Glycopeptides such as vancomycin have been used as the first-line therapy against MRSA infections for over half a century. Reduced susceptibility and emergence of resistance to first-generation glycopeptides has led to development of second-generation lipoglycopeptide derivatives such as dalbavancin which hold broader ranges of activity and enhanced pharmacokinetic properties.
KEYWORDS: combination therapy, dalbavancin, MRSA, PK/PD
ABSTRACT
Glycopeptides such as vancomycin have been used as the first-line therapy against MRSA infections for over half a century. Reduced susceptibility and emergence of resistance to first-generation glycopeptides has led to development of second-generation lipoglycopeptide derivatives such as dalbavancin which hold broader ranges of activity and enhanced pharmacokinetic properties. We evaluated the MIC values for a total of 100 isolates, including 25 methicillin-resistant Staphylococcus aureus (MRSA), 25 heterogeneus vancomycin-intermediate S. aureus, 25 daptomycin nonsusceptible (DNS), and 25 vancomycin-intermediate S. aureus strains against dalbavancin, ceftaroline, and vancomycin alone and in combination. Dalbavancin was highly active against hVISA, DNS, and MRSA strains, achieving 96 to 100% susceptibility and 72% susceptibility against VISA strains. The combination of dalbavancin plus ceftaroline reduced dalbavancin MICs 62.5-fold and demonstrated enhanced killing against all four phenotypes in pharmacokinetic/pharmacodynamic models. Four strains of the aforementioned phenotypes were randomly chosen for pharmacodynamic/pharmacokinetic simulation models. Of interest, while both dalbavancin and vancomycin in combination with ceftaroline demonstrated significant improvement in glycopeptide fAUC/MIC values against these four phenotypes, the dalbavancin-ceftaroline combinations exhibited a 44- to 11,270-fold higher fAUC/MIC value in comparison to vancomycin-ceftaroline combinations. In addition, the time to detection limit was reduced for this combination (24 to 32 h) versus the vancomycin-ceftaroline combination (24 to 72h). To our knowledge, this is the first comprehensive study of dalbavancin and vancomycin combinations with ceftaroline. These data provide a novel approach for combating recalcitrant MRSA infections.
INTRODUCTION
Glycopeptide antibiotics, including vancomycin (VAN) and teicoplanin are large rigid molecules that inhibit a late stage in bacterial cell wall peptidoglycan synthesis (1–4). Principally, VAN, has been the first line therapy against methicillin-resistant Staphylococcus aureus (MRSA). However, S. aureus strains have now developed reduced susceptibility to common glycopeptides (5, 6). Low-level VAN resistance is an emerging problem in hospital settings, and it is associated with both VAN-intermediate S. aureus (VISA; VAN MIC, 4 to 8 μg/ml) and heterogeneous VAN-intermediate S. aureus (these pre-VISA strains have MIC values in the susceptible range of ≤2 μg/ml, but they contain resistant subpopulations) (7, 8). In addition, antibiotics developed to overcome glycopeptide resistance such as daptomycin and linezolid have also been associated with the emergence of multidrug resistance (MDR) strains which harbor daptomycin nonsusceptible (DNS) or linezolid-resistant S. aureus (LRSA) related mutations (9–13).
Increased emergence of resistance has led to the discovery and clinical improvement of second generation semisynthetic lipoglycopeptides, such as dalbavancin (DAL). DAL has increased antibacterial activity against Gram-positive pathogens, including MRSA, and improved pharmacokinetic properties compared to VAN (3, 14). Unlike VAN, DAL’s disposition is minimally affected by renal function (14, 15). Despite high protein binding, DAL has favorable tissue penetration (8, 16, 17). Furthermore, the long lipophilic lateral chain in DAL’s structure (VAN lacks this side chain) extends its half-life, promotes its anchoring to the cell membrane and improves the drug’s affinity for the d-alanyl-d-alanine subgroup, which leads to enhancement of its antibacterial activity (18). The half-life of DAL is 147 to 258 h, which facilitates a single-dose or two-dose administration for the treatment of acute bacterial skin and skin structure infections. This simple dosing strategy is an alternative to more resource demanding intravenously administered antibiotics used to treat serious infections in the hospital and community setting (3, 9, 14, 15).
Lipoglycopeptides such as DAL exhibit concentration-dependent activity and, according to previous research, the AUC/MIC is the best parameter for describing their activity (3, 14), where hydrophobic side chains of lipoglycopeptides bound to the cytoplasmic membranes of Gram-positive microorganisms (19–21). Dalbavancin was proven to be noninferior to vancomycin, followed by linezolid in acute bacterial skin and skin structure infections in the pivotal DISCOVER1 and DISCOVER2 trials (22, 23). Patients with catheter-related bloodstream infection had higher overall success rates when treated with dalbavancin once weekly in comparison to vancomycin (24).
While a considerable amount of data are available on susceptibility testing for this agent, information regarding the potential for an enhanced CFU/ml reduction in combination with β-lactams is lacking. It has been previously demonstrated that combination of VAN with various β-lactams, including ceftaroline (CPT), are synergistic against MRSA (25). The use of CPT in combination with glycopeptides is of particular interest since CPT also has activity against MRSA (26–28). Our objective was to evaluate the impact of DAL or VAN in combination CPT against MRSA strains with various VAN phenotypes via susceptibility testing and in a one-compartment pharmacokinetic/pharmacodynamic (PK/PD) model.
RESULTS
Susceptibility testing.
In order to evaluate the activity of DAL, VAN and CPT against MRSA, including MDR strains, we selected clinical strains of MRSA that had various susceptibilities to VAN and DAP. To determine the impact of CPT on DAL and VAN susceptibility, we evaluated the MIC to DAL and VAN in the presence of CPT. Since CPT is active against MRSA, we selected a fixed subinhibitory concentration of 0.5 × CPT MIC. Susceptibility testing results are shown in Table 1. Of the strains tested, 100% of MRSA, 72% of VISA, 96% of hVISA, and 100% of DNS strains were susceptible to DAL. Four strains representing MRSA and various susceptibilities to VAN and daptomycin were selected from this group to evaluate in the in vitro PK//PD models. The MIC values for the four strains used in an in vitro PK/PD models are listed in Table 2. Combination DAL-CPT resulted in the greatest reduction of MIC values compared to all other treatments (DAL, CPT, VAN, or VAN-CPT). When we compared DAL, CPT, and VAN alone to VAN-CPT or DAL-CPT combinations, a significant difference (P = 0.02) in MIC values was observed when different treatment groups were tested by analysis of variance (ANOVA; related variables). Tukey’s post hoc analysis showed that the combination of DAL-CPT was significantly (P < 0.05) different from all other treatments.
TABLE 1.
Summary of MIC values in 25 MRSA, 25 VISA, 25 hVISA, and 25 DNS strains (n = 100 strains)a
| Antibiotic(s) | Breakpoint (μg/ml) | MIC range (μg/ml) |
MIC50 (μg/ml) |
% susceptible |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRSA | VISA | hVISA | DNS | MRSA | VISA | hVISA | DNS | MRSA | VISA | hVISA | DNS | ||
| DAL | 0.25 | 0.016–0.25 | <0.008–1 | 0.0313–0.5 | 0.0156–0.25 | 0.0625 | 0.125 | 0.125 | 0.0625 | 100 | 72 | 96 | 100 |
| VAN | 2 | 0.5–2 | 4 | 0.5–2 | 1–4 | 1 | 4 | 1 | 1 | 100 | 0 | 100 | 96 |
| CPT | 1 | 0.25–2 | 0.25–2 | 0.25–2 | 0.25–1 | 0.5 | 0.5 | 1 | 1 | 96 | 96 | 96 | 100 |
| DAL-CPT | NA | 0.002–0.0156 | <0.002–0.5 | <0.002–0.125 | <0.002–0.0625 | <0.002 | 0.002 | 0.002 | 0.002 | NA | NA | NA | NA |
| VAN-CPT | NA | 0.0625–0.5 | 0.0625–2 | 0.0625–0.5 | 0.0625–2 | 0.125 | 0.25 | 0.25 | 0.5 | NA | NA | NA | NA |
NA, not applicable.
TABLE 2.
MIC values for the four strains used in PK/PD models using microbroth dilution method
| Treatment | MIC (μg/ml) |
|||
|---|---|---|---|---|
| 494 (MRSA) | D712 (DNS-VISA) | MU3 (hVISA) | 8015 (VISA) | |
| VAN | 1 | 4 | 2 | 4 |
| DAL | 0.0625 | 0.125 | 0.0625 | 0.125 |
| CPT | 1 | 1 | 1 | 2 |
| VAN-CPTa | 0.25 | 1 | 1 | 1 |
| DAL-CPTb | 0.0313 | <0.0039 | 0.0313 | <0.0313 |
MIC for VAN in presence of CPT.
MIC for DAL in presence of CPT.
Pharmacokinetics.
In order to simulate the antibiotic exposures obtained by the various treatment regimens, we humanized the concentrations of DAL, VAN, and CPT and their combinations in the PK/PD in vitro model. The achieved versus targeted pharmacokinetic parameters for all three single regimens in the in vitro models are shown in Table 3. The average time above the MIC was 100% of the dosing interval for all regimens.
TABLE 3.
Achieved versus targeted pharmacokinetic parameters in one-compartment models
| Parameter | Mean ± SD of achieved pharmacokinetic parameters (targeted value) |
||
|---|---|---|---|
| VAN | DAL | CPT | |
| fCmax (μg/ml) | 36.71 ± 0.43 (36.00) | 31.72 ± 0.56 (30.10) | 17.33 ± 0.22 (17.04) |
| t1/2 (h) | 5.97 ± 0.05 (6.00) | 187.06 ± 0.19 (187.40) | 2.64 ± 0.01 (2.66) |
DAL was administered as a continuous infusion to simulate its extremely long elimination half-life (147 to 258 h) in the one-compartment PK/PD model (14). In the DAL-CPT models, we achieved the targeted DAL concentrations in the presence of ceftaroline. In the VAN-CPT model, the fAUC0–24h and Cmax were within ±2.1% for the fAUC and ±3.7% for the fCmax of the monotherapy models.
Pharmacodynamics.
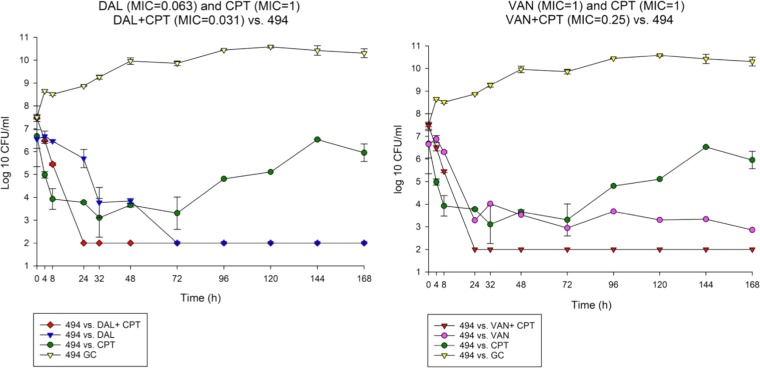
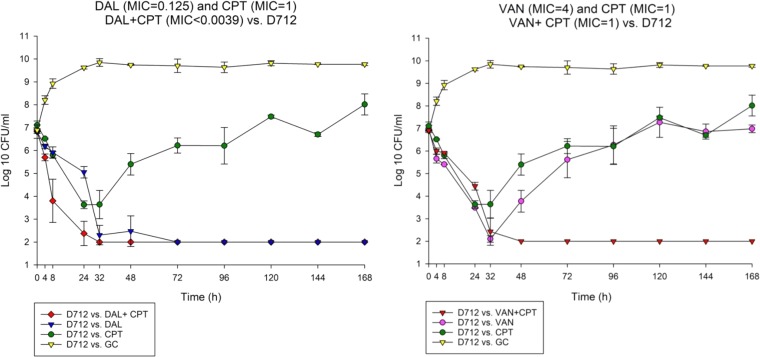
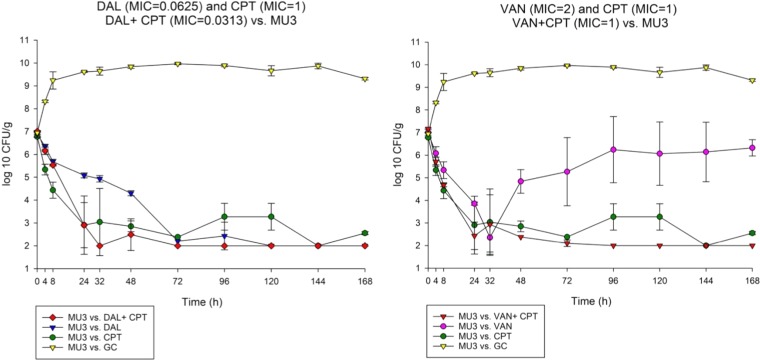
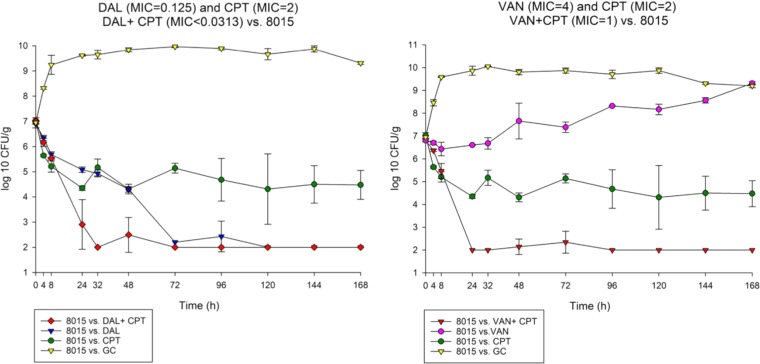
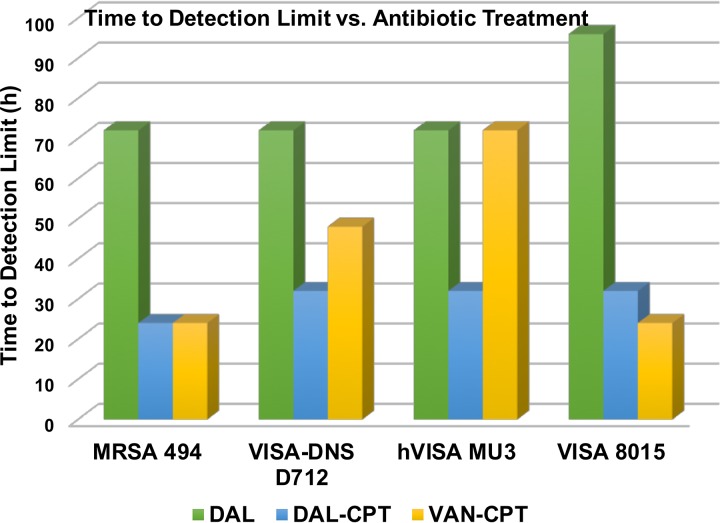
To determine the impact of monotherapy and combination therapy on strains with various VAN and DAP susceptibility phenotypes, we evaluated DAL, VAN, and CPT and their combination regimens over 7 days in the in vitro PK/PD model. The combination DAL-CPT significantly reduced the bacterial burden >5 log10CFU/ml from initial inoculum and reached detection limits in 24 to 32 h, while the combination VAN-CPT required 24 to 72 h to achieve the same reduction in bacterial burden (Fig. 1 to 4). While DAL, VAN, and CPT were bactericidal within the first 72 h, treatment with CPT or VAN alone did not achieve kill to the model detection limits within this timeframe and was associated with significant regrowth without resistance. Mean log10CFU/ml for DAL models reached detection limits in 72 to 96 h, with no regrowth detected after this time. VAN exposure resulted in a temporary CFU reduction at 24 of 32 h except for VISA strain 8015 (no activity was observed for the VAN model versus 8015), which was followed by regrowth for all PK/PD models. Figure 5 displays a comparison (in time-to-detection limits) between the three different treatment regimens where DAL, DAL-CPT, or VAN-CPT resulted in kill-to-detection limits with no further regrowth. While regrowth was observed in the monotherapy regimens (e.g., VAN versus 8015), we did not detect any emergence of increased resistance. The fAUC/MIC values are demonstrated in Table 4.
FIG 1.
Comparison between dalbavancin combination with ceftaroline versus vancomycin ceftaroline combination against 494.
FIG 2.
Comparison between dalbavancin combination with ceftaroline versus vancomycin ceftaroline combination against D712.
FIG 3.
Comparison between dalbavancin combination with ceftaroline versus vancomycin ceftaroline combination against MU3.
FIG 4.
Comparison between dalbavancin combination with ceftaroline versus vancomycin ceftaroline combination against 8015.
FIG 5.
Comparison of time-to-detection limit versus different antibiotic treatments and different MRSA phenotypes.
TABLE 4.
AUC/MIC values for different treatments versus each strain
| Strain |
fAUC/MIC |
||||
|---|---|---|---|---|---|
| VAN | DAL | CPT | VAN-CPTa | DAL-CPTb | |
| 494 | 314 | 27,396 | 66 | 1,257 | 55,141 |
| D712 | 79 | 13,807 | 66 | 314 | 3,540,349 |
| MU3 | 157 | 27,615 | 66 | 314 | 55,141 |
| 8015 | 79 | 13,807 | 33 | 314 | >55,141 |
VAN MIC in presence of CPT.
DAL MIC in presence of CPT.
Combination of DAL-CPT demonstrated 43.88- to 11,269.97-fold higher fAUC/MIC values in comparison to the VAN-CPT combination. While combination of DAL-CPT demonstrated a 1.99- to 256.41-fold enhancement in fAUC/MIC values in comparison to DAL alone, VAN-CPT had a 2- to 4-fold improvement in fAUC/MIC values compared to VAN alone.
DISCUSSION
This study evaluated the activity of DAL, VAN, and their combination with CPT against difficult-to-treat antibiotic-resistant phenotypes of MRSA. The combinations DAL-CPT and VAN-CPT offer encouraging results in the PK/PD models simulating humanized dosing, as well as the potential for reduced DAL and VAN MIC values in the four selected phenotypes of S. aureus. Most of the selected strains had elevated VAN MICs while they were susceptible to DAL alone and demonstrated reduced DAL MIC values in combination with CPT. Our results demonstrated that DAL is highly active against MRSA, DNS, and hVISA and has slightly reduced activity against VISA strains, which is in line with previous literature reporting on DAL activity against these phenotypes (14). The mechanism of glycopeptide resistance in Staphylococcus is not fully understood. However, reduced susceptibility has been linked to increased cell wall thickness and reduced cross linking which is accompanied by amplified production/activity of the penicillin-binding proteins (PBPs) (5, 8, 29). In addition, altered peptidoglycan composition demonstrates the potential for existence of factors other than PBP2a, which can lead to methicillin resistance (8, 29–31). We have previously demonstrated that the thickened cell wall of VISA is reduced in the presence of as little as 1 μg/ml of CPT, which may improve the activity of vancomycin against these strains (27).
Since the mechanism of action of DAL bears similarities to the mechanism of action of VAN, the so-called “seesaw effect” hypothesis, where β-lactam activity is improved in the presence of glycopeptides or lipopeptides, may partially explain the enhanced activity of DAL-CPT combinations and VAN-CPT combinations (28, 32–35). Although the mechanism for the seesaw effect is not fully understood, it has been demonstrated that the β-lactams that specifically target PBP1 and/or PBP2 which localizes in the septum of S. aureus are more likely to demonstrate this effect (33, 36, 37). Although there have been no reports on a direct relationship between the extent of expressed PBP2a protein and β-lactam MIC (38), researchers have shown elevated CPT affinity (up to 256-fold higher affinity than other tested β-lactams) toward PBP2, especially PBP2a in MRSA strains (39). Thus, the MRSA activity of CPT makes it an ideal candidate to take advantage of the seesaw effect when combined with glycopeptides and lipopeptide antibiotics.
This study had some limitations, including the limited representative strains that were used in the PK/PD model simulations. Regrowth without resistance was observed for monotherapy regimens of CPT and VAN. It is possible that there may have been some shift in population susceptibility that did not result in a meaningful change in the MIC. However, population analysis of these samples was not performed. In addition, we did not evaluate the ability to deescalate therapy from combination to monotherapy DAL or VAN. We have previously demonstrated using DAP combinations with either CPT or trimethoprim-sulfamethoxazole that it may be possible to deescalate after a few days of combination therapy once the inoculum is low enough without significant regrowth or the emergence of resistance (40–42).
In order to evaluate the impact of the presence CPT on DAL and VAN susceptibility, we tested the susceptibility of the MRSA strains to DAL and VAN in the presence of CPT at a fixed subinhibitory concentration of 0.5× the MIC. While the presence of CPT reduced the MIC of DAL and VAN considerably and therefore improved the DAL and VAN fAUC/MIC ratios, it is difficult to say whether this was responsible for the enhanced activity of the combination therapy using humanized antibiotic PK in the one-compartment PK/PD model. Our research has demonstrated that the combination of DAL-CPT and VAN-CPT has improved activity against S. aureus strains with various susceptibilities to VAN and DAP. Further research exploring these combinations is warranted in difficult-to-treat MRSA infections.
MATERIALS AND METHODS
Bacterial strains.
Various MRSA VAN phenotypes (total of 100 strains), including MRSA (n = 25), hVISA (n = 25), VISA (n = 25), and DNS (n = 25) strains, from the Anti-Infective Research Laboratory (ARL) library were used for susceptibility testing, and one strain of each MRSA phenotype (MRSA 494, VISA-DNS D712, hVISA MU3, VISA 8015) was randomly selected for evaluation in a one-compartment PK/PD model. All hVISA strains were verified by the gold standard, modified population analysis profile (PAP) (43).
Antimicrobials and media.
DAL and CPT were provided by their manufacturers (Allergan, Parsippany, NJ, and Actavis, Parsippany, NJ). VAN, was commercially obtained from Sigma Chemical Company (St. Louis, MO). In vitro experiments were performed in Mueller-Hinton broth (MHB; Difco, Detroit, MI) supplemented with 25 mg/liter calcium and 12.5 mg/liter magnesium. According to recent CLSI guidelines (44), MHB was supplemented with 0.002% polysorbate 80 (Tween; Sigma) for DAL studies due to the propensity of DAL binding to plastic present within in vitro testing modalities. Brain heart infusion agar (Difco Laboratories, San Jose, CA) supplemented with VAN (1 mg/liter) was used to subculture VISA strains in order to maintain this phenotype.
Susceptibility testing.
MIC values of DAL, VAN, and CPT were determined in duplicate by broth microdilution at approximately 106 CFU/ml according to CLSI guidelines (44). In addition, DAL and VAN MICs were performed in combination with CPT. MICs were determined on 25 MRSA, 25 hVISA, 25 VISA, and 25 DNS strains. All MIC values were determined in duplicate. DAL MIC values were determined in the presence of 0.002% polysorbate 80, as described above. For combination MIC testing, DAL MIC was determined in the presence of the second antimicrobial (CPT) at 0.5× the MIC of the respective organisms. The same procedure applied to combinations of VAN and CPT. All samples were incubated at 35°C for 18 to 24 h before reading according to CLSI guidelines (44).
In vitro PK/PD model.
Four representative strain phenotypes of MRSA, VISA, hVISA, and DNS were chosen for evaluation in a one-compartment, in vitro PK/PD model. A PK/PD model with a 250-ml capacity and input and output ports was used. The apparatus was prefilled with media, and DAL (1,500 mg, single dose), VAN (2 g, every 12 h), and CPT (600 mg, every 12 h) were administered as boluses over a time period of 168 h. A starting inoculum of 107 CFU/ml was targeted for each experiment by injecting a 1-ml portion of freshly prepared and appropriately diluted overnight suspension into each model. Fresh medium was continuously supplied and removed from the compartment, along with the drug, via a peristaltic pump (Masterflex; Cole-Palmer Instrument Company, Chicago, IL) at an appropriate rate to target the average human half-lives of DAL, CPT, and VAN. Drug exposures targeted free drug concentrations. Owing to the possible binding of DAL to model structures, the medium was supplemented with 0.002% polysorbate 80 according to CLSI guidelines (44). For combination models, the model clearance was set for the antibiotic with the shortest half-life, and the antibiotic with the longer half-life was supplemented (45). DAL was supplied as a continuous infusion. A total of six regimens were evaluated on each isolate over a 7-day treatment period. The free antibiotic concentrations for model experiments were determined from the estimated protein binding of 93% for DAL, 50% for VAN, and 20% CPT (46–49). Table 3 depicts a summary of targeted half-lives and peak concentrations for each antibiotic (14, 46, 50–53).
Pharmacodynamic analysis.
Samples were collected in duplicate from each model at 0, 4, 8, 24, 32, 48, 72, 96, 120, 144, and 168 h. In order to eliminate antibiotic carryover for DAL samples we have experimented various strategies, such as the addition of microbeads, charcoal, or albumin. Consequently, we have demonstrated that centrifuging the samples for a minimum of two times is necessary to eliminate drug carryover for samples containing DAL. Samples were centrifuged, 0.9 ml of supernatant was withdrawn after each centrifugation, and 0.9 ml of normal saline (0.9% sodium chloride) was added to the remaining palate. The final sample was vortexed and serially diluted in normal saline. Bacterial counts were determined by spiral plating appropriate dilutions using an automatic spiral plater (easySpiral; Interscience, Woburn, MA) (54). We have previously determined these methods to have a lower limit of reliable detection of 100 CFU/ml. Bactericidal activity was defined as >3-log decrease in CFU/ml from the baseline, whereas bacteriostatic activity was outlined as a >2-log decrease in CFU/ml from the baseline. SigmaPlot (v10) was used for the graphs in Fig. 1 to 4.
Pharmacokinetic analysis.
Pharmacokinetic samples were obtained in duplicate (quadruplicate for DAL) through the injection port of each model at 0, 2, 4, 8, 24, 48, 72, 96, 120, 144, and 168 h for verification of target antibiotic concentrations and half-life determination. All samples were stored at –80°C until ready for analysis. Samples for experiments involving DAL were shipped to the manufacturer’s analytical source (Keystone Bioanalytical, North Wales, PA) for analysis by high-pressure liquid chromatography (HPLC). The HPLC assay demonstrated coefficient of variation between 1.32 and 5.21% for all DAL standards (standards were performed in quadruplicate).
VAN samples were analyzed using our developed HPLC method. The HPLC assay demonstrated coefficient of variation between 0.29 and 1.68% for all VAN standards (standards were performed in duplicate).
All CPT concentrations were determined by bioassay procedures as previously described using E. coli ATCC 25922 as the test organism (28, 41). The bioassay demonstrated coefficient of variation between 1.17 and 7.06% for all CPT standards (standards were performed in duplicate).
The half-life, area under the curve (AUC), and peak concentrations were determined using PK Analyst Software (v1.10; MicroMath Scientific Software, Salt Lake City, UT) using the trapezoidal method to calculate the fAUC.
Resistance.
Emergence of resistance was evaluated at the end of the models by plating 100-μl samples from the model on plates supplemented with DAL, CPT, or VAN at concentrations of 3× the respective MICs. Plates were examined for growth after 48 h of incubation at 35°C. Resistant colonies growing on screening plates were evaluated by broth microdilution method to determine the MIC. If resistance was detected at the end of the model, additional screening was performed to identify the first occurrence of resistance.
Statistical analysis.
Statistical analysis of MIC values was done using randomized block two-factor ANOVA for related variables (different strains versus antibiotic treatments), and Tukey’s post-hoc analysis was used to define which means were different.
ACKNOWLEDGMENTS
M.J.R. received funding support, consulted or participated in speaking bureaus for Allergan, Achaogen, Bayer, Melinta, Merck, and Theravance. R.K., K.C.S., and S.A.R. have nothing to disclose. This study was supported by an investigator-initiated grant from Allergan, Parsippany, NJ.
We thank George Sakoulas (Sharp Memorial Hospital, San Diego, CA, and School of Medicine, University of California—San Diego, La Jolla, CA) for providing the D712 strain.
References
- 1.Reynolds PE. 1989. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis 8:943–950. [DOI] [PubMed] [Google Scholar]
- 2.Binda E, Marinelli F, Marcone GL. 2014. Old and new glycopeptide antibiotics: action and resistance. Antibiotics (Basel) 3:572–594. doi: 10.3390/antibiotics3040572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler MS, Hansford KA, Blaskovich MA, Halai R, Cooper MA. 2014. Glycopeptide antibiotics: back to the future. J Antibiot (Tokyo) 67:631–644. doi: 10.1038/ja.2014.111. [DOI] [PubMed] [Google Scholar]
- 4.Chow AW, Azar RM. 1994. Glycopeptides and nephrotoxicity. Intensive Care Med 20(Suppl 4):S23–S29. [DOI] [PubMed] [Google Scholar]
- 5.Gardete S, Tomasz A. 2014. Mechanisms of vancomycin resistance in Staphylococcus aureus. J Clin Invest 124:2836–2840. doi: 10.1172/JCI68834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. 2010. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev 23:99–139. doi: 10.1128/CMR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh NB, Yim J, Jahanbakhsh S, Sakoulas G, Rybak MJ. 2018. Impact of cefazolin co-administration with vancomycin to reduce development of vancomycin-intermediate Staphylococcus aureus. Diagn Microbiol Infect Dis 91:363–370. doi: 10.1016/j.diagmicrobio.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Lowy FD. 2003. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest 111:1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott LJ. 2015. Dalbavancin: a review in acute bacterial skin and skin structure infections. Drugs 75:1281–1291. doi: 10.1007/s40265-015-0430-x. [DOI] [PubMed] [Google Scholar]
- 10.Quiles-Melero I, Gomez-Gil R, Romero-Gomez MP, Sanchez-Diaz AM, de Pablos M, Garcia-Rodriguez J, Gutierrez A, Mingorance J. 2013. Mechanisms of linezolid resistance among staphylococci in a tertiary hospital. J Clin Microbiol 51:998–1001. doi: 10.1128/JCM.01598-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roman F, Roldan C, Trincado P, Ballesteros C, Carazo C, Vindel A. 2013. Detection of linezolid-resistant Staphylococcus aureus with 23S rRNA and novel L4 riboprotein mutations in a cystic fibrosis patient in Spain. Antimicrob Agents Chemother 57:2428–2429. doi: 10.1128/AAC.00208-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firsov AA, Golikova MV, Strukova EN, Portnoy YA, Romanov AV, Edelstein MV, Zinner SH. 2015. In vitro resistance studies with bacteria that exhibit low mutation frequencies: prediction of “antimutant” linezolid concentrations using a mixed inoculum containing both susceptible and resistant Staphylococcus aureus. Antimicrob Agents Chemother 59:1014–1019. doi: 10.1128/AAC.04214-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cafini F, Nguyen le TT, Higashide M, Roman F, Prieto J, Morikawa K. 2016. Horizontal gene transmission of the cfr gene to MRSA and Enterococcus: role of Staphylococcus epidermidis as a reservoir and alternative pathway for the spread of linezolid resistance. J Antimicrob Chemother 71:587–592. doi: 10.1093/jac/dkv391. [DOI] [PubMed] [Google Scholar]
- 14.Zhanel GG, Calic D, Schweizer F, Zelenitsky S, Adam H, Lagace-Wiens PR, Rubinstein E, Gin AS, Hoban DJ, Karlowsky JA. 2010. New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin. Drugs 70:859–886. doi: 10.2165/11534440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Smith JR, Roberts KD, Rybak MJ. 2015. Dalbavancin: a novel lipoglycopeptide antibiotic with extended activity against gram-positive infections. Infect Dis Ther 4:245–258. doi: 10.1007/s40121-015-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dash RP, Babu RJ, Srinivas NR. 2017. Review of the pharmacokinetics of dalbavancin, a recently approved lipoglycopeptide antibiotic. Infect Dis (Lond) 49:483–492. doi: 10.1080/23744235.2017.1296968. [DOI] [PubMed] [Google Scholar]
- 17.Bennett JW, Lewis JS, Ellis MW. 2008. Dalbavancin in the treatment of complicated skin and soft-tissue infections: a review. Ther Clin Risk Manag 4:31–40. doi: 10.2147/TCRM.S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guskey MT, Tsuji BT. 2010. A comparative review of the lipoglycopeptides: oritavancin, dalbavancin, and telavancin. Pharmacotherapy 30:80–94. doi: 10.1592/phco.30.1.80. [DOI] [PubMed] [Google Scholar]
- 19.Kwun MJ, Hong HJ. 2014. The activity of glycopeptide antibiotics against resistant bacteria correlates with their ability to induce the resistance system. Antimicrob Agents Chemother 58:6306–6310. doi: 10.1128/AAC.03668-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steed ME, Vidaillac C, Rose WE, Winterfield P, Kaatz GW, Rybak MJ. 2011. Characterizing vancomycin-resistant Enterococcus strains with various mechanisms of daptomycin resistance developed in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 55:4748–4754. doi: 10.1128/AAC.00084-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SJ, Tanaka KS, Dietrich E, Rafai Far A, Schaefer J. 2013. Locations of the hydrophobic side chains of lipoglycopeptides bound to the peptidoglycan of Staphylococcus aureus. Biochemistry 52:3405–3414. doi: 10.1021/bi400054p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramdeen S, Boucher HW. 2015. Dalbavancin for the treatment of acute bacterial skin and skin structure infections. Expert Opin Pharmacother 16:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. 2014. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med 370:2169–2179. doi: 10.1056/NEJMoa1310480. [DOI] [PubMed] [Google Scholar]
- 24.Raad I, Darouiche R, Vazquez J, Lentnek A, Hachem R, Hanna H, Goldstein B, Henkel T, Seltzer E. 2005. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis 40:374–80. doi: 10.1086/427283. [DOI] [PubMed] [Google Scholar]
- 25.Tran KN, Rybak MJ. 2018. Beta-lactam combinations with vancomycin show synergistic activity against vancomycin-susceptible Staphylococcus aureus, vancomycin-intermediate S. aureus (VISA), and heterogeneous VISA. Antimicrob Agents Chemother 62:e00157-18. doi: 10.1128/AAC.00157-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werth BJ, Barber KE, Ireland CE, Rybak MJ. 2014. Evaluation of ceftaroline, vancomycin, daptomycin, or ceftaroline plus daptomycin against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model of simulated endocardial vegetations. Antimicrob Agents Chemother 58:3177–3181. doi: 10.1128/AAC.00088-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werth BJ, Sakoulas G, Rose WE, Pogliano J, Tewhey R, Rybak MJ. 2013. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 57:66–73. doi: 10.1128/AAC.01586-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werth BJ, Steed ME, Kaatz GW, Rybak MJ. 2013. Evaluation of ceftaroline activity against heteroresistant vancomycin-intermediate Staphylococcus aureus and vancomycin-intermediate methicillin-resistant S. aureus strains in an in vitro pharmacokinetic/pharmacodynamic model: exploring the “seesaw effect.” Antimicrob Agents Chemother 57:2664–2668. doi: 10.1128/AAC.02308-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreira B, Boyle-Vavra S, deJonge BL, Daum RS. 1997. Increased production of penicillin-binding protein 2, increased detection of other penicillin-binding proteins, and decreased coagulase activity associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother 41:1788–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyle-Vavra S, Carey RB, Daum RS. 2001. Development of vancomycin and lysostaphin resistance in a methicillin-resistant Staphylococcus aureus isolate. J Antimicrob Chemother 48:617–625. doi: 10.1093/jac/48.5.617. [DOI] [PubMed] [Google Scholar]
- 31.Daum RS, Gupta S, Sabbagh R, Milewski WM. 1992. Characterization of Staphylococcus aureus isolates with decreased susceptibility to vancomycin and teicoplanin: isolation and purification of a constitutively produced protein associated with decreased susceptibility. J Infect Dis 166:1066–1072. [DOI] [PubMed] [Google Scholar]
- 32.Barber KE, Ireland CE, Bukavyn N, Rybak MJ. 2014. Observation of “seesaw effect” with vancomycin, teicoplanin, daptomycin, and ceftaroline in 150 unique MRSA strains. Infect Dis Ther 3:35–43. doi: 10.1007/s40121-014-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renzoni A, Kelley WL, Rosato RR, Martinez MP, Roch M, Fatouraei M, Haeusser DP, Margolin W, Fenn S, Turner RD, Foster SJ, Rosato AE. 2017. Molecular bases determining daptomycin resistance-mediated resensitization to beta-lactams (seesaw effect) in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 61:e01634-16. doi: 10.1128/AAC.01634-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang SJ, Xiong YQ, Boyle-Vavra S, Daum R, Jones T, Bayer AS. 2010. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob Agents Chemother 54:3161–3169. doi: 10.1128/AAC.00487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jousselin A, Renzoni A, Andrey DO, Monod A, Lew DP, Kelley WL. 2012. The posttranslocational chaperone lipoprotein PrsA is involved in both glycopeptide and oxacillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 56:3629–3640. doi: 10.1128/AAC.06264-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berti AD, Sakoulas G, Nizet V, Tewhey R, Rose WE. 2013. beta-Lactam antibiotics targeting PBP1 selectively enhance daptomycin activity against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:5005–5012. doi: 10.1128/AAC.00594-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berti AD, Baines SL, Howden BP, Sakoulas G, Nizet V, Proctor RA, Rose WE. 2015. Heterogeneity of genetic pathways toward daptomycin nonsusceptibility in Staphylococcus aureus determined by adjunctive antibiotics. Antimicrob Agents Chemother 59:2799–2806. doi: 10.1128/AAC.04990-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parvez MA, Shibata H, Nakano T, Niimi S, Fujii N, Arakaki N, Higuti T. 2008. No relationship exists between PBP 2a amounts expressed in different MRSA strains obtained clinically and their beta-lactam MIC values. J Med Invest 55:246–253. doi: 10.2152/jmi.55.246. [DOI] [PubMed] [Google Scholar]
- 39.Kosowska-Shick K, McGhee PL, Appelbaum PC. 2010. Affinity of ceftaroline and other beta-lactams for penicillin-binding proteins from Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob Agents Chemother 54:1670–1677. doi: 10.1128/AAC.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barber KE, Werth BJ, Rybak MJ. 2015. The combination of ceftaroline plus daptomycin allows for therapeutic de-escalation and daptomycin sparing against MRSA. J Antimicrob Chemother 70:505–509. doi: 10.1093/jac/dku378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steed M, Vidaillac C, Rybak MJ. 2011. Evaluation of ceftaroline activity versus daptomycin (DAP) against DAP-nonsusceptible methicillin-resistant Staphylococcus aureus strains in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 55:3522–3526. doi: 10.1128/AAC.00347-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steed ME, Vidaillac C, Rybak MJ. 2010. Novel daptomycin combinations against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus in an in vitro model of simulated endocardial vegetations. Antimicrob Agents Chemother 54:5187–92. doi: 10.1128/AAC.00536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wootton M HR, Hillman R, Walsh TR, Bennett PM, MacGowan AP. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J Antimicrob Chemother 47:399–403. doi: 10.1093/jac/47.4.399. [DOI] [PubMed] [Google Scholar]
- 44.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing; 38th informational supplement. CLSI document MS100-S38. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 45.Blaser J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J Antimicrob Chemother 15:125–130. [DOI] [PubMed] [Google Scholar]
- 46.Bailey J, Summers KM. 2008. Dalbavancin: a new lipoglycopeptide antibiotic. Am J Health Syst Pharm 65:599–610. doi: 10.2146/ajhp070255. [DOI] [PubMed] [Google Scholar]
- 47.Das B, Sarkar C, Biswas R, Pandey S. 2008. Review: dalbavancin: a novel lipoglycopeptide antimicrobial for gram positive pathogens. Pak J Pharm Sci 21:78–87. [PubMed] [Google Scholar]
- 48.Butterfield JM, Patel N, Pai MP, Rosano TG, Drusano GL, Lodise TP. 2011. Refining vancomycin protein binding estimates: identification of clinical factors that influence protein binding. Antimicrob Agents Chemother 55:4277–4282. doi: 10.1128/AAC.01674-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhanel GG, Sniezek G, Schweizer F, Zelenitsky S, Lagace-Wiens PR, Rubinstein E, Gin AS, Hoban DJ, Karlowsky JA. 2009. Ceftaroline: a novel broad-spectrum cephalosporin with activity against methicillin-resistant Staphylococcus aureus. Drugs 69:809–831. doi: 10.2165/00003495-200969070-00003. [DOI] [PubMed] [Google Scholar]
- 50.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 51.Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC Jr, Craig WA, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 29:1275–1279. doi: 10.1592/phco.29.11.1275. [DOI] [PubMed] [Google Scholar]
- 52.Hall Snyder AD, Vidaillac C, Rose W, McRoberts JP, Rybak MJ. 2014. Evaluation of high-dose daptomycin versus vancomycin alone or combined with clarithromycin or rifampin against Staphylococcus aureus and S. epidermidis in a novel in vitro PK/PD model of bacterial biofilm. Infect Dis Ther 4:51–65. doi: 10.1007/s40121-014-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith JR, Barber KE, Raut A, Rybak MJ. 2015. beta-Lactams enhance daptomycin activity against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium in in vitro pharmacokinetic/pharmacodynamic models. Antimicrob Agents Chemother 59:2842–2848. doi: 10.1128/AAC.00053-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Interscience. 2018. easySpiral: technical specifications. Interscience, Woburn, MA: https://www.interscience.com/en/products/automatic-spiral-platers/automatic-easyspiral. [Google Scholar]