Abstract
Objective(s):
There is increasing evidence for the importance of gender in different diseases; however, the role of gender in response to treatments is still unknown. Therefore, this study investigated the impact of gender on the protective effects of celecoxib in ischemia reperfusion (IR)-induced acute kidney injury.
Materials and Methods:
In this experimental study, rats were randomly divided into 6 groups (n=6): IR, sham and celecoxib groups of males and females. In IR groups, after orally receiving saline for 5 days, renal pedicles were clamped for 55 min and then kidneys were reperfused for 24 hr. In the sham groups, clamping of renal pedicles was not performed. In the celecoxib groups, 30 mg/kg celecoxib was given orally for 5 days before induction of ischemia. Plasma was collected to determine creatinine (Cr) and blood urea nitrogen (BUN). Kidney tissue samples were also stored for examining the histopathology and measuring malondialdehyde (MDA) levels and superoxide dismutase (SOD) activities.
Results:
IR caused significant increases in plasma Cr (P<0.05), BUN (P<0.05) and renal histopathological damages in both genders. Also, induction of IR resulted in significant increase of MDA levels (P<0.05) and decrease of SOD activities (P<0.05) in the kidney in both genders. Celecoxib administration prevented the IR-induced functional, histopathological and oxidative changes in both genders by similar degrees.
Conclusion:
This study suggested that in similar pathological conditions, celecoxib improves renal function and histopathological damages and attenuates oxidative stress in both genders by the same degrees. These protective effects of celecoxib on IR-induced kidney injury are gender-independent.
Key Words: Acute kidney injury, Celecoxib, Gender difference, Oxidative stress, Reperfusion injury
Introduction
Acute kidney injury (AKI) is a common clinical disorder that is associated with significant morbidity and mortality (1). It is characterized by abrupt (within hr) decrease of function and structural damage to the kidneys (2).
Renal ischemia reperfusion injury (IRI) is one of the main causes of AKI, which is related to different clinical situations such as shock, low cardiac output and kidney transplantations (3). In these conditions, the restoration of blood flow after a period of deprived circulation leads to renal damages (4).
Oxidative stress, an imbalance between oxidant (e.g. reactive oxygen species, ROS) and anti-oxidant (e.g. superoxide dismutase, SOD) systems, is implicated in the pathophysiology of renal IRI and consequent AKI (5). One of the reactions involved in the oxidative stress process is initiated by the cyclooxygenase 2 (COX-2) enzyme (6). This enzyme produces ROS by indirectly activating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (7). Then, ROS formation leads to renal tubular cell damage by membrane lipid peroxidation, protein dysfunction and DNA breakdown. In addition, ROS formation may indirectly initiate several other pathophysiological processes of IRI such as neutrophil infiltration, endoplasmic reticulum stress and mitochondrial dysfunction (8).
Celecoxib, a member of non-steroidal anti-inflammatory drugs (NSAIDs) family, selectively inhibits COX-2 enzyme activity. There is no report on the increased risk of hematologic side effects and gastrointestinal tract disturbances with the use of NSAIDs (9). Therefore, in order to minimize the damages caused by oxidative stress on the reperfused organs, celecoxib can be a possible choice.
Gender difference in renal IRI has been well established in humans and experimental animals. Females are known to be more resistant to renal IRI than males (10). However, to understand the impact of gender on the effects of celecoxib, this study was designed to compare the protective effects of celecoxib on renal function, oxidative stress status and histopathology in IR-induced AKI between male and female rats with the same degree of injuries.
Materials and Methods
All experimental procedures (i.e. anesthesia and surgery) were performed according to the standards established by Tehran University of Medical Sciences and the institution’s ethics committee approved the study. Animals were maintained at room temperature (22 °C), 12 hr light-12 hr dark cycle with non-limited
water and food. Adult male and female Wistar rats were randomly divided into 6 groups (n=6) as previous work in our laboratory: IR, sham and celecoxib groups (11). Male and female rats in the IR and sham groups received saline by gavage for 5 days. Animals in the celecoxib groups were given 30 mg/kg celecoxib orally for 5 days (12). In the fifth day, rats in the IR and celecoxib groups were anesthetized by ketamine (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally (11). Then, they were placed supine and their abdomen was opened through a midline incision. Pedicle of kidneys was exposed and occluded by bulldog clamps bilaterally. After 55 min, renal clamps were released and the kidneys were reperfused for 24 hr. In the sham groups, all of the above processes were performed except the clamping of renal pedicles. At the end of the reperfusion time, the animals were anesthetized and their abdomen was opened. Blood samples were obtained from the inferior vena cava using a 5ml syringe. Then, the samples were centrifuged at 3000 g for 10 min at 4 °C and plasma was collected and stored at -70 °C until analysis for biochemical parameters. Kidneys were also resected and washed in cold phosphate-buffered saline on ice and their capsule was separated. For oxidative stress assays, part of the kidney tissues was frozen in liquid nitrogen quickly and the samples were kept at -70 °C until further study. Other part of the kidney tissues were fixed in %10 formalin for histological assessments (Figure 1).
Figure 1.
The experimental protocol of study
Biochemical assays
Plasma levels of creatinine (Cr) and blood urea nitrogen (BUN) were evaluated as renal functional indices and measured by colorimetric methods using a commercial kit (Pars Azmoon kit) and Hitachi 704 autoanalyser (Hitachi, Tokyo, Japan).
Measurement of renal oxidative stress indicators
Malondialdehyde (MDA) levels of renal tissues were determined according to Esterbauer and Cheeseman method. On the basis of this method, MDA reacts with thiobarbituric acid (TBA) (Sigma-Aldrich, USA) and creates a pink pigment, which has a maximum absorbance at 532 nm. The exact amount of MDA in each sample was calculated from the standard curve and presented as μmol/100 mg tissue (13). Renal SOD activities were measured by the method of Paoletti and Mocali. In this assay, superoxide anion is produced from molecular oxygen in the presence of Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, USA), manganese (II) chloride (Sigma-Aldrich, USA), and mercaptoethanol (Sigma-Aldrich, USA). NADPH oxidation is related to the activation of superoxide anions that are produced in the medium (14).
Evaluation of renal histopathology
Formalin (10% phosphate-buffered) was used to fix the renal tissues. They were then dehydrated by ethanol in different concentrations. Paraffin-embedded renal sections (4 µm) were stained by hematoxylin and eosin. Renal tubules were evaluated based on the extent of the destruction, cellular degeneration and vacuolization, tubular obstruction and formation of luminal debris and casts (11).
Statistical analysis
All results are expressed as the mean±SEM. Comparisons between groups were calculated by two-way ANOVA and Tukey’s post hoc test. Statistical significance was considered as P<0.05.
Results
Effect of gender on renal function
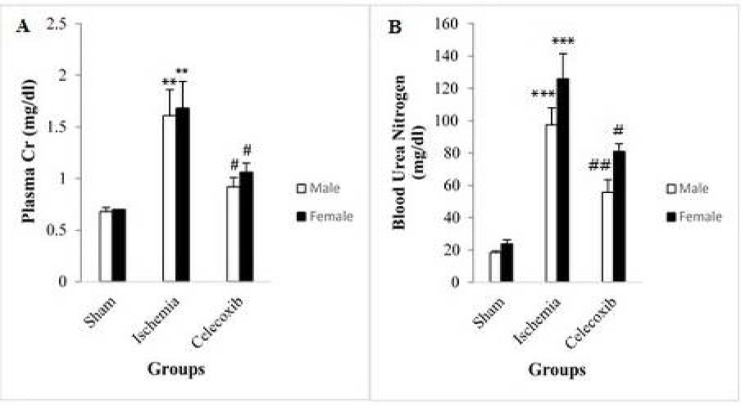
In male rats, plasma Cr level increased significantly in the IR group in comparison with the sham group (1.61±0.25 vs 0.68±0.04 mg/dl, P<0.003) (Figure 2A). In female rats, similar results were observed (1.68±0.26 vs. 0.70±0.00 mg/dl, P<0.005) (Figure 2A). In male rats, plasma Cr level decreased significantly in the celecoxib group in comparison with the IR group (0.92±0.09 vs. 1.61±0.25 mg/dl, P<0.02) (Figure 2A), and similar results were observed in female rats (1.06±0.09 vs. 1.68±0.26 mg/dl, P<0.04) (Figure 2A). Plasma Cr level difference was not statistically significant between the male and female groups with the same treatments (Figure 2A).
Figure 2.
Changes in plasma creatinine (Cr) (A) and blood urea nitrogen (BUN) (B) in different groups. The data are expressed as mean±SEM.
**P<0.01 versus the related sham group. ***P<0.001 versus the related sham group. #P<0.01 versus the related IR group. ##P<0.05 versus the male IR group. IR: Ischemia reperfusion
In male rats, plasma BUN level increased significantly in the IR group in comparison with the sham group (97.57±10.33 vs. 18.50±0.92 mg/dl, P<0.001) (Figure 2B). In female rats, similar results were observed (126.00±15.44 vs. 23.75±2.59 mg/dl, P<0.001) (Figure 2B). In addition, plasma BUN level in male rats decreased significantly in the celecoxib group in comparison with the IR group (55.63±7.91 vs. 97.57±10.33 mg/dl, P<0.003) (Figure 2B), and similar results were observed in female rats (81.00±4.70 vs 126.00±15.44 mg/dl, P<0.03) (Figure 2B). Plasma BUN level difference was not statistically significant between the male and female groups with the same treatments (Figure 2B).
Effect of gender on renal oxidative stress status
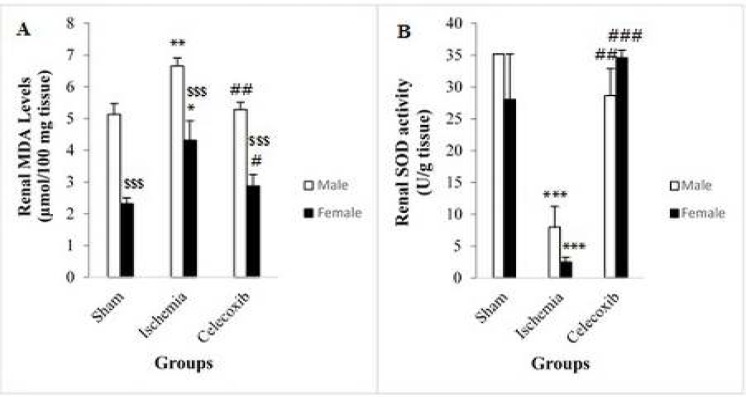
In male rats, renal MDA levels increased significantly in the IR group in comparison with the sham group (6.66±0.25 vs. 5.13±0.34 μmol/100 mg tissue, P<0.007) (Figure 3A). Similar results were also observed in female rats (4.32±0.61 vs 2.32±0.18 μmol/100 mg tissue, P<0.02) (Figure 3A). Renal MDA levels decreased significantly in the male rats of celecoxib group in comparison with the IR group (5.29±0.22 vs 6.66±0.25 μmol/100 mg tissue, P<0.01) (Figure 3A). In female rats, similar results were observed (2.88±0.35 vs 4.32±0.61 μmol/100 mg tissue, P<0.05) (Figure 3A). However, there were significant differences in renal MDA levels between male and female rats in the sham group (5.13±0.34 vs. 2.32±0.18 μmol/100 mg tissue, P<0.001), IR group (6.66±0.25 vs 4.32±0.61, P<0.001) (Figure 3A) and celecoxib group (5.29±0.22 vs 2.88±0.35 μmol/100 mg tissue, P<0.001) (Figure 3A).
Figure 3.
Changes in renal malondialdehyde (MDA) levels (A) and renal superoxide dismutase (SOD) activities (B) in different groups. Data are expressed as mean±SEM. *P<0.05 versus the female sham group. **P<0.01 versus the male sham group. # P<0.05 versus the female IR group. ## P<0.01 versus the male IR group. ###P<0.001 versus the female IR group. $$$P<0.001 versus the related male group. IR: Ischemia reperfusion
In male rats, renal SOD activities decreased significantly in the IR group in comparison with the sham group (7.96±3.26 vs. 35.18±0.00 U/g tissue, P<0.001) (Figure 3B). In female rats, similar results were found (2.50±0.76 vs. 28.06±7.12 U/g tissue, P<0.001) (Figure 3B). In male rats, renal SOD activities increased significantly in the celecoxib group in comparison with the IR group (28.66±4.22 vs 7.96±3.26 U/g tissue, P<0.003) (Figure 3B), and similar results were reported in female rats (34.59±1.16 vs. 2.50±0.76 U/g tissue, P<0.001) (Figure 3B). Renal SOD activity differences were not statistically significant between the male and female groups with the same treatments (Figure 3B).
Effect of gender on histopathological changes in the kidneys
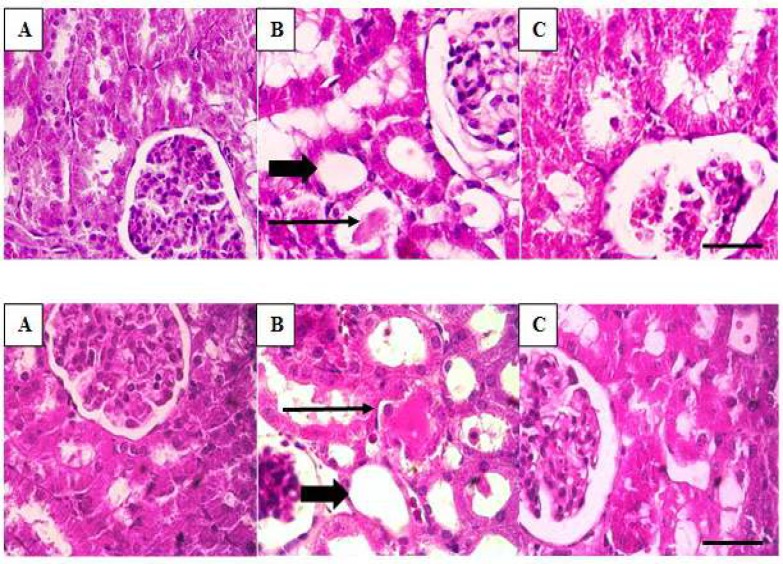
In both genders of rats, there was no detectable damage to the kidney tissues of the sham groups (Figure 4A). Renal tissues in the IR groups showed severe changes in the tubules compared to the sham group (Figure 4B). These changes include tubular destruction and disintegration of the tubular cells. Vacuolation, cast formation and tubular obstruction were frequent. Flattening of the tubular cells and necrosis were also observed. By administration of celecoxib, there were reductions in the amount of histopathological damages. In both females and males, tubular destruction and cast formation were reduced (Figure 4C).
Figure 4.
Changes in renal histology in different groups with hematoxylin and eosin staining by light microscopy. Top: (A): sham, (B): IR, (C): celecoxib group in male rats. Bottom: (A): sham, (B): IR, (C): celecoxib groups in female rats. In the rats of the sham groups (A), there was no detectable damage to the renal tissues. In the IR groups (B), some histological changes were observed including disintegration of the tubular cells vacuolation, cast formation and tubular obstruction (long black arrow), flattening of the tubular cells (thick arrow) and necrosis. Administration of celecoxib reduced the extent of these histopathological damages (C). Bar, 100 μm. IR: Ischemia reperfusion
Discussion
In experimental studies, one of the most used models for evaluation of the pathogenesis of renal IRI is occlusion of both renal pedicles (15). Therefore, in the present study, this model was used for induction of renal IRI.
The results of this study demonstrated that IR-induced AKI caused significant increases of plasma Cr and BUN levels and histopathological damages. Renal IR causes extensive alterations in structure and function of the tubular epithelial cells particularly the proximal tubule cells (16). It has been suggested that increased ROS production during renal IR is one of the important causes of renal damage such as extensive interstitial edema, tubular flattening with loss of brush border microvilli, tubular dilation, shedding of brush border, casts and obstruction (17, 18). Another significant event following renal IR is a reduction in the glomerular filtration rate (GFR) that is attributed to decrease of the transglomerular hydraulic pressure gradient as a result of tubular obstruction (19). The reduction of GFR causes impairment of water and electrolyte homeostasis and elevated plasma Cr and BUN levels (i.e. decrease of renal function) (20). Similar to our study, in the study of Liu et al., the IR group showed a significant increase in Cr and BUN levels and extensive morphological abnormalities (21).
However, there were not significant differences between the male and female rats in the IR groups. Several studies have shown that induction of renal IR in females causes less injuries compared to males (22). However, some studies indicated that the required ischemia time to induce IRI in female rats is much more than male rats (e.g. 60 min vs. 30 min) (23, 24). In the present study, the duration of ischemia was well-enough for the establishment of similar injuries in both genders. Therefore, we had an opportunity to investigate the protective effects of celecoxib on both genders with similar degrees of IRI.
Administration of celecoxib significantly prevented renal dysfunction and histopathological damages in IR male rats. Consistent with our results, it was reported that administration of celecoxib attenuated changes of renal functional indicators in a male rat model of cisplatin-induced nephrotoxicity (25). In another study, the authors demonstrated that celecoxib maintained physiological structure of the hepatic tissues in liver ischemia reperfusion in male rats (11). In our study, celecoxib was also capable of significant prevention of renal dysfunction and histopathological damages in female rats. Gender differences of kidneys under physiologic conditions are well demonstrated. For example, two studies independently showed that males have normally higher levels of ROS production compared to females (26, 27). In line with these studies, we also observed an enhanced physiological renal MDA levels in males compared to females in the sham groups.
The exact molecular mechanisms underlying IRI are not fully understood. Nevertheless, several causal factors have been shown to contribute to its pathogenesis. IR causes the liberation of arachidonic acid from membrane phospholipids by phospholipase A2 (PLA 2) enzymes. The released arachidonic acid is metabolized to prostaglandin (PG) H2 by either cyclooxygenase 1 (COX-1) or COX-2 (28, 29). COX-2 enzyme activates NADPH oxidase, which causes oxidative stress (2). In these conditions, it results in excessive production of ROS that is responsible for oxidative damage to biological molecules such as lipids, leading to tissue injury (30).
According to the vital role of COX-2 in renal IRI, several studies have examined the protective effect of selective COX-2 blockade (31). Feiotza et al., indicated that COX-2 blockade ameliorated renal damage induced by IRI (32).
Tissue MDA level is a valuable indicator of lipid peroxidation (33). The present study demonstrated that renal MDA levels were significantly higher in the IR group compared to the sham group in both genders. This observation is in accordance with another study that reported an increase in MDA levels in rat kidney after 60 min of ischemia and 15 min of reperfusion (34). However, there were significant differences in renal MDA levels between males and females in the IR groups. Female rats showed less injury compared to the male animals. One explanation for this result is due to the lower renal MDA levels in the sham-related groups. Similarly, it has been reported that renal IR-induced oxidative stress in female was lower than male rats (35).
To remove toxic ROS, cells have several natural defense systems, including SOD enzyme. Increased ROS that is generated during IR may lead to the depletion of these endogenous anti-oxidants (36). In this study, IR-induced AKI significantly caused a decrease of renal SOD activity in both genders. These findings are in good agreement with the study of Sedaghat et al. that found reduced SOD activity in renal IR (36).
Celecoxib is a selective inhibitor of COX-2 enzyme and a member of NSAIDs (9).
It prevents downhill pathways of oxidative stress by inhibition of COX-2 enzyme activity (Figure 5). Thus, it is reasonable to assume that celecoxib might provide protection in IRI.
Figure 5.
Protective effect of celecoxib against IRI. Celecoxib inhibits COX-2 enzyme activity resulting in the reduction of oxidative stress (ROS formation). PLA 2: Phospholipase A2, COX-1: Cyclooxygenase 1, COX-2: Cyclooxygenase 2, PGH 2: Prostaglandin H2, NADPH: Nicotinamide adenine dinucleotide phosphate, ROS: Reactive oxygen species, SOD: Superoxide dismutase, MDA: Malondialdehyde, DNA: Deoxyribonucleic acid, IRI: Ischemia reperfusion injury
Administration of celecoxib significantly prevented the increase of renal MDA levels and decrease of SOD activities in male rats. Similar to our results, in the study of Ozturk et al., celecoxib decreased MDA levels in the hepatic tissues, and in a study by Koul and Arora, administration of celecoxib augmented the anti-oxidant system in cigarette smoke-induced oxidative stress in mice (12, 37). In this study, celecoxib was also capable of significant suppression of the renal oxidative damages in female rats. Significant improvement of renal MDA levels by celecoxib in female rats may be due to the lower injury in them compared to males.
Limitation of the current study was that the authors did not evaluate the anti-inflammatory effects of celecoxib on both genders in IR-induced AKI. Therefore, we suggest to evaluate the impact of gender on the celecoxib effects on inflammatory indices in IR- induced AKI.
Clinical implications
Results of the present study indicate that in conditions such as shock, low cardiac output and kidney transplantation, celecoxib may show protective effects to prevent IR-induced AKI through amelioration of oxidative stress independent of gender.
Conclusion
In similar pathological conditions, celecoxib improves renal function and histopathological damages and attenuates oxidative damages in both genders by the same degrees independent of gender.
Acknowledgment
The results described in this paper are a part of student thesis with ID number 94. This study was supported by a grant from Tehran University of Medical Sciences, Tehran, Iran.
References
- 1.Bolisetty S, Zarjou A, Agarwal A. Heme oxygenase 1 as a therapeutic target in acute kidney injury. Am J Kidney Dis. 2017;69:531–545. doi: 10.1053/j.ajkd.2016.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makris K, Spanou L. Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev. 2016;37:85–98. [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira R, Brito M, Júnior R, Gonçalves R, Oliveira L, Monteiro A, et al. Influence of remote ischemic conditioning and tramadol hydrochloride on oxidative stress in kidney ischemia/reperfusion injury in rats. Acta Cir Bras. 2017;32:229–235. doi: 10.1590/S0102-865020170030000007. [DOI] [PubMed] [Google Scholar]
- 4.Menting T, Ergun M, Bruintjes M, Wever K, Lomme R, van Goor H, et al. Repeated remote ischemic preconditioning and isoflurane anesthesia in an experimental model of renal ischemia-reperfusion injury. BMC Anesthesiol. 2017;17:1–7. doi: 10.1186/s12871-017-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, Pinlaor S, et al. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci. 2014;16:193–217. doi: 10.3390/ijms16010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris RE. Inflammation in the pathogenesis of chronic diseases: the COX-2 controversy. Springer Science & Business Media; 2007. [Google Scholar]
- 7.Alzoghaibi M. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J Gastroenterol. 2013;19:6540–6547. doi: 10.3748/wjg.v19.i39.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Vicente A, Garvin J. Effects of reactive oxygen species on tubular transport along the nephron. Antioxidants. 2017;6:1–15. doi: 10.3390/antiox6020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu C, Gu K, Yasen Y, Hou Y. Efficacy and safety of celecoxib therapy in osteoarthritis: A meta-analysis of randomized controlled trials. Medicine. 2016;95:e3585. doi: 10.1097/MD.0000000000003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller V, Losonczy G, Heemann U, Vannay Á, Fekete A, Reusz G, et al. Sexual dimorphism in renal ischemia-reperfusion injury in rats: possible role of endothelin. Kidney Int. 2002;62:1364–1371. doi: 10.1111/j.1523-1755.2002.kid590.x. [DOI] [PubMed] [Google Scholar]
- 11.Azizi F, Seifi B, Kadkhodaee M, Ahghari P. Administration of hydrogen sulfide protects ischemia reperfusion-induced acute kidney injury by reducing the oxidative stress. Ir J Med Sci. 2016;185:649–654. doi: 10.1007/s11845-015-1328-z. [DOI] [PubMed] [Google Scholar]
- 12.Ozturk H, Gezici A, Ozturk H. The effect of celecoxib, a selective COX-2 inhibitor, on liver ischemia/reperfusion-induced oxidative stress in rats. Hepatol Res. 2006;34:76–83. doi: 10.1016/j.hepres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Esterbauer H, Schaur R, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 14.Paoletti F, Mocali A. Changes in CuZn-superoxide dismutase during induced differentiation of murine erythroleukemia cells. Cancer Res. 1988;48:6674–6677. [PubMed] [Google Scholar]
- 15.Patel N, Cuzzocrea S, Collino M, Chaterjee P, Mazzon E, Britti D, et al. The role of cycloxygenase-2 in the rodent kidney following ischaemia/reperfusion injury in vivo. Eur J Pharmacol. 2007;562:148–154. doi: 10.1016/j.ejphar.2007.01.079. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg J. The cell biology of ischemic renal injury. Kidney Int. 1991;39:476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Jang H, Park K. Reactive oxygen species generated by renal ischemia and reperfusion trigger protection against subsequent renal ischemia and reperfusion injury in mice. Am J Physiol Renal Physiol. 2009;298:F158–F166. doi: 10.1152/ajprenal.00474.2009. [DOI] [PubMed] [Google Scholar]
- 18.Shanley P, Rosen M, Brezis M, Silva P, Epstein F, Rosen S. Topography of focal proximal tubular necrosis after ischemia with reflow in the rat kidney. Am J Pathol. 1986;122:462–468. [PMC free article] [PubMed] [Google Scholar]
- 19.Myers B, Miller D, Mehigan J, Olcott C, Golbetz H, Robertson C, et al. Nature of the renal injury following total renal ischemia in man. J Clin Invest. 1984;73:329–341. doi: 10.1172/JCI111217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonventre J, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Liu X, Wang L, Du Y, Chen Z, Chen H, et al. Effects of apigenin on the expression levels of Bcell lymphoma2, Fas and Fas ligand in renal ischemiareperfusion injury in rats. Exp Ther Med. 2017;14:5345–5354. doi: 10.3892/etm.2017.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebrahimi S, Aboutaleb N, Nobakht M. Consequences of ischemic preconditioning of kidney: comparing between male and female rats. Iran J Basic Med Sci. 2012;15:1148–1153. [PMC free article] [PubMed] [Google Scholar]
- 23.Hu H, Wang G, Batteux F, Nicco C. Gender differences in the susceptibility to renal ischemia-reperfusion injury in BALB/c mice. Tohoku J Exp Med. 2009;218:325–329. doi: 10.1620/tjem.218.325. [DOI] [PubMed] [Google Scholar]
- 24.Park K, Kim J, Ahn Y, Bonventre A, Bonventre J. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem. 2004;279:52282–52292. doi: 10.1074/jbc.M407629200. [DOI] [PubMed] [Google Scholar]
- 25.Suddek G, El-kenawi A, Abdel-Aziz A, El-Kashef H. Celecoxib, a selective cyclooxygenase-2 inhibitor, attenuates renal injury in a rat model of cisplatin-induced nephrotoxicity. Chemotherapy. 2011;57:321–326. doi: 10.1159/000329529. [DOI] [PubMed] [Google Scholar]
- 26.Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, et al. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol. 2002;22:438–442. doi: 10.1161/hq0302.104515. [DOI] [PubMed] [Google Scholar]
- 27.Brandes R, Mügge A. Gender differences in the generation of superoxide anions in the rat aorta. Life Sci. 1997;60:391–396. doi: 10.1016/s0024-3205(96)00663-7. [DOI] [PubMed] [Google Scholar]
- 28.Sapirstein A, Bonventre J. Phospholipases A2 in ischemic and toxic brain injury. Neurochem Res. 2000;25:745–753. doi: 10.1023/a:1007583708713. [DOI] [PubMed] [Google Scholar]
- 29.Ueno N, Takegoshi Y, Kamei D, Kudo I, Murakami M. Coupling between cyclooxygenases and terminal prostanoid synthases. Biochem Biophys Res Commun. 2005;338:70–76. doi: 10.1016/j.bbrc.2005.08.152. [DOI] [PubMed] [Google Scholar]
- 30.Tabassum R, Vaibhav K, Shrivastava P, Khan A, Ahmed M, Ashafaq M, et al. Perillyl alcohol improves functional and histological outcomes against ischemia–reperfusion injury by attenuation of oxidative stress and repression of COX-2, NOS-2 and NF-κB in middle cerebral artery occlusion rats. Eur J Pharmacol. 2015;747:190–199. doi: 10.1016/j.ejphar.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Feitoza C, Câmara N, Pinheiro H, Gonçalves G, Cenedeze M, Pacheco-Silva A, et al. Cyclooxygenase 1 and/or 2 blockade ameliorates the renal tissue damage triggered by ischemia and reperfusion injury. Int Immunopharmacol. 2005;5:79–84. doi: 10.1016/j.intimp.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Feitoza C, Gonçalves G, Semedo P, Cenedeze M, Pinheiro H, Beraldo F, et al. Inhibition of COX 1 and 2 prior to renal ischemia/reperfusion injury decreases the development of fibrosis. Mol Med. 2008;14:724–730. doi: 10.2119/2008-00064.Feitoza. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahfoudh-Boussaid A, Zaouali M, Hadj-Ayed K, Miled A, Saidane-Mosbahi D, Rosello-Catafau J, et al. Ischemic preconditioning reduces endoplasmic reticulum stress and upregulates hypoxia inducible factor-1α in ischemic kidney: the role of nitric oxide. J Biomed Sci. 2012;19:1–8. doi: 10.1186/1423-0127-19-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paller M, Hoidal J, Ferris T. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahmoudi A, Kadkhodaee M, Golab F, Najafi A, Sedaghat Z. Postconditioning is protective in renal reperfusion injury only in male rats A gender difference study. Physiol Int. 2014;102:67–76. doi: 10.1556/APhysiol.101.2014.011. [DOI] [PubMed] [Google Scholar]
- 36.Sedaghat Z, Kadkhodaee M, Seifi B, Salehi E, Najafi A, Dargahi L. Remote per-conditioning reduces oxidative stress, downregulates cyclo-oxygenase-2 expression and attenuates ischaemia–reperfusion-induced acute kidney injury. Clin Exp Pharmacol Physiol. 2013;40:97–103. doi: 10.1111/1440-1681.12044. [DOI] [PubMed] [Google Scholar]
- 37.Koul A, Arora N. Celecoxib mitigates cigarette smoke induced oxidative stress in mice. Indian J Biochem Biophys. 2010;47:285–291. [PubMed] [Google Scholar]