Streptococcus pneumoniae is one of the leading pathogens that cause a variety of mucosal and invasive infections. With the increased emergence of multidrug-resistant S. pneumoniae, new antimicrobials with mechanisms of action different from conventional antibiotics are urgently needed.
KEYWORDS: CHAP domain, antibiotic resistance, bacteriophage, chimeric lysin, endolysin, lysin
ABSTRACT
Streptococcus pneumoniae is one of the leading pathogens that cause a variety of mucosal and invasive infections. With the increased emergence of multidrug-resistant S. pneumoniae, new antimicrobials with mechanisms of action different from conventional antibiotics are urgently needed. In this study, we identified a putative lysin (gp20) encoded by the Streptococcus phage SPSL1 using the LytA autolysin as a template. Molecular dissection of gp20 revealed a binding domain (GPB) containing choline-binding repeats (CBRs) that are high specificity for S. pneumoniae. By fusing GPB to the CHAP (cysteine, histidine-dependent amidohydrolase/peptidase) catalytic domain of the PlyC lysin, we constructed a novel chimeric lysin, ClyJ, with improved activity to the pneumococcal Cpl-1 lysin. No resistance was observed in S. pneumoniae strains after exposure to incrementally doubling concentrations of ClyJ for 8 continuous days in vitro. In a mouse bacteremia model using penicillin G as a control, a single intraperitoneal injection of ClyJ improved the survival rate of lethal S. pneumoniae-infected mice in a dose-dependent manner. Given its high lytic activity and safety profile, ClyJ may represent a promising alternative to combat pneumococcal infections.
INTRODUCTION
Streptococcus pneumoniae is an encapsulated Gram-positive bacterium usually found in the nasopharynx of healthy humans as part of the normal flora (1). However, in certain instances it can cause a variety of invasive diseases such as sepsis, community-acquired pneumonia, and meningitis, as well as mucosal infections such as acute otitis media and rhinosinusitis (2). As a challenging human pathogen that causes high morbidity and mortality in young children and immunocompromised patients (3), S. pneumoniae remains a critical target for new drug development. However, in recent years few new antibiotics have been approved by the U.S. Food and Drug Administration, and yet the emergence of antimicrobial-resistant bacteria continues to increase (4), which has led to a worldwide health care crisis, necessitating an urgent need for novel antibiotic alternatives (5).
Bacteriophage endolysins (also called lysins) have been increasingly recognized as a promising approach to combat multidrug-resistant pathogens. These enzymes are encoded by the genomes of bacteriophage and exhibit high species-specific degrading activity against the bacterial peptidoglycan. To fully perform their function, lysins usually contain two distinguished domains; an N-terminal catalytic domain (CD) and a C-terminal cell wall binding domain (CBD) (6). The CBD binds to a specific substrate (usually carbohydrate) found in the cell wall of a target bacterium (6, 7), promoting access of the CD to its substrate (8, 9). Adding purified lysins exogenously to susceptible Gram-positive bacteria can cause rapid cell lysis. Due to their high specificity and robust lytic activity, lysins offer an alternative antimicrobial approach to conventional antibiotics (10, 11). Currently, several lysins targeting Staphylococcus aureus have progressed to human clinical trials, such as the natural lysin CF-301 (https://clinicaltrials.gov/ct2/show/NCT03163446#wrapper) and the chimeric lysin P128 (https://www.clinicaltrials.gov/ct2/show/NCT01746654?termNCT01746654&rank1). Protein engineering strategies can be applied to naturally occurring lysins in order to achieve improved lytic activity (12), enhanced solubility and stability (13, 14), or an extended lytic spectrum (15). Furthermore, lysins can be engineered to breach the bacterial outer membrane (16), extending the utility of the lysin approach to target Gram-negative pathogens (17).
Several naturally occurring lysins have been reported to be active against S. pneumoniae in animal colonization and infection models. Among the most studied are Pal (18) and Cpl-1 (19), each possessing a CBD with six choline-binding repeats (CBRs) that are known to bind teichoic acids unique to the pneumococcal surface. The Cpl-1 lysin is encoded by the virulent pneumococcal bacteriophage Cp-1 and has been reported to be the most active natural pneumococcal lysin to date (20). In contrast, the Cpl-7 lysin, encoded by the pneumococcal phage Cp-7, has a CBD with three identical tandem repeats (CW_7 repeats) that are unrelated to choline-targeting motifs, but nonetheless specifically target the pneumococcal surface (21). Based on the CW_7 repeats, chimeric lysins engineered by domain swapping between Cpl-7 and other lysins demonstrated increased bactericidal activities in vitro and in colonization models (20, 22).
LytA is the major autolysin of S. pneumoniae essential for bacterial survival and cell wall synthesis (23). Recent structural elucidation of the full-length LytA and its complex with large peptidoglycan fragments reveals new insights into its function and lysis mechanism (24, 25), providing new clues for developing chimeric lysins against S. pneumoniae. PlyC, a multimeric streptococcal lysin composed of one PlyCA subunit and eight subunits of PlyCB, is specific for groups A, C, and E streptococci (26–28). Our previous study reported a chimeric lysin, ClyR, containing the CHAP (cysteine, histidine-dependent amidohydrolase/peptidase) domain from PlyCA and the CBD from the PlySs2 lysin (14). Although its CBD does not contain any known modules specific for S. pneumoniae (29), ClyR displayed an extended streptococcal host range, including S. pneumoniae (14), suggesting that the CHAP domain of PlyCA is active against S. pneumoniae.
In the present study, we identified a new lysin encoded by the Streptococcus phage SPSL1 (Gene ID 24725347, termed gp20 here) using SWISS-MODEL with LytA as the template molecule (30, 31). Molecular dissection of gp20 was performed and an N-acetylmuramic acid l-alanine amidase CD was identified (here known as GPC), as well as a CBD with six choline binding repeats (here known as GPB). We further created a novel chimeric lysin, ClyJ, by fusing the CHAP domain from PlyCA with the newly identified GPB from gp20 and confirmed the bactericidal activity of ClyJ against S. pneumoniae in vitro and in a mouse bacteremia model.
RESULTS
GPB is highly specific to S. pneumoniae.
By using the major autolysin of S. pneumoniae, LytA, as the template, we identified a new putative lysin (gp20) in the genome of the Streptococcus phage SPSL1 that shares 78% similarity with LytA (Fig. S1). Further analysis based on the amino acids of gp20 revealed that its catalytic domain (GPC) belongs to the PGRP superfamily and its binding domain (GPB) is composed of six typical CW_binding_1 repeats, also known as a choline binding module (CBM) that specifically recognizes and binds choline residues on the cell wall (Fig. S2).
As an attempt to assess the lytic activity of gp20, we purified the full-length lysin, as well as its catalytic domain from Escherichia coli (Fig. S3), and tested their activities against two clinical S. pneumoniae strains. Results showed that the lytic activity of the full-length lysin, gp20, is very weak in comparison to that of Cpl-1, while the single catalytic domain, GPC, has negligible activity (Fig. 1a and b). Because the CBD of gp20 (GPB) has a typical CBM similar to that of LytA (Fig. S1), we hypothesized that it should maintain a specific binding ability for S. pneumoniae. To visualize this, we fused enhanced green fluorescent protein (EGFP) with GPB and purified the recombinant protein, EGPB, from E. coli (Fig. S3). Fluorescence microscopy analysis showed that EGPB binds to the cell surface of both S. pneumoniae strains, but not other streptococcal strains tested (Fig. 1c), suggesting a specific recognition of S. pneumoniae.
FIG 1.
Molecular dissection of gp20. (a and b) Lytic activities of gp20 and GPC against S. pneumoniae strains in comparison with Cpl-1 lysin. S. pneumoniae NS26 (a) and S. pneumoniae NS63 (b) cells are treated with an equal molar amount of each lysin and monitored by a microplate reader at OD600 for 60 min. (c) Binding specificity of EGPB. Various streptococcal strains, including S. pneumoniae (Spn), S. agalactiae (Sag), S. dysgalactiae (Sdy), S. pyogenes (Spy), and S. mutans (Smu), were incubated with an equal concentration of EGPB or EGFP protein for 60 min at 37°C. After three washes with phosphate-buffered saline (PBS), the bacteria were imaged by a Nikon fluorescence microscope (TS2-FL). Scale bar, 5 μm.
ClyJ is active against S. pneumoniae.
Based on the specificity of GPB to S. pneumoniae, we created a novel chimeric lysin, ClyJ, by fusing GPB with the CHAP domain of PlyCA (Fig. 2a). We purified this chimera from E. coli (Fig. S4) and tested its activity against S. pneumoniae in comparison to Cpl-1, the best natural lysin against S. pneumoniae, and ClyR, another chimeric lysin that shares the same CHAP domain with ClyJ (Fig. 2a). Results showed that ClyJ displays higher lytic activities than either Cpl-1 or ClyR against S. pneumoniae strains NS26 and NS63 (Fig. 2b). Accordingly, a greater decrease in viable cell number of S. pneumoniae NS26 was observed in the ClyJ-treated group compared to groups treated with an equal molar amount of ClyR or Cpl-1 (Fig. 2c). A dose-dependent comparison also confirmed that the lytic activity of ClyJ is significantly higher than that of Cpl-1 (Fig. 2d). Meanwhile, an obvious synergism was observed between ClyJ and Cpl-1. For instance, under an equal molar concentration of 2.76 μM, Cpl-1 and ClyJ alone caused a reduction of 2.3 and 4.2 logs, respectively, while, a reduction of 6.3 logs was achieved in groups treated with an equal (1.38 μM each) combination of Cpl-1 and ClyJ (Fig. 2d). Moreover, an MIC assay showed that S. pneumoniae NS26 and NS63 are more sensitive to ClyJ than to Cpl-1 (Table S1), specifically, the MIC of Cpl-1 against either strain is about 2-fold higher than that of ClyJ.
FIG 2.
Overall features of ClyJ. (a) Schematic representation of ClyJ and closely related lysins. Conserved domains were analyzed by SMART and are depicted by colors. The CHAP and CBM domain of ClyJ is linked by GS amino acids. CHAP, cysteine, histidine-dependent amidohydrolases/peptidases; PlyCA CHAP, the CHAP domain from PlyCA (amino acids 314 to 465); PlyCA GyH, the glycosyl hydrolase domain from PlyCA (amino acids 1 to 205); PlySs2 SH3b, the SH3b domain from PlySs2 lysin (amino acids 147 to 245); CBM, choline binding module. (b) Lytic activity of ClyJ against S. pneumoniae NS26 and NS63 compared to that of Cpl-1 and ClyR at the same concentration (25 μg/ml). (c) Log killing activity of ClyJ against S. pneumoniae NS26 compared to that of Cpl-1 and ClyR at the same molar concentration (0.69 μM). (d) Synergism between ClyJ and Cpl-1. S. pneumoniae NS26 was treated with an equal molar concentration (0, 0.69, 1.38, and 2.76 μM) of either Cpl-1, ClyJ, or an equal combination of Cpl-1 and ClyJ for 30 min. The viable cells were detected by dilution and plating on THY agar. The result was analyzed by two-tailed Student t test. The data are shown as means ± the standard deviations (***, P < 0.001).
Expanded host-range testing was conducted on an additional 13 S. pneumoniae strains representing multiple serovars, as well as S. mutans, S. gordonii, S. mitis, S. rattus, S. salivarius, S. parasanguis, S. intermedius, and several representative bacilli. It was revealed that ClyJ was active against all S. pneumoniae strains tested, similar to Cpl-1, but had no activity against other streptococcus or bacillus species (Fig. 3), suggesting the host range of ClyJ is highly pneumococcus specific.
FIG 3.
Host range of ClyJ. The susceptibility of various strains to ClyJ was tested in a turbidity assay in comparison to the Cpl-1 lysin. Each strain was treated with 25 μg/ml ClyJ or Cpl-1 for 20 min, and the decrease in the OD600 was recorded by a microplate reader. The data are shown as means ± the standard deviations.
Characteristics of ClyJ.
Because the initial data showed high lytic activity for ClyJ, we further ascertained its biochemical properties. First, we examined the time- and dose-dependent lytic activity of ClyJ against S. pneumoniae NS26 and found 25 μg/ml ClyJ caused ∼3 log10 decrease in viable S. pneumoniae cells after 60 min, with a reduction of >1 log10 noted within the first minute (Fig. 4a). Notably, treatment with 100 μg/ml ClyJ caused a reduction of >4 log10 within 60 min (Fig. 4a). Next, we tested the influence of various environment factors on the lytic activity of ClyJ, including pH, EDTA, NaCl, and temperature. Results showed that ClyJ is active in a pH range of 5 to 9, with an optimum pH of 7 (Fig. S5a). EDTA displayed little effect on the activity of ClyJ, with over 90% activity remaining under EDTA concentrations up to 500 μM (Fig. S5b). Likewise, NaCl had little effect on the lytic activity of ClyJ (Fig. S5c). A thermal stability test showed that while ClyJ is stable at room temperature, displaying 97% activity after being stored at 25°C for 60 min (Fig. S5d), activity dropped to 66.7% after being stored at 37°C for 60 min, and activity was nearly abolished after 60 min at 42°C (Fig. S5d). Choline enhanced the thermal stability of ClyJ, with 97.8 and 46.3% activity remained after being incubated at 37 and 40°C for 1 h in the presence of choline, respectively (Fig. S5d), suggesting the enzymes are stabilized once bound to the choline-containing pneumococcal surface. More importantly, direct examination of the lytic activity of ClyJ at various temperatures showed minimal loss of lytic activity up to temperature of 45°C (Fig. S5e), presumably due to the rapid lytic nature of these enzymes. It has been demonstrated that CBM-containing lysins, such as Cpl-1 and LytA, dimerize upon binding choline present in the wall teichoic acids of pneumococci, and this monomer-to-dimer switch is necessary for cell wall hydrolytic activity of these enzymes (32, 33). Therefore, we further investigated the impact of choline on ClyJ activity, hypothesizing low levels may activate the enzyme, but high levels may inhibit the enzyme from binding to its cellular receptor. Results demonstrated that low concentrations of choline (<20 mM) improved the lytic activity of ClyJ on S. pneumoniae NS26, but the activity gradually dropped as the choline concentration increased (Fig. 4b). Under the same conditions, Cpl-1 displayed less activity stimulation at low choline concentrations and a more dramatic decrease in activity at higher choline concentrations. However, both enzymes displayed very similar responses to choline concentration, i.e., initial increase and subsequent decrease in lytic activity, when tested with S. pneumoniae NS63 (Fig. S5f). These observations are also consistent with previously published choline response curves of LytA (34, 35).
FIG 4.
Biochemical characteristics of ClyJ. (a) Time- and dose-dependent killing capacity of ClyJ. S. pneumoniae NS26 was treated with 25 μg/ml ClyJ for 0, 1, 5, 15, 30, or 60 min (black lines) or treated with 0, 2.5, 5, 10, 25, 50, and 100 μg/ml ClyJ for 60 min (blue lines) before dilution and plating on THY agar. (b) Relative activities of ClyJ and Cpl-1 against S. pneumoniae NS26 in the presence of various concentrations of choline. (c) Morphology of S. pneumoniae NS26 after exposure to 100 μg/ml ClyJ for 0, 30, and 60 s, as observed by transmission electron microscopy. Scale bar, 2 μm.
The rapid lytic activity of ClyJ against S. pneumoniae was also confirmed by transmission electron microscopy thin-section imaging (Fig. 4c), which showed cells beginning to lyse and lose their cytoplasmic contents after exposure to ClyJ for 30 s, with lysis almost complete by 60 s (Fig. 4c).
No resistance development to ClyJ.
It is well known that bacterial pathogens can easily develop tolerance or resistance to the immediate life-or-death pressure placed by selected antibiotics. However, because the killing mechanism of lysins is different from that of antibiotics, we assessed the rate of resistance development against ClyJ for S. pneumoniae. First, we examined the susceptibility of S. pneumoniae NS26 and NS63 to ClyJ and penicillin G using the broth microdilution method (Table S1). Next, we cultured these two strains under incrementally doubling concentrations of ClyJ or penicillin G (from 1/32× to 4× the initial MIC) and checked their susceptibility daily for 8 days. The results showed that both strains acquire resistance, defined as a 4-fold increase over the original MIC, to penicillin G, with 8- and 16-fold increases in MIC after 8 days of exposure (Fig. 5). In contrast, no resistance to ClyJ was observed under identical conditions.
FIG 5.

In vitro resistance development assay. S. pneumoniae strains were exposed to 1/32× to 4× the initial MIC of ClyJ and penicillin G over 8 days, and the daily MICs of ClyJ and penicillin G were examined and compared to the initial MIC for each strain.
ClyJ protects mice from lethal S. pneumoniae infection.
The in vivo efficacy of ClyJ was evaluated in a systemic S. pneumoniae infection model. First, we assessed the virulence of S. pneumoniae NS26 and found that a single intraperitoneal injection of 2.68 × 107 CFU/mouse causes 100% death within 24 h (Fig. 6a). Next, we examined the protective efficacy of a single dose of ClyJ administered 1 h after the infection with 2.68 × 107 CFU/mouse S. pneumoniae NS26. As shown in Fig. 6b, a large amount of bacteria could be detected in the blood (1.5 × 106 CFU/ml) and organs (2.4 × 106 CFU/g tissue, 1.4 × 106 CFU/g tissue, and 2.2 × 106 CFU/g tissue in kidney, liver, and lung, respectively) in infected mice at 1 h postinfection, suggesting rapid migration and dissemination from the site of intraperitoneal injection. Administration of penicillin G at 1 h postinfection showed 40% (4/10) protective efficacy (Fig. 6c), while a dose-dependent protection was observed in ClyJ-treated groups, with 90% (9/10) of the mice surviving 10 days in the group that received 0.3 mg/mouse and 100% (10/10) protection in the group that received 0.4 mg/mouse (Fig. 6c). Moreover, a single high dose of ClyJ (1 mg/mouse) did not cause a noticeable change in health or affect the survival rate of mice (Fig. 6c). At 3 h after bacterial challenge with a lethal dose of S. pneumoniae NS26, the kidney and liver maintained high pneumococcal loads, i.e., 2.2 × 106 CFU/g tissue and 1.3 × 106 CFU/g tissue, respectively (Fig. 6b). Compared to the number of bacteria detected at 1 h postinfection, the bacterial burden 3 h postinfection significantly decreased to an average of 6.8 × 105 CFU/ml in the blood and increased significantly to 5.3 × 106 CFU/g tissue in the lung, consistent with dissemination of the infection to sensitive organs (Fig. 6b). Protective efficacies administered 3 h postinfection dropped to 20% (2/10) and 50% (5/10) with single doses of 0.4 and 0.8 mg/mouse of ClyJ, respectively (Fig. 6d), presumable due to the extent of bacteremia. Notably, none (0/10) of the mice given 0.25 mg/mouse of penicillin G at 3 h postinfection survived (Fig. 6d).
FIG 6.
ClyJ protects mice from lethal S. pneumoniae infection. (a) Virulence of S. pneumoniae NS26 in vivo. Mice were intraperitoneally injected with different concentrations of S. pneumoniae NS26, and the survival rate of each group (n = 5 each) was recorded for 5 days. (b) Bacterial burden in blood and organs at 1 and 3 h after inoculation of a lethal dose of S. pneumoniae NS26. The numbers of bacteria in the blood and lungs were compared between groups euthanized at 1 and 3 h postinfection and analyzed by a two-tailed Student t test. The data are shown as means ± the standard deviations (*, P < 0.05; ***, P < 0.001). (c and d) Protective efficacy of ClyJ. Female BALB/c mice were infected with 2.68 × 107 CFU/mouse S. pneumoniae NS26 and divided into eight groups randomly after infection. Three groups (n = 10) intraperitoneally received a single dose of 0.3 mg/mouse ClyJ, 0.4 mg/mouse ClyJ, or 0.25 mg/mouse penicillin G at 1 h postinfection, respectively (c). Three other groups (n = 10) intraperitoneally received a single dose of 0.4 mg/mouse ClyJ, 0.8 mg/mouse ClyJ, or 0.25 mg/mouse penicillin G at 3 h postinfection, respectively (d). The final two groups (n = 10) were injected with PBS and served as controls. Another group (n = 5) without S. pneumoniae infection was intraperitoneally administered a single high dose of 1 mg/mouse ClyJ to assess its toxicity (c). The survival rates in all groups were recorded for 10 days. (e and f) Inflammatory cytokines in mouse blood. The mice were intraperitoneally injected with one ClyJ dose of 0.4 mg/mouse (n = 5), 3,000 EU/mouse LPS (n = 5), or PBS (n = 5); 6 h later, the expression levels of IL-1β (e) and IL-6 (f) in each group were detected by ELISA. The expression level in the ClyJ-treated group was compared to that of the PBS- and LPS-treated controls by two-tailed Student t test. The data are shown as a minimum-to-maximum boxplot, where the box represents the standard deviations, and the medians are marked by a bar. ns, not significant; **, P < 0.01; ***, P < 0.001.
To further confirm the safety of ClyJ, we scanned the hematological parameters and cytokine expression levels in mice receiving a single high therapeutic dose of ClyJ (0.4 mg/mouse, n = 5). Compared with the PBS-treated group, no significant changes of hematological parameters were observed in the ClyJ-treated group, whereas a higher content of monocytes was observed in the lipopolysaccharide (LPS)-treated group (from 4.66 to 11.58%, Table S2). Although a slight increase in the MCHC (mean corpuscular hemoglobin concentration) was observed in the ClyJ-treated group, it is still within the normal range of 30 to 38 g/dl for mouse serum (36). Meanwhile, no significant difference was observed in expression levels of IL-1β (Fig. 6e) or IL-6 (Fig. 6f) in sera from groups treated with ClyJ and PBS, suggesting that no significant inflammatory response is evoked by ClyJ under the present experimental conditions.
Cytotoxicity of ClyJ.
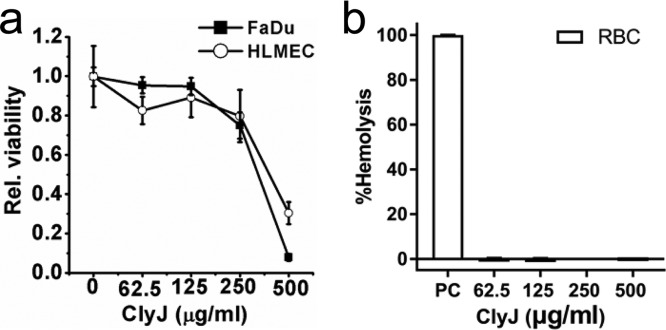
The SRB assay showed that FaDu and HLMEC cell lines remained healthy at ClyJ concentrations less than 250 μg/ml (Fig. 7a) but had increasing cytotoxicity at ClyJ concentrations of >250 μg/ml. Furthermore, no hemolysis was observed in human red blood cells (RBCs) exposed to high concentrations of ClyJ up to 500 μg/ml (Fig. 7b). In addition, the overall analysis of gene expression profiles in FaDu cells cocultured with ClyJ (50 μg/ml) revealed no significant differences compared to these cells cocultured with albumin (Fig. S6).
FIG 7.
Effects of ClyJ on viability of human cell lines. (a) Relative viabilities of FaDu and HLMEC cells exposed to various concentrations of ClyJ. Cells were cocultured with a series of concentrations of ClyJ (0, 62.5, 125, 250, and 500 μg/ml) for 48 h, and the relative viabilities of cells after each treatment were determined by an SRB assay. (b) Hemolysis effect of ClyJ on RBCs. Cells were incubated with different concentrations of ClyJ (62.5, 125, 250, and 500 μg/ml) for 60 min at 37°C, and the degree of hemolysis was estimated by measuring the absorbance at 540 nm. Groups treated with endotoxin-free water and PBS with 10 mM glucose served as positive controls (PC).
DISCUSSION
The increased emergence of multidrug-resistant pathogens has posed a challenging medical threat to the public health worldwide (37), which has renewed interest in alternative therapies such as phage-derived lysin therapy (10). As an attempt to develop novel antimicrobials against S. pneumoniae, the leading pathogen responsible for bacterial pneumonia in young children and the elderly (1), we identified a new lysin, gp20, encoded by the Streptococcus phage SPSL1. In the present study, we dissected the functional domains of gp20 and created a novel chimeric lysin, ClyJ, by fusing the pneumococcus-specific binding domain from gp20 to the CHAP catalytic domain derived from the PlyC lysin. The bactericidal activity of ClyJ against S. pneumoniae was further assessed both in vitro and in a mouse bacteremia model.
Interestingly, CHAP domains are usually found in staphylococcal phage lysins (38), while pneumococcal phage lysins typically consist of a lysozyme catalytic domain belonging to the GH-25 superfamily, as is the case in Cpl-1 (39) and Cpl-7 (21), or an amidase catalytic domain as seen in Pal (40) and LytA (25). Moreover, the lysozyme catalytic domain from Cpl-1 or Cpl-7 (20, 22) and the amidase catalytic domain from Pal (41) have been used as potential donors in construction of chimeric lysins targeting S. pneumoniae. Therefore, ClyJ is the first chimeric lysin specific for S. pneumoniae that contains a CHAP domain.
The binding domain of gp20 is homologous to that of LytA, Pal, and Cpl-1, showing a typical choline binding module (CBM). The increase of ClyJ activity under low choline concentrations suggests that ClyJ may undergo a structural switch from monomer to dimer after binding the choline containing wall teichoic acids on the pneumococcal surface, a structural change that has been observed in LytA (42), Pal (43), and Cpl-1 (44). However, the conformational dynamics of ClyJ and the structural relationship of its CBM with its lytic activity require further studies.
There are several characteristics that warrant further development of ClyJ as an anti-pneumococcal lysin despite numerous manuscripts already devoted to the merits of Cpl-1 for the same purpose. First, ClyJ could overcome the threat posed by lysozyme-resistant S. pneumoniae considering the different peptidoglycan bonds targeted by ClyJ and Cpl-1. Many S. pneumoniae strains are resistant to lysozyme because of the lack of acetylation of glucosamine in their peptidoglycan (45, 46). It is therefore possible in theory for S. pneumoniae to develop resistance to lysins with lysozyme-like enzymatic activities, such as Cpl-1 and Cpl-7, although, to date, there have been no reports of resistance developed to these enzymes. Second, ClyJ has improved activity compared to Cpl-1. The MIC of ClyJ was about 2-fold lower than that of Cpl-1 against two pneumococcal strains tested, indicating that ClyJ is about 2 times more active than Cpl-1. However, ClyJ and Cpl-1 have yet to be benchmarked against each other in vivo. Third, ClyJ and Cpl-1 could work synergistically to achieve improved bactericidal efficacy. The CHAP domain of PlyC, which is reported to contain an N-acetylmuramoyl-l-alanine amidase activity, has been shown to demonstrate intrinsic synergy with the glycosidase activity of the PlyCA GyH domain (26). In addition, the amidase domain from the Pal lysin has been shown to work synergistically with Cpl-1 both in vitro and in vivo (47, 48). Taken together, the evidence provided here supports further developmental studies for ClyJ.
One major concern in the development of antimicrobials is their resistance, a key question that has not been fully resolved. In this context, bacteriophage lysins have been reported to possess a very low possibility of developing resistance compared to traditional antibiotics (6). As expected, no resistance was observed in S. pneumoniae strains after exposure to incrementally doubling concentrations of ClyJ for 8 continuous days, while high resistance to penicillin G occurred in both S. pneumoniae strains. Nonetheless, how these strains respond to ClyJ with longer induction cycles or with more selective pressure, i.e., stable mutants, still needs to be established. In addition, there exist mutants (i.e., tacF) that have significantly reduced choline on the cell surface (49, 50). Although our screen did not detect any of these mutants, in theory such mutants would be resistant to ClyJ and other lysins that contain CBMs, although it should be noted that pneumococcal mutants lacking choline have a significantly diminished virulence capacity (49).
Aside from resistance development, there are other potential hurdles to be taken into consideration for future translational development of ClyJ. First, ClyJ is sensitive to elevated temperatures. A residual activity of 66.7% was observed for ClyJ after being stored at 37°C for 1 h, and activity was almost lost after exposure at 42°C for the same amount of time. The fact that choline enhanced the thermal stability of ClyJ indicates that ClyJ would gain thermal stability upon bound to the S. pneumoniae surface. Further, addition of sugars, amino acids, poloxamers, ions, or other excipients are routinely used to stabilize pharmaceutical preparations of lysins (51), but these thermal stabilization tests have yet to be performed for ClyJ. Second, ClyJ shows potential cytotoxicity at high concentrations. Although no hemolysis was observed in RBCs at ClyJ doses up to 500 μg/ml, toxicity to FaDu and HLMEC cells was observed at this concentration. However, this concentration is more than an order of magnitude higher than the actual doses described for the in vivo studies. We also did not evaluate the use of ClyJ in combination with conventional antibiotics, an approach that has demonstrated synergism and would require lower concentrations of either the lysin or the antibiotic, which may address toxicity issues if they arise (13, 52).
In summary, we report here a novel chimeric lysin, ClyJ, consisting of a unique CHAP domain and a classical CBM, exhibits robust lytic activity against S. pneumoniae both in vitro and in vivo. Taking into account its high lytic activity, low resistance occurrence, and safety profile, ClyJ may represent a promising alternative to combat pneumococcal infections.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study are listed in Table S3. S. pneumoniae strains were cultured in THY (Todd-Hewitt broth containing 0.5% yeast extract) medium statically at 37°C with 5% CO2. Other Gram-positive bacteria were grown in BHI (brain heart infusion; Becton, Dickinson and Company, Sparks, MD) broth at 37°C. Escherichia coli BL21(DE3), used for gene cloning and protein expression, was grown in LB (Luria broth) medium. Kanamycin (50 μg/ml) was added when necessary.
Sequence screening and cloning.
The putative sequence coding a LytA-like murein hydrolase (Gene ID 24725347) was identified in the genome of the Streptococcus phage SPSL1 by SWISS-MODEL using LytA as the template. The candidate gene was chemically synthesized by GenScript (Nanjing, China) and cloned into a pET28b(+) vector using primers GP-F/GP-R (Table S4), and the corresponding gene product was termed gp20. The CD of gp20 (GPC, amino acids 1 to 158), and the CBD of gp20 (GPB, amino acids 148 to 316) fused with EGFP were amplified by primer-specific PCR and cloned into the NcoI and XhoI restriction sites of a pET28b(+) plasmid (Table S4). In addition, the chimeric lysin, ClyJ, was constructed by fusing GPB coding sequence to the C terminus of the CHAP domain of PlyCA (amino acids 314 to 465) using sequence-specific primers (Table S4). The pneumococcal Cpl-1 lysin coding sequence was chemically synthesized by GenScript (Nanjing, China) and cloned into a pET28b(+) vector using primers Cpl-1-F/Cpl-1-R (Table S4). All constructs were transformed into E. coli BL21(DE3) cells and verified by direct DNA sequencing analysis.
Protein expression and purification.
For production of recombinant proteins, E. coli BL21(DE3) cells were incubated at 37°C to mid-log phase and then induced with 0.2 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and incubated at 16°C for 18 h to allow for protein expression. Cells were collected by centrifugation at 8,000 × g for 10 min and lysed by using a cell disrupter (JNBIO, JN-10C) on ice. His-tagged proteins were purified by nickel nitrilotriacetic acid columns according to the supplier’s instructions. Briefly, columns were equilibrated with 20 mM imidazole before loading the protein samples. Target proteins were collected by washing and elution with 60 and 250 mM imidazole, respectively. Collected proteins were dialyzed against PBS buffer (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4·H2O, 1.4 mM KH2PO4 [pH 7.4]) and then passed through a Detoxi-Gel endotoxin removing gel (Thermo Scientific), and quantified by a EndoLISA (enzyme-linked immunosorbent assay [ELISA]-based endotoxin detection assay; Hyglos, GmbH) according to the manufacturer’s instructions. After quantitation by the Bradford assay, the protein solution was filter sterilized and stored at 4°C until used.
Bactericidal assays.
Lytic activity of ClyJ was measured via the turbidity reduction assay as described previously (53). Bacteria were cultured to an optical density at 600 nm (OD600) of 0.8 to 1, washed once with PBS (pH 7.4), and resuspended in PBS to a final OD600 of 0.5 to 0.8. Next, 190 μl of the bacterial suspension was mixed with 10 μl of purified proteins (at a final concentration of 25 μg/ml) in 96-well plates (Corning). The decrease in absorbance at OD600 was monitored for 60 min at 37°C by using a Synergy H1 microplate reader (BioTek) at 1-min intervals or was measured every 15 s for 20 min on a SpectraMax 190 spectrophotometer (Molecular Devices, San Jose, CA). Controls were run in parallel and used an equal volume of PBS buffer in place of the enzyme. The activity of each lysin was expressed as the difference in the percent decrease in OD600 (% decrease in OD600) from lysin-treated wells and the PBS-treated control wells.
To examine the biochemical properties of ClyJ, S. pneumoniae NS26 was resuspended in PBS with various concentrations of NaCl (0, 50, 100, 150, 300, 500, and 750 mM) or EDTA (0, 50, 100, 200, 300, 400, and 500 μM) or was resuspended in phosphate buffer with different pHs (3.5, 5, 6, 6.5, 7, 8, 8.5, 9, 9.5 10, 11, and 11.5). ClyJ (25 μg/ml) was then added to bacterial suspensions, and the change in turbidity (OD600) under each condition was monitored by using a microplate reader at 37°C for 60 min. The stability of ClyJ at different temperatures was evaluated by testing the residual activity of each lysin (at a final concentration of 25 μg/ml) against S. pneumoniae NS26 after incubation at various temperatures (25, 37, 42, and 45°C) for 60 min in the presence or absence of 50 mM choline. The lytic activity of ClyJ (0, 4, 8, 16, 32, 64, and 128 μg/ml) against S. pneumoniae NS26 under different temperatures (37, 42, and 45°C) was further assessed by a microplate reader (BioTek) for 60 min. The effect of each factor (NaCl, EDTA, pH, and temperature) on the lytic activity of ClyJ was normalized by comparison to the maximum activity of ClyJ among different levels of that factor and was expressed as the relative activity.
The effect of choline on the lytic activity of ClyJ was assessed by adding different concentrations of choline (0, 5, 10, 20, 40, 80, and 160 mM) to the reaction mixture of ClyJ (at a final concentration 25 μg/ml) and S. pneumoniae NS26. Identical experiments were performed with Cpl-1 as a control. The relative activities of ClyJ and Cpl-1 under different choline conditions were normalized by comparison to the lytic activity of each lysin in the absence of choline.
The decrease of S. pneumoniae in viable cell numbers after treatment with different lysins was determined by plating series of dilutions on THY agar. Specifically, a series of 10-fold dilutions was prepared in PBS, and a 10-μl aliquot of each dilution was spotted on THY agar to visualize viable cells after an overnight incubation at 37°C. In the time- and dose-dependent bactericidal assay for ClyJ, S. pneumoniae NS26 was treated with 25 μg/ml ClyJ for 0, 1, 5, 15, 30, or 60 min, or treated with 0, 2.5, 5, 10, 25, 50, or 100 μg/ml ClyJ for 60 min, respectively, before dilution and plating on THY agar. In comparing the log killing capacity of ClyJ to other lysins, S. pneumoniae NS26 or S. pneumoniae NS63 were treated with an equal molar concentrations (0, 0.69, 1.38, and 2.76 μM) of either ClyR, ClyJ, Cpl-1, or an equal combination of ClyJ and Cpl-1 for 30 min at 37°C, and residual CFU were counted on THY agar. All experiments included at least three biological replicates.
Fluorescence microscopy.
The binding specificity of GPB to various bacterial strains was examined by fluorescence microscopy after incubation with an EGFP-fused GPB protein, EGFP-GPB (EGPB). Briefly, various bacterial strains, including S. pneumoniae NS26, S. pneumoniae NS63, S. agalactiae, S. dysgalactiae, S. pyogenes, and S. mutans, were grown overnight, washed and suspended in PBS to an OD600 of 0.5 to 0.8. Next, 100 μl of each bacterial suspension was incubated with an equal concentration (20 μM) of EGPB or EGFP protein for 60 min at 37°C. After three washes with PBS, the samples were imaged by a Nikon fluorescence microscope (TS2-FL) at ×100 magnification and with a 0.4-s exposure time.
Transmission electron microscopy.
The change in morphology of S. pneumoniae after exposure to ClyJ was observed by using a transmission electron microscope. S. pneumoniae NS26 was washed twice with PBS, resuspended in PBS to an OD600 of 0.8 to 1.0, and treated with 100 μg/ml ClyJ for 0, 30, or 60 s. The bacterial suspensions were then cross-linked with 2.5% glutaraldehyde and analyzed by a transmission electron microscope (Tecnai G2 20 Twin; Fei).
MIC determination.
The MICs of Cpl-1, ClyJ, and penicillin against S. pneumoniae NS26 and NS63 were determined by the broth dilution method as described by the Clinical and Laboratory Standards Institute (54), with some modifications. Briefly, S. pneumoniae was inoculated at a final concentration of 1 × 105 cells/well in a 96-well microtiter plate and allowed to incubate in cation-adjusted Mueller-Hinton broth (CAMHB) for 24 h at 37°C in the presence of various concentrations of ClyJ (0, 1, 2, 4, 8, 16, 32, and 64 μg/ml), Cpl-1 (0, 1, 2, 4, 8, 16, 32, and 64 μg/ml), or penicillin G (0, 0.25, 0.5, 1, 2, 4, 8, and 16 μg/ml). The resulting cell viability within each well was determined by adding 0.04% resazurin, followed by incubation for 4 h at 37°C for colorimetrical detection as described previously (55). The MIC was defined as the lowest concentration of antimicrobial that completely abolished the bacterial viability (corresponding to lowest concentration that causes the color from blue to red).
In vitro resistance development assay.
The resistance development for S. pneumoniae NS26 and NS63 to ClyJ and penicillin G were performed according to established methods (56, 57), with minor modifications. Briefly, bacterial cells at a concentration of 5 × 108 CFU/ml were cultured overnight in THY broth in the presence of 1/32× initial MIC of ClyJ at 37°C with 5% CO2. The cells were pelleted and subdivided into two aliquots. One aliquot was diluted 10-fold into fresh THY medium and allowed to grow to log phase with 1/16× initial MIC of ClyJ (2-fold higher than the former day); the other portion was applied to determine the actual MIC of ClyJ. The above procedure was repeated over an 8-day period, and the concentration of ClyJ in the liquid culture was serially doubled from 1/32× to 4× the initial MIC. The resistance development of these two S. pneumoniae strains to penicillin G was also conducted according to the same method.
Toxicity of ClyJ.
The cytotoxicity of ClyJ was evaluated against two different cell lines. Briefly, HLMEC cells (Institute of Immunology and Experimental Therapy Cell Culture Collection) and FaDu cells (ATCC HTB-43) were seeded on 96-well plated at 104 cells per well in 100 μl of medium (recommended by the manufacturer) and allowed to adhere to the surface for 3 h. Next, the medium was removed, and new medium containing various concentrations of ClyJ (0, 62.5, 125, 250, and 500 μg/ml) was added and allowed to coculture for 48 h at 37°C in 5% CO2. Subsequently, a sulforhodamine B (SRB) assay was conducted as described previously (58). Briefly, 50 μl/well of cold 50% (wt/vol) trichloroacetic acid was added to each well, followed by incubation for 1 h at 4°C. Wells were washed with PBS, and 50 μl/well of a 0.05% (wt/vol) SRB solution was added and allowed to incubate for 1 h at room temperature. Wells were washed with 1% (vol/vol) acetic acid and then incubated with 10 mM Tris base solution (pH 10.5, 100 μl/well) for 30 min at room temperature. Finally, the absorbance was measured at 510 nm in a Synergy H4 microplate reader (BioTek). The relative viability of cells after each treatment was normalized and compared to PBS-treated control wells.
The effects of ClyJ on the gene expression profile of FaDu cells was further evaluated using the SurePrint G3 Human Gene Expression v3 8x60K microarrays (Agilent Technologies). Briefly, FaDu cells were cocultured with 50 μg/ml ClyJ for 5 h before detection, using 50 μg/ml albumin-treated cells as a control. For detailed information, see the supplemental material.
Hemolysis assay.
In vitro assessment of ClyJ cytotoxicity was also studied using human RBCs as described previously (59). Briefly, fresh human blood was obtained from healthy volunteers (n = 3) to tubes containing EDTA to prevent coagulation and centrifuged at 3,000 × g for 10 min at 4°C. Plasma and leukocytes were discarded, and erythrocytes were washed three times (3,000 × g for 10 min at 4°C) with PBS (pH 7.2) supplemented with 10 mM glucose. Erythrocyte suspensions (1.6 × 108 cells/ml) were incubated with different concentrations of ClyJ (62.5, 125, 250, and 500 μg/ml) for 60 min at 37°C with gentle shaking. Endotoxin free water served as the positive control (PC), while PBS with 10 mM glucose served as the negative control (NC). After incubation, the erythrocytes were centrifuged at 3,000 × g for 10 min, and the degree of hemolysis was estimated by measuring the absorbance at 540 nm of hemoglobin in the supernatant. The percentage of hemolysis (% hemolysis) was calculated as follows: % hemolysis = 100 × (ODsample – ODNC)/(ODPC – ODNC).
Mouse infection models.
All mouse infection experiments were carried out in an ABSL-2 lab, and all experimental methods were carried out in accordance with the regulations and guidelines set forth by the Animal Experiments Committee of Wuhan Institute of Virology, Chinese Academy of Sciences. All experimental protocols were approved by the Animal Experiments Committee of Wuhan Institute of Virology, Chinese Academy of Sciences (WIVA17201602). For hematological parameter detection, animal experiments were performed in the Animal Breeding Centre of the Institute of Immunology and Experimental Therapy according to EU directive 2010/63/EU for animal experiments and were approved by the 1st Local Committee for Experiments with the Use of Laboratory Animals, Wrocław, Poland. Animals were randomized and cared in individually ventilated cages, following a set of animal welfare and ethical criteria during the experiment, and euthanized at the end of observation.
In the mouse systemic infection model, the virulence of S. pneumoniae NS26 was determined initially. Female BALB/c mice (6 to 8 weeks old) were randomly separated into four groups (n = 5), three of which were injected intraperitoneally with different concentrations of S. pneumoniae NS26 (6.7 × 106 to 26.8 × 106 CFU/mouse). The fourth group was challenged with PBS only. To test the bacterial burden in blood and organs, female BALB/c mice (6 to 8 weeks old) were injected intraperitoneally with a bacterial dose that caused 100% mortality within 24 h. Animals were euthanized at 1 or 3 h after infection (n = 5). Blood was collected and diluted for the enumeration of CFU on THY agar. Kidney, liver, and lungs were aseptically removed from each mouse and homogenized in 1 ml PBS with 0.1% Triton X-100 by a Qiagen TissueLyse II homogenizer at 1/20 frequency for 90 s, and serial dilutions of each organ were plated on THY agar for enumeration of CFU. To test the protective efficacy of ClyJ, female BALB/c mice (6 to 8 weeks old) were injected intraperitoneally with a bacterial dose that caused 100% mortality within 24 h and divided randomly into eight groups. Three groups (n = 10) intraperitoneally received a single dose of 0.3 mg/mouse of ClyJ, 0.4 mg/mouse of ClyJ, or 0.25 mg/mouse of penicillin G at 1 h postinfection, respectively. Another three groups (n = 10) intraperitoneally received a single dose of 0.4 mg/mouse of ClyJ, 0.8 mg/mouse of ClyJ, or 0.25 mg/mouse of penicillin G at 3 h postinfection, respectively. The final two groups (n = 10) were intraperitoneally injected with PBS buffer at 1 or 3 h postinfection and served as controls. To evaluate the potential toxicity of ClyJ, another group of mice (n = 5, without S. pneumoniae infection) were intraperitoneally injected with a high dose of ClyJ (1 mg/mouse). The survival rates for all groups were recoded for 10 days.
For detection of hematological parameters, 16-week-old female BALB/c mice (n = 5 each) were inoculated intraperitoneally with a single ClyJ dose of 0.4 mg/mouse (the residual LPS content was 5 EU/mouse) and an equal volume of PBS containing 5 EU/mouse of LPS (negative control) or 3,000 EU/mouse of LPS (inflammatory control). Six hours later, the mice were sacrificed, blood was collected into EDTA-containing tubes, and blood parameters were tested by a computerized Mythic 18 hemoanalyzer (C2 Diagnostics, France). Serum was separated from the blood by double centrifugation (2,250 × g for 5 min and 10,000 × g for 10 min) and subjected to interleukin-1β (IL-1β) and IL-6 detection using commercial ELISA kits (PeproTech).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Youth Innovation Promotion Association CAS (to H.Y.), the National Natural Science Foundation of China (31770192 to H.Y. and 31570175 to H.W.), and the National Science Centre in Poland (UMO-2015/18/M/NZ6/00412 to K.D.).
We are grateful to Xuefang An, Jia Wu, and Pei Zhang from the Core Facility and Technical Support, Wuhan Institute of Virology, for their assistance in animal experiment and image studies.
The authors declare no competing financial interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02043-18.
REFERENCES
- 1.Deng X, Church D, Vanderkooi OG, Low DE, Pillai DR. 2013. Streptococcus pneumoniae infection: a Canadian perspective. Expert Rev anti Infect Ther 11:781–791. doi: 10.1586/14787210.2013.814831. [DOI] [PubMed] [Google Scholar]
- 2.Andrade AL, Toscano CM, Minamisava R, Costa PS, Andrade JG. 2011. Pneumococcal disease manifestation in children before and after vaccination: what’s new? Vaccine 29:C2–14. doi: 10.1016/j.vaccine.2011.06.096. [DOI] [PubMed] [Google Scholar]
- 3.Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, Damaske B, Stefonek K, Barnes B, Patterson J, Zell ER, Schuchat A, Whitney CG. Active Bacterial Core Surveillance/Emerging Infections Program N. 2001. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995-1998: Opportunities for prevention in the conjugate vaccine era. JAMA 285:1729–1735. doi: 10.1001/jama.285.13.1729. [DOI] [PubMed] [Google Scholar]
- 4.Reinert RR. 2009. The antimicrobial resistance profile of Streptococcus pneumoniae. Clin Microbiol Infect 15:7–11. doi: 10.1111/j.1469-0691.2009.02724.x. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy M. 2013. CDC calls for urgent action to combat rise of drug resistant pathogens. BMJ 347:f5649. doi: 10.1136/bmj.f5649. [DOI] [PubMed] [Google Scholar]
- 6.Fischetti VA. 2008. Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol 11:393–400. doi: 10.1016/j.mib.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischetti VA. 2011. Exploiting what phage have evolved to control gram-positive pathogens. Bacteriophage 1:188–194. doi: 10.4161/bact.1.4.17747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osipovitch DC, Griswold KE. 2015. Fusion with a cell wall binding domain renders autolysin LytM a potent anti-Staphylococcus aureus agent. FEMS Microbiol Lett 362:1–7. doi: 10.1093/femsle/fnu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Tassell ML, Angela Daum M, Kim JS, Miller MJ. 2016. Creative lysins: Listeria and the engineering of antimicrobial enzymes. Curr Opin Biotechnol 37:88–96. doi: 10.1016/j.copbio.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Sharma U, Vipra A, Channabasappa S. 2018. Phage-derived lysins as potential agents for eradicating biofilms and persisters. Drug Discov Today 23:848–856. doi: 10.1016/j.drudis.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Haddad Kashani H, Schmelcher M, Sabzalipoor H, Seyed Hosseini E, Moniri R. 2018. Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: current status of research and novel delivery strategies. Clin Microbiol Rev 31:e00071-17. doi: 10.1128/CMR.00071-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H, Zhang Y, Yu J, Huang Y, Zhang XE, Wei H. 2014. Novel chimeric lysin with high-level antimicrobial activity against methicillin-resistant Staphylococcus aureus in vitro and in vivo. Antimicrob Agents Chemother 58:536–542. doi: 10.1128/AAC.01793-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel A, Euler C, Collin M, Chahales P, Gorelick KJ, Fischetti VA. 2010. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 54:1603–1612. doi: 10.1128/AAC.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Linden SB, Wang J, Yu J, Nelson DC, Wei H. 2015. A chimeolysin with extended-spectrum streptococcal host range found by an induced lysis-based rapid screening method. Sci Rep 5:17257. doi: 10.1038/srep17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Q, Wang J, Yang H, Wei C, Yu J, Zhang Y, Huang Y, Zhang XE, Wei H. 2015. Construction of a chimeric lysin Ply187N-V12C with extended lytic activity against staphylococci and streptococci. Microb Biotechnol 8:210–220. doi: 10.1111/1751-7915.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briers Y, Lavigne R. 2015. Breaking barriers: expansion of the use of endolysins as novel antibacterials against Gram-negative bacteria. Future Microbiol 10:377–390. doi: 10.2217/fmb.15.8. [DOI] [PubMed] [Google Scholar]
- 17.Gerstmans H, Criel B, Briers Y. 2018. Synthetic biology of modular endolysins. Biotechnol Adv 36:624–640. doi: 10.1016/j.biotechadv.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Loeffler JM, Nelson D, Fischetti VA. 2001. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170–2172. doi: 10.1126/science.1066869. [DOI] [PubMed] [Google Scholar]
- 19.Loeffler JM, Djurkovic S, Fischetti VA. 2003. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect Immun 71:6199–6204. doi: 10.1128/IAI.71.11.6199-6204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Díez-Martínez R, De Paz HD, García-Fernández E, Bustamante N, Euler CW, Fischetti VA, Menendez M, García P. 2015. A novel chimeric phage lysin with high in vitro and in vivo bactericidal activity against Streptococcus pneumoniae. J Antimicrob Chemother 70:1763–1773. doi: 10.1093/jac/dkv038. [DOI] [PubMed] [Google Scholar]
- 21.Bustamante N, Campillo NE, Garcia E, Gallego C, Pera B, Diakun GP, Saiz JL, Garcia P, Diaz JF, Menendez M. 2010. Cpl-7, a lysozyme encoded by a pneumococcal bacteriophage with a novel cell wall-binding motif. J Biol Chem 285:33184–33196. doi: 10.1074/jbc.M110.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corsini B, Diez-Martinez R, Aguinagalde L, Gonzalez-Camacho F, Garcia-Fernandez E, Letrado P, Garcia P, Yuste J. 2018. Chemotherapy with phage lysins reduces pneumococcal colonization of the respiratory tract. Antimicrob Agents Chemother doi: 10.1128/AAC.02212-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellroth P, Daniels R, Eberhardt A, Ronnlund D, Blom H, Widengren J, Normark S, Henriques-Normark B. 2012. LytA, major autolysin of Streptococcus pneumoniae, requires access to nascent peptidoglycan. J Biol Chem 287:11018–11029. doi: 10.1074/jbc.M111.318584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandalova T, Lee M, Henriques-Normark B, Hesek D, Mobashery S, Mellroth P, Achour A. 2016. The crystal structure of the major pneumococcal autolysin LytA in complex with a large peptidoglycan fragment reveals the pivotal role of glycans for lytic activity. Mol Microbiol 101:954–967. doi: 10.1111/mmi.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Cheng W, Morlot C, Bai XH, Jiang YL, Wang W, Roper DI, Vernet T, Dong YH, Chen Y, Zhou CZ. 2015. Full-length structure of the major autolysin LytA. Acta Crystallogr D Biol Crystallogr 71:1373–1381. doi: 10.1107/S1399004715007403. [DOI] [PubMed] [Google Scholar]
- 26.McGowan S, Buckle AM, Mitchell MS, Hoopes JT, Gallagher DT, Heselpoth RD, Shen Y, Reboul CF, Law RH, Fischetti VA, Whisstock JC, Nelson DC. 2012. X-ray crystal structure of the streptococcal specific phage lysin PlyC. Proc Natl Acad Sci U S A 109:12752–12757. doi: 10.1073/pnas.1208424109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson D, Schuch R, Chahales P, Zhu S, Fischetti VA. 2006. PlyC: a multimeric bacteriophage lysin. Proc Natl Acad Sci U S A 103:10765–10770. doi: 10.1073/pnas.0604521103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson D, Loomis L, Fischetti VA. 2001. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc Natl Acad Sci U S A 98:4107–4112. doi: 10.1073/pnas.061038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Yang H, Yu J, Wei H. 2015. Molecular dissection of phage lysin PlySs2: integrity of the catalytic and cell wall binding domains is essential for its broad lytic activity. Virol Sin 30:45–51. doi: 10.1007/s12250-014-3535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bienert S, Waterhouse A, de Beer TA, Tauriello G, Studer G, Bordoli L, Schwede T. 2017. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res 45:D313–D319. doi: 10.1093/nar/gkw1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede T. 2014. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varea J, Saiz JL, López-Zumel C, Monterroso B, Medrano FJ, Arrondo JL, Iloro I, Laynez J, Garcia JL, Menéndez M. 2000. Do sequence repeats play an equivalent role in the choline-binding module of pneumococcal LytA amidase? J Biol Chem 275:26842–26855. doi: 10.1074/jbc.M004379200. [DOI] [PubMed] [Google Scholar]
- 33.Buey RM, Monterroso B, Menendez M, Diakun G, Chacon P, Hermoso JA, Diaz JF. 2007. Insights into molecular plasticity of choline binding proteins (pneumococcal surface proteins) by SAXS. J Mol Biol 365:411–424. doi: 10.1016/j.jmb.2006.09.091. [DOI] [PubMed] [Google Scholar]
- 34.Briese T, Hakenbeck R. 1985. Interaction of the pneumococcal amidase with lipoteichoic acid and choline. Eur J Biochem 146:417–427. [DOI] [PubMed] [Google Scholar]
- 35.Maestro B, Gonzalez A, Garcia P, Sanz JM. 2007. Inhibition of pneumococcal choline-binding proteins and cell growth by esters of bicyclic amines. FEBS J 274:364–376. doi: 10.1111/j.1742-4658.2006.05584.x. [DOI] [PubMed] [Google Scholar]
- 36.O’Connell KE, Mikkola AM, Stepanek AM, Vernet A, Hall CD, Sun CC, Yildirim E, Staropoli JF, Lee JT, Brown DE. 2015. Practical murine hematopathology: a comparative review and implications for research. Comp Med 65:96–113. [PMC free article] [PubMed] [Google Scholar]
- 37.Pendleton JN, Gorman SP, Gilmore BF. 2013. Clinical relevance of the ESKAPE pathogens. Expert Rev anti Infect Ther 11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 38.Zou Y, Hou C. 2010. Systematic analysis of an amidase domain CHAP in 12 Staphylococcus aureus genomes and 44 staphylococcal phage genomes. Comput Biol Chem 34:251–257. doi: 10.1016/j.compbiolchem.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Sanz JM, Garcia JL, Laynez J, Usobiaga P, Menendez M. 1993. Thermal stability and cooperative domains of CPL1 lysozyme and its NH2- and COOH-terminal modules. Dependence on Choline Binding J Biol Chem 268:6125–6130. [PubMed] [Google Scholar]
- 40.Sheehan MM, Garcia JL, Lopez R, Garcia P. 1997. The lytic enzyme of the pneumococcal phage Dp-1: a chimeric lysin of intergeneric origin. Mol Microbiol 25:717–725. [DOI] [PubMed] [Google Scholar]
- 41.Blázquez B, Fresco-Taboada A, Iglesias-Bexiga M, Menéndez M, García P. 2016. PL3 amidase, a tailor-made lysin constructed by domain shu ffl ing with potent killing activity against pneumococci and related species. Front Microbiol 7:1156. doi: 10.3389/fmicb.2016.01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mellroth P, Sandalova T, Kikhney A, Vilaplana F, Hesek D, Lee M, Mobashery S, Normark S, Svergun D, Henriques-Normark B, Achour A. 2014. Structural and functional insights into peptidoglycan access for the lytic amidase LytA of Streptococcus pneumoniae. mBio 5:e01120-13. doi: 10.1128/mBio.01120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varea J, Monterroso B, Sáiz JL, López-Zumel C, García JL, Laynez J, García P, Menéndez M. 2004. Structural and thermodynamic characterization of Pal, a phage natural chimeric lysin active against pneumococci. J Biol Chem 279:43697–43707. doi: 10.1074/jbc.M407067200. [DOI] [PubMed] [Google Scholar]
- 44.Monterroso B, Saiz JL, Garcia P, Garcia JL, Menendez M. 2008. Insights into the structure-function relationships of pneumococcal cell wall lysozymes, LytC and Cpl-1. J Biol Chem 283:28618–28628. doi: 10.1074/jbc.M802808200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vollmer W, Tomasz A. 2000. The pgdA gene encodes for a peptidoglycan N-acetylglucosamine deacetylase in Streptococcus pneumoniae. J Biol Chem 275:20496–20501. doi: 10.1074/jbc.M910189199. [DOI] [PubMed] [Google Scholar]
- 46.Davis KM, Weiser JN. 2011. Modifications to the peptidoglycan backbone help bacteria to establish infection. Infect Immun 79:562–570. doi: 10.1128/IAI.00651-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loeffler JM, Fischetti VA. 2003. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob Agents Chemother 47:375–377. doi: 10.1128/AAC.47.1.375-377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jado I, Lopez R, Garcia E, Fenoll A, Casal J, Garcia P. Spanish pneumococcal infection study N. 2003. Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J Antimicrob Chemother 52:967–973. doi: 10.1093/jac/dkg485. [DOI] [PubMed] [Google Scholar]
- 49.Damjanovic M, Kharat AS, Eberhardt A, Tomasz A, Vollmer W. 2007. The essential tacF gene is responsible for the choline-dependent growth phenotype of Streptococcus pneumoniae. J Bacteriol 189:7105–7111. doi: 10.1128/JB.00681-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzalez A, Llull D, Morales M, Garcia P, Garcia E. 2008. Mutations in the tacF gene of clinical strains and laboratory transformants of Streptococcus pneumoniae: impact on choline auxotrophy and growth rate. J Bacteriol 190:4129–4138. doi: 10.1128/JB.01991-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jun SY, Jung GM, Yoon SJ, Oh MD, Choi YJ, Lee WJ, Kong JC, Seol JG, Kang SH. 2013. Antibacterial properties of a pre-formulated recombinant phage endolysin, SAL-1. Int J Antimicrob Agents 41:156–161. doi: 10.1016/j.ijantimicag.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Schuch R, Lee HM, Schneider BC, Sauve KL, Law C, Khan BK, Rotolo JA, Horiuchi Y, Couto DE, Raz A, Fischetti VA, Huang DB, Nowinski RC, Wittekind M. 2014. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J Infect Dis 209:1469–1478. doi: 10.1093/infdis/jit637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Etobayeva I, Linden S, Alem F, Harb L, Rizkalla L, Mosier P, Johnson A, Temple L, Hakami R, Nelson D. 2018. Discovery and biochemical characterization of PlyP56, PlyN74, and PlyTB40-Bacillus specific endolysins. Viruses 10:276. doi: 10.3390/v10050276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 10th ed Approved standard M07-A10 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 55.Sarker SD, Nahar L, Kumarasamy Y. 2007. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42:321–324. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rouse MS, Rotger M, Piper KE, Steckelberg JM, Scholz M, Andrews J, Patel R. 2005. In vitro and in vivo evaluations of the activities of lauric acid monoester formulations against Staphylococcus aureus. Antimicrob Agents Chemother 49:3187–3191. doi: 10.1128/AAC.49.8.3187-3191.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pastagia M, Euler C, Chahales P, Fuentes-Duculan J, Krueger JG, Fischetti VA. 2011. A novel chimeric lysin shows superiority to mupirocin for skin decolonization of methicillin-resistant and -sensitive Staphylococcus aureus strains. Antimicrob Agents Chemother 55:738–744. doi: 10.1128/AAC.00890-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vichai V, Kirtikara K. 2006. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 59.Szczeszak A, Ekner-Grzyb A, Runowski M, Szutkowski K, Mrówczyńska L, Kaźmierczak Z, Grzyb T, Dąbrowska K, Giersig M, Lis S. 2016. Spectroscopic, structural and in vitro cytotoxicity evaluation of luminescent, lanthanide doped core@shell nanomaterials GdVO4:Eu(3+)5%@SiO2@NH2. J Colloid Interface Sci 481:245–255. doi: 10.1016/j.jcis.2016.07.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.