Abstract
Background
Ginsenoside Rg3 (G-Rg3) is the major bioactive ingredient of Panax ginseng and has many pharmacological effects, including antiadipogenic, antiviral, and anticancer effects. However, the effect of G-Rg3 on mast cell–mediated allergic inflammation has not been investigated.
Method
The antiallergic effects of G-Rg3 on allergic inflammation were evaluated using the human and rat mast cell lines HMC-1 and RBL-2H3. Antiallergic effects of G-Rg3 were detected by measuring cyclic adenosine monophosphate (cAMP), detecting calcium influx, and using real-time reverse transcription polymerase chain reaction, enzyme-linked immunosorbent assay, Western blotting, and in vivo experiments.
Results
G-Rg3 decreased histamine release from activated mast cells by enhancing cAMP levels and calcium influx. Proinflammatory cytokine production was suppressed by G-Rg3 treatment via regulation of the mitogen-activated protein kinases/nuclear factor-kappa B and receptor-interacting protein kinase 2 (RIP2)/caspase-1 signaling pathway in mast cells. Moreover, G-Rg3 protected mice against the IgE-mediated passive cutaneous anaphylaxis reaction and compound 48/80-induced anaphylactic shock.
Conclusion
G-Rg3 may serve as an alternative therapeutic agent for improving allergic inflammatory disorders.
Keywords: Allergic inflammation, Ginsenoside Rg3, Mast cells, Mitogen-activated protein kinase, Nuclear factor-κB
1. Introduction
Mast cells are the main immune effector cells involved in allergic responses [1]. They circulate in the blood as progenitors and are involved in allergic diseases including asthma and atopic dermatitis, also known as eczema, as initiators. During allergic responses, they trigger degranulation by various stimuli and release several bioactive substances, such as histamine and proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6. These mediators promote inflammatory progression through the migration and activation of immune effector cells [2]. Therefore, inhibition of degranulation and proinflammatory cytokines release may be an efficient therapeutic strategy for allergic inflammatory disorders.
The mitogen-activated protein kinase (MAPK) signaling pathway regulates a wide range of cellular processes. This signaling pathway is involved in the activation, differentiation, and survival of immune cells. Extracellular signal–regulated kinase (ERK), c-jun N-terminal kinase (JNK), and p38 are included in the MAPK pathway [3]. In allergic responses, MAPK signaling is associated with nuclear factor-kappa B (NF-κB) activation. NF-κB controls the transcription of proinflammatory cytokine genes, and the secretion of these cytokines has been observed in the progression of allergic responses [4], [5].
The cysteine protease caspase-1 is related to the production of proinflammatory cytokines in allergic inflammation [6]. Caspase-1 activation induces proinflammatory cytokine production via NF-κB translocation to the nucleus [7]. Particularly, caspase-1 can convert pro-forms of IL-1β into a mature form via the IL-1β–converting enzyme [8]. RIP2 binds to the caspase-1 prodomain and subsequently regulates the activation of caspase-1. RIP2 depletion decreases the release of proinflammatory cytokines such as TNF-α and IL-6 [9]. Therefore, blockage of RIP2/caspase-1 activation reduces the severity of inflammatory diseases.
Ginsenosides are active pharmaceutical constituents isolated from Panax ginseng. Among the approximately 40 types of ginsenosides, ginsenoside Rg3 (G-Rg3) is derived from red ginseng. Red ginseng is a type of root prepared by steaming and drying [10]. Red ginseng extract improves skin lesions in atopic dermatitis models by inhibiting chemokine and proinflammatory cytokine expression in mast cells and keratinocytes [11]. G-Rg3 has shown positive therapeutic effects on cancer, diabetes mellitus, viral diseases, and inflammation [12], [13], [14], [15]. Particularly, G-Rg3 inhibits oxazolone-induced contact dermatitis by decreasing the expression of lipopolysaccharide (LPS)-induced cyclooxygenase-2 and cytokines in macrophages [16]. This compound also ameliorates 2,4,6-trinitrochlorobenzene-induced skin lesions by inhibiting the infiltration of mast cells and expression of proinflammatory cytokines [17]. However, the effects and mechanisms of G-Rg3 on allergic inflammation in activated mast cells are unclear.
In this study, we examined the effects of G-Rg3 on allergic inflammation using HMC-1 and RBL-2H3 mast cells. The inhibitory effect of G-Rg3 on degranulation of mast cells and its related mechanisms were confirmed in HMC-1 and RBL-2H3 cells. Systemic anaphylactic shock and the passive cutaneous anaphylaxis (PCA) reaction after G-Rg3 administration were conducted to confirm the antiallergic effect of G-Rg3.
2. Materials and methods
2.1. Antibodies and reagents
Prednisolone (PDN) and antibodies against phospho-ERK, phospho-JNK, phospho-p38, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα), and phospho-NF-κB were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-ERK, p38, JNK, α-tubulin, Lamin B, RIP2, and caspase-1 antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). G-Rg3 (CAS no. 14197-60-5, purity: 95–99%) was purchased from Chengdu Biopurify Phytochemical Ltd. (Chengdu, Sichuan, China). Water Soluble Tetrazolium Salt (WST) reagent (EZ-cytox) was purchased from DoGen (Seoul, Korea). Fluo-4 acetoxymethyl (AM) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). 3-(4 5-dimethylthiazol-2-yl)-2 5-diphenyltetrazolium bromide (MTT), Compound 48/80, Evans blue, Dinitrophenyl (DNP)-IgE, DNP-Bovine Serum Albumin (BSA), phorbol 12-myristate 13-acetate (PMA), and A23187 were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Cell culture
The human mast cell line HMC-1 and rat basophilic leukemia mast cell line RBL-2H3 were maintained in Iscove's Modified Dulbecco's Medium and Dulbecco's Modified Eagle's Medium, respectively. Fetal bovine serum and penicillin-streptomycin were added to the medium, and the cells were maintained in a cell culture incubator.
2.3. Cell viability
The viability of G-Rg3-treated mast cells was quantified by the MTT and WST assays. After 24 h incubation with G-Rg3, MTT and WST reagents were added to HMC-1 and RBL-2H3 cells, respectively. The absorbance after MTT and WST treatment were measured at 540 nm and 405 nm.
2.4. Measurement of histamine
Histamine release was measured by the histamine enzyme-linked immunosorbent assay kit according to the manufacturer's protocols (ALPCO, Salem, NH, USA).
2.5. cAMP and intracellular calcium (Ca2+) levels
To measure cyclic adenosine monophosphate (cAMP) levels, a cAMP direct immunoassay kit (BioVision, Inc., Milpitas, CA, USA) was used. Cells (5 × 104 cells/well) were added to a 24-well plate and treated with G-Rg3 at the indicated times. After treatment, the cells were harvested, and cAMP levels were measured according to the manufacturer's protocol.
Intracellular Ca2+ levels were detected with a fluorescence microscope. Cells were cultured in an 8-well chamber slide and incubated with Fluo-4 AM (5 μM) for 30 min. G-Rg3 was used to treat the cells for 30 min, and stimulator was treated to slides. The results were visualized with a fluorescence microscope (Observer A1 microscope, Carl Zeiss, Oberkochen, Germany).
2.6. Measurement of proinflammatory cytokines
An OptEIA ELISA kit (BD Pharmingen, San Jose, CA, USA) was used to detect proinflammatory cytokines. Briefly, the capture antibody was diluted and pre-coated in 96-well plates at 4°C overnight. Fetal bovine serum (5%) in phosphate-buffered saline was added to the plate for blocking for 1 h. The plate was washed with 0.05% phosphate-buffered saline containing Tween 20, and then samples and cytokine standards were added. After 3 h of incubation, biotinylated detection antibodies plus streptavidin–horseradish peroxidase solution were added and incubated for 1 h. Absorbance was measured within 30 min after stop solution treatment.
2.7. Real-time reverse transcription polymerase chain reaction
Total RNA extraction and cDNA synthesis were carried out with QIAzol lysis reagent (Qiagen, Hilden, Germany) and the High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturers' instructions. SYBR Green Realtime PCR Master Mix (Toyobo, Osaka, Japan) and StepOnePlus Real-Time PCR Systems (Applied Biosystems) were used for polymerase chain reaction (PCR). Table 1 shows the sequences of the primers used for real-time reverse transcription (RT) PCR.
Table 1.
Sequences of the real-time RT-PCR primers
| Genes | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| IL-1β (h) | TACCTGTCCTGCGTGTTGAA | TDTTTGGGTAATTTTTGGGATCT |
| IL-6 (h) | GATGAGTACAAAAGTCCTGATCCA | CTGCAGCCACTGGTTCTGT |
| TNF-α (h) | CGCTCCCCAAGAAGACAG | AGAGGCTGAGGAACAAGCAC |
| β-actin (h) | CCAACCGCGAGAAGATGA | CCAGAGGCGTACAGGGATAG |
| IL-1β (m) | CACAGCAGCACATCAACAAG | GTGCTCATGTCCTCATCCTG |
| IL-6 (m) | CTCTGGGAAATCGTGGAAAT | CCAGTTTGGTAGCATCCATC |
| TNF-α (m) | ATGAGAAGTTCCCAAATGGC | CTCCACTTGGTGGTTTGCTA |
| GAPDH (m) | GACATGCCGCCTGGAGAAAC | AGCCCAGGATGCCCTTTAGT |
IL, interleukin; RT-PCR, reverse transcription polymerase chain reaction; TNF, tumor necrosis factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
2.8. Western blotting
Cells were lysed, and cell lysates were used for protein quantification. An NE-PER Nuclear and Cytoplasmic Extraction kit (Thermo Fisher Scientific, Rockford, IL, USA) was used to prepare the nuclear extracts. Proteins were separated using electrophoresis, transferred to membranes, and detected using primary antibodies. Specific primary antibodies were captured using secondary antibodies and were detected using the FluorChem M system (ProteinSimple, San Jose, CA, USA).
2.9. Systemic anaphylactic shock
All in vivo experiments were carried out with Institute of Cancer research (ICR) mice (5 weeks, male) and approved by the Wonkwang University Institutional Animal Care and Use Committee (WKU16-12). Mice were purchased from Samtaco Korea (Osan, Korea) and housed in a ventilated cage system. To establish the systemic anaphylactic model, compound 48/80 (10 mg/kg) was intraperitoneally injected into the mice (n = 6). G-Rg3 (10 mg/kg, 25 mg/kg, and 50 mg/kg) was mixed in 0.5% carboxymethylcellulose (CMC) solution, while control mice were administered a 0.5% CMC solution. G-Rg3 and 0.5% CMC solution were used to treat the mice by oral administration 1 h before injecting compound 48/80.
2.10. Passive cutaneous anaphylaxis
To induce the PCA reaction, back hair was removed using hair clippers before the experiments. DNP-IgE (10 μg/spot) was intradermally injected into mice 24 h before antigen injection. G-Rg3 was orally administered 1 h before antigen challenge. DNP-BSA (1 μg/mL) containing 1% Evans blue was injected into the mouse tail vein. Mice were sacrificed, and stained back skin was incubated in formaldehyde overnight. Dissolved blue dye was measured using a microplate reader at 620 nm.
2.11. Statistical analysis
The obtained data were expressed as the mean ± SD from a minimum of three independent experiments. The results were analyzed by Student t test using the SPSS statistical program (SPSS, Inc., Chicago, IL, USA). Differences with p < 0.05 were regarded as statistically significant.
3. Results
3.1. Effects of G-Rg3 on histamine release
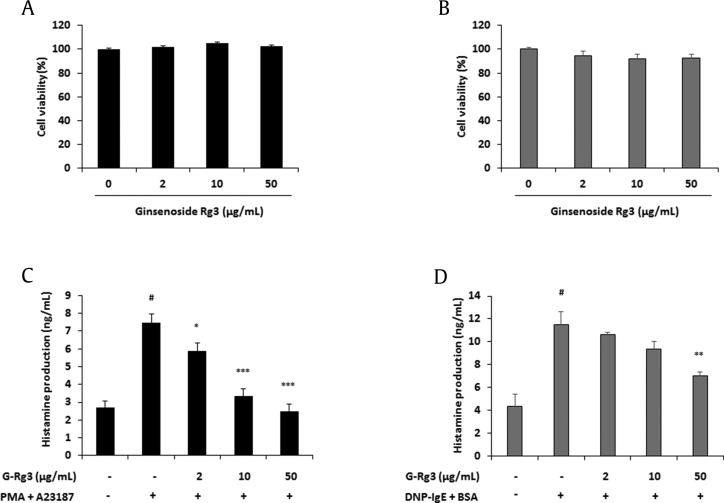
As shown in Figs. 1A, 1B, the maximum treatment of 50 μg/mL of G-Rg3 did not change the viability of both cell lines. During degranulation of mast cells, inflammatory mediators are released and cause allergic reactions. Among inflammatory mediators, histamine is an important protein involved in allergic reactions [18]. Because G-Rg3 reduces β-hexosaminidase secretion from activated RBL-2H3 cells [16], we predicted that G-Rg3 can suppress the release of histamine from mast cells. G-Rg3 (2–50 μg/mL) dose-dependently decreased histamine release from activated HMC-1 and RBL-2H3 cells (Figs. 1C, 1D).
Fig. 1.
Ginsenoside Rg3 (G-Rg3) inhibited histamine production from activated mast cells. (A) HMC-1 cells (1 × 104 cells/well) were treated with G-Rg3 (2-50 μg/mL) for 24 h. (B) RBL-2H3 cells (5 × 103 cells/well) were treated with G-Rg3 (2-50 μg/mL) for 24 h. Cell viability was determined by MTT assay and WST assay, respectively. The production level of histamine was measured using the ELISA kit. (C) HMC-1 cells (2 × 105 cells/well) were pretreated with G-Rg3 and PDN (10 μM) for 30 min and stimulated with PMA (50 nM) + A23187 (1 μM) for 24 h. (D) DNP-IgE (100 ng/mL)–sensitized RBL-2H3 cells (2 × 105 cells/well) were pretreated with G-Rg3 and PDN (10 μM) for 30 min and stimulated with DNP-BSA (10 μg/mL) for 24 h. Results are the mean ± SD. #p < 0.05 versus the blank; *p < 0.05, **p < 0.01, and ***p < 0.001 versus stimulator-treated group.
ELISA, enzyme-linked immunosorbent assay; PDN, prednisolone; PMA, phorbol 12-myristate 13-acetate; SD, standard deviation.
3.2. Effects of G-Rg3 on intracellular cAMP and Ca2+ levels in mast cells
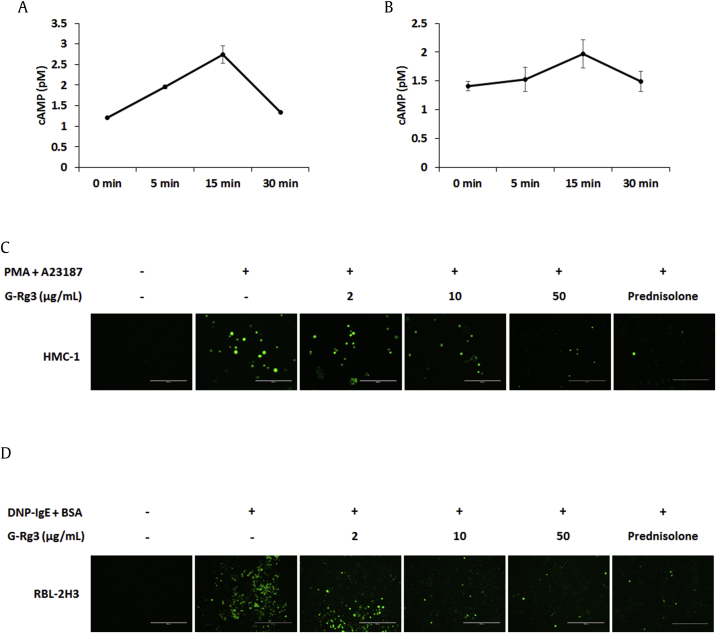
During the degranulation of mast cells, the cAMP concentration and Ca2+ level affect histamine release [19]. Blocking Ca2+ influx and increasing cAMP concentration are effective strategies for treating allergic diseases. Intracellular cAMP and Ca2+ levels were measured using a cAMP ELISA kit and Fluo-4 AM, respectively. The intracellular cAMP concentration was increased at 5 min and remained at an increased level until 15 min by G-Rg3 treatment in HMC-1 and RBL-2H3 cells (Figs. 2A, 2B). Ca2+ influx into mast cells was elevated by PMA + A23187 or DNP-BSA stimulation, whereas G-Rg3 (10 μg/mL and 50 μg/mL) reduced intracellular Ca2+ concentrations (Figs. 2C, 2D). Therefore, G-Rg3 may inhibit histamine release by blocking Ca2+ influx and enhancing intracellular cAMP content.
Fig. 2.
Ginsenoside Rg3 (G-Rg3) increased the concentration of cAMP levels and blocked calcium influx into mast cells. cAMP levels were measured using a colorimetric cAMP ELISA kit. (A) HMC-1 cells were treated with G-Rg3 (50 μg/mL) at the indicated time. (B) RBL-2H3 cells were treated with G-Rg3 (50 μg/mL) at the indicated time. Intracellular calcium levels were detected by fluorescence microscopy (magnification ×200). (C) HMC-1 cells were treated with G-Rg3 and PDN (10 μM) 30 min before stimulation using PMA + A23187 for 5 min. (D) IgE-sensitized RBL-2H3 cells were treated with G-Rg3 and PDN (10 μM) 30 min before stimulation using DNP-BSA for 5 min. Results are the mean ± SD.
ELISA, enzyme-linked immunosorbent assay; PDN, prednisolone; PMA, phorbol 12-myristate 13-acetate; SD, standard deviation.
3.3. Effects of G-Rg3 on the production of proinflammatory cytokines in mast cells
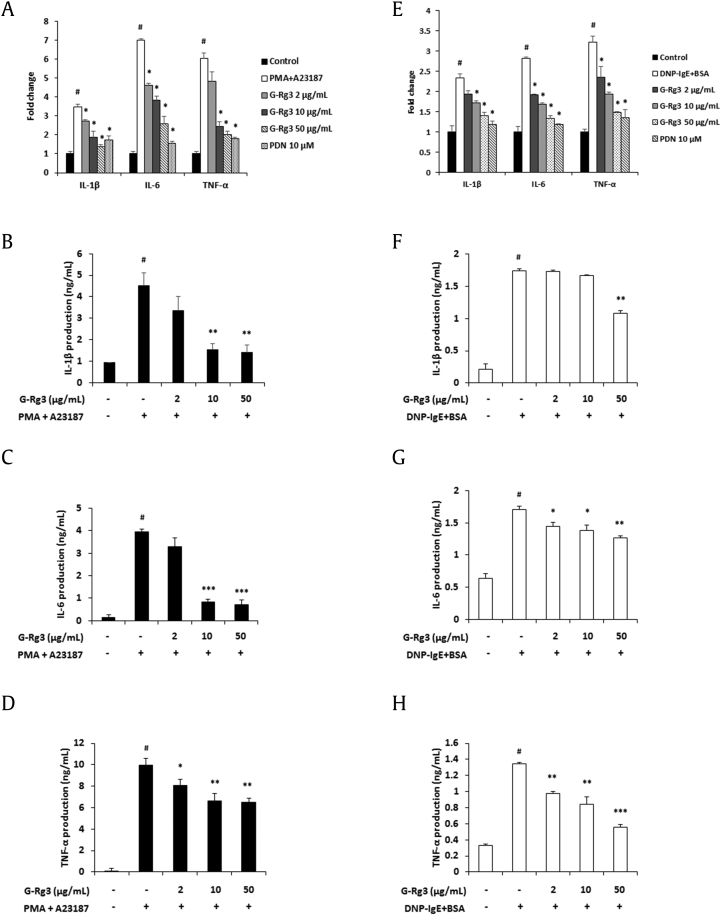
In response to various stimulation pathogens, mast cells release proinflammatory cytokines, which cause allergic and inflammatory diseases [1]. Therefore, inhibition of proinflammatory cytokine secretion may assist in ameliorating allergic diseases. In this study, we investigated whether G-Rg3 modulates the secretion of proinflammatory cytokines in HMC-1 and RBL-2H3 cells. IL-1β, IL-6, and TNF-α messenger RNA expression levels were decreased by G-Rg3 treatment (Figs. 3A, 3E). In addition, G-Rg3 significantly suppressed the release of these cytokines in PMA + A23187-stimulated HMC-1 cells (Figs. 3B–3D). Similarly, G-Rg3 treatment decreased the production of these cytokines in IgE-sensitized and antigen-stimulated RBL-2H3 cells (Figs. 3F–3H).
Fig. 3.
Ginsenoside Rg3 (G-Rg3) decreased the production of proinflammatory cytokines IL-1β, IL-6, and TNF-α in activated mast cells. HMC-1 cells (5 × 105 cells/well) were treated with G-Rg3 (2–50 μg/mL) and PDN (10 μM) for 30 min and stimulated with PMA + A23187 for 24 h. (A) mRNA expression of proinflammatory cytokines was analyzed by real-time RT-PCR. (B) Release of proinflammatory cytokines IL-1β was measured by ELISA. (C) Release of proinflammatory cytokines IL-6 was measured by ELISA. (D) Release of proinflammatory cytokines TNF-α was measured by ELISA. (E–H) DNP-IgE (100 ng/mL)–sensitized RBL-2H3 cells (5 × 105 cells/well) were treated with G-Rg3 (2–50 μg/mL) and PDN (10 μM) for 30 min and stimulated with DNP-BSA (10 μg/mL) for 24 h. (E) mRNA expression of proinflammatory cytokines was analyzed by real-time RT-PCR. (F) Production of proinflammatory cytokines IL-1β. (G) Production of proinflammatory cytokines IL-6. (H) Production of proinflammatory cytokines TNF-α. Results are the mean ± SD. #p < 0.05 versus the control group; *p < 0.05, **p < 0.01, and ***p < 0.001 versus stimulator-treated group.
ELISA, enzyme-linked immunosorbent assay; IL, interleukin; mRNA, messenger RNA; PDN, prednisolone; PMA, phorbol 12-myristate 13-acetate; RT-PCR, reverse transcription polymerase chain reaction; SD, standard deviation; TNF, tumor necrosis factor.
3.4. Effects of G-Rg3 on MAPK and NF-κB activation in mast cells
The MAPK-NF-κB signaling pathway is crucial for regulating inflammatory responses as proinflammatory cytokine production is induced by allergen stimulus [3], [4], [5]. Therefore, the effect of G-Rg3 on the phosphorylation of MAPKs including ERK, JNK, and p38 was evaluated to determine which molecule is regulated by G-Rg3 in activated mast cells. G-Rg3 dose-dependently blocked ERK, JNK, and p38 activation (Fig. 4). Moreover, degradation of IκBα and NF-κB nuclear translocation were suppressed by G-Rg3 in activated mast cells (Fig. 5).
Fig. 4.
Ginsenoside Rg3 (G-Rg3) inhibited phosphorylation of MAPKs in activated mast cells. Phosphorylation of ERK, JNK, and p38 was detected by Western blotting. (A and B) HMC-1 cells (1 × 106 cells/well) were incubated with G-Rg3 and PDN (10 μM) for 30 min and stimulated with PMA (50 nM) + A23187 (1 μM) for 30 min. (C and D) DNP-IgE (100 ng/mL)–sensitized RBL-2H3 cells (1 × 106 cells/well) were incubated with G-Rg3 and PDN (10 μM) for 30 min and stimulated with DNP-BSA (10 μg/mL) for 30 min. Relative levels of MAPKs (B and D) were calculated using the Image J program (NIH, Bethesda, MD, USA). Results are the mean ± SD. #p < 0.05 versus the control group; *p < 0.05 versus stimulator-treated group.
ERK, extracellular signal–regulated kinase; JNK, c-jun N-terminal kinase; MAPK, mitogen-activated protein kinase; PDN, prednisolone; PMA, phorbol 12-myristate 13-acetate; SD, standard deviation.
Fig. 5.
Ginsenoside Rg3 (G-Rg3) inhibited the degradation of IκBα and NF-κB translocation in activated mast cells. Cytosol IκBα and nuclear NF-κB levels were detected by Western blotting. (A and B) HMC-1 cells (1 × 106 cells/well) were pretreated with G-Rg3 and PDN (10 μM) for 30 min, and PMA (50 nM) + A23187 (1 μM) was added to the cells for 2 h. (C and D) DNP-IgE (100 ng/mL)–sensitized RBL-2H3 cells (1 × 106 cells/well) were incubated with G-Rg3 and PDN (10 μM) for 30 min and stimulated with DNP-BSA (10 μg/mL) for 2 h. α-Tubulin and Lamin B were used as loading controls. Relative levels of IκBα and NF-κB (B and D) were calculated using an Image J program. Results are the mean ± SD. #p < 0.05 versus the control group; *p < 0.05 versus stimulator-treated group.
C, cytosol extracts; N, nuclear extracts; NF-κB, nuclear factor-kappa B; PDN, prednisolone; PMA, phorbol 12-myristate 13-acetate; SD, standard deviation.
3.5. Effect of G-Rg3 on RIP2/caspase-1 activation in mast cells
RIP2 interacts with caspase-1 and subsequently induces NF-κB activation. Activated caspase-1 induces cleavage of pro-IL-1β, which is a proinflammatory cytokine IL-1β precursor [20]. Active IL-1β promotes allergic disorders including asthma, atopic dermatitis, and contact hypersensitivity [21]. To determine the effect of G-Rg3 on RIP2/caspase-1 activation in mast cells, protein expression levels of RIP2 and caspase-1 were determined by Western blotting. G-Rg3 dose-dependently suppressed RIP2 activation and cleavage of caspase-1 (Figs. 6A–6D). In addition, the effects of G-Rg3 on antigen-induced caspase-1 activation were confirmed. As shown in Figs. 6E, 6F, G-Rg3 treatment also significantly reduced caspase-1 activity. These data demonstrate that blockage of the MAPK/NF-κB and RIP2/caspase-1 signaling pathways by G-Rg3 treatment suppressed proinflammatory cytokine release.
Fig. 6.
Ginsenoside Rg3 (G-Rg3) suppressed the activation of RIP-2 and caspase-1 in activated mast cells. HMC-1 cells (1 × 106 cells/well) were incubated with G-Rg3 and PDN (10 μM) for 30 min and stimulated with PMA (50 nM) + A23187 (1 μM) for 1 h. DNP-IgE (100 ng/mL)–sensitized RBL-2H3 cells (1 × 106 cells/well) were incubated with G-Rg3 and PDN (10 μM) for 30 min and stimulated with DNP-BSA (10 μg/mL) for 1 h. (A) The protein levels of RIP2 and caspase-1 in HMC-1 cells. (B) Quantification of band intensities in HMC-1 cells was determined using Image J program. (C) The protein levels of RIP2 and caspase-1 in RBL-2H3 cells. α-Tubulin was used as a loading control. (D) Quantification of band intensities in RBL-2H3 cells was determined using Image J program. (E) The activity of caspase-1 in HMC-1 cells was measured with a caspase-1 assay kit. (F) The activity of caspase-1 in RBL-2H3 cells was measured with a caspase-1 assay kit. Results are the mean ± SD. #p < 0.05 versus the control group; *p < 0.05 versus stimulator-treated group.
PDN, prednisolone; PMA, phorbol 12-myristate 13-acetate; SD, standard deviation.
3.6. Effects of G-Rg3 on compound 48/80–induced anaphylactic shock and IgE-mediated PCA reaction
Injection of DNP-IgE and antigen-induced PCA is a suitable in vivo model of local allergic reaction [22]. Oral and intraperitoneal administration of G-Rg3 (25 mg/kg) to mice inhibits the IgE-antigen complex–induced PCA reaction [16]. However, the dose–response effects of G-Rg3 on the antigen-induced PCA reaction are unclear. Compound 48/80 is used as a reagent to trigger a fatal allergic reaction by activating mast cells [23]. In this study, each mouse was administered 0.5% CMC solution as a control or G-Rg3 by oral administration 2 h before compound 48/80 injection. As shown in Table 2, the mortality rate of the 50 mg/kg G-Rg3 group was 27.7%, whereas those of the 10 mg/kg and 25 mg/kg G-Rg3 groups were 66.7% and 33.3%, respectively.
Table 2.
Effect of ginsenoside Rg3 (G-Rg3) on compound 48/80-induced anaphylactic shock
| G-Rg3 (mg/kg)1) | Compound 48/80 (10 mg/kg) | Mortality (%)2) |
|---|---|---|
| 0 | − | 0 |
| 0 | + | 100 |
| 10 | + | 66.7* |
| 25 | + | 33.3* |
| 50 | + | 27.7* |
| Prednisolone (3 mg/kg) | + | 22.1* |
*p < 0.05; significantly different from control group.
The groups of mice (n = 6) were orally administrated with 0.05% carboxymethylcellulose solution or G-Rg3 1 h before injection of compound 48/80.
Mortality (%) is presented as the ‘number of dead mice × 100/total number of experimental mice’. This result was represented from three independent experiments.
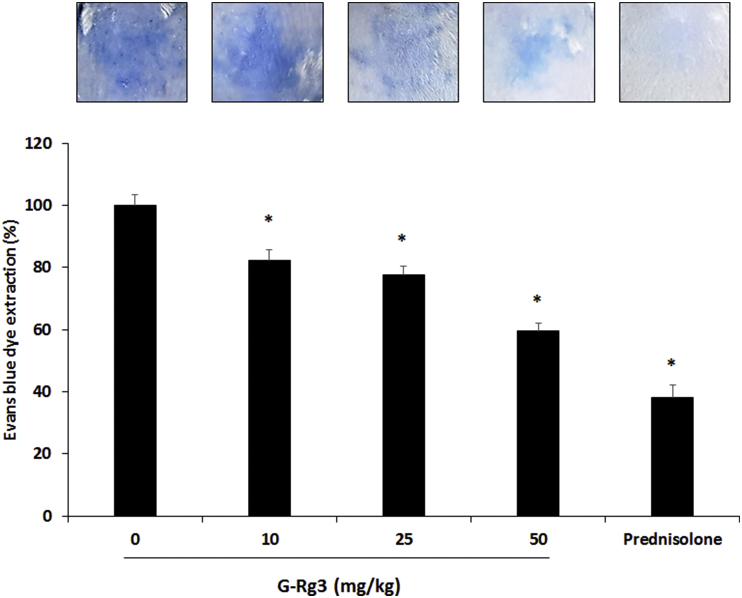
We investigated the dose-dependent effect of G-Rg3 (10–50 mg/kg) on the PCA reaction. The inhibition rate of the 10 mg/kg G-Rg3 group was 17.7%, 25 mg/kg G-Rg3 group was 22.4%, and 50 mg/kg G-Rg3 group was 40.5% (Fig. 7). These results demonstrate that compound 48/80 and antigen-induced allergic reactions are dose-dependently decreased by G-Rg3 treatment.
Fig. 7.
Ginsenoside Rg3 (G-Rg3) inhibited the IgE-mediated PCA reaction. Anti-DNP-IgE (0.5 μg) was injected into the mouse dorsal skin 24 h before antigen challenge. DNP-BSA (1 μg) containing 1% Evans blue was injected by intravascular injection into the mouse tail vein (n = 6). G-Rg3, PDN (3 mg/kg), or saline was administered 1 h before the challenge with DNP-BSA. Dorsal skin was excised, and blue dye was dissolved in formaldehyde. Colorimetric changes were measured using a microplate reader at 620 nm. Results are the mean ± SD. *p < 0.05.
PCA, passive cutaneous anaphylaxis; PDN, prednisolone; SD, standard deviation.
4. Discussion
Mast cells are involved in allergic reactions through the release of various inflammatory mediators [24]. HMC-1 cells show similar characteristics to human mast cells which were established from leukemia patients [25]. The rat basophilic leukemia cell line RBL-2H3 was isolated from Wistar rat basophilic cells. This cell line is a proper model for studying cellular events in mast cell activation as it displays characteristics of mucosal-type mast cells [26]. Therefore, these cell lines are commonly used in inflammation, allergy, and immunological research.
Mast cell degranulation is triggered by increasing intracellular calcium concentration. Increased calcium influx subsequently activates calcium-dependent processes for the degranulation of mast cells. Calcium ionophores can activate mast cells to induce degranulation through high-affinity immunoglobulin E receptor–mediated signaling, transporting extracellular calcium into the cytosol [27]. In contrast, degranulation of mast cells is inhibited by increasing intracellular cAMP levels [19]. Therefore, the regulation of Ca2+ influx and intracellular cAMP concentration in mast cells plays an important role in degranulation. G-Rg3 treatment increased intracellular cAMP levels within 15 min and decreased antigen-induced calcium influx in mast cells (Fig. 2).
During inflammatory and allergic responses, mast cells activated by allergens release several inflammatory mediators, such as histamine, proinflammatory cytokines, and chemokines. Ginsenoside Rb1, Re, and Rh1 treatment significantly decreased histamine release from activated mast cells [28], [29], [30]. In addition, G-Rg5 and G-Rh3 suppressed degranulation of mast cells by decreasing histamine, TNF-α, and IL-6 in RBL-2H3 cells [31]. Moreover, G-Rg3 inhibits the release of TNF-α, IL-1β, and IL-6 in LPS-stimulated neutrophils and macrophages in bronchoalveolar lavage fluids [32]. Our results showed that the release of proinflammatory cytokines including IL-1β, IL-6, and TNF-α in mast cells was decreased by G-Rg3 treatment (Fig. 3).
The activation of MAPKs, including ERK, JNK, and p38, is related to mast cell degranulation and subsequent cytokine production [33]. NF-κB is a major transcription factor that is a critical mediator of the inflammatory reaction. MAPKs activated by various stimuli degrade IκB proteins; the separated NF-κB is translocated into the nucleus, and its target genes are transcribed [4]. RIP2/caspase-1 activation is also related to the NF-κB signaling pathway and production of proinflammatory cytokines. It has been reported that Korean Red Ginseng extract suppresses proinflammatory cytokine release by inhibiting MAPK/NF-κB activation [11]. Active compounds in Korean Red Ginseng including G-Re inhibited activation of MAPKs including p38 ERK, and JNK in the LPS-induced lung inflammation model [34]. In addition, G-Rg3 inhibits receptor activator of nuclear factor kappa-Β ligand-induced osteoclast differentiation and epithelial–mesenchymal transition of lung cancer cells via MAPK/NF-κB signaling pathways [12], [35].
In this study, we investigated the antiallergic effects of G-Rg3 on mast cells and in a mouse model using maximum doses of 50 μg/mL and 50 mg/kg G-Rg3, respectively. It has been reported that a G-Rg3 concentration of 50 μg/mL is reached in 1–2 h after oral administration of 50 mg/kg G-Rg3 in normal rats [36]. In addition, oral administration of 50 mg/kg of G-Rg3 showed hepatoprotective effects in mice with tert-butyl hydroperoxide–induced liver injury [37]. Therefore, we investigated the antiallergic effects using 2–50 μg/mL of G-Rg3 in an in vitro experiment and 10–50 mg/kg of G-Rg3 in the in vivo model.
PDN, a corticosteroid drug used to treat inflammatory diseases, was used as a positive control in this study. PDN suppresses LPS-induced TNF-α, IL-6, IL-8, and monocyte chemotactic protein 1 release in the serum and IL-1β-induced cyclooxygenase-2 expression via the ERK, JNK, and activator protein 1 signaling pathway in house ear institute-organ of corti 1 murine auditory cells [38], [39]. Particularly, PDN significantly decreased 2,4-dinitrofluorobenzene–induced scratching behavior and the edematous reaction in IgE-sensitized mice [40]. Although PDN showed the greatest effects in the experimental groups, G-Rg3 also significantly suppressed anaphylactic shock and the PCA reaction (Table 2 and Fig. 7).
In conclusion, this study demonstrated that G-Rg3 inhibits mast cell–mediated allergic responses by blocking degranulation. G-Rg3 decreases histamine and proinflammatory cytokine release via the MAPK/NF-κB and RIP2/caspase-1 pathway. Compound 48/80-induced anaphylactic shock and the IgE-mediated PCA reaction were suppressed by G-Rg3 administration in the mouse model. Therefore, G-Rg3 is a potential agent for treating allergic diseases.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2015R1C1A1A02036733 and NRF-2016R1A2B2013921).
References
- 1.Galli S.J., Kalesnikoff J., Grimbaldeston M.A., Piliponsky A.M., Williams C.M., Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 2.Galli S.J., Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immunol. 2010;40:1843–1851. doi: 10.1002/eji.201040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson G.L., Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 4.May M.J., Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 5.Barnes P.J., Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 6.Kuida K., Lippke J.A., Ku G., Harding M.W., Livingston D.J., Su M.S., Flavell R.A. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 7.Lamkanfi M., Kalai M., Saelens X., Declercq W., Vandenabeele P. Caspase-1 activates nuclear factor of the kappa-enhancer in B cells independently of its enzymatic activity. J Biol Chem. 2004;279:24785–24793. doi: 10.1074/jbc.M400985200. [DOI] [PubMed] [Google Scholar]
- 8.Thornberry N.A., Bull H.G., Calaycay J.R., Chapman K.T., Howard A.D., Kostura M.J., Miller D.K., Molineaux S.M., Weidner J.R., Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi K., Inohara N., Hernandez L.D., Galán J.E., Nú˜nez G., Janeway C.A., Medzhitov R., Flavell R.A. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 10.Chen C.F., Chiou W.F., Zhang J.T. Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacol Sin. 2008;29:1103–1108. doi: 10.1111/j.1745-7254.2008.00868.x. [DOI] [PubMed] [Google Scholar]
- 11.Kee J.Y., Jeon Y.D., Kim D.S., Han Y.H., Park J., Youn D.H., Kim S.J., Ahn K.S., Um J.Y., Hong S.H. Korean Red Ginseng improves atopic dermatitis-like skin lesions by suppressing expression of proinflammatory cytokines and chemokines in vivo and in vitro. J Ginseng Res. 2017;41:134–143. doi: 10.1016/j.jgr.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian L., Shen D., Li X., Shan X., Wang X., Yan Q., Liu J. Ginsenoside Rg3 inhibits epithelial-mesenchymal transition (EMT) and invasion of lung cancer by down-regulating FUT4. Oncotarget. 2016;7:1619–1632. doi: 10.18632/oncotarget.6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K.S., Jung Yang H., Lee I.S., Kim K.H., Park J., Jeong H.S., Kim Y., Ahn K.S., Na Y.C., Jang H.J. The aglycone of ginsenoside Rg3 enables glucagon-like peptide-1 secretion in enteroendocrine cells and alleviates hyperglycemia in type 2 diabetic mice. Sci Rep. 2015;5:18325. doi: 10.1038/srep18325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang L.J., Choi Y.J., Lee S.G. Stimulation of TRAF6/TAK1 degradation and inhibition of JNK/AP-1 signalling by ginsenoside Rg3 attenuates hepatitis B virus replication. Int J Biochem Cell Biol. 2013;45:2612–2621. doi: 10.1016/j.biocel.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Shin Y.M., Jung H.J., Choi W.Y., Lim C.J. Antioxidative, anti-inflammatory, and matrix metalloproteinase inhibitory activities of 20(S)-ginsenoside Rg3 in cultured mammalian cell lines. Mol Biol Rep. 2013;40:269–279. doi: 10.1007/s11033-012-2058-1. [DOI] [PubMed] [Google Scholar]
- 16.Bae E.A., Han M.J., Shin Y.W., Kim D.H. Inhibitory effects of Korean red ginseng and its genuine constituents ginsenosides Rg3, Rf, and Rh2 in mouse passive cutaneous anaphylaxis reaction and contact dermatitis models. Biol Pharm Bull. 2006;29:1862–1867. doi: 10.1248/bpb.29.1862. [DOI] [PubMed] [Google Scholar]
- 17.Kim H.S., Kim D.H., Kim B.K., Yoon S.K., Kim M.H., Lee J.Y., Kim H.O., Park Y.M. Effects of topically applied Korean red ginseng and its genuine constituents on atopic dermatitis-like skin lesions in NC/Nga mice. Int Immunopharmacol. 2011;11:280–285. doi: 10.1016/j.intimp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Gould H.J., Sutton B.J., Beavil A.J., Beavil R.L., McCloskey N., Coker H.A., Fear D., Smurthwaite L. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 19.Tasaka K. Role of Ca2+ and cAMP in histamine release from mast cells. New Adv Histamine Res. 1994:97–167. [Google Scholar]
- 20.Sarkar A., Duncan M., Hart J., Hertlein E., Guttridge D.C., Wewers M.D. ASC directs NF-kappaB activation by regulating receptor interacting protein-2 (RIP2) caspase-1 interactions. J Immunol. 2006;176:4979–4986. doi: 10.4049/jimmunol.176.8.4979. [DOI] [PubMed] [Google Scholar]
- 21.Krause K., Metz M., Makris M., Zuberbier T., Maurer M. The role of interleukin-1 in allergy-related disorders. Curr Opin Allergy Clin Immunol. 2012;12:477–484. doi: 10.1097/ACI.0b013e3283574d0c. [DOI] [PubMed] [Google Scholar]
- 22.Braga F., Mota I. Homologous passive cutaneous anaphylaxis (PCA) in mice and heterologous PCA induced in rats with mouse IgE. Immunology. 1976;30:655–669. [PMC free article] [PubMed] [Google Scholar]
- 23.Tasaka K., Mio M., Okamoto M. Intracellular calcium release induced by histamine releasers and its inhibition by some antiallergic drugs. Ann Allergy. 1986;56:464–469. [PubMed] [Google Scholar]
- 24.Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012 Jan;106(1):9–14. doi: 10.1016/j.rmed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Hamann K., Grabbe J., Welker P., Haas N., Algermissen B., Czarnetzki B.M. Phenotypic evaluation of cultured human mast and basophilic cells and of normal human skin mast cells. Arch Dermatol Res. 1994;286:380–385. doi: 10.1007/BF00371797. [DOI] [PubMed] [Google Scholar]
- 26.Passante E., Frankish N. The RBL-2H3 cell line: its provenance and suitability as a model for the mast cell. Inflamm Res. 2009;58:737–745. doi: 10.1007/s00011-009-0074-y. [DOI] [PubMed] [Google Scholar]
- 27.Dedkova E.N., Sigova A.A., Zinchenko V.P. Mechanism of action of calcium ionophores on intact cells: ionophore-resistant cells. Membr Cell Biol. 2000;13(3):357–368. [PubMed] [Google Scholar]
- 28.Bae H.M., Cho O.S., Kim S.J., Im B.O., Cho S.H., Lee S., Kim M.G., Kim K.T., Leem K.H., Ko S.K. Inhibitory effects of ginsenoside re isolated from ginseng berry on histamine and cytokine release in human mast cells and human alveolar epithelial cells. J Ginseng Res. 2012;36:369–374. doi: 10.5142/jgr.2012.36.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park E.K., Choo M.K., Han M.J., Kim D.H. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol. 2004;133:113–120. doi: 10.1159/000076383. [DOI] [PubMed] [Google Scholar]
- 30.Ro J.Y., Ahn Y.S., Kim K.H. Inhibitory effect of ginsenoside on the mediator release in the Guinea pig lung mast cells activated by specific antigen-antibody reactions. Int J Immunopharmacol. 1998;20:625–641. doi: 10.1016/s0192-0561(98)00062-9. [DOI] [PubMed] [Google Scholar]
- 31.Shin Y.W., Bae E.A., Han M.J., Kim D.H. Metabolism of Ginsenoside Rg5, a main constituent isolated from red ginseng, by human intestinal microflora and their antiallergic effect. J Microbiol Biotechnol. 2006;16:1791–1798. [Google Scholar]
- 32.Cheng Z., Li L. Ginsenoside Rg3 ameliorates lipopolysaccharide-induced acute lung injury in mice through inactivating the nuclear factor-κB (NF-κB) signaling pathway. Int Immunopharmacol. 2016;34:53–59. doi: 10.1016/j.intimp.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy–from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Lee J.H., Min D.S., Lee C.W., Song K.H., Kim Y.S., Kim H.P. Ginsenosides from Korean Red Ginseng ameliorate lung inflammatory responses: inhibition of the MAPKs/NF-κB/c-Fos pathways. J Ginseng Res. 2018;42:476–484. doi: 10.1016/j.jgr.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siddiqi M.H., Siddiqi M.Z., Kang S., Noh H.Y., Ahn S., Simu S.Y., Aziz M.A., Sathishkumar N., Jiménez Pérez Z.E., Yang D.C. Inhibition of osteoclast differentiation by ginsenoside Rg3 in RAW264.7 cells via RANKL, JNK and p38 MAPK pathways through a modulation of cathepsin K: an in silico and in vitro study. Phytother Res. 2015;29:1286–1294. doi: 10.1002/ptr.5374. [DOI] [PubMed] [Google Scholar]
- 36.Fan H., Xiao-Ling S., Yaliu S., Ming-Ming L., Xue F., Xian-Sheng M., Li F. Comparative pharmacokinetics of ginsenoside Rg3 and ginsenoside Rh2 after oral administration of ginsenoside Rg3 in normal and Walker 256 tumor-bearing rats. Pharmacogn Mag. 2016;12:21–24. doi: 10.4103/0973-1296.176014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H.U., Bae E.A., Han M.J., Kim D.H. Hepatoprotective effect of 20(S)-ginsenosides Rg3 and its metabolite 20(S)-ginsenoside Rh2 on tert-butyl hydroperoxide-induced liver injury. Biol Pharm Bull. 2005;28:1992–1994. doi: 10.1248/bpb.28.1992. [DOI] [PubMed] [Google Scholar]
- 38.Hong H., Jang B.C. Prednisone inhibits the IL-1β-induced expression of COX-2 in HEI-OC1 murine auditory cells through the inhibition of ERK-1/2, JNK-1 and AP-1 activity. Int J Mol Med. 2014;34:1640–1646. doi: 10.3892/ijmm.2014.1967. [DOI] [PubMed] [Google Scholar]
- 39.de Kruif M.D., Lemaire L.C., Giebelen I.A., van Zoelen M.A., Pater J.M., van den Pangaart P.S., Groot A.P., de Vos A.F., Elliott P.J., Meijers J.C. Prednisolone dose-dependently influences inflammation and coagulation during human endotoxemia. J Immunol. 2007;178:1845–1851. doi: 10.4049/jimmunol.178.3.1845. [DOI] [PubMed] [Google Scholar]
- 40.Musoh K., Nakamura N., Sakurai T., Inagaki N., Nagai H. Scratching behavior in mice associated with IgE-mediated allergic cutaneous reaction and its pharmacological characterization. Allergol Int. 1997;46:117–124. [Google Scholar]