WCK 5222 is a combination of cefepime and the high-affinity PBP2-binding β-lactam enhancer zidebactam. The cefepime-zidebactam combination is active against multidrug-resistant Gram-negative bacteria, including carbapenemase-expressing Acinetobacter baumannii.
KEYWORDS: Acinetobacter baumannii, in vivo mouse model, WCK 5222, zidebactam
ABSTRACT
WCK 5222 is a combination of cefepime and the high-affinity PBP2-binding β-lactam enhancer zidebactam. The cefepime-zidebactam combination is active against multidrug-resistant Gram-negative bacteria, including carbapenemase-expressing Acinetobacter baumannii. The mechanism of action of the combination involves concurrent multiple penicillin binding protein inhibition, leading to the enhanced bactericidal action of cefepime. The aim of the present study was to assess the impact of the zidebactam-mediated enhanced in vitro bactericidal action in modulating the percentage of the time that the free drug concentration remains above the MIC (percent fT>MIC) for cefepime required for the in vivo killing of A. baumannii. Cefepime and cefepime-zidebactam MICs were comparable and ranged from 2 to 16 mg/liter for the A. baumannii strains (n = 5) employed in the study. Time-kill studies revealed the improved killing of these strains by the cefepime-zidebactam combination compared to that by the constituents alone. Employing a neutropenic mouse lung infection model, exposure-response analyses for all the A. baumannii strains showed that the cefepime fT>MIC required for 1-log10 kill was 38.9%. In the presence of a noneffective dose of zidebactam, the cefepime fT>MIC requirement dropped significantly to 15.5%, but it still rendered a 1-log10 kill effect. Thus, zidebactam mediated the improvement in cefepime’s bactericidal effect observed in time-kill studies, manifested in vivo through the lowering of cefepime’s pharmacodynamic requirement. This is a first-ever study demonstrating a β-lactam enhancer role of zidebactam that helps augment the in vivo activity of cefepime by reducing the magnitude of its pharmacodynamically relevant exposures against A. baumannii.
INTRODUCTION
The emergence of diverse carbapenemases in Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii poses a heightened therapeutic challenge (1). In the United States, the scenario is even more difficult when considering A. baumannii infections, since >50% of A. baumannii isolates are reported to be carbapenem resistant due to acquisition of carbapenem-hydrolyzing class D β-lactamases, such as OXA-23, OXA-24/40, and OXA-58. The percentage of multidrug-resistant (MDR) Acinetobacter spp. causing hospital-acquired infections was found to range from 5.0% to 88.1% across U.S. states, with the national average being 54.8% (2).
The recently approved drugs ceftazidime-avibactam, meropenem-vaborbactam, and plazomicin, which promise clinical coverage of certain carbapenem-resistant Gram-negative bacteria, however, are not able to offer therapeutically relevant activity against MDR A. baumannii strains. The pharmacophores of β-lactamase inhibitors employed in ceftazidime-avibactam and meropenem-vaborbactam are structurally quite different (diazabicyclooctane and cyclic boronic acid, respectively), yet both lack broad-spectrum β-lactamase-inhibitory activity; specifically, they lack activity against the class B carbapenemases expressed in Enterobacteriaceae and P. aeruginosa and the class D carbapenemases encountered in A. baumannii (3, 4). Thus, discovery of a β-lactamase inhibitor that inhibits all four classes of β-lactamases remains elusive, pointing toward the limitation of a β-lactamase inhibitor-based approach. Therefore, alternative approaches independent of β-lactamase inhibition are required to resurrect well-accepted β-lactam agents.
WCK 5222 is a combination of cefepime (FEP) and the β-lactam enhancer antibiotic zidebactam (ZID), which is based on a novel bicycloacyl hydrazide pharmacophore derived from diazabicyclooctane. The combination operates through a novel mechanism of action involving the concomitant inactivation of multiple penicillin binding proteins (PBPs) (5, 6). As a result, the FEP-ZID combination circumvents the need for β-lactamase inhibition and has been shown to be active against all four classes of β-lactamase producers (7). Zidebactam, being a non-β-lactam, is stable to β-lactamases, including those of class B and D, and therefore provides unhindered PBP2 binding even in β-lactamase-producing strains. Though ZID inhibits certain class A and C β-lactamases, however, it is not an inhibitor of class B β-lactamases and the A. baumannii-associated class D carbapenemases. Zidebactam’s PBP2 binding in A. baumannii was evidenced through spheroplast formation upon exposure to low concentrations of ZID (5, 8, 9), as well as through cell-free PBP-binding studies (5, 6, 8, 9). Studies have shown that FEP causes PBP3 inactivation at sublethal concentrations, even in the presence of FEP-hydrolyzing β-lactamases, which is manifested as cell elongation. Upon concurrent PBP2 and PBP3 inactivation by FEP-ZID, a rapid bactericidal action is triggered even against isolates producing ZID-noninhibitable β-lactamases (5, 6, 8, 9). As a result, the FEP-ZID combination is associated with bactericidal action faster than that of FEP alone. Enhanced cell killing by combinations of β-lactam agents that provide multiple PBP occupancy was previously described (10). Even carbapenems that are known to possess a high affinity to multiple PBPs display rapid bactericidal action and have been reported to require a lower percentage of the time that the free drug concentration remains above the MIC (percent fT>MIC) of 20% to 30% for efficacy. In contrast, penicillins and cephalosporins are associated with a higher percent fT>MIC requirement owing to their sole PBP3-mediated slow bactericidal action.
In a large surveillance study, the MIC90 of FEP-ZID against Enterobacteriaceae, P. aeruginosa, and A. baumannii was 0.12, 4, and 32 mg/liter, respectively (11, 12). The in vivo efficacy of the FEP-ZID combination against MDR Acinetobacter strains has been established through several studies (13). Recently, Avery et al. (14) and Abuhussain et al. (15) demonstrated the 2- to 4-log10 kill of several carbapenem-resistant A. baumannii strains (meropenem MICs, 8 to >64 mg/liter) by a simulated human regimen of FEP-ZID in neutropenic murine lung and thigh infection models. Interestingly, most of these MDR A. baumannii isolates expressed a carbapenem-hydrolyzing oxacillinase (OXA-23 or OXA-24), and FEP-ZID MICs ranged from 16 to 64 mg/liter.
The objective of the present study was to explore the significance of ZID-mediated enhancement of FEP’s in vitro bactericidal activity in modulating FEP’s in vivo pharmacodynamic (PD) activity. A standard neutropenic mouse lung infection model was employed, wherein the efficacy of FEP alone and of FEP-ZID against five A. baumannii strains with FEP MICs ranging from 2 to 16 mg/liter was determined. Employing strains with lower FEP MICs enabled us to determine the magnitude of the percent fT>MIC for FEP monotherapy, which could then be compared with the FEP requirement in the presence of ZID. Demonstration of an impact of ZID on the FEP percent fT>MIC would not have been possible by employing MDR A. baumannii (FEP-resistant) strains, as FEP would fail to provide an antibacterial response at clinically relevant doses. In order to investigate the impact of ZID on the FEP percent fT>MIC required for 1-log10 kill, ZID was combined with various fractionated dose regimens of FEP. The percent fT>MIC for cefepime as monotherapy and in combination with ZID required for 1-log10 kill was derived from exposure-response analyses.
(This study was presented in part at ASM Microbe, New Orleans, LA, 1 to 5 June 2017 [16].)
RESULTS
The MICs of the antibacterial agents against five A. baumannii strains were determined by broth microdilution. Zidebactam MICs were >512 mg/liter, consistent with the reported lack of intrinsic growth-inhibitory activity of zidebactam against A. baumannii (13). Cefepime and FEP-ZID (1:1 ratio) modal MICs were identical for all the strains and ranged from 2 to 16 mg/liter. All these strains showed meropenem MICs in the range of 0.5 to 2 mg/liter (Table 1).
TABLE 1.
Modal MICs of cefepime, zidebactam, cefepime-zidebactam, and meropenem against A. baumannii strains evaluated in time-kill and in vivo studies
| A. baumannii strain | β-Lactamases | MIC (mg/liter)a |
|||
|---|---|---|---|---|---|
| FEP | ZID | FEP-ZID (1:1) | MEM | ||
| ATCC 19606 | ADC, TEM, PER, OXA-51 | 16 | >512 | 16 | 2 |
| SL1 | ADC, TEM, PER, OXA-51 | 16 | >512 | 16 | 1 |
| NCTC 10303 | ADC, TEM, PER, OXA-51 | 2 | >512 | 2 | 1 |
| ATCC BAA 747 | ADC, TEM, OXA-51 | 2 | >512 | 2 | 0.5 |
| S 630 | ADC, TEM, OXA-51 | 4 | >512 | 4 | 1 |
FEP, cefepime; ZID, zidebactam; MEM, meropenem.
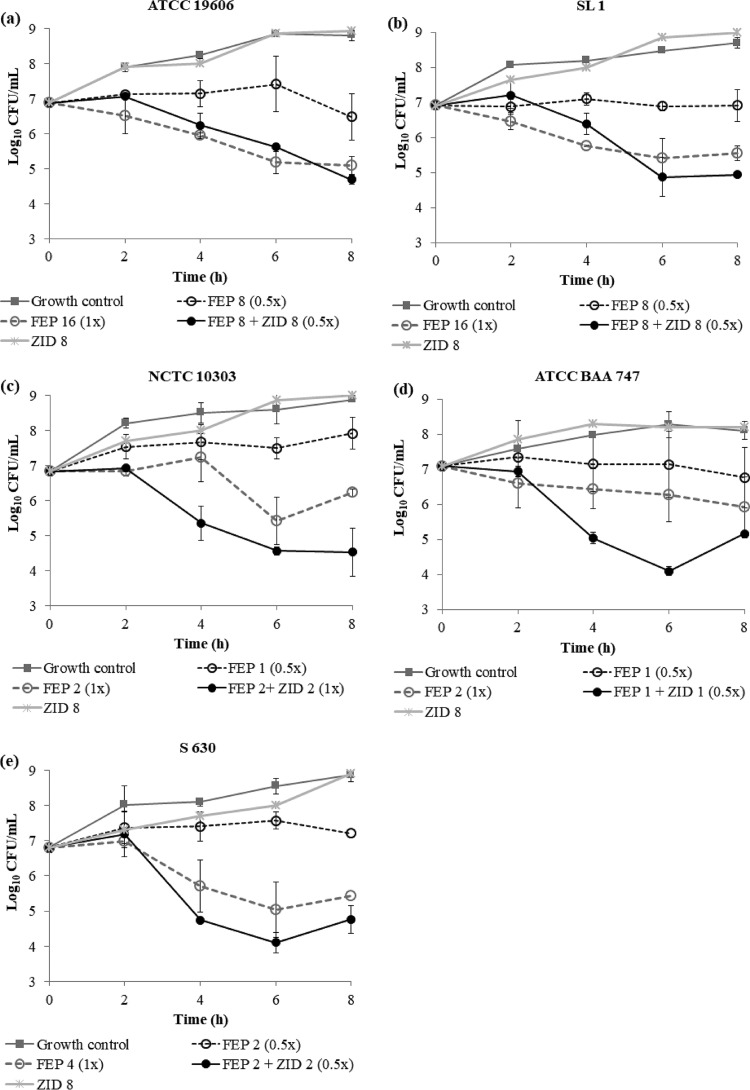
Time-kill studies were undertaken for FEP, ZID, and FEP-ZID against all A. baumannii strains. ZID alone did not show killing activity, even at the highest concentration of 64 mg/liter. FEP alone at 0.5× MIC was largely static, while 1× MIC induced 0.5- to 1.5-log10 kill across the strains studied. However, in the presence of ZID, 0.5× MIC of FEP turned bactericidal and caused ≥2-log10 kill for all strains except A. baumannii NCTC 10303. For A. baumannii NCTC 10303, enhanced killing to the extent of 2 log10 was observed with FEP-ZID at 1× MIC. Thus, although no MIC-based synergistic effect was observed, the FEP-ZID combination showed enhanced kill compared to that of FEP and ZID alone (Fig. 1a to e).
FIG 1.
Time-kill study results for FEP-ZID against various A. baumannii strains (a to e). ZID alone at 64 mg/liter did not elicit even a bacteriostatic response. The numbers after the abbreviations FEP and ZID are concentrations of drug (in milligrams per kilogram) and the multiple of the MIC (which is in parentheses).
Table 2 describes the plasma pharmacokinetic (PK) parameters of FEP and ZID in healthy mice at doses ranging from 25 to 200 mg/kg of body weight for FEP and 12.5 to 100 mg/kg for ZID administered through the subcutaneous route. For both FEP and ZID, a dose-dependent increase in the maximum concentration in plasma and the area under the concentration-time curve (AUC) was observed. The half-life for both FEP and ZID was in the range of 0.2 to 0.4 h. The mouse plasma protein binding of both FEP and ZID was <10%. Combination PK studies showed a lack of an interaction between FEP and ZID, as reported previously (data not shown) (17). The study comparing the PK profiles of FEP and ZID in healthy and infected mice showed no significant difference between the two groups (Fig. 1; see also Table S1 in the supplemental material).
TABLE 2.
Mouse plasma pharmacokinetic parameters of single doses of cefepime or zidebactama
| Druga | Dose (mg/kg) | Cmax (mg/liter) | Tmax (h) | AUC (μg·h/ml) | CL (liters/h/kg) | V (liters/kg) | t1/2 (h) |
|---|---|---|---|---|---|---|---|
| FEP | 25 | 31.9 ± 3.6 | 0.1 ± 0.0 | 15.7 ± 0.9 | 1.6 ± 0.1 | 0.6 ± 0.1 | 0.2 ± 0.0 |
| 50 | 58.2 ± 0.3 | 0.25 ± 0.0 | 38.8 ± 0.0 | 2.6 ± 0.0 | 1.2 ± 0.6 | 0.3 ± 0.2 | |
| 100 | 151.8 ± 11.6 | 0.25 ± 0.0 | 68.42 ± 4.38 | 1.47 ± 0.1 | 0.56 ± 0.15 | 0.27 ± 0.1 | |
| 200 | 229.00 ± 0.85 | 0.25 ± 0.0 | 142.9 ± 18.0 | 1.41 ± 0.18 | 0.67 ± 0.2 | 0.34 ± 0.1 | |
| ZID | 12.5 | 19.8 ± 3.4 | 0.3 ± 0.0 | 12.8 ± 0.3 | 1.0 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 |
| 25 | 38.2 ± 0.6 | 0.3 ± 0.0 | 24.4 ± 2.1 | 1.0 ± 0.1 | 1.9 ± 0.4 | 0.3 ± 0.0 | |
| 50 | 93.8 ± 19.9 | 0.1 ± 0.1 | 43.2 ± 4.3 | 1.2 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.0 | |
| 100 | 201.6 ± 55.9 | 0.1 ± 0.1 | 90.0 ± 11.0 | 1.1 ± 0.2 | 0.47 ± 0.2 | 0.28 ± 0.1 |
FEP, cefepime; ZID, zidebactam; Cmax, maximum concentration of drug in plasma; Tmax, time to Cmax; AUC, area under the concentration-time curve; CL, clearance; V, volume of distribution; t1/2, half-life.
In order to determine the total plasma/epithelial lining fluid (ELF) penetration ratio, mouse ELF PK were determined by employing 50- and 100-mg/kg doses of FEP and 25- and 50-mg/kg doses of ZID. The FEP and ZID ELF exposures in terms of the AUC for these doses were in the range of 13.66 ± 1.47 to 31.68 ± 3.54 μg·h/ml and 6.69 ± 0.42 to 15.49 ± 1.64 μg·h/ml, respectively (Table S2). Thus, the total plasma/ELF penetration ratio for FEP and ZID was in the range of 0.3 to 0.5. The PK/PD analyses were undertaken based on the plasma concentrations, as PK/PD requirements for β-lactams are invariably expressed by employing readily and robustly measurable plasma concentrations.
Table 3 illustrates the plasma percent fT>MIC for FEP and ZID, taking into account the FEP-ZID MIC range of 2 to 16 mg/liter and employing FEP at a dose of 25 mg/kg administered every 2 h (q2h) and ZID doses of 12.5 and 37.5 mg/kg q2h. The ZID dose of 12.5 mg/kg q2h in the mouse yields about 1/3 of the clinical exposure, while the higher dose of 37.5 mg/kg q2h is equivalent to the clinical exposures attained with a selected dose regimen of ZID at 1 g every 8 h (q8h) in FEP-ZID. Our previous studies have shown that varying the in vivo exposure of one constituent of FEP-ZID modulates the efficacy-linked exposure of the second constituent. In this context, it was envisaged that use of ZID at 12.5 mg/kg q2h would impose a considerable PK/PD stretch on to FEP and, in turn, on the enhancer mechanism.
TABLE 3.
Percent fT>MIC for cefepime and zidebactam in mouse plasma for an MIC range of 2 to 16 mg/liter
| MIC (mg/liter) | % fT>MIC for: |
||
|---|---|---|---|
| Cefepime at 25 mg/kg q2h | Zidebactam at 12.5 mg/kg q2h | Zidebactam at 37.5 mg/kg q2h | |
| 2 | 73.8 | 63.5 | 79.8 |
| 4 | 63.6 | 48.7 | 66.5 |
| 8 | 48.4 | 33.1 | 51.2 |
| 16 | 31.5 | 10.1 | 37.3 |
In neutropenic mouse lung infection studies, across the strains, the mean ± standard deviation bacterial lung burden at the initiation of treatment (0 h) was 7.12 ± 0.43 log10 CFU/lung. At the 25-h endpoint, net growth of >2 log10 CFU/lung was observed in untreated control mice and mice treated with ZID alone (12.5 and 37.5 mg/kg q2h), indicating a lack of in vivo efficacy of ZID.
Figure 2 illustrates the pharmacodynamic response observed with FEP monotherapy and FEP-ZID combination therapy at a total daily dose of 600 mg/kg for A. baumannii SL1. Based on the mouse plasma PK, FEP monotherapy at a total dose of 600 mg/kg fractionated into regimens administered every 2 h (q2h), every 4 h (q4h), every 6 h (q6h), and every 12 h (q12h) provides an FEP fT>MIC of 48.3%, 29.7%, 22.9%, and 12.3%, respectively. Cefepime at the most frequent regimen of 50 mg/kg q2h produced 1.03 ± 0.4-log10 kill, which was associated with an fT>MIC of 48.3%. Regimen-dependent erosion in the FEP bactericidal action was observed with less frequent fractionation of q4h (6 doses), q6h (4 doses), and q12h (2 doses). For instance, q4h, q6h, and q12h fractionations of FEP provided bacteriostatic, 0.5 ± 0.5-log10, and 1.48 ± 0.42-log10 net growth effects, respectively, due to lowering of the percent fT>MIC with these infrequent regimens. Interestingly, addition of ZID (12.5 mg/kg q2h) to such infrequent FEP regimens of q4h and q6h enabled FEP to achieve 1.74 ± 0.5-log10 kill and 1.02 ± 0.52-log10 kill, respectively, which were associated with FEP fT>MIC of 29.7% and 22.9%, respectively. These requirements are significantly lower than those for FEP monotherapy, which is associated with an fT>MIC of 48.3%, which is linked with 1.03-log10 kill, thereby demonstrating ZID’s enhancer action. Notably, ZID rendered a bacteriostatic effect even at the most infrequent q12h FEP regimen, which otherwise was inefficacious. A similar trend was noted for the other strains as well.
FIG 2.
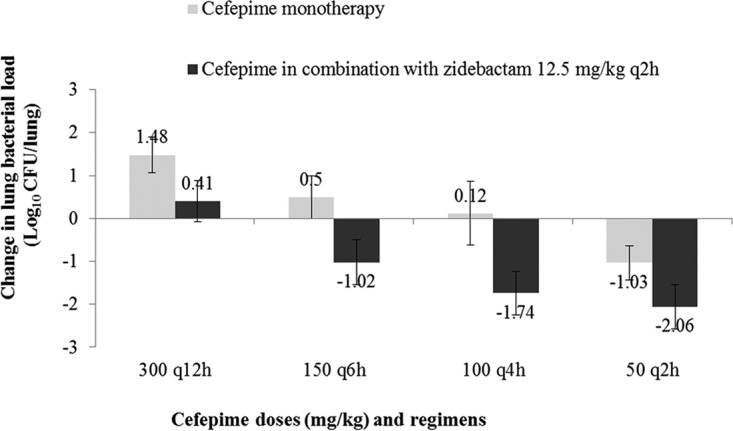
Pharmacodynamic efficacy of cefepime monotherapy and the cefepime-zidebactam combination against A. baumannii SL1.
For all five A. baumannii strains (strain-wise total daily FEP dose range, 50 to 600 mg/kg), bacterial eradication ranging from 0.67 to 1.41 log10 was observed with the regimen of FEP alone q2h. However, addition of an inefficacious dose of ZID (12.5 mg/kg q2h) to the less frequent FEP regimens of q6h or q12h yielded 1.02- to 2.96-log10 kill, respectively (Fig. 3). Thus, ZID was able to substantially reduce the number of FEP doses (without altering the total daily dose) and still provide a favorable pharmacodynamic effect.
FIG 3.
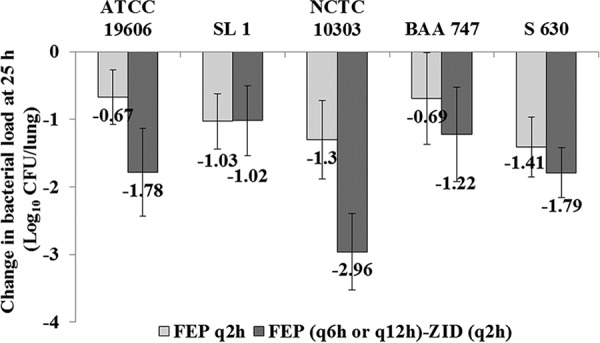
Pharmacodynamic response of A. baumannii strains to FEP and FEP-ZID in various dose regimens in an immunocompromised murine lung infection model. The lung bacterial load is represented as the mean number of log10 CFU per lung ± standard deviation. The strain-wise total daily doses of FEP were 600 mg/kg (ATCC 19606), 600 mg/kg (SL1), 50 mg/kg (NCTC 10303), 50 mg/kg (BAA 747), and 200 mg/kg (S 630).
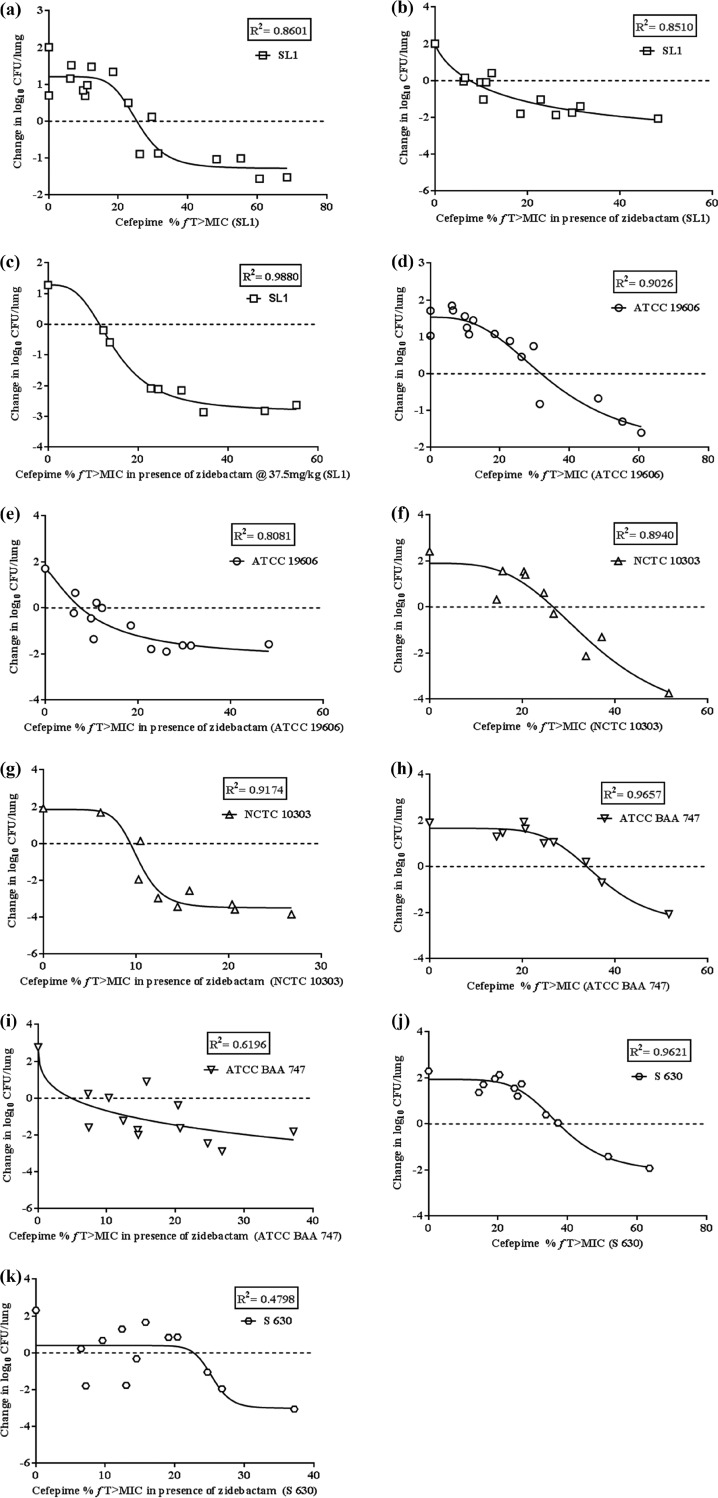
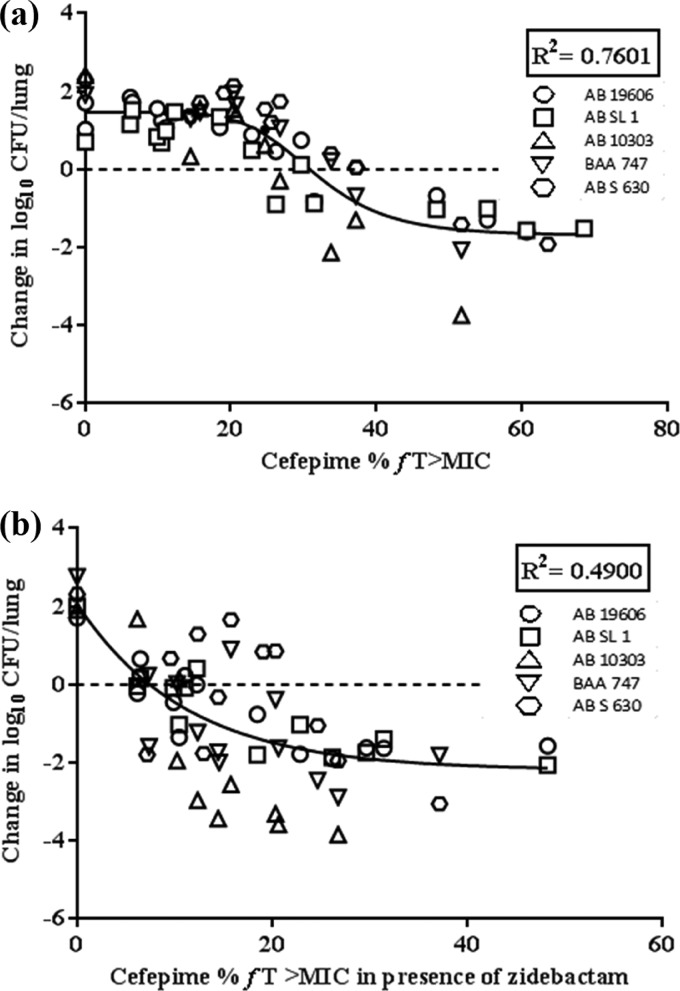
Table 4 provides the percent fT>MIC of FEP as monotherapy and in combination with ZID, identified from a nonlinear sigmoidal maximum-effect (Emax) model for each of the five strains. The exposure-response curves obtained for these strains are provided in Fig. 4a to k. For 1-log10 kill, the fT>MIC for FEP monotherapy ranged from 32 to 47.8%, but in the presence of ZID, this value was significantly reduced to 10.4 to 24.9% (Table 4), signifying the role of the enhancer action of ZID. Further, the pharmacodynamic responses obtained for all the strains were combined using the nonlinear sigmoidal Emax model (Fig. 5a and b). For 1-log10 kill, this composite fit-based exposure-response analyses showed an fT>MIC requirement of 38.9% for FEP alone, which dropped to 15.5% in combination with ZID (Table 4).
TABLE 4.
Percent fT>MIC of cefepime alone and in the presence of zidebactam for bacteriostatic and 1-log10-kill effects against five A. baumannii strains
| A. baumannii strain | % fT>MIC requirement for the indicated effecta |
|||
|---|---|---|---|---|
| Cefepime |
Cefepime in presence of zidebactam |
|||
| Bacteriostatic | 1-log10 kill | Bacteriostatic | 1-log10 kill | |
| NCTC 10303 | 26.7 | 32 | 9.9 | 10.4 |
| ATCC 19606 | 31.9 | 47.8 | 7.7 | 16.6 |
| SL1 | 25.4 | 35.5 | 7.6 | 17.7 |
| BAA 747 | 34 | 39.9 | 4.9 | 13.5 |
| S630 | 37.4 | 45.4 | 22.7 | 24.9 |
| Composite fit for all strains | 30.9 | 38.9 | 7.3 | 15.5 |
Identified from a sigmoidal nonlinear Emax model (GraphPad Prism [version 7] software)-based exposure-response analysis.
FIG 4.
Exposure-response analysis for identifying the percent fT>MIC of cefepime alone and in the presence of zidebactam for static and 1-log10 kill effects. (a) Cefepime alone for A. baumannii SL1; (b) cefepime in the presence of zidebactam for A. baumannii SL1; (c) cefepime in the presence of zidebactam (at a higher dose of 37.5 mg/kg q2h) for A. baumannii SL1; (d) cefepime alone for A. baumannii ATCC 19606; (e) cefepime in the presence of zidebactam for A. baumannii ATCC 19606; (f) cefepime alone for A. baumannii NCTC 10303; (g) cefepime in the presence of zidebactam for A. baumannii NCTC 10303; (h) cefepime alone for A. baumannii ATCC BAA 747; (i) cefepime in the presence of zidebactam for A. baumannii ATCC BAA 747; (j) cefepime alone for A. baumannii S 630.
FIG 5.
(a) Comodeling-based cefepime exposure-response analysis for all A. baumannii strains. (b) Comodeling-based cefepime-zidebactam exposure-response analysis for all A. baumannii strains.
For A. baumannii SL1, the impact of a higher ZID dose providing clinically equivalent exposures was also assessed. Figure 4c shows an exposure (percent fT>MIC)-response curve based on the pharmacodynamic responses obtained for FEP in combination with ZID at 37.5 mg/kg q2h. The lower-dose regimen of 12.5 mg/kg q2h reduced the FEP requirement from 35.5% (Fig. 4a and Table 4) to 17.7% (Fig. 4b and Table 4) for 1-log10 kill. Likewise, the higher ZID dose regimen of 37.5 mg/kg q2h resulted in a reduction to 15.8%. This shows that even the lower ZID dose was able to provide a significant reduction in the FEP percent fT>MIC and that the higher dose maintained the enhancer effect.
DISCUSSION
The objective of the present study was to investigate the effect of PBP2-binding ZID on the pharmacodynamic activity of PBP3-binding FEP against five A. baumannii strains. The cefepime MIC against these strains ranged from 2 to 16 mg/liter, suggesting a lack of significant expression of FEP-impacting β-lactamases. The addition of ZID did not lower the MIC of FEP against any of these A. baumannii strains. However, time-kill studies revealed that ZID mediated the enhancement of bactericidal activity at sub-MICs of FEP. Even though high concentrations of ZID (64 mg/liter) were not bactericidal on a stand-alone basis, the ZID concentrations of 1 to 8 mg/liter in combination with FEP induced a higher degree of killing. This corroborates the previous observation demonstrating the induction of pleomorphic spheroplasts by ZID at concentrations as low as 0.5 to 1 mg/liter, an indicator of efficient PBP2 binding in A. baumannii (5). Previous studies have also shown that ZID alone does not bring about consistent bactericidal action, an observation also reported for the PBP2-binding penicillin amdinocillin alone (18). In view of the reported high affinity of ZID to PBP2 and FEP to PBP3, the augmented killing observed with FEP in the presence of ZID is attributable to the simultaneous inactivation of PBP3 and PBP2 by FEP and ZID, respectively. Satta et al. previously showed that the simultaneous saturation of PBP2 and PBP3 by a combination of amdinocillin and aztreonam, respectively, produced an enhanced killing of Escherichia coli compared to that of single PBP2 or PBP3 saturation by amdinocillin and aztreonam alone, respectively (10).
The zidebactam-mediated enhancement of FEP killing activity observed in time-kill studies was also manifested in neutropenic mouse lung infection studies. For each strain, FEP monotherapy administered q2h was associated with 1-log10 kill, and less frequent q4h, q6h, and q12h regimens did not consistently result in a bactericidal effect. However, combining less frequent FEP dose regimens (q4h, q6h, or q12h) with ZID consistently resulted in a ≥1-log10 kill, despite the significantly reduced percent fT>MIC of FEP with these regimens. The composite fit of the FEP pharmacodynamic responses for all five A. baumannii strains revealed that the fT>MIC linked with a 1-log10 kill was 38.9%, which in the presence of ZID dropped to 15.5%, thus demonstrating ZID’s enhancer effect in vivo. A similar reduction in the FEP percent fT>MIC was also associated with bacteriostasis as the efficacy endpoint. Thus, ZID mediated enhancement of FEP’s in vitro bactericidal activity duly translated in vivo in terms of a significant reduction of the FEP pharmacodynamic requirement for efficacy. It is notable that despite the limited availability of ZID, a significant enhancement of FEP pharmacodynamic activity was observed. For instance, ZID at a dose of 12.5 mg/kg administered q2h provides a ZID fT>MIC of merely 10.1% for a FEP-ZID MIC of 16 mg/liter (Table 3). Such a short-duration requirement for ZID indicates that, possibly, for enhancer action, even sub-MICs drive pharmacodynamic action.
The demonstration of FEP-ZID efficacy in the lung, despite lower ELF exposure requirements, is in agreement with the findings of a previous study, wherein A. baumannii strains with even higher FEP-ZID MICs were employed (14). In this study, by Avery et al., the authors refer to spheroplast- and elongation-inducing concentrations of ZID and FEP, respectively, as the pharmacodynamic drivers of efficacy (14). The fact that ZID brought down the percent fT>MIC of FEP, despite not being able to lower the FEP MICs, signifies the relevance of time-kill studies in revealing the enhancer mechanism of action. Though the sub-MIC killing eventually diminished by 24 h in vitro, bacterial eradication in vivo was sustained due to the replenishment of FEP-ZID through repeat dose administration in mice, a scenario similar to clinical dosing.
Previously, for multiple-PBP-binding carbapenems, MacVane et al. (19) reported an fT>MIC of 23.67% and 32.82% for bacteriostatic and bactericidal effects, respectively, against A. baumannii in a neutropenic mouse thigh infection model. These values are lower than those reported for cephalosporins and penicillins. The characteristic features of carbapenems, such as multiple PBP binding, rapid bactericidal action, and low percent fT>MIC requirements, are also evident with FEP-ZID, which shows a mechanism of action similar to carbapenems. For instance, in recently reported studies involving MDR A. baumannii strains with an FEP-ZID MIC in the range of 16 to 64 mg/liter, FEP-ZID in a simulated human regimen showed 2- to 3-log10 kill against class D carbapenemase-producing A. baumannii strains in neutropenic mouse lung and thigh infection models (14, 15). Interestingly, in these studies, FEP availability of as low as an ∼20% fT>MIC in combination with ZID was adequate to exert 2- to 4-log10 kill effects. Even for ZID, availability of as low as an ∼4% fT>MIC was able to evoke enhancer action (14, 15).
This study demonstrated that ZID exerted a reduction in the percent fT>MIC of FEP, and in conjunction with the high FEP-ZID clinical doses selected, this feature could impart a substantial PK/PD leeway to the combination. This PK/PD attribute could be helpful in providing consistent clinical efficacy even for therapeutically challenging patients, such as those with reduced drug exposures.
The β-lactam enhancer action of ZID in augmenting FEP efficacy is distinct from the action of β-lactamase inhibitors, as the latter are not known to alter the PK/PD attributes of the partner β-lactam drug. This is the first study to reveal the augmentation of FEP’s pharmacodynamic action by the β-lactam enhancer ZID against A. baumannii.
MATERIALS AND METHODS
Antimicrobial test agents.
Cefepime hydrochloride with arginine analytical powder (batch no. 7040DK86DA; Qilu Antibiotics Pharmaceutical, China) and ZID (batch no. ESS10928, synthesized by Wockhardt, India) were used throughout the studies. A commercially available formulation of meropenem (batch no. ZID17018; Zuventus, India) was used. For in vivo studies, drug stock solutions (20 mg/ml) were prepared with sterile water for injection, and subsequent working dilutions were prepared in sterile 0.9% saline solution. All doses were administered as milligrams per kilogram of body weight. Control animals were administered the vehicle at a volume equivalent to the highest volume used in the study group. FEP and ZID were given as two separate injections.
Bacterial isolates and in vitro susceptibility testing.
Five carbapenemase-negative A. baumannii isolates (ATCC 19606, SL1, NCTC 10303, ATCC BAA 747, and S 630) that showed meropenem MICs of ≤2 mg/liter were employed. A. baumannii ATCC 19606 and ATCC BAA 747 were procured from ATCC. A. baumannii NCTC 10303 was procured from Public Health England, UK. The other two A. baumannii strains, SL1 and S 630, were clinical isolates obtained from Indian tertiary care hospitals. For all isolates, the modal MICs of FEP, ZID, and FEP-ZID were determined from multiple replicates, each from a separate subculture, by broth microdilution, in accordance with Clinical Laboratories and Standards Institute procedure M07-A10 (20). Quality control isolate A. baumannii NCTC 13304 was used for validation of FEP-ZID MICs (quality control range, 4 to 16 mg/liter) (21). Since both the constituents of FEP-ZID are antibacterially active, the MIC determination method involves the use of a 1:1 FEP-ZID ratio, which has been accepted by CLSI and published in the 28th edition of the CLSI M100 document (22). The M23 study, which involves independent MIC determinations in multiple replicates undertaken in eight U.S. laboratories, showed that the FEP-ZID MICs determined at a 1:1 ratio are highly reproducible, and the quality control ranges were successfully established (21). Moreover, the use of a 1:1 ratio for MIC determination eliminates activity bias due to either of the components.
Time-kill studies.
Time kill studies were initiated by adding drugs to cation-adjusted Mueller-Hinton broth (CA-MHB) medium containing exponentially growing cultures at the targeted inoculum, and the cultures were incubated at 37°C under shaking conditions (120 rpm). Viability counts were determined at various time points by plating serial dilutions (1:10) of the cultures. Time-kill studies were initiated with an inoculum (∼7 log10 CFU/ml) 1 log10 higher than the generally employed cell density. The change in the number of log10 CFU over that in the starting inoculum was monitored for up to 8 h upon exposure to FEP, ZID, or FEP-ZID. All time-kill studies were performed in duplicate, and mean values from two experiments with standard deviations are shown.
Animal infection model.
(i) Laboratory animals. Male and female Swiss albino mice weighing approximately 22 to 24 g were obtained from the Wockhardt animal breeding facility. Mice were allowed to acclimate to the laboratory environment for at least 48 h prior to all experimental procedures and were provided food and water ad libitum. The study protocol was reviewed and approved by Wockhardt’s Institutional Animal Ethics Committee.
(ii) Neutropenic mouse lung infection model.
Male and female Swiss albino mice weighing 25 to 27 g were rendered neutropenic by intraperitoneal (i.p.) injections of cyclophosphamide at 150 and 100 mg/kg 4 days and 1 day, respectively, prior to infection. At 2 h prior to the initiation of antibacterial therapy, each animal was infected, while under anesthesia (xylazine at 8 mg/kg and thiopental sodium at 40 mg/kg i.p.), by the intranasal route with 0.08 ml of a bacterial suspension containing approximately 108 log10 CFU/ml of the infecting pathogen.
Pharmacokinetic studies.
The PK of FEP and ZID alone and FEP-ZID in combination (2:1 ratio) were determined in healthy Swiss albino mice following subcutaneous administration of FEP single doses ranging from 25 mg/kg to 200 mg/kg and ZID single doses ranging from 12.5 to 100 mg/kg. For determination of the PK of the combination, FEP and ZID doses were combined at a 2:1 ratio. Following administration of the doses, blood samples were collected from groups of four mice each at 6 to 10 time points ranging from 0.25 to 24 h. Plasma FEP and ZID concentrations were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS), using a validated method. PK parameters for FEP and ZID were calculated using first-order input and elimination, by using a nonlinear least-squares technique with the help of Phoenix WinNonlin (version 6.4) software. The PK parameters obtained from these studies were used to derive the percent fT>MIC for FEP administered in different regimens. In a separate experiment, PK in infected and healthy mice were comparatively determined at a dose of 100 mg/kg for FEP and 50 mg/kg for zidebactam. The infection was induced as described above in “(ii) Neutropenic mouse lung infection model.” Pulmonary PK studies were conducted in Swiss albino mice following subcutaneous administration of single doses of FEP alone at 25 mg/kg and ZID alone at 12.5 mg/kg. Following administration of the compounds (n = 3 mice/time point), samples of blood and bronchoalveolar lavage (BAL) fluid were concomitantly collected at various predetermined time points ranging from 0.0 to 8 h. Blood samples were collected through the retro-orbital sinus and placed in heparin-containing tubes, and the mice were later humanely sacrificed by cervical dislocation while they were under CO2 anesthesia. The mice were then placed on a comfortable platform, and the trachea was exposed by making a 1-cm-long incision on the ventral neck skin for insertion of the cannula, which was secured in place. The lungs were instilled 3 times with 0.5 ml of sterile normal saline solution (0.9% NaCl), and the fluid was immediately aspirated. Aspirates thus recovered were pooled in a single microcentrifuge tube and directly placed on ice. Collected blood and BAL fluid samples were centrifuged at 10,000 rpm for 10 min. Plasma and BAL fluid samples from the respective mice were split (according to matrix), arranged with respect to the scheduled time points, and stored at –70°C until bioanalysis. Plasma FEP and ZID concentrations were determined by LC-MS/MS using a validated method.
Bacterial density studies.
In order to determine the exposure requirements of FEP alone, infected animals were treated with various FEP fractionated dose regimens that allowed creation of a range of percent fT>MIC values and identify the magnitude of the percent fT>MIC required for bacteriostasis and a 1-log10 drop for each strain. To assess the pharmacodynamic enhancement, FEP dose regimens were combined with a fixed ZID dose of 12.5 mg/kg, which produced about 1/3 of the clinical exposure of ZID in the FEP-ZID combination (23).
Bacterial eradication studies were performed to identify the exposures of FEP alone and FEP-ZID that provided bacteriostasis and a 1-log10 drop in the number of CFU of each of the five A. baumannii strains. At 2 h following infection, over a 24-h period, groups of 6 mice received subcutaneous doses (0.25 ml) of FEP alone in various fractionated regimens or FEP in combination with a nonefficacious 12.5-mg/kg q2h regimen of ZID. An additional ZID dose of 37.5 mg/kg q2h, which provides a clinically equivalent ZID exposure, was also tested against the SL1 strain. The control group included 6 animals that were untreated at 0 h and another 6 animals that received equivalent (0.25 ml) saline dosing at each time point for the period of 24 h. To assess the lung bacterial load at the initiation (0 h) and end (25 h) of the treatment, animals were humanely euthanized, and all lung lobes were harvested in sterile phosphate-buffered saline and homogenized. The normal practice is to determine the viable count at 24 h after the initiation of dosing; however, we delayed it by 1 h (25 h). The lung viable bacterial load (number of log10 CFU per lung) was determined by plating the serial dilutions of lung homogenates on Trypticase soya agar (TSA) plates, followed by incubation at 37°C for approximately 16 to 20 h. The efficacy was estimated as the mean change in the lung bacterial load at 25 h in the treatment groups compared to that at 0 h (at the initiation of therapy) in the control group.
The percent fT>MIC of FEP for each dose was determined from PK studies and employed in a nonlinear sigmoidal Emax model (GraphPad Prism [version 7] software) to derive the magnitude of the percent fT>MIC of FEP linked to bacteriostatic and 1-log10 kill effects against each strain. A comodeling approach was also undertaken, wherein the pharmacodynamic responses obtained from all strains were combined for the nonlinear sigmoidal Emax model to identify a unified exposure requirement for FEP alone and in the presence of ZID.
Supplementary Material
ACKNOWLEDGMENTS
We thank Rajesh Chavan and Prashant Joshi for providing technical support in conducting the in vitro and in vivo studies.
We are all employees of the Wockhardt Research Centre. S. S. Bhagwat and M. V. Patel are employees and shareholders of Wockhardt Ltd.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02146-18.
REFERENCES
- 1.Meletis G. 2016. Carbapenem resistance: overview of the problem and future perspectives. Ther Adv Infect Dis 3:15–21. doi: 10.1177/2049936115621709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2011. Antibiotic resistance patient safety atlas. Antibiotic resistance (AR) data. Centers for Disease Control and Prevention, Atlanta, GA: https://gis.cdc.gov/grasp/PSA/MapView.html. [Google Scholar]
- 3.Wang X, Zhang F, Zhao C, Wang Z, Nichols WW, Testa R, Li H, Chen H, He W, Wang Q, Wang H. 2014. In vitro activities of ceftazidime-avibactam and aztreonam-avibactam against 372 Gram-negative bacilli collected in 2011 and 2012 from 11 teaching hospitals in China. Antimicrob Agents Chemother 58:1774–1778. doi: 10.1128/AAC.02123-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfaller MA, Huband MD, Mendes RE, Flamm RK, Castanheira M. 2018. In vitro activity of meropenem/vaborbactam and characterisation of carbapenem resistance mechanisms among carbapenem-resistant Enterobacteriaceae from the 2015 meropenem/vaborbactam surveillance programme. Int J Antimicrob Agents 52:144–150. doi: 10.1016/j.ijantimicag.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Oliver A. 2017. Potent beta-lactam enhancer activity of zidebactam and WCK 5153 against Acinetobacter baumannii, including carbapenemase-producing clinical isolates. Antimicrob Agents Chemother 61:e01238-17. doi: 10.1128/AAC.01238-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Oliver A. 2017. WCK 5107 (zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “beta-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-beta-lactamase-producing high-risk clones. Antimicrob Agents Chemother 61:e02529-16. doi: 10.1128/AAC.02529-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papp-Wallace KM, Nguyen NQ, Jacobs MR, Bethel CR, Barnes MD, Kumar V, Bajaksouzian S, Rudin SD, Rather PN, Bhavsar S, Ravikumar T, Deshpande PK, Patil V, Yeole R, Bhagwat SS, Patel MV, van den Akker F, Bonomo RA. 2018. Strategic approaches to overcome resistance against Gram-negative pathogens using beta-lactamase inhibitors and beta-lactam enhancers: activity of three novel diazabicyclooctanes WCK 5153, zidebactam (WCK 5107), and WCK 4234. J Med Chem 61:4067–4086. doi: 10.1021/acs.jmedchem.8b00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palwe SR, Biniwale SS, Khande HN, Joshi PR, Bhagwat SS, Patel MV. 2016. Cefepime (FEP) and WCK 5107 (zidebactam, ZID) mediated dual PBP engagement at sub-MIC concentrations drive cidality against diverse β-lactamases expressing Gram-negatives, poster no. Sunday 448 ASM Microbe, Boston, MA. American Society for Microbiology, Washington, DC. [Google Scholar]
- 9.Palwe SR, Joshi PR, Khande HN, Biniwale SS, Bhagwat SS, Patel MV. 2016. WCK 5107 (zidebactam, ZID): a pan Gram-negative β-lactam enhancer augmenting β-lactam pharmacodynamics in wild type and carbapenemase producers (CP), poster no. Sunday 438. ASM Microbe, Boston, MA. American Society for Microbiology, Washington, DC. [Google Scholar]
- 10.Satta G, Cornaglia G, Mazzariol A, Golini G, Valisena S, Fontana R. 1995. Target for bacteriostatic and bactericidal activities of beta-lactam antibiotics against Escherichia coli resides in different penicillin-binding proteins. Antimicrob Agents Chemother 39:812–818. doi: 10.1128/AAC.39.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sader HS, Castanheira M, Huband M, Jones RN, Flamm RK. 2017. WCK 5222 (cefepime-zidebactam) antimicrobial activity against clinical isolates of Gram-negative bacteria collected worldwide in 2015. Antimicrob Agents Chemother 61:e00072-17. doi: 10.1128/AAC.00072-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sader HS, Rhomberg PR, Flamm RK, Jones RN, Castanheira M. 2017. WCK 5222 (cefepime/zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant beta-lactamases. J Antimicrob Chemother 72:1696–1703. doi: 10.1093/jac/dkx050. [DOI] [PubMed] [Google Scholar]
- 13.Takalkar SS, Kulkarni AM, Chavan RP, Zope VS, Joshi PR, Khande HS, Satav JS, Bhagwat SS, Patel MV. 2016. WCK 5222 [cefepime (FEP)-WCK 5107 (zidebactam, ZID)]: thigh and lung PK/PD studies against higher MIC OXA carbapenemase-expressing A. baumannii (AB), poster no. Sunday 441. ASM Microbe, Boston, MA. American Society for Microbiology, Washington, DC. [Google Scholar]
- 14.Avery LM, Abdelraouf K, Nicolau DP. 2018. Assessment of the in vivo efficacy of WCK 5222 (cefepime-zidebactam) against carbapenem-resistant Acinetobacter baumannii in the neutropenic murine lung infection model. Antimicrob Agents Chemother 62:e00948-18. doi: 10.1128/AAC.00948-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abuhussain SA, Avery LM, Abdelraouf K, Nicolau P. 2018. Assessment of the in vivo efficacy of WCK 5222 (cefepime-zidebactam) against carbapenem-resistant Acinetobacter baumannii in the neutropenic murine thigh infection model, poster no. 1336. ID Week, San Francisco, CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhagwat SS, Takalkar SS, Chavan RP, Friedland HD, Patel MV. 2017. WCK 5222 [cefepime (FEP) + WCK 5107 (zidebactam, ZID)]: in vivo demonstration of ZID-mediated β-lactam enhancer effect leading to lowering of FEP %fT>MIC against P. aeruginosa (PA) and A. baumannii (AB), poster no. Saturday 284. ASM Microbe, New Orleans, LA. American Society for Microbiology, Washington, DC. [Google Scholar]
- 17.Chugh R, Lakdavala F, Friedland HD, Bhatia A. 2017. Safety and pharmacokinetics of multiple ascending doses of WCK 5107 (zidebactam) and WCK 5222 (cefepime and zidebactam), poster no. P1301. ECCMID, Vienna, Austria. [Google Scholar]
- 18.Fass RJ. 1980. Activity of mecillinam alone and in combination with other beta-lactam antibiotics. Antimicrob Agents Chemother 18:906–912. doi: 10.1128/AAC.18.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacVane SH, Crandon JL, Nicolau DP. 2014. Characterizing in vivo pharmacodynamics of carbapenems against Acinetobacter baumannii in a murine thigh infection model to support breakpoint determinations. Antimicrob Agents Chemother 58:599–601. doi: 10.1128/AAC.02029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 10th ed CLSI document M07-A10 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.Traczewski MM, Bhagwat SS. 2017. Cefepime-zidebactam (FEP-ZID) or WCK 5222 tier 2 broth microdilution MIC quality control versus E. coli ATCC 25922, K. pneumoniae ATCC 700603, P. aeruginosa ATCC 27853, A. baumannii NCTC 13304 and E. coli NCTC 13353, poster no. Saturday 284. ASM Microbe, New Orleans, LA. American Society for Microbiology, Washington, DC. [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 28th ed CLSI document M100 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 23.Rodvold KA, Gotfried MH, Chugh R, Gupta M, Patel A, Chavan R, Yeole R, Friedland HD, Bhatia A. 2018. Plasma and intrapulmonary concentrations of cefepime and zidebactam following intravenous administration of WCK 5222 to healthy adult subjects. Antimicrob Agents Chemother 62:e00682-18. doi: 10.1128/AAC.00682-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.