Abstract
Background
Ginsenosides of Korean Red Ginseng extracts (RGE) and its saponin components suppress secretion of inflammasome-mediating cytokines, whereas the nonsaponin fraction (NS) of RGE oppositely stimulates cytokine secretion. Although direct exposure of NS to macrophages in mice induces cytokine production, oral administration of NS has not been studied in inflammasome-related disease in animal models.
Methods
Mice were fed RGE or NS for 7 days and then developed peritonitis. Peritoneal cytokines were measured, and peritoneal exudate cells (PECs) were collected to assay expression levels of a set of toll-like receptors (TLRs) and cytokines in response to NS ingestion. In addition, the role of intestinal bacteria in NS-fed mice was assessed. The effect of preexposure to NS in bone marrow–derived macrophages (BMDMs) on cytokine production was further confirmed.
Results
NS ingestion attenuated secretion of peritoneal cytokines resulting from peritonitis. In addition, the isolated PECs from NS-fed mice presented lower TLR transcription levels than PECs from control diet–fed mice. BMDMs treated with NS showed downregulation of TLR4 mRNA and protein expression, which was mediated by the TLR4-MyD88-NFκB signal pathway. BMDMs pretreated with NS produced less cytokines in response to TLR4 ligands.
Conclusion
NS administration directly inhibits TLR4 expression in inflammatory cells such as macrophages, thereby reducing secretion of cytokines during peritonitis.
Keywords: Cytokine, Korean Red Ginseng extracts, Nonsaponin fraction, Peritonitis, TLR4
Abbreviations: Alum, aluminum potassium sulfate; BMDMs, bone marrow–derived macrophages; HKST, heat-killed Salmonella typhimurium; IL, interleukin; ip, intraperitoneally; LB, Luria-Bertani; LCCM, L929 cell-conditioned medium; LPS, lipopolysaccharide; Lys, lysate; MSU, monosodium urate crystal; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3, (NOD)2-like receptor protein 3; NOD, nucleotide-binding and oligomerization domain; Non, nontreatment; NS, nonsaponin fraction; PECs, peritoneal exudate cells; RGE, Korean Red Ginseng extracts; SF, saponin fraction; Sup, supernatant; TLRs, toll-like receptors
1. Introduction
Ginseng is a root of Panax ginseng Meyer of the family Araliaceae and is cultivated in northeastern Asian countries such as Korea and parts of China. Although the various pharmacological properties of ginseng have been verified, its ability to modulate immune systems is continuously studied [1]. Ginsenoside, a saponin of ginseng, is mostly noted as an immune modulator. Korean Red Ginseng is made by repetitive steaming and drying cycles of ginseng root and contains more ginsenosides than fresh ginseng [2], [3]. Ginsenosides attenuate production of cytokines of immune cells through inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling and maturation of cytokines through attenuation of inflammasome activation [4], [5]. In addition to saponin components of ginseng acting as immune modulators, nonsaponin (NS) ingredients such as polysaccharides possess immunomodulatory properties [6], [7], [8], [9]. Ginsan and red ginseng acidic polysaccharide have been shown to induce inflammatory cytokines through toll-like receptor (TLR)/NF-κB signaling [6], [7], [8], [9].
The effects of ginsenosides and NSs isolated from Korean Red Ginseng extracts (RGEs) on inflammasome activation have been progressively studied [4], [10]. Inflammasome, an intracellular surveillance factor that detects dangerous molecules derived from pathogens and endogenous metabolites, mediates maturation of interleukin (IL)-1β and IL-18 through cleavage of caspase-1 and induces pyroptosis by forming membrane pores of gasdermin D [11], [12]. RGE and its fractions, saponin fraction (SF) and nonsaponin fraction (NS), present contrasting effects on inflammasome activation [4], [10]. SF containing ginsenosides attenuates cytokine secretion by inhibiting the priming and activation steps of inflammasome activation, whereas NS induces upregulation of IL-1β precursor and nucleotide-binding and oligomerization domain (NOD)2–like receptor protein 3 (NLRP3), which are key inflammasome components, via interaction with TLR4 [4], [10]. In addition, a high dose of RGE presents a more SF predominant effect (antiinflammasome), whereas low concentration of RGE shows NS-like efficacy (proinflammasome) in macrophages [10]. In this study, we elucidated the roles of SF and NS of RGE on inflammasome activation in animal models. In addition, we assessed whether or not NS ingestion alters cytokine secretion as an inflammatory response during peritonitis. We further confirmed how NS ingestion regulates cytokine secretion during peritonitis by observing expression of TLRs using in vivo and in vitro systems.
2. Materials and methods
2.1. Preparation of SF and NS of RGE
RGE was manufactured from roots of 6-year-old fresh Panax ginseng provided by Korea Ginseng Corporation (Daejeon, Korea). Subfractions of RGE, SF and NS, were prepared according to previous studies [10], [13]. Briefly, RGE (2.0 Kg) was subjected sequentially to adsorption chromatography using H2O, 20% ethyl alcohol (EtOH), and absolute EtOH (Daejung Chemicals and Materials Co., Siheung-si, Gyeonggi-do, Korea) as eluents. No ginsenosides were detected on H2O or 20% EtOH elution, which was combined and evaporated to dryness in vacuo (NS, 1.1 kg). Absolute EtOH yield was 135.4 g of SF. According to the results of the component analysis (Supplementary data), SF contained higher saponin content (223.4 mg/g) than NS (5.5 mg/g). NS showed fourfold higher acidic polysaccharide content and sixfold higher arginine–fructose–glucose content than SF. Generally, the ginsenoside content of Korean Red Ginseng powder was 18.5 mg/g. The contents of SF, NS, and RGE are summarized in Table 1.
Table 1.
Major contents of saponin and nonsaponin fractions of RGE
| Components | RGE (mg/g ± SD) | Saponin fraction (SF, mg/g ± SD) | Nonsaponin fraction (NS, mg/g± SD) | |
|---|---|---|---|---|
| Ginsenoside | Rg1 | 1.06 ± 0.02 | 13.33 ± 0.70 | 0.35 ± 0.61 |
| Re | 1.21 ± 0.03 | 15.68 ± 0.77 | 0.40 ± 0.70 | |
| Rf | 1.04 ± 0.02 | 13.39 ± 0.89 | 0.35 ± 0.6 | |
| Rh1 | 0.96 ± 0.01 | 11.54 ± 1.05 | 0.32 ± 0.56 | |
| Rg2s | 1.43 ± 0.08 | 15.75 ± 0.96 | 0.48 ± 0.82 | |
| Rb1 | 5.19 ± 0.03 | 64.20 ± 5.02 | 1.73 ± 3.00 | |
| Rc | 2.02 ± 0.05 | 25.98 ± 2.54 | 0.67 ± 1.17 | |
| Rb2 | 1.88 ± 0.02 | 22.96 ± 1.63 | 0.63 ± 1.08 | |
| Rd | 0.67 ± 0.00 | 8.29 ± 0.75 | 0.22 ± 0.39 | |
| Rg3s | 2.10 ± 0.03 | 21.69 ± 2.95 | 0.70 ± 1.21 | |
| Rg3r | 0.96 ± 0.01 | 10.60 ± 1.30 | 0.32 ± 0.55 | |
| Arginine–fructose–glucose | 28.18 ± 1.06 | 6.75 ± 2.97 | 39.78 ± 10.06 | |
| Acidic polysaccharides | 63.10 ± 0.53 | 21.40 ± 2.53 | 95.43 ± 28.05 | |
| Water | 4.42 ± 1.22 | 3.38 ± 1.30 | 5.64 ± 1.24 | |
RGE, Red ginseng extract; SD, standard deviation.
2.2. Animals study
Male C57BL/6 mice (8 weeks old) obtained from Narabio Co. (Seoul, Korea) were maintained under a 12-h light/dark cycle at 24°C. Mice were supplied standard sterile food and water ad libitum, after which they were allowed to adjust to the environment for 1 week. Dosage of RGE to mice was elevated threefold [2 mg/mouse (20 g)/day] than the recommended daily intake of Cheongkwanjang [2 g/human (60 kg)/day], and the concentrations of NS and SF were based on the ratio of elution (RGE: NS: SF = 2: 1.1: 0.1354). Mice were orally administered 200 μL of filtered water as vehicle (Non; nontreatment), RGE (2 mg/mouse/day), NS (1.1 mg/mouse/day), or SF (135.4 μg/mouse/day) for 7 days and then intraperitoneally (ip) injected with monosodium urate crystal (MSU, 10 mg/mouse; U2875; Sigma-Aldrich Co., St. Louis, MO, USA), aluminum potassium sulfate (Alum, 5 mg/mouse; 039-4404; Daejung Chemicals & Materials Co.), or lipopolysaccharide (LPS, 100 μg/mouse; L4130; Sigma-Aldrich Co.). After 6 h, mice were anesthetized with ether inhalation and sacrificed by decapitation. Peritoneal cavities were washed with 5 mL of phosphate-buffered saline, and peritoneal exudate cells (PECs) were collected for further analysis. To eliminate intestinal bacteria, mice were daily fed 200 μL of antibiotic cocktail containing vancomycin (50 μg/mL), ampicillin (100 μg/mL), kanamycin (100 μg/mL), neomycin (100 μg/mL), streptomycin (50 μg/mL), penicillin (100 U/mL), gentamycin (150 μg/mL), cefazolin (100 μg/mL), and cefradine (50 μg/mL) for 7 days. For assessment of bacterial burden, peritoneal lavage (10 μL) was dropped onto Luria-Bertani (LB; Laboratories Conda, Madrid, Spain) plates and incubated at 37°C overnight. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Kangwon National University (IACUC; approval no. KW-170110-1).
2.3. Cell culture and treatment
Unless otherwise indicated, all materials for cell culture were purchased from GenDEPOT Inc. (Barker, TX, USA). Bone marrow–derived macrophages (BMDMs) were obtained by differentiation of bone marrow progenitors from tibia and femur bones of C57BL/6 mice (8- to 15-weeks old; Narabio Co.) in L929 cell-conditioned medium as a source of macrophage colony-stimulating factor [14]. The progenitors were cultured in Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10% fetal bovine serum, 50% L929 cell-conditioned medium, 100 U/mL of penicillin, and 100 μg/mL of streptomycin. Cells were seeded in nontissue culture–treated Petri dishes (SPL life science Co., Pocheon-si, Gyeonggi-do, Korea) and incubated at 37°C in 5% CO2 atmosphere for 7 days. For inflammasome activation, BMDMs (1.0 × 106 cells per well) were primed with 1 μg/mL of LPS (Sigma-Aldrich Co.) and treated with MSU (0.8 mg/mL) with/without RGE, NS or SF, for 3 h [15]. Cellular supernatant (Sup) and lysate (Lys) were collected for further analysis.
For gene expression, BMDMs (2.0 × 106 cells per well for RNA extraction or 1.0 × 106 cells per well for protein analysis) were plated on 6- or 12-well plates (SPL Life Science Co.) and treated with NS (1 mg/mL). In addition, BMDMs were treated with MSU (0.5 mg/mL) or NS (1 mg/mL) with/without CU-CPT22 (5 mM, 4884; Tocris Bioscience, Minneapolis, MN, USA), Anti-mTLR2-IgG (100 ng/mL; InvivoGen, San Diego, CA, USA), TAK-242 (5 μM, CLI-095; InvivoGen), polymyxin B (100 μg/mL; tlrl-pmb, InvivoGen), MyD88 inhibitor peptide (50 μM, tlrl-pimyd, InvivoGen), and Bay 11-7082 (5 μM; 1744, Tocris Bioscience) for 3 h or 9 h. BMDMs were pretreated with NS (1 mg/mL) for 3 h and then replaced with fresh media. After 30 h, cells were treated with LPS (5 ng/mL) or heat-killed Salmonella typhimurium (HKST, 0.001%) for 3 h. Total RNA and cellular lysates were prepared for further analysis.
2.4. Reverse transcription polymerase chain reaction and quantitative real-time polymerase chain reaction
Total RNA was extracted using NucleoZOL (MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany) and reverse transcribed to first-strand complementary DNA (cDNA) using an M-MLV cDNA Synthesis kit (Enzynomics, Daejeon, Korea) [16]. For reverse transcription polymerase chain reaction (RT-PCR), transcription was amplified by a SimpliAmp Thermal Cycler (Thermo Fisher Scientific Inc. Grand Island, NY, USA) and nTaq polymerase (Enzynomics). PCR products were visualized by agarose gel electrophoresis and ethidium bromide staining. For quantitative real-time PCR (qPCR), gene expression was quantified using an Eco Real-Time PCR system (Illumina, San Diego, CA, USA) and TOPreal qPCR 2X PreMIX containing SYBR Green (Enzynomics). Quantitation was normalized with glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Gene-specific primers are listed below. Pro-IL-1β (Genebank ID: NM_008361) primers 5′-CCC AAG CAA TAC CCA AAG AA-3′ and 5′-GCT TGT GCT CTG CTT GTG AG-3′; TNFα (NM_013693) 5′-ACG GCA TGG ATC TCA AAG AC-3′ and 5′-GTG GGT GAG GAG CAC GTA GT-3′; IL-1α (NM_010554) 5′-CCG ACC TCA TTT TCT TCT GG-3′ and 5′-GTG CAC CCG ACT TTG TTC TT-3′; IL-6 (NM_031168) 5′-GTT CTC TGG GAA ATC GTG GA-3′ and 5′-GGA AAT TGG GGT AGG AAG GA-3′; IL-10 (NM_010548) 5′-TCA TTT CCG ATA AGG CTT GG-3′ and 5′-TGC TAT GCT GCC TGC TCT TA-3′; TLR1 (NM_003263) 5′-GCA CGA TTC TTT CTG GGT GA-3′ and 5′-GGA ATG GGT TCC AGC AAG AT-3′; TLR2 (NM_001318787) 5′-GAC CGC AAT GGT ATC TGC AA-3′ and 5′-CTG CCC AGG GAA GAA AAA GA-3’; TLR3 (NM_003265) 5′-TTA GCA CGG CTC TGG AAA CA′ and 5′-GCC CGA AAA CCT TCT TCT CA-3′; TLR4 (NM_007482) 5′-GCT TTC ACC TCT GCC TTC AC-3′ and 5′-AGG CGA TAC AAT TCC ACC TG-3′; TLR5 (NM_003268) 5′-GGC CGA ATA GCC TTT TAT CG-3′ and 5′-AAG GTT GGG CAG GTT TCT GA-3′; TLR6 (NM_006068) 5′-CTG CCT GGG TGA AAA GTG AA-3′ and 5′-GGG AAT GCT GTT CTG TGG AA-3′; TLR7 (NM_016562) 5′-GGA AAA CCT TTC CCA GAG CA-3′ and 5′-GTT TGT TGG CCA CTC AAG GA-3′; TLR8 (NM_016610) 5′-GGA AAC CCC TTT GAA TGC AC-3′ and 5′-AGG GCA GCC AAC ATA ACC AT-3′; TLR9 (NM_017442) 5′-TCT TGA AGG CCT GGT GTT GA-3′ and 5′-GGC AAA GGA CAC CCT CTT TT-3′; GAPDH (NM_001289726) 5′-AAC TTT GGC ATT GTG GAA GG-3′ and 5′-ACA CAT TGG GGG TAG GAA CA-3′.
2.5. Western blot analysis
Sup and Lys samples were separated on a sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE) gel (10% or 16%), transferred onto a polyvinylidene difluoride (PVDF) membrane (Pall Corporation, Port Washington, NY, USA), and blocked with 3% skim milk. The membrane was probed with primary antibodies against antimouse IL-1β antibody (AF-401-NA; R&D Systems, MN, USA), anticaspase-1 antibody (AG-20B-0042; AdipoGen Co., San Diego, CA, USA), anti-TLR4 antibody (sc-293072; Santa Cruz Biotechnology, Santa Cruz, CA, USA), or antiactin antibody (sc-1615; Santa Cruz Biotechnology) overnight at 4°C. The membranes were further probed with Horseradish Peroxidase (HRP)-conjugated second antisera (Santa Cruz Biotechnology) and visualized using Power-Opti ECL solution (BioNote Co., Gyeonggi-do, Korea) and a cooled CCD camera System (AE-9150, EZ-Capture II; ATTO Technology, Tokyo, Japan). Band intensity was measured by CS Analyzer, version 3.00 (ATTO Technology).
2.6. Cytokine assay
To quantify secretion of IL-1β, IL-6, and IL-18, peritoneal lavage fluids or cell culture supernatants were measured by a mouse IL-1beta/IL-1F2 Salmonella typhimurium ELISA Kit (MTA00B; R&D Systems), mouse IL-6 Quantikine ELISA Kit (M6000B; R&D Systems), and mouse IL-18 platinum ELISA (eBioscience, San Diego, CA, USA). The plates were read out using a Synergy H1 microplate reader (BioTek, Winooski, VT, USA).
2.7. Statistical analyses
Statistical analyses were performed using a t test (Mann–Whitney test) for the two groups or one-way analysis of variance (Tukey's multiple comparisons test) for multiple groups using GraphPad Prism 6 (GraphPad Software, San Diego CA).
3. Results
3.1. Oral administration of NS inhibits peritoneal IL-1β and IL-6 secretion in MSU-induced peritonitis
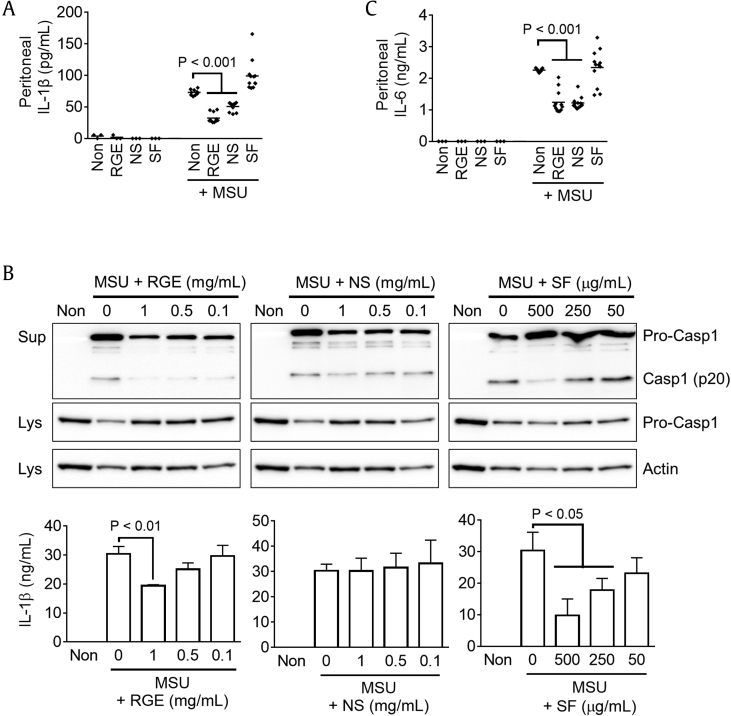
To elucidate the in vivo properties of NS of RGE in an inflammasome-mediated disease model of MSU-induced peritonitis [17], [18], mice were fed Korean RGE, SF, or NS for 7 days and then ip injected with MSU. Peritoneal IL-1β secretion was measured as a readout of inflammasome activation. As seen in Fig. 1A, MSU treatment induced peritoneal IL-1β secretion, whereas secretion was significantly attenuated in RGE- or NS-fed mice. This result implies that NS has antiinflammasome properties. Then, we treated RGE, NS, or SF on LPS-primed BMDMs to confirm their antiinflammasome properties in an in vitro system (Fig. 1B). RGE or SF treatments blocked MSU-mediated caspase-1 and IL-1β secretions, whereas NS did not. This result suggests that NS does not have antiinflammasome properties. We then observed peritoneal IL-6 secretion in MSU-injected mice fed RGE, NS, or SF (Fig. 1C). Interestingly, IL-6 secretion levels in RGE- or NS-fed mice were significantly lower than those in control and SF-fed mice, similar to the results for IL-1β secretion. Based on these data, NS ingestion might inhibit inflammatory cytokine secretion resulting from MSU-induced peritonitis independent of inflammasome activation.
Fig. 1.
Effects of RGE, NS, and SF on peritoneal cytokine secretion and inflammasome activation in response to MSU injection. Mice (total n = 57) were fed Non (nontreatment, 200 μL of water, n = 12), Korean Red Ginseng extracts (RGE, 2 mg/mouse/day, n = 15), nonsaponin fraction (NS, 1.1 mg/mouse/day, n = 15), or saponin fraction (SF, 135.4 μg/mouse/day, n = 15) for 7 days and then intraperitoneally (ip) injected with PBS (3 mice for each group) or monosodium urate crystal (MSU, 10 mg/mouse) to induce peritonitis. After 6 h, peritoneal (A) IL-1β and (C) IL-6 secretions were measured. (B) LPS-primed BMDMs were treated with MSU (0.8 mg/mL) in the presence of RGE, NS, or SF as indicated. Secretions of caspase-1 (Casp1) and IL-1β were assayed by immunoblotting and ELISA. Bar graph presents the mean ± SD. All data shown are representative of at least two independent experiments.
BMDM, bone marrow–derived macrophages; ELISA, enzyme-linked immunosorbent assay; IL, interleukin; LPS, lipopolysaccharide; Lys, lysate; PBS, phosphate-buffered saline; SD, standard deviation; Sup, supernatent.
3.2. NS intake inhibits cytokine production resulting from peritonitis
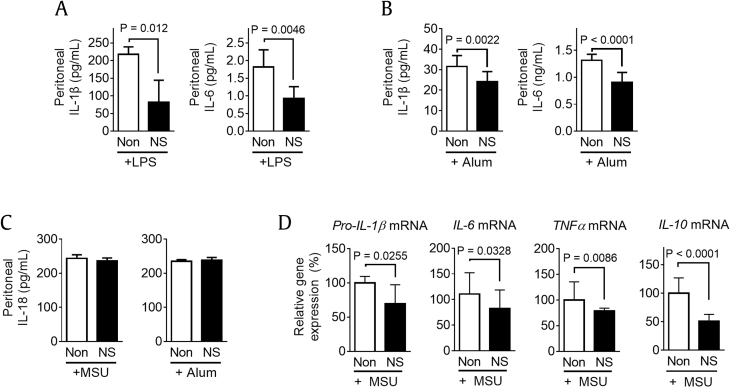
We further confirmed the inhibitory effect of NS intake on peritoneal IL-1β secretion in the context of inflammasome-mediating peritonitis. Mice ingested NS for 7 days and were then ip injected with LPS, a TLR4 ligand and noncanonical inflammasome trigger [16], [19], [20], or alum, an inflammasome trigger [21]. In our results, NS ingestion attenuated peritoneal IL-1β secretion and peritoneal IL-6 secretion in both LPS- and alum-injected mice (Fig. 2A and B). Then, we tested peritoneal IL-18 production, another readout of inflammasome activation [12], [22]. As seen in Fig. 2C, IL-18 secretion in response to MSU or alum was not altered by NS feeding. This result implies that NS attenuated peritoneal production of IL-1β and IL-6 independent of inflammasome activation. In addition, we elucidated the mRNA expression levels of pro-IL-1β, IL-6, TNFα, and IL-10 in PECs isolated from MSU-injected mice fed with/without NS (Fig. 2D). Similar to peritoneal IL-1β and IL-6 secretions, gene expression levels were downregulated by NS ingestion. Based on these data, we can conclude that NS intake inhibits expression of inflammatory cytokines in peritonitis.
Fig. 2.
Effects of NS intake on peritoneal cytokine secretion in peritonitis. Mice (n = 6 per group) were fed Non (200 μL of water) or NS (1.1 mg/mouse/day) for 7 days and then ip injected with (A) LPS (100 μg/mouse) or (B) alum (5 mg/mouse). (C) After 6 h, peritoneal IL-1β, IL-6, and IL-18 secretions were measured. (D) PECs were collected, and mRNA expression levels of pro-IL-1β, IL-6, TNFα, and IL-10 were analyzed. Bar graph presents the mean ± SD.
IL, interleukin; ip, intraperitoneally; LPS, lipopolysaccharide; MSU, monosodium urate; NS, nonsaponin fraction; PEC, peritoneal exudate cell; SD, standard deviation.
3.3. NS ingestion inhibits TLR4 expression via TLR4-MyD88-NF-κB signaling
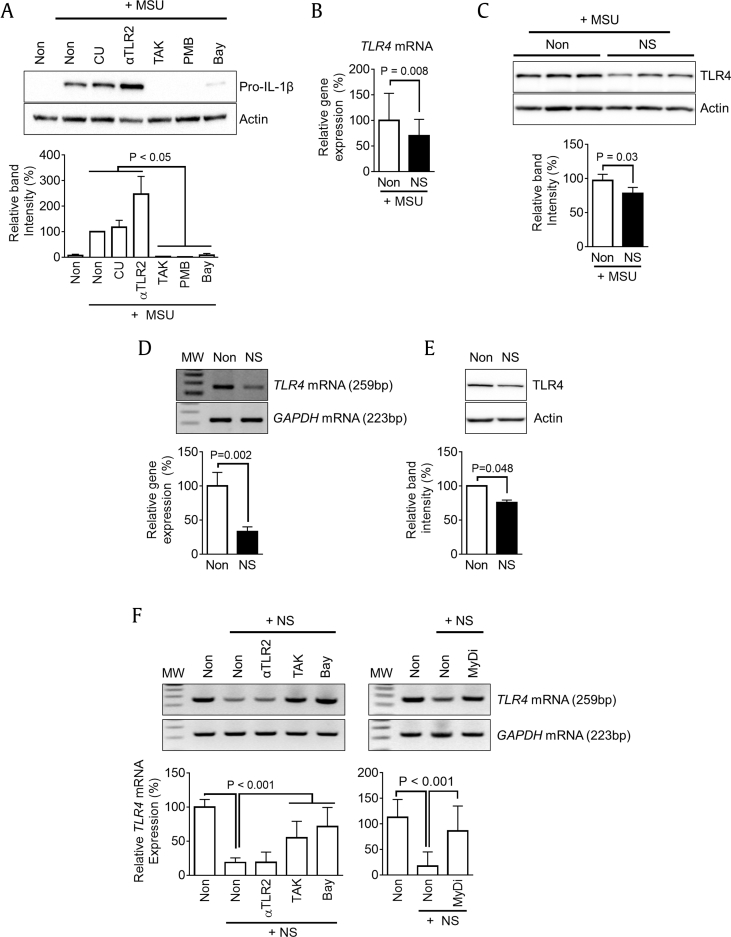
Although MSU crystals are known to induce production of IL-1β via inflammasome activation [17], they also induce production of inflammatory cytokines by interacting with TLR2 and TLR4 [23]. Therefore, we focused on cytokine production by TLR signaling and tested which TLR is involved in pro-IL-1β expression in response to MSU. BMDMs were treated with MSU in the presence of CU-CPT22 (CU, TLR1/2 antagonist), anti-mTLR2-IgG (αTLR2, TLR2 neutralizing antibody), TAK-242 (TAK, TLR4 inhibitor), polymyxin B (PMB, TLR4 inhibitor), and Bay 11-7082 (Bay, NF-κB inhibitor). As seen in Fig. 3A, upregulation of pro-IL-1β expression by MSU treatment was not altered by the TLR2 blockers, whereas expression was significantly reduced by inhibition of TLR4 and NF-κB signaling. This result implies that MSU induces cytokine expression via TLR4. Then, we confirmed the transcriptional and translational levels of TLR4 in PECs isolated from MSU-injected mice (Fig. 3B and C). Interestingly, the expression of TLR4 was significantly attenuated in NS-fed mice. In sequence, we assessed whether or not NS directly inhibits TLR4 expression in an in vitro system. BMDMs were treated with NS, and expression of TLR4 mRNA and protein was measured (Fig. 3D and E). NS treatment significantly downregulated TLR4 expression in macrophages. Furthermore, we tested which downstream pathway transmits NS signaling for downregulation of TLR4 expression. BMDMs were treated with NS in the presence of inhibitors for TLR2 (αTLR2), TLR4 (TAK), NF-κB (Bay), or MyD88 (MyDi, MyD88 inhibitor peptide). As shown in Fig. 3F, decreased TLR4 mRNA expression by NS treatment was recovered by TLR4 and NF-κB inhibitors but not by TLR2 blocker. In addition, MyD88 inhibitor interrupted NS-mediated TLR4 downregulation. Taken together, NS attenuates the expression of TLR4 via the TLR4-MyD88-NF-κB signal pathway, in which the receptor mediates cytokine production in response to MSU injection.
Fig. 3.
Effects of MSU on TLR signaling and NS on TLR4 expression. (A) BMDMs were treated with MSU (0.5 mg/mL) in the presence of TLR inhibitors (CU for TLR1/2, αTLR2 for TLR2, and TAK and PMB for TLR4) or NF-κB inhibitor (Bay) for 3 h. The protein levels of pro-IL-1β were measured as a readout of MSU–TLR interaction. The below bar graph indicates band intensity. Mice (n = 6 per group) fed Non (200 μL of water) or NS (1.1 mg/mouse/day) for 7 days and then ip injected with MSU (10 mg/mouse) to induce peritonitis. PECs were isolated from mice, and (B) TLR4 mRNA and (C) protein expression was analyzed by qPCR and immunoblotting. BMDMs were treated with NS for 3 h, and (D) mRNA and (E) protein expression levels of TLR4 were measured using RT-PCR/qPCR and immunoblotting. (F) BMDMs were treated with NS in the presence of TLR2, TLR4, NF-κB, or MyD88 (MyDi) inhibitor, and TLR4 mRNA expression was assayed by RT-PCR/qPCR. Bar graph presents the mean ± SD. All data shown are representative of at least two independent experiments. MW, molecular weight (100 bp ladder).
BMDM, bone marrow–derived macrophages; IL, interleukin; ip, intraperitoneally; MSU, monosodium urate; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NS, nonsaponin fraction; PEC, peritoneal exudate cell; qPCR, quantitative real-time polymerase chain reaction; RT-PCR, reverse transcription polymerase chain reaction; SD, standard deviation; TLR, toll-like receptor.
3.4. Intestinal bacteria are a key factor for peritoneal IL-1β secretion in response to MSU injection
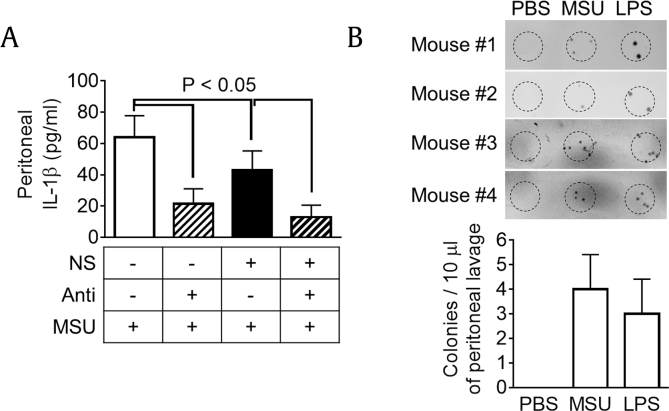
Then, we assessed the effects of intestinal bacteria, which might modify the chemical components of NS [24] and/or directly stimulate TLR4 signaling. To eliminate intestinal bacteria, mice were supplied an antibiotic cocktail with/without NS, and peritonitis was induced by MSU injection (Fig. 4A). In the results, antibiotic administration significantly reduced peritoneal IL-1β secretion in both mice fed with/without NS. Although NS ingestion significantly reduced IL-1β production, antibiotic administration showed much more potent effects than NS intake alone, implying that intestinal bacteria directly stimulates peritoneal IL-1β secretion rather than chemical modification of NS. We further tested whether or not bacteria invade the peritoneal cavity during peritonitis. We collected peritoneal lavage from MSU- or LPS-injected mice and cultured the lavage to assess bacterial burden (Fig. 4B). Interestingly, bacterial burden was observed in the peritoneal cavity of MSU- or LPS-injected mice, implying that intestinal bacteria invade into the peritoneal cavity during peritonitis and stimulate TLR4 signaling of peritoneal immune cells to produce cytokines.
Fig. 4.
Effects of intestinal bacteria on IL-1β secretion in MSU-induced peritonitis. (A) Mice (n = 6 per group) were fed Non (200 μL of water) or NS (1.1 mg/mouse/day) with/without antibiotic cocktail for 7 days and then ip injected with MSU (10 mg/mouse) to induce peritonitis. Peritoneal IL-1β secretion was measured. (B) Mice (n = 4 per group) were ip injected with PBS (200 μL), MSU (10 mg/mouse), or LPS (100 μg/mouse). After 6 h, peritoneal lavage was collected and cultured onto an LB plate to observe bacterial burden. Bar graph presents the mean ± SD.
IL, interleukin; ip, intraperitoneally; LB, Luria-Bertani; LPS, lipopolysaccharide; MSU, monosodium urate; NS, nonsaponin fraction; PBS, phosphate-buffered saline; SD, standard deviation.
3.5. NS attenuates cytokine production via inhibition of TLR4 expression
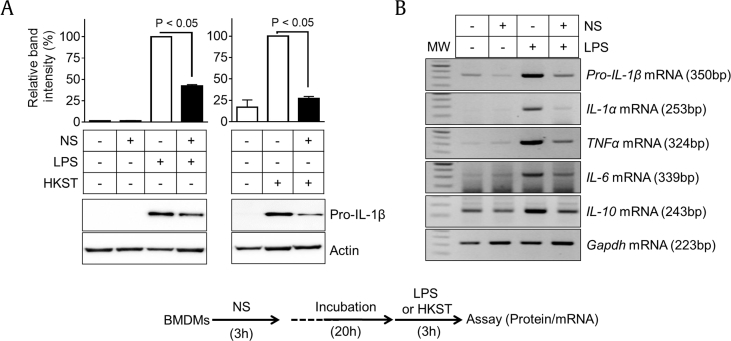
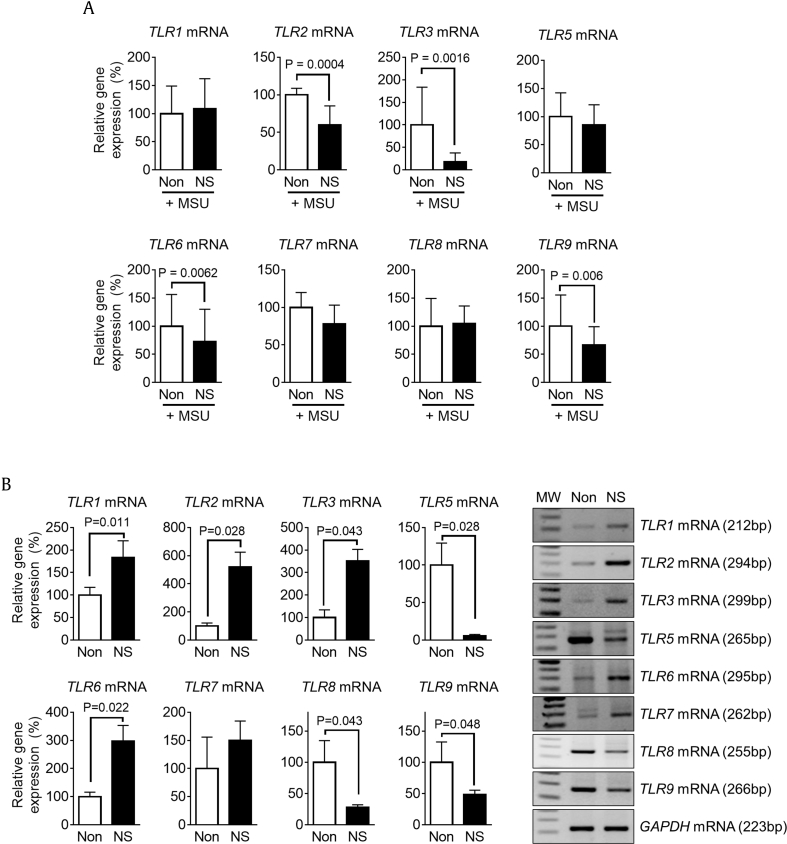
We then elucidated whether or not NS pretreatment attenuates TLR4-mediated cytokine expression. Macrophages were treated with NS 1 day before treatment with LPS, a TLR4 ligand, or HKST, a TLR4 and TLR5 ligand. In the results, both TLR ligands induced pro-IL-1β expression in BMDMs, whereas increased pro-IL-1β expression in BMDMs pretreated with NS was significantly lower than that in intact BMDMs (Fig. 5A). In addition, NS pretreatment interrupted the upregulation of LPS-mediated cytokine transcription (Fig. 5B). This implies that the efficacy of LPS- and HKST-mediated cytokine production was abrogated because NS pretreatment downregulated TLR4 expression in BMDMs. In addition, we determined the expression levels of other TLRs in PECs (Fig. 3B and C) and BMDMs (Fig. 3D and E). PECs isolated from NS-fed mice presented reduced transcription levels of TLR2, TLR3, TLR6, and TLR9, whereas there was no increase in TLR transcription (Fig. 6A). NS treatment in BMDMs downregulated TLR5, TLR8, and TLR9 gene expression but upregulated mRNA expression of TLR1, TLR2, TLR4, and TLR6 (Fig. 6B). Although NS differentially regulated TLR expression between PECs and BMDMs, NS constantly downregulated mRNA expression of TLR4 and TLR9. Taken together, we conclude that the inhibitory effects of NS on cytokine secretion in peritonitis (Fig. 1, Fig. 2B) can be attributed to downregulation of TLR4 because MSU and LPS are TLR4 ligands. In addition, reduction of TLRs expression in PECs might attenuate cytokine production stimulated by invading bacteria.
Fig. 5.
Effects of NS pretreatment on LPS- or HKST-mediated cytokine expression. BMDMs were pretreated with NS (1 mg/mL) for 3 h and then further administered LPS (TLR4 ligand) or HKST (TLR4/5 ligand) for 3 h as indicated in the below schematic graph. (A) Protein levels of pro-IL-1β were measured by immunoblotting. (B) Gene expression levels of pro-IL-1β, IL-1α, TNFα, IL-6, and IL-10 mRNAs were analyzed by RT-PCR. Bar graph presents the mean ± SD. All data shown are representative of at least two independent experiments. BMDM, bone marrow–derived macrophages; HKST, heat-killed Salmonella typhimurium.
IL, interleukin; LPS, lipopolysaccharide; MW, molecular weight; NS, nonsaponin fraction; RT-PCR, reverse transcription polymerase chain reaction; SD, standard deviation.
Fig. 6.
Effects of NS pretreatment on LPS- or HKST-mediated cytokine expression. (A) Mice (n = 6 per group) fed Non (200 μL of water) or NS (1.1 mg/mouse/day) for 7 days and then ip injected with MSU (10 mg/mouse) to induce peritonitis. PECs were isolated from mice, and TLR mRNA expression was analyzed by qPCR. (B) BMDMs were treated with NS for 3 h, and expression levels of TLR mRNAs were measured using RT-PCR and qPCR. Bar graph presents the mean ± SD. All data shown are representative of at least two independent experiments. MW, molecular weight (100 bp ladder). BMDM, bone marrow–derived macrophages; HKST, heat-killed Salmonella typhimurium.
IL, interleukin; ip, intraperitoneally; LPS, lipopolysaccharide; MSU, monosodium urate; NS, nonsaponin fraction; PEC, peritoneal exudate cell; qPCR, quantitative real-time polymerase chain reaction; RT-PCR, reverse transcription polymerase chain reaction; SD, standard deviation.
4. Discussion
Based on the results of this study, we conclude that NS intake downregulated TLR4 expression in inflammatory cells, resulting in attenuation of cytokine production in response to peritonitis. Mice fed RGE or NS presented lower peritoneal IL-1β and IL-6 secretions resulting from MSU, alum, and LPS injection. However, inflammasomes were not involved in NS-mediated cytokine inhibition because peritoneal IL-18 secretion was unchanged in NS-fed mice, and NS treatment did not alter IL-1β or caspase-1 secretion in BMDMs. In addition to being inflammasome triggers, MSU and LPS are ligands for TLR4. Expression of TLR4 in PECs isolated from peritonitis mice was inhibited by NS ingestion, and TLR4 expression was also attenuated by NS treatment in BMDMs. Cytokine secretion during peritonitis is not only regulated by NS ingestion but also dependent on intestinal penetration into the peritoneum. Thus, NS ingestion inhibits cytokine production through inhibition of TLR4 expression, which is activated by MSU, LPS, and intestinal bacteria.
Red ginseng contains several ginsenosides, such as Rg1, Re, Rb1, Rc, Rb2, and Rd [25]. These saponin contents are regarded as major pharmaceutical effectors on biological activities. However, Red ginseng also has nutritional components such as saccharides and amino acids [25]. Among them, arginine with sugars such as arginine–fructose–glucose and arginine–fructose have been shown to inhibit protein glycosylation in the blood and attenuate harmful signs of metabolic disease [25]. Furthermore, acid polysaccharides, a well-defined content of NS, have various inhibitory effects on fatigue, tumor growth, oxidation production, ulcers, and hyperlipidemia [25]. In addition, acid polysaccharides act as immunomodulatory agents by altering nitric oxide production, transcription factors, and phosphorylating enzymes. Based on previous literature, acid polysaccharides interact with TLR2 of macrophages, resulting in induction of immune responses [9]. Other known components of red ginseng extracts include polyactylene compounds such as panaxynol and panaxydol. These compounds present toxicity and antitumor activities in vitro but not in vivo because polyactylenes are chemically unstable [25]. Although we could not characterize exactly which NS component downregulated TLR4 expression, we speculate that several TLR4 ligands present in NS downregulate transcription of TLR4.
Ginseng is well characterized to modulate the immune response via TLRs, especially TLR4. Ginseng extracts interact with TLR4 and induce expression of proinflammatory cytokines [26]. In addition, our previous report suggests that NS interacts with TLR4 to induce expression of inflammatory cytokines [27]. These reports suggest that RGE itself and its components are tightly involved in TLR4 signaling in the immune response.
Several studies have investigated the effects of ginseng extracts on expression of TLR4. Oral administration of Panax ginseng extracts to mice was shown to attenuate increased hepatic TLR4 expression in alcoholic liver disease [28]. In addition, the extracts were shown to inhibit expression of TLR4 in murine RAW 264.7 macrophages [29]. Saponin of ginseng, ginsenoside Rg1, attenuated TLR4 expression in neuroglial cells in response to amyloid beta peptide [30]. Ginsa, a polysaccharide extracted from Panax ginseng, downregulated TLR4, which was induced by Staphylococcus aureus inoculation in mouse peritoneal macrophages [31]. However, opposite data have been reported [32]. Ginseng extracts were fed to mice, and TLR4 expression in their peritoneal macrophages was measured for 4 weeks [32]. TLR4 expression peaked during the second and third weeks and then returned to levels of the untreated group [32]. The authors insisted that the levels of TLR4 were consistent with the levels of proinflammatory cytokines [32]. Based on these data, we speculate that ginseng and its components modulated levels of TLR4 in the in vivo and in vitro experiments.
Conflicts of interest
The authors have no conflicts of interest.
Acknowledgments
This research was supported by a grant (2016) from the Korean Society of Ginseng funded by the Korean Ginseng Corporation and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2016R1A4A1010115).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jgr.2018.03.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Ru W., Wang D., Xu Y., He X., Sun Y.E., Qian L., Zhou X., Qin Y. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.) Drug Discov Ther. 2015;9:23–32. doi: 10.5582/ddt.2015.01004. [DOI] [PubMed] [Google Scholar]
- 2.Baek S.H., Bae O.N., Park J.H. Recent methodology in ginseng analysis. J Ginseng Res. 2012;36:119–134. doi: 10.5142/jgr.2012.36.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohn E.H., Jang S.A., Lee C.H., Jang K.H., Kang S.C., Park H.J., Pyo S. Effects of Korean red ginseng extract for the treatment of atopic dermatitis-like skin lesions in mice. J Ginseng Res. 2011;35:479–486. doi: 10.5142/jgr.2011.35.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J., Ahn H., Han B.C., Lee S.H., Cho Y.W., Kim C.H., Hong E.J., An B.S., Jeung E.B., Lee G.S. Korean red ginseng extracts inhibit NLRP3 and AIM2 inflammasome activation. Immunol Lett. 2014;158:143–150. doi: 10.1016/j.imlet.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Kee J.Y., Jeon Y.D., Kim D.S., Han Y.H., Park J., Youn D.H., Kim S.J., Ahn K.S., Um J.Y., Hong S.H. Korean red ginseng improves atopic dermatitis-like skin lesions by suppressing expression of proinflammatory cytokines and chemokines in vivo and in vitro. J Ginseng Res. 2017;41:134–143. doi: 10.1016/j.jgr.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim D.S., Bae K.G., Jung I.S., Kim C.H., Yun Y.S., Song J.Y. Anti-septicaemic effect of polysaccharide from Panax ginseng by macrophage activation. J Infect. 2002;45:32–38. doi: 10.1053/jinf.2002.1007. [DOI] [PubMed] [Google Scholar]
- 7.Shin J.Y., Song J.Y., Yun Y.S., Yang H.O., Rhee D.K., Pyo S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 2002;24:469–482. doi: 10.1081/iph-120014730. [DOI] [PubMed] [Google Scholar]
- 8.Choi H.S., Kim K.H., Sohn E., Park J.D., Kim B.O., Moon E.Y., Rhee D.K., Pyo S. Red ginseng acidic polysaccharide (RGAP) in combination with IFN-gamma results in enhanced macrophage function through activation of the NF-kappaB pathway. Biosci Biotechnol Biochem. 2008;72:1817–1825. doi: 10.1271/bbb.80085. [DOI] [PubMed] [Google Scholar]
- 9.Byeon S.E., Lee J., Kim J.H., Yang W.S., Kwak Y.S., Kim S.Y., Choung E.S., Rhee M.H., Cho J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediat Inflamm. 2012;2012 doi: 10.1155/2012/732860. 732860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han B.C., Ahn H., Lee J., Jeon E., Seo S., Jang K., Lee S.H., Kim C.H., Lee G.S. Nonsaponin fractions of Korean red ginseng extracts prime activation of NLRP3 inflammasome. J Ginseng Res. 2017;41:513–523. doi: 10.1016/j.jgr.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V.G., Wu H., Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee G.S. Inflammasomes, multi-cellular protein complex in myeloid cells, induce several metabolic diseases via interleukin-1β maturation. J Biomed Res. 2013;14:195–200. [Google Scholar]
- 13.Larina L., Cho B.G., Ten L., Park H. Isolation of saponin-free fraction from Ginseng (Panax ginseng C.A. Meyer) and its effects on the function of neutrophils. Korean J Chem Eng. 2001;18:986–991. [Google Scholar]
- 14.Ahn H., Lee G.S. Isorhamnetin and hyperoside derived from water dropwort inhibits inflammasome activation. Phytomed Int J Phytother Phytopharmacol. 2017;24:77–86. doi: 10.1016/j.phymed.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Ahn H., Kang S.G., Yoon S.I., Kim P.H., Kim D., Lee G.S. Poly-gamma-glutamic acid from Bacillus subtilis upregulates pro-inflammatory cytokines while inhibiting NLRP3, NLRC4 and AIM2 inflammasome activation. Cell Mol Immunol. 2016 doi: 10.1038/cmi.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn H., Jeon E., Kim J.C., Kang S.G., Yoon S.I., Ko H.J., Kim P.H., Lee G.S. Lentinan from shiitake selectively attenuates AIM2 and non-canonical inflammasome activation while inducing pro-inflammatory cytokine production. Sci Reports. 2017;7:1314. doi: 10.1038/s41598-017-01462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinon F., Petrilli V., Mayor A., Tardivel A., Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 18.Lee J., Ahn H., Hong E.J., An B.S., Jeung E.B., Lee G.S. Sulforaphane attenuates activation of NLRP3 and NLRC4 inflammasomes but not AIM2 inflammasome. Cell Immunol. 2016;306–307:53–60. doi: 10.1016/j.cellimm.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Kayagaki N., Wong M.T., Stowe I.B., Ramani S.R., Gonzalez L.C., Akashi-Takamura S., Miyake K., Zhang J., Lee W.P., Muszynski A. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y.C., Yeh W.C., Ohashi P.S. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Li H., Willingham S.B., Ting J.P., Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Veerdonk F.L., Netea M.G., Dinarello C.A., Joosten L.A. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Liu-Bryan R., Scott P., Sydlaske A., Rose D.M., Terkeltaub R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthr Rheumat. 2005;52:2936–2946. doi: 10.1002/art.21238. [DOI] [PubMed] [Google Scholar]
- 24.Yang X.D., Yang Y.Y., Ouyang D.S., Yang G.P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia. 2015;100:208–220. doi: 10.1016/j.fitote.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of Korean red ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39:384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakaya T.A., Kita M., Kuriyama H., Iwakura Y., Imanishi J. Panax ginseng induces production of proinflammatory cytokines via toll-like receptor. J Interferon Cytokine Res. 2004;24:93–100. doi: 10.1089/107999004322813336. [DOI] [PubMed] [Google Scholar]
- 27.Han B.C., Ahn H., Lee J., Jeon E., Seo S., Jang K.H., Lee S.H., Kim C.H., Lee G.S. Nonsaponin fractions of Korean red ginseng extracts prime activation of NLRP3 inflammasome. J Ginseng Res. 2017;41:513–523. doi: 10.1016/j.jgr.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bang C.S., Hong S.H., Suk K.T., Kim J.B., Han S.H., Sung H., Kim E.J., Kim M.J., Kim M.Y., Baik S.K. Effects of Korean red ginseng (Panax ginseng), urushiol (Rhus vernicifera Stokes), and probiotics (Lactobacillus rhamnosus R0011 and Lactobacillus acidophilus R0052) on the gut-liver axis of alcoholic liver disease. J Ginseng Res. 2014;38:167–172. doi: 10.1016/j.jgr.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen C.T., Rhee D.K. Panax ginseng as a potential modulator of macrophages. Macrophage. 2016;3:1. [Google Scholar]
- 30.Zhao B.S., Liu Y., Gao X.Y., Zhai H.Q., Guo J.Y., Wang X.Y. Effects of ginsenoside Rg1 on the expression of toll-like receptor 3, 4 and their signalling transduction factors in the NG108-15 murine neuroglial cell line. Molecules. 2014;19:16925–16936. doi: 10.3390/molecules191016925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn J.Y., Choi I.S., Shim J.Y., Yun E.K., Yun Y.S., Jeong G., Song J.Y. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur J Immunol. 2006;36:37–45. doi: 10.1002/eji.200535138. [DOI] [PubMed] [Google Scholar]
- 32.Pannacci M., Lucini V., Colleoni F., Martucci C., Grosso S., Sacerdote P., Scaglione F. Panax ginseng C.A. Mayer G115 modulates pro-inflammatory cytokine production in mice throughout the increase of macrophage toll-like receptor 4 expression during physical stress. Brain Behav Immun. 2006;20:546–551. doi: 10.1016/j.bbi.2005.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.