Lantibiotics present an attractive scaffold for the development of novel antibiotics. We report here a novel lantibiotic for the treatment of Clostridium difficile infection.
KEYWORDS: antibiotic, antibiotic resistance, antimicrobial peptide, bacteriocin, bioavailability, lanthipeptide, lantibiotic, mutacin, mutagenesis, nisin
ABSTRACT
Lantibiotics present an attractive scaffold for the development of novel antibiotics. We report here a novel lantibiotic for the treatment of Clostridium difficile infection. The lead compounds were selected from a library of over 700 single- and multiple-substitution variants of the lantibiotic mutacin 1140 (MU1140). The best performers in vitro and in vivo were further used to challenge Golden Syrian hamsters orally in a Golden Syrian hamster model of Clostridium difficile-associated disease (CDAD) in a dose-response format, resulting in the selection of OG716 as the lead compound. This lantibiotic was characterized by a 50% effective dose of 23.85 mg/kg of body weight/day (10.97 μmol/kg/day) in this model. Upon oral administration of the maximum feasible dose (≥1,918 mg/kg/day), no observable toxicities or side effects were noted, and no effect on intestinal motility was observed. Compartmentalization to the gastrointestinal tract was confirmed. MU1140-derived variants offer a large pipeline for the development of novel antibiotics for the treatment of several indications and are particularly attractive considering their novel mechanism of action. Based on the currently available data, OG716 has an acceptable profile for further development for the treatment of CDAD.
INTRODUCTION
The prototype lantibiotic nisin was described in 1928 (the same year that penicillin was described) and has been primarily used as a food preservative (1, 2). Structurally, all lantibiotics are polycyclic lanthipeptides with antimicrobial properties (3). As shown in Fig. 1, mutacin 1140 (MU1140) and its variants derive their names from the thioether ring containing amino acids lanthionine (Lan; Ala–S-Ala) and/or 3-methyl-lanthionine (MeLan; aminobutyric acid–S-Ala). As reviewed elsewhere, lantibiotics often incorporate posttranslationally modified amino acids, such as 2,3-didehydroalanine (Dha), 2,3-didehydrobutyrine (Dhb), and the unsaturated lanthionine derivatives aminovinyl-d-cysteine (AviCys), at their C termini (3–5). Several lantibiotics may be efficacious and have been shown to be tolerated as human therapeutic agents, including NVB-333 (which is in preclinical testing) NVB-302 (which has completed a phase I trial) and duramycin (which has completed a phase II trial for use in patients with cystic fibrosis) (6–9).
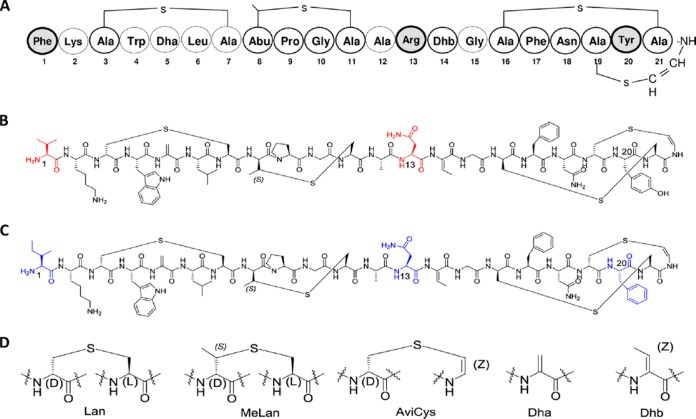
FIG 1.
Structure of MU1140 and analogs. (A) Primary amino acid sequence of MU1140. The residues of MU1140 modified in its variants are highlighted in gray. (B) Chemical structure of OG716 (F1V-R13N). Residue modifications are highlighted in red. (C) Chemical structure of OG718 (F1I-R13N-Y20F). Residue modifications are highlighted in blue. (D) Structure of unusual amino acids. Lan, lanthionine; MeLan, methyl-lanthionine; AviCys, aminovinyl-d-cysteine; Dha, 2,3-didehydroalanine; Dhb, 2,3-didehydrobutyrine.
It is striking that nisin has been used in the food industry for over 60 years and in over 50 countries with the limited development of resistance. One of the most clinically relevant characteristics of nisin, MU1140, and related compounds is their mechanism of action: lipid II abduction (10, 11). This novel mechanism of action is thought to lead to less resistance development due to the nature of the pyrophosphate moiety of its target, lipid II, an essential constituent of cell wall synthesis (10, 12). It is well-known that vancomycin also targets lipid II but does so at the terminal part of the pentapeptide (13). Bacteria can develop a mutated lipid II pentapeptide in response to selective pressure in the presence of vancomycin. The best-characterized example of this phenomenon is the acquisition of vanA by vancomycin-resistant enterococci (VREs). VRE strains that acquire vanA replace the d-Ala–d-Ala moiety of lipid II with a d-Ala–d-lactate moiety (14). In contrast, several lantibiotics bind to the pyrophosphate moiety of lipid II, which is thought to confer a much lower frequency of resistance development because bacteria are less likely to develop mutations of the nonproteinaceous pyrophosphate binding site.
While mutacin 1140 (MU1140) is characterized by an excellent overall therapeutic profile (5, 12, 15), it suffers from limitations that impede its drugability and further clinical development. Building on the lessons learned from previous structure-function studies, key amino acid substitutions were pyramided into compounds that were further characterized in vitro and in vivo (16–18). Thirty-four years after the initial discovery of MU1140 by Hillman’s group (19) and after the screening of over 700 compounds engineered with single and multiple (up to eight) amino acid substitutions in MU1140, two compounds with improved physicochemical, pharmacological, and therapeutic properties, OG716 and OG718, targeting Clostridium difficile in C. difficile-associated disease (CDAD) (Fig. 1) emerged (16, 17, 18). This infectious agent continues to be an urgent threat in the hospital setting, according to the CDC (20). The incidence rate of Clostridium difficile infection (CDI) varies markedly from country to country, with many countries detecting increasing incidence rates above the current high rates of CDI (21). New therapies that range from antibodies to antibiotics and from vaccines to fecal transplants are becoming available (22, 23). Unfortunately, the high cost of some of those new therapies may limit broad utilization (24) and create opportunities for cost-effective alternatives.
In this report, we further characterize the therapeutic profile of OG716 and OG718 using a dose-response format of CDAD infection in the Golden Syrian hamster model. The pharmacological and toxicological properties of the lead compound OG716 were further characterized by performing a maximum tolerated dose (MTD) assay in Wistar Han rats. In addition, the systemic bioavailability in and the compartmentalization of OG716 to the gastrointestinal (GI) tract when administered orally at the maximum feasible dose were determined.
RESULTS
Characterization of test compounds.
Lyophilized OG716 and OG718 were produced as acetate salts of lanthipeptides, as previously described (17, 18). The purity and identity of the two compounds were characterized and confirmed using several analytical methods, including high-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS). The purities of the two compounds were >90% by HPLC, and the identity of the product peak was confirmed by LC-MS for both molecules (data not shown). Elemental analysis was used to calculate the net peptide contents from lyophilized peptide powder. Both peptides contained 84% ± 1% of the net peptide content by mass, determined following a method modified from that of Vemuri (25) (data not shown). Proton (1H) nuclear magnetic resonance (NMR) was used to calculate the amount of acetate counterion, and it was found that OG716 and OG718 had 2.7 eq and 1.9 eq of acetate counterion, respectively (data not shown).
Efficacy dose range finding.
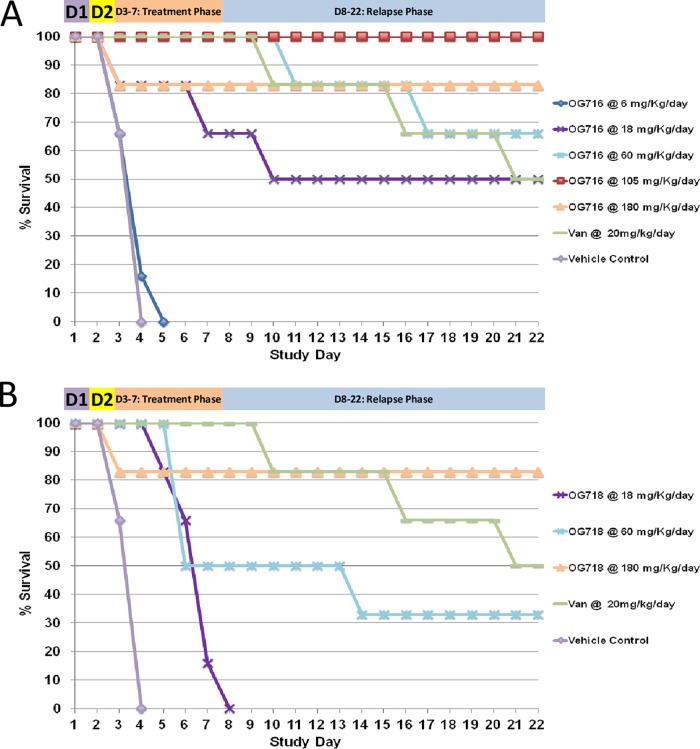
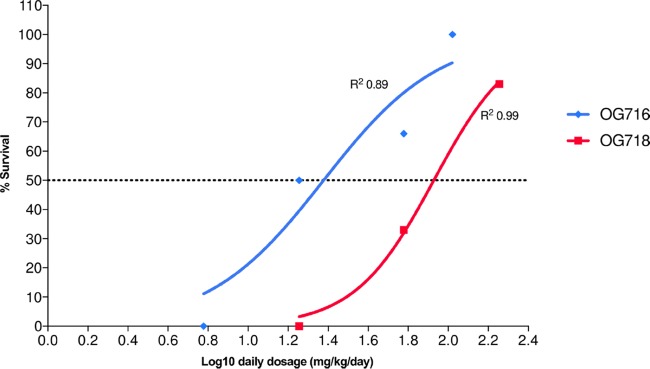
The experimental scheme used to investigate the dose-response of both drugs under investigation in the hamster C. difficile infection model is presented in Table 1, and the resulting data are shown in Fig. 2. All untreated animals (negative-control animals treated with vehicle only) were dead by day 4. Analysis of the survival results revealed that most of the treatment regimens enhanced the survival of infected animals (according to the overall survival or time to mortality) compared to that of the animals in the vehicle control group. The survival of animals treated with OG716 at a dose of 6 mg/kg of body weight/day was not statistically significantly different from that of the vehicle controls, and although animals treated with OG718 at an 18-mg/kg/day dose exhibited 0% survival by day 8, OG718 provided a significant delay in the observed mortality compared to that for the controls. All other active treatment regimens were statistically significantly more effective than the vehicle control regimen (P ≤ 0.01). The 50% effective doses (ED50) of OG716 and OG718 were calculated from the in vivo dose-response curve presented in Fig. 3. OG716 presented with an ED50 of 23.85 mg/kg/day, while the ED50 of OG718 was 84.72 mg/kg/day. Comparative analysis of the ED50 values determined that OG716 was significantly more efficacious than OG718 in the hamster C. difficile infection model (P = 0.0423). The 20 mg/kg/day (13.80 μmol/kg/day) of vancomycin chosen as a positive control for this study resulted in 50% survival (Fig. 2).
TABLE 1.
Dose ranging study design in the Syrian hamster model of CDADa
| Group | Test article | Regimen | Dose (mg/kg/dose) | Daily dose (mg/kg/day) |
|---|---|---|---|---|
| 1 | OG716 | TID for 5 days | 2 | 6 |
| 2 | OG716 | TID for 5 days | 6 | 18 |
| 3 | OG716 | TID for 5 days | 20 | 60 |
| 4 | OG716 | TID for 5 days | 35 | 105 |
| 5 | OG716 | TID for 5 days | 60 | 180 |
| 6 | OG718 | TID for 5 days | 6 | 18 |
| 7 | OG718 | TID for 5 days | 20 | 60 |
| 8 | OG718 | TID for 5 days | 60 | 180 |
| 9 | Vancomycin | QD for 5 days | 20 | 20 |
| 10 | Infection (vehicle) control | TID for 5 days | NA | NA |
All regimens were administered by oral gavage, and each group contained six mice. NA, not applicable.
FIG 2.
Survival plots of hamsters in the dose ranging study. (A and B) Survival data for hamsters treated with OG716 (A) and OG718 (B). Golden Syrian hamsters (n = 6 per group) were infected on day 1 (D1) and received a single subcutaneous injection of clindamycin (10 mg/kg) on day 2. Test compounds at various doses (6 to 180 mg/kg/day) in 5% d-mannitol were administered by oral gavage 3 times per day (TID), starting on day 2 at 18 h after clindamycin treatment, for 5 consecutive days (days 2 through 6). Vancomycin (the positive control) was administered at 20 mg/kg/day (QD) in parallel, and the infection control group was dosed with vehicle alone. See Materials and Methods for details. Van, vancomycin.
FIG 3.
Calculation of the 50% effective dose (ED50). Representation of the survival rate against the log10(daily dose). ED50 values were calculated with the variable slope model (y = 100/{1 + 10[(log10 EC50 − x)·Hill slope]}, where y values are % survival and x values are log10 daily dosage in mg/kg/day) and were compared for statistical significance using the extra sum-of-squares F test (P < 0.05).
Cecum-associated CFU count and toxin titers.
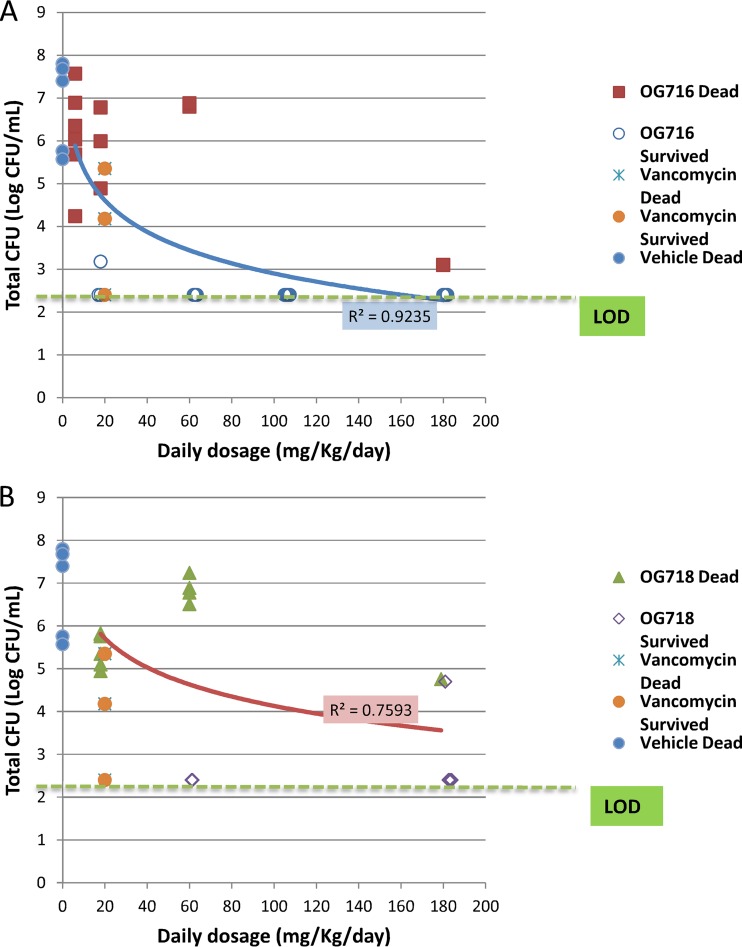
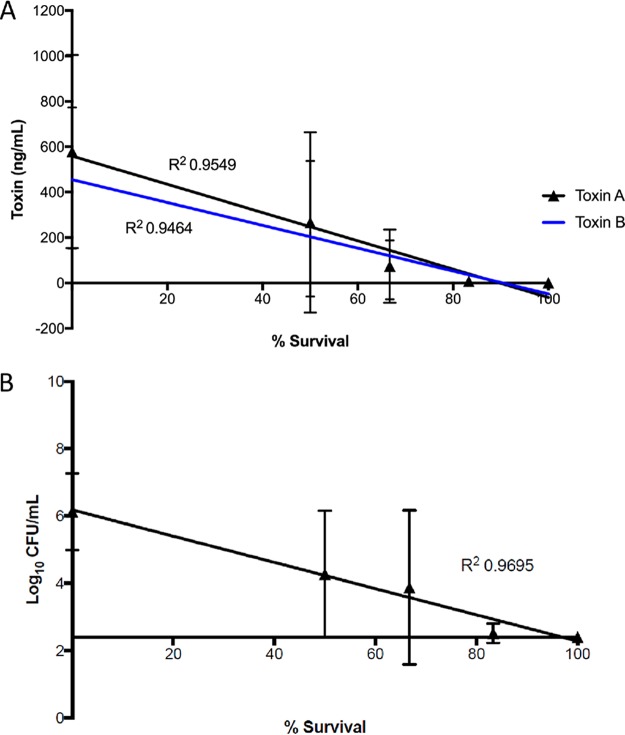
The results for the C. difficile CFU levels (including the levels of both the vegetative and the spore forms) found in cecal samples from treated animals are presented in Fig. 4 and Table 2, and the results of intragroup analysis are presented in Table S1 in the supplemental material. The mean cecal CFU count for the vehicle control group was 6.66 log10 CFU/ml. Animals treated with OG716 at 105 mg/kg/day demonstrated 100% survival and had CFU counts below the limit of detection (2.40 log10 CFU/ml). All other groups, which showed rates of survival of from 33 to 83%, exhibited CFU counts ranging from 2.51 to 5.37 log10 CFU/ml, and these counts paralleled the clinical signs. Additionally, the groups that were administered the top two doses of OG716 (105 and 180 mg/kg/day) had significantly lower mean CFU counts in their cecal contents than the mean CFU counts associated with the cecal contents of the vehicle control group. The strength of the linear relatedness between cecum-associated CFU titers and the survival of groups treated with OG716 was evaluated using the Pearson correlation coefficient or Pearson’s r test. The log10 CFU counts per milliliter of cecal fluid taken from the OG716-treated groups were significantly related to the survival of these groups (P < 0.05; n = 6), with an R squared value of 0.97.
FIG 4.
Distribution of total C. difficile counts based on dose group versus surviving or dead animals. The cecal contents from all hamsters that died on study or from hamsters euthanized at the end of the observation period (day 21; Fig. 2) were collected and tested for total cecum-associated CFU counts (see the Materials and Methods section for details). The limit of detection (LOD) was 2.40 log10 CFU/ml. Blue and red curves, logarithmic regression of the OG716 mean (A) and OG718 mean (B), respectively.
TABLE 2.
Mean cecum-associated toxin A and B and C. difficile titers per treatment
| Lantibiotic and treatment (no. of hamsters)c | Toxin A |
Toxin B |
Cecal content |
|||
|---|---|---|---|---|---|---|
| Mean titer (ng/ml of cecal contents) | P valuea | Mean titer (ng/ml of cecal contents) | P value (toxin B) | Mean ± SD no. of log10 CFU/ml | P value | |
| OG716 | ||||||
| 6 mg/kg q8h for 5 days (n = 6) | 578.5 | >0.999 | 462.7 | >0.999 | 6.13 | >0.999 |
| 18 mg/kg q8h for 5 days (n = 6) | 266.2 | 0.224 | 239.1 | 0.613 | 4.27 | >0.999 |
| 60 mg/kg q8h for 5 days (n = 6) | 74.2 | 0.011b | 57.8 | 0.026b | 3.88 | 0.789 |
| 105 mg/kg q8h for 5 days (n = 6) | <0.8 | 0.0003b | <0.8 | 0.0009b | <2.4 | 0.008b |
| 180 mg/kg q8h for 5 days (n = 6) | 7.3 | 0.001b | 5.5 | 0.043b | 2.51 | 0.02b |
| OG718 | ||||||
| 18 mg/kg q8h for 5 days (n = 6) | 215.5 | >0.999 | 242.2 | >0.999 | 5.47 | >0.999 |
| 60 mg/kg q8h for 5 days (n = 6) | 165.5 | 0.292 | 272.3 | >0.999 | 5.37 | >0.999 |
| 180 mg/kg q8h for 5 days (n = 6) | 47.6 | 0.009b | 108.7 | 0.069 | 3.18 | 0.075 |
| Vancomycin, 20 mg/kg q24h for 5 days (n = 6) | 292.6 | 0.292 | 71.1 | 0.323 | 5.15 | >0.999 |
| Vehicle (control), q8h for 5 days (n = 6) | 2,857.1 | 1,852.8 | 6.66 | |||
P values were generated by comparing the mean cecum-associated toxin A and B and CFU titers in the OG716-, OG718-, and vancomycin-treated groups to those in the vehicle-treated control group (Kruskal-Wallis with Dunn’s multiple-comparison test). The limits of detection (LOD) were 0.8 ng/ml for the toxin assay and 2.4 log10 CFU/ml for the C. difficile counts.
The mean value is significantly different from the mean value for the vehicle control (P < 0.05).
q8h, every 8 h; q24h, every 24 h.
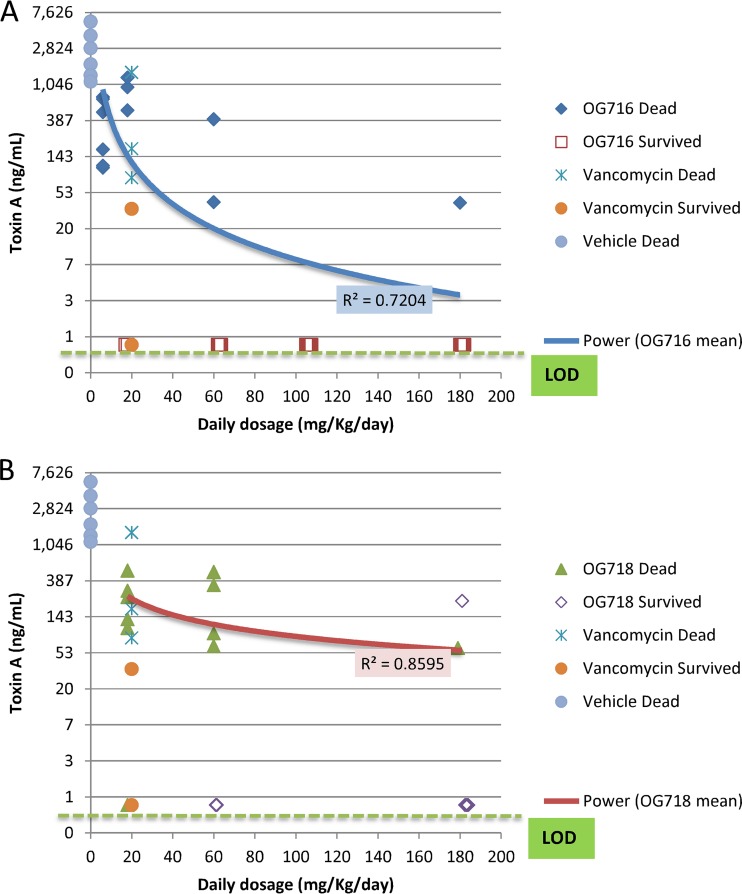
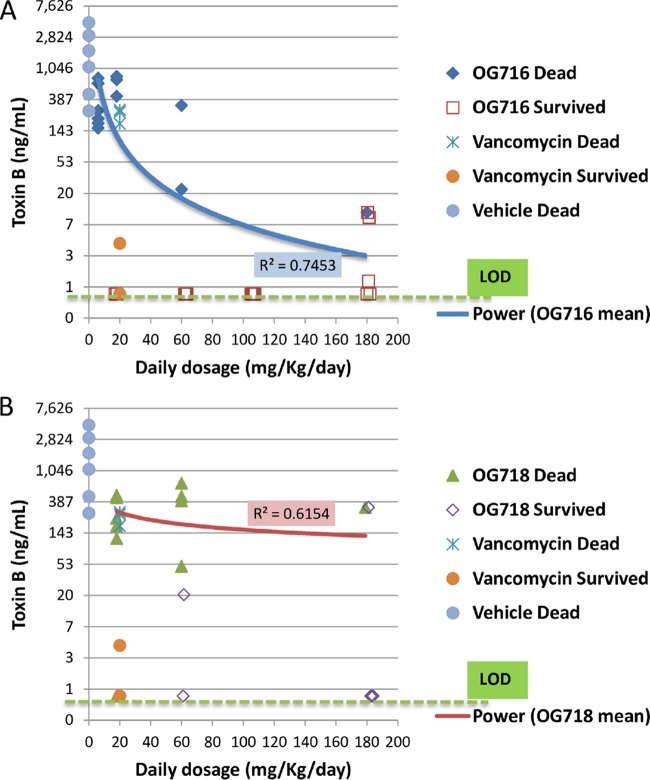
The toxin titers determined in matching cecal samples are presented in Fig. 5 and 6 and Table 2, and the results of the intragroup analysis are presented in Tables S1 and S2. The levels of toxins A and B in the cecal contents tracked with both the measured CFU counts and survival during the study and normalized per milliliter of cecal fluid with limits of detection for the toxin assay as 8 ng/ml and for the C. difficile counts as 2.4 log10 CFU/ml. All of the groups treated with OG716, OG718, or vancomycin had 4- to 3,000-fold lower mean toxin A and B concentrations in their cecal contents than the mean toxin titers in the vehicle-treated group. However, only the groups administered the top three doses of OG716 (60, 105, and 180 mg/kg/day) had mean toxin A and B titers significantly lower than those in the vehicle control group. The highest dose of OG718 (180 mg/kg/day) resulted in a mean toxin A titer significantly lower than that in the vehicle control group, but the mean toxin B titers were determined not to be significantly different with the same comparison. Similarly, vancomycin treatment resulted in cecum-associated toxin concentrations lower than those in the vehicle control group, but those differences were not significant. The total cecum-associated CFU count results from the study were comparable to the toxin results, in that the groups administered the top two doses of OG716 (105 and 180 mg/kg) had significantly lower mean CFU counts in their cecal contents than the mean CFU counts associated with the cecal contents of the vehicle control group. More specifically, the mean toxin A and B levels for animals that died on study were 867 and 650 ng/ml, respectively. The titers in surviving animals were ≤0.8 ng/ml for both toxins. With the exception of one hamster in group 8 of the study (hamster 10495), animals that survived 21 days after infection had toxin A and B titers near or below the limit of detection (≤0.8 ng/ml for both toxins). Even though animal 10495 survived the full 21 days after infection, it had cecal fluid-associated toxin A and B titers of 222 ng/ml and 325 ng/ml, respectively, and a C. difficile count of 4.7 log10 CFU/ml of cecal fluid. As with the CFU data, the Pearson correlation coefficient or Pearson’s r test was used to determine the strength of the linear relatedness between cecum-associated toxin titers and the survival of groups treated with OG716, which were found to be significantly related (P < 0.05; n = 6), with R squared values of 0.95 for both analyses (Fig. 7).
FIG 5.
Distribution of toxin A based on dose group versus surviving or dead animals. The cecal contents from all hamsters that died on study or from hamsters euthanized at the end of the observation period (day 21; Fig. 2) were collected and tested for C. difficile toxin A analysis (see the Materials and Methods section for details). The limit of detection (LOD) was 0.8 ng/ml. Blue and red curves, power regression of the OG716 mean (A) and the OG718 mean (B), respectively.
FIG 6.
Distribution of toxin B based on dose group versus surviving or dead animals. The cecal contents from all hamsters that died on study or from hamsters euthanized at the end of the observation period (day 21; Fig. 2) were collected and tested for C. difficile toxin B analysis (see the Materials and Methods section for details). The limit of detection (LOD) was 0.8 ng/ml. Blue and red curves, power regression of OG716 mean (A) and OG718 mean (B), respectively.
FIG 7.
Linear relationships of survival and postmortem levels of C. difficile and toxins for OG716-treated animals. Both cecal content-associated mean toxin A and B titers (A) and mean C. difficile CFU counts (B) were significantly related to the survival outcome of each OG716-treated group, as determined by the Pearson correlation coefficient or Pearson’s r test (P < 0.05; n = 6).
Altogether, these results suggest that even though other factors, like gut microbiome dysbiosis and recovery, may affect overall survival in the hamster C. difficile infection model, the survival of animals treated with OG716 in this study was significantly impacted by the amounts of toxins and C. difficile in the ceca of infected hamsters at the time of death. Therefore, the efficacy of OG716 in this study is represented as a dose-dependent survival of infected hamsters, which is directly correlated to the decrease in the number of cecum-associated CFU and toxin levels in animals treated with OG716. Such a correlation was not observed in OG718-treated animals. Intragroup statistical analyses were performed, and the results are included in Tables S1 to S3.
MTD, GI motility and bioavailability.
The in vivo maximum tolerated dose (MTD) and toxicology study designs are described in Table 3. There were no unanticipated deaths during the course of this study. A hunched posture was noted in one animal following administration of a single dose of OG716 at 639 mg/kg on the day of dose administration. There were no additional clinical signs noted in this animal, and there were no clinical signs noted in the remaining treated animals. No test-related changes in body weights or food consumption were noted in group 5 and 6 animals or through the study observation period (3 days).
TABLE 3.
Experimental design for MTD, GI tract motility, and bioavailability studies in Wistar Han rats
| Group no | Test material | Dose level |
Dose concn (mg/ml/dose)a | No. of animals |
||||
|---|---|---|---|---|---|---|---|---|
| GI study |
PK study |
|||||||
| mg/kg/daya | mg/kg/dose | Md | Fe | M | F | |||
| 1 | Vehicle control | 0b | 0 | 0 | 6 | |||
| 2 | Vehicle control | 0c | 0 | 0 | 6 | |||
| 3 | OG716 | 639b | 639 | 63.9 | 6 | |||
| 4 | OG716 | 1,917c | 639 | 63.9 | 6 | |||
| 5 | OG716 | 1,000c | 333.3 | 33.33 | 6 | 6 | ||
| 6 | OG716 | 1,917c | 639 | 63.9 | 6 | 6 | ||
Dose levels and concentrations are expressed as the amount of OG716 free peptide. Dose volume was based on body weights, at 10 ml/kg.
Administered once daily.
Administered three times daily (every 8 h).
M, male rats.
F, female rats.
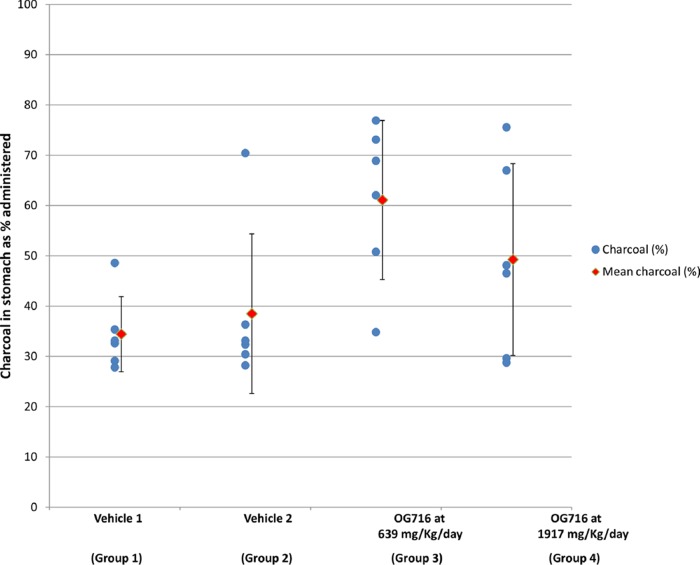
A decrease in gastric emptying was noted in group 3 animals following administration of a single dose of OG716 at 639 mg/kg as well as in group 4 animals following administration of three doses of OG716 at 639 mg/kg/dose for a total dose of 1,917 mg/kg. As presented in Fig. 8, the charcoal content in the stomach was approximately 77% greater in group 3 animals than in vehicle control group 1 animals and 30% greater in group 4 animals than in control group 2 animals. In group 3 animals, the decrease was mainly noted in 4 out of 6 animals, with 2 animals not showing any significant changes. For group 4 animals, the decrease was mainly noted in 2 out of 6 animals, with the 4 remaining animals not showing any significant changes. The length and location of the trace amounts of charcoal in the intestinal tract were comparable across groups, indicating that there were no effects on intestinal motility (data not shown).
FIG 8.
Trace of charcoal in stomach. Wistar Han rats were administered OG716 solutions/suspensions by oral gavage as a single dose once daily for 1 day for groups 1 and 3 and three times a day (every 8 h) for 1 day for groups 2 and 4. Animals were administered an activated charcoal suspension as the gastrointestinal marker, which was administered 1 h following the last dosing to the appropriate animals by oral gavage. At the end of a 20-min waiting period, the rats were euthanized, the abdominal cavity was opened, and the stomach and intestines were removed. The presence or absence of charcoal in the stomach and intestine was documented (see the Materials and Methods section for details). Blue circles, individual animals; red diamonds, mean value (standard deviation).
Based on the pharmacokinetic (PK) evaluation of plasma collected as shown in Table 4, no systemic exposure was noted following administration of OG716 at 1,000 or 1,917 mg/kg/day. The concentrations in all samples from the control and treated groups analyzed for the plasma concentration of OG716 were below the level of quantification, with the exception of 2 samples. One animal had a plasma concentration of OG716 of 10.3 ng/ml at 1 h after the second dose (9 h after the first dose of 1,000 mg/kg/day), and one animal had a plasma concentration of 13.6 ng/ml at 1 h after the first dose of 1,917 mg/kg/day (data not shown). It should be noted that the levels detected were at the bottom range of the assay precision, as the lower limit of quantification (LLOQ) was 10 ng/ml in the bioanalytical assay used in this study.
TABLE 4.
PK sample collection schedule
| Group no. | Sample collection time point postdose on day 1 |
||||
|---|---|---|---|---|---|
| 0 ha | 1 hb | 9 hb (1 h after 2nd dose) | 17 hb (1 h after 3rd dose) | 24 hb | |
| 5 | Xc | X | X | X | X |
| 6 | X | X | X | X | X |
The sample was collected before the first daily dose.
The sample was collected relative to the first daily dose (1 h after each dose).
X, sample collected.
DISCUSSION
As previously reviewed, lantibiotics are an attractive class of molecules that present interesting properties and structural features for the development of novel antibiotics that may be less susceptible to the development of microbial resistance (2, 5, 6, 8). We recently had an opportunity to build on the lessons learned from the structure-function analysis of MU1140 homologs produced by saturation mutagenesis to identify those residues that hindered the drugability of MU1140 (16). Several residues of the primary MU1140 peptide sequence can be mutated, enabling productive multiple-amino-acid substitutions that can alleviate each of MU1140’s shortcomings by improving specific physicochemical or pharmacological properties of the molecule while preserving its distinctive therapeutic characteristics (16). Using those seminal studies as blueprints for the subsequent development of the second generation of MU1140 variants, we pyramided multiple substitutions that led to several compounds with better overall pharmacokinetic (PK)/pharmacodynamic (PD) properties (18). The top performers identified in those studies combined Phe1 substitutions and Arg13 substitutions, which, taken together, enhanced the half-life of MU1140 in simulated GI fluids by substitution of amino acid residues that are inherently more stable and not prone to proteolytic degradation (18). This pharmacological improvement was engineered without negatively impacting potency, in vivo efficacy, or manufacturability (18). In the current study, we further characterized the dose-response of two of the top performers and assessed the maximum tolerated dose, the potential impact of high doses on GI motility, and the bioavailability of the lead compound, OG716.
In vitro, both OG716 and OG718 were previously shown to be very comparable in terms of their potency and basic physicochemical properties (18). In particular, the MIC range of OG716 (0.25 to 0.5 μg/ml) was not significantly different from the MIC range of OG718 (0.25 to 1 μg/ml) for a small subset of C. difficile strains tested (n = 8). Similarly, the MIC mode and MIC50 were identical for both molecules (0.5 μg/ml). The MIC90 was also very similar between OG716 (0.5 μg/ml) and OG718 (1 μg/ml). The MIC range and MIC90 of both molecules were still superior to the MIC range (0.5 to 4 μg/ml) and MIC90 (4 μg/ml) of vancomycin, the comparator used in those studies (18). The intrinsic stability of both OG716 and OG718, based on forced degradation studies performed at 37°C and 50°C, also revealed a favorable degree of stability for both compounds. The half-life of both compounds in simulated gastric fluid was identical and immeasurable (>1,440 min), while the half-life of OG718 in simulated intestinal fluid (>720 min) was longer than that of OG716 (552 min). The solubility of OG718 in a 5% d-mannitol formulation also was greater than that of OG716 under the same conditions (≥24 mg/ml versus 12 to 18 mg/ml, respectively) (18).
The in vivo efficacy of OG716 and OG718 when they were tested at a single dose (20 mg/kg, orally, three times a day [TID]) in previous studies (18) suggested that the efficacy of OG716 is better that of OG718, based on 100% survival, no relapse, and undetectable C. difficile counts and toxin titers within cecal fluid. In contrast, animals treated with OG718 presented 83% survival, with the total CFU and toxin levels correlating with the clinical outcomes (18). The difference in survival between OG716- and OG718-treated animals (1/6 animals tested) was not statistically significantly different (18). In the current study, we aimed to further characterize the dose-response of the top two leading candidates side by side. The survival results for C. difficile-infected hamsters treated with a range of OG716 and OG718 concentrations (Fig. 3) of between 2 and 60 mg/kg TID (6 to 180 mg/kg/day for 5 days) resulted in dose-dependent survival curves through day 21 for both compounds. Statistical analysis of the survival plots for the group treated with a test article versus the vehicle control group indicated statistical significance for OG716 (P < 0.05) for all doses except 2 mg/kg (Fig. 2). The corresponding dose-responses were plotted for each compound and compared (Fig. 3). Dose-response analysis suggested that OG716 was superior to OG718 (ED50, 23.85 mg/kg/day [10.97 μmol/kg/day] and 84.72 mg/kg/day [39.00 μmol/kg/day], respectively). The cecum-associated total CFU and toxin levels correlated with the clinical outcomes (Fig. 4 to 6). In particular, the data presented suggest that even though the gut microbiome dysbiosis and/or recovery may affect overall survival in the hamster C. difficile infection model, the survival of animals treated with OG716 is significantly impacted by the amounts of toxins and the total number of CFU in the ceca of infected hamsters at the time of death. Under the experimental conditions used, the vehicle control group exhibited 66% mortality on day 3 and 100% mortality on day 4 (2 days after treatment initiation). In the vancomycin treatment group (20 mg/kg, orally, once a day [QD] for 5 days), all animals survived the treatment phase and started to relapse in a stepwise fashion until survival reached 50% by day 21 of the study. Altogether, the in vivo efficacy data set supports the concept that OG716 is sufficient to confer a greater degree of survival as well as a sufficient degree of stability in the GI tract to be efficacious when administered by oral gavage, despite the longer half-life of OG718 in simulated intestinal fluids. It has previously been shown that mutacin 1140 exhibits rapid initial killing against multidrug-resistant Staphylococcus aureus and Streptococcus pneumoniae, whereas bacteriostatic activity against vancomycin-resistant enterococci was reported (12). While we cannot rule out the possibility that OG716 may be bacteriostatic with C. difficile, regimens with which treated animals presented with a cure rate of 100% and the organism (and its spores/toxins) was completely eradicated, an effect that persisted for at least 2 weeks after the cessation of therapy, were identified (18). It is unclear at this time what the local concentrations of compounds in the colon may be and what the bactericidal/bacteriostatic properties of OG716 are for C. difficile in vitro or during infection. Whether or not this compound has sporicidal properties, whether it can inhibit germination, or even whether it can directly modulate toxin levels remains to be elucidated and is under investigation. Taking into account the higher molecular weight of OG716 (∼2.2 kDa) than of vancomycin (∼1.4 kDa) and the fact that they both target the same molecular target (lipid II), albeit at a different moiety (pyrophosphate versus pentapeptide, respectively), OG716 treatment compared favorably to vancomycin treatment on a micromole-per-kilogram-per-day basis, despite the fact that the OG716 dosage was not fully optimized; in that model, the ED50 of OG716 and vancomycin were 10.97 μmol/kg/day and 13.3 μmol/kg/day, respectively.
The maximum tolerated dose of OG716 was further investigated to characterize the safety profile and unveil any potential toxicities of this new compound when administered up to 3 times daily following a single-day oral administration regimen. The tolerability was assessed at the highest dose that the regulatory authorities recommend be tested for a therapeutic peptide (1,000 mg/kg/day) and at the maximum feasible dose for OG716 (1,917 mg/kg/day, or 639 mg/kg/dose TID). The dose levels were selected in an attempt to produce graded responses to OG716. The higher dose level (1,917 mg/kg/day) was considered the maximum feasible dose, based on the allowable dose volume for single-dose administration and what can be produced as a homogeneous suspension (higher concentrations jellify and are not dosable). This dose was expected to produce some toxic effects but not excessive morbidity that would prevent meaningful evaluation. The lower dose levels in the GI study (639 mg/kg/day QD) and PK study (1,000 mg/kg/day, or 333.3 mg/kg/dose TID) were expected to produce no detectable indications of toxicity and were selected to assess the effects of a single dose compared to those of 3 doses in 1 day on the GI transit time or a lower dose on systemic exposure levels, respectively.
Under the conditions tested, no adverse clinical signs or differences in body weights or food consumption were noted, and no OG716-related gross findings were noted by pathological assessment. However, a decrease in gastric emptying was noted following administration of a single dose of OG716 at 639 mg/kg, as well as in animals challenged by administration of three doses of OG716 at 639 mg/kg/dose for a total dose of 1,917 mg/kg. A change in gastric emptying can impact drug absorption and the rate of absorption. In most cases, a decrease in gastric emptying correlates with a decrease in the rate of absorption (26). In contrast, the same doses did not seem to have any measurable effect on intestinal motility. The absence of obvious signs of toxicities, determined using several in vitro assays, has been previously reported (17, 18). In two reports, low cytotoxicity was observed using HepG2 hepatocytes (17) and other cell types (5). The extremely low levels of cytotoxicity that were previously observed at high concentrations of variants of MU1140 (millimolar range) remain approximately 3 orders of magnitude higher than the expected therapeutic concentration (low micromolar to high nanomolar) (17). Similarly, off-target pharmacological profiling was previously performed on several variants of MU1140 using the Safety Screen 44 (17, 27). This profiling panel has been successfully used to provide the early identification of significant off-target interactions for the optimization of safety margins (27). The results also suggested that this class of compounds possesses relatively low overall toxicity and is not likely to present inadvertent off-target effects (17).
The single-day oral administration regimen (QD versus TID) to Wistar Han rats at doses of 1,000 and 1,917 mg/kg/day showed that there was no consistent systemic exposure to OG716 at these dose levels. These findings were expected, considering that the molecular weight of OG716 (∼2.2 kDa) far exceeds the maximum molecular weight cutoff (∼500 Da) of compounds through the GI barrier (28). It remains to be seen whether or not infected GI tracts, often resulting in “leaky guts,” affect the bioavailability in the bloodstream.
In conclusion, OG716 emerged as the best compound from a library of over 700 MU1140-derived compounds with single and multiple substitutions to treat C. difficile enteritis in hamsters. This lead compound was characterized by a relatively low EC50 (23.85 mg/kg/day [10.97 μmol/kg/day]), which compares favorably to the current standard of care in human infections, vancomycin. The administration of OG716 by oral gavage to Wistar Han rats at the highest feasible daily dose resulted in a decrease in gastric emptying with no effect on intestinal motility. Administration of OG716 at doses of up to 1,917 mg/kg/day resulted in no test article-related mortality, clinical signs, changes in body weight or food consumption, or gross findings and showed that there was no consistent systemic exposure to OG716 at these dose levels. Based on these results, OG716 is a reasonable candidate to advance toward clinical testing for its activity against CDI.
MATERIALS AND METHODS
Bacterial strains and drug manufacturing.
Amino acid substitutions were introduced into MU1140; Streptococcus mutans strain SM716 produces the compound OG716 (MU1140 Phe1Val-Arg13Asn), and S. mutans strain SM718 produces the compound OG718 (MU1140 Phe1Ile-Arg13Asn-Tyr20Phe). The construction of strains expressing OG716 and OG718 have been previously described in detail and was performed by Intrexon Corp. (16, 18). Briefly, both strains were constructed in S. mutans JH1140 (29) by allelic replacement, where the native chromosomal lanA gene was replaced with lanA variants encoding codon substitutions. Splicing by overlap extension (SOE) PCR was used to construct DNA vectors for integration of lanA variants into the JH1140 chromosome using a selectable erythromycin resistance marker (from pVA891 [30]). Strains were routinely grown on tryptic soy agar or broth containing yeast extract and 3 μg/ml erythromycin and incubated in a candle jar for 3 days at 37°C. PCR and Sanger DNA sequencing were utilized to confirm replacement of the chromosomal copy of lanA with the lanA variant carried by the integration vector. The PCR primers and probes used in strain engineering are proprietary to Intrexon Corp., and their design took into account the S. mutans codon preference.
OG716 and OG718 were manufactured at the gram scale using proprietary methods. Briefly, fermentations were performed in 1,500-liter bioreactors using fed-batch fermentations under aerobic stirred tank conditions with automated temperature/pH/dissolved oxygen controls. Purifications of crude peptide were carried out using conventional column chromatography with acetic acid-containing buffers. Purified fractions were lyophilized to obtain acetate salts of lanthipeptides (Oragenics, unpublished data).
Characterization by HPLC, LC-MS, NMR, and elemental analysis.
The purity, quantity, and identity of each compound during manufacturing were determined by HPLC and LC-MS, as previously reported (18). A Waters XBridge C18 column (particle size, 3.5 μm; 4.6 by 70 mm) was used. Buffer A was 0.1% formic acid in H2O, and buffer B was 0.1% formic acid in acetonitrile (ACN). The gradient was 20% buffer B to 95% buffer B over 20 min at a flow rate of 0.4 ml/min. The injection volume was 25 μl. The mass of the peak of interest was analyzed by electrospray ionization mass spectrometry with a source temperature of 250°C, and the cone gas flow was 60 liters/h. The NMR spectra of OG716 (MU1140 F1V and R13N) were acquired and analyzed as previously reported (16, 18). They were acquired on an Agilent NMRS instrument operating at 600 MHz for proton NMR and equipped with a high-temperature superconductor (HTS) 1.5-mm probe (University of Florida NMR Core Facility, Gainesville, FL). The water signal was suppressed in a wet one-dimensional experiment. The precedent parameters were used to set up (i) a total correlation spectroscopy (TOCSY) experiment with a mixing time of 150 ms, (ii) a rotating-frame nuclear Overhauser enhancement spectroscopy (ROESY) experiment with a mixing time of 200 ms, (iii) a heteronuclear single quantum coherence (HSQC) experiment optimized for a one-bond coupling constant of 146 Hz, and (iv) a heteronuclear multiple bond correlation (HMBC) experiment optimized for a long-range coupling constant of 8 Hz. A 5-mg sample of OG253 was dissolved in 60 μl of a mixture of deuterated acetonitrile-water, 3:1, yielding a 37 mM solution. Elemental analysis was performed by Peptide International (Louisville, KY, USA).
Efficacy and dose-response assessment.
The in vivo efficacy and dose-response assessment was performed as previously described in detail (18) via oral gavage. This study was carried out in accordance with protocol 2016-0015, approved by the Institutional Animal Care and Use Committee (IACUC) at the University of North Texas Health Science Center (UNTHSC). IACUC has established guidelines ensuring that approved protocols are in compliance with federal and state laws regarding animal care and use activity at UNTHSC. The UNTHSC animal program is USDA registered (74-R0081) and fully AAALAC accredited. Briefly, male Golden Syrian hamsters (Charles River Laboratories, Wilmington, MA) with a body weight of 87 to 105 g on day 1 were housed at 1 hamster per cage and were given free access to food and water in accordance with NIH guidelines (35). C. difficile UNT103-1 (VA11, a nonepidemic cdtB-negative, REA group J strain) was received from Curtis Donskey, Cleveland VA Hospital (Cleveland, OH), and cultured as previously described for use in the hamster CDI model (17, 18, 31). The isolate has been previously utilized for the hamster model (31) and is part of the UNTHSC culture collection. Prior to infection, the inoculum was tested to determine the percentage of spores in relation to the number of vegetative cells. In accordance with the details in Table 1, a total of 60 animals were used in this study, and animals were randomized into the 10 study groups (n = 6 per group) prior to treatment. On day 1, 24 h after infection, all animals received a single subcutaneous injection of clindamycin (10 mg/kg) to suppress the natural flora. Test articles in 5% d-mannitol were administered orally 3 times per day (TID), starting 18 h after the dose of clindamycin, for 5 consecutive days (days 2 through 6). Vancomycin (positive control) was orally administered at 20 mg/kg once per day (QD) on days 2 through 6, and the infection control group was dosed with the test article vehicle in the same manner. During the study, the investigators were kept blind to the identity and concentration of all the test articles. Following completion of the study, the stability, purity, and activity were confirmed for all of the OG716 and OG718 test material used in the study. The survival of hamsters infected with C. difficile UNT103-1 was recorded for a period of 21 days postinfection to collect data on initial survival and relapse rates. Kaplan-Meier survival plots were generated for each group, and survival rates were compared between groups using the log-rank test. The effective dose at 50% (ED50) of the dose-response was generated for OG716 and OG718 using a variable-slope model, and the extra sum-of-squares F test was used to determine if the ED50 values were significantly different from each other (P < 0.05). The cecal contents from all of the hamsters were collected as they died on study or from euthanized hamsters at the end of the study. Toxin A and B titers per milliliter of cecal fluid were determined for each sample by use of an enzyme-linked immunosorbent assay kit (tgcBIOMICS GmbH, Bingen, Germany), the total viable C. difficile counts (vegetative and spore forms) were determined for each cecal fluid sample by plating serial dilutions onto cycloserine-cefoxitin-fructose agar containing 7% laked horse blood and 0.1% taurocholate (TCCFA), and the plates were anaerobically incubated at 37°C for 48 h to determine the total C. difficile counts per milliliter of cecal fluid (number of CFU per milliliter). Intragroup statistics were generated for the CFU and toxin results and included the calculated means, standard deviations, standard errors, 95% confidence intervals, coefficients of variation, and skewness for each study group (see Tables S1 to S3 in the supplemental material). Mean cecum-associated toxin A and B titers were calculated for all of the study groups and compared for statistically significant differences by the Kruskal-Wallis method with Dunn’s multiple-comparison test (P < 0.05; n = 6). Likewise, individual C. difficile CFU counts determined from harvested cecal contents were averaged for all study groups and compared for statistical significance by the Kruskal-Wallis method with Dunn’s multiple-comparison test (P < 0.05; n = 6). In order to further evaluate the impact of OG716 or OG718 treatment on survival as it relates to cecum-associated toxin and CFU titers, the Pearson correlation coefficient or Pearson’s r test was used to determine the strength of linear relatedness between the toxin or CFU titer and survival outcomes for the OG716- and OG718-treated groups. All data collected from the hamster C. difficile infection study were analyzed using GraphPad Prism (version 7.0d) software.
MTD assessment, impact on GI motility, and pharmacokinetic analysis.
The tolerability, pharmacokinetic profile, and potential pharmacological effects of OG716 on the gastrointestinal system were evaluated at Charles River Laboratories (Senneville, QC, Canada) following OECD and ICH guidelines (32–34). This study was carried out in accordance with protocol no. 6901324, approved by the Institutional Animal Care and Use Committee (IACUC) at Charles River Laboratories, Montreal, QC, Canada. This animal program was carried out in a USDA-registered (registration no. 000553) AAALAC-accredited facility, and the care and use of the animals were conducted with guidance from the U.S. National Research Council (35) and the Canadian Council on Animal Care (CCAC) (36). The drug was formulated as solutions or suspensions (when concentrations exceeded the maximum solubility) in 5% mannitol (free peptide, purity adjusted). The gastrointestinal marker contained a 10% (wt/vol) activated charcoal suspension in 5% (wt/vol) gum arabic in sterile water for injection. All chemicals were USP grade and were from Sigma-Aldrich (St. Louis, MO, USA). As described in the experimental scheme outlined in Table 3, a total of 41 male and 15 female 7- to 8-week old Wistar Han rats (Charles River Raleigh, Raleigh, NC, USA) were used in this study. Males weighed between 167 and 208 g and females weighed between 163 and 188 g at the initiation of dosing. The Wistar Han rat was chosen as the animal model for this study, as it is a rodent species accepted by regulatory agencies for preclinical toxicity testing and for evaluation of the effects of test compounds on the gastrointestinal system (Charles River Laboratories, personal communication). After an acclimation period of 5 days, animals were assigned to groups by a stratified randomization scheme designed to achieve similar group mean body weights. Males and females were randomized separately. Animals were housed at 3 animals per cage per sex and had free access to food and water in accordance with NIH guidelines. OG716 and negative controls (5% mannitol solution) were administered by oral gavage as a single dose once daily for 1 day for groups 1 and 3 and three times a day (every 8 h ± 15 min) for 1 day for groups 2, 4, 5, and 6. The dose volume for each animal was based on the most recent body weight measurement.
Animals in groups 1 to 4 of the GI study were food deprived overnight prior to dose administration and had access to water throughout the experiment, except for the 20-min period following administration of activated charcoal, during which the animals were also water deprived. The charcoal formulation (3 ml for each animal) was administered 1 h following the last dosing to the appropriate animals by oral gavage. At the end of the 20-min waiting period, the rats were euthanized, the abdominal cavity was opened, and the stomach and intestines were removed. The presence or absence of charcoal in the stomach was documented. The stomachs were weighed (with and without contents), and the values were recorded to give an indication of gastric emptying. The intestines were extended to their full length, and the intestines were opened. The charcoal was located, and the distances from the pyloric sphincter to the most proximal and distal traces of charcoal were measured and recorded, as was the total distance from the pyloric sphincter to the cecum (all distances were measured in millimeters). Samples were collected as indicated in Tables 3 and 4 and sent out for bioanalytical testing.
The animals (groups 5 and 6) used for PK analysis were subjected to a complete necropsy examination by a veterinary pathologist, which included evaluation of the carcass and musculoskeletal system; all external surfaces and orifices; the cranial cavity and the external surfaces of the brain; and the thoracic, abdominal, and pelvic cavities with their associated organs and tissues.
All statistical tests were conducted at the 5% significance level. All pairwise comparisons were conducted using two-sided tests and were reported at the 0.05, 0.01, and 0.001 levels. The body weights of the group 5 and group 6 animals in the PK analysis were compared using a two-sided t test if Levene’s test was not significant or the Wilcoxon rank-sum test if it was significant. The percentage distance along the length of the intestines from the pyloric sphincter to the cecum was calculated for the proximal and distal measures for each animal. Numerical data obtained from the parameters of interest during the conduct of the study were subjected to calculation of group mean values and standard deviations. Inferential statistics were performed when possible, but data for any group with less than 3 observations were excluded. Levene’s test was used to assess the homogeneity of group variances. Data sets with at least 3 groups were compared using an overall one-way analysis of variance F test if Levene’s test was not significant or the Kruskal-Wallis test if it was significant. If the overall F test or Kruskal-Wallis test was found to be significant, then the pairwise comparisons mentioned above were conducted using a two-sided t test or the Wilcoxon rank-sum test, respectively. Adjustments for a multiplicity of tests were made based on the square root of the number of pairwise comparisons. Data sets with 2 groups (both involved in one of the pairwise comparisons listed above) were compared using a two-sided t test if Levene’s test was not significant or the Wilcoxon rank-sum test if it was.
Development of an LC-MS/MS method for bioanalysis.
An LC-MS method was developed by AIT Bioscience (Indianapolis, IN, USA) to quantify OG716 in serum (Oragenics, unpublished). Briefly, OG716 was enriched from K2EDTA-treated plasma samples by solid-phase extraction followed by quantitation by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using a Thermo Scientific TSQ Quantiva mass spectrometer. The lower limit of quantification (LLOQ) was 10 ng/ml using this method. Statistical analyses, including regression analysis, and descriptive statistics, including arithmetic means and standard deviations, accuracy, and precision, were performed using Thermo Scientific LCquan software or the Watson laboratory information management system (LIMS) and Microsoft Excel software.
Supplementary Material
ACKNOWLEDGMENTS
Current and past employees of Oragenics and Intrexon are acknowledged for their contribution to the work described in this report. In particular, R. Eryl Sharp is acknowledged for his work on the improvements to methods to build, purify, and characterize MU1140 and variants; and Sheela Muley, Melissa Mayo, and Jeffrey Colbeck helped with the development of analytical methods to extract, purify, and characterize MU1140 and variants, designed DNA vectors and genetic constructions, and produced and characterized MU1140 variants. We also thank Albert G. Fosmoe, II, Bruce Tilley, Gabriela Philips, Mathoor (Siv) Sivaram, Joel Ngoje, Fernando Anazco, Zaxton Lamon, Emily Richeson, and Vicky DaSilva, who contributed to the production, purification, and testing/characterization of MU1140 and variants. M.H. is grateful to Charles River Laboratories (Nataliya Sadekova, Michelle King, Kevin Norton, and Andreanne Morency), AIT Biosciences (Brad King), and Synergy Partners (Shelley Ching and Jim MacDonald) for their help in the design and execution of several of the animal studies and analytical assays reported in this work. J.H.P. is grateful to the NMR facility at the University of Florida (Ion Ghiviriga) and Peptide International (Patricia Y. Coxon) for extended NMR study and elemental analysis, respectively. M.H. and J.H.P. thank Alan Joslyn for critical review of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01904-18.
REFERENCES
- 1.Rogers LA, Whittier EO. 1928. Limiting factors in the lactic fermentation. J Bacteriol 16:211–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Field D, Cotter PD, Hill C, Ross RP. 2015. Bioengineering lantibiotics for therapeutic success. Front Microbiol 6:1363. doi: 10.3389/fmicb.2015.01363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee C, Paul M, Xie L, van der Donk WA. 2005. Biosynthesis and mode of action of lantibiotics. Chem Rev 105:633–684. doi: 10.1021/cr030105v. [DOI] [PubMed] [Google Scholar]
- 4.Ross AC, Vederas JC. 2011. Fundamental functionality: recent developments in understanding the structure-activity relationships of lantibiotic peptides. J Antibiot 64:27–34. doi: 10.1038/ja.2010.136. [DOI] [PubMed] [Google Scholar]
- 5.Smith L, Hillman JD. 2008. Therapeutic potential of type A (I) lantibiotics, a group of cationic peptide antibiotics. Curr Opin Microbiol 11:401–408. doi: 10.1016/j.mib.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ongey EL, Yassi H, Pflugmacher S, Neubauer P. 2017. Pharmacological and pharmacokinetic properties of lanthipeptides undergoing clinical studies. Biotechnol Lett 39:473–482. doi: 10.1007/s10529-016-2279-9. [DOI] [PubMed] [Google Scholar]
- 7.Piper C, Casey PG, Hill C, Cotter PD, Ross RP. 2012. The lantibiotic lacticin 3147 prevents systemic spread of Staphylococcus aureus in a murine infection model. Int J Microbiol 2012:806230. doi: 10.1155/2012/806230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boakes S, Weiss WJ, Vinson M, Wadman S, Dawson MJ. 2016. Antibacterial activity of the novel semisynthetic lantibiotic NVB333 in vitro and in experimental infection models. J Antibiot 69:850–857. doi: 10.1038/ja.2016.47. [DOI] [PubMed] [Google Scholar]
- 9.Sandiford SK. 2015. Perspectives on lantibiotic discovery—where have we failed and what improvements are required? Expert Opin Drug Discov 10:315–320. doi: 10.1517/17460441.2015.1016496. [DOI] [PubMed] [Google Scholar]
- 10.Hasper HE, Kramer NE, Smith JL, Hillman JD, Zachariah C, Kuipers OP, de Kruijff B, Breukink E. 2006. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313:1636–1637. doi: 10.1126/science.1129818. [DOI] [PubMed] [Google Scholar]
- 11.De Kruijff B, van Dam V, Breukink E. 2008. Lipid II: a central component in bacterial cell wall synthesis and a target for antibiotics. Prostaglandins Leukot Essent Fatty Acids 79:117–121. doi: 10.1016/j.plefa.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Ghobrial OG, Derendorf H, Hillman JD. 2009. Pharmacodynamic activity of the lantibiotic MU1140. Int J Antimicrob Agents 33:70–74. doi: 10.1016/j.ijantimicag.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brötz H, Josten M, Wiedemann I, Schneider U, Götz F, Bierbaum G, Sahl HG. 1998. Role of lipid bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics. Mol Microbiol 30:317–327. doi: 10.1046/j.1365-2958.1998.01065.x. [DOI] [PubMed] [Google Scholar]
- 14.Hsu ST, Breukink E, Tischenko E, Lutters MA, de Kruijff B, Kaptein R, Bonvin AM, van Nuland NA. 2004. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat Struct Mol Biol 11:963–967. doi: 10.1038/nsmb830. [DOI] [PubMed] [Google Scholar]
- 15.Ghobrial O, Derendorf H, Hillman JD. 2010. Pharmacokinetic and pharmacodynamic evaluation of the lantibiotic MU1140. J Pharm Sci 99:2521–2528. doi: 10.1002/jps.22015. [DOI] [PubMed] [Google Scholar]
- 16.Kers JA, Sharp RE, Muley S, Mayo M, Colbeck J, Zhu Y, DeFusco AW, Park JH, Handfield M. 16 July 2018. Blueprints for the rational design of therapeutic mutacin 1140 variants. Chem Biol Drug Des doi: 10.1111/cbdd.13365. [DOI] [PubMed] [Google Scholar]
- 17.Kers JA, Sharp RE, Defusco AW, Park JH, Xu J, Pulse ME, Weiss WJ, Handfield M. 2018. Mutacin 1140 lantibiotic variants are efficacious against Clostridium difficile infection. Front Microbiol 9:415. doi: 10.3389/fmicb.2018.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kers JA, DeFusco AW, Park JH, Xu J, Pulse ME, Weiss WJ, Handfield M. 2018. OG716: designing a fit-for-purpose lantibiotic for the treatment of Clostridium difficile infections. PLoS One 13:e0197467. doi: 10.1371/journal.pone.0197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillman JD, Johnson KP, Yaphe BI. 1984. Isolation of a Streptococcus mutans strain producing a novel bacteriocin. Infect Immun 44:141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
- 21.Wilcox M. 2016. Interview with Professor Mark Wilcox. Future Microbiol 11:991–994. doi: 10.2217/fmb-2016-0123. [DOI] [PubMed] [Google Scholar]
- 22.Vaishnavi C. 2015. Fidaxomicin—the new drug for Clostridium difficile infection. Indian J Med Res 141:398–407. doi: 10.4103/0971-5916.159251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kufel WD, Devanathan AS, Marx AH, Weber DJ, Daniels LM. 2017. Bezlotoxumab: a novel agent for the prevention of recurrent Clostridium difficile infection. Pharmacotherapy 37:1298–1308. doi: 10.1002/phar.1990. [DOI] [PubMed] [Google Scholar]
- 24.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. 2013. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 108:478–498. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 25.Vemuri S. 2005. Comparison of assays for determination of peptide content for lyophilized thymalfasin. J Pept Res 65:433–439. doi: 10.1111/j.1399-3011.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 26.Nimmo WS. 1976. Drugs, diseases and altered gastric emptying. Clin Pharmacokinet 1:189–203. doi: 10.2165/00003088-197601030-00002. [DOI] [PubMed] [Google Scholar]
- 27.Bowes J, Brown AJ, Hamon J, Jarolimek W, Sridhar A, Waldron G, Whitebread S. 2012. Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nat Rev Drug Discov 11:909–922. doi: 10.1038/nrd3845. [DOI] [PubMed] [Google Scholar]
- 28.Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. 2002. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 29.Smith L, Novák J, Rocca J, McClung S, Hillman JD, Edison AS. 2000. Covalent structure of mutacin 1140 and a novel method for the rapid identification of lantibiotics. Eur J Biochem 267:6810–6816. doi: 10.1046/j.1432-1033.2000.01777.x. [DOI] [PubMed] [Google Scholar]
- 30.Macrina FL, Evans RP, Tobian JA, Hartley DL, Clewell DB, Jones KR. 1983. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene 25:145–150. [DOI] [PubMed] [Google Scholar]
- 31.Weiss W, Pulse M, Vickers R. 2014. In vivo assessment of SMT19969 in a hamster model of Clostridium difficile infection. Antimicrob Agents Chemother 58:5714–5718. doi: 10.1128/AAC.02903-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.OECD. 2010. Test no. 417: toxicokinetics. OECD Publishing, Paris, France. [Google Scholar]
- 33.ICH. 1994. Toxicokinetics: the assessment of systemic exposure in toxicity studies. ICH harmonised tripartite guideline S3a. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S3A/Step4/S3A_Guideline.pdf.
- 34.ICH. 2000. Guideline on safety pharmacology studies for human pharmaceuticals. ICH harmonised tripartite guideline S7A. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S7A/Step4/S7A_Guideline.pdf.
- 35.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 36.Canadian Council on Animal Care. 2017. Guide to the care and use of experimental animals, vol 1, 2nd ed Canadian Council on Animal Care, Ottawa, Ontario, Canada. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.