Albendazole is an effective anthelmintic intensively used for decades. However, profound pharmacokinetic (PK) characterization is missing in children, the population mostly affected by helminth infections.
KEYWORDS: Mitra, albendazole, dried-blood spot, pharmacokinetics
ABSTRACT
Albendazole is an effective anthelmintic intensively used for decades. However, profound pharmacokinetic (PK) characterization is missing in children, the population mostly affected by helminth infections. Blood microsampling would facilitate PK studies in pediatric populations but has not been applied to quantify albendazole’s disposition. Quantification methods were developed and validated using liquid chromatography-tandem mass spectrometry to analyze albendazole and its metabolites albendazole sulfoxide and albendazole sulfone in wet samples (plasma and blood) and blood microsamples (dried-blood spots [DBS]; Mitra). The use of DBS was limited by a matrix effect and poor recovery, but the extraction efficiency was constant throughout the concentration range. Hookworm-infected adolescents were venous and capillary blood sampled posttreatment with 400 mg albendazole and 25 mg/kg oxantel pamoate. Similar half-life (t1/2 = ∼1.5 h), time to reach the maximum concentration (tmax = ∼2 h), and maximum concentration (Cmax = 12.5 to 26.5 ng/ml) of albendazole were observed in the four matrices. The metabolites reached Cmax after ∼4 h with a t1/2 of ca. 7 to 8 h. A statistically significant difference in albendazole sulfone’s t1/2 as determined by using DBS and wet samples was detected. Cmax of albendazole sulfoxide (288 to 380 ng/ml) did not differ among the matrices, but higher Cmax of albendazole sulfone were obtained in the two microsampling devices (22 ng/ml) versus the wet matrices (14 ng/ml). In conclusion, time-concentration profiles and PK results of the four matrices were similar, and the direct comparison of the two microsampling devices indicates that Mitra extraction was more robust during validation and can be recommended for future albendazole PK studies.
INTRODUCTION
Albendazole, a benzimidazole derivative, was marketed in 1982 as an anthelmintic for humans. Besides mebendazole, it is the gold standard to treat infections with soil-transmitted helminths (STH) due its good safety and efficacy profile (1). STH are responsible for high disease burdens and are still endemic in more than 100 countries affecting mostly the African, South American, and Asian continents (2). In the framework of the World Health Organization’s preventive chemotherapy program, anthelmintic drugs are distributed to populations at high risk, mainly children to reduce the burden caused by STH (3).
Despite the intensive administration of albendazole over decades, surprisingly little information is known on the optimal albendazole dosages treating infections with Ascaris lumbricoides, hookworms, and Trichuris trichiura. In addition, pharmacokinetic (PK) studies have been mostly performed in healthy adults and in a few cases in adults infected with Wuchereria bancrofti or hydatid disease (4–11). Only three small PK studies have been performed with 5 to 10 children who received albendazole treatment and who were infected with hydatid disease, neurocysticercosis, or Giardia intestinalis (12–14). Albendazole is rapidly metabolized to albendazole sulfoxide and albendazole sulfone (15). The sulfoxide is majorly found in the blood compartment and thus is primarily characterized in the PK studies conducted to date. However, it is not known yet which of the molecules and whether systemic or local (in the gastrointestinal tract) drug concentrations are relevant for the anthelmintic activity. Larger PK studies and PK/pharmacodynamic (PD) analyses are required to identify optimal dosing regimens. Moreover, it is a well-known challenge to extrapolate PK from adults to children, and modeling approaches cannot fully replace clinical studies yet. Venous blood sampling is, however, a stressful approach, especially for children when multiple sampling is required. Hence, less invasive alternatives are required, such as blood microsampling. Two blood microsampling devices are currently available: dried-blood spot (DBS) cards and the Mitra microsampler. The procedures for both tools involve a capillary blood drop, produced via finger pricking, which is either taken with a glass capillary and spotted on DBS cards (20 to 60 μl per spot) or absorbed by the tip of the Mitra microsampler (10 to 20 μl). However, neither device has yet been evaluated for its suitability to quantify albendazole and its metabolites, and blood microsampling needs to be validated before applying it to a larger albendazole PK trial.
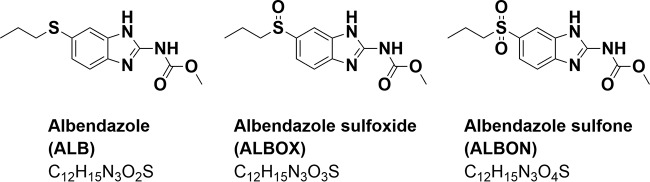
The aim of the present study was to investigate whether the two microsampling techniques, DBS and Mitra, are valuable tools in clinical PK studies on albendazole. First, the extraction and liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods were established and validated according to U.S. Food and Drug Administration (FDA) guidelines to quantify albendazole and its two metabolites albendazole sulfoxide and albendazole sulfone (Fig. 1) in blood, plasma, DBS, and Mitra. The methods were next applied to samples collected during a clinical study in rural Côte d’Ivoire. The primary aim of the clinical trial was to study drug combinations in hookworm-infected adolescents. A subsample of ten adolescents randomized to receive the standard doses of albendazole (400 mg) and oxantel pamoate (25 mg/kg) were venous and capillary blood sampled until 24 h posttreatment. The time-concentration profiles, as well as the PK parameters, were evaluated and compared among the matrices.
FIG 1.
Molecular structures of albendazole, albendazole sulfoxide, and albendazole sulfone.
RESULTS AND DISCUSSION
Method development and validation.
Methods to quantify albendazole and its metabolites have been previously established by HPLC or LC-MS/MS (4–7). Based on the published data, SRM scans were performed for 266.1 → 234.4 (albendazole), 298.1 → 159.3 m/z (albendazole sulfone), 282.1 → 240.4 m/z (albendazole sulfoxide), and 269.1 → 234.4 (albendazole-d3) m/z fragments (5, 7). Fragment-specific mass spectrometer parameters were evaluated by direct infusion of 1 μg/ml albendazole, albendazole sulfoxide, and albendazole sulfone dissolved in methanol to the mass spectrometer, followed by a stepwise optimization of source parameters (see Table S2 in the supplemental material).
LC conditions were tested on a C18 column with water and acetonitrile or methanol as mobile phases spiked with formic acid. First, an isocratic elution was performed, which caused insufficient compound separation. The best results were observed with a gradient eluent, including an initial trapping system to remove remaining matrix (Table S1). Water and methanol (both spiked with 0.05% formic acid) served best as mobile phases as already illustrated by Chhonker et al. (7). High carryover could be resolved by performing a needle wash after every injection with methanol-water (9:1) spiked with 0.1% formic acid.
The extraction methods of albendazole and its metabolites from plasma, blood, DBS, and Mitra were stepwise evaluated by testing the relative recovery (RRE) and matrix effect (ME) with three concentrations (low, middle, and high) (Table 1). A protein precipitation method was applied to plasma samples by adding acetonitrile or methanol purely or in an aqueous mixture of different ratios and volumes with or without formic acid. Best results were achieved with a mixture of acetonitrile/H2O (5:1 [vol/vol], extraction solvent 1 [ES 1], 240 μl) with 0.1% formic acid and a shaking time of 5 min at room temperature (RRE >93%). Longer shaking, a higher volume of ES 1, and the inclusion of sonication were tested, with no improvement. The same extraction method was tested for blood samples. A higher volume of ES 1 (300 μl) was required to entirely precipitate blood cells and proteins (RRE >95%; Table 1). Initially, an ME was observed in both wet matrices that could be resolved by evaporating and reconstituting the extracted samples in methanol (ME >93%). The lower limits of quantification (LLOQ) of both wet matrices were identified to be 1 ng/ml.
TABLE 1.
Relative recovery and matrix effect of albendazole, albendazole sulfone, and albendazole sulfoxide extracted from plasma, blood, DBS, and Mitra samplesa
| Matrix | Analyte | Cnominal (ng/ml) | RRE |
ME |
||
|---|---|---|---|---|---|---|
| RRE ± CV (%)* | Mean ± CV (%)† | ME ± CV (%)* | Mean ± CV (%)† | |||
| Plasma | ALB | 5 | 99.7 ± 6.20 | 98.0 ± 4.78 | 109 ± 4.31 | 102 ± 7.00 |
| 100 | 98.7 ± 2.56 | 96.8 ± 5.53 | ||||
| 150 | 95.6 ± 3.81 | 99.8 ± 3.21 | ||||
| ALBON | 5 | 93.9 ± 1.22 | 93.9 ± 2.94 | 94.0 ± 2.14 | 95.3 ± 3.24 | |
| 100 | 91.9 ± 1.87 | 96.3 ± 2.88 | ||||
| 150 | 96.0 ± 3.56 | 95.5 ± 3.98 | ||||
| ALBOX | 10 | 95.0 ± 0.56 | 93.6 ± 2.98 | 101 ± 1.30 | 99.1 ± 2.60 | |
| 500 | 90.9 ± 2.05 | 98.6 ± 1.25 | ||||
| 750 | 94.9 ± 3.38 | 97.4 ± 2.92 | ||||
| Blood | ALB | 5 | 96.6 ± 2.97 | 96.0 ± 8.47 | 96.8 ± 3.17 | 98.0 ± 5.45 |
| 100 | 100 ± 2.85 | 103 ± 1.28 | ||||
| 150 | 85.0 ± 9.84 | 93.8 ± 5.40 | ||||
| ALBON | 5 | 96.2 ± 9.47 | 95.6 ± 8.17 | 98.9 ± 6.30 | 93.2 ± 6.17 | |
| 100 | 102 ± 2.73 | 91.2 ± 3.53 | ||||
| 150 | 88.5 ± 2.93 | 89.5 ± 3.41 | ||||
| ALBOX | 10 | 99.1 ± 8.87 | 95.8 ± 6.32 | 109 ± 5.59 | 97.7 ± 9.83 | |
| 500 | 95.5 ± 4.05 | 89.3 ± 3.06 | ||||
| 750 | 92.8 ± 2.27 | 94.6 ± 6.16 | ||||
| DBS | ALB | 5 | 44.1 ± 3.44 | 41.6 ± 3.79 | 70.7 ± 3.77 | 72.1 ± 4.09 |
| 100 | 41.3 ± 3.93 | 70.2 ± 1.75 | ||||
| 150 | 39.4 ± 2.18 | 74.9 ± 4.83 | ||||
| ALBON | 5 | 52.1 ± 3.26 | 51.6 ± 4.51 | 82.6 ± 1.34 | 83.1 ± 2.05 | |
| 100 | 51.0 ± 5.99 | 81.4 ± 0.88 | ||||
| 150 | 51.9 ± 3.74 | 85.0 ± 1.79 | ||||
| ALBOX | 10 | 62.1 ± 2.74 | 55.0 ± 6.20 | 90.3 ± 0.85 | 86.6 ± 3.12 | |
| 500 | 53.0 ± 5.05 | 83.2 ± 1.12 | ||||
| 750 | 49.9 ± 1.37 | 86.3 ± 1.29 | ||||
| Mitra | ALB | 10 | 66.8 ± 4.96 | 63.1 ± 5.87 | 102 ± 7.44 | 101 ± 5.15 |
| 100 | 60.9 ± 5.45 | 101 ± 4.89 | ||||
| 150 | 61.6 ± 5.36 | 101 ± 0.49 | ||||
| ALBON | 5 | 102 ± 4.88 | 98.4 ± 4.84 | 93.9 ± 2.99 | 96.9 ± 5.46 | |
| 100 | 99.0 ± 2.92 | 95.2 ± 4.54 | ||||
| 150 | 93.9 ± 1.22 | 102 ± 4.93 | ||||
| ALBOX | 10 | 114 ± 3.69 | 105 ± 7.15 | 91.5 ± 4.09 | 95.5 ± 5.66 | |
| 500 | 102 ± 2.13 | 93.6 ± 3.02 | ||||
| 750 | 99.3 ± 2.74 | 99.4 ± 3.85 | ||||
RRE, relative recovery; ME, matrix effect; ALB, albendazole; ALBON, albendazole sulfone; ALBOX, albendazole sulfoxide. *, Values are means of n = 4 different human sources; †, values are means of n = 12 samples (three concentrations measured in four human sources).
Identical optimization steps were performed for Mitra samples. Best results were achieved with ES 2 of acetonitrile-methanol (1:1 [vol/vol], 300 μl) with 0.1% formic acid, and the ME was resolved by evaporating and reconstituting the extracts in methanol, but the RRE was still low. Longer sonication (1 h) and agitation (1 h) increased the RRE of the two albendazole metabolites in Mitra up to 100% (Table 1). Albendazole yielded lower RRE (60 to 70%), which was nevertheless reproducible (coefficient of variation [CV] <6%) and thus led to consistent results in the validation process. However, the LLOQ of albendazole for Mitra had to be raised to 5 ng/ml. The physiochemical properties of albendazole and its metabolites differ from each other and can affect the binding affinity to the Mitra device and thus the recovery of the molecules, as has been previously reported for other compounds (16).
The composition of the Mitra device differs from DBS filter cards, and thus the extraction method had to be separately established. The molecules were most effectively extracted from DBS with 200 μl of ACN/H2O (4:1) with 0.1% formic acid. The samples were first agitated (5 min) and then sonicated (1 h). DBS extracts were also evaporated and reconstituted in methanol, but an ME of 72% was still observed for albendazole (Table 1). In addition, all three compounds could not be fully recovered from DBS (RRE ∼40 to 50%). The RRE could not be improved with longer agitation, sonication, or other extraction solvents. The RRE and ME were consistent throughout the calibration line, and the validation could therefore be performed with an LLOQ of albendazole of 5 ng/ml. Nevertheless, the partially obtained ME and poor RRE of DBS samples is a disadvantage in comparison to the Mitra device, as has been already described in earlier studies (16–18).
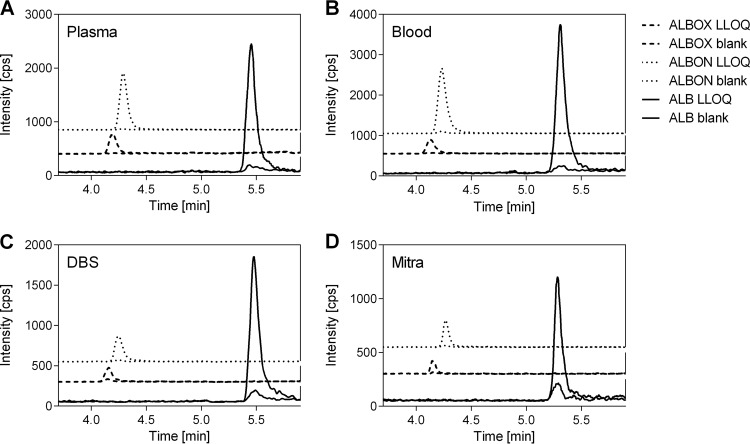
The selectivity and sensitivity of the methods were validated for albendazole and its two metabolites by extracting and analyzing six blank and six zero samples (without and with internal standard, respectively) and six LLOQs of each matrix. The results show that the respective molecules are selectively quantified, and neither a coelution of unidentified molecules extracted from the matrices nor impurities of the internal standard were observed (Fig. 2). In addition, pure oxantel pamoate was analyzed with the established method but failed to be detected, confirming the selective detection of albendazole and its metabolites. U.S. FDA guidelines demand a 5× larger result of the LLOQ than noise (extracted blank matrix) (19). Thus, an LLOQ of 1 ng/ml was determined for all three compounds extracted from plasma and blood and LLOQs of 2 ng/ml and 5 ng/ml were determined for the two metabolites and for albendazole, respectively, in DBS and Mitra (Fig. 2).
FIG 2.
Lower limits of quantification (LLOQ) of albendazole (ALB), albendazole sulfoxide (ALBOX), and albendazole sulfone (ALBON) extracted from plasma (A), blood (B), DBS (C), and Mitra (D) samples. The background signal of a zero sample matrix samples is shown for comparison. Zero samples included the internal standard (deuterated albendazole), which is not shown in the graphs. In plasma and blood samples, an LLOQ of 1 ng/ml was achieved for all three compounds. The LLOQs of ALBOX and ALBON were 2 and 5 ng/ml for ALB in DBS and Mitra.
The calibration lines were defined to cover the expected concentration range of patient samples. The highest concentration for albendazole and its two metabolites was based on earlier reported data of healthy volunteers, and the LLOQ for each matrix was determined as described above (7). The linearity of the methods was evaluated by the correlation coefficients (r2) of the calibration lines, which were >0.992. In addition, ≥75% of calibration line samples did not deviate more than ±15% (LLOQ, ±20%) from the nominal value according to U.S. FDA guidelines (19).
Accuracy and precision were measured in intra- and interday assessments, where three validation sets were analyzed on one day or on three individual days, respectively. Four quality control (QC) concentrations (LLOQ, low, medium, and high) were evaluated in six different replicates (ntot = 18 QC samples). At least 4/6 QC samples of each concentration did not deviate more than ±15% of the nominal value (±20% for the LLOQ) in all four matrices as required by U.S. FDA guidelines, and inter- and intraday assessments yielded similar results (Table 2). The accuracy of all three compounds in plasma and blood samples was similar (91.5 to 109%), and a precision of <10% supports the validity of the quantification methods. Similar results were achieved for the two blood microsampling devices. Accuracy of all three compounds ranged from 93.3 to 111%, with a precision of <8%. Moreover, various hematocrits (20 to 55%) of blood, DBS, and Mitra samples did not affect the results.
TABLE 2.
Intra- and interassay accuracy and precision of albendazole, albendazole sulfone, and albendazole sulfoxide extracted from plasma, blood, DBS, and Mitra samplesa
| Matrix | Analyte | Cnominal (ng/ml) | Interassay |
Intra-assay |
||
|---|---|---|---|---|---|---|
| cø (ng/ml) | Accuracy ± CV (%) | cø (ng/ml) | Accuracy ± CV (%) | |||
| Plasma | ALB | 1 | 1.07 | 107 ± 7.84 | 1.01 | 101 ± 1.90 |
| 5 | 4.60 | 92.0 ± 2.16 | 4.60 | 92.0 ± 2.99 | ||
| 100 | 101 | 101 ± 2.99 | 101 | 101 ± 3.81 | ||
| 150 | 159 | 106 ± 4.65 | 163 | 109 ± 2.01 | ||
| ALBON | 1 | 1.03 | 103 ± 2.86 | 1.01 | 101 ± 2.26 | |
| 5 | 4.60 | 103 ± 5.56 | 4.58 | 91.5 ± 4.65 | ||
| 100 | 98.6 | 101 ± 3.29 | 5.29 | 101 ± 5.29 | ||
| 150 | 153 | 98.3 ± 4.41 | 4.57 | 107 ± 3.05 | ||
| ALBOX | 1 | 1.03 | 103 ± 4.98 | 1.02 | 102 ± 3.50 | |
| 10 | 10.3 | 103 ± 5.55 | 9.91 | 99.1 ± 3.03 | ||
| 500 | 505 | 101 ± 3.29 | 521 | 104 ± 3.55 | ||
| 750 | 737 | 98.3 ± 4.41 | 779 | 104 ± 5.29 | ||
| Blood | ALB | 1 | 1.03 | 103 ± 8.82 | 1.02 | 102 ± 9.41 |
| 5 | 5.04 | 100 ± 9.18 | 4.97 | 99.4 ± 8.48 | ||
| 100 | 95.9 | 95.9 ± 6.04 | 93.2 | 93.2 ± 6.13 | ||
| 150 | 158 | 105 ± 7.26 | 160 | 107 ± 4.97 | ||
| ALBON | 1 | 1.03 | 103 ± 4.11 | 1.1 | 101 ± 5.26 | |
| 5 | 4.83 | 96.5 ± 4.14 | 4.57 | 91.5 ± 5.30 | ||
| 100 | 96.7 | 96.7 ± 5.44 | 97.1 | 97.1 ± 8.15 | ||
| 150 | 161 | 107 ± 5.28 | 164 | 109 ± 4.24 | ||
| ALBOX | 1 | 1.00 | 100 ± 6.18 | 0.98 | 97.9 ± 8.43 | |
| 10 | 10.0 | 100 ± 3.75 | 10.1 | 101 ± 3.34 | ||
| 500 | 517 | 103 ± 4.40 | 512 | 102 ± 4.70 | ||
| 750 | 796 | 106 ± 4.29 | 789 | 105 ± 3.33 | ||
| DBS | ALB | 5 | 5.25 | 105 ± 5.39 | 5.22 | 104 ± 5.45 |
| 10 | 9.66 | 96.6 ± 5.92 | 10.1 | 101 ± 8.32 | ||
| 100 | 102 | 102 ± 7.45 | 105 | 105 ± 6.14 | ||
| 150 | 152 | 101 ± 7.00 | 99.0 | 100 ± 7.15 | ||
| ALBON | 2 | 2.15 | 107 ± 7.94 | 2.03 | 101 ± 6.30 | |
| 5 | 5.01 | 100 ± 7.13 | 5.08 | 102 ± 7.20 | ||
| 100 | 99.6 | 99.6 ± 6.77 | 100 | 100 ± 4.88 | ||
| 150 | 153 | 102 ± 4.85 | 154 | 103 ± 4.12 | ||
| ALBOX | 2 | 2.19 | 109 ± 5.23 | 2.10 | 105 ± 6.11 | |
| 10 | 9.67 | 96.7 ± 4.60 | 9.93 | 99.3 ± 5.77 | ||
| 500 | 472 | 94.3 ± 5.91 | 494 | 98.9 ± 6.83 | ||
| 750 | 722 | 96.3 ± 6.30 | 740 | 98.6 ± 8.86 | ||
| Mitra | ALB | 5 | 5.34 | 107 ± 6.47 | 5.07 | 101 ± 6.93 |
| 10 | 9.57 | 95.7 ± 8.05 | 9.72 | 97.2 ± 7.26 | ||
| 100 | 96.3 | 96.3 ± 6.50 | 100 | 100 ± 4.82 | ||
| 150 | 150 | 100 ± 5.38 | 153 | 102 ± 3.06 | ||
| ALBON | 2 | 2.23 | 111 ± 4.62 | 2.07 | 104 ± 8.50 | |
| 5 | 4.73 | 94.5 ± 5.37 | 4.94 | 98.7 ± 5.67 | ||
| 100 | 93.3 | 93.3 ± 3.16 | 97.9 | 97.9 ± 6.08 | ||
| 150 | 146 | 97.5 ± 2.64 | 153 | 102 ± 4.54 | ||
| ALBOX | 2 | 2.16 | 108 ± 6.52 | 2.07 | 104 ± 7.20 | |
| 10 | 9.58 | 95.8 ± 4.13 | 10.1 | 101 ± 7.33 | ||
| 500 | 474 | 94.7 ± 3.41 | 491 | 98.2 ± 5.53 | ||
| 750 | 737 | 98.3 ± 1.85 | 753 | 100 ± 3.88 | ||
ALB, albendazole; ALBON, albendazole sulfone; ALBOX, albendazole sulfoxide; cø, mean concentration. The inter- and intra-assay values are means of n = 12 QC samples of three independent validation sets.
The stability of albendazole and its metabolites was evaluated in plasma, blood, DBS, and Mitra under different conditions (Table S3). Three QC concentrations (low, middle, and high) were extracted from plasma and blood and stored at room temperature for 4 h (room temperature stability). The accuracy of the three compounds ranged from 92.1 to 106% with a maximal deviation of 6.11% of both wet matrices. DBS and Mitra samples were prepared and stored at room temperature for 2 months. The analysis of analytes resulted in required accuracy (95.9 to 108%) and good precision (CV <6.71%). In addition, extracted samples of the four matrices were maintained at 4°C for 72 h to evaluate the autosampler stability. Valid accuracy (93.4 to 110%) and precision (CV <7.70%) was obtained for all three compounds in the four matrices. Three freeze-thaw cycles were performed with spiked blood, plasma, and prepared DBS samples. Individual Mitra patient samples do not undergo freeze-thaw cycles since the Mitra device is for single use only, whereas DBS filter cards, plasma, and blood can be used for several measurements. Albendazole and its two metabolites were stable under the latter condition with acceptable accuracy (±11% versus the nominal value) and precision (CV <8.2%). Long-term stability was evaluated by storing the samples at –80°C for 2 months. Albendazole and its two metabolites were extracted from all four matrices with an accuracy of 87.0 to 110% and a deviation of <8.04%. Overall, the three analytes retained stable under all tested conditions in plasma, blood, DBS, and Mitra.
Pharmacokinetics.
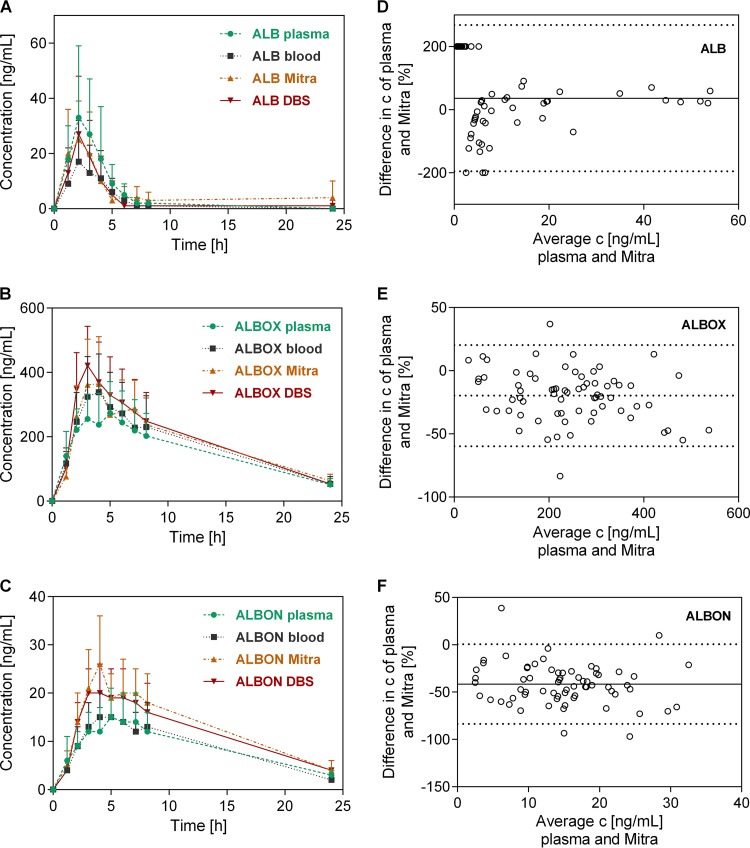
Plasma, blood, DBS, and Mitra samples were collected in the framework of a clinical trial in rural Côte d’Ivoire, where several drug combinations were tested for their efficacy against hookworm in adolescents. A subsample of ten participants randomly assigned to receive albendazole and oxantel pamoate were venous and capillary blood sampled until 24 h posttreatment. Samples were analyzed with the validated LC-MS/MS method to obtain the concentration-time profiles of albendazole, albendazole sulfoxide, and albendazole sulfone, which show similar behaviors in all four matrices (Fig. 3). The PK parameters of albendazole did not deviate significantly among the matrices, with a median half-life t1/2 of ∼1.5 h, a time to reach maximum concentration tmax of ∼2 h, a maximum concentration Cmax of 12.5 to 26.5 ng/ml, and an area under the concentration-time curve (AUC) of 44 to 78 ng ⋅ h/ml (Table 3). Albendazole sulfoxide, the main metabolite, exhibited a longer half-life (∼8 h) and tmax (∼4 h), a median Cmax of ∼300 ng/ml, and a median AUC of ∼4,000 ng ⋅ h/ml. No statistically significant difference was calculated for any PK parameter of albendazole sulfoxide among the four matrices. The second metabolite, albendazole sulfone, showed median half-life and tmax values (∼7 and ∼4 h, respectively) similar to those for albendazole sulfoxide. A greater half-life was observed in DBS samples (8.60 h, interquartile range [IQR] = 7.31 to 10.2), which differs significantly (P = 0.008) from blood samples (6.55 h, IQR = 5.55 to 7.53). The Cmax values of albendazole sulfone differ among wet and dried matrices (14 and 22 ng/ml, respectively), with a statistically significant difference among Mitra and blood or plasma results (P = 0.015). The median AUC of albendazole sulfone was similar in all four matrices (∼200 ng ⋅ h/ml). No difference of PK parameters was observed between Mitra and DBS samples in the presented study. However, other studies reported similar, significantly lower or higher Cmax for Mitra compared to DBS or plasma depending on the analyte (16, 18, 20, 21). Therefore, the applicability of the two microsampling devices to PK analyses has to be confirmed for each compound individually.
FIG 3.
Concentration-time profiles (n = 10, means + the SD) of albendazole ALB (A), albendazole sulfoxide ALBOX (B), and albendazole sulfone ALBON (C) extracted from plasma, blood, Mitra, and DBS samples, as well as Bland-Altman analysis comparing results of plasma and Mitra patient samples of ALB (D), ALBOX (E), and ALBON (F). The 95% confidence intervals are shown as dashed lines. Bias is illustrated as a solid line.
TABLE 3.
Pharmacokinetic parameters of albendazole, albendazole sulfoxide, and albendazole sulfone extracted from plasma, blood, DBS, and Mitra samplesa
| Molecule | Matrix | Median or IQR | t1/2 (h) | Tmax (h) | Cmax (ng/ml) | AUC (ng ⋅ h/ml) |
|---|---|---|---|---|---|---|
| ALB | Plasma | Median | 1.53 | 2.22 | 24.5 | 73.0 |
| IQR | 1.25–1.91 | 1.39–3.17 | 14.0–61.8 | 42.5–172 | ||
| Blood | Median | 1.34 | 2.19 | 12.5 | 44.4 | |
| IQR | 1.11–3.55 | 1.40–3.36 | 5.63–37.8 | 17.1–102 | ||
| DBS | Median | 1.22 | 2.22 | 22.0 | 55.7 | |
| IQR | 1.05–1.76 | 1.00–2.29 | 16.5–44.0 | 32.0–138 | ||
| Mitra | Median | 4.15 | 1.50 | 26.5 | 78.0 | |
| IQR | 1.47–11.9 | 1.00–2.47 | 20.0–36.8 | 53.0–150 | ||
| ALBOX | Plasma | Median | 8.56 | 4.21 | 288 | 3,418 |
| IQR | 7.37–9.26 | 3.83–5.26 | 229–347 | 2,629–4,547 | ||
| Blood | Median | 7.54 | 3.55 | 336 | 3695 | |
| IQR | 7.10–8.50 | 3.17–4.26 | 278–465 | 3,143–5,273 | ||
| DBS | Median | 7.00 | 3.17 | 380 | 4,579 | |
| IQR | 6.35–7.76 | 3.12–3.40 | 320–521 | 3,157–5,752 | ||
| Mitra | Median | 7.51 | 4.08 | 329 | 3,931 | |
| IQR | 6.54–8.33 | 3.18–5.24 | 295–554 | 2,251–5,762 | ||
| ALBON | Plasma | Median | 7.28 | 4.98 | 14.0 | 183 |
| IQR | 6.51–7.88 | 4.09–6.33 | 11.5–19.3 | 137–255 | ||
| Blood | Median | 6.55 | 4.06 | 14.0 | 171 | |
| IQR | 5.55–7.53 | 3.18–5.12 | 11.2–19.4 | 149–263 | ||
| DBS | Median | 8.60 | 4.08 | 22.0 | 286 | |
| IQR | 7.31–10.2 | 3.17–5.17 | 16.0–25.3 | 205–363 | ||
| Mitra | Median | 8.02 | 4.07 | 22.0 | 255 | |
| IQR | 6.69–8.75 | 3.97–4.48 | 20.0–37.0 | 186–394 |
ALB, albendazole; ALBON, albendazole sulfone; ALBOX, albendazole sulfoxide.
Based on the consistent results of the method validation and PK parameters Mitra was identified to be the better microsampling device in the presented study and was further evaluated by a Bland-Altmann analysis, which determines the agreement between two methods (Fig. 3) (22). Plasma is the gold standard for PK analysis and was therefore set as the comparator.
Good correlation between plasma and Mitra samples was obtained for all three compounds with no or few data points outside the confidence intervals. Albendazole sulfone yielded higher concentrations in Mitra compared to plasma samples, but the Bland-Altman analysis indicates good agreement of the two matrices (Fig. 3).
A total of 20% of the plasma, blood, and DBS samples were reanalyzed, and >2/3 of the samples did not deviate more than 20% from the initial sample result; thus, the analyses meet FDA requirements. However, Mitra samples were not collected in replicates so no second sample reanalysis could be performed. This is a limitation of our study, and the missing reanalysis of Mitra samples must be confirmed in a future study to prove the reproducibility of the results. Nevertheless, the comparable results of the concentration-time profiles and the PK parameters of the Mitra and wet samples support the validity of the Mitra device to quantify albendazole and its metabolites.
Conclusion.
Robust methods to quantify albendazole and its two metabolites extracted from plasma, blood, DBS, and Mitra samples were established by LC-MS/MS and validated according to U.S. FDA guidelines. The accuracy, precision, selectivity, sensitivity, and stability fulfilled the requirements for all four matrices. However, DBS samples presented poor RRE and high ME, which could not be resolved in the optimization process, and therefore limit the applicability of this tool. When the methods were applied to patient samples collected in a clinical trial with hookworm-infected adolescents, the time-concentration profiles of albendazole and the two metabolites appeared similar for the four matrices. The noncompartmental analysis supported the similarity since most of the PK parameters did not deviate significantly among the matrices. The plasma and blood samples yielded the same results, but the t1/2 of albendazole sulfone of the DBS differed significantly from blood. The Mitra results deviated significantly in the Cmax of albendazole sulfone from the wet matrices, but Bland-Altman analysis demonstrated good agreement among individual plasma and Mitra concentrations of all three compounds.
Given the above-mentioned limitations of DBS and the fact that PK parameters of Mitra samples resemble well the results of the wet matrices, Mitra represents the microsampling device of choice to quantify albendazole and its two metabolites. The reproducibility of Mitra results has to be verified in a future study by collecting replicates to fully ensure the validity of this microsampling technique. The application of a microsampling device to clinical trials offers a more ethical alternative to venous blood sampling and simplifies field and laboratory work, especially when working with children or a large cohort.
MATERIALS AND METHODS
Chemicals and materials.
Albendazole was purchased from Fluka (Steinheim, Germany), albendazole sulfoxide was obtained from Sigma-Aldrich (Steinheim, Germany), albendazole sulfone was synthesized by WITEGA Laboratorien (Berlin, Germany), and albendazole-d3 was purchased from C/D/N Isotopes, Inc. (Quebec, Canada). Albendazole tablets (chewable, 400 mg) were obtained from GlaxoSmithKline (Brentford, UK). Ultrapure water was prepared using a Millipore water purification system (Milli-Q Advantage A10; Merck KGaA, Darmstadt, Germany). Formic acid (LC-MS grade), acetonitrile and methanol (LC-MS grade), and Whatman protein saver cards 903 were purchased from Merck KGaA. Mitra devices (10 μl) were purchased from Neoteryx (Torrance, CA). Human blood was obtained from the local blood donation center (Basel, Switzerland) in heparin-coated Vacutainer tubes to prevent the coagulation of blood.
LC-MS/MS instrumentation and settings.
Analyte separation was performed on a 1260 Infinity liquid chromatography system, and compounds were identified by using a 6460 triple quad mass spectrometer (Agilent Technologies, Santa Clara, CA). The autosampler Thermostat (1200 series; Agilent Technologies) served for sample injection and cooling (4°C).
The analytes were first loaded onto a column trapping system (HALO C-18, 4.6 × 5 mm; Optimize Technologies, Oregon City, OR) to remove the remaining matrix and thereafter eluted to a Symmetry C18 column (4.6 × 100 mm, 3.5-μm particle size; Waters AG, Baden-Dättwil, Switzerland) for compound separation. A gradient elution program was applied (Table S1) with ultrapure water (mobile phase A) and methanol (mobile phase B), both spiked with 0.05% formic acid (vol/vol) as eluents. The autosampler syringe and injection valve were cleaned three times with methanol-H2O (9:1 [vol/vol]) with 0.1% formic acid (vol/vol) after each sample injection. The optimized fragment-specific mass spectrometer parameters of albendazole, albendazole sulfoxide, albendazole sulfone, and deuterated albendazole are summarized in Table S2. The positively charged fragments of albendazole, its metabolites, and the internal standard were detected by selected reaction monitoring. The general settings of the mass spectrometer were adapted as followed: gas temperature, 350°C; gas flow, 13 liters/min; nebulizer, 50 lb/in2; sheath gas temperature, 400°C; sheath gas flow, 11 liters/min; and capillary voltage, 2,500 V. Data analysis was performed using MassHunter quantitative analysis (Agilent Technologies).
Blood, plasma, DBS, and Mitra sample procession. (i) Preparation of calibration and quality control samples.
The stock solutions of albendazole, albendazole sulfoxide, and albendazole sulfone were prepared in dimethyl sulfoxide (DMSO; 1 mg/ml) and stored at –20°C. Working solutions of the analytes were obtained by serial dilutions in acetonitrile-water (1:1 [vol/vol]). Quality control (QC) and calibration line (CL) samples were prepared by spiking plasma and blood with working solutions (50:1 [vol/vol]). DBS and Mitra QC and CL samples were produced with spiked blood. The final concentration of organic solvent in the spiked plasma and blood samples was ≤3%. The CL of albendazole and albendazole sulfone covered a range of 1 to 200 ng/ml, and the CL of albendazole sulfoxide covered a range of 1 to 1,000 ng/ml for plasma and blood samples. The CL of DBS and Mitra samples were prepared from 5 to 200 ng/ml for albendazole, 2 to 200 ng/ml for albendazole sulfone, and 2 to 1,000 ng/ml for albendazole sulfoxide. CL samples consisted of eight concentrations, including the lower limit of quantification (LLOQ). QC samples were prepared at LLOQ, low, medium, and high concentrations to cover the entire CL. QC samples of plasma and blood for albendazole and albendazole sulfone consisted of 1, 5, 100, and 150 ng/ml, and 1, 10, 500, and 750 ng/ml were used as QC samples for albendazole sulfoxide. In contrast to blood and plasma, the LLOQ DBS and Mitra QC sample was 2 ng/ml for albendazole sulfoxide and sulfone, and the LLOQ and low QC of albendazole were 5 and 10 ng/ml, respectively.
QC samples were prepared in six replicates of different human sources. In addition to CL and QC samples, six blank samples (matrix without internal standard) and six zero samples (matrix with internal standard) of different human sources were additionally prepared. QC and CL samples derived from plasma were always spiked freshly on the day of analysis; blood, DBS, and Mitra samples were prepared and stored at –20°C until analyzed.
(ii) Sample extraction.
The stock solution of albendazole-d3 (1 mg/ml) was prepared in DMSO and stored at –20°C. The extraction solvent (acetonitrile/H2O [5:1, vol/vol] with 0.1% formic acid, ES 1) was spiked with 50 ng/ml of the internal standard. Portions (100 μl) of plasma or blood samples were mixed with 240 or 300 μl of ES 1, respectively, and agitated for 5 min at 2,000 rpm at room temperature. Mitra samples were placed in a Strata 96-well plate (2 ml; Phenomenex, Basel, Switzerland) prefilled with 300 μl of acetonitrile/methanol (1:1 [vol/vol]) with 0.1% formic acid and spiked with 16.7 ng/ml internal standard (ES 2). Samples were sonicated in an ultrasonic bath (TPC-280; M. Scherrer AG, Zuzwil, Switzerland) for 1 h and agitated for 1 h at room temperature at 1,200 rpm; the Mitra devices were then discarded. A circular spot (5 mm in diameter) was punched from each DBS filter card, and 200 μl of ACN/H2O (4:1) with 0.1% formic acid and 50 ng/ml internal standard was added (ES 3). The samples were mixed for 5 min at 1,200 rpm and sonicated in an ultrasonic bath for 1 h at room temperature.
After extraction, plasma and blood samples were centrifuged for 10 min at 3,300 × g. The supernatants and the DBS and Mitra extracts were evaporated and reconstituted in methanol (200 μl for plasma and blood and 100 μl for DBS and Mitra samples). Then, 10 μl of each sample was injected into the LC-MS/MS system for analysis.
(iii) Impact of hematocrit on blood, DBS, and Mitra sample analysis.
The hematocrit value can potentially cause variability of results in blood, DBS, and Mitra samples. To ensure that the hematocrit does not affect the quantification of albendazole and its metabolites extracted from blood, DBS, and Mitra, the hematocrit of the QC samples (n = 6 from different human donors) was adjusted with plasma to 20 to 55% to cover a broad hematocrit range. CL samples were prepared with 40 or 45% hematocrit.
Method validation.
According to U.S. FDA guidelines, the stability, selectivity, sensitivity, accuracy, precision, recovery, matrix effect, and linearity of the analytes’ quantification methods in plasma, blood, DBS, and Mitra were validated (19).
(i) Selectivity and sensitivity.
Six zero samples of plasma, blood, DBS, and Mitra of different human sources were processed. The analyte response of LLOQ must be >5× the zero sample response and is not allowed to deviate more than ±20% from the nominal concentration.
(ii) Linearity.
CL samples were accepted with a coefficient of determination (r2) ≥0.99 and when 75% of the CL samples were ±15% (±20 for LLOQ) of the nominal value.
(iii) Accuracy and precision.
The accuracy and precision of the methods was determined by processing four QC concentrations (LLOQ, low, medium, and high) each prepared in six replicates of different blood or plasma sources. The intra-assay was determined by analyzing three validation sets in a single analytical run, and the precision and accuracy of the interassay was assessed by processing three validation sets on three different days. The results for the QCs were compared to the nominal value evaluated with the CL samples. Successful validation was confirmed when 4/6 replicates were within ±15% of the nominal value and within ±20% of the nominal value for the LLOQ. Accuracy is presented as the percentage ratio of measured to the nominal concentration and precision is illustrated as the coefficient of variation (CV [%]).
(iv) Recovery and matrix effect.
QC sample concentrations (low, medium, and high) were prepared for analyzing relative recovery (RRE) and matrix effect (ME) in four replicates of different human sources. RRE is evaluated by comparing extracted QC samples and zero samples that are spiked with the corresponding analytes’ concentrations after extraction. The ME is determined by comparing the extracted zero sample matrix that is spiked after extraction to the corresponding analytes’ concentrations prepared in methanol.
(v) Stability.
Four conditions mimicking field and laboratory work were tested to ensure the stability of the analytes in plasma, blood, DBS, and Mitra samples: (i) extracted plasma and blood samples were incubated at room temperature for 4 h, or DBS and Mitra samples were stored at room temperature for 2 months (room temperature stability); (ii) extracted samples were incubated at 4°C for 72 h (autosampler stability); (iii) samples were stored at –80°C for 2 months (long-term stability); and (iv) samples were frozen three times for 24 h at –80°C, followed by thawing to room temperature (freeze-thaw cycles). QC samples (low, middle, and high) were prepared and kept under the specified conditions. The samples were analyzed and compared to the CL. The analytes were considered stable when the results did not deviate more than ±15% from the nominal value.
Pharmacokinetics.
Samples were collected in a clinical trial that primarily evaluated the efficacy of several anthelmintic combinations against hookworm infections in adolescents in rural Côte d’Ivoire (23). Ethical approval was obtained from the Ethical Committee of Northwestern and Central Switzerland (EKNZ UBE-15/35) and the Comité d’Ethique et de la Recherche of the Ministry of Health in Côte d’Ivoire (083/MSHP/CNER-kp). The study was registered at the ISRCTN registry (no. 14373201). Written informed consent was obtained from all participants and their legal guardians. Detailed information on patient recruitment, exclusion and inclusion criteria, and randomization procedure and efficacy and safety data can be found elsewhere (23).
In one treatment arm of the study, hookworm-infected adolescents (15 to 20 years of age) were orally treated with 400 mg albendazole and 25 mg/kg oxantel pamoate. Ten participants (15 to 18 years of age, 46 to 65 kg, 158 to 170 cm, all males) were selected for inclusion in the PK study. Volunteers received oily fish on bread as a standardized breakfast before treatment (8). Venous and capillary blood sampling was performed at 0, 1, 2, 3, 4, 5, 6, 7, 8, and 24 h posttreatment. Venous blood (∼4 ml) was collected in EDTA-covered Vacutainer tubes (BD, Allschwil, Switzerland) to prevent coagulation. Half of the collected blood was centrifuged to obtain plasma. Blood and plasma samples were stored at –20°C at the clinical trial site. In addition, blood microsampling was performed at the same time points by taking capillary blood from the same individuals. Sterile finger-prickers were used to puncture the tip of a finger to obtain a blood drop. For DBS sampling, lithium heparin-coated capillaries were loaded with capillary blood, which was dropped onto DBS cards (∼20 μl per spot). Four blood spots were collected for each patient and time point. Single Mitra sampling was performed by dipping the tip of the Mitra device into the capillary blood drop on the finger at each time point and patient. The DBS and Mitra samples were dried for at least 2 h and stored at room temperature at the clinical trial site. All samples were shipped to Basel, Switzerland, with dried ice and stored at –80°C until processed for analysis. Albendazole and its metabolites were quantified with the presented, validated LC-MS/MS method. Incurred sample reanalysis was performed for 20% of the samples, and two-thirds are not allowed to deviate more than 20% from the initial results.
Data analysis.
The PK parameters were evaluated by a noncompartmental analysis using WinNonlin (5.2; Certara, Princeton, NJ). The maximum concentration (Cmax), the time to reach Cmax (tmax), the time in which half of the absorbed drug is eliminated (half-life t1/2), and the area under the curve (until last positive concentration [AUC]) were determined. The concentration results of albendazole of four Mitra samples were excluded from data analysis. The results were identified as artifacts, with a >5-fold higher evaluated concentration compared to the respective plasma, blood, and DBS samples. Mitra sampling was not performed in replicates so that the sample analysis could not be repeated.
Statistical data analysis was performed with Prism 6.01 (GraphPad, San Diego, CA). A Kruskal-Wallis analysis, followed by a Dunn’s post hoc test, was applied to evaluate statistically significant differences among the PK parameters of the four matrices. Bland-Altman analysis was conducted to compare individual analyte concentrations obtained from plasma and Mitra samples. The difference (%) of the compound concentration extracted from the matrices was plotted versus the average concentration. The first time point (1 h posttreatment) was excluded from the Bland-Altman analysis due to a large time deviation among the matrices. Blood microsamples were collected at 1 h posttreatment, but wet sampling was delayed (≥30 min) due to technical issues.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to all of the volunteers who agreed to participate in the clinical trial and the local team in Azaguié that performed the PK study.
We thank the Swiss National Science Foundation (320030_14930/1 and 320030_175585) for financial support.
There are no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02489-18.
REFERENCES
- 1.World Health Organization. 2017. Preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 2.Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG. 2018. Soil-transmitted helminth infections. Lancet 391:252–265. doi: 10.1016/S0140-6736(17)31930-X. [DOI] [PubMed] [Google Scholar]
- 3.Schulz JD, Moser W, Hürlimann E, Keiser J. 2018. Preventive chemotherapy in the fight against soil-transmitted helminthiasis: achievements and limitations. Trends Parasitol 34:590–602. doi: 10.1016/j.pt.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Saraner N, Özkan GY, Güney B, Alkan E, Burul-Bozkurt N, Sağlam O, Fikirdeşici E, Yıldırım M. 2016. Determination of albendazole sulfoxide in human plasma by using liquid chromatography-tandem mass spectrometry. J Chromatogr B 1022:1–5. doi: 10.1016/j.jchromb.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Rathod DM, Patel KR, Mistri HN, Jangid AG, Shrivastav PS, Sanyal M. 2016. Liquid chromatography–tandem mass spectrometry method for simultaneous determination of albendazole and albendazole sulfoxide in human plasma for bioequivalence studies. J Pharm Anal 6:226–234. doi: 10.1016/j.jpha.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirfazaelian A, Dadashzadeh S, Rouini MR. 2002. A high performance liquid chromatography method for simultaneous determination of albendazole metabolites in human serum. J Pharm Biomed Anal 30:1249–1254. doi: 10.1016/S0731-7085(02)00482-X. [DOI] [PubMed] [Google Scholar]
- 7.Chhonker YS, Edi C, Murry DJ. 2018. LC–MS/MS method for simultaneous determination of diethylcarbamazine, albendazole and albendazole metabolites in human plasma: application to a clinical pharmacokinetic study. J Pharm Biomed Anal 151:84–90. doi: 10.1016/j.jpba.2017.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marriner SE, Morris DL, Dickson B, Bogan JA. 1986. Pharmacokinetics of albendazole in man. Eur J Clin Pharmacol 30:705–708. doi: 10.1007/BF00608219. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Zhao L, Xu H, Zhong D. 2004. Simultaneous determination of albendazole and its major active metabolite in human plasma using a sensitive and specific liquid chromatographic-tandem mass spectrometric method. J Pharm Biomed Anal 35:829–836. doi: 10.1016/j.jpba.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Wojnicz A, Cabaleiro-Ocampo T, Román-Martínez M, Ochoa-Mazarro D, Abad-Santos F, Ruiz-Nuño A. 2013. A simple assay for the simultaneous determination of human plasma albendazole and albendazole sulfoxide levels by high performance liquid chromatography in tandem mass spectrometry with solid-phase extraction. Clin Chim Acta 426:58–63. doi: 10.1016/j.cca.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Ceballos L, Krolewiecki A, Juárez M, Moreno L, Schaer F, Alvarez LI, Cimino R, Walson J, Lanusse CE. 2018. Assessment of serum pharmacokinetics and urinary excretion of albendazole and its metabolites in human volunteers. PLoS Negl Trop Dis 12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okelo GB, Hagos B, Ng’ang’a JN, Ogeto JO. 1993. Pharmacokinetics of albendazole in children with hydatid disease. East Afr Med J 70:643–645. [PubMed] [Google Scholar]
- 13.Jung H, Sánchez M, González-Astiazarán A, Martínez JM, Suástegui R, González-Esquivel DF. 1997. Clinical pharmacokinetics of albendazole in children with neurocysticercosis. Am J Ther 4:23–26. doi: 10.1097/00045391-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Pengsaa K, Na-Bangchang K, Limkittikul K, Kabkaew K, Lapphra K, Sirivichayakul C, Wisetsing P, Pojjaroen-Anant C, Chanthavanich P, Subchareon A. 2004. Pharmacokinetic investigation of albendazole and praziquantel in Thai children infected with Giardia intestinalis. Ann Trop Med Parasitol 98:349–357. doi: 10.1179/000349804225003398. [DOI] [PubMed] [Google Scholar]
- 15.Dayan AD. 2003. Albendazole, mebendazole, and praziquantel: review of nonclinical toxicity and pharmacokinetics. Acta Trop 86:141–159. doi: 10.1016/S0001-706X(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 16.Andersen IKL, Rosting C, Gjelstad A, Halvorsen TG. 2018. Volumetric absorptive MicroSampling versus other blood sampling materials in LC-MS-based protein analysis: preliminary investigations. J Pharm Biomed Anal 156:239–246. doi: 10.1016/j.jpba.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Protti M, Catapano MC, Samolsky Dekel BG, Rudge J, Gerra G, Somaini L, Mandrioli R, Mercolini L. 2018. Determination of oxycodone and its major metabolites in haematic and urinary matrices: comparison of traditional and miniaturised sampling approaches. J Pharm Biomed Anal 152:204–214. doi: 10.1016/j.jpba.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 18.Barco S, Castagnola E, Moscatelli A, Rudge J, Tripodi G, Cangemi G. 2017. Volumetric adsorptive microsampling-liquid chromatography tandem mass spectrometry assay for the simultaneous quantification of four antibiotics in human blood: method development, validation, and comparison with dried blood spot. J Pharm Biomed Anal 145:704–710. doi: 10.1016/j.jpba.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Food and Drug Administration. 2013. Guidance for industry: bioanalytical method validation. U.S. Food and Drug Administration, Bethesda, MD. [Google Scholar]
- 20.Kovač J, Panic G, Neodo A, Meister I, Coulibaly JT, Schulz JD, Keiser J. 2018. Evaluation of a novel micro-sampling device, MitraTM, in comparison to dried blood spots, for analysis of praziquantel in Schistosoma haematobium-infected children in rural Côte d’Ivoire. J Pharm Biomed Anal 151:339–346. doi: 10.1016/j.jpba.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 21.Kita K, Noritake K, Mano Y. 2018. Application of a volumetric absorptive microsampling device to a pharmacokinetic study of Tacrolimus in rats: comparison with wet blood and plasma. Eur J Drug Metab Pharmacokinet 2018:1–12. [DOI] [PubMed] [Google Scholar]
- 22.Giavarina D. 2015. Understanding Bland Altman analysis. Biochem Med (Zagreb) 25:141–151. doi: 10.11613/BM.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moser W, Coulibaly JT, Ali SM, Ame SM, Amour AK, Yapi RB, Albonico M, Puchkov M, Huwyler J, Hattendorf J, Keiser J. 2017. Efficacy and safety of tribendimidine, tribendimidine plus ivermectin, tribendimidine plus oxantel pamoate, and albendazole plus oxantel pamoate against hookworm and concomitant soil-transmitted helminth infections in Tanzania and Côte d’Ivoire: a randomised, controlled, single-blinded, non-inferiority trial. Lancet Infect Dis 17:1162–1171. doi: 10.1016/S1473-3099(17)30487-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.