Mycobacterium abscessus is a nontuberculous mycobacterium that causes invasive pulmonary infections in patients with structural lung disease. M. abscessus is intrinsically resistant to several classes of antibiotics, and an increasing number of strains isolated from patients exhibit resistance to most antibiotics considered for treatment of infections by this mycobacterium.
KEYWORDS: β-lactamase inhibitor, β-lactams, Mycobacterium abscessus, antibiotics, avibactam, rifamycins, synergy
ABSTRACT
Mycobacterium abscessus is a nontuberculous mycobacterium that causes invasive pulmonary infections in patients with structural lung disease. M. abscessus is intrinsically resistant to several classes of antibiotics, and an increasing number of strains isolated from patients exhibit resistance to most antibiotics considered for treatment of infections by this mycobacterium. Therefore, there is an unmet need for new regimens with improved efficacy to treat this disease. Synthesis of the essential cell wall peptidoglycan in M. abscessus is achieved via two enzyme classes, l,d- and d,d-transpeptidases, with each class preferentially inhibited by different subclasses of β-lactam antibiotics. We hypothesized that a combination of two β-lactams that comprehensively inhibit the two enzyme classes will exhibit synergy in killing M. abscessus. Paired combinations of antibiotics tested for in vitro synergy against M. abscessus included dual β-lactams, a β-lactam and a β-lactamase inhibitor, and a β-lactam and a rifamycin. Of the initial 206 combinations screened, 24 pairs exhibited synergy. A total of 13/24 pairs were combinations of two β-lactams, and 12/24 pairs brought the MICs of both drugs to within the therapeutic range. Additionally, synergistic drug pairs significantly reduced the frequency of selection of spontaneous resistant mutants. These novel combinations of currently available antibiotics may offer viable immediate treatment options against highly-resistant M. abscessus infections.
INTRODUCTION
Mycobacterium abscessus is considered to be among the most virulent of the rapidly growing nontuberculous mycobacteria (NTM). It may be environmentally or nosocomially acquired (1) and can lead to severe and invasive pulmonary infections in immunocompromised patients or those with structural lung diseases, such as bronchiectasis or cystic fibrosis (CF). In the CF population, invasive M. abscessus infections are associated with rapid lung function decline (2–4), so adequate treatment of these infections is paramount. M. abscessus is intrinsically resistant to several classes of antibiotics, and the percentage of clinical isolates exhibiting resistance to the few drugs currently available to treat this infection is steadily increasing (5–8). Sputum culture conversion rates as low as 25% have been described with antibiotic treatment alone (9), and the cure rate for M. abscessus pulmonary disease is only 30 to 50% (10).
The current treatment guidelines for M. abscessus pulmonary disease include at least 18 months of multidrug therapy, several of which require intravenous administration and may be associated with significant cytotoxicity (11, 12). These recommendations are largely based on empirical evidence, as few systematic clinical trials have been performed to elucidate the optimal therapeutic regimen against M. abscessus. It is also frequently necessary in clinical practice to tailor treatment regimens based on the resistance profiles of individual M. abscessus isolates, as the high degree of variability in antibiotic resistance observed among M. abscessus strains often precludes the use of a standardized regimen (10).
Macrolide antibiotics have historically been considered the backbone of treatment against many NTM, including M. abscessus (2, 12). However, two of the three M. abscessus subspecies, Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii, harbor a functional erm(41) gene, which confers inducible macrolide resistance and, thus, limits the effectiveness of this antibiotic class beyond the first 2 weeks of treatment (13, 14). Guidelines, therefore, recommend subspeciation of the M. abscessus complex, which many clinical laboratories are not equipped to perform routinely. Consequently, some CF centers prescribe initial treatment regimens that include a combination of intravenous amikacin and either cefoxitin or imipenem rather than a macrolide (15). Cefoxitin (a cephalosporin) and imipenem (a carbapenem) are the only two β-lactam antibiotics included in the current M. abscessus treatment guidelines (12, 16).
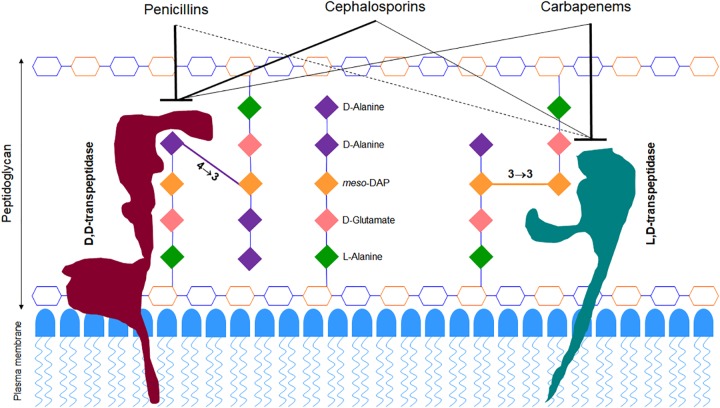
β-lactams function by inhibiting enzymes that catalyze synthesis of peptidoglycan, a three-dimensional macromolecule that forms the exoskeleton of bacterial cells (17). During the final step of peptidoglycan synthesis, M. abscessus utilizes two enzyme classes, the canonical d,d-transpeptidases (DDTs) (also known as penicillin-binding proteins) and the recently discovered l,d-transpeptidases (LDTs) (18), to generate 4→3 and 3→3 linkages between stem peptides, respectively (Fig. 1). Since as many as 80% of the linkages in M. abscessus peptidoglycan are of the 3→3 type (18), the LDTs that generate them are likely at least as important as DDTs for this organism. An initial survey of the M. abscessus genome identified five putative LDT-encoding genes (19). These LDTs are differentially susceptible to β-lactam subclasses, with most carbapenems exhibiting strong inhibitory activities, followed by cephalosporins and only a few penicillins exhibiting moderate inhibition of these enzymes (20, 21).
FIG 1.
Model of M. abscessus peptidoglycan depicting preferential binding of β-lactam subclasses. The hexagonal structures represent sugars N-acetylglucosamine (blue) and N-acetylmuramic acid (orange).
Inhibition of peptidoglycan synthesis is lethal to bacteria (22). As LDT and DDT activities are required for synthesis of M. abscessus peptidoglycan, simultaneous inhibition of both enzymes could be bactericidal. Since these enzymes exhibit differential susceptibilities to β-lactams (21, 23, 24), we hypothesize that a combination of β-lactam subclasses—one that optimally inhibits LDTs and another that specifically targets DDTs—will demonstrate synergy in killing M. abscessus. In this study, we have tested this hypothesis by assessing the potencies of combinations of 16 β-lactams consisting of cephalosporins and carbapenems against M. abscessus. Penicillins were not assessed, as many require frequent dosing, and we preferentially chose oral cephalosporins requiring once or twice daily dosing to simplify administration in patients.
M. abscessus exhibits robust β-lactamase activity via BlaMab, which significantly reduces the efficacy of β-lactams against this mycobacterium (25, 26). BlaMab degrades several β-lactams with much greater efficiency than BlaC in Mycobacterium tuberculosis (27). Among the known β-lactamase inhibitors, avibactam strongly inhibits BlaMab (28) and reduces the MICs of various β-lactams against M. abscessus (25, 29–31). Although clavulanate, tazobactam, and sulbactam are not potent inhibitors of BlaMab (27), clavulanate is the only orally bioavailable agent, and whether it exhibits synergy in combination with β-lactams against M. abscessus is not sufficiently documented. Therefore, we have included avibactam and clavulanate in our study. Rifamycins were also included based on prior demonstration of synergy between carbapenems and rifamycins against M. abscessus in vitro (32–34).
RESULTS
MICs of β-lactams against M. abscessus.
We evaluated the antimicrobial activity of several β-lactam antibiotics, including nine cephalosporins (cefadroxil, cefprozil, cefuroxime, cefixime, ceftibuten, cefdinir, cefditoren, cefpodoxime, and cefoxitin), six carbapenems (ertapenem, meropenem, imipenem, doripenem, biapenem, and tebipenem), and a penem (faropenem), by determining their MICs against M. abscessus (Table 1). We preferentially tested oral cephalosporins that did not require more than twice daily dosing, as their use in the clinical setting would be more convenient. MICs were also determined for three rifamycin antibiotics (rifabutin, rifapentine, and rifampin) and two β-lactamase inhibitors (clavulanate and avibactam), which were tested for synergy with β-lactams in subsequent experiments. The majority of cephalosporins exhibited high baseline MICs of 256 μg/ml, with the exception of cefoxitin and cefdinir at 64 μg/ml. The MICs of the carbapenems and penem were more variable, ranging from 8 μg/ml for imipenem to 256 μg/ml for ertapenem, tebipenem, and faropenem.
TABLE 1.
MICs of individual antibiotics tested against M. abscessus ATCC 19977 in vitro
| β-Lactam class | Drug | MIC (μg/ml) |
|---|---|---|
| Cephalosporins | Cefadroxil | 256 |
| Cefprozil | 256 | |
| Cefuroxime | 256 | |
| Cefixime | 256 | |
| Ceftibuten | 256 | |
| Cefdinir | 64 | |
| Cefditoren | 256 | |
| Cefpodoxime | 256 | |
| Cefoxitin | 64 | |
| Carbapenems | Ertapenem | 256 |
| Meropenem | 32 | |
| Imipenem | 8 | |
| Doripenem | 16 | |
| Biapenem | 16 | |
| Tebipenem | 256 | |
| Penem | Faropenem | 256 |
| Rifamycins | Rifabutin | 32 |
| Rifapentine | 128 | |
| Rifampin | 128 | |
| β-Lactamase inhibitors | Avibactam | 256 |
| Clavulanate | 256 |
Several β-lactam combinations exhibit synergy against M. abscessus.
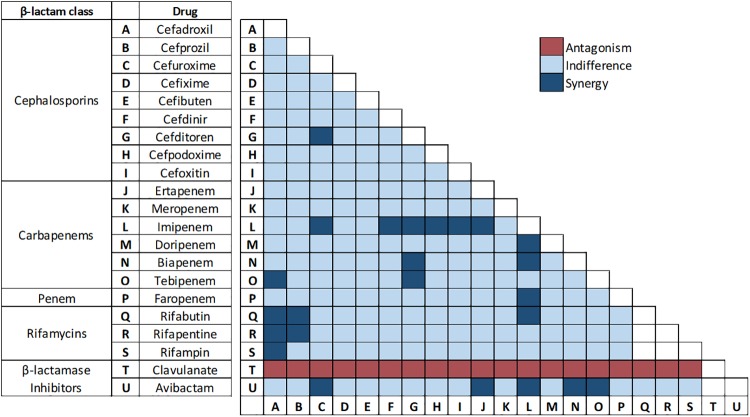
A total of 206 paired combinations of antibiotics were initially screened for synergy via a condensed version of the checkerboard assay as described in Materials and Methods. Combinations included all possible pairs of cephalosporins and carbapenems/penem, a rifamycin with either a cephalosporin or carbapenem/penem, and avibactam or clavulanate with a cephalosporin or carbapenem/penem (Fig. 2).
FIG 2.
Results representing potencies of dual drug combinations against M. abscessus using checkerboard assay. Each box represents a combination of drugs shown in the x axis and y axis. Inhibition of M. abscessus growth in samples containing drug pairs at one-fourth MIC or less of each drug is designated “Synergy” and growth at one-half to 1× MIC of each drug is designated “Indifference.” Growth of M. abscessus in samples containing drug pairs at 2× MIC of each drug is designated “Antagonism.” These designations are based on the published guidelines for interpreting checkerboard assay results (35–37). Combinations that were not assessed are represented by blank boxes.
Of the initial 206 combinations screened, 24 combinations showed no growth at one-fourth of the MIC or less for each drug and were further evaluated to verify synergy and determine the fractional inhibitory concentration index (FICI) using a checkerboard titration assay as described in Materials and Methods (Fig. 2). The FICI of a synergistic pair is a mathematical representation of the degree to which each drug contributes to synergy (35–37). These 24 synergistic combinations included cefuroxime and avibactam, biapenem and avibactam, cefoxitin and imipenem, cefditoren and imipenem, imipenem and doripenem, cefuroxime and cefditoren, cefditoren and biapenem, cefdinir and imipenem, cefuroxime and imipenem, cefpodoxime and imipenem, imipenem and biapenem, imipenem and avibactam, tebipenem and avibactam, ertapenem and avibactam, ertapenem and imipenem, imipenem and faropenem, imipenem and rifabutin, cefadroxil and tebipenem, cefditoren and tebipenem, cefadroxil and rifabutin, cefadroxil and rifapentine, cefadroxil and rifampin, cefprozil and rifabutin, and cefprozil and rifapentine (Table 2). Although substantial variability in the level of synergy among paired combinations was observed, there were a few notable trends. For example, imipenem was synergistic with the majority of drugs it was tested with, and the rifamycins were synergistic with select earlier generation cephalosporins. Of note, all of the combinations that included clavulanate failed to inhibit growth of M. abscessus in the presence of antibiotics at concentrations as high as 2× MIC. This antagonism was unexpected but was reliably reproducible when the experiment was repeated.
TABLE 2.
Fractional inhibitory concentration indices of synergistic drug pairsa
| Drug combination | MIC of single drug (μg/ml) | MIC in combination (μg/ml) | FICI |
|---|---|---|---|
| Cefuroxime and avibactam | 256/256 | 32/5 | 0.15 |
| Biapenem and avibactam | 16/256 | 4/4 | 0.27 |
| Cefoxitin and imipenem | 64/8 | 9/1 | 0.27 |
| Cefditoren and imipenem | 256/8 | 26/1 | 0.27 |
| Imipenem and doripenem | 8/16 | 2/2 | 0.30 |
| Cefuroxime and cefditoren | 256/256 | 33/44 | 0.30 |
| Cefditoren and biapenem | 256/16 | 26/4 | 0.32 |
| Cefdinir and imipenem | 64/8 | 9/2 | 0.35 |
| Cefuroxime and imipenem | 256/8 | 30/2 | 0.35 |
| Cefpodoxime and imipenem | 256/8 | 28/2 | 0.36 |
| Imipenem and biapenem | 8/16 | 2/3 | 0.42 |
| Imipenem and avibactam | 8/256 | 2/64 | 0.47 |
| Tebipenem and avibactam | 256/256 | 28/5 | 0.13 |
| Ertapenem and avibactam | 256/256 | 64/4 | 0.27 |
| Ertapenem and imipenem | 256/8 | 19/2 | 0.29 |
| Imipenem and faropenem | 8/256 | 1/29 | 0.29 |
| Imipenem and rifabutin | 8/32 | 2/3 | 0.31 |
| Cefadroxil and tebipenem | 256/256 | 40/48 | 0.34 |
| Cefditoren and tebipenem | 256/256 | 64/32 | 0.38 |
| Cefadroxil and rifabutin | 256/32 | 48/6 | 0.38 |
| Cefadroxil and rifapentine | 256/128 | 64/16 | 0.38 |
| Cefadroxil and rifampin | 256/128 | 64/32 | 0.50 |
| Cefprozil and rifabutin | 256/32 | 64/8 | 0.50 |
| Cefprozil and rifapentine | 256/128 | 64/32 | 0.50 |
Combinations capable of reducing MICs to within susceptible/intermediate range based on established/presumed breakpoints are listed on the top half of the table in order of ascending FICI. FICIs and MICs in combination were extrapolated using data averaged from 2 to 3 replicate experiments.
To determine if the degree of synergy between paired combinations was sufficient to reduce MICs to within the therapeutic range, the fractional inhibitory concentration (FIC) of each drug in combination was used to extrapolate expected MICs as a result of synergy. Although Clinical and Laboratory Standards Institute (CLSI) guidelines regarding MIC breakpoints against M. abscessus are not currently available for most of the antibiotics tested, they have been established for cefoxitin and imipenem (38). Therefore, MIC breakpoints for all cephalosporins and carbapenems/penem were assumed to be the same as those for cefoxitin (≤16 μg/ml, susceptible; ≤64 μg/ml, intermediately susceptible) and imipenem (≤4 μg/ml, susceptible; ≤8 μg/ml, intermediately susceptible), respectively. With regard to the rifamycins, a breakpoint MIC of ≤1 μg/ml has been established for rifampin and rifabutin against M. tuberculosis and Mycobacterium avium complex (38), and the same principle was applied. The most recent guidelines from the European Committee on Antimicrobial Susceptibility Testing (EUCAST) do not include clinical breakpoints of antibiotics against M. abscessus (39). Based on these presumed breakpoints, 5/24 combinations exhibited MICs within the fully susceptible range for both drugs. They are biapenem and avibactam, cefoxitin and imipenem, imipenem and doripenem, cefdinir and imipenem, and imipenem and biapenem. An additional 7/24 combinations showed MICs that were considered moderately susceptible or intermediate. They are cefuroxime and avibactam, cefditoren and imipenem, cefuroxime and cefditoren, cefditoren and biapenem, cefuroxime and imipenem, cefpodoxime and imipenem, and imipenem and avibactam. The remaining 12/24 combinations were unable to bring MICs below resistance breakpoints.
All of the 12 most synergistic combinations—those that brought MICs within the therapeutic range—were pairs of either two β-lactams or a β-lactam and avibactam. Several of the remaining 12/24 combinations also exhibited a high degree of synergy based on FICI; however, their initial MICs were so high that the synergistic effect was insufficient to reduce MICs to below presumed breakpoints. We also observed a limit to the degree of synergy achievable with drugs exhibiting relatively low initial MICs, such as imipenem, biapenem, and doripenem. Combinations with these drugs resulted in up to a 4-fold decrease in MIC, but this appeared to be the limit of reduction as MICs approached 2 μg/ml.
Additionally, the rifamycins showed synergy in combination with two of the earlier generation cephalosporins (cefprozil and cefadroxil), with at least a 4-fold reduction in MIC for each combination. However, given the relatively low presumed MIC breakpoint of ≤1 μg/ml for rifamycins, none of the combinations brought MICs within the therapeutic range.
β-lactam combinations reduce frequency of selection of spontaneous drug-resistant mutants.
The 24 synergistic combinations were also evaluated to determine the frequency at which spontaneous resistant mutants are selected in the presence of paired drug combinations compared to that for each drug alone (Table 3). The frequency of resistant mutant selection was lower for all 24 combinations than the frequency for each drug individually. The greatest decrease was noted among the cephalosporins at >4 log reduction for six out of seven agents, with cefdinir being the exception, as the mutation frequency of this drug alone was lower than that of the other cephalosporins. Also of note, rifapentine and rifabutin exhibited the lowest frequency of resistant mutant selection, with no mutant colonies observed on any of the individual drug plates.
TABLE 3.
Frequency of emergence of spontaneous drug-resistant mutants of M. abscessus when exposed to individual drugs and paired combinations that exhibit synergy in vitro
| Drug combination | Resistance frequency for: |
|
|---|---|---|
| Individual drug | Combination | |
| Cefuroxime and avibactam | >2 × 10−6/— | <1 × 10−10 |
| Biapenem and avibactam | 8.9 × 10−8/— | 3.3 × 10−9 |
| Cefoxitin and imipenem | >2 × 10−6/1.9 × 10−7 | <1 × 10−10 |
| Cefditoren and imipenem | >2 × 10−6/1.9 × 10−7 | <1 × 10−10 |
| Imipenem and doripenem | 1.9 × 10−7/9.9 × 10−8 | 9.1 × 10−9 |
| Cefuroxime and cefditoren | >2 × 10−6/>2 × 10−6 | <1 × 10−10 |
| Cefditoren and biapenem | >2 × 10−6/8.9 × 10−8 | <1 × 10−10 |
| Cefdinir and imipenem | 7.8 × 10−9/1.9× 10−7 | <1 × 10−10 |
| Cefuroxime and imipenem | >2 × 10−6/1.9 × 10−7 | <1 × 10−10 |
| Cefpodoxime and imipenem | >2 × 10−6/1.9 × 10−7 | <1 × 10−10 |
| Imipenem and biapenem | 1.9 × 10−7/8.9 × 10−8 | 1.1 × 10−9 |
| Imipenem and avibactam | 1.9 × 10−7/— | <1 × 10−10 |
| Tebipenem and avibactam | 1.1 × 10−7/— | <1 × 10−10 |
| Ertapenem and avibactam | 8.3 × 10−8/— | <1 × 10−10 |
| Ertapenem and imipenem | 8.3 × 10−8/1.9 × 10−7 | 4.3 × 10−9 |
| Imipenem and faropenem | 1.9 × 10−7/<1 × 10−10 | <1 × 10−10 |
| Imipenem and rifabutin | 1.9 × 10−7/<1 × 10−10 | <1 × 10−10 |
| Cefadroxil and tebipenem | >2 × 10−6/1.1 × 10−7 | 1.3 × 10−8 |
| Cefditoren and tebipenem | >2 × 10−6/1.1 × 10−7 | <1 × 10−10 |
| Cefadroxil and rifabutin | >2 × 10−6/<1 × 10−10 | <1 × 10−10 |
| Cefadroxil and rifapentine | >2 × 10−6/<1× 10−10 | <1 × 10−10 |
| Cefadroxil and rifampin | >2 × 10−6/>2 × 10−6 | <1 × 10−10 |
| Cefprozil and rifabutin | >2 × 10−6/<1 × 10−10 | <1 × 10−10 |
| Cefprozil and rifapentine | >2 × 10−6/<1 × 10−10 | <1 × 10−10 |
DISCUSSION
Given the growing prevalence of extensively drug-resistant M. abscessus infections, development of novel treatment strategies is imperative. Although our current understanding of β-lactam targets in M. abscessus is not comprehensive, we have leveraged the available data to begin developing synergistic treatment regimens with potential to treat M. abscessus infections that are resistant to standard therapies.
In this study, we tested a total of 206 paired combinations of antibiotics in vitro against M. abscessus reference strain ATCC 19977, 24 of which displayed synergy with FICIs of ≤0.5. Of these, 12 combinations achieved MIC reductions below presumed breakpoints for both drugs. Although a few studies have been published describing synergy between β-lactams and other antibiotic classes against M. abscessus in vitro (32, 34, 40, 41), only one has assessed synergy between dual β-lactams (21). Our current study encompasses a broader array of combinations and offers a more comprehensive analysis of the synergistic activity of the two major β-lactam subclasses currently used to treat M. abscessus infections: cephalosporins and carbapenems.
We hypothesize that the basis for synergy exhibited by β-lactam pairs is their nonredundant selective inhibition of distinct transpeptidases that synthesize peptidoglycan in M. abscessus. If only one type of enzyme existed as the target for β-lactams, the pairs would likely exhibit additive activity rather than synergy. Variation in the level of inhibition of LDTs of M. abscessus (21) and M. tuberculosis (20, 24, 42) by agents of this antibiotic class supports this hypothesis. We further hypothesize that, at the molecular level, the structures of β-lactams that exhibit synergy against M. abscessus most effectively complement the structure of the binding sites available in the transpeptidases of this organism, thereby favoring initial binding and subsequent interaction to bring about effective inhibition of the enzymes. Differences in binding affinities and kinetics of inhibition by different β-lactams against the LDTs of Enterococcus faecium (43) illustrate the basis for this hypothesis. By virtue of belonging to the β-lactam group, the penicillin subclass likely also interacts with the transpeptidases of M. abscessus, and some drugs in this class may potentially inhibit them as they are known to inhibit DDTs in other organisms. To develop a comprehensive understanding of interactions of the entire β-lactam class against their targets in M. abscessus, inclusion of the penicillins is necessary. The scope of the current study was limited to the β-lactam subclasses with demonstrated potential for clinical utility in treating M. abscessus infections. Penicillins were not included, as several members of this subclass require frequent dosing, making them less suitable for treating chronic diseases, such as M. abscessus infection. The number of proteins encoded by the M. abscessus genome with DDT activity and their identities are not known. Determination of this information would facilitate the assessment of the relationship between β-lactam subclasses and DDTs.
Synergy was also observed between the rifamycins and two of the earlier generation cephalosporins, cefadroxil and cefprozil. Although rifamycins tend to affect intracellular processes via inhibition of RNA polymerase activity, studies in M. tuberculosis have demonstrated synergy between rifampin and cephalosporins, especially early generation (44). It was proposed that, by damaging the cell wall, cephalosporins promote penetration by rifampin, leading to higher effective concentrations. Rifampin may also increase susceptibility to lower intracellular levels of β-lactams (44). However, rifabutin exhibited negligible synergy in combination with cephalosporins against M. tuberculosis, and it was hypothesized that rifabutin’s lipophilicity allows for rapid penetration into the cell, thus, bypassing synergistic cell wall interactions with β-lactams. A prior study evaluating rifamycin efficacy against M. abscessus (33) found that rifabutin alone was active against multiple M. abscessus isolates, whereas rifampin showed little activity. The reasons for the differential efficacies of rifamycins against M. abscessus have yet to be confirmed but may be related to differences in bacterial uptake/efflux or drug metabolism (33, 45–48).
Five out of 24 synergistic combinations included avibactam. In these combinations, the MICs of three β-lactams (cefuroxime, imipenem, and biapenem) were reduced to below therapeutic breakpoints. Therefore, avibactam appears to be a viable adjunct to β-lactam-based treatment regimens. However, its current coformulation with ceftazidime (which itself does not exhibit valuable activity against M. abscessus [28, 30]) and intravenous administration limit its usefulness. Relevant to the strategy of combining a carbapenem with a β-lactamase inhibitor against M. abscessus, several coformulated agents have recently been developed. These include FDA-approved meropenem-vaborbactam (Melinta Therapeutics) and imipenem-relebactam (Merck), which recently completed phase III trials. Coformulations of cefepime-zidebactam (Wockhardt) and meropenem-nacubactam (Roche) are currently in phase II of development. Although these compounds have yet to be tested against M. abscessus, our data suggest that they could be viable options for treatment of M. abscessus infections and would potentially simplify β-lactam-based regimens. Interestingly, clavulanate was found to be antagonistic in combination with all β-lactams tested in this study, although the mechanism for this is unclear. As there is no precedent for antagonism of β-lactam and clavulanate combinations against other bacteria, we speculate the following hypotheses. Clavulanate is metabolized by M. abscessus, and the resulting metabolite alters the rate of influx or efflux of β-lactams, thereby reducing the effective concentration available to bind target enzymes. It is also possible that binding of the clavulanate metabolite to target enzymes may alter their binding kinetics to β-lactams. In addition, the possibility of the clavulanate metabolite directly competing for binding sites associated with the 4-carbon core ring of β-lactams cannot be ruled out. Further study would be necessary to elucidate the underlying mechanism for this phenomenon, including possibilities not considered above.
Another measure of synergy is the frequency of selection of spontaneous resistant mutants. For a synergistic pair, the frequency of resistant mutant selection would ideally be lower than the product of frequencies associated with either drug alone. The majority of drug pairs with the lowest FICIs selected resistant mutants with a frequency of <1 × 10−10, which approaches the product of the individual drugs (Table 3). However, due to physical limitations of the number of M. abscessus CFU that could be used to identify resistant mutants, we were unable to obtain the exact frequency of mutant selection for several paired combinations. Based on these observations, we propose that the majority of pairs identified here exhibit synergy in both antimicrobial activity and reduction of selection of drug-resistant mutants.
Although significant variability in MIC exists among M. abscessus strains and in vitro drug susceptibility data do not always correlate with clinical efficacy (49), the novel β-lactam combinations identified here using the reference M. abscessus strain ATCC 19977 could be leveraged for further preclinical assessment, including in vivo efficacy against drug-resistant clinical isolates. As M. abscessus treatment generally necessitates a regimen consisting of at least 3 to 4 agents, the addition of other antibiotic classes or β-lactamase inhibitors to these synergistic β-lactam combinations may further potentiate MIC reduction and improve efficacy. This may also allow clinicians to avoid use of the more cytotoxic antibiotics, such as amikacin, especially in the context of prior adverse effects.
Based on current trends of M. abscessus strains isolated in clinics, resistance to an increasing number of drugs is likely to continue over the next several years, further compromising our ability to treat disease resulting from this pathogen. New antibiotics and coformulations are currently in development, but none are primarily intended for treatment of M. abscessus or other NTM infections, and it could take several years for efficacy studies to be completed. Repurposing currently available antibiotics in novel combinations, such as those identified here, may provide vital immediate therapeutic options for patients failing standard M. abscessus treatment regimens and facilitate rapid implementation in the clinical setting.
MATERIALS AND METHODS
Bacterial strains and in vitro growth conditions.
The M. abscessus reference strain ATCC 19977 (ATCC, Manassas, VA) was used for all experiments. Strains were grown in Middlebrook 7H9 broth (Difco) supplemented with 0.5% glycerol, 10% albumin-dextrose-catalase enrichment, and 0.05% Tween 80 at 37°C with constant shaking at 220 RPM in an orbital shaker. All drugs were obtained from the following commercial vendors: Toronto Research Chemicals (ertapenem) and Sigma-Aldrich (rifampin, meropenem, imipenem, doripenem, biapenem, faropenem, tebipenem, and all cephalosporins). To assess the quality of these compounds, meropenem, biapenem, and tebipenem were randomly selected and assessed by liquid chromatography-mass spectrometry. The purity of compounds ranged from 95% to 99%.
MIC.
The MIC of each drug against M. abscessus was determined using the standard broth dilution method (50, 51) in accordance with CLSI guidelines specific for this organism (38). In summary, powdered drug stocks were reconstituted in dimethyl sulfoxide (DMSO), and 2-fold serial dilutions were prepared in Middlebrook 7H9 broth to obtain final drug concentrations ranging from 256 μg/ml to 1 μg/ml in 96-well plates in a final volume of 200 μl. A total of 105 CFU of M. abscessus from exponentially growing culture was added to each well. M. abscessus culture without drug and 7H9 broth alone were included in each plate as positive and negative controls, respectively. Plates were incubated at 30°C for 72 h per CLSI guidelines. MIC was assessed via visual inspection to determine growth or lack thereof, and an MIC for each drug was recorded as the lowest concentration at which M. abscessus growth was not observed. All MIC assessments were repeated to verify results.
Checkerboard titration assay.
The checkerboard titration assay is a modified broth dilution assay and was performed as previously described (35–37). An initial synergy screen of two-drug combinations was performed at four different concentrations based on each drug’s respective MIC: 2× MIC, MIC, one-half MIC, and one-fourth MIC for each drug in combination via 2-fold serial dilutions in a 96-well plate. A total of 105 CFU of M. abscessus from exponentially growing culture was inoculated into each well, with positive and negative controls as described above for the MIC assay, and plates were incubated at 30°C for 72 h. Plates were visually inspected for M. abscessus growth or lack thereof. Drug combinations that inhibited M. abscessus growth at one-fourth MIC or less of each drug were considered to have some degree of synergy and were chosen for additional synergy testing.
To confirm the degree of synergy, two drugs were added to Middlebrook 7H9 broth in a 96-well plate, each starting at 2× MIC and serially diluted 2-fold up to 1/32× MIC, so all possible 2-fold dilution combinations from 2× to 1/32× MIC were assayed. A total of 105 CFU of M. abscessus was inoculated into each well. Plates were incubated at 30°C and evaluated for M. abscessus growth by visual inspection at 72 h. The fractional inhibitory concentration (FIC) of each drug in combination was determined as described previously (35–37). The FIC of a drug in a sample is calculated as the concentration of the drug divided by the MIC of the drug when used alone. The FIC Index (FICI) is the sum of the FIC of two drugs in a sample. The FICI was calculated for each combination of drugs that inhibited M. abscessus growth at less than one-half of the MIC of each individual drug. An FICI of ≤0.5 was interpreted as synergy, >0.5 to 2 as indifference, and >2 as antagonism. As an internal control, the MIC of each individual drug was also assessed via broth microdilution within each plate. All combinations with an FICI of ≤0.5 were tested in triplicate to confirm reproducibility, and an average FICI was calculated and reported here.
Determination of frequency of spontaneous drug resistance emergence.
Any drug combination with an FICI of ≤0.5 was further assessed for frequency of spontaneous drug resistance. The CFU per milliliter of M. abscessus in culture at an A600 of 1.0 was initially determined as follows. M. abscessus was grown to exponential phase, adjusted to an A600 of 1.0 in Middlebrook 7H9 broth, and was serially diluted 10-fold in this broth. A total of 100 μl of each dilution was plated onto Middlebrook 7H10 agar, which was incubated at 37°C for 72 h. Resultant CFU counts were used to determine M. abscessus CFU density in culture. This assessment was repeated three times, and the mean M. abscessus CFU density was used in calculations in subsequent experiments.
To determine frequency of spontaneous drug resistance emergence, 10 ml of M. abscessus culture grown to exponential phase in 7H9 broth was used to prepare a suspension at an A600 of 1.0, and 1.0 ml of this suspension was inoculated onto each of 10 total Middlebrook 7H10 agar plates, which were supplemented with either a single drug or a combination of two drugs. The input M. abscessus was ∼1 × 109 CFU per plate. Therefore, the limit of detection of resistant mutant is ∼1 × 1010. If we were unable to isolate a resistant CFU, we assigned a resistance frequency of <1 × 1010. These assessments were performed at the MIC for single-drug and combination plates to promote selection of resistant mutants. CFU were counted after 7 days of incubation at 37°C. The frequency of drug-resistant mutants was determined from the number of spontaneous mutants observed as a percentage of the input CFU inoculum.
ACKNOWLEDGMENTS
This work was supported by Cystic Fibrosis Foundation award LAMICH17GO and NIH award R21 AI121805 to G.L. E.S.-R. was supported by NIH grant T32 AI007291.
This content is solely the responsibility of the authors and does not necessarily represent the official views of the Cystic Fibrosis Foundation or the National Institutes of Health.
REFERENCES
- 1.Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, Peacock SJ, Parkhill J, Floto RA. 2013. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 381:1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 3.Esther CR Jr, Esserman DA, Gilligan P, Kerr A, Noone PG. 2010. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros 9:117–123. doi: 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benwill JL, Wallace RJ Jr. 2014. Mycobacterium abscessus: challenges in diagnosis and treatment. Curr Opin Infect Dis 27:506–510. doi: 10.1097/QCO.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 5.Brown-Elliott BA, Wallace RJ Jr. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev 15:716–746. doi: 10.1128/CMR.15.4.716-746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 7.van Ingen J, Boeree MJ, van Soolingen D, Mouton JW. 2012. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updat 15:149–161. doi: 10.1016/j.drup.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Flume PA. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. J Cyst Fibros 15:139–140. doi: 10.1016/S1569-1993(16)00018-7. [DOI] [PubMed] [Google Scholar]
- 9.Diel R, Ringshausen F, Richter E, Welker L, Schmitz J, Nienhaus A. 2017. Microbiological and clinical outcomes of treating non-Mycobacterium Avium complex nontuberculous mycobacterial pulmonary disease: a systematic review and meta-analysis. Chest 152:120–142. doi: 10.1016/j.chest.2017.04.166. [DOI] [PubMed] [Google Scholar]
- 10.Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. 2011. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 52:565–571. doi: 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- 11.Wallace RJ Jr, Swenson JM, Silcox VA, Bullen MG. 1985. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J Infect Dis 152:500–514. doi: 10.1093/infdis/152.3.500. [DOI] [PubMed] [Google Scholar]
- 12.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann JL, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 71:i1–i22. doi: 10.1136/thoraxjnl-2015-207360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nash KA, Brown-Elliott BA, Wallace RJ Jr. 2009. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 53:1367–1376. doi: 10.1128/AAC.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, Daley CL, Kwon OJ. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 15.Philley JV, DeGroote MA, Honda JR, Chan MM, Kasperbauer S, Walter ND, Chan ED. 2016. Treatment of non-tuberculous mycobacterial lung disease. Curr Treat Options Infect Dis 8:275–296. doi: 10.1007/s40506-016-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavollay M, Dubee V, Heym B, Herrmann JL, Gaillard JL, Gutmann L, Arthur M, Mainardi JL. 2014. In vitro activity of cefoxitin and imipenem against Mycobacterium abscessus complex. Clin Microbiol Infect 20:O297–O300. doi: 10.1111/1469-0691.12405. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann R, Holtje JV, Schwarz U. 1972. Targets of penicillin action in Escherichia coli. Nature 235:426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- 18.Lavollay M, Fourgeaud M, Herrmann JL, Dubost L, Marie A, Gutmann L, Arthur M, Mainardi JL. 2011. The peptidoglycan of Mycobacterium abscessus is predominantly cross-linked by l,d-transpeptidases. J Bacteriol 193:778–782. doi: 10.1128/JB.00606-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattoo R, Lloyd EP, Kaushik A, Kumar P, Brunelle JL, Townsend CA, Lamichhane G. 2017. LdtMav2, a nonclassical transpeptidase and susceptibility of Mycobacterium avium to carbapenems. Future Microbiol 12:595–607. doi: 10.2217/fmb-2016-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubee V, Triboulet S, Mainardi JL, Etheve-Quelquejeu M, Gutmann L, Marie A, Dubost L, Hugonnet JE, Arthur M. 2012. Inactivation of Mycobacterium tuberculosis l,d-transpeptidase LdtMt1 by carbapenems and cephalosporins. Antimicrob Agents Chemother 56:4189–4195. doi: 10.1128/AAC.00665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar P, Chauhan V, Silva JRA, Lameira J, d'Andrea FB, Li SG, Ginell SL, Freundlich JS, Alves CN, Bailey S, Cohen KA, Lamichhane G. 2017. Mycobacterium abscessus l,d-transpeptidases are susceptible to inactivation by carbapenems and cephalosporins but not penicillins. Antimicrob Agents Chemother 61:e00866-17. doi: 10.1128/AAC.00866-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh C, Wencewicz TA. 2016. Assembly of the peptidoglycan layer of bacterial cell walls, p 37–68. In Walsh C, Wencewicz TA (ed), Antiobiotics: challenges, mechanisms, opportunities. American Society for Microbiology, Washington, DC. [Google Scholar]
- 23.Triboulet S, Arthur M, Mainardi JL, Veckerle C, Dubee V, Nguekam-Moumi A, Gutmann L, Rice LB, Hugonnet JE. 2011. Inactivation kinetics of a new target of β-lactam antibiotics. J Biol Chem 286:22777–22784. doi: 10.1074/jbc.M111.239988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar P, Kaushik A, Lloyd EP, Li SG, Mattoo R, Ammerman NC, Bell DT, Perryman AL, Zandi TA, Ekins S, Ginell SL, Townsend CA, Freundlich JS, Lamichhane G. 2017. Non-classical transpeptidases yield insight into new antibacterials. Nat Chem Biol 13:54–61. doi: 10.1038/nchembio.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubee V, Soroka D, Cortes M, Lefebvre AL, Gutmann L, Hugonnet JE, Arthur M, Mainardi JL. 2015. Impact of β-lactamase inhibition on the activity of ceftaroline against Mycobacterium tuberculosis and Mycobacterium abscessus. Antimicrob Agents Chemother 59:2938–2941. doi: 10.1128/AAC.05080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rominski A, Schulthess B, Muller DM, Keller PM, Sander P. 2017. Effect of β-lactamase production and β-lactam instability on MIC testing results for Mycobacterium abscessus. J Antimicrob Chemother 72:3070–3078. doi: 10.1093/jac/dkx284. [DOI] [PubMed] [Google Scholar]
- 27.Soroka D, Ourghanlian C, Compain F, Fichini M, Dubee V, Mainardi JL, Hugonnet JE, Arthur M. 2017. Inhibition of β-lactamases of mycobacteria by avibactam and clavulanate. J Antimicrob Chemother 72:1081–1088. doi: 10.1093/jac/dkw546. [DOI] [PubMed] [Google Scholar]
- 28.Dubee V, Bernut A, Cortes M, Lesne T, Dorchene D, Lefebvre AL, Hugonnet JE, Gutmann L, Mainardi JL, Herrmann JL, Gaillard JL, Kremer L, Arthur M. 2015. β-lactamase inhibition by avibactam in Mycobacterium abscessus. J Antimicrob Chemother 70:1051–1058. doi: 10.1093/jac/dku510. [DOI] [PubMed] [Google Scholar]
- 29.Lefebvre AL, Dubee V, Cortes M, Dorchene D, Arthur M, Mainardi JL. 2016. Bactericidal and intracellular activity of β-lactams against Mycobacterium abscessus. J Antimicrob Chemother 71:1556–1563. doi: 10.1093/jac/dkw022. [DOI] [PubMed] [Google Scholar]
- 30.Kaushik A, Gupta C, Fisher S, Story-Roller E, Galanis C, Parrish N, Lamichhane G. 2017. Combinations of avibactam and carbapenems exhibit enhanced potencies against drug-resistant Mycobacterium abscessus. Future Microbiol 12:473–480. doi: 10.2217/fmb-2016-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lefebvre AL, Le Moigne V, Bernut A, Veckerle C, Compain F, Herrmann JL, Kremer L, Arthur M, Mainardi JL. 2017. Inhibition of the β-lactamase BlaMab by avibactam improves the in vitro and in vivo efficacy of imipenem against Mycobacterium abscessus. Antimicrob Agents Chemother 61:e02440-16. doi: 10.1128/AAC.02440-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaushik A, Makkar N, Pandey P, Parrish N, Singh U, Lamichhane G. 2015. Carbapenems and rifampin exhibit synergy against Mycobacterium tuberculosis and Mycobacterium abscessus. Antimicrob Agents Chemother 59:6561–6567. doi: 10.1128/AAC.01158-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aziz DB, Low JL, Wu ML, Gengenbacher M, Teo JWP, Dartois V, Dick T. 2017. Rifabutin is active against Mycobacterium abscessus complex. Antimicrob Agents Chemother 61:e00155-17. doi: 10.1128/AAC.00155-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Run E, Arthur M, Mainardi J-L. 2018. In vitro and intracellular activity of imipenem combined with tedizolid, rifabutin, and avibactam against Mycobacterium abscessus. bioRxiv doi: 10.1101/411181. [DOI] [PMC free article] [PubMed]
- 35.Krogstad DJ, Moellering RC. 1986. Antimicrobial combinations, p 537–595. In Lorian V. (ed), Antibiotics in laboratory medicine, 2nd ed Williams & Wilkins Co, Baltimore, MD. [Google Scholar]
- 36.Rohner P, Herter C, Auckenthaler R, Pechere JC, Waldvogel FA, Lew DP. 1989. Synergistic effect of quinolones and oxacillin on methicillin-resistant Staphylococcus species. Antimicrob Agents Chemother 33:2037–2041. doi: 10.1128/AAC.33.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh MH, Yu CM, Yu VL, Chow JW. 1993. Synergy assessed by checkerboard. A critical analysis. Diagn Microbiol Infect Dis 16:343–349. doi: 10.1016/0732-8893(93)90087-N. [DOI] [PubMed] [Google Scholar]
- 38.Clinical and Laboratory Standards Institute. 2011. Susceptibility testing of Mycobacteria, Nocardiae and other aerobic actinomycetes. CLSI document M24-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 39.European Committee on Antimicrobial Susceptibility Testing. 2019. Breakpoint tables for interpretation of MICs and zone diameters.
- 40.Cremades R, Santos A, Rodriguez JC, Garcia-Pachon E, Ruiz M, Royo G. 2009. Mycobacterium abscessus from respiratory isolates: activities of drug combinations. J Infect Chemother 15:46–48. doi: 10.1007/s10156-008-0651-y. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz M, Fisher S, Story-Roller E, Lamichhane G, Parrish N. 2018. Activities of dual combinations of antibiotics against multidrug-resistant nontuberculous mycobacteria recovered from patients with cystic fibrosis. Microb Drug Resist 24:1191–1197. doi: 10.1089/mdr.2017.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cordillot M, Dubee V, Triboulet S, Dubost L, Marie A, Hugonnet JE, Arthur M, Mainardi JL. 2013. In vitro cross-linking of peptidoglycan by Mycobacterium tuberculosis l,d-transpeptidases and inactivation of these enzymes by carbapenems. Antimicrob Agents Chemother 57:5940–5945. doi: 10.1128/AAC.01663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Triboulet S, Dubee V, Lecoq L, Bougault C, Mainardi JL, Rice LB, Etheve-Quelquejeu M, Gutmann L, Marie A, Dubost L, Hugonnet JE, Simorre JP, Arthur M. 2013. Kinetic features of l,d-transpeptidase inactivation critical for β-lactam antibacterial activity. PLoS One 8:e67831. doi: 10.1371/journal.pone.0067831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramon-Garcia S, Gonzalez Del Rio R, Villarejo AS, Sweet GD, Cunningham F, Barros D, Ballell L, Mendoza-Losana A, Ferrer-Bazaga S, Thompson CJ. 2016. Repurposing clinically approved cephalosporins for tuberculosis therapy. Sci Rep 6:34293. doi: 10.1038/srep34293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunin CM. 1996. Antimicrobial activity of rifabutin. Clin Infect Dis 22:S3–13. doi: 10.1093/clinids/22.Supplement_1.S3. [DOI] [PubMed] [Google Scholar]
- 46.Baysarowich J, Koteva K, Hughes DW, Ejim L, Griffiths E, Zhang K, Junop M, Wright GD. 2008. Rifamycin antibiotic resistance by ADP-ribosylation: structure and diversity of Arr. Proc Natl Acad Sci U S A 105:4886–4891. doi: 10.1073/pnas.0711939105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ripoll F, Pasek S, Schenowitz C, Dossat C, Barbe V, Rottman M, Macheras E, Heym B, Herrmann JL, Daffe M, Brosch R, Risler JL, Gaillard JL. 2009. Non mycobacterial virulence genes in the genome of the emerging pathogen Mycobacterium abscessus. PLoS One 4:e5660. doi: 10.1371/journal.pone.0005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rominski A, Roditscheff A, Selchow P, Bottger EC, Sander P. 2017. Intrinsic rifamycin resistance of Mycobacterium abscessus is mediated by ADP-ribosyltransferase MAB_0591. J Antimicrob Chemother 72:376–384. doi: 10.1093/jac/dkw466. [DOI] [PubMed] [Google Scholar]
- 49.Philley JV, Griffith DE. 2013. Management of nontuberculous mycobacterial (NTM) lung disease. Semin Respir Crit Care Med 34:135–142. doi: 10.1055/s-0033-1333575. [DOI] [PubMed] [Google Scholar]
- 50.Tilton RC, Lieberman L, Gerlach EH. 1973. Microdilution antibiotic susceptibility test: examination of certain variables. Appl Microbiol 26:658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cynamon MH, Speirs RJ, Welch JT. 1998. In vitro antimycobacterial activity of 5-chloropyrazinamide. Antimicrob Agents Chemother 42:462–463. [DOI] [PMC free article] [PubMed] [Google Scholar]