In sub-Saharan Africa (SSA), gentamicin is commonly used for severe infections in non-intensive-care-unit (ICU) settings, but pharmacokinetic and pharmacodynamic data for this specific population are lacking. We performed a population pharmacokinetic study in an adult Mozambican non-ICU hospital population treated with gentamicin (n = 48) and developed a pharmacokinetic model using nonlinear mixed-effects modeling.
KEYWORDS: aminoglycosides, gentamicin, pharmacodynamics, population pharmacokinetics, severe illness, sub-Saharan Africa
ABSTRACT
In sub-Saharan Africa (SSA), gentamicin is commonly used for severe infections in non-intensive-care-unit (ICU) settings, but pharmacokinetic and pharmacodynamic data for this specific population are lacking. We performed a population pharmacokinetic study in an adult Mozambican non-ICU hospital population treated with gentamicin (n = 48) and developed a pharmacokinetic model using nonlinear mixed-effects modeling. Simulations showed that non-ICU patient populations in SSA may be at substantial risk for underexposure to gentamicin during routine once-daily dosing.
TEXT
In sub-Saharan Africa (SSA), community-acquired bloodstream infection and sepsis are leading causes of morbidity and mortality among hospitalized adults (1–3). Acute infection-induced pathophysiological changes, such as organ dysfunction, increased capillary permeability, and hypoalbuminemia, are known to lead to alterations in antibiotic volume of distribution (V) and clearance (CL) (4). Pharmacokinetic (PK) changes can give rise to an inability to attain pharmacodynamic (PD) targets, especially among critically ill patients in an intensive care unit (ICU) setting (4, 5). In earlier studies with severely ill non-ICU SSA patients, we found that acute infection in this setting is likely to lead to underexposure to the β-lactams ceftriaxone and benzylpenicillin (6, 7). The specific aims of the current study were to describe the population PK (PPK) of gentamicin in an adult non-ICU SSA hospital population, identify sources of PK parameter variability, and assess the probability of PD target attainment (PTA) of gentamicin for the treatment of infections caused by Enterobacteriaceae.
From October 2014 to November 2015, we performed a prospective, observational PPK study of intravenously administered gentamicin among patients aged ≥18 years admitted to the Beira Central Hospital medicine ward (HCB) in Mozambique. The patient population was described previously (6, 7). The study was approved by the Mozambican National Committee for Bioethics in Health (118/CNBS/2013). Participants gave written informed consent. Those unable to read, write, and/or understand Portuguese gave a thumbprint, and an impartial literate witness observed the entire informed-consent process and subsequently cosigned the informed-consent form.
Baseline characteristics and gentamicin dosing information were captured. Any gentamicin dosing regimen prescribed by an HCB physician was accepted. Gentamicin 40-mg/ml solution for injection (Nitin Lifesciences Ltd., Haryana, India) was intravenously administered through bolus injection via a venous catheter in half a minute, according to the physician’s prescription. Gentamicin concentration sampling times were predose, 30 to 120 min after intravenous administration, and two random time points during the dosing interval. One sample was used to measure biochemical markers. Creatinine clearance (CLCR) was estimated using the Cockcroft and Gault formula (8). EDTA-anticoagulated blood samples were refrigerated immediately after collection until laboratory processing within 2 h of collection and stored at −80°C until shipment on dry ice to the Netherlands for analysis.
Total gentamicin concentrations were measured using a validated high-performance liquid chromatography-mass spectrometry assay (LC30 UPLC, Shimadzu, Kyoto, Japan; Qtrap 5500 system, Sciex, Framingham, MA). The lower limit of quantification (LLQ) was 0.3 mg/liter and the higher LLQ was 50 mg/liter. Higher concentrations were diluted and reanalyzed.
The PPK analysis was performed using the nonlinear mixed-effects modeling software package NONMEM (7.1.2; Icon Development Solutions, Ellicott City, MD). Gentamicin concentration data that were either not in line with previous concentrations of the same patient (e.g., near LLQ within 2 h after dosing, where previous administrations resulted in concentrations of >3 mg/liter in around the same time span) or not in line with a patient’s estimated CLCR (e.g., near LLQ within 3 h after dosing, with a low CLCR) were removed from analysis because they were considered to have resulted from erroneous drug administration and/or data capture. Model building was performed using a stepwise approach (for a full explanation, see Methods in the supplemental material). In brief, first, a structural compartmental PPK model was developed in which the PK of gentamicin was described, including the between-patient variability (BPV) of the PK parameter estimates of V and CL. The so-called M3 method was used to handle concentrations below the LLQ (9). In the second step, an effort was made to explain the BPV by building a covariate model in which patient demographics and biochemical markers were tested for their associations with the estimated PK parameters. Improvement of the model by adding a parameter or by introducing a correlation between a covariate and a PK parameter was evaluated using the likelihood ratio test, in which the difference between the minimum objective-function value generated by NONMEM for two hierarchical models was determined. Model performance was also evaluated by visual inspection of diagnostic goodness-of-fit plots (10). In the last step, the robustness and validity of the model resulting from the second step were tested using bootstrap and visual predictive check (VPC) analyses. Using the final model, gentamicin concentration versus time profiles were generated for 1,000 virtual patients by Monte Carlo simulation of the following five dosing regimens: 1.5 mg/kg every 8 h (q8h), which is a recommended regimen according to Mozambique’s 2007 national formulary (11); 4 mg/kg once daily (q24h), commonly recommended for non-ICU patients; and 5, 6, and 7 mg/kg q24h, commonly recommended as a starting regimen for septic ICU patients. Gentamicin peak (Cmax) and trough (Cmin) concentrations were evaluated, with Cmax defined as the predicted gentamicin concentration 0.5 min after bolus administration of a gentamicin dose and Cmin as the predicted gentamicin concentration 8 or 24 h after bolus administration for the q8h- and q24h-dosing regimens, respectively. Based on these data, the PTA, i.e., the percentage of patients with a Cmax/MIC of ≥8, was calculated (12–14) The choices of target MICs were based on EUCAST clinical breakpoint tables for susceptibility to gentamicin of Enterobacteriaceae (MIC clinical susceptibility breakpoint, 2 mg/liter) and on regional reports on antimicrobial susceptibility of Enterobacteriaceae obtained from clinical specimens (15–18). The gentamicin Cmin was considered adequate for reducing the risk for nephro- and ototoxicity when <1.0 mg/liter (14).
Forty-eight participants yielded 141 gentamicin concentrations (see Fig. S1 in the supplemental material). Patient characteristics and dosing schedules are presented in Table 1. A total of 47 gentamicin samples (33.3%) had concentrations below the LLQ. Twenty samples were removed because they were considered to have resulted from erroneous drug administration and/or data capturing. A one-compartment model best fitted the data, and the estimated BPVs for CL and V were 91% and 44%, respectively. Residual variabilities were estimated to be 31% and 0.095 mg/liter, respectively. Parameter estimates from the structural model are summarized in Table 2. The covariate analysis yielded one significant association between gentamicin CL and CLCR (see Fig. S2 in the supplemental material). Incorporation of this linear association in the structural model explained 19% of the BPV. Yet, a substantial part (74%) of the BPV in CL remained unexplained. The final model had an adequate fit (Fig. 1). The bootstrap estimates were similar to the estimates from the structural and final models (Table 2).
TABLE 1.
Baseline characteristics and dosing schedule of study population
| Characteristica | Median baseline value (range)b |
|---|---|
| Female sex (n [%]) | 24 (49) |
| Age (years) | 40 (20–86) |
| Body weight (kg) | 51 (33–76) |
| Body mass index (kg/m2) | 19.2 (10.4–29.0) |
| Hemoglobin (g/dl) | 10.0 (6.7–14.6) |
| Albumin (g/liter) | 29 (13–40) |
| GGT (U/liter) | 40 (11–372) |
| ALT (U/liter) | 16 (4–116) |
| AST (U/liter) | 33 (12–258) |
| Creatinine (μmol/liter) | 76 (37–1192) |
| CLCR (ml/min)c | 74 (4–144) |
| Gentamicin dosing regimen (n [%]) | |
| q24h; dose range, 80–240 mg/kg | 29 (60.4) |
| q12h; dose range, 80–240 mg/kg | 16 (33.3) |
| q8h; dose range, 80–160 mg/kg | 3 (6.3) |
GGT, γ-glutamyl transferase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Results expressed as median (range) unless specified otherwise. n = 48.
Estimated with the Cockcroft and Gault equation (8).
TABLE 2.
Parameter estimates
| Parametera | Structural model |
Final model |
||
|---|---|---|---|---|
| Estimate | Bootstrap estimate (95% CI)b | Estimate | Bootstrap estimate (95% CI)b | |
| CL (liter/h) | 5.5 | 5.5 (4.0–7.0) | 5.7 | 5.7 (5.2–6.2) |
| V (liter) | 20 | 21 (16–24) | 19 | 20 (18–21) |
| BPV | ||||
| CL (%CV) | 91 | 90 (49–130) | 74 | 70 (58–89) |
| V (%CV) | 44 | 47 (2.2–66) | 49 | 48 (38–59) |
| Correlation between CL and V (%) | 35 | 31 (10–56) | 46 | 40 (11–62) |
| Residual variability | ||||
| Proportional error (%) | 31 | 31 (22–41) | 32 | 32 (28–37) |
| Additive error (mg/liter) | 0.095 | 0.072 (0.035–0.16) | 0.056 | 0.055 (0.023–0.089) |
| Covariate effect | ||||
| CLCR on CL | 0.0091 | 0.0093 (0.0077–0.010) | ||
BPV, between-patient variability; CL, clearance; V, volume of distribution; CV, coefficient of variation; CLCR, creatinine clearance; CI, confidence interval.
Minimization was successful for both the structural and the final models, but it failed for the covariance step, yielding no estimates for the relative standard errors of the parameter estimates. Instead, a bootstrap with 1,000 replicates was done for the structural and final models to obtain 95% CIs of the parameter estimates. The shrinkages in the final model in BPV for CL and V were 8% and 29%, respectively.
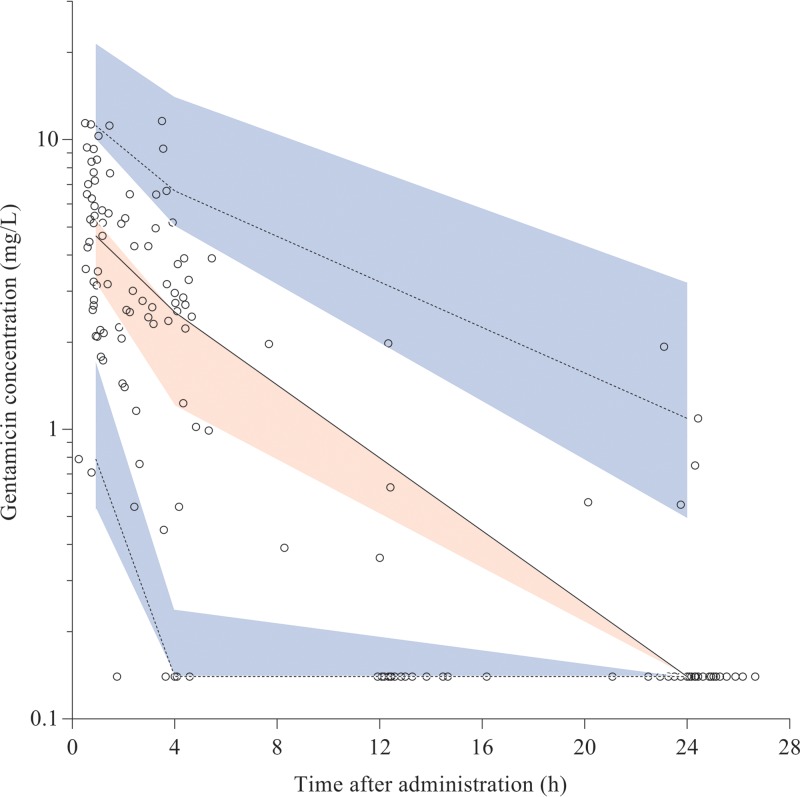
FIG 1.
Observed gentamicin concentration-time data and visual predictive check (VPC) of the final model. Open circles are observed concentrations. Solid line is observed median and dashed lines are 5th and 95th percentiles of the observed data. Red shaded area is the 95% confidence interval (CI) of the model-predicted median; blue shaded areas are the 95% CI of the model-predicted 5th and 95th percentiles. In preparing the plot, the observed and simulated concentrations below the LLQ were set to 0.14 mg/liter (0.5 × LLQ) to promote visual inspection of the figure. Solid and dashed lines run within their respective shaded areas, thereby demonstrating adequate fit of the model.
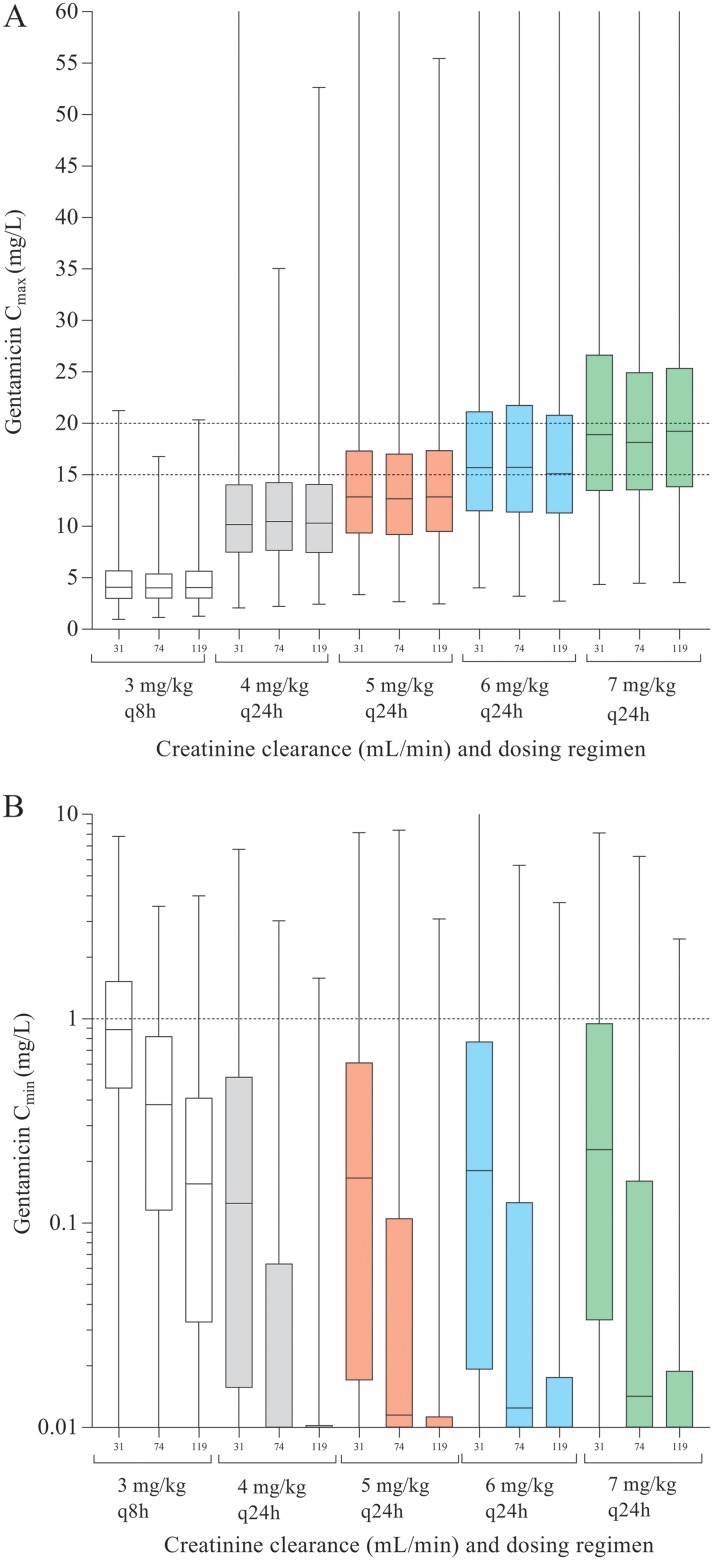
All dosing regimens were simulated by using the observed median CLCR (74 ml/min), the 10th percentile (31 ml/min), and the 90th percentile (119 ml/min). Simulations showed that the performance of the 1.5-mg/kg-q8h dosing regimen was poor, with a PTA of 7.5% for patients with the median CLCR and an infection with a susceptible pathogen with an MIC of 1.0 mg/liter, whereas 17.4% of patients were predicted to have a Cmin of ≥1.0 mg/liter after the first dose (Fig. 2 and Table S1 in the supplemental material). Use of the 4-mg/kg-q24h regimen resulted in PTAs of 71.7% and 17.9% when assuming an infection with a pathogen with MICs of 1.0 and 2.0 mg/liter, respectively. In this scenario, 14.5% of patients with a CLCR of 31 ml/min (observed 10th percentile of CLCR) were predicted to have a Cmin of ≥1.0 mg/liter after the first dose. The 7-mg/kg-q24h regimen had the highest PTAs, at 96.5% and 61.7% for pathogens with MICs of 1.0 and 2.0 mg/liter, respectively. For patients with a CLCR of 31 ml/min, 24.4% were predicted to have a Cmin of ≥1.0 mg/liter.
FIG 2.
Simulations of gentamicin peak concentrations (A) and trough concentrations (B) for 1,000 virtual patients with all median characteristics of the population but five different dosing schedules (white, 1.5 mg q8h; gray, 4 mg q24h; red, 5 mg q24h; blue, 6 mg q24h; green, 7 mg q24h) and three CLCR levels (10th percentile, 31 ml/min; median, 74 ml/min; and 90th percentile, 119 ml/min).
A one-compartment model adequately described gentamicin PK, and comparable to what can be found in septic ICU patients, the estimated V and CL in this study's non-ICU population were high, as were the BPVs of these PK parameters (19, 20). Once-daily gentamicin dosing with the commonly recommended initial regimen for non-ICU patients in the SSA setting is likely to lead to insufficient peak concentrations. Higher once-daily dosing regimens of gentamicin, such as those routinely recommended for septic ICU patients, seem to have higher PTAs, but this comes with a substantial risk of toxic through levels, especially for patients with impaired renal function. In the absence of a therapeutic drug-monitoring infrastructure, gentamicin may therefore not be a rational antibiotic choice for severely ill populations in SSA hospital settings.
Supplementary Material
ACKNOWLEDGMENTS
In Mozambique: we thank the study participants for their participation and trust. We thank Ivete Meque and Kajal Chhaganlal, subsequent FCS-UCM Research Centre for Infectious Diseases (CIDI) managers, and CIDI support staff for their share in training, finances, and daily transport of blood samples. We thank Janneke van de Wijgert, University of Liverpool, for advice with regard to local contracts and agreements, and Susan Mason, U.S. Military HIV Research Program (MHRP), for programming the CIDI laboratory’s study database. We are grateful to the executive board of the HCB and the late Carlos de Oliveira, internist and former head of the HCB medicine department, for making research office space available on the HCB wards, and to the entire nursing staff from the HCB medicine wards for their collaboration. In the Netherlands: we thank Femke Schrauwen, trial coordinator of the AMC biochemistry laboratory, and Marloes van der Meer and Marcel Pistorius, research analysts of the AMC pharmacology laboratory, for input in the interpretation of biochemistry and gentamicin concentration results.
This work was internally funded by AMC. It was indirectly supported by the Gilead Foundation (IA 356007) and a Dutch private donor who wants to stay anonymous (CA 356001) but whose professional activities do not create a conflict of interest. Both funding parties supported the local presence of J.C.B. in Mozambique in the context of a long-running medical educational capacity building project with the Faculty of Health Sciences of the Catholic University of Mozambique (FCS-UCM).
R.V.H. received personal fees from Nordic Pharma. We have no conflicts of interest to declare.
J.C.B., R.M., and J.P. designed the study. J.C.B. performed the literature search and obtained ethical approval. J.C.B., M.M., G.N., and M.D. implemented the study, and J.C.B. supervised data collection and study progress on a daily basis. J.C.B. and R.V.H. analyzed the data and drafted the manuscript. J.P. and R.M. critically examined the analysis and findings. We all critically read and commented on draft versions of the report and approved the final version.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02328-18.
REFERENCES
- 1.Ford N, Shubber Z, Meintjes G, Grinsztejn B, Eholie S, Mills EJ, Davies MA, Vitoria M, Penazzato M, Nsanzimana S, Frigati L, O'Brien D, Ellman T, Ajose O, Calmy A, Doherty M. 2015. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV 2:e438–e444. doi: 10.1016/S2352-3018(15)00137-X. [DOI] [PubMed] [Google Scholar]
- 2.Meintjes G, Kerkhoff AD, Burton R, Schutz C, Boulle A, Van Wyk G, Blumenthal L, Nicol MP, Lawn SD. 2015. HIV-related medical admissions to a South African district hospital remain frequent despite effective antiretroviral therapy scale-up. Medicine (Baltimore) 94:e2269. doi: 10.1097/MD.0000000000002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huson MAM, Kalkman R, Stolp SM, Janssen S, Alabi AS, Beyeme JO, van der Poll T, Grobusch MP. 2015. The impact of HIV on presentation and outcome of bacterial sepsis and other causes of acute febrile illness in Gabon. Infection 43:443–451. doi: 10.1007/s15010-015-0753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jager NG, van Hest RM, Lipman J, Taccone FS, Roberts JA. 2016. Therapeutic drug monitoring of anti-infective agents in critically ill patients. Expert Rev Clin Pharmacol 9:961–979. doi: 10.1586/17512433.2016.1172209. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL. 2014. Individualized antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bos JC, Prins JM, Mistício MC, Nunguiane G, Lang CN, Beirão JC, Mathôt RAA, van Hest RM. 2018. Pharmacokinetics and pharmacodynamics target attainment of ceftriaxone in adult severely ill sub-Saharan African patients: a population pharmacokinetic modelling study. J Antimicrob Chemother 73:1620–1629. doi: 10.1093/jac/dky071. [DOI] [PubMed] [Google Scholar]
- 7.Bos JC, van Hest RM, Mistício MC, Nunguiane G, Lang CN, Beirão JC, Mathôt RAA, Prins JM. 2018. Pharmacokinetics and pharmacodynamics target attainment of benzylpenicillin in an adult sub-Saharan African patient population. Clin Infect Dis 66:1261–1269. doi: 10.1093/cid/cix961. [DOI] [PubMed] [Google Scholar]
- 8.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nehphron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 9.Ahn JE, Karlsson MO, Dunne A, Ludden TM. 2008. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn 35:401–421. doi: 10.1007/s10928-008-9094-4. [DOI] [PubMed] [Google Scholar]
- 10.Ette EI, Ludden TM. 1995. Population pharmacokinetic modelling: the importance of informative graphics. Pharm Res 12:1845–1855. doi: 10.1023/A:1016215116835. [DOI] [PubMed] [Google Scholar]
- 11.Ministério da Saúde da Republica de Moçambique. 2007. Formulário nacional de medicamentos. 5a ed. http://apps.who.int/medicinedocs/en/d/Js19267pt/.
- 12.Moore RD, Lietman PS, Smith CR. 1987. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 13.Kashuba AD, Nafziger AN, Drusano GL, Bertino JS Jr.. 1999. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by Gram-negative bacteria. Antimicrob Agents Chemother 43:623–629. doi: 10.1128/AAC.43.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NVZA. 2018. TDM monografieën. http://tdm-monografie.org/monografie/gentamicine.
- 15.European Committee on Antimicrobial Susceptibility Testing. 2018. Breakpoint tables for interpretation of MICs and zone diameters, version 8.1. http://www.eucast.or/clinical_breakpoints/.
- 16.Van der Meeren BT, Chhaganlal KD, Pfeiffer A, Gomez E, Ferro JJ, Hilbink M, Macome C, van der Vondervoort FJ, Steidel K, Wever PC. 2013. Extremely high prevalences of multi-resistance among uropathogens from hospitalized children in Beira, Mozambique. S Afr Med J 103:382–386. doi: 10.7196/samj.5941. [DOI] [PubMed] [Google Scholar]
- 17.Tuem KB, Gebre AK, Atey TM, Bitew H, Yimer EM, Berhe DF. 2018. Drug resistance of Escherichia coli in Ethiopia: a meta-analysis. Biomed Res Int 2018:1. doi: 10.1155/2018/4536905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrol M, Rangaiahagari A, Musabeyezu E, Singer D, Ogbuagu O. 2016. Five-year antimicrobial susceptibility trends among bacterial isolates from a tertiary health-care facility in Kigali, Rwanda. Am J Trop Med Hyg 95:1277–1283. doi: 10.4269/ajtmh.16-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llanos-Paez CC, Hennig S, Staatz CE. 2017. Population pharmacokinetic modeling, Monte Carlo simulation and semi-mechanistic pharmacodynamics modeling as tools to personalize gentamicin therapy. J Antimicrob Chemother 72:639–667. doi: 10.1093/jac/dkw461. [DOI] [PubMed] [Google Scholar]
- 20.Hodiamont CJ, Juffermans NP, Bouman CS, de Jong MD, Mathôt RA, van Hest RM. 2017. Determinants of gentamicin concentrations in critically ill patients: a population pharmacokinetic analysis. Int J Antimicrob Agents 49:2014–2011. doi: 10.1016/j.ijantimicag.2016.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.