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 97.Zhang X., Shi M., Bjørås M., Wang W., Zhang G., Han J., Liu Z., Zhang Y., Wang B., Chen J. Ginsenoside Rd promotes glutamate clearance by up-regulating glial glutamate transporter GLT-1 via PI3K/AKT and ERK1/2 pathways. Front Pharmacol. 2013;4:152. doi: 10.3389/fphar.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Y., Zhang R.-Y., Zhao J., Dong Z., Feng D.-Y., Wu R., Shi M., Zhao G. Ginsenoside Rd protects SH-SY5Y cells against 1-methyl-4-phenylpyridinium induced injury. Int J Mol Sci. 2015;16(7):14395–14408. doi: 10.3390/ijms160714395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang X., Wang C., Wang J., Zhao S., Zhang K., Wang J., Zhang W., Wu C., Yang J. Pseudoginsenoside-F11 (PF11) exerts anti-neuroinflammatory effects on LPS-activated microglial cells by inhibiting TLR4-mediated TAK1/IKK/NF-κB, MAPKs and Akt signaling pathways. Neuropharmacology. 2014;79:642–656. doi: 10.1016/j.neuropharm.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 100.Gao Q.G., Chen W.F., Xie J.X., Wong M.S. Ginsenoside Rg1 protects against 6-OHDA-induced neurotoxicity in neuroblastoma SK-N-SH cells via IGF-I receptor and estrogen receptor pathways. J Neurochem. 2009;109(5):1338–1347. doi: 10.1111/j.1471-4159.2009.06051.x. [DOI] [PubMed] [Google Scholar]
- 101.Xu L., Chen W.F., Wong M.S. Ginsenoside Rg1 protects dopaminergic neurons in a rat model of Parkinson's disease through the IGF-I receptor signalling pathway. Br J Pharmacol. 2009;158(3):738–748. doi: 10.1111/j.1476-5381.2009.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Choi J.H., Lee M.J., Jang M., Kim H.J., Lee S., Lee S.W., Kim Y.O., Cho I.H. Panax ginseng exerts antidepressant-like effects by suppressing neuroinflammatory response and up-regulating Nrf2 signaling in the amygdala. J Ginseng Res. 2018;42(1):107–115. doi: 10.1016/j.jgr.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ye J., Yao J.P., Wang X., Zheng M., Li P., He C., Wan J.B., Yao X., Su H. Neuroprotective effects of ginsenosides on neural progenitor cells against oxidative injury. Mol Med Rep. 2016;13(4):3083–3091. doi: 10.3892/mmr.2016.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ni N., Liu Q., Ren H., Wu D., Luo C., Li P., Wan J.B., Su H. Ginsenoside Rb1 protects rat neural progenitor cells against oxidative injury. Molecules. 2014;19(3):3012–3024. doi: 10.3390/molecules19033012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Du X., Xu H., Jiang H., Xie J. Akt/Nrf2 activated upregulation of heme oxygenase-1 involves in the role of Rg1 against ferrous iron-induced neurotoxicity in SK-N-SH cells. Neurotox Res. 2013;24(1):71–79. doi: 10.1007/s12640-012-9362-3. [DOI] [PubMed] [Google Scholar]
- 106.Lee Y.Y., Park J.S., Lee E.J., Lee S.Y., Kim D.H., Kang J.L., Kim H.S. Anti-inflammatory mechanism of ginseng saponin metabolite Rh3 in lipopolysaccharide-stimulated microglia: critical role of 5′-adenosine monophosphate-activated protein kinase signaling pathway. J Agric Food Chem. 2015;63(13):3472–3480. doi: 10.1021/jf506110y. [DOI] [PubMed] [Google Scholar]
- 107.Gao Y., Chu S.F., Li J.P., Zhang Z., Yan J.Q., Wen Z.L., Xia C.Y., Mou Z., Wang Z.Z., He W.B. Protopanaxtriol protects against 3-nitropropionic acid-induced oxidative stress in a rat model of Huntington's disease. Acta Pharmacol Sin. 2015;36(3):311–322. doi: 10.1038/aps.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hussein J., Refaat E., Medhat D., El-Bana M., Latif Y.A., Farrag A.R., Nazeef N., Moify M. Panax ginseng attenuates experimental brain injury by increasing brainderived neurotrophic factor and inhibition of neuroinflammation. J Chem Pharm Res. 2016;8(1):186–195. [Google Scholar]
- 109.Si Y., Zhu J., Huang X., Zhu P., Xie C. Effects of Panax notoginseng saponins on proliferation and differentiation of rat embryonic cortical neural stem cells. J Chin Med Assoc. 2016;79(5):256–263. doi: 10.1016/j.jcma.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 110.Li F., Wu X., Li J., Niu Q. Ginsenoside Rg1 ameliorates hippocampal long-term potentiation and memory in an Alzheimer's disease model. Mol Med Rep. 2016;13(6):4904–4910. doi: 10.3892/mmr.2016.5103. [DOI] [PubMed] [Google Scholar]
- 111.Zhu G., Wang Y., Li J., Wang J. Chronic treatment with ginsenoside Rg1 promotes memory and hippocampal long-term potentiation in middle-aged mice. Neuroscience. 2015;292:81–89. doi: 10.1016/j.neuroscience.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 112.Gao X.Q., Yang C.X., Chen G.J., Wang G.Y., Chen B., Tan S.K., Liu J., Yuan Q.L. Ginsenoside Rb1 regulates the expressions of brain-derived neurotrophic factor and caspase-3 and induces neurogenesis in rats with experimental cerebral ischemia. J Ethnopharmacol. 2010;132(2):393–399. doi: 10.1016/j.jep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 113.Jiang Z., Wang Y., Zhang X., Peng T., Lu Y., Leng J., Xie Q. Preventive and therapeutic effects of ginsenoside Rb1 for neural injury during cerebral infarction in rats. Am J Chin Med. 2013;41(02):341–352. doi: 10.1142/S0192415X13500250. [DOI] [PubMed] [Google Scholar]
- 114.Cui J., Jiang L., Xiang H. Ginsenoside Rb3 exerts antidepressant-like effects in several animal models. J Psychopharmacol. 2012;26(5):697–713. doi: 10.1177/0269881111415735. [DOI] [PubMed] [Google Scholar]
- 115.Jiang B., Xiong Z., Yang J., Wang W., Wang Y., Hu Z.L., Wang F., Chen J.G. Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br J Pharmacol. 2012;166(6):1872–1887. doi: 10.1111/j.1476-5381.2012.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu X., Gao R., Liu Z., Cheng Z., Qi Y., Fan C., Yu S.Y. Ginsenoside Rg1 reverses stress-induced depression-like behaviours and brain-derived neurotrophic factor expression within the prefrontal cortex. Eur J Neurosci. 2016;44(2):1878–1885. doi: 10.1111/ejn.13255. [DOI] [PubMed] [Google Scholar]
- 117.You Z., Yao Q., Shen J., Gu Z., Xu H., Wu Z., Chen C., Li L. Antidepressant-like effects of ginsenoside Rg3 in mice via activation of the hippocampal BDNF signaling cascade. J Nat Med. 2016:1–13. doi: 10.1007/s11418-016-1066-1. [DOI] [PubMed] [Google Scholar]
- 118.Guo J., Chang L., Zhang X., Pei S., Yu M., Gao J. Ginsenoside compound K promotes β-amyloid peptide clearance in primary astrocytes via autophagy enhancement. Exp Ther Med. 2014;8(4):1271–1274. doi: 10.3892/etm.2014.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou T., Zu G., Zhang X., Wang X., Li S., Gong X., Liang Z., Zhao J. Neuroprotective effects of ginsenoside Rg1 through the Wnt/β-catenin signaling pathway in both in vivo and in vitro models of Parkinson's disease. Neuropharmacology. 2016;101:480–489. doi: 10.1016/j.neuropharm.2015.10.024. [DOI] [PubMed] [Google Scholar]