SAL200 is derived from a phage endolysin and is a novel candidate drug for the treatment of Staphylococcus aureus infection. We investigated the efficacy of the recombinant endolysin SAL200 in a lethal murine pneumonia model.
KEYWORDS: SAL200, Staphylococcus aureus, mice, phage endolysin, pneumonia
ABSTRACT
SAL200 is derived from a phage endolysin and is a novel candidate drug for the treatment of Staphylococcus aureus infection. We investigated the efficacy of the recombinant endolysin SAL200 in a lethal murine pneumonia model. Lethal pneumonia was established by intranasally administering a methicillin-susceptible (Newman) or methicillin-resistant (LAC) S. aureus strain into BALB/c mice. The mice were treated with a single intranasal administration of SAL200 or phosphate-buffered saline at 2 h after S. aureus infection. The survival rates were recorded until 60 h after the bacterial challenge. The bacterial loads in the lungs and blood, histopathology of lung tissues, and serum cytokine levels were evaluated following the S. aureus challenge. The SAL200-treated group and control group exhibited 90% to 95% and 10% to 40% survival rates, respectively. The bacterial loads in the lungs of the SAL200-treated group were significantly lower by ∼10-fold than those of the control group as early as 1 h after treatment. Histopathologic recovery of pneumonia was observed in the SAL200-treated mice. The cytokine levels were comparable between groups. These results suggest that direct administration of SAL200 into the lungs could be a potential adjunct treatment against severe pneumonia caused by S. aureus.
INTRODUCTION
Phage endolysins or phage lysins are peptidoglycan-degrading enzymes encoded in bacteriophages, which act by rapidly breaking down bacterial cell walls and releasing the phage progeny (1). The use of phage endolysins as alternatives to conventional antibiotics has been proposed because of their highly specific antibiotic activity and distinct mode of action that evades the resistance mechanisms against conventional antibiotics (2). SAL200, which is derived from a phage endolysin, is a novel investigational drug for treating Staphylococcus aureus infections. Its active pharmaceutical ingredient is a recombinant product of SAL-1, which is a phage lysin encoded in the staphylococcal phage SAP-1 (3).
Previous studies reported rapid and effective bactericidal activity of SAL200 against both encapsulated and biofilm-forming S. aureus cells as well as against planktonic S. aureus cells (4). Intravenous injection of SAL200 improved the survival of methicillin-resistant Staphylococcus aureus (MRSA)-infected mice and substantially decreased bacterial loads in the blood and spleen (4). Additionally, favorable results were reported in studies that investigated the safety of intravenous SAL200 in dogs, rats, and monkeys (5, 6). Recently, SAL200 became the first endolysin-based drug administered to humans in a phase 1 clinical trial. There were no serious adverse events or clinically significant changes in any of the participants after the infusion of a single ascending, intravenous dose up to 10 mg/kg (7).
These previous studies may be valid for evaluating the in vivo efficacy and safety of SAL200 administered intravenously for treating systemic S. aureus infections. However, the efficacy of SAL200 administered by other methods and in the treatment of organ-specific infections has not yet been studied. Here, using a lethal pneumonia murine model, we evaluated the efficacy of intranasally administered SAL200 in treating pneumonia due to S. aureus.
RESULTS
Effect of SAL200 on survival in mice with S. aureus pneumonia.
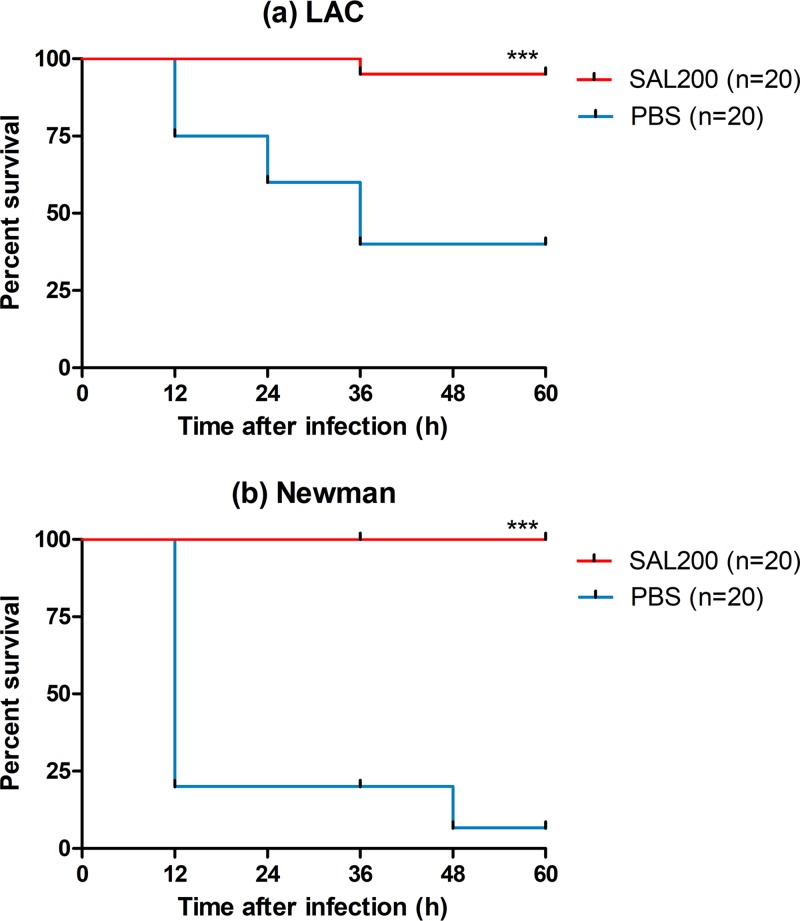
We established LAC or Newman strain-infected lethal pneumonia models in mice and administered SAL200 or phosphate-buffered saline (PBS) intranasally at 2 h after infection. In both pneumonia models, a significant survival benefit was observed in the SAL200-treated group. In the LAC strain-infected model, the SAL200-treated group and PBS-treated control group exhibited 95% and 40% survival rates, respectively (P < 0.001, log rank test). In the Newman strain-infected model, the survival rates of the SAL200-treated group and control group were 100% and 6.7%, respectively (P < 0.001) (Fig. 1).
FIG 1.
Intranasal administration of SAL200 improved survival in a murine model of S. aureus pneumonia. BALB/c mice were infected intranasally with the LAC (a) or Newman (b) strain and then treated intranasally with SAL200 or phosphate-buffered saline (PBS) solution at 2 h postinfection (n = 20 mice/group). A significant survival benefit was observed in the SAL200-treated group in both the LAC and Newman pneumonia models. Data were analyzed by the log rank test; ***, P < 0.001.
Bacterial loads in the lungs and blood after SAL200 treatment.
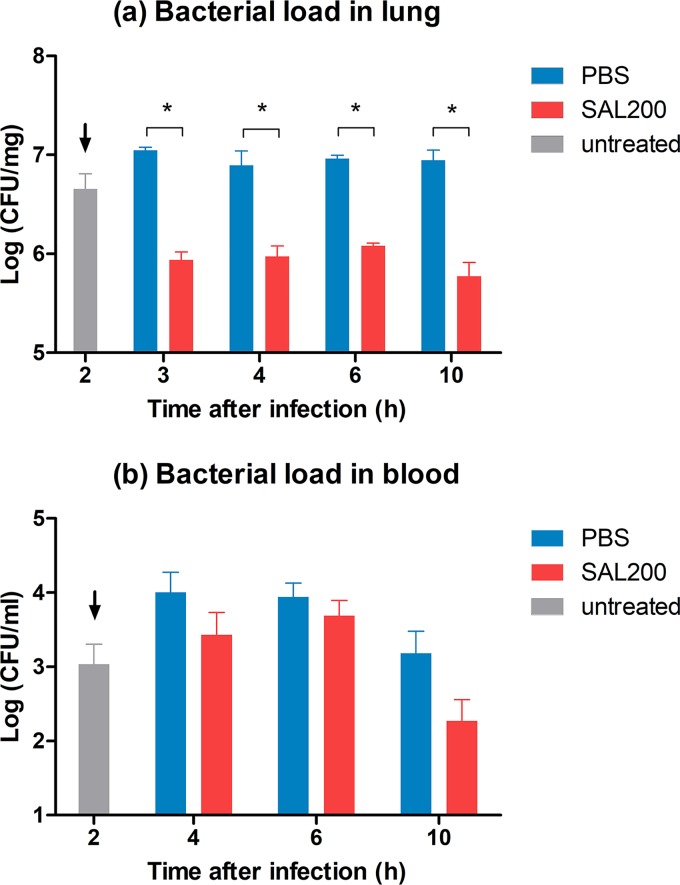
The bacterial loads in the lungs of the SAL200-treated mice were significantly lower by ∼1 log than those of the control group as early as 1 h after treatment (P = 0.021, Mann-Whitney U test). The bacterial loads in blood were decreased by ∼1 log in the SAL200-treated group compared with the control at 10 h after infection, although the difference was not statistically significant (Fig. 2).
FIG 2.
Intranasal administration of SAL200 reduced bacterial loads in the lungs and blood in a murine model of S. aureus pneumonia. BALB/c mice were infected intranasally with the LAC strain and then treated intranasally with SAL200 or phosphate-buffered saline (PBS) solution at 2 h postinfection. (a) The SAL200-treated group had a significantly lower lung bacterial load by ∼1 log than the PBS control as early as 1 h after treatment. Each column represents the mean + standard error of the mean (SEM) of two separate experiments with two replicates (n = 4). (b) Bacterial loads in blood tended to increase initially during 4 to 6 h after infection in both groups. However, in the SAL200-treated group, the load decreased and was ∼1 log lower than the PBS control group at 10 h after infection. Each column represents the mean + SEM of three separate experiments with two replicates (n = 6). Data were analyzed by Mann-Whitney U test; *, P < 0.05. Arrows indicate the time of treatment onset.
Pathologic changes in lung tissue after SAL200 treatment.
Histopathologic changes of lung tissues in SAL200- or PBS-treated mice were observed at 0, 2, 4, 6, and 10 h after the LAC challenge (n = 3 mice/group at each time point). Significant inflammatory cell infiltrations were observed at the time of treatment onset (2 h after the LAC challenge). In the PBS-treated control group, the lung pathology deteriorated over time. At 4 and 6 h postinfection, the lungs in the PBS group had high cellularity consisting of immune cell infiltrates and alveolar hemorrhage. Alveolar spaces were filled with inflammatory exudates at 10 h after infection in the PBS group. In contrast, the histopathologic findings of lungs in the SAL200-treated group revealed reductions in the immune cell infiltrations at 4 h postinfection. At 10 h postinfection, there was minimal evidence of damage in the SAL200-treated group (Fig. 3a). The pathological difference between the two groups was also demonstrated by semiquantitative score (SQS). SAL200-treated mice revealed significantly lower SQS than the PBS group at both 4 and 10 h after infection (Fig. 3b) (P < 0.05).
FIG 3.
Intranasal administration of SAL200 prevented the progression of inflammatory changes in lung tissues and improved histopathologic findings in a murine model of S. aureus pneumonia. BALB/c mice were infected intranasally with the LAC strain and then treated intranasally with SAL200 or phosphate-buffered saline (PBS) solution at 2 h postinfection. (a) Histopathologic examination of the lungs revealed significant inflammatory cell infiltration at the time of treatment onset (2 h postinfection). These changes deteriorated significantly over time in PBS-treated mice, resulting in immune cell infiltration and hemorrhage into alveolar spaces. In contrast, SAL200-treated mice had reduced inflammatory cell infiltration at 4 h postinfection and minimal evidence of inflammation by 10 h postinfection. All images are representative of the pathology from 3 mice (hematoxylin and eosin [H&E] staining; magnification, ×200). (b) Histopathologic scoring of the lungs (0, no significant changes; 1, slight damage; 2, mild to moderate damage; 3, moderate to severe damage; and 4, severe damage) for three categories (structure/congestion, hemorrhage, and cellularity). Semiquantitative score (SQS) is expressed as the sum of the scores of the three categories. SAL200-treated group revealed significantly lower SQS than the control group at both 4 and 10 h after infection. Each column represents the mean + SEM (n = 6). Data were analyzed by Mann-Whitney U test; *, P < 0.05.
Serum cytokine levels after SAL200 treatment.
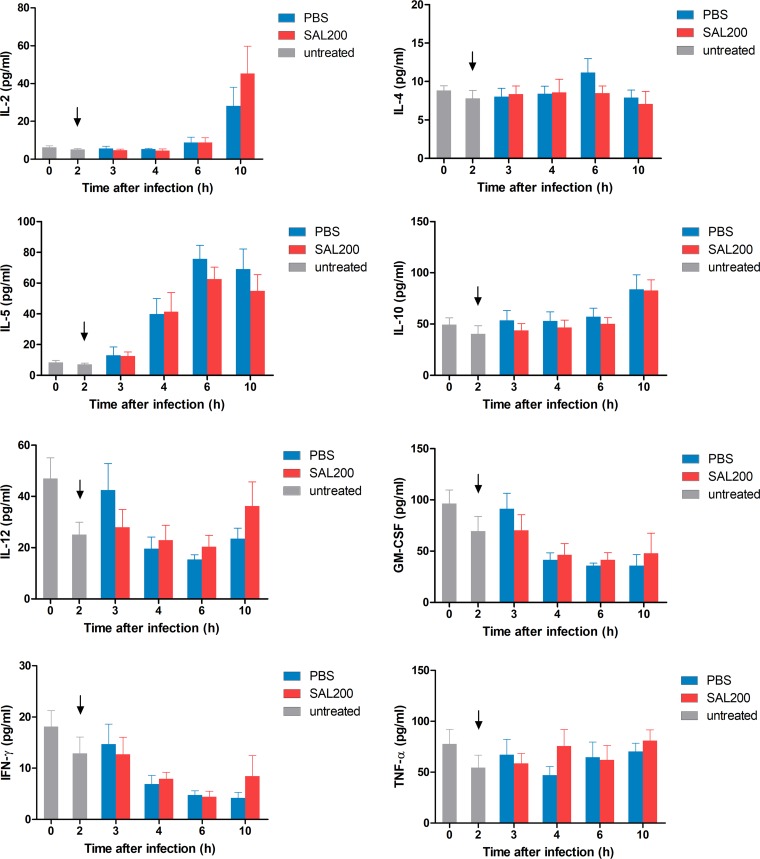
We investigated the serum interleukin 2 (IL-2), IL-4, IL-5, IL-10, IL-12, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α) levels at 0, 2, 3, 4, 6, and 10 h after the infection in a murine model of S. aureus pneumonia. There was no significant difference in cytokine levels between the SAL200-treated group and control group at all time points (Fig. 4).
FIG 4.
Changes in serum cytokine levels after the intranasal administration of SAL200 in a murine model of S. aureus pneumonia. BALB/c mice were infected intranasally with the LAC strain and then treated intranasally with SAL200 or phosphate-buffered saline (PBS) solution at 2 h postinfection. The levels of interleukin 2 (IL-2), IL-4, IL-5, IL-10, IL-12, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α) were determined at 2, 4, 6, and 10 h after the LAC challenge. At each time point, no significant difference in cytokine levels was noted between the SAL200 and PBS control group using the Mann-Whitney U test. Each column represents the mean + SEM of three separate experiments with two replicates (n = 6). Arrows indicate the time of treatment onset.
DISCUSSION
The present study demonstrated that intranasally administered SAL200 improved survival, decreased the bacterial load in the lungs and blood, promoted histopathologic improvement of lung tissue, and did not significantly increase cytokine release in a mouse S. aureus pneumonia model.
Endolysins are considered a potential alternative for conventional antibiotics (8). They are promising antimicrobials against diverse bacterial pathogens, including the multidrug-resistant organisms, because of their excellent antibacterial activity and specificity and the low possibility of developing resistance.
In recent years, S. aureus has been a major target for endolysin development because of their resistance problems. Characterization, cloning, and recombinant production of native and engineered antistaphylococcal endolysins have been described in vitro and in animal models over the past years (9, 10). The best-studied staphylococcal phage endolysins to date are LysK and its close homologues, LysGH15 and SAL-1. In spite of the amino acid sequence similarity between SAL-1 and LysK, SAL-1 was reported to exhibit higher hydrolyzing activity and reduced MICs against multiple S. aureus strains compared with LysK (3, 11–13).
In a previous study that investigated the efficacy of LysGH15 in treating pneumonia in mice, LysGH15 was intranasally administered at 1 h postinfection; the treated group exhibited a survival benefit, and lung tissue obtained 24 h postinfection revealed less inflammatory cell infiltration than the control (14). However, it is unclear whether the pneumonia was induced at the time treatment was initiated because direct histopathologic investigation of the lung tissue was not performed at 1 h postinfection. In contrast, we administered SAL200 at 2 h postinfection and established pathological confirmation of pneumonia at the time treatment was initiated. This suggests that SAL200 has potential as a therapeutic agent for pneumonia rather than preventing invasive infection at the prepneumonia stage.
The induction of the inflammatory response triggered by the degraded bacterial component may cause a safety issue of the lysin treatment. The degradation of S. aureus by lysin causes the rapid release of abundant peptidoglycans, lipoteichoic acid, and exotoxins and results in an inflammatory response and cytokine release (15–19). In a previous study that evaluated the inflammatory response caused by LysGH15 given concurrently with MRSA using an intraperitoneal route, mRNA levels of IFN-γ, IL-4, and IL-6 peaked at 12 h after the MRSA injection. In addition, the levels were higher than that of MRSA-infected mice without lysin treatment. The cytokine mRNA levels subsequently declined to normal at 24 h in the lysin-treated group (15). These peak increases in cytokines within the first 6 to 12 h of infection are consistent with the rapid reduction of bacterial cells (15, 19).
Although the cytokine studies of intranasal lysin therapy to date were limited to measuring levels in bronchoalveolar lavage fluid, it is necessary to investigate serum cytokine levels as they reflect the potential risk of immediate systemic inflammatory reactions caused by intranasal lysin treatment in the pneumonia model (14, 19). We measured cytokine levels in serum and found that inhaled SAL200 did not significantly increase serum inflammatory cytokines in mice with pneumonia until 10 h postinfection, even though the rapid reduction in bacterial load occurred during this time. This finding suggests that SAL200 could be administered safely to treat pneumonia without the additional risk of inducing severe proinflammatory reactions, including septic shock and multiorgan failure (20).
Because a substantial number of mice in the control group were dead within 12 h of infection in the survival study, the bacterial load in the lungs and blood were investigated only until 12 h postinfection to exclude potential selection bias. Complete eradication of S. aureus in lung tissue was not observed after the single SAL200 administration used in this study. This result is consistent with previous studies on intranasal lysin treatment in pneumonia, where the bacterial load in the lungs was detectable until 24 to 48 h after the treatment; although, the relatively short duration of observation in our study makes a direct comparison difficult (14, 19). In a recent study that investigated the effect of SAL200 combined with standard-of-care antibiotics (SOC) in vitro and in mouse models, the combination of intravenous SAL200 with SOC significantly reduced the blood bacterial density in bacteremic mice and achieved lower levels of bacteremia than SAL200 or SOC treatment alone, indicating the synergistic effect of SAL200 and SOC treatment (21). This synergism indicates that further studies should investigate the effect of inhaled SAL200 in combination with intravenous SOC treatment.
Several issues need to be resolved to use intranasal SAL200 therapy to treat S. aureus pneumonia in practice. First, the therapeutic safety of inhaled SAL200 has to be assessed in human safety testing. Second, appropriate pharmacokinetic and pharmacodynamic studies are needed to optimize the inhalation treatment dose and time.
In this study, a single intranasal administration of SAL200 significantly reduced the pulmonary bacterial burden in lethal murine S. aureus pneumonia, leading to highly increased survival without a significant increase in inflammatory cytokines. Our data suggest that direct administration of SAL200 into the lungs may be a potential treatment against severe pneumonia caused by S. aureus.
MATERIALS AND METHODS
Bacterial preparation.
A lethal model of mouse S. aureus pneumonia was established using methicillin-susceptible (Newman) and methicillin-resistant (LAC) S. aureus strains. The Newman strain is a human S. aureus isolate extensively used for murine infection models (22). LAC USA300 is a resistant community-acquired MRSA isolate obtained from the Network on Antimicrobial Resistance in S. aureus program. Each strain was cultured to a logarithmic phase into tryptic soy broth (Becton, Dickinson, and Company, Sparks, MD, USA) with shaking at 37°C. The bacteria were pelleted by centrifugation and then suspended in phosphate-buffered saline (PBS). Serial dilutions were performed to reach the appropriate concentration.
Mouse model of lethal pneumonia.
Six-week-old female BALB/c mice (SamtakoBioKorea, Osan-si, Gyeonggi-do, Republic of Korea) weighing between 17 and 19 g were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved housing facility. The animals adjusted to their environment for 7 days prior to procedures and received free access to food and water throughout the study. All procedures were performed humanely with minimal suffering under the approval of the Seoul National University Hospital Institutional Animal Care and Use Committee. To establish the lethal pneumonia model, mice were anesthetized with intraperitoneal ketamine and xylazine. A total of 30 μl, containing 1.8 × 109 to 2.2 × 109 CFU, of the LAC or Newman strain was administered at the anterior of nares using a micropipette, and the animals inhaled the bacterial suspension.
Survival study.
The protective effects of SAL200 on the mice with pneumonia were investigated with survival studies. Following infection with S. aureus, the mice were treated with single intranasal administration of SAL200 (0.3 mg/mouse) or PBS (30 μl/mouse) at 2 h postinfection. SAL200 or PBS was administered at the anterior of nares using a micropipette, and the mice inhaled the solutions. The survival rates of the mice were recorded every 12 h until 60 h after the bacterial challenge. The experiments were repeated twice for each strain of S. aureus.
Bacterial load, lung pathology, and cytokine study.
To determine the bacterial load in the lungs and blood and analyze the histopathology, the mice were treated intranasally with SAL200 (0.3 mg/mouse) or PBS (30 μl/mouse) at 2 h following the challenge with the LAC strain. Two mice from each group were euthanized by cardiac puncture at 2, 3, 4, 6, and 10 h following the challenge. Blood samples were collected from two mice from each group and were investigated for bacterial load and cytokines. The cytokine concentrations in the serum were measured by multiplex immunoassay using a Bio-Plex multiplex system (Bio-Rad Laboratories, Hercules, CA, USA), according to the manufacturer’s instructions. The lower limit of detecting each cytokine was 0.8 to 4.0 pg/ml. Lung tissue samples collected from a mouse in each group were suspended in filter-sterilized PBS and homogenized with a tissue homogenizer (Omni TH-2; Omni International, USA). Serial dilutions were made and plating performed to measure the bacterial loads of the lungs. The lungs of the other euthanized mice were harvested and placed immediately in 4% formalin. The formalin-fixed lungs were processed and stained with hematoxylin and eosin and then analyzed using an Eclipse Ci-L upright microscope (Nikon Instruments, Inc., New York, USA). A semiquantitative scoring system was used to compare the pathological changes in SAL200-treated and control mice at 4 and 10 h after infection. The lung section for each condition was analyzed by two blind observers according to the following categories: structural abnormalities/congestion, hemorrhaging, and cellularity. Each lung section was given a score ranging from 0 to 4 for each of the three categories, as follows: 0, no significant changes; 1, slight damage; 2, mild to moderate damage; 3, moderate to severe damage; and 4, severe damage. The semiquantitative score (SQS) is presented as the sum of the scores for all three categories. The experiments were repeated 2 to 3 times.
Statistical analysis.
The IBM SPSS statistics software package (version 22.0; SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. Kaplan-Meier survival curves were analyzed by the log-rank test; other experimental data were analyzed by a repeated measures analysis of variance or Mann-Whitney U test. A P value of <0.05 was considered significant.
ACKNOWLEDGMENTS
This work was supported by grants from the Yuhan Corporation.
SAL200 was provided by iNtRON Biotechnology, Inc. (Seongnam, Republic of Korea), which was not involved in designing and performing experiments or writing the manuscript. We are grateful to Su Jin Choi and Young Mi Jo (Seoul National University College of Medicine, Seoul, Republic of Korea) for their technical support throughout the experiments.
REFERENCES
- 1.Young R, Wang I-N, Roof WD. 2000. Phages will out: strategies of host cell lysis. Trends Microbiol 8:120–128. [DOI] [PubMed] [Google Scholar]
- 2.Loessner MJ. 2005. Bacteriophage endolysins—current state of research and applications. Curr Opin Microbiol 8:480–487. doi: 10.1016/j.mib.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Jun SY, Jung GM, Son JS, Yoon SJ, Choi YJ, Kang SH. 2011. Comparison of the antibacterial properties of phage endolysins SAL-1 and LysK. Antimicrob Agents Chemother 55:1764–1767. doi: 10.1128/AAC.01097-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jun SY, Jung GM, Yoon SJ, Oh MD, Choi YJ, Lee WJ, Kong JC, Seol JG, Kang SH. 2013. Antibacterial properties of a pre-formulated recombinant phage endolysin, SAL-1. Int J Antimicrob Agents 41:156–161. doi: 10.1016/j.ijantimicag.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Jun SY, Jung GM, Yoon SJ, Choi YJ, Koh WS, Moon KS, Kang SH. 2014. Preclinical safety evaluation of intravenously administered SAL200 containing the recombinant phage endolysin SAL-1 as a pharmaceutical ingredient. Antimicrob Agents Chemother 58:2084–2088. doi: 10.1128/AAC.02232-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jun SY, Jung GM, Yoon SJ, Youm SY, Han HY, Lee JH, Kang SH. 2016. Pharmacokinetics of the phage endolysin-based candidate drug SAL200 in monkeys and its appropriate intravenous dosing period. Clin Exp Pharmacol Physiol 43:1013–1016. doi: 10.1111/1440-1681.12613. [DOI] [PubMed] [Google Scholar]
- 7.Jun SY, Jang IJ, Yoon S, Jang K, Yu KS, Cho JY, Seong MW, Jung GM, Yoon SJ, Kang SH. 2017. Pharmacokinetics and tolerance of the phage endolysin-based candidate drug SAL200 after a single intravenous administration among healthy volunteers. Antimicrob Agents Chemother 61:e02629-16. doi: 10.1128/AAC.02629-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haddad Kashani H, Schmelcher M, Sabzalipoor H, Seyed Hosseini E, Moniri R. 2018. Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: current status of research and novel delivery strategies. Clin Microbiol Rev 31:e00071-17. doi: 10.1128/CMR.00071-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roach DR, Donovan DM. 2015. Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage 5:e1062590. doi: 10.1080/21597081.2015.1062590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmelcher M, Donovan DM, Loessner MJ. 2012. Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7:1147–1171. doi: 10.2217/fmb.12.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Flaherty S, Coffey A, Meaney W, Fitzgerald GF, Ross RP. 2005. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J Bacteriol 187:7161–7164. doi: 10.1128/JB.187.20.7161-7164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmelcher M, Shen Y, Nelson DC, Eugster MR, Eichenseher F, Hanke DC, Loessner MJ, Dong S, Pritchard DG, Lee JC, Becker SC, Foster-Frey J, Donovan DM. 2015. Evolutionarily distinct bacteriophage endolysins featuring conserved peptidoglycan cleavage sites protect mice from MRSA infection. J Antimicrob Chemother 70:1453–1465. doi: 10.1093/jac/dku552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Flaherty S, Ross RP, Meaney W, Fitzgerald GF, Elbreki MF, Coffey A. 2005. Potential of the polyvalent anti-Staphylococcus bacteriophage K for control of antibiotic-resistant staphylococci from hospitals. Appl Environ Microbiol 71:1836–1842. doi: 10.1128/AEM.71.4.1836-1842.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia F, Li X, Wang B, Gong P, Xiao F, Yang M, Zhang L, Song J, Hu L, Cheng M, Sun C, Feng X, Lei L, Ouyang S, Liu ZJ, Li X, Gu J, Han W. 2016. Combination therapy of LysGH15 and apigenin as a new strategy for treating pneumonia caused by Staphylococcus aureus. Appl Environ Microbiol 82:87–94. doi: 10.1128/AEM.02581-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu J, Zuo J, Lei L, Zhao H, Sun C, Feng X, Du C, Li X, Yang Y, Han W. 2011. LysGH15 reduces the inflammation caused by lethal methicillin-resistant Staphylococcus aureus infection in mice. Bioeng Bugs 2:96–99. doi: 10.4161/bbug.2.2.14883. [DOI] [PubMed] [Google Scholar]
- 16.Henderson B, Poole S, Wilson M. 1996. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev 60:316–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timmerman CP, Mattsson E, Martinez-Martinez L, De Graaf L, Van Strijp JA, Verbrugh HA, Verhoef J, Fleer A. 1993. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun 61:4167–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Entenza JM, Loeffler JM, Grandgirard D, Fischetti VA, Moreillon P. 2005. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob Agents Chemother 49:4789–4792. doi: 10.1128/AAC.49.11.4789-4792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doehn JM, Fischer K, Reppe K, Gutbier B, Tschernig T, Hocke AC, Fischetti VA, Loffler J, Suttorp N, Hippenstiel S, Witzenrath M. 2013. Delivery of the endolysin Cpl-1 by inhalation rescues mice with fatal pneumococcal pneumonia. J Antimicrob Chemother 68:2111–2117. doi: 10.1093/jac/dkt131. [DOI] [PubMed] [Google Scholar]
- 20.Nau R, Eiffert H. 2002. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 15:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim NH, Park WB, Cho JE, Choi YJ, Choi SJ, Jun SY, Kang CK, Song KH, Choe PG, Bang JH, Kim ES, Park SW, Kim NJ, Oh MD, Kim HB. 2018. Effects of phage endolysin SAL200 combined with antibiotics on Staphylococcus aureus infection. Antimicrob Agents Chemother 62:e00731-18. doi: 10.1128/AAC.00731-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duthie ES, Lorenz LL. 1952. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol 6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]