Nacubactam is a novel, broad-spectrum, β-lactamase inhibitor that is currently under development as combination therapy with meropenem. This study evaluated the efficacy of human-simulated epithelial lining fluid (ELF) exposures of meropenem, nacubactam, and the combination of meropenem and nacubactam against class A serine carbapenemase-producing Enterobacteriaceae isolates in the neutropenic murine lung infection model.
KEYWORDS: β-lactam, β-lactamase inhibitor, Gram negative, OP0595, RG6080, carbapenemase, lung epithelial lining fluid, nacubactam
ABSTRACT
Nacubactam is a novel, broad-spectrum, β-lactamase inhibitor that is currently under development as combination therapy with meropenem. This study evaluated the efficacy of human-simulated epithelial lining fluid (ELF) exposures of meropenem, nacubactam, and the combination of meropenem and nacubactam against class A serine carbapenemase-producing Enterobacteriaceae isolates in the neutropenic murine lung infection model. Twelve clinical meropenem-resistant Klebsiella pneumoniae, Escherichia coli, and Enterobacter cloacae isolates, all harboring KPC or IMI-type β-lactamases, were utilized in the study. Meropenem, nacubactam, and meropenem-nacubactam (1:1) combination MICs were determined in triplicate via broth microdilution. At 2 h after intranasal inoculation, neutropenic mice were dosed with regimens that provided ELF profiles mimicking those observed in humans given meropenem at 2 g every 8 h and/or nacubactam at 2 g every 8 h (1.5-h infusions), alone or in combination. Efficacy was assessed as the change in bacterial growth at 24 h, compared with 0-h controls. Meropenem, nacubactam, and meropenem-nacubactam MICs were 8 to >64 μg/ml, 2 to >256 μg/ml, and 0.5 to 4 μg/ml, respectively. The average bacterial density at 0 h across all isolates was 6.31 ± 0.26 log10 CFU/lung. Relative to the 0-h control, the mean values of bacterial growth at 24 h in the untreated control, meropenem human-simulated regimen treatment, and nacubactam human-simulated regimen treatment groups were 2.91 ± 0.27, 2.68 ± 0.42, and 1.73 ± 0.75 log10 CFU/lung, respectively. The meropenem-nacubactam combination human-simulated regimen resulted in reductions of −1.50 ± 0.59 log10 CFU/lung. Meropenem-nacubactam human-simulated ELF exposure produced enhanced efficacy against all class A serine carbapenemase-producing Enterobacteriaceae isolates tested in the neutropenic murine lung infection model.
INTRODUCTION
Enterobacteriaceae is a family of Gram-negative bacteria that is implicated in various infections, including nosocomial pneumonia. Respiratory tract infections caused by drug-resistant Enterobacteriaceae strains are difficult to treat and a major public health burden (1–3). Resistance to β-lactams among Enterobacteriaceae strains occurs primarily through production of β-lactamases, with carbapenemases representing the most challenging β-lactamase family owing to their ability to hydrolyze almost all β-lactams (4). Furthermore, the worldwide emergence of Ambler class A serine carbapenemases, particularly Klebsiella pneumoniae carbapenemases (KPCs), is a cause of concern, given the limited selection of treatment options for carbapenemase-producing Enterobacteriaceae infections (5–8).
Nacubactam is a novel, non-β-lactam, diazabicyclooctane, β-lactamase inhibitor with in vitro activity against class A β-lactamases such as KPC, class C, and some class D β-lactamases, and it can thus restore the activity of β-lactam antibiotics against β-lactamase-producing organisms. In addition to β-lactamase inhibition, nacubactam possesses the following mechanisms: (i) intrinsic antimicrobial activity against Enterobacteriaceae via penicillin-binding protein 2 (PBP2) inhibition and (ii) synergy with various β-lactam agents (enhancer effect) (9–13). Nacubactam is being developed as a combination therapy with meropenem for the treatment of serious Gram-negative bacterial infections, including lung infections.
The purpose of this study was to evaluate the efficacy of human-simulated epithelial lining fluid (ELF) exposures of meropenem, nacubactam, and a meropenem-nacubactam combination against class A serine carbapenemase-producing Enterobacteriaceae strains in a murine neutropenic lung infection model. Assessing drug concentrations at the site of action is advisable for investigational agents (14, 15), as it allows the simulation of the observed human drug profiles in animal models and thus yields insights into antimicrobial efficacy at clinically relevant exposures. (This study was presented in part at IDWeek 2018, San Francisco, CA, 3 to 6 October 2018 [16].)
RESULTS
In vitro susceptibility studies.
All 12 Enterobacteriaceae isolates utilized in the study demonstrated in vitro resistance to meropenem using Clinical and Laboratory Standards Institute (CLSI) breakpoints (17). Meropenem and nacubactam MICs ranged from 8 to >64 μg/ml and from 2 to >256 μg/ml, respectively. The MICs of meropenem-nacubactam (concentration ratio of 1:1) varied from 0.5 to 4 μg/ml. The MICs of meropenem, nacubactam, and meropenem-nacubactam, as well as the β-lactamase profiles of the 12 isolates examined, are shown in Table 1.
TABLE 1.
Phenotypic and β-lactamase profiles of the K. pneumoniae, E. coli, and E. cloacae isolates utilized in in vivo efficacy studies
| Isolate |
MIC (μg/ml) for: |
||||
|---|---|---|---|---|---|
| Identification | Bacterial species | β-Lactamase(s) encoded | Meropenem | Nacubactam | Meropenem-nacubactama |
| EC 548b | E. coli | KPC-3, TEM-1 | 8 | 2 | 0.5 |
| KP 652b | K. pneumoniae | KPC-3 | 64 | >256 | 1 |
| ECL 72 | E. cloacae | AmpC, KPC-3, TEM-1 | 32 | >256 | 1 |
| KP 651b | K. pneumoniae | KPC-2 | 64 | >256 | 2 |
| KP 599c | K. pneumoniae | KPC-2, SHV-11 | >64 | 64 | 2 |
| KP 604c | K. pneumoniae | KPC-3, TEM-1, SHV-11 | >64 | 2 | 2 |
| KP C4-10 | K. pneumoniae | CTX-M-15, SHV-11, TEM-1, OXA-9, KPC-3 | >64 | >256 | 2 |
| KP C8-9 | K. pneumoniae | SHV-12, TEM-1, KPC-2 | >64 | >256 | 2 |
| ECL 119b | E. cloacae | NMC-A | 32 | 128 | 2 |
| KP C30-27 | K. pneumoniae | SHV-11, TEM-1, KPC-2 | >64 | >256 | 4 |
| ECL 118b | E. cloacae | NMC-A | 64 | >256 | 4 |
| KP 648b | K. pneumoniae | KPC-3 | >64 | >256 | 4 |
Meropenem/nacubactam concentration ratio of 1:1.
Clinical isolates obtained from FDA-CDC Antimicrobial Resistance Isolate Bank (Atlanta, GA).
Clinical isolates obtained from F. Hoffmann-La Roche Ltd. (Basel, Switzerland).
Lung ELF drug exposure studies.
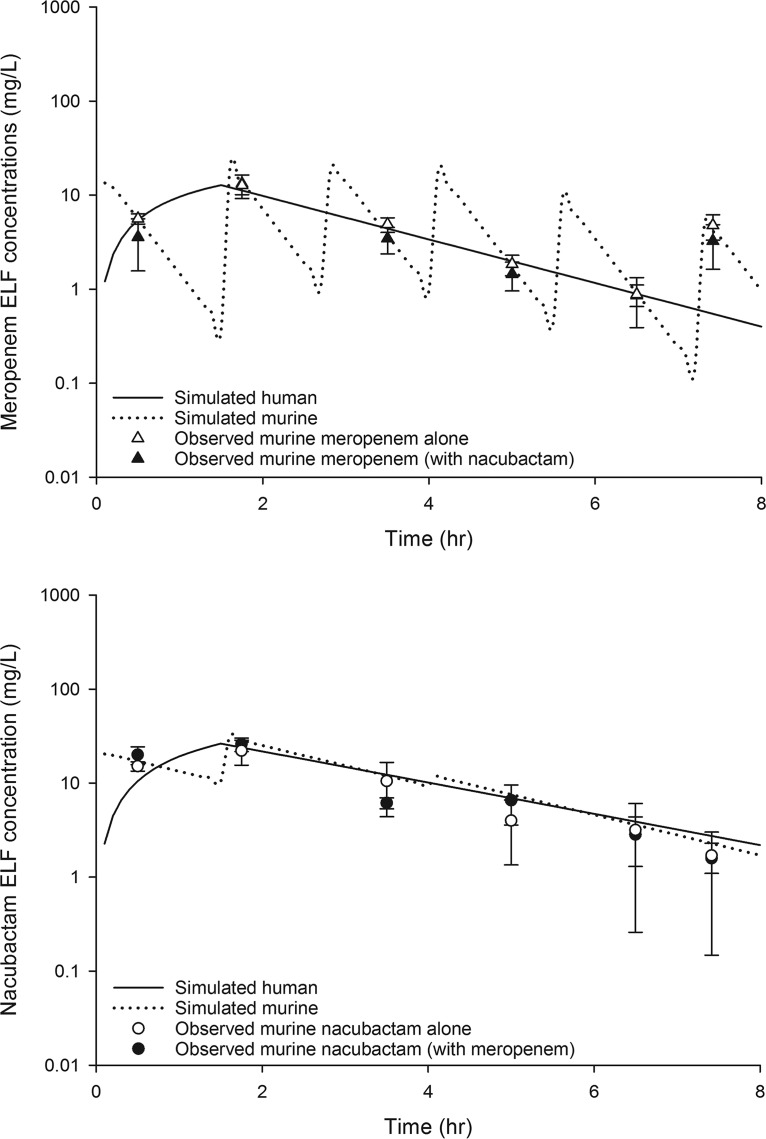
Meropenem and nacubactam were detected in mouse bronchoalveolar lavage (BAL) fluid after subcutaneous administration of two different single doses of each agent (meropenem, 50 mg/kg and 100 mg/kg; nacubactam, 10 mg/kg and 20 mg/kg). The ELF pharmacokinetics of meropenem and nacubactam single doses were well characterized using a one-compartment model; the best-fit pharmacokinetic parameters for nacubactam in the neutropenic lung infection model were as follows: volume of distribution (V) (conditioned on the unknown bioavailability in ELF), 0.42 liters/kg; rate constant for input into ELF (k01), 40.64 h−1; rate constant for elimination from ELF (k10), 0.50 h−1. The ELF profile of meropenem was utilized to develop a murine meropenem monotherapy regimen consisting of 6 doses during each 8-h dosing interval (i.e., a total of 18 doses for the 24-h study duration), as follows: 0 h, 15 mg/kg; 1.5 h, 19 mg/kg; 2.75 h, 19 mg/kg; 4 h, 17 mg/kg; 5.5 h, 9 mg/kg; 7.25 h, 5 mg/kg (repeated every 8 h); this provided ELF exposure similar to that achieved in humans following a dose of meropenem of 2 g every 8 h, as a 1.5-h infusion. Likewise, a murine nacubactam monotherapy regimen that provided ELF exposure similar to that achieved in humans following a dose of nacubactam of 2 g every 8 h, as a 1.5-h infusion, consisted of 3 doses during each 8-h dosing interval, as follows: 0 h, 9 mg/kg; 1.5 h, 9 mg/kg; 4 h, 1.25 mg/kg (repeated every 8 h). Confirmatory pharmacokinetic studies showed that the selected murine regimens simulated the exposures in humans on the basis of the percentage of the dosing interval during which the ELF drug concentration exceeded the ELF concentration threshold (%T > ELF) for a concentration range of 0.5 to 64 μg/ml, as well as the ELF area under the concentration-time curve (AUC) profile (Table 2). Figure 1 depicts the confirmatory murine ELF pharmacokinetic profiles of the nacubactam human-simulated regimen and the meropenem human-simulated regimen, compared with the respective human ELF profiles.
TABLE 2.
Meropenem and nacubactam %T > ELF concentration values estimated in humans and in mice
| Drug and species | %T > ELF concentration of: |
AUC0–24 (μg·h/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.5 μg/ml | 1 μg/ml | 2 μg/ml | 4 μg/ml | 8 μg/ml | 16 μg/ml | 32 μg/ml | 64 μg/ml | ||
| Meropenem | |||||||||
| Human (2 g every 8 h, 1.5-h infusion) | 94.58 | 78.33 | 60.83 | 42.08 | 20.83 | 0.00 | 0.00 | 0.00 | 104 |
| Mousea | 92.50 | 81.25 | 61.25 | 40.00 | 21.25 | 5.83 | 0.00 | 0.00 | 121 |
| Mouseb | 88.75 | 75.83 | 55.00 | 33.75 | 18.83 | 0.00 | 0.00 | 0.00 | 94 |
| Nacubactam | |||||||||
| Human (2 g every 8 h, 1.5-h infusion) | 100.00 | 100.00 | 100.00 | 80.42 | 55.42 | 26.67 | 0.00 | 0.00 | 265 |
| Mousec | 100.00 | 100.00 | 88.75 | 72.08 | 53.33 | 16.67 | 0.00 | 0.00 | 220 |
| Moused | 100.00 | 99.58 | 86.25 | 73.75 | 55.00 | 19.58 | 0.00 | 0.00 | 240 |
Meropenem human-simulated regimen administered alone.
Meropenem human-simulated regimen coadministered with nacubactam human-simulated regimen.
Nacubactam human-simulated regimen administered alone.
Nacubactam human-simulated regimen coadministered with meropenem human-simulated regimen.
FIG 1.
Observed meropenem (top) and nacubactam (bottom) ELF concentrations in the neutropenic lung infection model, compared with human ELF profiles of meropenem (2 g every 8 h, as 1.5-h infusion) and nacubactam (2 g every 8 h, as 1.5-h infusion). Data are presented as mean ± standard deviation.
When the meropenem and nacubactam murine human-simulated regimens were administered concomitantly, the meropenem exposure achieved in mice was unaltered, as shown in Fig. 1. However, the nacubactam elimination from ELF was enhanced and the ELF exposure was observed to be slightly reduced, which necessitated an increase in the nacubactam dose for animals receiving the combination in order to attain the target human ELF exposure. As a result, the nacubactam human-simulated regimen for combination treatment with meropenem was as follows: 0 h, 9 mg/kg; 1.5 h, 9 mg/kg; 4 h, 3 mg/kg (during each 8-h dosing interval). Following dose adjustment, the target nacubactam exposure upon meropenem coadministration was confirmed, as shown in Fig. 1. The mechanism of the pharmacokinetic interaction between meropenem and nacubactam was not studied in this investigation.
In vivo efficacy studies.
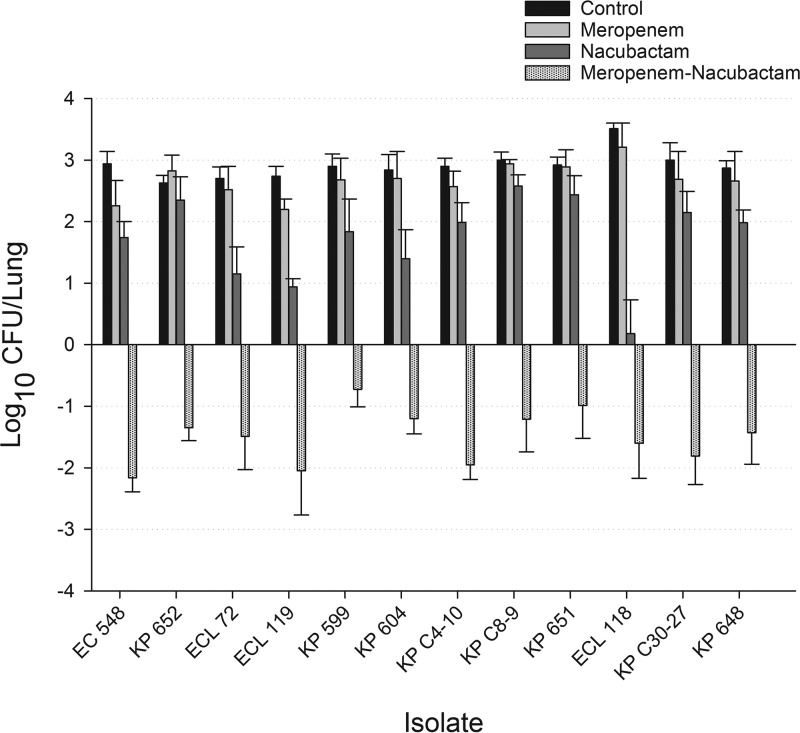
In this lung infection model, 0-h control mice displayed an overall growth value of 6.31 ± 0.26 log10 CFU/lung (mean ± standard deviation) across all isolates examined, which increased by an average of 2.91 ± 0.27 log10 CFU/lung in untreated mice after 24 h. Relative to the 0-h control, the mean values for bacterial growth at 24 h in the meropenem monotherapy and nacubactam monotherapy treatment groups were 2.68 ± 0.42 and 1.73 ± 0.75 log10 CFU/lung, respectively. In comparison to meropenem monotherapy, nacubactam monotherapy resulted in a lower bacterial burden (P < 0.001) for all isolates studied. The combination of meropenem and nacubactam resulted in bacterial reductions ranging from −0.73 ± 0.26 to −2.16 ± 0.21 log10 CFU/lung, with 10 of the 12 isolates studied achieving >1-log10 CFU/lung reduction at 24 h. The results of the bacterial density studies for each isolate are depicted in Fig. 2.
FIG 2.
Changes in bacterial growth (mean ± SD) at 24 h, relative to 0-h controls, with meropenem, nacubactam, and meropenem-nacubactam against Enterobacteriaceae strains harboring class A serine carbapenemases.
DISCUSSION
The Centers for Disease Control and Prevention estimates that more than 9,000 health care-associated infections each year are caused by carbapenemase-resistant Enterobacteriaceae (CRE), resulting in approximately 600 deaths (18). Currently, there are limited therapeutic options available for patients with CRE infections. Given that the predominant antimicrobial resistance mechanism among Enterobacteriaceae strains is β-lactamase production, the combination of a β-lactamase inhibitor with activity against carbapenemases and an existing broad-spectrum β-lactam agent is an attractive therapeutic strategy. Nacubactam, a novel β-lactamase inhibitor under clinical development, demonstrates a potent spectrum of in vitro activity against CRE in combination with meropenem (9, 11, 12). In vivo, antimicrobial activity requires the achievement of sufficient drug levels at the site of infection. For pulmonary infections, assessment of ELF drug concentrations enables more robust predictions of the exposure-response relationships, compared with plasma concentrations (19–24), but oftentimes data on the drug exposures at these critical sites are limited. In the present study, the availability of data on the meropenem-nacubactam bronchopulmonary pharmacokinetics in healthy adults provided the ability to evaluate the efficacy of the combination using the human-simulated ELF exposures in a murine lung infection model, which improves the translation application of the outcomes from this study to the clinic.
Meropenem-nacubactam MICs, determined using a 1:1 methodology, were 16- to 256-fold lower than meropenem MICs against CRE isolates in this study. These findings are supported by a similar result from an in vitro assessment by Morrisey et al., demonstrating that nacubactam in combination with meropenem had better in vitro activity against extended-spectrum β-lactamase (ESBL)-, class B-, class C-, class D-, and KPC-producing Enterobacteriaceae strains, compared with meropenem alone (11).
Focusing on class A carbapenemases, particularly KPC, the current study demonstrated enhanced in vivo activity, i.e., >1-log-unit bacterial density reductions, in 10 of 12 isolates with human-simulated ELF exposures of the meropenem-nacubactam combination, compared with either agent alone, in a lung infection model. The observation of nacubactam monotherapy resulting in a significantly lower bacterial burden, relative to meropenem monotherapy, among the Enterobacteriaceae isolates studied is attributed to the intrinsic antimicrobial activity of nacubactam mediated via PBP2 inhibition, as observed in a PBP-binding assay study (12).
Previously reported in vitro experiments demonstrated the activity of nacubactam in combination with either piperacillin, cefepime, or meropenem against a variety of β-lactamases, including CTX-M-15- and KPC-expressing Enterobacteriaceae strains (24, 25). Additionally, the activity of nacubactam (previously OP0595) in combination with cefepime, as the β-lactam backbone, against CTX-M-15-positive E. coli (n = 2) and KPC-positive K. pneumonia (n = 2) isolates was evaluated by Morinaka et al. in a thigh infection model (25). Treatment with either cefepime alone or nacubactam alone did not decrease the bacterial density in the murine thigh; however, combination treatment with cefepime and nacubactam decreased the bacterial density by 3 to 4 log10 CFU/thigh, relative to the untreated 24-h control (25). In comparison, human-simulated ELF exposures of meropenem-nacubactam in the current study resulted in reductions in the bacterial burden of 4 to 5 log10 CFU/lung, relative to the untreated 24-h control. Against contemporary clinical Enterobacteriaceae isolates (n = 317), meropenem and meropenem-nacubactam MIC90 values were 8 mg/liter and 0.25 mg/liter, respectively, while cefepime and cefepime-nacubactam MIC90 values were >64 mg/liter and 0.5 mg/liter, respectively (26). In addition, our observations are in agreement with the recent findings of Monogue and colleagues (27). Utilizing a murine urinary tract infection model, the authors demonstrated that human-simulated meropenem-nacubactam plasma exposure had potent activity against meropenem- and ceftazidime-avibactam-resistant Enterobacteriaceae strains. Isolates in that study harbored a range of β-lactamases, including ESBL, KPC, OXA, and NDM enzymes (27).
The need for new antimicrobial agents has contributed to renewed interest in β-lactam/β-lactamase inhibitor combinations. The recently approved agents ceftazidime-avibactam and meropenem-vaborbactam both have activity against class A (i.e., ESBL and KPC) and class C β-lactamases (10, 13, 28). However, recent real-world reports of resistance developing with ceftazidime-avibactam therapy are concerning (29). In contrast to avibactam and vaborbactam, nacubactam possesses a dual mechanism of action in addition to a synergistic effect in combination with β-lactams (12, 13). Future studies are needed to compare the activity of meropenem-nacubactam to those of ceftazidime-avibactam and meropenem-vaborbactam and to examine whether meropenem-nacubactam offers a potential alternative to ceftazidime-avibactam for resistant strains.
In conclusion, this study demonstrated that, in lung-infected mice administered a meropenem-nacubactam regimen that achieved exposures in pulmonary ELF comparable to those observed in humans following the currently examined clinical doses of the combination, the regimen resulted in enhanced efficacy against a variety of clinical isolates harboring KPC-2, KPC-3, or IMI-type β-lactamases. These translational data support the potential role of nacubactam in combination with meropenem for treatment of human lung infections due to class A carbapenemase-producing Enterobacteriaceae strains, and further studies are warranted.
MATERIALS AND METHODS
Antimicrobial test agent.
Analytical grade nacubactam (batch no. R07079901-001-009; Roche Laboratories, Basel, Switzerland) was used for all in vitro and in vivo testing. Analytical grade meropenem (lot no. M0608A; Tecoland Corp., Irvine, CA) and commercially available meropenem in 1-g vials (lot no. 0017D61; Fresenius Kabi USA) were utilized for in vitro and in vivo testing, respectively. Commercial vials of meropenem were reconstituted as described in the prescribing information, with dilution in sterile normal saline (Hospira, Inc., Lake Forest, IL) as appropriate to achieve the desired concentrations.
Bacterial isolates.
Eight Klebsiella pneumoniae, 1 Escherichia coli, and 3 Enterobacter cloacae clinical isolates were utilized in the studies. Of these 12 Enterobacteriaceae isolates, 6 isolates were obtained from the FDA-CDC Antimicrobial Resistance Isolate Bank (Atlanta, GA), 2 isolates were obtained from F. Hoffmann-La Roche Ltd. (Basel, Switzerland), and the remaining 4 isolates were from the Center for Anti-Infective Research and Development isolate repository. All isolates were maintained in skim milk (BD Biosciences, Sparks, MD) at −80°C. Each isolate was subcultured twice on Trypticase soy agar with 5% sheep blood (BD Biosciences) and grown for 18 to 20 h at 37°C under 5% CO2 prior to use in the experiments.
Susceptibility testing.
The MICs of meropenem, nacubactam, and meropenem-nacubactam were determined for all isolates using the broth microdilution methodology outlined by the CLSI (17). For meropenem-nacubactam MICs, doubling dilutions of meropenem and nacubactam were utilized at a 1:1 concentration ratio. MIC values were determined in triplicate, and the modal MIC was reported.
Neutropenic lung infection model.
Pathogen-free, female ICR mice (20 to 22 g) were obtained from Envigo RMS, Inc. (Indianapolis, IN). Animals were provided food and water ad libitum and were maintained and used in accordance with National Research Council recommendations. Mice were rendered transiently neutropenic with intraperitoneal injections of cyclophosphamide (250 mg/kg on day −4 and 100 mg/kg on day −1). Uranyl nitrate (5 mg/kg on day −3) was administered to produce a controlled degree of renal impairment, to assist with the development of human-simulated drug exposures. After 18 to 20 h of incubation of the isolate second transfer, a bacterial suspension of approximately 107 CFU/ml in 3% hog gastric mucin was made for inoculation. The mice were anesthetized using vaporized isoflurane (2 to 3% [vol/vol] in an oxygen carrier), and lung infection was produced by intranasal inoculation of 50 μl of inoculum 2 h prior to therapy initiation. This study was approved by the Hartford Hospital Institutional Animal Care and Use Committee.
Bronchopulmonary pharmacokinetics and human-simulated ELF exposures.
Human-simulated dosing regimens in mice that provided a percentage of the dosing interval above the ELF concentration and an ELF AUC similar to those achieved in a nonrandomized, open-label, one-treatment, one-group study to investigate the intrapulmonary lung penetration of RO7079901 in 21 healthy volunteers, conducted by F. Hoffmann-La Roche (ClinicalTrials registration no. NCT03182504), were developed. In the clinical trial, study participants received a single dose of nacubactam (2-g intravenous infusion of nacubactam over 1.5 h) coadministered with meropenem (2-g intravenous infusion of meropenem over 1.5 h).
Initially, single-dose ELF pharmacokinetic studies of meropenem (50 mg/kg and 100 mg/kg) and nacubactam (10 mg/kg and 20 mg/kg) were performed in the murine infection model. Using the pharmacokinetic parameter estimates derived from the single-dose pharmacokinetic studies, human-simulated ELF regimens of meropenem and nacubactam were developed. Confirmatory pharmacokinetic studies in which mice received meropenem or nacubactam human-simulated regimens alone or in combination were undertaken to ascertain whether the meropenem and nacubactam regimens resulted in the expected ELF exposures. All single-dose and confirmatory pharmacokinetic studies were conducted in lung-infected mice (n = 36 mice) to examine meropenem and nacubactam ELF profiles. Following intracardiac blood collection, BAL fluid was collected at 6 sampling time points, with 6 mice contributing to each time point; a catheter was inserted into the trachea of the mice, and the lungs were lavaged with 4 aliquots of 0.4 ml of normal saline. Plasma and BAL fluid were analyzed for drug and urea concentrations by F. Hoffmann-La Roche Ltd. (Basel, Switzerland) via high-performance liquid chromatography-mass spectrometry. The ELF concentrations (CELF) of nacubactam or meropenem were determined using the equation: CELF = CBAL × Ureaplasma/UreaBAL, where CBAL, UreaBAL,, and Ureaplasma are the concentration of drug in BAL fluid, the concentration of urea in BAL fluid, and the concentration of urea in plasma, respectively. Pharmacokinetic parameters for single-doses studies were calculated using Phoenix 64 (WinNonlin 6.4, NLME 1.3).
In vivo efficacy studies.
The 12 Enterobacteriaceae strains were used to infect cohorts of 30 mice each. Treatment was initiated 2 h following bacterial inoculation. Treated mice (6 mice per group) received subcutaneous injections (0.1 ml/agent) of either human-simulated meropenem alone, human-simulated nacubactam alone, a meropenem-nacubactam combination, or saline (24-h controls) at each treatment time point. The lung tissue harvesting procedure for all study mice began with euthanization by CO2 exposure, followed by cervical dislocation. Lungs from all animals were harvested 24 h after the initiation of therapy. After sacrifice, the lungs were removed aseptically and individually homogenized in normal saline. Tenfold serial dilutions of the lung homogenates were plated on Trypticase soy agar with 5% sheep blood for CFU determination. Untreated control mice (6 mice per group) were sacrificed 2 h postinoculation, to serve as the 0-h control animals. Efficacy was quantified by the change in bacterial density in the mice after 24 h, relative to the 0-h untreated controls.
ACKNOWLEDGMENTS
We thank Christina Sutherland, Deborah Santini, Jennifer Tabor-Rennie, Sara Giovagnoli, Elizabeth Cyr, Kimelyn Greenwood, Alissa Padgett, Janice Cunningham, Michelle Insignares, Lauren McLellan, Elias Mullane, Sean Stainton, Safa Abuhussain, Lindsay Avery, and James Kidd from the Center for Anti-Infective Research and Development (Hartford, CT) for their assistance with the conduct of the study.
This study was funded by F. Hoffmann-La Roche Ltd. (Basel, Switzerland). This project was funded in whole or in part with federal funds from the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under contract no. HHSO100201600038C.
D.P.N. has received research grants from F. Hoffmann-La Roche Ltd. C.B. and C.Z. are employees of F. Hoffmann-La Roche Ltd. All other authors have no conflicts to declare.
REFERENCES
- 1.Rodrigo-Troyano A, Sibila O. 2017. The respiratory threat posed by multidrug resistant Gram-negative bacteria. Respirology 22:1288–1299. doi: 10.1111/resp.13115. [DOI] [PubMed] [Google Scholar]
- 2.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neidell MJ, Cohen B, Furuya Y, Hill J, Jeon CY, Glied S, Larson EL. 2012. Costs of healthcare- and community-associated infections with antimicrobial-resistant versus antimicrobial-susceptible organisms. Clin Infect Dis 55:807–815. doi: 10.1093/cid/cis552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Queenan AM, Bush K. 2007. Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev 20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshpande LM, Rhomberg PR, Sader HS, Jones RN. 2006. Emergence of serine carbapenemases (KPC and SME) among clinical strains of Enterobacteriaceae isolated in the United States medical centers: report from the MYSTIC Program (1999–2005). Diagn Microbiol Infect Dis 56:367–372. doi: 10.1016/j.diagmicrobio.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. 2015. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis 2:ofv050. doi: 10.1093/ofid/ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potter RF, D’Souza AW, Dantas G. 2016. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist Updat 29:30–46. doi: 10.1016/j.drup.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porreca AM, Sullivan KV, Gallagher JC. 2018. The epidemiology, evolution, and treatment of KPC-producing organisms. Curr Infect Dis Rep 20:13. doi: 10.1007/s11908-018-0617-x. [DOI] [PubMed] [Google Scholar]
- 9.Livermore DM, Warner M, Mushtaq S, Woodford N. 2016. Interactions of OP0595, a novel triple-action diazabicyclooctane, with β-lactams against OP0595-resistant Enterobacteriaceae mutants. Antimicrob Agents Chemother 60:554–560. doi: 10.1128/AAC.02184-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush K, Page MGP. 2017. What we may expect from novel antibacterial agents in the pipeline with respect to resistance and pharmacodynamic principles. J Pharmacokinet Pharmacodyn 44:113–132. doi: 10.1007/s10928-017-9506-4. [DOI] [PubMed] [Google Scholar]
- 11.Morrissey I, Magnet S, Hawser S, Louvel S, Okujava R, Zampaloni C, Bradley K. 2018. In vitro activity of nacubactam, a novel dual action diazabicyclooctane, alone and with meropenem, against beta-lactamase-positive Enterobacteriaceae, abstr P1039 Abstr 28th Eur Cong Clin Microbiol Infect Dis, Madrid, Spain. [Google Scholar]
- 12.Morinaka A, Tsutsumi Y, Yamada M, Suzuki K, Watanabe T, Abe T, Furuuchi T, Inamura S, Sakamaki Y, Mitsuhashi N, Ida T, Livermore DM. 2015. OP0595, a new diazabicyclooctane: mode of action as a serine β-lactamase inhibitor, antibiotic and β-lactam ‘enhancer.’ J Antimicrob Chemother 70:2779–2786. doi: 10.1093/jac/dkv166. [DOI] [PubMed] [Google Scholar]
- 13.Docquier JD, Mangani S. 2018. An update on β-lactamase inhibitor discovery and development. Drug Resist Updat 36:13–29. doi: 10.1016/j.drup.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Food and Drug Administration. 2014. Hospital-acquired bacterial pneumonia and ventilator associated bacterial pneumonia: developing drugs for treatment. Center for Drug Evaluation and Research, Rockville, MD. [Google Scholar]
- 15.European Medicines Agency. 2016. Guideline on the use of pharmacokinetics and pharmacodynamics in the development of antimicrobial medicinal products. Committee for Medicinal Products for Human Use, London, UK. [Google Scholar]
- 16.Asempa TE, Motos A, Abdelraouf K, Bissantz C, Zampaloni C, Nicolau DP. 2018. Assessment of the in vivo efficacy of human-simulated epithelial lining fluid (ELF) exposure of meropenem/nacubactam (MEM/NAC) combination against β-lactamase-producing Enterobacteriaceae in neutropenic lung infection model. Open Forum Infect Dis 5(Suppl 1):S419. doi: 10.1093/ofid/ofy210.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2018. M100. Performance standards for antimicrobial susceptibility testing, 28th ed Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. 2013. Antibiotic/antimicrobial resistance: biggest threats and data. https://www.cdc.gov/drugresistance/biggest_threats.html. Accessed 22 September 2018.
- 19.Nix DE, Goodwin SD, Peloquin CA, Rotella DL, Schentag JJ. 1991. Antibiotic tissue penetration and its relevance: impact of tissue penetration on infection response. Antimicrob Agents Chemother 35:1953–1959. doi: 10.1128/AAC.35.10.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiem S, Schentag JJ. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother 52:24–36. doi: 10.1128/AAC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodvold KA, George JM, Yoo L. 2011. Penetration of anti-infective agents into pulmonary epithelial lining fluid. Clin Pharmacokinet 50:637–664. doi: 10.2165/11594090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Rizk ML, Zou L, Savic RM, Dooley KE. 2017. Importance of drug pharmacokinetics at the site of action. Clin Transl Sci 10:133–142. doi: 10.1111/cts.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tessier PR, Keel RA, Hagihara M, Crandon JL, Nicolau DP. 2012. Comparative in vivo efficacies of epithelial lining fluid exposures of tedizolid, linezolid, and vancomycin for methicillin-resistant Staphylococcus aureus in a mouse pneumonia model. Antimicrob Agents Chemother 56:2342–2346. doi: 10.1128/AAC.06427-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdelraouf K, Nicolau DP. 2017. Comparative in vivo efficacies of tedizolid in neutropenic versus immunocompetent murine Streptococcus pneumoniae lung infection models. Antimicrob Agents Chemother 61:e01957-16. doi: 10.1128/AAC.01957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morinaka A, Tsutsumi Y, Yamada K, Takayama Y, Sakakibara S, Takata T, Abe T, Furuuchi T, Inamura S, Sakamaki Y, Tsujii N, Ida T. 2016. In vitro and in vivo activities of OP0595, a new diazabicyclooctane, against CTX-M-15-positive Escherichia coli and KPC-positive Klebsiella pneumoniae. Antimicrob Agents Chemother 60:3001–3006. doi: 10.1128/AAC.02704-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aboklaish AF, Okujava R, El-Bouseary M, Zampaloni C, Najera I, Bradley K, Walsh TR, Tyrrell JM. 2018. Nacubactam antibacterial activity alone and in combination with beta-lactam antibiotics against contemporary Enterobacteriaceae clinical isolates, abstr P1034 Abstr 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain. [Google Scholar]
- 27.Monogue ML, Giovagnoli S, Bissantz C, Zampaloni C, Nicolau DP. 2018. In vivo efficacy of meropenem with a novel non-β-lactam-β-lactamase inhibitor, nacubactam, against Gram-negative organisms exhibiting various resistance mechanisms in a murine complicated urinary tract infection model. Antimicrob Agents Chemother 62:e02596-17. doi: 10.1128/AAC.02596-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pogue JM, Bonomo RA, Kaye KS. 2019. Ceftazidime/avibactam, meropenem/vaborbactam, or both? Clinical and formulary considerations. Clin Infect Dis 68:519–524. doi: 10.1093/cid/ciy576. [DOI] [PubMed] [Google Scholar]
- 29.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]