Abstract
Background
Oxidative stress induces the production of reactive oxygen species (ROS), which play important causative roles in various pathological conditions. Black ginseng (BG), a type of steam-processed ginseng, has drawn significant attention due to its biological activity, and is more potent than white ginseng (WG) or red ginseng (RG).
Methods
We evaluated the protective effects of BG extract (BGE) against oxidative stress-induced cellular damage, in comparison with WG extract (WGE) and RG extract (RGE) in a cell culture model. Ethanolic extracts of WG, RG, and BG were used to evaluate ginsenoside profiles, total polyphenols, flavonoid contents, and antioxidant activity. Using AML-12 cells treated with H2O2, the protective effects of WGE, RGE, and BGE on cellular redox status, DNA, protein, lipid damage, and apoptosis levels were investigated.
Results
BGE exhibited significantly enhanced antioxidant potential, as well as total flavonoid and polyphenol contents. ATP levels were significantly higher in BGE-treated cells than in control; ROS generation and glutathione disulfide levels were lower but glutathione (GSH) and NADPH levels were higher in BGE-treated cells than in other groups. Pretreatment with BGE inhibited apoptosis and therefore protected cells from oxidative stress-induced cellular damage, probably through ROS scavenging.
Conclusion
Collectively, our results demonstrate that BGE protects AML-12 cells from oxidative stress-induced cellular damages more effectively than WGE or RGE, through ROS scavenging, maintenance of redox status, and activation of the antioxidant defense system.
Keywords: antioxidant, black ginseng, chemoprotection, oxidative stress, Panax ginseng
1. Introduction
Oxidative stress involves elevated intracellular levels of ROS, which damage various biological molecules [1]. ROS produced during cellular respiration is eliminated regularly to maintain cellular homeostasis and support physiological functions [2]. A variety of antioxidant defense systems have been developed by cells to protect themselves against toxic free radicals and ROS [3]. Overproduction or insufficient elimination of ROS is highly correlated with various pathological processes such as neurodegenerative [4], cardiovascular [5], and liver diseases [6]. Oxidative stress can induce cellular damage, particularly of nucleic acids, proteins, and lipids [7], ultimately leading to cell death. The occurrence of oxidative stress-induced diseases has increased tremendously in recent years. Because of increasing cost of health-care services worldwide, there is a growing public interest in natural food products with elevated antioxidant activity and other health-promoting effects [8].
Korean ginseng (Panax ginseng Meyer) is one of the most widely used traditional medicines that has been reported to have health-promoting benefits effects, such as exhibiting anticancer, antidiabetic, and anti-inflammatory effects [9], [10], [11]. Steam processing of fresh ginseng produces red ginseng (RG), and further repeated steam and drying will produce black ginseng (BG) [12]. In addition to modified antioxidant potential, ginsenosides profile, and phenolic compounds [13], repeated steaming imparts further health-promoting effects [14]. Repeated heat processing causes the release of phenolic compounds and Maillard reaction products, which enhances the anti-inflammatory and free radical scavenging activity of BG compared with RG [15]. Furthermore, without any known side effects, ginseng has been used to protect against stress-induced cellular damage [16]. RG has protective potential against oxidative stress-induced cellular damage [17]. Similarly, white ginseng extract (WGE) has shown hepatoprotective potential against H2O2-induced side effect [18]. Cultured wild ginseng root extract has been reported to have a positive effect on male reproductive function by suppressing ROS production [19]. BG has been reported to alleviate acetaminophen-induced hepatotoxicity due to antioxidant, antiapoptotic, anti-inflammatory activity [20]. A previous study demonstrated that BG extract (BGE) shows elevated ginsenoside contents, antioxidant activity, and phenolic compounds compared with WGE and RG extract (RGE) [13]. However, fermented ginseng, which also contains many less polar ginsenosides, was reported to have detoxifying effect against H2O2-induced hepatotoxicity in HepG2 cells [21]; however, the protective effect of BGE on H2O2-induced liver injury had not been reported so far. Therefore, it is of great interest to investigate the effect of BGE on H2O2-induced liver injuries, particularly in comparison with WGE and RGE, in relation to maintenance of cellular redox status and prevention of cellular damage.
In this study, we compared the antioxidant activity and protective effects of WGE, RGE, and BGE against oxidative stress-induced cellular damage in AML-12 cell. We investigated the antioxidant properties of three types of ginseng extract at biochemical and cellular levels, and compared their ability to inhibit ROS production in mouse hepatocytes.
2. Materials and methods
2.1. Chemicals and materials
Six-yr-old WG, RG, and BG produced from same source of fresh ginseng were purchased from a local market (Misunne, Ganghwa, South Korea). Antihuman 4-hydroxynonenal (4-HNE)-Michael adduct antibody and antihuman dinitrophenyl (DNP) antibody were obtained from Calbiochem (La Jolla, CA, USA). 2,7-Dichlorodihydrofluorescein diacetate (DCF-DA) and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], gallic acid, quercetin, and rutin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Cell Tracker Green 5-chloromethylfluorescein diacetate (CMF-DA) was purchased from Molecular Probes (Life Technologies, Carlsbad, CA, USA) and In Situ Cell Death Detection Kit POD from Roche Diagnostics (Basel, Switzerland).
2.2. Sample preparation
Samples were extracted in 10 volume of 70% ethanol at 80°C with 4 h influx. Filtered extracts were concentrated, freeze-dried, and kept at −20°C until further use. For cell treatment, samples were dissolved in phosphate-buffered saline (PBS) unless otherwise indicated.
2.3. Ginsenoside analysis
An HPLC system (UltiMate 3000, Dionex Co., Sunnyvale, CA, USA) equipped with a C18 column (2.1 mm × 75 mm, Poroshell 120; Agilent, Santa Clara, CA, USA) was used for ginsenoside analysis. Gradient elution was maintained by flow of solvents A and B at a ratio of 90:10, 79:21, 78:22, 77:23, 76:24, 63:37, 55:45, 54:46, 52:48, 0:100, and 90:10 [10% acetonitrile (Solvent A) and 90% acetonitrile (Solvent B)]. Flow was maintained at a rate of 1.3 mL/min with a run time of 0 min, 22 min, 23 min, 40 min, 45 min, 53 min, 61 min, 66 min, 75 min, 80 min, 95 min, and 105 min; a detection wavelength of 203 nm was used.
2.4. Quantification of total phenolics and flavonoids
Total polyphenols and flavonoids were analyzed using a previously reported method [22] with some modifications. For polyphenol analysis, each extract aliquot (50 μL) was oxidized using Folin–Ciocalteu's phenol reagent (0.5 mL). About 2.5 mL of 10% Na2CO3 was added and the mixture was allowed to stand at room temperature for 20 min. After 20 min, absorbance was recorded at 720 nm. The total polyphenol content in each sample was calculated using a gallic acid standard curve; results were expressed as micrograms of gallic acid equivalent (GAE)/milligram of extract.
For flavonoid content analysis, the following method was adopted: an aliquot of each extract (1 mL) was mixed with 5% NaNO2 (75 μL) and incubated for 5 min. A 10% AlCl3 solution (150 μL) was added and incubated for 6 min. Finally, 500 μL of 1M NaOH was added and the absorbance was recorded at 510 nm. Rutin was used to create a standard curve; the results were expressed as microgram of rutin equivalent (RE)/milligram of extract.
2.5. ABTS and cellular antioxidant activity assay
The ABTS [2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)] radical cation decolorization assay was performed as described previously, with minor modifications [23]. In brief, an ABTS radical solution was prepared in PBS and allowed to stand at room temperature for 12–16 h with continuous stirring. The radical solution was diluted until an absorbance of 0.70 ± 0.020 at 734 nm was reached. The samples (20 μL) were mixed with 980 μL of ABTS radical solution for 10 min at room temperature in the dark; blank solution was mixed with an equal amount of water. The absorbance was measured at 734 nm, and the inhibitory percentage was calculated. Vitamin C was used as a positive control.
Cellular antioxidant activity (CAA) assay was performed as described previously [24]. All samples were analyzed in triplicate. After subtracting the blank, the area under the curve for fluorescence versus time was integrated to calculate the CAA value of each sample at the same concentration using the following equation:
where ∫SA and ∫CA are integrated under the fluorescence versus time curve of the test sample and the control, respectively.
2.6. Cell culture
AML-12 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). AML-12 cells were cultured according to supplier instructions and were maintained in a humidified incubator with 5% CO2 at 37°C.
2.7. Immunocytochemistry
Cells grown on glass coverslips until 90% confluence were treated with H2O2 and ginseng extract. After washing with PBS, cells were fixed with 4% paraformaldehyde for 10 min. Immunocytochemistry staining for 4-HNE, NADPH, glutathione disulfide (GSSG), and DNP was performed according to the manufacturer's instructions. In brief, endogenous peroxidase level was inhibited after fixation and cells were incubated in 0.2% H2O2 with PBS for 5 min at 20°C. Cells were washed three times with PBS and incubated overnight with 1:200 dilution of primary antibody at 4°C. After washing with PBS three times, the cells were incubated with respective secondary antibody for 30 min at room temperature. All treatment groups were incubated under the same conditions with same concentration of antibodies and at the same time; thus, the results of immunostaining were comparable between different treatment groups.
2.8. Fluorescence microscopy and spectrophotometry
The intracellular GSH level was determined using the GSH-sensitive fluorescent dye CMF-DA. AML-12 cells were grown until 90% confluence on coverslips. After treatment, cells were incubated with Cell Tracker Green CMF-DA (5 μM) for 30 min in the dark. The fluorescence caused by conjugation of GSH with the CMF-DA Cell Tracker was analyzed using an Axio Vert. A1 microscope. Levels of 8-hydroxy-2′-deoxyguanosine (8-OH-dG) and ATP in AML-12 cells were estimated using a fluorescence binding assay. After exposure of AML-12 cells to oxidative stress, cells were fixed and permeabilized with ice-cold methanol for 15 min. DNA damage was visualized using avidin-conjugated tetramethylrhodamine isothiocyanate (TRITC). For ATP detection, an FITC-linked ATP antibody was used for fluorescence microscopy with excitation and emission wavelengths of 540 nm and 588 nm, respectively. Intracellular ROS production was measured according to the instruction manual for the DCF-DA cellular ROS detection kit (113851 Abcam KK, Tokyo, Japan) and fluorescence was recorded using a microplate reader (excitation, 488 nm; emission, 535 nm).
2.9. Analysis of cell viability and apoptosis
Cell viability was assessed using an MTT assay as described previously [24]. Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) staining was conducted according to the instruction manual provided by the manufacturer. Images were captured using a light microscope.
2.10. Statistical analysis
Statistically significant differences between groups were determined using one-way analysis of variance using SAS software for Windows release 9.2 (SAS Institute Inc., Cary, NC, USA) on the W32_VSHOME platform. The least-squares means option using a Tukey–Kramer adjustment was used for the multiple comparisons among the treatment groups. Data are expressed as means ± standard error of mean from triplicate independent experiments and p < 0.05 was regarded as statistically significant.
3. Results
3.1. Content of ginsenosides, total phenolics, flavonoids, and antioxidant activity
To determine the amounts of different ginsenosides in the extracts, we performed HPLC analysis. The results are presented in Table 1 and Fig. S1 (see the Supplemental Information online). The total amount (milligram/gram) of ginsenosides differed significantly between the three extracts. BGE contained more ginsenosides than RGE or WGE. Rg5, Rh1, Cpk, and Rg3 were significantly higher in BGE than in the other two extracts; their quantities were very low in RGE and absent in WGE.
Table 1.
Ginsenoside contents (mg/g) of white, red, and black ginseng extracts
| Ginsenoside | WGE (mg/g) | RGE (mg/g) | BGE (mg/g) |
|---|---|---|---|
| Rg1 | 2.73 ± 0.06 | 2.45 ± 0.05 | 1.11 ± 0.06 |
| Re | 3.60 ± 0.08 | 2.37 ± 0.05 | 1.34 ± 0.09 |
| Rf | 0.83 ± 0.02 | 0.76 ± 0.02 | 1.36 ± 0.06 |
| Rh1 | n.d | 0.09 ± 0.01 | 1.06 ± 0.03 |
| Rg2(s) | 0.33 ± 0.01 | 0.27 ± 0.01 | 1.10 ± 0.03 |
| Rb1 | 4.13 ± 0.08 | 6.18 ± 0.03 | 4.33 ± 0.07 |
| Rc | 2.27 ± 0.06 | 2.56 ± 0.01 | 1.48 ± 0.03 |
| Rb2 | 1.97 ± 0.04 | 2.12 ± 0.01 | 1.22 ± 0.03 |
| Rd | 0.54 ± 0.01 | 0.71 ± 0.00 | 0.80 ± 0.01 |
| F2 | n.d | n.d | n.d |
| Rg3 | n.d | 0.15 ± 0.01 | 2.27 ± 0.04 |
| Cpk | 0.11 ± 0.01 | 0.49 ± 0.01 | 6.13 ± 0.15 |
| Rg5 | n.d | 0.24 ± 0.00 | 4.98 ± 0.13 |
| Rk1 | n.d | n.d | n.d |
| Rh2 | n.d | n.d | 0.03 ± 0.00 |
| Total | 16.51 ± 0.4 | 18.38 ± 0.2 | 27.19 ± 0.7 |
Values are presented as means ± standard error of mean; n = 3. BGE, black ginseng extract; n.d, not detected; RGE, red ginseng extract; WGE, white ginseng extract
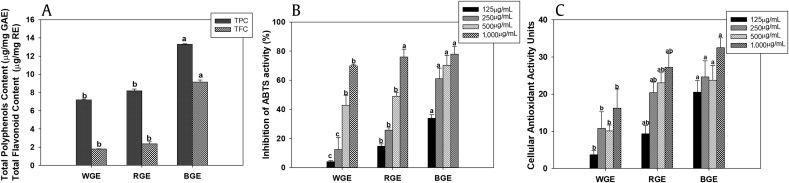
Total polyphenol and flavonoid contents decreased in the following order: BGE > RGE > WGE (Fig. 1A). The total contents of polyphenol compounds in WG, RG, and BG were 7.20 μg/mg of GAE, 8.19 μg/mg of GAE, and 13.29 μg/mg of GAE, respectively, and the total flavonoid contents were 1.79 μg/mg of RE, 2.37 μg/mg of RE, and 9.14 μg/mg of RE, respectively. BG showed a significantly higher content of phenolic compounds than RGE or WGE (p < 0.05).
Fig. 1.
Total polyphenol, flavonoid contents, and cellular antioxidant activity of ginseng extracts. (A) Change in total polyphenol and flavonoid contents of ginseng extracts after steam processing. (B) ABTS and (C) CAA assays of ginseng extracts. Significant differences between the means of various treatment groups are represented by different letters. A p value of less than 0.05 was considered statistically significant. Values are expressed as means ± standard error of mean; n = 3. ABTS, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid); BGE, black ginseng extract; CAA, cellular antioxidant activity; GAE, gallic acid equivalent; RGE, red ginseng extract; RE, rutin equivalent; TFC, total flavonoid content; TPC, total polyphenols; WGE, white ginseng extract.
To evaluate antioxidant activity, we used ABTS radical scavenging activity and CAA assays. Figs. 1B, 1C show the ABTS activity and CAA of ginseng extracts. BGE showed significantly higher ABTS scavenging activity and CAA than two other extracts; the effect was dose dependent (Figs. 1B, 1C). Ascorbic acid and quercetin were used as positive controls in the ABTS and CAA assays, respectively (data not shown).
3.2. Intracellular ROS production and ATP levels
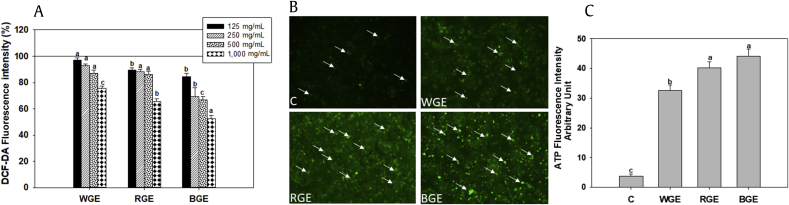
To measure the protective potential of the three different ginseng extracts under oxidative stress conditions, we used H2O2 as an oxidative stress inducer in AML-12 cells. During oxidative stress, ROS damage normal mitochondrial function, which ultimately results in ATP depletion and eventually cell death [25]; therefore, we also determined whether ATP levels were replenished by the ginseng extract. H2O2 challenge resulted in enhanced ROS production and depletion of ATP levels (Fig. 2). Pretreatment with all three ginseng extracts prevented ROS production in a dose-dependent manner (Fig. 2A). BGE showed significantly higher ROS quenching activity than WGE and RGE. At 1,000 μg/mL, BGE significantly inhibited ROS production (p < 0.05) compared with the same dose of WGE and RGE; this dose was used for further experiments. H2O2 exposure resulted in ATP depletion in AML-12 cells. As shown in Fig. 2B, treatment with all ginseng extracts (1,000 μg/mL), particularly BGE, restored green fluorescence, indicating that pre-exposure to ginseng extract restored intracellular ATP levels under oxidative stress conditions (p < 0.05; Figs. 2B, 2C).
Fig. 2.
Effect of the ginseng extracts on intracellular ROS and ATP levels in AML-12 cells exposed to H2O2. (A) Intracellular ROS scavenging activity of the ginseng extracts was determined using DCF-DA. AML-12 cells pretreated with ginseng extract were exposed to 0.2mM H2O2. Data are shown as the mean of replicate DCF-DA inhibitory effect (%) values ± standard error of mean (n = 3). (B) Effect of ginseng extract on intracellular ATP levels was measured using FITC-labeled antibodies; (C) ATP fluorescence intensity was quantified using ImageJ. Significant differences between the means of various treatment groups are represented by different letters. A p value less than 0.05 was considered statistically significant. All values are shown as means ± standard error of mean; n = 3. BGE, black ginseng extract; C, control; DFC-DA, 2,7-dichlorodihydrofluorescein diacetate; FITC, fluorescein isothiocyanate; RGE, red ginseng extract; ROS, reactive oxygen species; WGE, white ginseng extract.
3.3. Cellular redox status
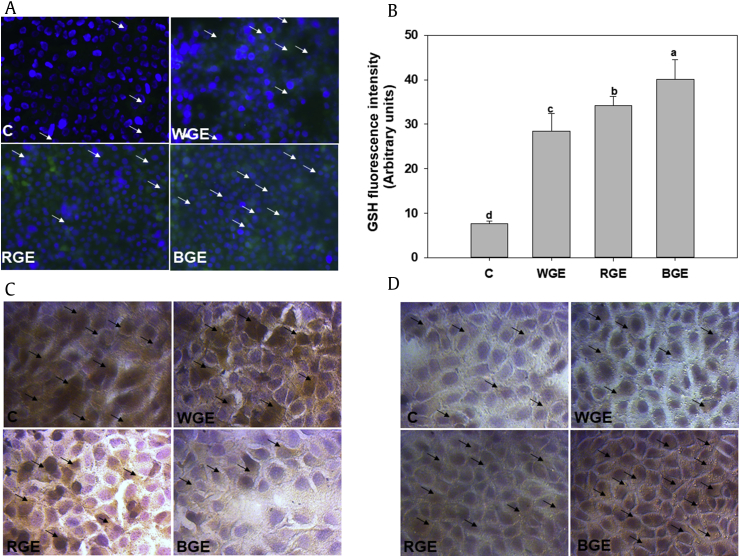
Steam processing significantly increased the antioxidant potential of RGE and BGE compared with WGE. We wanted to confirm whether the difference in antioxidant potential diminished oxidative stress and maintained normal cellular redox status. Under oxidative stress conditions, GSH is oxidized to GSSH, and NADPH is used to generate GSH; therefore, we also measured GSSH and NADPH levels under the same exposure conditions [26]. The GSSG level was elevated following exposure to H2O2. Treatment of cells with steam-processed ginseng samples, especially BGE, effectively decreased the GSSG level and restored the NADPH level (Fig. 3).
Fig. 3.
Intracellular redox status of AML-12 cells. (A) The GSH level in AML-12 cell was measured using the fluorescence probe CMF-DA. Images were obtained via fluorescence microscopy from three separate experiments. (B) Fluorescence intensity of GSH was quantified using the ImageJ software. (C) GSSG and (D) NADPH levels were quantified via immunocytochemistry and images were captured via light microscopy. Significant differences between the means of various treatment groups are represented by different letters. A p value of less than 0.05 was considered statistically significant. All values are shown as means ± standard error of mean; n = 3. BGE, black ginseng extract; C, control; CMF-DA, chloromethylfluorescein diacetate; GSH, glutathione; GSSG, glutathione disulfide; RGE, red ginseng extract; WGE, white ginseng extract.
3.4. Cellular oxidative damage
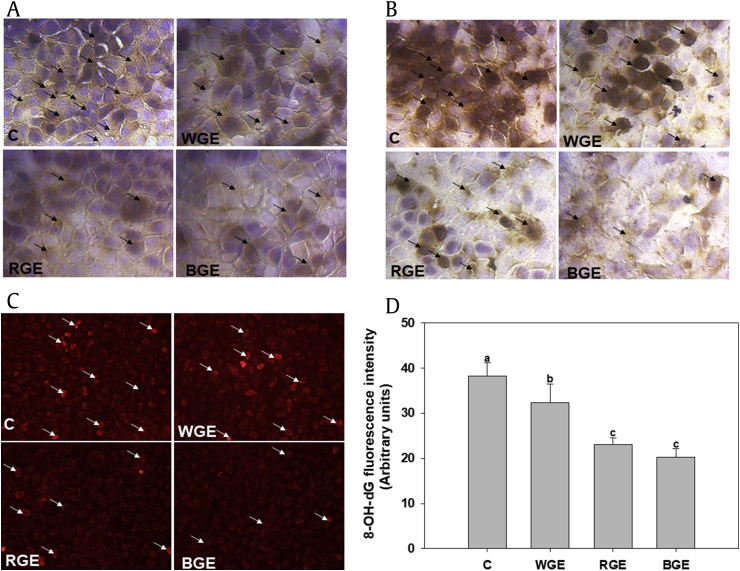
Oxidative stress-induced ROS production leads to lipid peroxidation, protein carbonylation, DNA damage, and eventually to cell death via apoptosis. Lipid peroxidation results in the accumulation of the lipid peroxide 4-HNE. We measured lipid peroxidation and protein carbonyl content by immunocytochemistry using HNE and DNP primary antibodies. 8-OH-dG, a well-known cause of DNA damage, was analyzed using avidin-conjugated TRIF antibody via fluorescence microscopy. Oxidative stress significantly damaged lipids, proteins, and DNA (Fig. 4). The ginseng extracts inhibited oxidative stress-induced cellular damage in the following order: BGE > RGE > WGE. BGE significantly reduced the cellular damage induced by H2O2 exposure compared with RGE.
Fig. 4.
Effect of ginseng extracts against cellular oxidative damage. (A) Level of lipid peroxidation measured using anti-HNE antibody and counterstained with DAB. (B) Protein carbonyl contents were measured using a DNP-specific antibody. (C) 8-OH-dG levels were analyzed using avidin-conjugated TRITC and fluorescence microscopy, and (D) the fluorescence intensity was quantified using the ImageJ software. Significant differences between the means of various treatment groups are represented by different letters. A p value of less than 0.05 was considered statistically significant. All values are shown as means ± standard error of mean; n = 3. 8-OH-dG, 8-hydroxy-2′-deoxyguanosine; BGE, black ginseng extract; DAB, 3′-diaminobenzidine; DNP, dinitrophenyl; HNE, 4-hydroxynonenal; RGE, red ginseng extract; WGE, white ginseng extract.
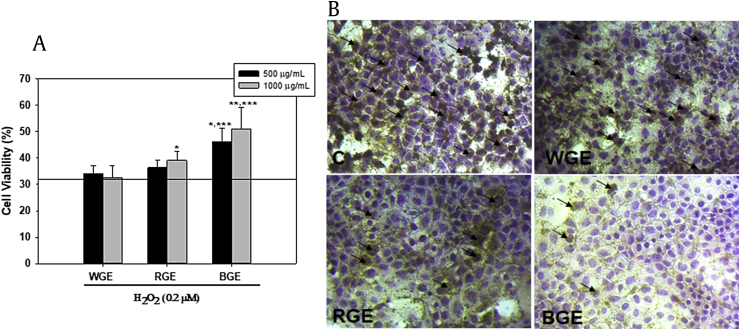
3.5. Cytotoxicity and apoptosis
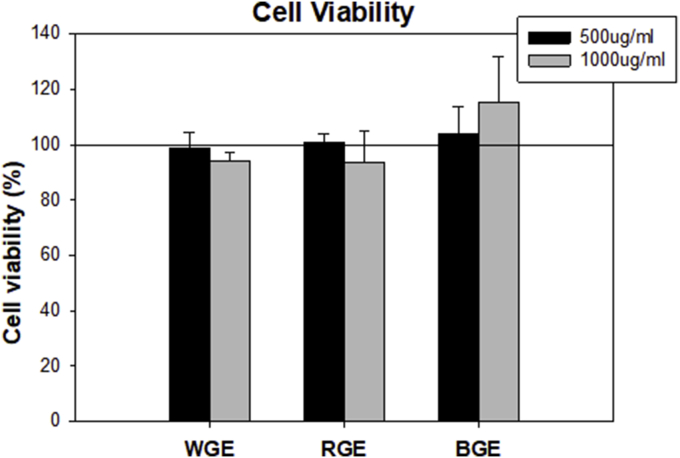
To further investigate the protective potential of BGE, we measured cell viability and level of apoptosis in AML-12 cells under the same experimental conditions. As shown in Fig. 5A, H2O2 exposure caused cell death, but BGE treatment significantly prevented cell death compared with WGE and RGE. Ginseng extract alone did not cause cell death (Fig. S2 in the supplemental information online). To further investigate whether BGE has a cytoprotective effect against H2O2, apoptosis was detected using the TUNEL assay. H2O2 induce apoptotic cell death. However, pretreatment with BGE inhibited H2O2-induced apoptosis in AML-12 cells (Fig. 5B).
Fig. 5.
Inhibitory effect of ginseng extract on H2O2-induced cytotoxicity in AML-12 cells. (A) Cell viability of AML-12 cells co-treated with H2O2 and ginseng extract for 12 h, determined by MTT assay. The reference line shows H2O2-induced cytotoxicity. (B) TUNEL staining of AML-12 cells treated with H2O2 alone or with ginseng extract for 12 h. ***p < 0.05 versus H2O2.*p < 0.05; **p < 0.01 versus BGE. All values are shown as means ± standard error of mean; n = 3. BGE, black ginseng extract; C, control; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; RGE, red ginseng extract; TUNEL, terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling; WGE, white ginseng extract.
4. Discussion
There were two main goals of this present study. The first was to determine whether WGE, RGE, and BGE exert comparable antioxidant potential and protection against H2O2-induced cellular damage. The second was to evaluate maintenance of cellular redox status and prevention of cellular damage (lipids, proteins, and DNA) specific to oxidative stress. Although a previous study [13] has indicated that steam processing enhances antioxidant activity and phenolic compounds in Korean ginseng, little is known about the effect of ginseng extracts, especially BGE, on H2O2-induced oxidative stress and cellular damage and its ROS-scavenging ability. In this study, we have demonstrated, for the first time, that ginseng extracts show CAA in AML-12 cells, via ROS scavenging activity and maintenance of cellular redox status.
The extracts of steam-processed ginseng, especially BGE, showed unique ginsenoside profiles and enhanced phenolic content and antioxidant activity (Fig. 1; Table 1). Prolonged heat treatment enhances antioxidant activity, with increased radical scavenging activity due to the release of phenolic compounds and Maillard reaction products during heat processing [15]. In addition, many pharmacological studies have shown that the ginsenosides CpK, Rg-3, and Rg-5 have several health-promoting activities, such as anti-inflammatory, antitumor, and antioxidant potential [27], [28], [29]. However, their contents in naturally available ginseng are insufficient for such activities. Increasing the content of these ginsenosides and phenolic compounds via steam processing enhanced the antioxidant potential of BGE, as shown herein (Fig. 1; Table 1).
We further investigated the protective role of the processed ginseng extracts against oxidative stress by adopting an oxidative stress model using AML-12 cells treated with H2O2. Since oxidative stress ultimately leads to increased ROS production, ATP depletion, and eventual cell death [5], [25], we also measured the ROS and ATP levels in AML-12 cells under oxidative stress. The results were in a good agreement with the antioxidant data (Fig. 2). BGE showed a significantly stronger protective effect against ROS depletion and ATP restoration compared with WGE and RGE (p < 0.05). H2O2 exposure resulted in an imbalance in cellular redox products. Cells are equipped with antioxidants such as GSH to combat oxidative stress [30], however, when the level of oxidative stress overwhelms the cellular antioxidant defense system, then the cellular redox status is disturbed, ultimately leading to cellular damage. Ginseng extract alone did not induce toxicity in AML-12 cells after 12 h of exposure (Fig. S2 in the supplemental information online). To determine the role of ginseng extract, especially BGE, in maintaining the cellular redox status and protecting against oxidative stress-induced cellular damage, we measured the levels of GSH, GSSG, and NADPH to determine the cellular redox status. Under oxidative stress, significantly reduced GSH and increased GSSG levels lead to cellular damage [26], [31], [32]. BGE significantly shifted the cellular balance to antioxidant condition and maintained the cellular redox status by restoring the NADPH and GSH levels (Fig. 3).
Oxidative stress ultimately leads to cell death by damaging lipids, proteins, and DNA [33], [34]. Therefore, we also measured protein carbonylation, lipid peroxidation, DNA damage, cytotoxicity, and apoptosis in AML-12 cells pretreated with ginseng extracts. Steam processing not only enhanced the cellular antioxidant potential of BGE but also blocked H2O2-induced cell death and cytotoxicity by inhibiting lipid peroxidation, protein carbonylation, and DNA damage (Fig. 4, Fig. 5). Taken together, these findings indicate that steam processing increased the antioxidant potential by changing the compositional profile of BGE and altering its biological activity. Thus, the antioxidant potential of BGE was significantly enhanced compared with that of RGE and WGE, which may be due to steam processing. BGE functioned as a ROS scavenger and maintained the oxidative balance in AML-12 cells during oxidative stress, significantly preventing cellular damage and protecting against the apoptosis-induced cell death compared with RGE and WGE. To best of our knowledge, in this study, we report for the first time the modulatory role of BGE in oxidative stress-induced liver cell damage. However, further studies are required using in vivo model to determine the optimal conditions of administration.
In conclusion, BGE supplementation might be useful for the protection against oxidative stress-induced liver damage by suppression of oxidative stress and maintenance of cellular redox status. BGE may be a potential natural agent for cellular defense in liver cells. Importantly, these data strongly suggest that BGE supplementation could be effective for treatment and prevention of liver cell damage induced during oxidative stress.
Acknowledgments
This research was supported by High Value-added Food Technology Development Program, funded by iPET (Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries) (114037-3), Ministry of Agriculture, Food and Rural Affairs (114037-3).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jgr.2017.10.003.
Contributor Information
Jin Hyup Lee, Email: jinhyuplee@korea.ac.kr.
Young Jun Kim, Email: yk46@korea.ac.kr.
Conflicts of interest
The authors declare no competing financial interest.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supp. Fig. 1.
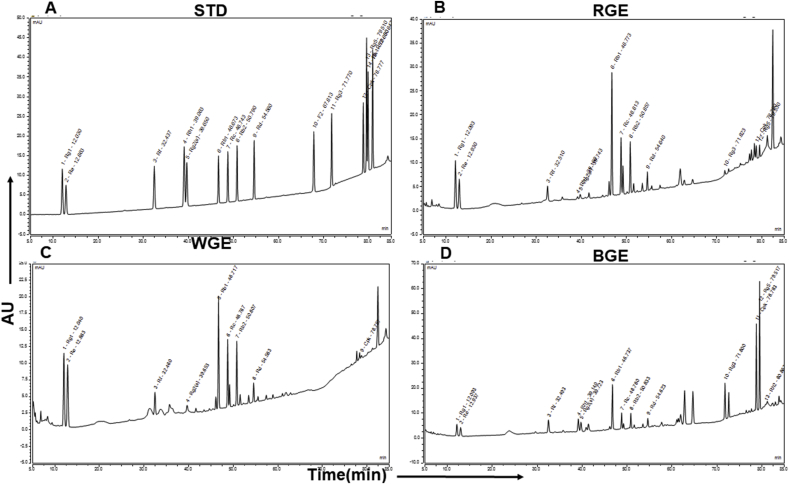
High-performance liquid chromatography chromatogram of ginsenosides standards (A) compared with the chromatogram of the ginsenoside in the white (B), red (C) and black (D) ginseng extracts (WGE, RGE & BGE).
Supp. Fig. 2.
Cytotoxicity. Cells were exposed to 500 and 1,000 μg/mL of WGE, RGE and BGE for 12 h. Cell viability was determined by MTT assay.
References
- 1.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mates J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 4.Islam M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res. 2017;39:73–82. doi: 10.1080/01616412.2016.1251711. [DOI] [PubMed] [Google Scholar]
- 5.Mondal N.K., Roychoudhury S., Mukherjee S., Siddique S., Banerjee M., Slaughter M.S., Lahiri T., Ray M.R. Increased risk of cardiovascular disease in premenopausal female ragpickers of Eastern India: involvement of inflammation, oxidative stress, and platelet hyperactivity. Mol Cell Biochem. 2016;419:193–203. doi: 10.1007/s11010-016-2773-3. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed J., Nazratun Nafizah A.H., Zariyantey A.H., Budin S.B. Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ Med J. 2016;16:e132–e141. doi: 10.18295/squmj.2016.16.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S.H., Kwon C.H., Nakano I. Detoxification of oxidative stress in glioma stem cells: mechanism, clinical relevance, and therapeutic development. J Neurosci Res. 2014;92:1419–1424. doi: 10.1002/jnr.23431. [DOI] [PubMed] [Google Scholar]
- 8.Safaeian L., Sajjadi S.E., Javanmard S.H., Montazeri H., Samani F. Protective effect of Melissa officinalis extract against H2O2-induced oxidative stress in human vascular endothelial cells. Res Pharm Sci. 2016;11:383–389. doi: 10.4103/1735-5362.192488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung S.Y., Kim C., Kim W.S., Lee S.G., Lee J.H., Shim B.S., Kim S.H., Ahn K.S., Ahn K.S. Korean Red Ginseng extract enhances the anticancer effects of imatinib mesylate through abrogation p38 and STAT5 Activation in KBM-5 cells. Phytother Res. 2015;29:1062–1072. doi: 10.1002/ptr.5347. [DOI] [PubMed] [Google Scholar]
- 10.Yun S.W., Kim M.R. Antidiabetic activity of Korean traditional candied ginseng in type I diabetic mouse induced by Alloxan. Diabetes Res Clin Pract. 2014;106:S244–S245. [Google Scholar]
- 11.Lee J., Cho J.Y., Kim W.K. Anti-inflammation effect of exercise and Korean Red Ginseng in aging model rats with diet-induced atherosclerosis. Nutr Res Pract. 2014;8:284–291. doi: 10.4162/nrp.2014.8.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun B.S., Gu L.J., Fang Z.M., Wang C.Y., Wang Z., Lee M.R., Li Z., Li J.J., Sung C.K. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC-ELSD. J Pharm Biomed Anal. 2009;50:15–22. doi: 10.1016/j.jpba.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Jin Y., Kim Y.J., Jeon J.N., Wang C., Min J.W., Noh H.Y., Yang D.C. Effect of white, red and black ginseng on physicochemical properties and ginsenosides. Plant Foods Hum Nutr. 2015;70:141–145. doi: 10.1007/s11130-015-0470-0. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.H., Pan J.H., Cho H.T., Kim Y.J. Black ginseng extract counteracts streptozotocin-induced diabetes in mice. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang K.S., Yamabe N., Kim H.Y., Okamoto T., Sei Y., Yokozawa T. Increase in the free radical scavenging activities of American ginseng by heat processing and its safety evaluation. J Ethnopharmacol. 2007;113:225–232. doi: 10.1016/j.jep.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Park H.R., Lee S.E., Yang H., Son G.W., Jin Y.H., Park Y.S. Induction of thioredoxin reductase 1 by Korean Red Ginseng water extract regulates cytoprotective effects on human endothelial cells. Evid Based Complement Alternat Med. 2015;2015:972040. doi: 10.1155/2015/972040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han J.Y., Lee S., Yang J.H., Kim S., Sim J., Kim M.G., Jeong T.C., Ku S.K., Cho I.J., Ki S.H. Korean Red Ginseng attenuates ethanol-induced steatosis and oxidative stress via AMPK/Sirt1 activation. J Ginseng Res. 2015;39:105–115. doi: 10.1016/j.jgr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parthasarathi S., Hong S.C., Oh M.H., Park Y.S., Yoo J.H., Seol S.Y., Lee H., Park J.D., Pyo M.K. Anti-oxidant and hepatoprotective effect of white ginsengs in H2O2-treated HepG2 cells. Nat Prod Sci. 2015;21:210–218. [Google Scholar]
- 19.Yun S.J., Bae G.S., Park J.H., Song T.H., Choi A., Ryu B.Y., Pang M.G., Kim E.J., Yoon M., Chang M.B. Antioxidant effects of cultured wild ginseng root extracts on the male reproductive function of boars and Guinea pigs. Anim Reprod Sci. 2016;170:51–60. doi: 10.1016/j.anireprosci.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Hu J.N., Liu Z., Wang Z., Li X.D., Zhang L.X., Li W., Wang Y.P. Ameliorative effects and possible molecular mechanism of action of black ginseng (Panax ginseng) on acetaminophen-mediated liver injury. Molecules. 2017;22 doi: 10.3390/molecules22040664. E664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bak M.J., Jeong W.S., Kim K.B. Detoxifying effect of fermented black ginseng on H2O2-induced oxidative stress in HepG2 cells. Int J Mol Med. 2014;34:1516–1522. doi: 10.3892/ijmm.2014.1972. [DOI] [PubMed] [Google Scholar]
- 22.Liu S.C., Lin J.T., Hu C.C., Shen B.Y., Chen T.Y., Chang Y.L., Shih C.H., Yang D.J. Phenolic compositions and antioxidant attributes of leaves and stems from three inbred varieties of Lycium chinense Miller harvested at various times. Food Chem. 2017;215:284–291. doi: 10.1016/j.foodchem.2016.06.072. [DOI] [PubMed] [Google Scholar]
- 23.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 24.Choudhry Q.N., Kim M.J., Kim T.G., Pan J.H., Kim J.H., Park S.J., Lee J.H., Kim Y.J. Saponin-based nanoemulsification improves the antioxidant properties of vitamin A and E in AML-12 cells. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17091406. E1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiwari B.S., Belenghi B., Levine A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol. 2002;128:1271–1281. doi: 10.1104/pp.010999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korge P., Calmettes G., Weiss J.N. Increased reactive oxygen species production during reductive stress: the roles of mitochondrial glutathione and thioredoxin reductases. Biochim Biophys Acta. 2015;1847:514–525. doi: 10.1016/j.bbabio.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hossen M.J., Hong Y.D., Baek K.S., Yoo S., Hong Y.H., Kim J.H., Lee J.O., Kim D., Park J., Cho J.Y. In vitro antioxidative and anti-inflammatory effects of the compound K-rich fraction BIOGF1K, prepared from Panax ginseng. J Ginseng Res. 2017;41:43–51. doi: 10.1016/j.jgr.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh S.J., Oh Y., Ryu I.W., Kim K., Lim C.J. Protective properties of ginsenoside Rb3 against UV-B radiation-induced oxidative stress in HaCaT keratinocytes. Biosci Biotechnol Biochem. 2015;80:95–103. doi: 10.1080/09168451.2015.1075862. [DOI] [PubMed] [Google Scholar]
- 29.Yun T.K. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat Res. 2003;523–524:63–74. doi: 10.1016/s0027-5107(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 30.Dunning S., Ur Rehman A., Tiebosch M.H., Hannivoort R.A., Haijer F.W., Woudenberg J., van den Heuvel F.A., Buist-Homan M., Faber K.N., Moshage H. Glutathione and antioxidant enzymes serve complementary roles in protecting activated hepatic stellate cells against hydrogen peroxide-induced cell death. Biochim Biophys Acta. 1832;2013:2027–2034. doi: 10.1016/j.bbadis.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Kavitha S., Chandra T.S. Oxidative stress protection and glutathione metabolism in response to hydrogen peroxide and menadione in riboflavinogenic fungus. Ashbya Gossypii, Appl Biochem Biotechnol. 2014;174:2307–2325. doi: 10.1007/s12010-014-1188-4. [DOI] [PubMed] [Google Scholar]
- 32.Presnell C.E., Bhatti G., Numan L.S., Lerche M., Alkhateeb S.K., Ghalib M., Shammaa M., Kavdia M. Computational insights into the role of glutathione in oxidative stress. Curr Neurovasc Res. 2013;10:185–194. doi: 10.2174/1567202611310020011. [DOI] [PubMed] [Google Scholar]
- 33.Bhardwaj M., Kim N.H., Paul S., Jakhar R., Han J., Kang S.C. 5-Hydroxy-7-methoxyflavone triggers mitochondrial-associated cell death via reactive oxygen species signaling in human colon carcinoma cells. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqui T., Zia M.K., Ali S.S., Rehman A.A., Ahsan H., Khan F.H. Reactive oxygen species and anti-proteinases. Arch Physiol Biochem. 2016;122:1–7. doi: 10.3109/13813455.2015.1115525. [DOI] [PubMed] [Google Scholar]