The International Network for Optimal Resistance Monitoring (INFORM) global surveillance program collected clinical isolates of Enterobacteriaceae (n = 7,665) and Pseudomonas aeruginosa (n = 1,794) from 26 medical centers in six Latin American countries from 2012 to 2015. The in vitro activity of ceftazidime-avibactam and comparators was determined for the isolates using the Clinical and Laboratory Standards Institute (CLSI) reference broth microdilution method.
KEYWORDS: Enterobacteriaceae, Gram negative, Latin America, Pseudomonas aeruginosa, ceftazidime-avibactam, surveillance
ABSTRACT
The International Network for Optimal Resistance Monitoring (INFORM) global surveillance program collected clinical isolates of Enterobacteriaceae (n = 7,665) and Pseudomonas aeruginosa (n = 1,794) from 26 medical centers in six Latin American countries from 2012 to 2015. The in vitro activity of ceftazidime-avibactam and comparators was determined for the isolates using the Clinical and Laboratory Standards Institute (CLSI) reference broth microdilution method. Enterobacteriaceae were highly susceptible (99.7%) to ceftazidime-avibactam, including 99.9% of metallo-β-lactamase (MBL)-negative isolates; 87.4% of all P. aeruginosa isolates and 92.8% of MBL-negative isolates were susceptible to ceftazidime-avibactam. Susceptibility to ceftazidime-avibactam ranged from 99.4% to 100% for Enterobacteriaceae and from 79.1% to 94.7% for P. aeruginosa when isolates were analyzed by country of origin. Ceftazidime-avibactam inhibited 99.6% to 100% of Enterobacteriaceae isolates that carried serine β-lactamases, including extended-spectrum β-lactamases (ESBLs), AmpC cephalosporinases, and carbapenemases (KPC and OXA-48-like) as well as 99.7%, 99.6%, 99.5%, and 99.2% of MBL-negative isolates demonstrating ceftazidime-nonsusceptible, multidrug-resistant (MDR), meropenem-nonsusceptible, and colistin-resistant phenotypes, respectively. Among carbapenem-nonsusceptible isolates of P. aeruginosa (n = 750), 14.7% carried MBLs with or without additional acquired serine β-lactamases, while in the majority of isolates (70.0%), no acquired β-lactamase was identified. Ceftazidime-avibactam inhibited 89.5% of carbapenem-nonsusceptible P. aeruginosa isolates in which no acquired β-lactamase was detected. Overall, clinical isolates of Enterobacteriaceae collected in Latin America from 2012 to 2015 were highly susceptible to ceftazidime-avibactam, including isolates that exhibited resistance to ceftazidime, meropenem, colistin, or an MDR phenotype. Country-specific variations were noted in the susceptibility of P. aeruginosa isolates to ceftazidime-avibactam.
INTRODUCTION
The threat of increasing resistance to β-lactams (penicillins, cephalosporins, β-lactam/β-lactamase inhibitor combinations, carbapenems, and monobactams) among clinical isolates of Enterobacteriaceae and Pseudomonas aeruginosa in Latin American countries and elsewhere is of great concern because agents in this antimicrobial class reliably demonstrate both efficacy and safety and are widely used (1–9). β-Lactam resistance mechanisms include β-lactamase production, porin mutation or porin loss, membrane-associated efflux pumps, and structural alterations in penicillin-binding proteins. Resistance determinants may occur individually or in combination and can confer a multitude of phenotypes upon in vitro testing. Among the mechanisms of β-lactam resistance identified to date, β-lactamase production is the most common in Gram-negative bacilli. The Ambler molecular classification system divides β-lactamases into four classes, A through D. The emergence and spread of carbapenemases, which are included among class A (e.g., KPC), class B (e.g., NDM, IMP, VIM, and SPM), and class D (e.g., OXA) β-lactamases, are of greatest concern because these enzymes frequently generate resistance to all β-lactams, and because isolates carrying carbapenemases often demonstrate multidrug-resistant (MDR) phenotypes that limit therapeutic options and are associated with increased risk of morbidity and mortality for patients (1, 3, 6, 10).
Avibactam is a newer non-β-lactam β-lactamase inhibitor that has been paired with ceftazidime to restore its activity and/or to improve its potency against isolates of Gram-negative bacilli carrying Ambler class A β-lactamases, including extended spectrum β-lactamases (ESBLs) (e.g., TEM-type, SHV-type, and CTX-M-type) and KPC carbapenemases, AmpC cephalosporinases (Ambler class C β-lactamases), and some class D β-lactamases (e.g., OXA-48), including isolates carrying ESBL and AmpC enzymes in combination with impaired permeability (1, 6, 10–14). Ceftazidime-avibactam is not active against Enterobacteriaceae and P. aeruginosa isolates producing class B metallo-β-lactamases (MBLs) (6, 13).
To date, only a limited number of surveillance studies to determine rates of antimicrobial resistance in clinical isolates from patients in Latin America has been conducted (2, 4–9, 15). Previous surveillance studies have reported that the prevalence of β-lactam-resistant and MDR Enterobacteriaceae and nonfermentative Gram-negative bacilli varies within Latin America. The data are diverse, study dependent and difficult to compare (1–9, 15). To date, the majority of published studies have neither included molecular characterization of β-lactamases specifically from the Latin American region nor provided ceftazidime-avibactam susceptibility data for Gram-negative bacilli isolated from patients in many Latin American countries (2–9, 15). The intent of the current study is to augment currently published phenotypic data by determining in vitro susceptibilities to ceftazidime-avibactam and comparators for clinical isolates of Enterobacteriaceae and P. aeruginosa specifically collected from hospitalized patients in six Latin American countries over a recent 4-year time period (2012 to 2015), as well as to analyze the activity of ceftazidime-avibactam against antimicrobial-resistant and molecularly characterized β-lactamase-producing subsets. These data were collected as part of the International Network for Optimal Resistance Monitoring (INFORM) global surveillance program. The INFORM global surveillance program was established in 2012 to benchmark and track the in vitro activity of ceftazidime-avibactam and comparative agents against clinical isolates of β-lactamase-producing Enterobacteriaceae and nonfermentative Gram-negative bacilli, including P. aeruginosa.
RESULTS
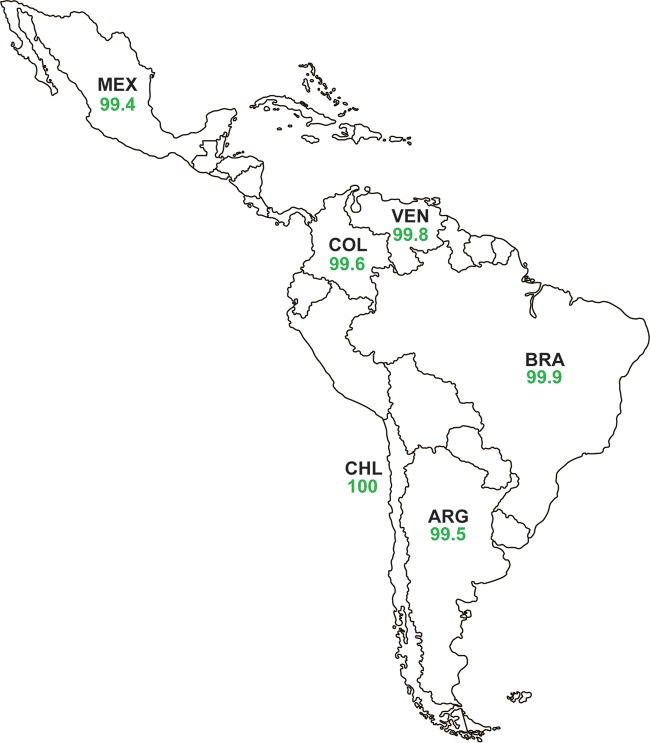
Of the 7,665 isolates of Enterobacteriaceae tested, 99.7% were susceptible to ceftazidime-avibactam (MIC90, 0.5 μg/ml). Percentages of susceptibility to all other antimicrobial agents tested, including doripenem (95.5%), meropenem (94.9%), amikacin (94.9%), tigecycline (93.7%), imipenem (83.9%), colistin (83.0%), and piperacillin-tazobactam (81.1%), were lower than for ceftazidime-avibactam (Table 1). Ceftazidime-avibactam MIC90 values for individual species or species groups within the Enterobacteriaceae family ranged from 0.12 μg/ml (tribe Proteeae) to 1 μg/ml (Enterobacter spp. and Klebsiella pneumoniae), with percent susceptibility to ceftazidime-avibactam varying by only 0.7%, from 99.2% susceptible for Enterobacter spp. to 99.9% susceptible for E. coli and Proteeae isolates (Table 1). Percent susceptibility to ceftazidime-avibactam was higher for isolates of Enterobacteriaceae that did not carry MBLs (99.9% susceptible) (Table 1) than for MBL-positive isolates (5.9% susceptible; 1/17 isolates) (Table 2). Due to the low percentage of MBL-positive isolates collected in this region, percent susceptibilities to ceftazidime-avibactam were only marginally higher (≤0.5%) for MBL-negative isolates of individual species or species groups of Enterobacteriaceae (99.5% to 100% susceptible) than for data sets of individual species or species groups that included all isolates (99.2% to 99.9%) (Table 1). The lower susceptibility of Enterobacteriaceae isolates to imipenem (83.9%) compared to doripenem (95.5%) and meropenem (94.9%) was attributable to the presence of 939 isolates of Proteeae (12.3% of all Enterobacteriaceae tested), as species within the tribe Proteeae intrinsically demonstrate elevated MICs for imipenem (16). Percent susceptibility to ceftazidime-avibactam for all isolates of Enterobacteriaceae from each of the six countries in Latin America surveyed ranged from 99.4% to 100% (MIC90, 0.25-1 μg/ml) (Fig. 1; Tables S2A to S7A).
TABLE 1.
In vitro activities of ceftazidime-avibactam and comparator antimicrobial agents tested against Enterobacteriaceae and P. aeruginosa isolatesa
| Organism, phenotype/genotype (no. of isolates)b | Antimicrobial agent | MIC (μg/ml) |
% Susceptiblec | ||
|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | |||
| Enterobacteriaceae (7,665) | Ceftazidime-avibactam | 0.12 | 0.5 | ≤0.015 to >128 | 99.7 |
| Ceftazidime | 0.25 | 64 | ≤0.015 to >128 | 69.9 | |
| Cefepime | ≤0.12 | >16 | ≤0.12 to >16 | 70.4 | |
| Aztreonam | 0.12 | 128 | ≤0.015 to >128 | 68.0 | |
| Piperacillin-tazobactam | 4 | >128 | ≤0.25 to >128 | 81.1 | |
| Doripenem | 0.06 | 0.25 | ≤0.008 to >4 | 95.5 | |
| Imipenem | 0.25 | 2 | ≤0.03 to >8 | 83.9 | |
| Meropenem | 0.03 | 0.12 | ≤0.004 to >8 | 94.9 | |
| Amikacin | 2 | 8 | ≤0.25 to >32 | 94.9 | |
| Colistin (n = 4,516)d | 0.5 | >4 | ≤0.12 to >4 | 83.0 | |
| Tigecycline | 0.5 | 2 | ≤0.015 to >8 | 93.7 | |
| Levofloxacin | 0.25 | >4 | ≤0.03 to >4 | 67.5 | |
| Enterobacteriaceae, MBL negative (7,648) | Ceftazidime-avibactam | 0.12 | 0.5 | ≤0.015 to >128 | 99.9 |
| Ceftazidime | 0.25 | 64 | ≤0.015 to >128 | 70.0 | |
| Cefepime | ≤0.12 | >16 | ≤0.12 to >16 | 70.5 | |
| Aztreonam | 0.12 | 128 | ≤0.015 to >128 | 68.0 | |
| Piperacillin-tazobactam | 4 | 128 | ≤0.25 to >128 | 81.3 | |
| Doripenem | 0.06 | 0.25 | ≤0.008 to >4 | 95.7 | |
| Imipenem | 0.25 | 2 | ≤0.03 to >8 | 84.1 | |
| Meropenem | 0.03 | 0.12 | ≤0.004 to >8 | 95.1 | |
| Amikacin | 2 | 8 | ≤0.25 to >32 | 95.0 | |
| Colistin (n = 4,500)d | 0.5 | >4 | ≤0.12 to >4 | 83.1 | |
| Tigecycline | 0.5 | 2 | ≤0.015 to >8 | 93.8 | |
| Levofloxacin | 0.25 | >4 | ≤0.03 to >4 | 67.6 | |
| Escherichia coli (2,705) | Ceftazidime-avibactam | 0.12 | 0.25 | ≤0.015 to 32 | 99.9 |
| Ceftazidime | 0.25 | 64 | ≤0.015 to >128 | 70.9 | |
| Cefepime | ≤0.12 | >16 | ≤0.12 to >16 | 67.7 | |
| Aztreonam | 0.12 | 64 | ≤0.015 to >128 | 67.7 | |
| Piperacillin-tazobactam | 2 | 32 | ≤0.25 to >128 | 88.9 | |
| Doripenem | 0.03 | 0.06 | ≤0.008 to >4 | 99.5 | |
| Imipenem | 0.12 | 0.25 | ≤0.03 to >8 | 99.2 | |
| Meropenem | 0.03 | 0.06 | ≤0.004 to >8 | 99.4 | |
| Amikacin | 4 | 8 | 0.5 to >32 | 97.1 | |
| Colistin (n = 1,515)d | 0.5 | 1 | ≤0.12 to >4 | 99.5 | |
| Tigecycline | 0.25 | 0.5 | ≤0.015 to 4 | 99.9 | |
| Levofloxacin | 1 | >4 | ≤0.03 to >4 | 51.9 | |
| Escherichia coli, MBL negative (2,703) | Ceftazidime-avibactam | 0.12 | 0.25 | ≤0.015 to 8 | 100 |
| Ceftazidime | 0.25 | 64 | ≤0.015 to >128 | 71.0 | |
| Cefepime | ≤0.12 | >16 | ≤0.12 to >16 | 67.8 | |
| Aztreonam | 0.12 | 64 | ≤0.015 to >128 | 67.7 | |
| Piperacillin-tazobactam | 2 | 32 | ≤0.25 to >128 | 89.0 | |
| Doripenem | 0.03 | 0.06 | ≤0.008 to >4 | 99.6 | |
| Imipenem | 0.12 | 0.25 | ≤0.03 to >8 | 99.2 | |
| Meropenem | 0.03 | 0.06 | ≤0.004 to >8 | 99.5 | |
| Amikacin | 4 | 8 | 0.5 to >32 | 97.1 | |
| Colistin (n = 1,513)d | 0.5 | 1 | ≤0.12 to >4 | 99.5 | |
| Tigecycline | 0.25 | 0.5 | ≤0.015 to 4 | 99.9 | |
| Levofloxacin | 1 | >4 | ≤0.03 to >4 | 51.9 | |
| Klebsiella pneumoniae (2,128) | Ceftazidime-avibactam | 0.25 | 1 | ≤0.015 to >128 | 99.5 |
| Ceftazidime | 2 | 128 | ≤0.015 to >128 | 52.9 | |
| Cefepime | 1 | >16 | ≤0.12 to >16 | 54.5 | |
| Aztreonam | 1 | >128 | ≤0.015 to >128 | 51.9 | |
| Piperacillin-tazobactam | 8 | >128 | ≤0.25 to >128 | 63.3 | |
| Doripenem | 0.06 | 2 | ≤0.008 to >4 | 86.7 | |
| Imipenem | 0.25 | 4 | 0.06 to >8 | 87.8 | |
| Meropenem | 0.06 | 4 | 0.008 to >8 | 84.7 | |
| Amikacin | 2 | 16 | ≤0.25 to >32 | 90.7 | |
| Colistin (n = 1,347)d | 1 | 1 | 0.25 to >4 | 93.9 | |
| Tigecycline | 0.5 | 2 | 0.06 to >8 | 96.4 | |
| Levofloxacin | 0.5 | >4 | ≤0.03 to >4 | 64.9 | |
| Klebsiella pneumoniae, MBL negative (2,119) | Ceftazidime-avibactam | 0.12 | 1 | ≤0.015 to >128 | 100 |
| Ceftazidime | 1 | 128 | ≤0.015 to >128 | 53.1 | |
| Cefepime | 0.5 | >16 | ≤0.12 to >16 | 54.7 | |
| Aztreonam | 1 | >128 | ≤0.015 to >128 | 52.0 | |
| Piperacillin-tazobactam | 8 | >128 | ≤0.25 to >128 | 63.5 | |
| Doripenem | 0.06 | 2 | ≤0.008 to >4 | 87.0 | |
| Imipenem | 0.25 | 4 | 0.06 to >8 | 88.2 | |
| Meropenem | 0.06 | 4 | 0.008 to >8 | 85.0 | |
| Amikacin | 2 | 16 | ≤0.25 to >32 | 90.9 | |
| Colistin (n = 1,338)d | 1 | 1 | 0.25 to >4 | 94.2 | |
| Tigecycline | 0.5 | 2 | 0.06 to >8 | 96.5 | |
| Levofloxacin | 0.5 | >4 | ≤0.03 to >4 | 64.9 | |
| Klebsiella oxytoca (393)e | Ceftazidime-avibactam | 0.12 | 0.25 | ≤0.015 to 16 | 99.8 |
| Ceftazidime | 0.12 | 8 | 0.03 to >128 | 89.6 | |
| Cefepime | ≤0.12 | 4 | ≤0.12 to >16 | 88.0 | |
| Aztreonam | 0.12 | 64 | ≤0.015 to >128 | 81.9 | |
| Piperacillin-tazobactam | 2 | 128 | ≤0.25 to >128 | 86.5 | |
| Doripenem | 0.06 | 0.12 | 0.03 to >4 | 98.5 | |
| Imipenem | 0.25 | 0.5 | 0.06 to >8 | 96.7 | |
| Meropenem | 0.03 | 0.06 | 0.015 to >8 | 98.0 | |
| Amikacin | 2 | 4 | 0.5 to >32 | 98.2 | |
| Colistin (n = 239)d | 0.5 | 1 | ≤0.12 to 4 | 99.2 | |
| Tigecycline | 0.25 | 1 | 0.06 to 4 | 99.8 | |
| Levofloxacin | 0.06 | 1 | ≤0.03 to >4 | 93.4 | |
| Enterobacter spp. (855)f | Ceftazidime-avibactam | 0.25 | 1 | ≤0.015 to 128 | 99.2 |
| Ceftazidime | 0.5 | 128 | 0.03 to >128 | 66.7 | |
| Cefepime | ≤0.12 | >16 | ≤0.12 to >16 | 78.2 | |
| Aztreonam | 0.12 | 128 | ≤0.015 to >128 | 66.2 | |
| Piperacillin-tazobactam | 4 | >128 | ≤0.25 to >128 | 75.6 | |
| Doripenem | 0.06 | 0.25 | ≤0.008 to >4 | 97.3 | |
| Imipenem | 0.5 | 2 | ≤0.03 to >8 | 83.4 | |
| Meropenem | 0.06 | 0.12 | 0.008 to >8 | 97.3 | |
| Amikacin | 2 | 8 | ≤0.25 to >32 | 96.1 | |
| Colistin (n = 496)d | 0.5 | 1 | ≤0.12 to >4 | 93.5 | |
| Tigecycline | 0.5 | 1 | 0.06 to 8 | 97.8 | |
| Levofloxacin | 0.06 | >4 | ≤0.03 to >4 | 86.4 | |
| Enterobacter spp., MBL negative (851) | Ceftazidime-avibactam | 0.25 | 1 | ≤0.015 to 32 | 99.5 |
| Ceftazidime | 0.5 | 128 | 0.03 to >128 | 66.9 | |
| Cefepime | ≤0.12 | >16 | ≤0.12 to >16 | 78.5 | |
| Aztreonam | 0.12 | 128 | ≤0.015 to >128 | 66.0 | |
| Piperacillin-tazobactam | 4 | >128 | ≤0.25 to >128 | 75.8 | |
| Doripenem | 0.06 | 0.25 | ≤0.008 to >4 | 97.8 | |
| Imipenem | 0.5 | 2 | ≤0.03 to >8 | 83.8 | |
| Meropenem | 0.06 | 0.12 | 0.008 to >8 | 97.6 | |
| Amikacin | 2 | 8 | ≤0.25 to >32 | 96.2 | |
| Colistin (n = 493)d | 0.5 | 1 | ≤0.12 to >4 | 93.5 | |
| Tigecycline | 0.5 | 1 | 0.06 to 8 | 97.8 | |
| Levofloxacin | 0.06 | >4 | ≤0.03 to >4 | 86.4 | |
| Citrobacter spp. (394)g | Ceftazidime-avibactam | 0.12 | 0.5 | ≤0.015 to 128 | 99.5 |
| Ceftazidime | 0.5 | 128 | ≤0.015 to >128 | 74.6 | |
| Cefepime | ≤0.12 | 8 | ≤0.12 to >16 | 86.3 | |
| Aztreonam | 0.12 | 64 | ≤0.015 to >128 | 72.3 | |
| Piperacillin-tazobactam | 4 | 64 | 0.5 to >128 | 81.5 | |
| Doripenem | 0.06 | 0.12 | 0.015 to >4 | 98.7 | |
| Imipenem | 0.5 | 2 | 0.06 to >8 | 88.1 | |
| Meropenem | 0.03 | 0.06 | ≤0.004 to >8 | 98.0 | |
| Amikacin | 2 | 4 | ≤0.25 to >32 | 94.9 | |
| Colistin (n = 237)d | 0.5 | 1 | ≤0.12 to 2 | 100 | |
| Tigecycline | 0.25 | 1 | 0.06 to 4 | 99.5 | |
| Levofloxacin | 0.06 | >4 | ≤0.03 to >4 | 86.8 | |
| Citrobacter spp., MBL negative (393) | Ceftazidime-avibactam | 0.12 | 0.5 | ≤0.015 to 128 | 99.8 |
| Ceftazidime | 0.5 | 128 | ≤0.015 to >128 | 74.8 | |
| Cefepime | ≤0.12 | 8 | ≤0.12 to >16 | 86.5 | |
| Aztreonam | 0.12 | 64 | ≤0.015 to >128 | 72.5 | |
| Piperacillin-tazobactam | 4 | 64 | 0.5 to >128 | 81.7 | |
| Doripenem | 0.06 | 0.12 | 0.015 to >4 | 99.0 | |
| Imipenem | 0.5 | 2 | 0.06 to >8 | 88.3 | |
| Meropenem | 0.03 | 0.06 | ≤0.004 to >8 | 98.2 | |
| Amikacin | 2 | 4 | ≤0.25 to >32 | 94.9 | |
| Colistin (n = 236)d | 0.5 | 1 | ≤0.12 to 2 | 100 | |
| Tigecycline | 0.25 | 1 | 0.06 to 4 | 99.5 | |
| Levofloxacin | 0.06 | >4 | ≤0.03 to >4 | 87.0 | |
| Proteeae (939)h | Ceftazidime-avibactam | 0.06 | 0.12 | ≤0.015 to 64 | 99.9 |
| Ceftazidime | 0.06 | 4 | ≤0.015 to >128 | 93.6 | |
| Cefepime | ≤0.12 | 8 | ≤0.12 to >16 | 88.2 | |
| Aztreonam | ≤0.015 | 1 | ≤0.015 to >128 | 95.0 | |
| Piperacillin-tazobactam | 0.5 | 2 | ≤0.25 to >128 | 98.5 | |
| Doripenem | 0.25 | 0.5 | 0.015 to >4 | 98.8 | |
| Imipenem | 2 | 4 | 0.06 to >8 | 23.2 | |
| Meropenem | 0.12 | 0.25 | 0.03 to 8 | 99.8 | |
| Amikacin | 4 | 8 | ≤0.25 to >32 | 96.3 | |
| Colistin (n = 532)d | >4 | >4 | 0.5 to >4 | 0.8 | |
| Tigecycline | 2 | 4 | ≤0.015 to >8 | 60.9 | |
| Levofloxacin | 0.12 | >4 | ≤0.03 to >4 | 75.4 | |
| Proteeae, MBL negative (938) | Ceftazidime-avibactam | 0.06 | 0.12 | ≤0.015 to 8 | 100 |
| Ceftazidime | 0.06 | 2 | ≤0.015 to >128 | 93.7 | |
| Cefepime | ≤0.12 | 4 | ≤0.12 to >16 | 88.3 | |
| Aztreonam | ≤0.015 | 1 | ≤0.015 to >128 | 95.0 | |
| Piperacillin-tazobactam | 0.5 | 2 | ≤0.25 to >128 | 98.6 | |
| Doripenem | 0.25 | 0.5 | 0.015 to 4 | 98.9 | |
| Imipenem | 2 | 4 | 0.06 to >8 | 23.2 | |
| Meropenem | 0.12 | 0.12 | 0.03 to 2 | 99.9 | |
| Amikacin | 4 | 8 | ≤0.25 to >32 | 96.3 | |
| Colistin (n = 531)d | >4 | >4 | 0.5 to >4 | 0.8 | |
| Tigecycline | 2 | 4 | ≤0.015 to >8 | 61.0 | |
| Levofloxacin | 0.12 | >4 | ≤0.03 to >4 | 75.4 | |
| Other Enterobacteriaceae (251)e,i | Ceftazidime-avibactam | 0.25 | 0.5 | ≤0.015 to >128 | 99.6 |
| Ceftazidime | 0.25 | 16 | 0.03 to >128 | 86.0 | |
| Cefepime | ≤0.12 | 16 | ≤0.12 to >16 | 87.2 | |
| Aztreonam | 0.12 | 64 | 0.03 to >128 | 84.0 | |
| Piperacillin-tazobactam | 2 | 16 | ≤0.25 to >128 | 92.8 | |
| Doripenem | 0.12 | 0.25 | 0.03 to >4 | 98.0 | |
| Imipenem | 0.5 | 1 | 0.06 to >8 | 90.0 | |
| Meropenem | 0.06 | 0.12 | 0.03 to >8 | 98.0 | |
| Amikacin | 2 | 8 | ≤0.25 to >32 | 92.4 | |
| Colistin (n = 150)d | >4 | >4 | ≤0.12 to >4 | 23.3 | |
| Tigecycline | 1 | 2 | 0.06 to 8 | 95.2 | |
| Levofloxacin | 0.12 | 2 | ≤0.03 to >4 | 93.6 | |
| Pseudomonas aeruginosa (1,794) | Ceftazidime-avibactam | 2 | 16 | 0.12 to >128 | 87.4 |
| Ceftazidime | 4 | 64 | 0.25 to >128 | 70.0 | |
| Cefepime | 4 | >16 | ≤0.12 to >16 | 73.0 | |
| Aztreonam | 8 | 64 | 0.12 to >128 | 55.9 | |
| Piperacillin-tazobactam | 8 | >128 | ≤0.25 to >128 | 62.5 | |
| Doripenem | 1 | >4 | ≤0.015 to >4 | 66.0 | |
| Imipenem | 2 | >8 | 0.12 to >8 | 56.9 | |
| Meropenem | 1 | >8 | 0.03 to >8 | 64.2 | |
| Amikacin | 4 | >32 | ≤0.25 to >32 | 81.6 | |
| Colistin (n = 1,301)d | 2 | 2 | 0.25 to >8 | 94.9 | |
| Levofloxacin | 1 | >4 | ≤0.03 to >4 | 65.7 | |
| Pseudomonas aeruginosa, MBL negative (1,684) | Ceftazidime-avibactam | 2 | 8 | 0.12 to >128 | 92.8 |
| Ceftazidime | 4 | 64 | 0.25 to >128 | 74.4 | |
| Cefepime | 4 | >16 | ≤0.12 to >16 | 77.1 | |
| Aztreonam | 8 | 64 | 0.12 to >128 | 58.1 | |
| Piperacillin-tazobactam | 8 | >128 | ≤0.25 to >128 | 66.1 | |
| Doripenem | 1 | >4 | ≤0.015 to >4 | 70.1 | |
| Imipenem | 2 | >8 | 0.12 to >8 | 60.6 | |
| Meropenem | 1 | >8 | 0.03 to >8 | 68.1 | |
| Amikacin | 4 | >32 | ≤0.25 to >32 | 86.1 | |
| Colistin (n = 1,213)d | 2 | 2 | 0.25 to 8 | 94.8 | |
| Levofloxacin | 1 | >4 | ≤0.03 to >4 | 69.1 | |
Isolates of Enterobacteriaceae (n = 7,665) and P. aeruginosa (n = 1,794) were collected in the Latin American region as part of the INFORM global surveillance program in 2012 to 2015.
MBL negative; no gene encoding a metallo-β-lactamase was detected by PCR assay.
Percent susceptibility was determined according to CLSI 2016 breakpoints, with the exception of those for ceftazidime-avibactam and tigecycline, where U.S. FDA breakpoints were applied, and for colistin, where EUCAST breakpoints were applied.
Values are for colistin tested without 0.002% polysorbate-80; isolates collected in 2014 to 2015 only.
All isolates were MBL negative.
Enterobacter spp. included Enterobacter aerogenes (n = 272), Enterobacter amnigenus (n = 1), Enterobacter asburiae (n = 48), Enterobacter cloacae (n = 512), Enterobacter kobei (n = 18), and Enterobacter ludwigii (n = 4).
Citrobacter spp. included Citrobacter amalonaticus (n = 5), Citrobacter braakii (n = 19), Citrobacter farmeri (n = 1), Citrobacter freundii (n = 274), and Citrobacter koseri (n = 95).
Proteeae included Morganella morganii (n = 213), Proteus mirabilis (n = 496), Proteus penneri (n = 13), Proteus vulgaris (n = 152), Providencia alcalifaciens (n = 2), Providencia rettgeri (n = 29), and Providencia stuartii (n = 34).
Other Enterobacteriaceae included Escherichia vulneris (n = 1), Klebsiella variicola (n = 1), Kluyvera ascorbata (n = 1), Pantoea agglomerans (n = 1), Pluralibacter gergoviae (n = 2), Raoultella ornithinolytica (n = 33), Raoultella planticola (n = 1), Serratia liquefaciens (n = 2), Serratia marcescens (n = 206), and Serratia ureilytica (n = 3).
TABLE 2.
In vitro activities of ceftazidime-avibactam and comparator antimicrobial agents tested against β-lactamase-positive Enterobacteriaceae and P. aeruginosa isolatesa
| Organism or genotype (no. of isolates)a | Antimicrobial agent | MIC (μg/ml)b |
% Susceptiblec | ||
|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | |||
| Enterobacteriaceae (2,321) | |||||
| OSBL positive (55) | Ceftazidime-avibactam | 0.25 | 0.5 | ≤0.03 to 2 | 100 |
| Ceftazidime | 4 | 32 | ≤0.015 to >128 | 58.2 | |
| Cefepime | 1 | 16 | ≤0.12 to >16 | 80.0 | |
| Aztreonam | 1 | 64 | ≤0.015 to 128 | 72.7 | |
| Piperacillin-tazobactam | 128 | >128 | ≤0.25 to >128 | 43.6 | |
| Doripenem | 0.06 | 0.5 | 0.03 to 2 | 96.4 | |
| Imipenem | 0.25 | 4 | 0.06 to 8 | 80.0 | |
| Meropenem | 0.06 | 0.12 | 0.03 to 2 | 96.4 | |
| Amikacin | 2 | 16 | ≤0.25 to >32 | 90.9 | |
| Colistin (n = 24)d | 1 | >4 | 0.25 to >4 | 83.3 | |
| Tigecycline | 0.5 | 2 | 0.25 to 4 | 92.7 | |
| Levofloxacin | 0.5 | >4 | ≤0.03 to >4 | 63.6 | |
| Spectrum undefined (2)e | Ceftazidime-avibactam | —b | — | 0.12 to 1 | 100 |
| Ceftazidime | — | — | 16 to 32 | 0 | |
| Cefepime | — | — | ≤0.12 to 16 | 50.0 | |
| Aztreonam | — | — | 4 to 32 | 50.0 | |
| Piperacillin-tazobactam | — | — | 4 to 64 | 50.0 | |
| Doripenem | — | — | 0.03 to 0.06 | 100 | |
| Imipenem | — | — | 0.25 to 0.25 | 100 | |
| Meropenem | — | — | 0.03 to 0.06 | 100 | |
| Amikacin | — | — | 0.5 to 1 | 100 | |
| Colistin (n = 0)d | — | — | NDd | ND | |
| Tigecycline | — | — | 0.5 to 2 | 100 | |
| Levofloxacin | — | — | 0.5 to >4 | 50.0 | |
| ESBL positive (1,701)f | Ceftazidime-avibactam | 0.25 | 1 | ≤0.015 to 128 | 99.9 |
| Ceftazidime | 32 | >128 | 0.25 to >128 | 14.8 | |
| Cefepime | >16 | >16 | ≤0.12 to >16 | 5.9 | |
| Aztreonam | 64 | >128 | ≤0.25 to >128 | 5.9 | |
| Piperacillin-tazobactam | 16 | >128 | ≤0.25 to >128 | 62.4 | |
| Doripenem | 0.06 | 0.25 | ≤0.008 to >4 | 96.4 | |
| Imipenem | 0.25 | 0.5 | 0.06 to >8 | 96.8 | |
| Meropenem | 0.06 | 0.12 | ≤0.004 to >8 | 94.2 | |
| Amikacin | 4 | 32 | ≤0.25 to >32 | 89.8 | |
| Colistin (n = 988)d | 0.5 | 1 | 0.12 to >4 | 94.5 | |
| Tigecycline | 0.5 | 2 | ≤0.015 to >8 | 95.4 | |
| Levofloxacin | >4 | >4 | ≤0.03 to >4 | 27.9 | |
| AmpC positive (199)g | Ceftazidime-avibactam | 0.25 | 1 | 0.03 to 32 | 99.0 |
| Ceftazidime | 16 | >128 | 0.03 to >128 | 45.7 | |
| Cefepime | 0.25 | 8 | ≤0.12 to >16 | 81.4 | |
| Aztreonam | 4 | 64 | ≤0.015 to >128 | 52.3 | |
| Piperacillin-tazobactam | 4 | >128 | ≤0.25 to >128 | 70.4 | |
| Doripenem | 0.12 | 0.5 | ≤0.008 to >4 | 96.5 | |
| Imipenem | 2 | 4 | 0.12 to >8 | 45.7 | |
| Meropenem | 0.06 | 0.25 | 0.015 to >8 | 95.5 | |
| Amikacin | 2 | 8 | ≤0.25 to >32 | 96.5 | |
| Colistin (n = 94)d | 1 | >4 | 0.25 to >4 | 86.2 | |
| Tigecycline | 0.5 | 2 | 0.06 to 4 | 95.5 | |
| Levofloxacin | 0.12 | >4 | ≤0.03 to >4 | 72.4 | |
| ESBL positive + AmpC positive (64)h | Ceftazidime-avibactam | 0.5 | 2 | 0.03 to 2 | 100 |
| Ceftazidime | 128 | >128 | 0.12 to >128 | 6.3 | |
| Cefepime | >16 | >16 | ≤0.12 to >16 | 12.5 | |
| Aztreonam | 128 | >128 | 0.25 to >128 | 4.7 | |
| Piperacillin-tazobactam | 128 | >128 | 2 to >128 | 31.3 | |
| Doripenem | 0.12 | 1 | 0.03 to >4 | 90.6 | |
| Imipenem | 0.5 | 4 | 0.06 to >8 | 78.1 | |
| Meropenem | 0.06 | 1 | 0.03 to >8 | 90.6 | |
| Amikacin | 4 | 32 | 0.5 to >32 | 76.6 | |
| Colistin (n = 26)d | 0.5 | 4 | 0.25 to >4 | 88.5 | |
| Tigecycline | 1 | 2 | 0.12 to 4 | 90.6 | |
| Levofloxacin | >4 | >4 | 0.06 to >4 | 17.2 | |
| KPC positive (269)i | Ceftazidime-avibactam | 0.5 | 2 | 0.03 to 16 | 99.6 |
| Ceftazidime | 64 | >128 | 0.5 to >128 | 8.2 | |
| Cefepime | >16 | >16 | ≤0.12 to >16 | 10.8 | |
| Aztreonam | >128 | >128 | 4 to >128 | 1.5 | |
| Piperacillin-tazobactam | >128 | >128 | 8 to >128 | 0.7 | |
| Doripenem | >4 | >4 | 0.06 to >4 | 10.4 | |
| Imipenem | >8 | >8 | 0.12 to >8 | 1.5 | |
| Meropenem | >8 | >8 | 0.03 to >8 | 5.9 | |
| Amikacin | 8 | >32 | 0.5 to >32 | 68.4 | |
| Colistin (n = 181)d | 1 | >4 | 0.25 to >4 | 79.0 | |
| Tigecycline | 1 | 2 | 0.12 to 8 | 94.8 | |
| Levofloxacin | >4 | >4 | ≤0.03 to >4 | 25.3 | |
| OXA-48-like positive (14)j | Ceftazidime-avibactam | 1 | 2 | 0.06 to 8 | 100 |
| Ceftazidime | 128 | >128 | 16 to >128 | 0 | |
| Cefepime | >16 | >16 | 2 to >16 | 7.1 | |
| Aztreonam | 64 | 128 | 1 to >128 | 7.1 | |
| Piperacillin-tazobactam | >128 | >128 | 4 to >128 | 7.1 | |
| Doripenem | 0.12 | >4 | 0.03 to >4 | 85.7 | |
| Imipenem | 0.5 | 2 | 0.12 to 2 | 85.7 | |
| Meropenem | 0.12 | >8 | 0.015 to >8 | 85.7 | |
| Amikacin | 8 | 32 | 1 to >32 | 78.6 | |
| Colistin (n = 10)d | 1 | 1 | 0.5 to 1 | 100 | |
| Tigecycline | 0.5 | 2 | 0.12 to 2 | 100 | |
| Levofloxacin | >4 | >4 | 0.06 to >4 | 21.4 | |
| MBL positive (17)k | Ceftazidime-avibactam | 64 | >128 | 0.12 to >128 | 5.9 |
| Ceftazidime | >128 | >128 | 0.25 to >128 | 5.9 | |
| Cefepime | >16 | >16 | 1 to >16 | 5.9 | |
| Aztreonam | 16 | 128 | 0.03 to >128 | 47.1 | |
| Piperacillin-tazobactam | >128 | >128 | 1 to >128 | 5.9 | |
| Doripenem | >4 | >4 | 2 to >4 | 0 | |
| Imipenem | 8 | >8 | 4 to >8 | 0 | |
| Meropenem | 8 | >8 | 1 to >8 | 5.9 | |
| Amikacin | 16 | >32 | 2 to >32 | 64.7 | |
| Colistin (n = 93)d | 1 | >4 | 0.25 to >4 | 68.8 | |
| Tigecycline | 1 | 4 | 0.5 to 4 | 82.4 | |
| Levofloxacin | 1 | >4 | 0.25 to >4 | 58.8 | |
| P. aeruginosa (750) | |||||
| OSBL positive (4)l | Ceftazidime-avibactam | —b | — | 4 to 16 | 75.0 |
| Ceftazidime | — | — | 4 to 64 | 50.0 | |
| Cefepime | — | — | 8 to >16 | 25.0 | |
| Aztreonam | — | — | 16 to 64 | 0 | |
| Piperacillin-tazobactam | — | — | 64 to >128 | 0 | |
| Doripenem | — | — | 1 to >4 | 50.0 | |
| Imipenem | — | — | 2 to >8 | 25.0 | |
| Meropenem | — | — | 2 to >8 | 25.0 | |
| Amikacin | — | — | >32 to >32 | 0 | |
| Colistin (n = 1)d | — | — | 1 | 100 | |
| Levofloxacin | — | — | >4 to >4 | 0 | |
| ESBL positive (29)l,m | Ceftazidime-avibactam | 16 | 64 | 4 to >128 | 31.0 |
| Ceftazidime | >128 | >128 | 8 to >128 | 3.4 | |
| Cefepime | >16 | >16 | 0.25 to >16 | 3.4 | |
| Aztreonam | >128 | >128 | 8 to >128 | 3.4 | |
| Piperacillin-tazobactam | 64 | >128 | 8 to >128 | 20.7 | |
| Doripenem | >4 | >4 | 2 to >4 | 13.8 | |
| Imipenem | >8 | >8 | >8 to >8 | 0 | |
| Meropenem | >8 | >8 | 4 to >8 | 0 | |
| Amikacin | >32 | >32 | 8 to >32 | 27.6 | |
| Colistin (n = 19)d | 1 | 4 | 0.5 to >4 | 89.5 | |
| Levofloxacin | >4 | >4 | 1 to >4 | 24.1 | |
| KPC positive (48)l | Ceftazidime-avibactam | 8 | 32 | 1 to 128 | 77.1 |
| Ceftazidime | 64 | 128 | 8 to >128 | 2.1 | |
| Cefepime | >16 | >16 | 0.5 to >16 | 2.1 | |
| Aztreonam | >128 | >128 | 64 to >128 | 0 | |
| Piperacillin-tazobactam | >128 | >128 | 32 to >128 | 0 | |
| Doripenem | >4 | >4 | >4 to >4 | 0 | |
| Imipenem | >8 | >8 | >8 to >8 | 0 | |
| Meropenem | >8 | >8 | >8 to >8 | 0 | |
| Amikacin | 8 | >32 | 1 to >32 | 77.1 | |
| Colistin (n = 31)d | 2 | 2 | 0.5 to 4 | 93.5 | |
| Levofloxacin | >4 | >4 | 0.25 to >4 | 8.3 | |
| GES carbapenemase positive (33)l,n | Ceftazidime-avibactam | 32 | 128 | 0.12 to >128 | 27.3 |
| Ceftazidime | >128 | >128 | 0.25 to >128 | 0 | |
| Cefepime | >16 | >16 | 1 to >16 | 0 | |
| Aztreonam | 128 | >128 | 0.03 to >128 | 9.1 | |
| Piperacillin-tazobactam | >128 | >128 | 1 to >128 | 0 | |
| Doripenem | >4 | >4 | 2 to >4 | 0 | |
| Imipenem | >8 | >8 | 4 to >8 | 15.2 | |
| Meropenem | >8 | >8 | 1 to >8 | 0 | |
| Amikacin | >32 | >32 | 2 to >32 | 15.2 | |
| Colistin (n = 27)d | 2 | 2 | 0.25 to >4 | 92.6 | |
| Levofloxacin | >4 | >4 | 0.25 to >4 | 0 | |
| GES, spectrum-undefined positive (1)l | Ceftazidime-avibactam | —b | — | >128 | 0 |
| Ceftazidime | — | — | >128 | 0 | |
| Cefepime | — | — | >16 | 0 | |
| Aztreonam | — | — | >128 | 0 | |
| Piperacillin-tazobactam | — | — | >128 | 0 | |
| Doripenem | — | — | >4 | 0 | |
| Imipenem | — | — | >8 | 0 | |
| Meropenem | — | — | >8 | 0 | |
| Amikacin | — | — | >32 | 0 | |
| Colistin (n = 1)d | — | — | 1 | 100 | |
| Levofloxacin | — | — | >4 | 0 | |
| MBL-positive (110)l,o | Ceftazidime-avibactam | 32 | >128 | 2 to >128 | 5.5 |
| Ceftazidime | 32 | >128 | 4 to >128 | 2.7 | |
| Cefepime | 16 | >16 | 4 to >16 | 10.0 | |
| Aztreonam | 16 | 64 | 2 to >128 | 21.8 | |
| Piperacillin-tazobactam | 64 | >128 | 4 to >128 | 7.3 | |
| Doripenem | >4 | >4 | 1 to >4 | 2.7 | |
| Imipenem | >8 | >8 | 4 to >8 | 0 | |
| Meropenem | >8 | >8 | 1 to >8 | 3.6 | |
| Amikacin | >32 | >32 | 2 to >32 | 13.6 | |
| Colistin (n = 88)d | 2 | 2 | 0.5 to 4 | 95.5 | |
| Levofloxacin | >4 | >4 | 0.25 to >4 | 13.6 | |
| No acquired β-lactamase detected (525)l | Ceftazidime-avibactam | 4 | 16 | 0.5 to >128 | 89.5 |
| Ceftazidime | 8 | 64 | 1 to >128 | 61.1 | |
| Cefepime | 8 | >16 | 0.25 to >16 | 61.5 | |
| Aztreonam | 16 | 64 | 0.25 to >128 | 33.9 | |
| Piperacillin-tazobactam | 32 | >128 | ≤0.25 to >128 | 45.7 | |
| Doripenem | 4 | >4 | 0.12 to >4 | 25.5 | |
| Imipenem | >8 | >8 | 1 to >8 | 6.9 | |
| Meropenem | 8 | >8 | ≤0.06 to >8 | 19.4 | |
| Amikacin | 8 | >32 | 0.5 to >32 | 74.9 | |
| Colistin (n = 369)d | 2 | 2 | 0.25 to 8 | 95.1 | |
| Levofloxacin | 4 | >4 | ≤0.03 to >4 | 45.9 | |
Isolates (n = 3,071) of β-lactamase-positive Enterobacteriaceae and P. aeruginosa were collected in the Latin American region as part of the INFORM global surveillance program in 2012 to 2015. OSBL, original-spectrum β-lactamase (e.g., TEM-1, SHV-1, SHV-11); ESBL, extended-spectrum β-lactamase; MBL, metallo-β-lactamase.
—, MIC50 and MIC90 were not calculated for n <10 isolates.
Percent susceptibility was determined according to CLSI 2016 breakpoints, with the exception of those for ceftazidime-avibactam and tigecycline, where U.S. FDA breakpoints were applied, and for colistin, where EUCAST breakpoints were applied.
Values are for colistin tested without 0.002% polysorbate-80; isolates collected in 2014 to 2015 only. ND, not determined; MIC range and % susceptible were not determined for n = 0 isolates.
“Spectrum undefined” refers to SHV-type and/or TEM-type β-lactamases with undefined spectrum of activity.
Included isolates carrying the chromosomal ESBL common to K. oxytoca, SHV-type and/or TEM-type β-lactamases with undefined spectrum of activity, and/or OSBLs.
Included isolates carrying the chromosomal AmpCs common to Citrobacter spp., Enterobacter spp., M. morganii, and Serratia spp.; plasmid-mediated AmpCs; and isolates cocarrying OSBLs.
Included isolates carrying the chromosomal β-lactamases common to Citrobacter spp., Enterobacter spp., Providencia spp., Serratia spp., and K. oxytoca, and isolates cocarrying OSBLs.
Included isolates carrying ESBLs, plasmidic and chromosomal AmpC β-lactamases, β-lactamases with unknown spectrum of activity, OSBLs, and OXA-48-like β-lactamases (KPC-2 and OXA-163, 1 isolate; KPC-2 and OXA-370, 1 isolate).
Included isolates carrying OXA-163 (10 isolates), OXA-48 (1 isolate), OXA-232 (1 isolate), OXA-370 (1 isolate), OXA-439 (1 isolate), and ESBLs, AmpC, and/or OSBLs. OXA-163 possesses weak carbapenemase activity that impacts the activity of carbapenems when combined with additional mechanisms of resistance, such as porin deficiencies. OXA-439 has not been confirmed to possess carbapenemase activity.
Included isolates cocarrying ESBLs, chromosomal AmpC β-lactamases, and/or OSBLs.
Assumed to carry the chromosomal AmpC common to P. aeruginosa.
Included one isolate carrying a GES β-lactamase with an undefined spectrum of activity.
Included isolates carrying GES β-lactamases with ESBL activity (GES-19 and GES-1).
Included 1 isolate cocarrying an OSBL and 5 isolates cocarrying VIM-2 and KPC-2.
FIG 1.
Percent susceptibility to ceftazidime-avibactam for isolates of Enterobacteriaceae collected in 2012 to 2015, by Latin American country. Ceftazidime-avibactam-susceptible, ≤8 μg/ml; ceftazidime-avibactam-resistant, ≥16 μg/ml, by U.S. FDA criteria. The green font indicates that >90% of isolates were ceftazidime-avibactam-susceptible. ARG, Argentina; BRA, Brazil; CHL, Chile; COL, Colombia; MEX, Mexico; VEN, Venezuela.
Table 2 depicts the in vitro activity of ceftazidime-avibactam and comparator agents against isolates of Enterobacteriaceae molecularly characterized for β-lactamase content. Ceftazidime-avibactam inhibited 99.9%, 99.6%, and 99.0% of ESBL-positive, KPC-positive, and AmpC-positive isolates, respectively, as well as all isolates that carried both ESBL and AmpC enzymes, isolates carrying only original-spectrum β-lactamases (OSBLs; e.g., TEM-1, SHV-1, and SHV-11), and those carrying OXA-48-like class D carbapenemases. The MIC90 values for ceftazidime-avibactam against these subsets of β-lactamase-producing isolates ranged from 0.5 μg/ml for OSBL-positive isolates to 2 μg/ml for ESBL-positive and AmpC-positive, KPC-positive, and OXA-48-like-positive isolates. The percentages of susceptibility to doripenem (90.6% to 96.5%) and meropenem (90.6% to 96.4%) were 2.5% to 9.4% lower than observed for ceftazidime-avibactam among ESBL-positive, AmpC-positive, ESBL-positive and AmpC-positive, and OSBL-positive isolates. The activities of all β-lactams tested (0% to 10.8% susceptible) were significantly reduced compared to that of ceftazidime-avibactam against KPC-positive and OXA-48-like-positive isolates, with the notable exception of the activities of doripenem, imipenem, and meropenem against OXA-48-like-positive isolates (85.7% susceptible). As anticipated, ceftazidime-avibactam, similarly to all other β-lactams, was poorly active against isolates carrying MBLs (MIC90, >128 μg/ml; 5.9% susceptible); only tigecycline (MIC90, 4 μg/ml; 82.4% susceptible) retained in vitro activity against >80% of MBL-positive isolates.
The distributions of serine-based β-lactamases and MBLs among molecularly characterized Enterobacteriaceae isolates from each of the six countries surveyed in Latin America are summarized in the supplemental material (Tables S2B to S7B; Fig. S1A to D). CTX-M-type ESBLs accounted for 88.7% (1,797/2,025) of all ESBLs identified in the region, with CTX-M-15 being the most common ESBL identified, accounting for 60.1% (1,217/2,025) of all ESBLs found. However, the prevalence of ESBL types and individual enzyme variants differed across countries (Fig. S1B). CMY-2 (77.1% [74/96]) and DHA-1 (17.7% [17/96]) comprised the majority of AmpC enzymes identified, with differences in the prevalence of these and other variants observed among countries (Fig. S1C). ESBLs and AmpC β-lactamases were identified in isolates from all six countries as were KPC carbapenemases, which comprised 89.1% of detected carbapenemases. MBLs were only identified in isolates from Colombia (NDM-1), Mexico (NDM-1, VIM-23), and Venezuela (NDM-1), while OXA-48-like β-lactamases were found only in Argentina (OXA-163, OXA-439), Mexico (OXA-163, OXA-232), and Brazil (OXA-48, OXA-370) (Fig. S1D). KPC-2 (80.5% [243/302]), KPC-3 (8.6% [26/302]), and NDM-1 (3.3% [10/302]) were the most common carbapenemases identified, and OXA-163 (68.8% [11/16]), a variant with attenuated carbapenemase activity, was the most common OXA-48-like β-lactamase (17, 18). The types and relative prevalence of carbapenemases differed among the surveyed countries, with both the greatest variety of carbapenemases and the majority of MBL-positive isolates (64.7%; 11/17) collected in Mexico (Fig. S1D). The in vitro activity of ceftazidime-avibactam against Enterobacteriaceae isolates in each country was affected by the proportion of isolates carrying an MBL.
Table S8 describes the in vitro activity of ceftazidime-avibactam and comparator agents against the 2,310 isolates of Enterobacteriaceae identified with a ceftazidime-nonsusceptible phenotype (30.1% of all isolates). Overall, 99.0% of ceftazidime-nonsusceptible isolates were susceptible to ceftazidime-avibactam (MIC90, 2 μg/ml), with MIC90 values against individual species of Enterobacteriaceae ranging from 0.5 to 2 μg/ml (97.1% to 99.8% susceptible). Across the Latin American region, the percentage of Enterobacteriaceae isolates that tested as nonsusceptible to ceftazidime ranged from 23.6% in Venezuela to 38.6% in Mexico (Fig. S2), while the percentage of ceftazidime-nonsusceptible isolates that tested as susceptible to ceftazidime-avibactam was >98% (MIC90, 1 to 2 μg/ml) in all six countries (Tables S2A to S7A).
Table S9 depicts the in vitro activity of ceftazidime-avibactam and comparator agents against the 389 isolates of Enterobacteriaceae identified with a meropenem-nonsusceptible phenotype (5.1% of all isolates); 95.4% of all meropenem-nonsusceptible isolates of Enterobacteriaceae were susceptible to ceftazidime-avibactam. MIC90 values for ceftazidime-avibactam were 4 to 32 μg/ml for all meropenem-nonsusceptible isolates and isolates of individual species or species groups of Enterobacteriaceae. However, MIC90 values decreased by up to 16-fold and susceptibilities increased to 99.7% to 100% for Escherichia coli, Enterobacter spp., and K. pneumoniae (the species with data for >10 isolates tested) when only MBL-negative, meropenem-nonsusceptible isolates were considered. The percentages of isolates that tested as nonsusceptible to meropenem differed by <10% among the six Latin American countries, ranging from 1.2% in Mexico to 10.2% in Brazil (Fig. S3).
Table S10 shows the in vitro activity of ceftazidime-avibactam and comparator agents against 124 colistin-resistant isolates of Enterobacteriaceae (excluding isolates of Proteeae and Serratia spp., which are intrinsically resistant to colistin). The percentage of isolates per country that were resistant to colistin ranged from 1.1% in Venezuela to 2.3% in Chile (Fig. S4). Ceftazidime-avibactam inhibited 96.0% of colistin-resistant Enterobacteriaceae isolates (MIC90, 2 μg/ml) and 99.2% of MBL-negative colistin-resistant isolates (MIC90, 2 μg/ml). Percentages of susceptibility to ceftazidime-avibactam against colistin-resistant isolates were higher than those for all other agents tested.
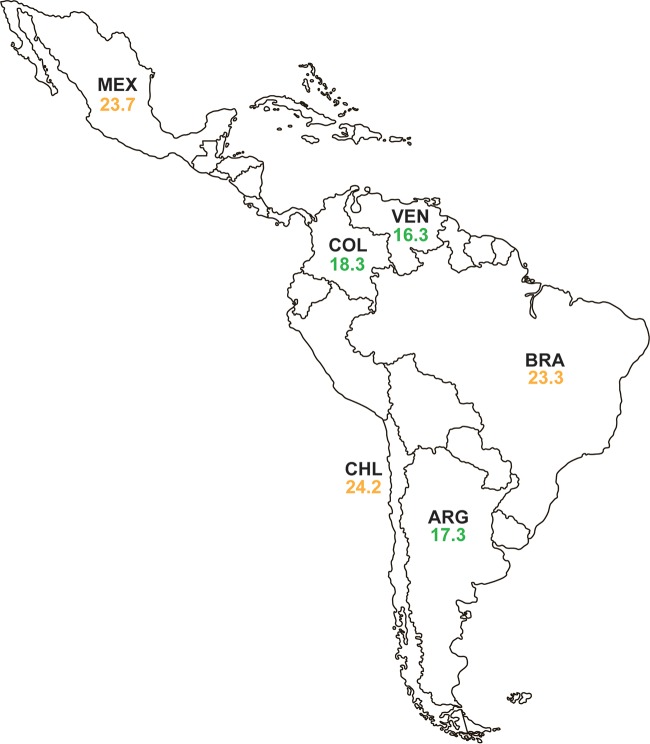
Table S11 describes the in vitro activity of ceftazidime-avibactam and comparator agents against 1,596 isolates of MDR Enterobacteriaceae. Susceptibility to ceftazidime-avibactam was 98.8% (MIC90, 2 μg/ml), exceeding susceptibility to all other agents tested. The MIC90 values of ceftazidime-avibactam against MDR isolates varied from 0.25 to 4 μg/ml for different species of Enterobacteriaceae, and ≥95% of MDR isolates of each species were susceptible to ceftazidime-avibactam. Susceptibility to ceftazidime-avibactam was greater than that to tigecycline (92.9%), colistin (85.7%), and carbapenems (77.1% to 79.7%) for the overall set of MDR Enterobacteriaceae isolates. MDR rates ranged across the region from 16.3% (Venezuela) to 24.2% (Chile) (Fig. 2), with ≥97.7% of MDR isolates collected in each country testing as susceptible to ceftazidime-avibactam. Ceftazidime-avibactam demonstrated the highest activity compared to those of all other agents tested against MDR isolates collected in all six individual countries (Tables S2A to S7A).
FIG 2.
Percentage of isolates of Enterobacteriaceae collected in 2012 to 2015 that were multidrug-resistant, by Latin American country. “Multidrug resistant” (MDR) isolates were defined as resistant, according to 2016 CLSI criteria, to three or more sentinel antimicrobial agents from different classes. The green font indicates that <20% of isolates were MDR, the orange font indicates that 20% to 29.9% of isolates were MDR, and the red font indicates that ≥30% of isolates were MDR. ARG, Argentina; BRA, Brazil; CHL, Chile; COL, Colombia; MEX, Mexico; VEN, Venezuela.
Of the 1,794 P. aeruginosa isolates collected from 2012 to 2015, 87.4% were susceptible to ceftazidime-avibactam (MIC90, 16 μg/ml) (Table 1). Percent susceptibilities to other agents tested were lower than those for ceftazidime-avibactam, with the exception of that of colistin (94.9% susceptible). The percent susceptibility to ceftazidime-avibactam increased to 92.8% when only MBL-negative isolates of P. aeruginosa were considered.
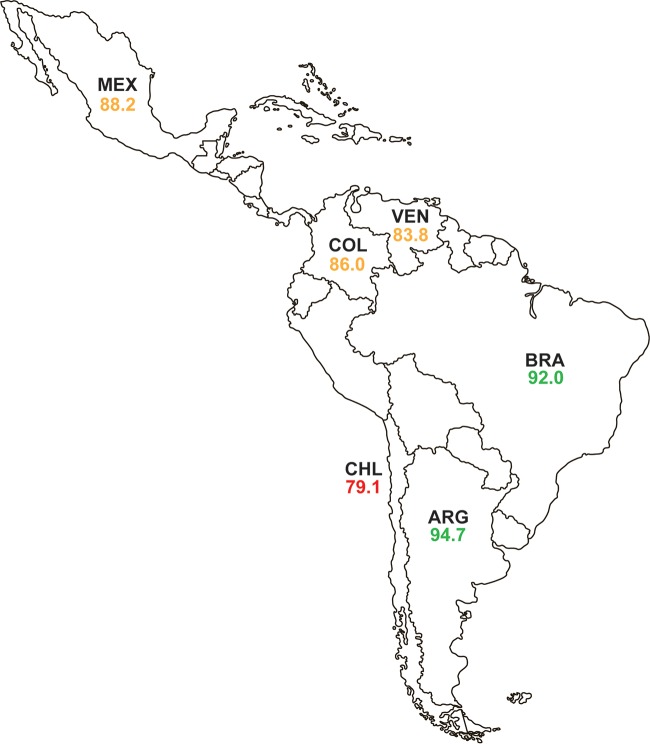
There were 750 isolates of P. aeruginosa testing with a doripenem, imipenem, or meropenem MIC of ≥4 μg/ml that were screened for β-lactamase genes. Of these 750 isolates, 110 isolates (14.7%) carried MBLs with or without additional acquired serine β-lactamases (Table 2). No acquired β-lactamase was identified in the majority of screened isolates (70.0% [525/750]) (Table 2; Fig. S5A), which were assumed to possess alterations in OprD or efflux pump expression, likely combined with hyperproduction of the intrinsic chromosomal AmpC β-lactamase (19). ESBLs were found primarily in isolates from Mexico (65.5% [36/55]) and differed in their distribution across Latin America, with PER-type enzymes found in isolates from Brazil, Chile, and Venezuela, and GES-type enzymes, comprising 67.3% ([37/55]) of ESBLs identified in P. aeruginosa isolates collected in the region, found in isolates from Argentina and Mexico (Fig. S5B). Considerable differences in the distribution of carbapenemases were also observed. VIM-2 (49.5% [97/196]), KPC-2 (27.0% [53/196]), and GES-20 (12.8% [25/196]) were the most common carbapenemases identified (Fig. S5C). VIM-2 was found in isolates from all six countries surveyed, while other MBLs were identified in Argentina, Brazil, and Mexico (IMP-type), Brazil (SPM-1), and Venezuela (VIM-50). KPC-2, which is rarely found in P. aeruginosa isolates collected outside Latin America, was identified in isolates from Argentina, Chile, and Colombia; all GES-type carbapenemases were identified in isolates collected in Mexico (Fig. S5C). In contrast to the Enterobacteriaceae, carbapenem nonsusceptibility in P. aeruginosa was not as reliant on the presence of carbapenemase genes (Fig. S5A). Ceftazidime-avibactam was not active against isolates carrying MBLs (5.5% susceptible), as expected, but it also demonstrated reduced activity against MBL-negative, ESBL-positive isolates (31.0% susceptible; composed of PER- and GES-type enzymes) and GES carbapenemase-positive isolates (27.3%) (Table 2). All of these isolates may have carried additional β-lactamases that were not included in the molecular testing algorithm and that were not inhibited by avibactam, or may have contained nonenzymatic resistance mechanisms. In contrast, 77.1% of KPC-positive isolates (MIC90, 32 μg/ml) and 89.5% of carbapenem-nonsusceptible isolates with no acquired β-lactamase detected were susceptible to ceftazidime-avibactam (MIC90, 16 μg/ml) (Table 2). Susceptibility to ceftazidime-avibactam among isolates of P. aeruginosa ranged from 79.1% (Chile) to 94.7% (Argentina) across the six countries of the Latin American region (Fig. 3).
FIG 3.
Percent susceptibility to ceftazidime-avibactam for isolates of P. aeruginosa collected in 2012 to 2015, by Latin American country. Ceftazidime-avibactam-susceptible, ≤8 μg/ml; ceftazidime-avibactam-resistant, ≥16 μg/ml by U.S. FDA criteria. The green font indicates that >90% of isolates were ceftazidime-avibactam susceptible. The orange font indicates that 80% to 89.9% of isolates were ceftazidime-avibactam susceptible. The red font indicates that <80% of isolates were ceftazidime-avibactam susceptible ARG, Argentina; BRA, Brazil; CHL, Chile; COL, Colombia; MEX, Mexico; VEN, Venezuela.
Among ceftazidime-nonsusceptible P. aeruginosa isolates (n = 538), 58.0% of isolates were susceptible to ceftazidime-avibactam (Table S8); the percent susceptibility to ceftazidime-avibactam ranged from 34.3% (Venezuela) to 77.6% (Argentina) across the countries surveyed (Tables S2A to S7A). Percentages of susceptibility to comparator agents other than colistin (95.0% susceptible) were lower than susceptibility to ceftazidime-avibactam among this subset of isolates. The activity of ceftazidime-avibactam was improved against the subsets of ceftazidime-nonsusceptible, MBL-negative isolates collected in the overall region (71.7% susceptible; Table S8) and in the individual countries (56.6% to 92.0% susceptible; Tables S2A to S7A).
The percentage of isolates of P. aeruginosa that were meropenem-nonsusceptible in Latin American countries was 35.8% (Table 1, Table S9) and ranged from 32.3% (Venezuela) to 46.8% (Chile) (Fig. S7, Tables S2A–S7A). Overall, 67.5% of meropenem-nonsusceptible isolates collected in the Latin American region remained susceptible to ceftazidime-avibactam, which was higher than the susceptibilities observed to all other agents tested except colistin (95.1% susceptible) (Table S9). Susceptibility of meropenem-nonsusceptible isolates to ceftazidime-avibactam was lowest in Venezuela (50.0% susceptible) and highest in Argentina (84.7% susceptible); percentages of susceptibility increased 1.8% to 45.8%, to 71.1% to 95.8% susceptible across the 6 countries and to 79.7% for the overall region, when isolates carrying MBLs were excluded (Table S9, Tables S2A–S7A).
Only two P. aeruginosa isolates collected in Latin America in 2012 to 2015 tested as resistant to colistin (MIC ≥8 μg/ml, Fig. S8) (16). These isolates were collected in Argentina and Mexico and were susceptible to ceftazidime-avibactam (Tables S2A, S6A, and S10).
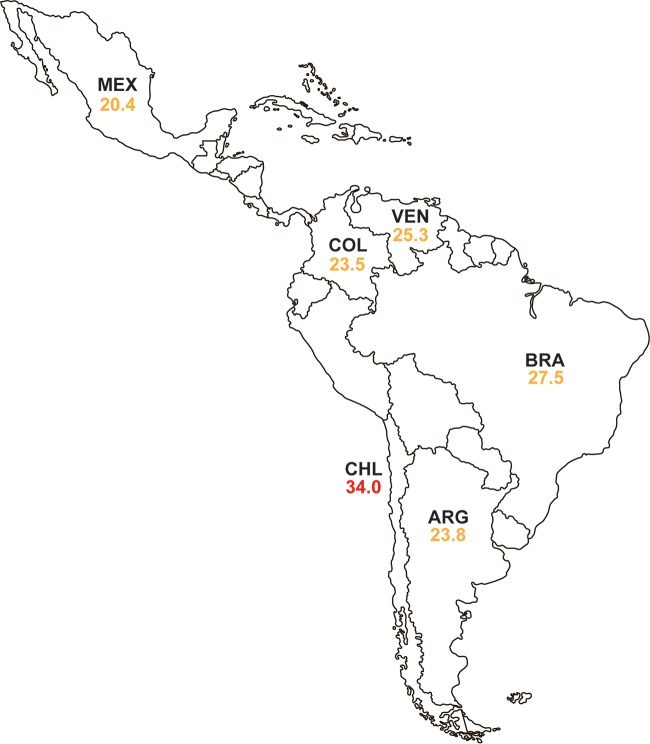
An MDR phenotype was present in 454 isolates of P. aeruginosa (25.3% of all isolates), with percentages of isolates testing as MDR varying from 20.4% (Mexico) to 34.0% (Chile) (Fig. 4). Of these MDR isolates, 57.1% were susceptible to ceftazidime-avibactam, a higher percent susceptibility than that observed for all other agents tested except colistin (94.9% susceptible) (Table S11). Ceftazidime-avibactam was least active against MDR isolates from Venezuela (37.3% susceptible; MIC90, 32 μg/ml) and Mexico (45.2% susceptible, MIC90, >128 μg/ml), and it was most active against MDR isolates from Argentina (77.6% susceptible; MIC90, 16 μg/ml) and Brazil (72.4% susceptible; MIC90, 32 μg/ml) (Tables S2A to S7A). Activity was improved 2.4% to 55.8% against subsets of MBL-negative, MDR isolates, but the associated MIC90 value decreased into the susceptible range only for isolates collected in Venezuela (MIC90, 8 μg/ml) (Tables S2A to S7A). Ceftazidime-avibactam remained the second most active agent after colistin, and in one case was the third most active agent (after amikacin and colistin) against all MDR and MBL-negative MDR isolates in each of the six countries and in the region as a whole (Table S11).
FIG 4.
Percentage of isolates of P. aeruginosa collected in 2012 to 2015 that were multidrug-resistant, by Latin American country. “Multidrug resistant” (MDR) isolates were defined as resistant, according to 2016 CLSI criteria, to three or more sentinel antimicrobial agents from different classes. The green font indicates that <20% of isolates were MDR. The orange font indicates that 20% to 29.9% of isolates were MDR. The red font indicates that ≥30% of isolates were MDR. ARG, Argentina; BRA, Brazil; CHL, Chile; COL, Colombia; MEX, Mexico; VEN, Venezuela.
DISCUSSION
In the current study, we found that 99.7% of isolates of Enterobacteriaceae were susceptible to ceftazidime-avibactam, with a MIC90 of 0.5 μg/ml, and that ceftazidime-avibactam MIC90 values for individual species or species groups within the family Enterobacteriaceae ranged from 0.12 μg/ml (Proteeae) to 1 μg/ml (Enterobacter spp. and K. pneumoniae), with only minor variation (0.7%) observed in the percent susceptibility to ceftazidime-avibactam, from 99.2% (Enterobacter spp.) to 99.9% (E. coli and Proteeae) (Table 1). Our results are in agreement with those of earlier studies by Rossi et al., who reported that 99.1% (339/342) of clinical isolates of Enterobacteriaceae collected at a teaching hospital in São Paulo, Brazil in 2014 to 2015 were susceptible to ceftazidime-avibactam (2), and by Flamm et al., who observed an MIC90 of 0.25 μg/ml for ceftazidime-avibactam tested against 130 urinary isolates of Enterobacteriaceae collected in seven Latin American countries in 2011 (4).
In the current study, we also noted that 87.4% of all isolates of P. aeruginosa tested were susceptible to ceftazidime-avibactam, with an MIC90 of 16 μg/ml (Table 1). Our results confirm earlier observations made regarding clinical isolates of P. aeruginosa from Latin American laboratories (2, 4, 6). Rossi et al. reported 84.0% (21/25) of P. aeruginosa isolates to be susceptible to ceftazidime-avibactam (2), Flamm et al. reported a ceftazidime-avibactam MIC90 of 16 μg/ml for 13 isolates of P. aeruginosa (4), and Nichols et al. reported 88.7% of 1,088 isolates of P. aeruginosa collected in six Latin American countries to be susceptible to ceftazidime-avibactam (MIC90, 16 μg/ml) (6). In isolate subset analysis, Nichols et al. reported that 60.3% of ceftazidime-nonsusceptible (n = 310) and 70.2% of meropenem-nonsusceptible (n = 382) isolates of P. aeruginosa were susceptible to ceftazidime-avibactam (6).
ESBL production and carbapenem resistance among Enterobacteriaceae isolates are important concerns across all Latin American countries. Previous surveillance studies testing clinical isolates of Enterobacteriaceae have consistently reported ESBL rates in many Latin American countries to be 20% to >40% for both E. coli and K. pneumoniae, and have also reported rates of carbapenem-resistant Enterobacteriaceae that approach and often exceed 10%, particularly for K. pneumoniae and Enterobacter spp. (5, 7–9). The distribution of ESBL and carbapenemase types observed in the current study was in general agreement with previous reports for Latin America (3, 15, 20). In the current study, we found that ceftazidime-avibactam inhibited 99.9% of ESBL-positive isolates, 99.7% of ceftazidime-nonsusceptible isolates, 99.5% of meropenem-nonsusceptible isolates, 99.0% of AmpC-positive isolates, 99.6% of MDR isolates, and 99.2% of colistin-resistant isolates of Enterobacteriaceae that were concurrently MBL-negative. Among clinical isolates of P. aeruginosa from Latin American countries, other investigators have reported country-specific percentages of susceptibility to ceftazidime that ranged from 50% to 80%, while 60% to 70% of isolates were carbapenem susceptible (7, 9), similar to our findings in the current study.
The current study identified only 24 isolates of Enterobacteriaceae (0.3% of all isolates) that were resistant to ceftazidime-avibactam; 16 (66.7%) of these 24 isolates were MBL positive (Table 2). For eight isolates (four Enterobacter cloacae and one each of Citrobacter freundii, Klebsiella oxytoca, K. pneumoniae, and Raoultella ornithinolytica), reduced ceftazidime-avibactam susceptibility could not be attributed to MBL production. These eight isolates were comprised of one CTX-M-2-positive isolate, one KPC-2-positive isolate, and six isolates that did not meet the criteria for molecular characterization or in which no acquired β-lactamase or only an OSBL was identified. Of these, two isolates were resistant to carbapenems, all were nonsusceptible or resistant to aztreonam, ceftazidime, and cefepime, and all displayed elevated MICs to another tested avibactam-cephalosporin combination. The mechanism(s) of reduced susceptibility remain to be determined for these isolates but may reflect the presence of an avibactam-insensitive β-lactamase that was not detected using the current molecular algorithm (20, 21) or a combination of mechanisms, such as increased KPC production with porin deficiency and altered efflux (22–24). Sequence insertions in penicillin-binding protein 3 have also been reported to result in reduced susceptibility to avibactam-cephalosporin combinations, although ceftazidime-avibactam remained active (MIC ≤8 μg/ml) against the isolates reported to date (25, 26). Upregulation of efflux transport systems or porin loss alone were not implicated in reduced susceptibility to ceftazidime-avibactam in a previous direct test of those mechanisms (27). Recently, isolated clinical cases in which ceftazidime-avibactam was prescribed to treat patients infected with KPC-producing K. pneumoniae have resulted in the emergence of resistance to ceftazidime-avibactam during treatment (23, 28–31). In each case, resistance to ceftazidime-avibactam was reported to be the result of mutations within a plasmid-borne blaKPC-3, which was associated with a porin OmpK35 deficiency in one instance (23) and which coincidently restored carbapenem susceptibility in some isolates (28).
In general, the presence of an MBL gene was associated with in vitro resistance to ceftazidime-avibactam among isolates of Enterobacteriaceae (Table 2) (13, 14). Carbapenem-nonsusceptible isolates without MBLs, such as those carrying KPC (12–14, 32) or OXA-48-like (13, 32) carbapenemases, were generally susceptible to ceftazidime-avibactam (Table 2). Intrinsic imipenem resistance among Proteeae species (16) did not affect susceptibility to ceftazidime-avibactam (Table 1).
Among isolates of P. aeruginosa tested in the current study, we observed that 58.0% of ceftazidime-nonsusceptible isolates (range, 34.3% [Venezuela] to 77.6% [Argentina]) and 67.5% of meropenem-nonsusceptible isolates (range, 50.0% [Venezuela] to 84.7% [Argentina]) were susceptible to ceftazidime-avibactam. If only MBL-negative isolates of P. aeruginosa were considered, 71.7% of ceftazidime-nonsusceptible isolates (range, 56.6% [Mexico] to 92.0% [Venezuela]) and 79.7% of meropenem-nonsusceptible isolates (range, 71.1% [Mexico] to 95.8% [Venezuela]) were susceptible to ceftazidime-avibactam. Only 110 isolates (14.7% of molecularly characterized isolates; 6.1% of all isolates tested) were found to carry genes encoding an MBL, and the majority of MBLs identified were VIM-type (90.0% [99/110]) and IMP-type (9.1% [10/110]) enzymes (Fig. S5C). Previous studies also identified VIM-type MBLs among isolates of P. aeruginosa from Argentina, Chile, Colombia, Mexico, and Venezuela, and IMP-type MBLs among isolates of P. aeruginosa from Brazil and Mexico (33, 34). Isolates carrying blaSPM have been reported to be endemic among clinical isolates of P. aeruginosa in Brazil (35); however, we identified only one isolate carrying blaSPM, collected in Brazil, in the current study. PER- and GES-type β-lactamases were heavily represented in P. aeruginosa isolates collected in Latin America, especially in isolates from Mexico (as reported previously [36]). Although MBL-negative subsets of ESBL- and GES-producing isolates showed reduced susceptibility to ceftazidime-avibactam, the enzyme variants found in these isolates are expected to be susceptible to inhibition by avibactam (37), suggesting the presence of additional undetermined resistance mechanisms.
We conclude that clinical isolates of Enterobacteriaceae from six Latin American countries in 2012 to 2015 were highly susceptible to ceftazidime-avibactam (99.0% susceptible) and that ceftazidime-avibactam was more active than currently available antimicrobial agents of last resort (e.g., amikacin, colistin, and tigecycline). Only 17 isolates of Enterobacteriaceae (0.2% of all isolates tested) carried an MBL. The current study demonstrated ceftazidime-avibactam to be a potent agent against ceftazidime-nonsusceptible, meropenem-nonsusceptible, colistin-resistant, and MDR isolates of Enterobacteriaceae. Ceftazidime-avibactam (87.4% susceptible) was the second most potent agent tested against isolates of P. aeruginosa from six Latin American countries in 2012 to 2015, after colistin (94.9% susceptible). Based on the in vitro susceptibilities and proven clinical efficacy (30, 31, 38–41), ceftazidime-avibactam should be considered in the treatment of indicated infections caused by susceptible Enterobacteriaceae and P. aeruginosa strains.
MATERIALS AND METHODS
Clinical isolates of Enterobacteriaceae and P. aeruginosa.
The INFORM global surveillance program collected and confirmed the identities of 9,459 nonduplicate clinical isolates of Gram-negative bacilli (7,665 isolates of Enterobacteriaceae and 1,794 isolates of P. aeruginosa) from 26 medical center laboratories in six countries in Latin America from 2012 to 2015. The INFORM global surveillance program annually requested that each participating medical center laboratory collect predefined quotas of selected bacterial pathogens isolated from patients with specific types of infection (6, 10). Collection was limited to one isolate per patient. All isolates were determined to be clinically significant by algorithms used by the participating laboratories and were collected irrespective of antimicrobial susceptibility profile (6, 10). The demographic information associated with the 9,459 isolates is summarized in Table S1 in the supplemental material. All isolates were transported to International Health Management Associates, Inc. (IHMA; Schaumburg, IL) which served as the central reference laboratory for the INFORM global surveillance study. At IHMA, the identity of each isolate was confirmed using a Bruker Biotyper matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF) instrument (Bruker Daltonics, Billerica, MA).
Antimicrobial susceptibility testing.
The Clinical and Laboratory Standards Institute (CLSI)-defined broth microdilution antimicrobial susceptibility testing was performed using 96-well broth microdilution panels prepared in-house at IHMA (16, 42). Avibactam was tested at a fixed concentration of 4 μg/ml in combination with doubling dilutions of ceftazidime (16). MICs were interpreted using 2016 CLSI breakpoints (16) with the following exceptions. Ceftazidime-avibactam MICs were interpreted using U.S. FDA MIC breakpoints for Enterobacteriaceae and P. aeruginosa (susceptible, ≤8 μg/ml; resistant, ≥16 μg/ml) (38), as CLSI MIC interpretative breakpoints were not published at that time. U.S. FDA MIC interpretative breakpoints were also used for tigecycline (43). EUCAST MIC interpretative breakpoints were used for colistin tested against Enterobacteriaceae (44), as CLSI criteria are not available.
Isolates of Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, and Proteus mirabilis that tested with MICs of ≥2 μg/ml to ceftazidime or aztreonam were subjected to phenotypic combination testing with clavulanic acid to confirm the presence of an ESBL (16). An MDR phenotype was defined, according to the criteria of Magiorakos et al., as resistance to sentinel agents from three or more antimicrobial agent classes, including cephalosporins (sentinel agent cefepime), monobactams (aztreonam), β-lactam–β-lactamase inhibitor combinations (piperacillin-tazobactam), carbapenems (meropenem), fluoroquinolones (levofloxacin), aminoglycosides (amikacin), glycylcyclines (tigecycline), and polymyxins (colistin) (45).
Screening of clinical isolates of Enterobacteriaceae and P. aeruginosa for β-lactamase genes.
All isolates of Enterobacteriaceae with MICs of ≥2 μg/ml to doripenem, imipenem, or meropenem and all isolates of E. coli, K. pneumoniae, K. oxytoca and P. mirabilis demonstrating a positive ESBL confirmatory test or MICs of ≥16 μg/ml to ceftazidime were screened for β-lactamase content using a combination of the microarray-based Check-MDR CT101 kit (Check-Points, Wageningen, Netherlands) and published multiplex PCR assays (46). These assays were intended to detect genes encoding carbapenemases (KPC, GES, NDM, IMP, VIM, SPM, GIM, and OXA-48-like), ESBLs (TEM, SHV, CTX-M, VEB, PER, and GES), original-spectrum β-lactamases (OSBLs; TEM and SHV enzymes that do not contain substitutions at amino acid positions 104, 164, or 238 in TEM or at 146, 238, or 240 in SHV, which are associated with ESBL activity) (47), and plasmid-mediated AmpC β-lactamases (ACC, ACT, CMY, DHA, FOX, MIR, and MOX) as previously described (46). All isolates of P. aeruginosa testing with MICs of ≥4 μg/ml to doripenem, imipenem, or meropenem were screened for the genes encoding carbapenemases, ESBLs, and OSBLs listed above, plus OXA-24/40-like β-lactamases, as described previously (6). Enzyme variants were identified by amplification of full-length β-lactamase genes followed by DNA sequencing, and comparison of the sequences generated to the National Center for Biotechnology Information database (www.ncbi.nlm.nih.gov) and the Lahey Clinic website (www.lahey.org/studies).
Data availability.
The sequences of two new β-lactamase variants identified during this study were deposited in GenBank under accession no. KP727573 (OXA-439) and KU663375 (VIM-50).
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge and thank all INFORM participants and IHMA laboratory personnel for their contributions to the INFORM program.
This study was performed at IHMA and was supported financially by AstraZeneca Pharmaceuticals LP (AZ). AZ financial support included compensation paid to IHMA for preparing the manuscript.
The AZ product ceftazidime-avibactam was acquired by Pfizer in December 2016. K.M.K., S.K.B., and D.F.S. are employees of IHMA. G.G.S. and B.L.M.D. were employees of, and shareholders in, AZ at the time of this study, and are currently employees of Pfizer, Inc. J.A.K. is an employee of the University of Manitoba and Diagnostic Services Manitoba and is a consultant to IHMA. The IHMA authors and J.A.K. do not have personal financial interests in the sponsor of this study.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01814-18.
REFERENCES
- 1.de Jonge BLM, Karlowsky JA, Kazmierczak KM, Biedenbach DJ, Sahm DF, Nichols WW. 2016. In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible Enterobacteriaceae isolates collected during the INFORM global surveillance study (2012 to 2014). Antimicrob Agents Chemother 60:3163–3169. doi: 10.1128/AAC.03042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossi F, Cury AP, Franco MRG, Testa R, Nichols WW. 2017. The in vitro activity of ceftazidime-avibactam against 417 gram-negative bacilli collected in 2014 and 2015 at a teaching hospital in São Paulo, Brazil. Braz J Infect Dis 21:569–573. doi: 10.1016/j.bjid.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlowsky JA, Lob SH, Kazmierczak KM, Badal RE, Young K, Motyl MR, Sahm DF. 2017. In vitro activity of imipenem against carbapenemase-positive Enterobacteriaceae isolates collected by the SMART global surveillance program from 2008 to 2014. J Clin Microbiol 55:1638–1649. doi: 10.1128/JCM.02316-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flamm RK, Sader HS, Farrell DJ, Jones RN. 2014. Ceftazidime-avibactam and comparator agents tested against urinary tract isolates from a global surveillance program (2011). Diagn Microbiol Infect Dis 80:233–238. doi: 10.1016/j.diagmicrobio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Jones RN, Guzman-Blanco M, Gales AC, Gallegos B, Castro ALL, Martino MDV, Vega S, Zurita J, Cepparulo M, Castanheira M. 2013. Susceptibility rates in Latin American nations: report from a regional resistance surveillance program (2011). Braz J Infect Dis 17:672–681. doi: 10.1016/j.bjid.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichols WW, de Jonge BLM, Kazmierczak KM, Karlowsky JA, Sahm DF. 2016. In vitro susceptibility of global surveillance isolates of Pseudomonas aeruginosa to ceftazidime-avibactam (INFORM 2012 to 2014). Antimicrob Agents Chemother 60:4743–4749. doi: 10.1128/AAC.00220-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlowsky JA, Hoban DJ, Hackel MA, Lob SH, Sahm DF. 2017. Resistance among gram-negative ESKAPE pathogens isolated from hospitalized patients with intra-abdominal and urinary tract infections in Latin American countries: SMART 2013–2015. Braz J Infect Dis 21:343–348. doi: 10.1016/j.bjid.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sader HS, Castanheira M, Farrell DJ, Flamm RK, Mendes RE, Jones RN. 2016. Tigecycline antimicrobial activity tested against clinical bacteria from Latin American medical centres: results from SENTRY antimicrobial surveillance program (2011-2014). Int J Antimicrob Agents 48:144–150. doi: 10.1016/j.ijantimicag.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Canigia L, Dowzicky MJ. 2012. Susceptibility of important gram-negative pathogens to tigecycline and other antibiotics in Latin America between 2004 and 2010. Ann Clin Microbiol Antimicrob 11:29. doi: 10.1186/1476-0711-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlowsky JA, Biedenbach DJ, Kazmierczak KM, Stone GG, Sahm DF. 2016. Activity of ceftazidime-avibactam against extended-spectrum- and AmpC β-lactamase-producing Enterobacteriaceae collected in the INFORM global surveillance study from 2012 to 2014. Antimicrob Agents Chemother 60:2849–2857. doi: 10.1128/AAC.02286-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazmierczak KM, Biedenbach DJ, Hackel M, Rabine S, de Jonge BLM, Bouchillon SK, Sahm DF, Bradford PA. 2016. Global dissemination of blaKPC into bacterial species beyond Klebsiella pneumoniae and in vitro susceptibility to ceftazidime-avibactam and aztreonam-avibactam. Antimicrob Agents Chemother 60:4490–4500. doi: 10.1128/AAC.00107-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papp-Wallace KM, Bajaksouzian S, Abdelhamed AM, Foster AN, Winkler ML, Gatta JA, Nichols WW, Testa R, Bonomo R, Jacobs MR. 2015. Activities of ceftazidime, ceftaroline and aztreonam alone and combined with avibactam against isogenic Escherichia coli strains expressing selected single β-lactamases. Diagn Microbiol Infect Dis 82:65–69. doi: 10.1016/j.diagmicrobio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 55:390–394. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Estabrook M, Jacoby GA, Nichols WW, Testa RT, Bush K. 2015. In vitro susceptibility of characterized β-lactamase-producing strains tested with avibactam combinations. Antimicrob Agents Chemother 59:1789–1793. doi: 10.1128/AAC.04191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazmierczak KM, Lob SH, Hoban DJ, Hackel MA, Badal RE, Bouchillon SK. 2015. Characterizaton of extended-spectrum beta-lactamases and antimicrobial resistance of Klebsiella pneumoniae in intra-abdominal infection isolates in Latin America, 2008–2012. Results of the Study for Monitoring Antimicrobial Resistance Trends. Diagn Microbiol Infect Dis 82:209–214. doi: 10.1016/j.diagmicrobio.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing, 26th informational supplement. CLSI document M100-S26. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Oueslati S, Nordmann P, Poirel L. 2015. Heterogeneous hydrolytic features for OXA-48-like β-lactamases. J Antimicrob Chemother 70:1059–1063. doi: 10.1093/jac/dku524. [DOI] [PubMed] [Google Scholar]
- 18.Stojanoski V, Adamski CJ, Hu L, Mehta SC, Sankaran B, Zwart P, Prasad BV, Palzkill T. 2016. Removal of the side chain at the active-site serine by a glycine substitution increases the stability of a wide range of serine β-lactamases by relieving steric strain. Biochemistry 55:2479–2490. doi: 10.1021/acs.biochem.6b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lister PD, Wolter DJ, Hanson ND. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan LK, Weinstein RA. 2017. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 215:S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahiri SD, Giacobbe RA, Johnstone MR, Alm RA. 2014. Activity of avibactam against Enterobacter cloacae producing an extended spectrum class C β-lactamase enzyme. J Antimicrob Chemother 69:2942–2946. doi: 10.1093/jac/dku237. [DOI] [PubMed] [Google Scholar]
- 22.Nelson K, Hemarajata P, Sun D, Rubio-Aparicio D, Tsivkovski R, Yang S, Sebra R, Kasarskis A, Nguyen H, Hanson BM, Leopold S, Weinstock G, Lomovskaya O, Humphries RM. 2017. Resistance to ceftazidime-avibactam is due to transposition of KPC in a porin-deficient strain of Klebsiella pneumoniae with increased efflux activity. Antimicrob Agents Chemother 61:e0098917. doi: 10.1128/AAC.00989-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen Z, Ding B, Ye M, Wang P, Bi Y, Wu S, Xu X, Guo Q, Wang M. 2017. High ceftazidime hydrolysis activity and porin OmpK35 deficiency contribute to the decreased susceptibility to ceftazidime/avibactam in KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother 72:1930–1936. doi: 10.1093/jac/dkx066. [DOI] [PubMed] [Google Scholar]
- 24.Castanheira M, Mendes RE, Sader HS. 2017. Low frequency of ceftazidime-avibactam resistance among Enterobacteriaceae isolates carrying blaKPC collected in hospitals from the United States from 2012 to 2015. Antimicrob Agents Chemother 61:e02369-16. doi: 10.1128/AAC.02369-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alm RA, Johnstone MR, Lahiri SD. 2015. Characterization of Escherichia coli NDM isolates with decreased susceptibility to aztreonam/avibactam: role of a novel insertion in PBP3. J Antimicrob Chemother 70:1420–1428. doi: 10.1093/jac/dku568. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Kashikar A, Brown CA, Denys G, Bush K. 2017. Unusual Escherichia coli PBP 3 insertion sequence identified from a collection of carbapenem-resistant Enterobacteriaceae tested in vitro with a combination of ceftazidime-, ceftaroline-, or aztreonam-avibactam. Antimicrob Agents Chemother 61:e00389-17. doi: 10.1128/AAC.00389-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagès J-M, Peslier S, Keating TA, Lavigne J-P, Nichols WW. 2016. The role of the outer membrane and porins in the susceptibility of β-lactamase-producing Enterobacteriaceae to ceftazidime-avibactam. Antimicrob Agents Chemother 60:1349–1359. doi: 10.1128/AAC.01585-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shields RK, Chen L, Cheng S, Chavada KD, Press EG, Synder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. 2017. In vitro selection of meropenem resistance among ceftazidime-avibactam-resistant, meropenem-susceptible Klebsiella pneumoniae isolates with variant KPC-3 carbapenemases. Antimicrob Agents Chemother 61:e00079-17. doi: 10.1128/AAC.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 63:1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krapp F, Grant JL, Sutton SH, Ozer EA, Barr VO. 2017. Treating complicated carbapenem-resistant Enterobacteriaceae infections with ceftazidime/avibactam: a retrospective study with molecular strain characterization. Int J Antimicrob Agents 49:770–773. doi: 10.1016/j.ijantimicag.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Castanheira M, Mills JC, Costello SE, Jones RN, Sader HS. 2015. Ceftazidime-avibactam activity tested against Enterobacteriaceae isolates from U.S. hospitals (2011 to 2013) and characterization of β-lactamase-producing strains. Antimicrob Agents Chemother 59:3509–3517. doi: 10.1128/AAC.00163-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sader HS, Castanheira M, Mendes RE, Toleman M, Walsh TR, Jones RN. 2005. Dissemination and diversity of metallo-β-lactamases in Latin America: report from the SENTRY Antimicrobial Surveillance Program. Int J Antimicrob Agents 25:57–61. doi: 10.1016/j.ijantimicag.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Hong DJ, Bae IK, Jang IH, Jeong SH, Kang HK, Lee K. 2015. Epidemiology and characteristics of metallo-β-lactamase-producing Pseudomonas aeruginosa. Infect Chemother 47:81–97. doi: 10.3947/ic.2015.47.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silveira MC, Albano RM, Asensi MD, Carvalho-Assef AP. 2016. Description of genomic islands associated to the multidrug-resistant Pseudomonas aeruginosa clone ST227. Infect Genet Evol 42:60–65. doi: 10.1016/j.meegid.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 36.Garza-Ramos U, Barrios H, Reyna-Flores F, Tamayo-Legorreta E, Catalan-Najera JC, Morfin-Otero R, Rodríguez-Noriega E, Volkow P, Cornejo-Juarez P, González A, Gaytan-Martinez J, Del Rocío Gónzalez-Martínez M, Vazquez-Farias M, Silva-Sanchez J. 2015. Widespread of ESBL- and carbapenemase GES-type genes on carbapenem-resistant Pseudomonas aeruginosa clinical isolates: a multicenter study in Mexican hospitals. Diagn Microbiol Infect Dis 81:135–137. doi: 10.1016/j.diagmicrobio.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 37.Lahiri SD, Bradford PA, Nichols WW, Alm RA. 2016. Structural and sequence analysis of class A β-lactamases with respect to avibactam inhibition: impact of Ω-loop variations. J Antimicrob Chemother 71:2848–2855. doi: 10.1093/jac/dkw248. [DOI] [PubMed] [Google Scholar]
- 38.Allergan. 2017. Avycaz® (ceftazidime and avibactam) for injection, for intravenous use, prescribing information. Allergan USA, Inc., Irvine, CA. [Google Scholar]
- 39.Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, Doi Y, Kreiswirth BN, Clancy CJ. 2017. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 61:e00883-17. doi: 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, Rank D, Llorens L, Newell P, Pachl J. 2016. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, Phase 3 program. Clin Infect Dis 62:1380–1389. doi: 10.1093/cid/ciw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stone GG, Bradford PA, Newell P, Wardman A. 2017. In vitro activity of ceftazidime-avibactam against isolates in a Phase 3 open-label clinical trial for complicated intra-abdominal and urinary tract infections caused by ceftazidime-nonsusceptible Gram-negative pathogens. Antimicrob Agents Chemother 61:e01820-16. doi: 10.1128/AAC.01820-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 10th ed CLSI document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 43.Pfizer. 2016. Tygacil® (tigecycline) injection, powder, lyophilized, for solution, prescribing information. Pfizer, Inc, Philadelphia, PA. [Google Scholar]
- 44.The European Committee on Antimicrobial Susceptibility Testing. 2016. Breakpoint tables for interpretation of MICs and zone diameters, version 6.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf.
- 45.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microb Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 46.Lob SH, Kazmierczak KM, Badal RE, Hackel MA, Bouchillon SK, Biedenbach DJ, Sahm DF. 2015. Trends in susceptibility of Escherichia coli from intra-abdominal infections to ertapenem and comparators in the United States according to data from the SMART Program, 2009 to 2013. Antimicrob Agents Chemother 59:3606–3610. doi: 10.1128/AAC.05186-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradford PA. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev 14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequences of two new β-lactamase variants identified during this study were deposited in GenBank under accession no. KP727573 (OXA-439) and KU663375 (VIM-50).