Avibactam is a non-β-lactam β-lactamase inhibitor that has been approved in combination with ceftazidime for the treatment of complicated intra-abdominal infections, complicated urinary tract infections, and nosocomial pneumonia, including ventilator-associated pneumonia. In Europe, ceftazidime-avibactam is also approved for the treatment of Gram-negative infections with limited treatment options.
KEYWORDS: PK/PD, breakpoints, ceftazidime-avibactam, dose selection, probability of target attainment
ABSTRACT
Avibactam is a non-β-lactam β-lactamase inhibitor that has been approved in combination with ceftazidime for the treatment of complicated intra-abdominal infections, complicated urinary tract infections, and nosocomial pneumonia, including ventilator-associated pneumonia. In Europe, ceftazidime-avibactam is also approved for the treatment of Gram-negative infections with limited treatment options. Selection and validation of the ceftazidime-avibactam dosage regimen was guided by an iterative process of population pharmacokinetic (PK) modelling, whereby population PK models for ceftazidime and avibactam were developed using PK data from clinical trials and updated periodically. These models were used in probability of target attainment (PTA) simulations using joint pharmacodynamic (PD) targets for ceftazidime and avibactam derived from preclinical data. Joint PTA was calculated based on the simultaneous achievement of the individual PK/PD targets (50% free time above the ceftazidime-avibactam MIC for ceftazidime and free time above a critical avibactam threshold concentration of 1 mg/liter for avibactam). The joint PTA analyses supported a ceftazidime-avibactam dosage regimen of 2,000 + 500 mg every 8 h by 2-h intravenous infusion for patients with creatinine clearance (CLCR) >50 ml/min across all approved indications and modified dosage regimens for patients with CLCR ≤50 ml/min. Subgroup simulations for individual phase 3 patients showed that the dosage regimen was robust, with high target attainment (>95%) against MICs ≤8 mg/liter achieved regardless of older age, obesity, augmented renal clearance, or severity of infection. This review summarizes how the approved ceftazidime-avibactam dosage regimens were developed and validated using PK/PD targets, population PK modeling, and PTA analyses.
INTRODUCTION
Infections caused by multidrug-resistant (MDR) Gram-negative bacteria affect increasing numbers of patients worldwide and are associated with high rates of morbidity and mortality (1). Of particular concern is the continued and growing resistance to β-lactam antibiotics, which in Gram-negative bacteria is primarily mediated by the production of β-lactamases. Avibactam is a novel non-β-lactam β-lactamase inhibitor that restores the in vitro activity of ceftazidime against Ambler class A, class C, and some class D β-lactamase-producing pathogens (2–5), including those producing Klebsiella pneumoniae carbapenemase (KPC) and OXA-48 carbapenemases (6–9). The addition of avibactam thus expands the spectrum of activity of ceftazidime to encompass a broad range of Gram-negative bacteria, including non-metallo-β-lactamase (MBL)-producing carbapenemase-resistant Enterobacteriaceae and some MDR Pseudomonas aeruginosa (7, 10, 11).
Dosage regimen selection and validation for ceftazidime-avibactam was guided by an iterative population pharmacokinetic (PK) modeling process in which models for ceftazidime and avibactam were built from the ground up using patient PK data from early phase 1 and 2 trials (12, 13) and updated periodically with new PK data as phase 3 studies for each indication were completed (14–16). Using ceftazidime and avibactam PK/pharmacodynamic (PD) targets derived from preclinical studies, these models were used to calculate probability of target attainment (PTA) for simulated patients receiving the proposed ceftazidime-avibactam dosage regimen, including dosage adjustments for renal impairment (15–19). Such analyses are commonly used to optimize dosage regimens for new antibiotics and to support determination of MIC interpretative criteria (clinical susceptibility breakpoints) (20–22). The ceftazidime-avibactam dosage regimen evaluated in the phase 3 trial program was 2,000 + 500 mg given as a 2-h intravenous (i.v.) infusion, every 8 h (q8h), with adjustments for renal function in patients with a creatinine clearance (CLCR) of <50 ml/min. Five randomized, active-comparator phase 3 studies have demonstrated the clinical efficacy and safety of this ceftazidime-avibactam dosage regimen in patients with complicated intra-abdominal infections (cIAI), complicated urinary tract infections (cUTI), and nosocomial pneumonia (NP), including ventilator-associated pneumonia (VAP) (23–27).
Ceftazidime avibactam has been approved in the United States and in Europe for the treatment of adults with cIAI (in combination with metronidazole), cUTI, and hospital-acquired pneumonia (HAP), including VAP (28, 29). In Europe, ceftazidime-avibactam is also approved for the treatment of infections due to aerobic Gram-negative organisms in patients with limited treatment options (29). PK/PD analyses were of particular importance in supporting these regulatory approvals. The original U.S. Food and Drug Administration (FDA) approval in 2015 for cIAI and cUTI was based on phase 2 data (30, 31) and population PK/PD analyses as part of the Qualified Infectious Disease Product program (32). Results from the phase 3 trials in cIAI and cUTI confirmed the efficacy of the selected dosage regimens, expanded the available safety data, and provided key data on patient subgroups (e.g., based on disease severity and renal function). PK/PD analyses also supported modifications to the dosage adjustments used in phase 3 studies for patients with CLCR values of <50 ml/min. In 2018, the FDA approved ceftazidime-avibactam for the treatment of HAP and VAP following results from the phase 3 trial in NP (28). In Europe, initial approval of ceftazidime-avibactam for cIAI and cUTI followed the completion of the pivotal phase 3 trials in these indications. Population PK analyses and data from the phase 3 cIAI and cUTI studies also supported the European approval of the same ceftazidime-avibactam dosage regimen for HAP (including VAP), before full results were available from the phase 3 trial in NP/VAP (29, 33). This dosage regimen was also approved for the fourth European indication (infections due to aerobic Gram-negative organisms in adults with limited treatment options), based on experience with ceftazidime alone and on analyses of the ceftazidime-avibactam PK/PD relationship (29). Of note, the ceftazidime component of the ceftazidime-avibactam dose is comparable (i.e., up to 2,000 mg q8h) to the approved ceftazidime labeling (34, 35) and for which there is a well-established safety profile (36). Avibactam has low potential for drug-drug interactions (37), and its safety and tolerability have been established in multiple clinical studies in the development of ceftazidime-avibactam, including in subjects with renal impairment, and in healthy volunteers with exposures up to 1,000 mg q8h (38, 39).
In this review, we summarize how the approved ceftazidime-avibactam dosage regimens were selected, modified, and validated and briefly discuss their application in breakpoint determination.
Using PK/PD analyses to guide antibiotic dosage selection.
While the MIC is a standard measure of the in vitro activity of antimicrobial agents, the antibacterial efficacy of antibiotics in vivo is not only dependent on MIC but also on the exposure at the site of infection (i.e., PK) and the effect of the drug on the bacteria (i.e., PD). The relationships between antimicrobial exposures and antibacterial effects are usually described by PK/PD indices. These are typically either time dependent (i.e., related to the duration of time that the free drug concentration remains above the MIC [fT>MIC]), concentration dependent (related to the ratio of the free maximal drug concentration to the MIC [fCmax/MIC]), or related to the ratio of the area under the free drug concentration-time curve to the MIC (fAUC/MIC) (40). In vitro and in vivo models of infection are commonly used to establish antibiotic exposure-response relationships and to determine the target PK/PD index required for antimicrobial efficacy (21). Confirming these PK/PD index targets in clinical trials is challenging because there are often not enough response failures to perform exposure-response analyses (21). The other challenge is that there are typically few clinical trial isolates with MICs toward the upper end of the wild-type MIC distribution (41). Thus, the PK/PD index values are not well distributed, and most patients in the trial will have a fT>MIC close to 100%. For antibiotics, as the effect of the drug is directly on the bacteria (rather than, for example, a host receptor), antimicrobial effects observed in preclinical models are generally predictive of effects in infected patients, and there is good concordance between PK/PD indices derived from animal models and those derived clinically (40, 42, 43).
Due to the variability of PK characteristics between patients, a population PK modeling approach using Monte Carlo simulation is required to predict whether a particular antibiotic dosage regimen will result in a high probability of achievement of the PK/PD target (PTA). The requirements for using Monte Carlo simulation to guide dose selection are (i) a robust population PK model with defined distribution and covariance of PK parameters, ideally using human PK data from patients with the target infection; (ii) a covariate model describing how patient characteristics influence the PK parameters; and (iii) PK/PD targets associated with antimicrobial efficacy (44). Monte Carlo simulation can then be used to simulate PK for a large patient population and to calculate the percentage of patients predicted to achieve the PK/PD target, i.e., the PTA. As ceftazidime and avibactam are separate drugs that are coadministered in a fixed dose ratio, it was necessary to define separate PK/PD targets for each agent and to conduct PTA analyses based on the joint attainment of both targets simultaneously, referred to as the “joint PTA.” The following sections describe the PK/PD targets for ceftazidime and avibactam and the use of population PK modeling and PTA analyses to guide dose selection and breakpoint setting.
Ceftazidime PK/PD target.
The achievement of 50% fT>MIC is well established as the PK/PD target associated with efficacy for ceftazidime and other cephalosporins (42, 43, 45, 46) and has been used to determine ceftazidime breakpoints (22, 47, 48). The ceftazidime-avibactam MIC for the PK/PD target was determined from global surveillance studies of commonly causative pathogens (Enterobacteriaceae and P. aeruginosa) isolated from patients with the intended indications. The Clinical and Laboratory Standards Institute (CLSI)-and European Committee on Antimicrobial Susceptibility Testing (EUCAST)-approved methods of measuring the ceftazidime-avibactam MIC employ a fixed concentration of avibactam (4 mg/liter), while the concentration of ceftazidime is varied in 2-fold increments (49, 50). This concentration of avibactam inhibits the β-lactamases against which avibactam is active (i.e., not metallo-β-lactamases) such that the underlying antibacterial activity of ceftazidime can be assessed (51). Of note, the in vitro activity of ceftazidime-avibactam was assessed specifically against isolates from patients with pneumonia, since reduced susceptibility to some antibiotics has been reported for such isolates compared to those from other infection sources (52, 53).
At the time of dosage selection, ceftazidime-avibactam MIC90 values for samples of clinical isolates of P. aeruginosa not preselected for particular resistance phenotypes or genetic markers, ranged from 4 to 8 mg/liter across several surveillance studies (11, 54–58). Depending on the region, 89 to 96% of unselected clinical isolates of P. aeruginosa tested had ceftazidime-avibactam MIC values of ≤8 mg/liter. For Enterobacteriaceae, ceftazidime-avibactam MIC90 values in global surveillance studies were generally between 0.5 and 1 mg/liter for phenotypically and genotypically unselected Enterobacteriaceae (54, 55, 59), and ≥99% of unselected clinical isolates of Enterobacteriaceae tested yielded ceftazidime-avibactam MIC values of ≤8 mg/liter. In certain highly resistant subgroups, such as carbapenem-nonsusceptible and MDR Enterobacteriaceae isolates, the ceftazidime-avibactam MIC distribution was shifted to the right toward higher MICs than those for unselected isolates (7). For example, in 816 meropenem-nonsusceptible MBL-negative isolates, the 90th percentile ceftazidime-avibactam MIC was 4 mg/liter (7), and in 2,821 MDR Klebsiella spp. isolates, 90% tested with a ceftazidime-avibactam MIC of ≤2 mg/liter (60). Among the few isolates against which MICs of ceftazidime-avibactam were >8 mg/liter, most were MBL producers, for which avibactam is not expected to have any effect on the ceftazidime MIC (7, 60). In a surveillance study of isolates from hospitalized patients with pneumonia, the ceftazidime-avibactam MICs were ≤8 mg/liter against 92 to 96% of P. aeruginosa isolated from nonventilated patients and 79.2 to 95.4% of P. aeruginosa from ventilated patients (61). A ceftazidime-avibactam PK/PD target MIC of 8 mg/liter was therefore considered suitable for both Enterobacteriaceae and P. aeruginosa and was selected for the ceftazidime component of the joint PK/PD target (50% fT > 8 mg/liter) for dose selection and breakpoint setting.
Avibactam PK/PD target.
Using in vitro hollow fiber and in vivo mouse models of infection, the PK/PD index for avibactam in combination with ceftazidime was shown to be best described by the %fT that avibactam exceeded a required critical concentration threshold (CT) (i.e., %fT>CT) (62). In the first set of studies, Coleman and colleagues used a hollow-fiber infection model with ceftazidime-resistant Enterobacteriaceae that tested with a range of ceftazidime-avibactam MICs (≤0.125 to 4 mg/liter) and expressing different β-lactamase types. Isolates used in these studies included K. pneumoniae producing SHV-5, CTX-M-15, or KPC-2; an Enterobacter cloacae isolate producing derepressed AmpC; and a Citrobacter freundii isolate producing stably derepressed AmpC (63). From these studies, a minimum CT of 0.5 mg/liter avibactam was shown to be appropriate for the avibactam Enterobacteriaceae PK/PD target (63). In a second set of studies, Berkhout et al. used neutropenic murine thigh and lung infection models to determine the avibactam PK/PD target for P. aeruginosa (64). Seven well-characterized ceftazidime-resistant P. aeruginosa strains producing stably derepressed AmpC and/or TEM-24 β-lactamases were used in dose fractionation experiments. The results from these studies suggested that the contribution of the inhibitory effect of avibactam to the antibacterial effect of the combination was related primarily to %fT>CT, rather than Cmax or AUC, and that a CT of 1 mg/liter avibactam was the best predictor of efficacy. The %fT>CT (1 mg/liter) associated with efficacy ranged from 20% to 50% across the thigh and lung infection models, consistent with the 50% fT>MIC required for efficacy of ceftazidime alone. Based on these studies, and taking a conservative approach to ensure that the CT was appropriate for both Enterobacteriaceae and P. aeruginosa, 50% fT>CT of 1 mg/liter was considered a robust avibactam target for use in dose selection (62–64). In combination with the previously established ceftazidime target, the joint PK/PD target for dosage selection was defined as ceftazidime 50% fT > 8 mg/liter and avibactam 50% fT > 1 mg/liter (65).
Patient population PK modeling of ceftazidime and avibactam and PTA analyses to guide dosage selection.
Population PK models were developed for ceftazidime and avibactam using patient PK data from clinical trials in an iterative process throughout the clinical development program. Early population PK models developed using phase 1 and phase 2 study data were used to support the dose selection for the phase 3 studies (12, 19). These population PK models were updated to include additional data from the ceftazidime-avibactam clinical trial program and used to confirm that the phase 3 clinical dose selection provided sufficient exposure in more than 90% of patients across different patient subgroups (13, 15–18). The ceftazidime-avibactam phase 2 and 3 studies and dosage regimens evaluated are summarized in Table 1 , and the model iterations and patient data sets used to develop the population PK models are summarized in Table 2.
TABLE 1.
Summary of ceftazidime-avibactam phase 2 and 3 clinical trialsa
| ClinicalTrials.gov identifier (reference) | Patient population (n) | Ceftazidime-avibactam dosage regimen evaluated | Dosage modifications for patients with CLCR <50 ml/minb |
|---|---|---|---|
| Phase 2 | |||
| NCT00752219 (31) | cIAI (203) | 2,000 + 500 mg, 30-min i.v. infusion q8h | NA; only included patients with CLCR ≥50 ml/min |
| NCT00690378 (30) | cUTI (135) | 500 + 125 mg, 30-min i.v. infusion q8h | NA; only included patients with CLCR ≥50 ml/min |
| Phase 3 | |||
| RECLAIM, NCT01499290 (23) | cIAI (1,066) | 2,000 + 500 mg 2-h i.v. infusion q8h | Original dosage adjustments specified in protocol |
| REPRISE, NCT01644643 (25) | cIAI and cUTI caused by ceftazidime-nonsusceptible Enterobacteriaceae or P. aeruginosa (333) | 2,000 + 500 mg 2-h i.v. infusion q8h | Original dosage adjustments specified in protocol |
| RECLAIM 3, NCT01726023 (26) | Asian patients with cIAI (441) | 2,000 + 500 mg 2-h i.v. infusion q8h | Original dosage adjustments specified in protocol |
| RECAPTURE, NCT01595438 and NCT01599806 (24) | cUTI (1,033) | 2,000 + 500 mg 2-h i.v. infusion q8h | Original dosage adjustments specified in protocol |
| REPROVE, NCT01808092 (27) | NP including VAP (879) | 2,000 + 500 mg 2-h i.v. infusion q8h | Protocol amended to reflect the modified adjusted dosage regimens following results from RECLAIM |
cIAI, complicated intra-abdominal infection; CLCR, creatinine clearance; cUTI, complicated urinary tract infection; i.v., intravenous; q8h, every 8 h; NA, not applicable.
See Table 3 for details of adjusted ceftazidime-avibactam dosage regimens for patients with a CLCR of ≤50 ml/min.
TABLE 2.
Summary of the population pharmacokinetic models used to guide ceftazidime-avibactam dosage regimen selection and validation in adultsa
| Population PK model (reference) | Clinical trials included | No. of subjects in population PK model dataset |
|---|---|---|
| Early population PK model (12) | Five phase 1 studies, one phase 2 study (cIAI) | Ceftazidime, 103; avibactam, 288 |
| Iteration 1b (13) | Ten phase 1 studies, two phase 2 studies (cIAI and cUTI) | Ceftazidime, 227; avibactam. 486 |
| Iteration 2 (14) | Studies included in iteration 1, and the following added studies: one phase 1 study, two phase 3 studies (RECLAIM, REPRISE first data-cutoff)c | Ceftazidime, 780; avibactam, 1,057 |
| Iteration 3 (15) | Studies included in iteration 2, final data from REPRISE and the following added studies: two phase 3 studies (RECAPTURE and RECLAIM 3) | Ceftazidime, 1,563; avibactam, 1,836 |
| Iteration 4 (16) | Studies included in iteration 3 and the REPROVE phase 3 study | Ceftazidime, 1,975; avibactam, 2,249 |
cIAI, complicated intra-abdominal infection; cUTI, complicated urinary tract infection; PK, pharmacokinetic.
The ceftazidime and avibactam population PK models in iteration 1 were built from the ground up using available clinical data and did not use the prior early population PK model as a basis for development.
The first data cutoff for REPRISE comprised 126 patients of the 333 patients eventually randomized.
Overall, the population PK models described the PK of ceftazidime and avibactam well; prediction-corrected visual predictive checks confirmed that the models reflected the observed data, and were suitable to use in Monte Carlo simulations for PTA analyses (13–16). The PK data for both ceftazidime and avibactam were well described by a two-compartment PK model with first-order elimination from the central compartment. A wide range of patient covariates were evaluated for their impact on ceftazidime and avibactam PK, including CLCR, age, sex, body weight, race, geographical region, APACHE II score >10, and infection source. All population PK iterations to date have consistently demonstrated that CLCR is the key covariate that impacts the clearance of both ceftazidime and avibactam (13–16). Several other covariate effects on clearance and volume of the central compartment were included in the ceftazidime and avibactam population PK models. Noteworthy covariate effects included infection site and racial origin on ceftazidime clearance, and APACHE II score >10 and Asian race (non-Japanese or Chinese) on avibactam clearance (16). However, only CLCR had sufficiently large effects on drug exposures to warrant dosage adjustments and only for patients with a CLCR of <50 ml/min (16). Through their various iterations, these population PK models supported key decisions in the development of ceftazidime-avibactam, including phase 3 dose selection and validation, dose adjustments for renal impairment, and breakpoint determination. These models have also been adapted to support ceftazidime-avibactam dose selection for phase 2 trials in pediatric patients (66).
Clinical exposure-response PK/PD targets for ceftazidime and avibactam could not be identified using the phase 2 or phase 3 clinical data, since clinical and microbiological failure rates were low in these studies (16, 23–27, 30, 31). The PK/PD targets for ceftazidime and avibactam determined from the nonclinical studies described above (50% fT>MIC 8 mg/liter and avibactam 50% fT>CT of 1 mg/liter) were therefore deemed appropriate for the PTA analyses to select ceftazidime-avibactam dosage regimens. In all of the PTA analyses described below, a target MIC of 8 mg/liter was used. The PTA was determined as the percentage of simulated patients (5,000 patients per simulation) who met the PK/PD targets for both ceftazidime and avibactam simultaneously (referred to as the “joint PTA”). The joint PTA considers the concentrations of both the β-lactam and the β-lactamase inhibitor simultaneously, which is a more explicitly inclusive approach than estimating PTA based on β-lactam concentrations alone, as has been done for other β-lactam/β-lactamase inhibitor combinations (67–69). Covariate values for simulations in the different indications were obtained by sampling with replacement from the corresponding set of phase 2 or phase 3 study patients for each indication. The covariates used in the simulations were matched so that any given value of the patient identification variable had exactly the same covariates (and random effects) in the simulation data sets for both compounds, thereby preserving any underlying correlations. For patients with renal impairment, a uniform distribution for CLCR in the designated range for each category of renal impairment was used. Dosage selection and validation was based on the achievement of a high (>90%) joint PTA.
(i) Phase 3 dose selection for cIAI and cUTI. In the phase 2 trial in patients with cIAI and normal renal function (31), ceftazidime-avibactam 2,000 + 500 mg was given as a 30-min i.v. infusion q8h, based on the manufacturer’s recommendations for ceftazidime alone. Using early population PK models of ceftazidime and avibactam developed from phase 1 and 2 study data (13, 70), PTA simulations predicted that this regimen would provide inadequate joint PTA (i.e., <90%) at the ceftazidime-avibactam MIC of 8 mg/liter. Given that both ceftazidime and avibactam exhibit time-dependent PD, extending the infusion duration would be expected to increase the fT>MIC and fT>CT and hence the joint PTA; accordingly, the infusion duration was increased from 30 min to 2 h for phase 3 studies.
For patients with cUTI, a lower dose was used in the phase 2 trial (500 mg ceftazidime + 125 mg avibactam given as a 30-min i.v. infusion q8h) since, at the time, 500 mg q8h was the approved dose of ceftazidime alone for the treatment of cUTI, and avibactam was given in a fixed dose ratio to ceftazidime. This lower dose was also based on an assumption of high concentrations of ceftazidime and avibactam in the urine due to their predominantly renal excretion (39, 71). For the phase 3 studies in patients with cUTI, the same dosage regimen was selected as for the phase 3 studies in patients with cIAI (2,000 + 500 mg by 2-h i.v. infusion q8h). There were two key reasons for this decision. First, the dose was intended to provide sufficient exposures for the treatment of cUTI involving upper urinary tract infections, pyelonephritis and bacteremia; thus, a dose that would be adequate based on plasma concentrations and not just urinary concentrations of ceftazidime and avibactam was deemed important. Second, it was necessary to select a dosage regimen suitable to treat patients infected by P. aeruginosa, against which a ceftazidime-avibactam MIC90 of 8 mg/liter had already been observed among clinical isolates (54, 56, 57). The high PTAs of ≥90% (70) predicted following the administration of 2,000 + 500 mg 2-h i.v. infusion q8h for MICs up to 8 mg/liter supported the use of this dosage regimen in the phase 3 cIAI and cUTI trials.
(ii) Phase 3 dose selection for NP, including VAP. A phase 2 study evaluating ceftazidime-avibactam in patients with NP was not conducted; however, for the phase 3 dose selection, certain factors specific to lung infection were considered. Adequate availability of ceftazidime and avibactam at the site of infection was assessed by measuring penetration into epithelial lining fluid (ELF) in mice and humans (72, 73). Plasma exposures were shown to be related to efficacy in mouse pneumonia models for both ceftazidime and avibactam (64) and clinically for ceftazidime (42, 43), which demonstrated that plasma is a suitable surrogate for ELF. Moreover, in a phase 1 study, ceftazidime and avibactam penetration into ELF was shown to be at sufficient levels, with slightly higher penetration in humans compared to mice (72–74). These experiments indicated that ceftazidime and avibactam PD targets based on plasma levels that were associated with efficacy in mice with lung infections could also be used to predict efficacy in humans with lung infections.
The PK of many drugs, including antibiotics, are known to be affected by the presence of critical illness (75–78). Since NP is associated with a higher severity of disease compared to cIAI or cUTI, any impact on PK needs to be taken into consideration in the PTA analyses for dosage selection. The PK of ceftazidime in patients with NP has previously been reported (79–81), and comparison of those data to the data from the ceftazidime-avibactam population modeling in patients with cIAI (13) demonstrated that ceftazidime PK and exposure are similar between patients with cIAI and NP. The impact of covariates on the PK of avibactam is comparable to ceftazidime, and the disposition is largely the same; thus, an assumption was made that avibactam exposure in patients with NP would, as with ceftazidime, be similar to that of patients with cIAI. It has been reported that there is a larger proportion of patients with NP who have augmented renal clearance (ARC) compared with patients with other infections (82). This can impact patients who are ventilated to a greater extent than those who are nonventilated. Since both ceftazidime and avibactam are cleared almost exclusively renally (39, 71) and the relationship between exposure and CLCR is well understood, this was taken into account in the simulation settings for the PTA analyses to ensure that dose selection took account of any potential higher renal clearance in patients with NP (33). CLCR distributions from patients with NP and VAP reported in the literature were used in the simulations (52), along with a CLCR distribution from patients in phase 3 trials of ceftaroline fosamil (83, 84), which was truncated to include only patients with high CLCR.
Greater than 90% joint PTA at an MIC of 8 mg/liter was predicted for patients with NP, including VAP, receiving ceftazidime-avibactam 2,000 + 500 mg 2-h i.v. infusions q8h (33). These analyses “bridged the gap” in the available exposure and PK/PD data and supported the decision to use the same ceftazidime-avibactam dosage regimen that had been evaluated in the cIAI and cUTI trials in the phase 3 NP/VAP study, REPROVE (27). On the basis of this approach, and supported by the patient-rich population PK models, ceftazidime-avibactam was approved in Europe for the treatment of adults with HAP, including VAP, prior to the completion of the REPROVE study (29). Given the complexity of the analyses, a more detailed discussion of the studies supporting ceftazidime-avibactam dose selection in NP is to be presented elsewhere.
Ceftazidime-avibactam for patients with limited treatment options.
Ceftazidime-avibactam 2,000 + 500 mg by 2-h i.v. infusion q8h is also approved in Europe for the treatment of patients with other aerobic Gram-negative infections and limited treatment options (i.e., patients with infections at sites other than the approved indications) (29). The efficacy of ceftazidime-avibactam 2,000 + 500 mg as a 2-h i.v. infusion q8h was extrapolated to these patients based on experience with ceftazidime alone and on analyses of ceftazidime and avibactam PK/PD. Of particular note, the disposition of avibactam is similar to that of ceftazidime; both drugs have a similar volume of distribution, and their respective rates and extent of penetration into ELF are similar (72–74). Penetration of these drugs into other sites of infection is likely to be comparable, and in some cases the presence of infection and inflammation may increase the extent of tissue penetration. For example, penetration of ceftazidime across the blood-brain barrier is increased in patients with meningitis (85); that of avibactam has not been studied clinically, but it penetrated the meninges to a similar extent as ceftazidime in a rabbit model of meningitis (86). The ceftazidime and avibactam PK/PD targets are well validated in nonclinical models; since the activity of ceftazidime-avibactam occurs directly on the infecting pathogens, the PK/PD targets should be the same regardless of the infection site. The clinical efficacy demonstrated across patients with cIAI, cUTI, and NP validates the selected dosage regimen and the resulting plasma exposures required to achieve efficacy (23–27). Taken together with the comparable plasma exposures across the three indications, it is expected that ceftazidime-avibactam would achieve sufficient exposure and target attainment in patients with other infections due to aerobic Gram-negative organisms, for which ceftazidime is already used. Based on this rationale, ceftazidime-avibactam 2,000 + 500 mg as a 2-h i.v. infusion q8h was approved in Europe for the treatment of patients with aerobic Gram-negative infections and limited treatment options (29).
Dosage selection for patients with renal impairment.
Due to the predominantly renal excretion of both ceftazidime and avibactam, the exposures of both drugs increase with increasing severity of renal impairment (39, 71). Ceftazidime-avibactam dosage adjustments are therefore required to minimize the risk of overexposure in patients with a CLCR of ≤50 ml/min. At the time of dose selection for the phase 3 study program, the labeled ceftazidime dose adjustments for patients with a CLCR of ≤50 ml/min were used as a guide, with avibactam maintained at a quarter of the ceftazidime dose (Table 3) (19). Dosage adjustments were selected based on achieving >90% PTA and on the assumption that renal function would remain stable (19).
TABLE 3.
Ceftazidime-avibactam dosage adjustments for renal impairment employed in the phase 3 trials and approved modifications to original dosage adjustments by renal function categorya
| Renal function category | Original ceftazidime-avibactam dosage regimen included in protocol for phase 3 trialsb | Modified dosage regimenc | Joint PTA for a target MIC of 8 mg/liter in patients with cIAI receiving approved modified dosage adjustments (%)d |
|---|---|---|---|
| Normal CLCR >80 ml/min | 2,000 + 500 mg q8h | NA | 94.9 |
| Mild renal impairment CLCR 51–80 ml/min | 2,000 + 500 mg q8h | NA | 99.0 |
| Moderate renal impairment CLCR 31–50 ml/min | 1,000 + 250 mg q12h | 1,000 + 250 mg q8h | 99.3 |
| Severe renal impairment (upper range of CLCR) CLCR 16–30 ml/min | 1,000 + 250 mg q24h | 750 + 187.5 mg q12h | 99.0 |
| Severe renal impairment (lower range of CLCR) CLCR 6–15 ml/min | 500 + 125 mg q24h | 750 + 187.5 mg q24h | 99.3 |
| End-stage renal disease CLCR <6 ml/min | 500 + 125 mg q48h | 750 + 187.5 mg q48h | 99.6 |
All ceftazidime-avibactam dosages were administered by 2-h i.v. infusions. cIAI, complicated intra-abdominal infection; CLCR, creatinine clearance; i.v., intravenous; PK, pharmacokinetic; PTA, probability of target attainment; q8h, every 8 h; q12 h, every 12 h; q48h, every 48 h.
Not all trials allowed all degrees of renal impairment; in REPROVE, the protocol was amended to reflect the modified dosage regimens following results from RECLAIM.
Approved in the United States and Europe.
Following completion of the RECLAIM 1&2 phase 3 cIAI studies, it was found that despite an overall noninferiority result in the primary analysis, patients with moderate renal impairment at baseline (CLCR, 31 to 50 ml/min) in the ceftazidime-avibactam treatment arm had a lower clinical cure rate than the comparator, meropenem (23). A period of underdosing in a proportion of renally impaired cIAI patients who had rapidly improving renal function (due to volume repletion, fluid shifts, etc.) during the early treatment period was thought to have contributed to this finding (23). Although there was no evidence of reduced efficacy in patients with moderate renal impairment in any of the other phase 3 clinical trials, revised ceftazidime-avibactam dosage adjustments for patients with a CLCR of ≤50 ml/min were proposed to address the potential risk of underdosing in patients with rapidly improving renal function (Table 3) (17). Selection of these dosage adjustments was based on achievement of >90% PTA with the dosage regimen for the intended renal function group and maintaining comparable exposures to patients with normal renal function or mild renal impairment receiving the standard dosage regimen. Joint PTAs for simulated patients with cIAI for each renal function category receiving the modified dosages are shown in Table 3. Compared with the original dosages, the modified regimens provide higher PTA in the event of a shift in renal function into a higher renal function group without concomitant increase in dose (17). These modified dosage regimens are now included in U.S. and Europe product labels, which advise close monitoring of CLCR in patients with renal impairment (28, 29).
Validation of the phase 3 dosage regimens.
(i) Clinical efficacy of the selected ceftazidime-avibactam dose. The ceftazidime-avibactam adult phase 3 clinical trial program consisted of five trials which evaluated the selected ceftazidime-avibactam dosage regimen in patients with cIAI (RECLAIM 1&2, RECLAIM 3, and REPRISE), cUTI (REPRISE and RECAPTURE), and NP, including VAP (REPROVE) (Table 1). These trials have all demonstrated the efficacy and safety of ceftazidime-avibactam with respect to currently standard or best-available treatments (23–27), confirming that the selected dosage regimen for patients with a CLCR of >50 ml/min provides adequate exposure at the site of infection for clinical efficacy. Of note, in RECLAIM 1&2, the clinical cure rates in the subgroup of patients with moderate renal impairment (CLCR, 31 to 50 ml/min) at baseline were reduced in patients treated with ceftazidime-avibactam compared to those treated with meropenem, and this was thought to have resulted from a period of potential underdosing of some patients with rapidly improving renal function (23). Although this finding was not replicated across the other phase 3 studies, including a cIAI study in patients in Asia and the NP study (24–27), it did prompt the reevaluation of ceftazidime-avibactam dosage adjustments for renal impairment, which have been incorporated in U.S. and European product labeling (28, 29). The efficacy observed in the REPRISE study of ceftazidime-avibactam in patients with cIAI or cUTI caused by ceftazidime-nonsusceptible Gram-negative bacteria supports the hypothesis that the avibactam plasma exposure achieved with this dose is adequate to protect ceftazidime from hydrolysis by β-lactamases in a clinical setting (25). This is also supported by results from the other phase 3 studies, which reported similar efficacy of ceftazidime-avibactam versus carbapenems against ceftazidime-nonsusceptible pathogens (23, 24, 26, 27).
These results highlight the value of the population PK modeling and Monto Carlo simulation used for ceftazidime-avibactam development, which guided selection of dosage regimens that have now demonstrated clinical efficacy across cIAI, cUTI, and NP indications. The clinical validation of the PK/PD modeling used for ceftazidime-avibactam dosage selection has important regulatory implications. Specifically, ceftazidime-avibactam was first approved in the United States and in Europe based on preclinical data, PK/PD analyses, and limited clinical data. The clinical efficacy demonstrated in the ceftazidime-avibactam phase 3 program validates the initial benefit/risk assessment based on the streamlined drug development programs in the United States and Europe. Thus, the ceftazidime-avibactam program is an important prototype for the process of streamlining drug development programs which address unmet needs for serious bacterial infections, as outlined in the 2017 FDA guidance (87).
(ii) Assessment of the suitability of ceftazidime-avibactam dosage regimens following inclusion of phase 3 data in the population PK models. The ceftazidime and avibactam population PK models used to guide phase 3 dose selection were updated periodically with additional patient PK data from the phase 3 trials (Table 2). The final ceftazidime and avibactam population PK models (iteration 4 in Table 2) included PK data from all phase 3 trials and were used to confirm the suitability of the selected ceftazidime-avibactam dosage regimens. The inclusion of PK sampling in all the phase 3 studies resulted in final population PK models which comprised data from over 1,900 subjects (16). Since patient and disease characteristics such as older age, obesity, ARC and disease severity can impact the exposures of some antibiotics, further analyses were undertaken to ensure the selected dose provided robust exposure and joint PK/PD target attainment in these patient subgroups. The final models were used to calculate individual exposures for phase 3 patients and determine the percentage of patients achieving the joint PK/PD target (i.e., the rate of joint target attainment at a MIC of 8 mg/liter) for different patient subgroup categories of interest (16) (Table 4). From the large patient database included in the final population PK models, there was a very wide spread of the above-mentioned patient and disease characteristics which allowed these extra analyses.
TABLE 4.
Individual ceftazidime and avibactam steady-state exposures (geometric mean [CV%]) and joint PK/PD target attainment at an MIC of 8 mg/liter for ceftazidime and avibactam for subgroups of actual phase 3 patientsa
| Parameter | n | Ceftazidime |
Avibactam |
Joint PK/PD target attainment rate, % (95% CI)b | ||
|---|---|---|---|---|---|---|
| Cmax,ss (mg/liter) | AUCss,0–24 (mg ⋅ h/liter) | Cmax,ss (mg/liter) | AUCss,0–24 (mg ⋅ h/liter) | |||
| Indication | ||||||
| cIAI | 703 | 66.9 (105.0) | 749 (114.0) | 12.8 (155.3) | 132 (152.0) | 98.6 (97.7–99.5) |
| cUTI | 648 | 77.9 (114.2) | 979 (119.7) | 12.1 (161.9) | 138 (164.1) | 98.5 (97.5–99.4) |
| NP | 413 | 72.9 (125.2) | 950 (131.0) | 14.2 (166.1) | 169 (168.5) | 99.0 (98.1–100.0) |
| Non-VAP | 275 | 79.0 (120.0) | 1,016 (122.0) | 15.5 (166.9) | 183 (168.7) | 99.6 (98.9–100.0) |
| VAP | 138 | 61.9 (127.0) | 830 (142.7) | 12 (157.6) | 146 (163.0) | 97.8 (95.4–100.0) |
| Age (yr) | ||||||
| 18–65 | 1,192 | 70.0 (113.5) | 800 (122.7) | 12.5 (167.1) | 131 (166.8) | 98.4 (97.7–99.1) |
| >65–75 | 284 | 77.1 (109.4) | 997 (107.6) | 13.2 (119.0) | 156 (118.4) | 99.6 (99.0–100.0) |
| >75–89 | 288 | 76.8 (120.5) | 1,102 (120.6) | 14.0 (169.6) | 180 (164.7) | 98.6 (97.3–100.0) |
| BMI (kg/m2) | ||||||
| <29.9 | 1,441 | 73.0 (115.5) | 878 (124.2) | 13.0 (160.2) | 144 (161.4) | 98.7 (98.1–99.3) |
| ≥29.9–<34.9 | 208 | 67.9 (111.8) | 841 (125.2) | 12.0 (178.4) | 136 (179.1) | 97.6 (95.5–99.7) |
| ≥34.9–<39.9 | 74 | 73.7 (109.6) | 894 (115.2) | 13.2 (139.7) | 141 (140.0) | 100.0 (NA) |
| ≥39.9 | 32 | 64.2 (93.6) | 806 (119.4) | 9.7 (116.9) | 115 (128.5) | 100.0 (NA) |
| Missing | 9 | 70.9 (87.0) | 959 (106.5) | 14.2 (83.4) | 172 (112.8) | 100.0 (NA) |
| Day 3 CLCR (ml/min)d | ||||||
| 8–15 | 4 | 34.3 (173.3) | 551 (121.9) | 6.3 (305.6) | 86.3 (220.6) | 75.0 (32.6–100.0) |
| >15–30 | 20 | 50.4 (139.5) | 789 (116.5) | 10.9 (174.1) | 155 (143.6) | 100.0 (NA) |
| >30–50 | 128 | 58.8 (120.5) | 938 (122.9) | 10.2 (147.6) | 148 (153.3) | 98.4 (96.3–100.0) |
| >50–80 | 418 | 90.0 (108.0) | 1,213 (110.4) | 15.3 (142.9) | 186 (144.5) | 99.0 (98.1–100.0) |
| >80–150 | 955 | 72.9 (105.9) | 828 (112.4) | 13.2 (165.5) | 138 (163.4) | 99.0 (98.3–99.6) |
| >150–180 | 123 | 58.5 (93.0) | 652 (112.8) | 9.9 (124.5) | 103 (137.5) | 98.4 (96.1–100.0) |
| >180–610 | 116 | 51.2 (109.6) | 542 (108.1) | 9.9 (171.6) | 96 (155.9) | 95.7 (92.0–99.4) |
| Bacteraemia at baseline | ||||||
| No | 1,465 | 71.9 (116.1) | 881 (125.5) | 12.6 (157.3) | 141 (161.2) | 98.6 (98.0–99.2) |
| Yes | 88 | 73.6 (102.8) | 919 (120.1) | 14.2 (164.1) | 161 (161.3) | 100.0 (NA) |
| Baseline APACHE II score | ||||||
| ≤10 | 677 | 67.0 (105.0) | 748 (113.8) | 12.7 (154.3) | 131 (150.6) | 98.5 (97.6–99.4) |
| >10 | 438 | 72.3 (124.3) | 938 (130.9) | 14.3 (167.0) | 170 (168.7) | 99.1 (98.2–100.0) |
| Missingc | 649 | 77.9 (114.1) | 979 (119.7) | 12.1 (161.8) | 138 (164.0) | 98.5 (97.5–99.4) |
| SIRS at baseline | ||||||
| No | 770 | 72.3 (108.9) | 895 (120.5) | 12.8 (159.2) | 143 (162.0) | 99.1 (98.4–99.8) |
| Yes | 773 | 71.5 (121.3) | 869 (129.7) | 12.6 (157.1) | 142 (161.3) | 98.3 (97.4–99.2) |
| Missing | 10 | 83.5 (130.2) | 977 (123.7) | 12.1 (115.1) | 129 (116.4) | 100.0 (NA) |
| Baseline WBC count (cells/μl) | ||||||
| ≤12,000 | 876 | 74.6 (110.9) | 923 (118.9) | 12.8 (159.1) | 145 (161.7) | 98.9 (98.2–99.6) |
| >12,000 | 486 | 67.6 (119.4) | 801 (128.4) | 12.5 (160.4) | 136 (161.5) | 98.6 (97.5–99.6) |
| Missing | 191 | 72.0 (121.4) | 924 (136.8) | 12.3 (145.3) | 147 (158.6) | 98.4 (96.7–100.0) |
| Fever at baseline | ||||||
| No | 1,166 | 71.9 (113.4) | 888 (123.9) | 12.9 (154.5) | 146 (159.2) | 99.1 (98.5–99.6) |
| Yes | 343 | 72.1 (121.8) | 859 (130.3) | 12.2 (165.7) | 134 (167.4) | 98.3 (96.9–99.6) |
| Missing | 44 | 75.1 (117.3) | 929 (118.4) | 11.8 (180.9) | 132 (164.7) | 93.2 (85.7–100.0) |
Table adapted from Li et al. (16). APACHE, Acute Physiology and Chronic Health Evaluation; AUCss,0–24; area under the curve over 24 h at steady state; BMI, body mass index; CI, confidence interval; cIAI, complicated intra-abdominal infection; Cmax,ss, maximum concentration at steady state; CLCR, creatinine clearance; cUTI, complicated urinary tract infection; NP, nosocomial pneumonia; q8h, every 8 h; q24 h, every 24 h; PD, pharmacodynamic; PK, pharmacokinetic; SIRS, systemic inflammatory response syndrome; WBC, white blood cell; NA, not applicable.
The joint PK/PD target was defined as 50% fT>MIC of 8 mg/liter for ceftazidime and 50% fT>CT of 1 mg/liter for avibactam.
APACHE II scores were collected for cIAI and NP patients only, hence these data were not available for the 648 cUTI patients. Data were missing for one cIAI patient.
Patients with CLCR <50 ml/min were assumed to receive the labeled dosage regimen appropriate to their level of renal insufficiency.
There was a modest increase in exposure in patients >65 years, which corresponded to decreased CLCR (16). For both patients with ARC (CLCR, >150 ml/min) and morbidly obese patients (body mass index [BMI], >39.9 kg/m2), modest decreases in the exposures of ceftazidime and avibactam were observed relative to those with normal renal function and nonobese patients, respectively (16). Critically, in these patients the joint target attainment achieved was >95%. Although exposure is lower in obese patients, joint target attainment is higher compared to nonobese patients (16). The primary effect of obesity is to increase the volume of distribution, which leads to longer plasma half-lives and tends to increase the time above a given threshold and hence target attainment.
Disease severity, as measured by the presence of bacteremia, APACHE II score >10, systemic inflammatory response syndrome (SIRS), fever, or high white blood cell count (>12,000/mm3) at baseline, did not adversely affect exposures of ceftazidime or avibactam, and high joint target attainment (>95%) was achieved (16). An assessment of the impact of disease severity was made on an earlier population PK model at the end of the cIAI and cUTI phase 3 studies, prior to the inclusion of NP data (15). The same conclusion from that analysis was supportive of extrapolation of dose and efficacy to patients with NP. Overall, the high joint target attainment, which was maintained across all subgroup categories despite the observed effects on exposure, confirmed that ceftazidime-avibactam dosage adjustments are not required for patients with older age, obesity, or markers of more severe infection.
The final models were also used to simulate exposures and joint PTA for patient populations representative of different indications (5,000 patients in each indication and renal function category). More than 90% joint PTA at an MIC of 8 mg/liter was predicted in each indication for patients with normal renal function (CLCR, >80 ml/min) receiving the standard dosage regimen (Table 5) and for patients with a CLCR of ≤50 ml/min receiving dosage adjustments for renal impairment (see Table 3 for cIAI patients, data not shown for other indications) (16). This validation step confirmed the suitability of the ceftazidime-avibactam dosage regimen selected for patients with a CLCR of > 50 ml/min in the phase 3 study program, and the approved dosage adjustments for patients with a CLCR of ≤50 ml/min across all approved indications (16).
TABLE 5.
Geometric mean (%CV) steady-state exposures for ceftazidime and avibactam and joint PTA at an MIC of 8 mg/liter summarized by indication (n = 5,000 simulated patients per cohort)a
| Cohort | Ceftazidime |
Avibactam |
Joint PTA, % | ||
|---|---|---|---|---|---|
| Cmax,ss (mg/liter) | AUCss,0–24 (mg ⋅ h/liter) | Cmax,ss (mg/liter) | AUCss,0–24 (mg ⋅ h/liter) | ||
| cIAI | 61.1 (44) | 683 (45) | 11.5 (83) | 121 (72) | 94.9 |
| cUTI | 73.0 (47) | 880 (49) | 11.2 (87) | 126 (82) | 95.2 |
| NP | 65.4 (53) | 805 (55) | 12.8 (94) | 147 (89) | 98.3 |
| VAP | 55.1 (59) | 719 (64) | 10.7 (85) | 129 (79) | 96.1 |
| Non-VAP | 75.7 (43) | 894 (48) | 14.7 (92) | 164 (93) | 100 |
This table adapted from Li et al. (16). The final population PK models included PK data from 1,975 subjects for ceftazidime and 2,249 subjects for avibactam. Simulations were conducted for 5,000 patients with normal renal function (CLCR >80 ml/min) for each indication, receiving ceftazidime-avibactam 2,000 + 500 mg, q8h, as a 2-h infusion (16). AUC and Cmax values are based on total plasma concentrations for ceftazidime and avibactam. AUCss,0–24, area under the curve over 24 h at steady state; cIAI, complicated intra-abdominal infection; Cmax,ss, maximum concentration at steady state; CLCR, creatinine clearance; cUTI, complicated urinary tract infection; CV, coefficient of variation; NP, nosocomial pneumonia; PK, pharmacokinetic; PTA; probability of target attainment; q8h, every 8 h; VAP, ventilator-associated pneumonia.
To further explore the performance of the ceftazidime-avibactam dosage regimens, additional PTA simulations were conducted for more conservative ceftazidime and avibactam PK/PD targets with longer required durations above the threshold concentrations (65). When the required duration during which ceftazidime and avibactam concentrations were above target thresholds was extended from 50 to 60% of the interdose interval, the joint PTA rates remained >90% for all indications. These results suggest that the approved ceftazidime-avibactam dosage regimens should support treatment of infections that may require longer durations of threshold achievement, such as severe infections or patients who are critically ill.
Ceftazidime-avibactam clinical MIC breakpoints.
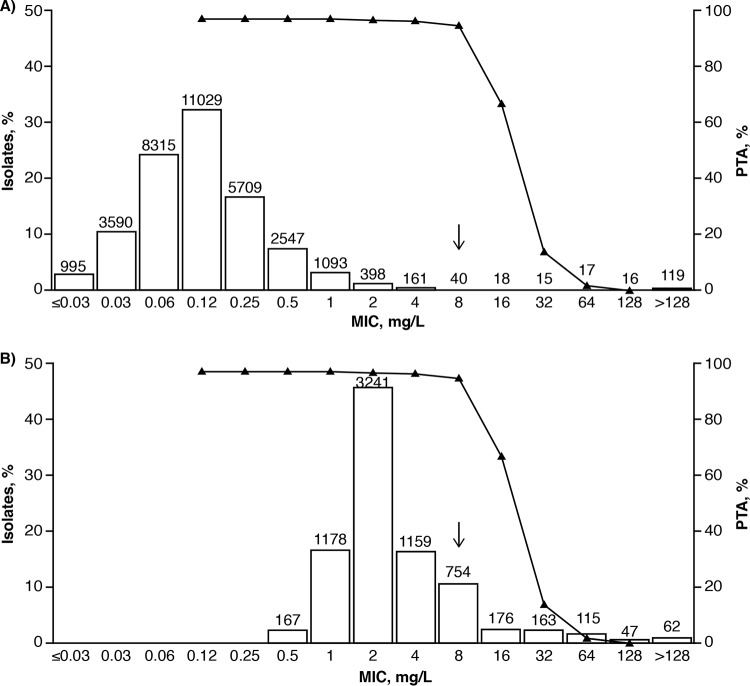
Determination of clinical MIC breakpoints for new antimicrobial therapies typically involves: evaluation of PK/PD targets derived from preclinical experiments; population PK modeling and PTA analyses; clinical and microbiological efficacy data; and MIC distributions from global surveillance studies of target pathogens (20–22). As discussed above, global surveillance studies have consistently reported that clinical isolates of Enterobacteriaceae and P. aeruginosa (including many MDR phenotypes) have yielded ceftazidime-avibactam MIC90 values of ≤8 mg/liter (11, 60, 88), and high PTA values (>90%) were reported for Enterobacteriaceae and P. aeruginosa with ceftazidime-avibactam MICs ≤8 mg/liter using the joint PK/PD target. The joint PTA for patients with cIAI receiving ceftazidime-avibactam 2,000 + 500 mg q8h plotted as a function of ceftazidime-avibactam MIC and overlaid with the MIC distributions for Enterobacteriaceae and P. aeruginosa from the INFORM global surveillance study (2012 to 2014) are shown in Fig. 1 (89). These data, along with molecular characterization of isolates with specific resistance mechanisms from microbiological surveillance studies and per-pathogen clinical and microbiological response by MIC data from the phase 3 studies (89), supported the FDA, EUCAST, and CLSI decisions to establish clinical MIC breakpoints for the approved ceftazidime-avibactam dosage regimens against Enterobacteriaceae and P. aeruginosa of susceptible ≤8 mg/liter and resistant >8 mg/liter (28, 29, 49, 70).
FIG 1.
Joint PTA for patients with cIAI receiving ceftazidime-avibactam 2,000 + 500 mg q8h plotted as a function of ceftazidime-avibactam MIC overlaying the ceftazidime-avibactam MIC distributions against Enterobacteriaceae (n = 34,062) (A) and Pseudomonas aeruginosa (n = 7,062) (B) from the INFORM global surveillance study (2012 to 2014). The figure is from Nichols et al. (89). Joint PTA is defined as simultaneous attainment of 50% fT>MIC of ceftazidime-avibactam for ceftazidime and 50% fT>CT of 1 mg/liter for avibactam, with both targets having to be achieved for a simulated patient to be categorized as achieving the joint target. The joint PTA was calculated using iteration 4 of the population PK models (see Table 2). Ceftazidime-avibactam MIC distributions were obtained from the INFORM 2012–2014 global surveillance study (7, 11, 60, 88). Values above the bars are the numbers of isolates tested at each MIC. The arrows show the position of the approved ceftazidime-avibactam susceptible clinical breakpoint of MIC ≤8 mg/liter.
Potential for resistance to ceftazidime-avibactam.
In preclinical studies, the frequency of spontaneous resistance to ceftazidime-avibactam varied depending on the genetic constitution of the bacterial isolate tested. In Enterobacteriaceae carrying blaKPC-3, the frequency of mutation to ≥16-fold MIC in vitro was ca. 10−9 (90). Similar rates were found for ceftazidime-resistant ESBL producers and ceftazidime-susceptible Enterobacteriaceae (91). The frequency of selection of single-step mutants that tested with ≥8-fold increased MIC of ceftazidime-avibactam among three ceftazidime-resistant, ceftazidime-avibactam-susceptible, isolates of P. aeruginosa in vitro ranged between <6 × 10−10 and 4 × 10−9 (92). All of these frequencies are low and actually lower than frequencies that have been observed for ceftazidime alone in ceftazidime-susceptible isolates (93–96).
There have been isolated reports that the same shift from susceptible to resistant has also been observed as a result of mutation in blaKPC-3 in carbapenem-resistant ST258-clone K. pneumoniae in high-risk patients (97, 98), despite the fact that development of resistance was not observed during the ceftazidime-avibactam clinical trials (23–27, 30, 31). It should be noted that the MIC of ceftazidime-avibactam against the resistant isolates recovered in vitro or from patients in the above clinical reports varied up to >512 and >256 mg/liter, respectively (90, 97). The high values of these MICs make it unlikely that a higher dose of ceftazidime-avibactam could have prevented resistance selection or, alternatively, that altered PK in the patients concerned had predisposed them to resistance selection, particularly noting that in two of the three patients from whom ceftazidime-avibactam-resistant K. pneumoniae were isolated during therapy the initial isolations were made from colonization sites (97).
In summary, in patients infected with carbapenem-resistant bacteria that carry blaKPC-3, there is the possibility that a spontaneous ceftazidime-avibactam-resistant variant could arise against which the MIC of ceftazidime-avibactam is so high that it would be resistant to ceftazidime-avibactam therapy. The frequency of this mutation appears to be low, but one can calculate that the risk of a resistant isolate arising in a particular medical unit will increase if multiple patients are colonized and infected by the same carbapenem-resistant isolate, and then treated with ceftazidime-avibactam, because of the high numbers of bacteria being exposed to drug.
Conclusions.
In common with ceftazidime, avibactam PD is primarily time dependent. In vitro and in vivo data support a joint PK/PD target of ceftazidime 50% fT>MIC 8 mg/liter and avibactam 50% fT>CT 1 mg/liter for use in PTA analyses for ceftazidime-avibactam dose selection, validation, and breakpoint determination. PTA analyses using these targets enabled selection of a ceftazidime-avibactam dosage regimen for the phase 3 trial program which corresponded with clinical efficacy and supported determination of susceptible MIC breakpoints against key target pathogens (≤8 mg/liter for Enterobacteriaceae and P. aeruginosa). The final population PK models, including data from all phase 3 trials, confirmed that high PTAs were predicted with this dosage regimen across all indications and that dosage adjustments are only required for patients with a CLCR of ≤50 ml/min. Considered together, these data support ceftazidime-avibactam 2,000 + 500 mg given as a 2-h i.v. infusion q8h for the approved indications, with modified dosage adjustments for patients with a CLCR of ≤50 ml/min. The clinical validation of the initial approvals of ceftazidime-avibactam in the United States and Europe based primarily on PK/PD analyses is an important prototype for the process of streamlining drug development programs which address unmet needs for serious bacterial infections.
ACKNOWLEDGMENTS
This review was sponsored by AstraZeneca and Pfizer. AstraZeneca’s rights to ceftazidime-avibactam were acquired by Pfizer in December 2016.
J.L., P.N., S.D., and G.G.S. are former employees of AstraZeneca. G.G.S. is currently an employee of and shareholder in Pfizer. J.L., S.D., and G.G.S. have held shares in AstraZeneca. P.N. still retains shares in AstraZeneca. W.W.N is a former employee of and shareholder in AstraZeneca. T.R. and T.C. are employees of and shareholders in Allergan. D.M. and I.C. are former employees of and shareholders in Allergan.
Medical writing support was provided by Risha Bulusu and Mark Waterlow, at Prime, Knutsford, Cheshire, UK, funded by AstraZeneca and Pfizer. The opinions, conclusions, and interpretation of the data in this review are the responsibility of the authors.
REFERENCES
- 1.Falcone M, Paterson D. 2016. Spotlight on ceftazidime/avibactam: a new option for MDR Gram-negative infections. J Antimicrob Chemother 71:2713–2722. doi: 10.1093/jac/dkw239. [DOI] [PubMed] [Google Scholar]
- 2.Lahiri S, Mangani S, Durand-Reville T, Benvenuti M, De Luca F, Sanyal G, Docquier JD. 2013. Structural insight into potent broad-spectrum inhibition with reversible recyclization mechanism: avibactam in complex with CTX-M-15 and Pseudomonas aeruginosa AmpC β-lactamases. Antimicrob Agents Chemother 57:2496–2505. doi: 10.1128/AAC.02247-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagace-Wiens PR, Denisuik A, Rubinstein E, Gin AS, Hoban DJ, Lynch IIIJP, Karlowsky JA. 2013. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Drugs 73:159–177. doi: 10.1007/s40265-013-0013-7. [DOI] [PubMed] [Google Scholar]
- 4.Lagacé-Wiens P, Walkty A, Karlowsky JA. 2014. Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of Gram-negative bacterial infections. Core Evid 9:13–25. doi: 10.2147/CE.S40698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lahiri S, Bradford P, Nichols WW, Alm R. 2016. Structural and sequence analysis of class A β-lactamases with respect to avibactam inhibition: impact of Ω-loop variations. J Antimicrob Chemother 71:2848–2855. doi: 10.1093/jac/dkw248. [DOI] [PubMed] [Google Scholar]
- 6.Stachyra T, Levasseur P, Péchereau M-C, Girard A-M, Claudon M, Miossec C, Black MT. 2009. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J Antimicrob Chemother 64:326–329. doi: 10.1093/jac/dkp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jonge BL, Karlowsky JA, Kazmierczak KM, Biedenbach DJ, Sahm DF, Nichols WW. 2016. In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible Enterobacteriaceae isolates collected during the INFORM global surveillance study (2012 to 2014). Antimicrob Agents Chemother 60:3163–3169. doi: 10.1128/AAC.03042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aktaş Z, Kayacan C, Oncul O. 2012. In vitro activity of avibactam (NXL104) in combination with β-lactams against Gram-negative bacteria, including OXA-48 β-lactamase-producing Klebsiella pneumoniae. Int J Antimicrob Agents 39:86–89. doi: 10.1016/j.ijantimicag.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Kazmierczak KM, Biedenbach DJ, Hackel M, Rabine S, de Jonge BL, Bouchillon SK, Sahm DF, Bradford PA. 2016. Global dissemination of blaKPC into bacterial species beyond Klebsiella pneumoniae and in vitro susceptibility to ceftazidime-avibactam and aztreonam-avibactam. Antimicrob Agents Chemother 60:4490–4500. doi: 10.1128/AAC.00107-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sader H, Castanheira M, Mendes RE, Flamm RK, Farrell DJ, Jones RN. 2015. Ceftazidime-avibactam activity against multidrug-resistant Pseudomonas aeruginosa isolated in U.S. medical centers in 2012 and 2013. Antimicrob Agents Chemother 59:3656–3659. doi: 10.1128/AAC.05024-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols WW, de Jonge BL, Kazmierczak KM, Karlowsky JA, Sahm DF. 2016. In vitro susceptibility of global surveillance isolates of Pseudomonas aeruginosa to ceftazidime-avibactam: INFORM 2012-2014. Antimicrob Agents Chemother 22:4743–4749. doi: 10.1128/AAC.00220-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Knebel W, Riggs M, Zhou D, Nichols W, Das S. 2019. Population pharmacokinetic modelling of ceftazidime (CAZ) and avibactam (AVI) in healthy volunteers and patients with complicated intra-abdominal infection (cIAI), abstr A-634. 52nd Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC). ICAAC, San Francisco, CA. [Google Scholar]
- 13.Carrothers TJ, Green M, Chiu J, Riccobene T, Lovern M. 2014. Population pharmacokinetic modeling of combination treatment of intravenous ceftazidime and avibactam, abstr T-071. 5th American Conference on Pharmacometrics, Las Vegas, NV. [Google Scholar]
- 14.Li J, Zhou D, Das S, Lovern M, Green M, Chiu J, Riccobene T, Carrothers T, Al-Huniti N. 2015. Population PK modeling for ceftazidime-avibactam (CAZ-AVI) in patients with complicated intra-abdominal infection (cIAI) and complicated urinary tract infection (cUTI), abstr 2472. American Association of Pharmaceutical Scientists Annual Meeting and Exposition, Orlando, FL. [Google Scholar]
- 15.Das S, Wright J, Riccobene T, Macpherson M, Carrothers T, Lovern M. 2016. Comparison of ceftazidime-avibactam (CAZ-AVI) exposure and PK/PD target attainment (TA) across patient subgroups, abstr 500. ASM Microbe 2016, Boston, MA. [Google Scholar]
- 16.Li J, Lovern M, Green ML, Chiu J, Zhou D, Comisar C, Xiong Y, Hing J, MacPherson M, Wright JG, Riccobene T, Carrothers TJ, Das S. 2018. Ceftazidime-avibactam population pharmacokinetic modeling and pharmacodynamic target attainment across adult indications and patient subgroups. Clin Transl Sci doi: 10.1111/cts.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Zhou D, Nichols WW, Al-Huniti N, Lovern MR, Green ML, Chiu JS, Riccobene TA, Carrothers TJ, Das S. 2015. PK/PD target attainment analyses and assessment of dose adjustments for renal insuffiency for ceftazidime-avibactam (CAZ-AVI) in patients with complicated intra-abdominal infection (cIAI), complicated urinary tract infection (cUTI) or nosocomial pneumonia (NP), abstr 2459. American Association of Pharmaceutical Scientists Annual Meeting and Exposition, Orlando, FL. [Google Scholar]
- 18.Li J, Nichols WW, Zhou D, Das S. 2015. Population pharmacokinetic modelling of ceftazidime and avibactam and probability of target attainment to support the dosing regimen in patients with nosocomial pneumonia including ventilator-associated pneumonia, poster 2459. American Association of Pharmaceutical Scientists Annual Meeting and Exposition, Orlando, FL. [Google Scholar]
- 19.Li J, Zhou D, Nichols W, Das S. 2012. Evaluation of ceftazidime-avibactam (CAZ-AVI) dose regimens for Phase III study in patients with different renal function, abstr A-635. 52nd Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC). ICAAC, San Francisco, CA. [Google Scholar]
- 20.Turnidge J, Paterson DL. 2007. Setting and revising antibacterial susceptibility breakpoints. Clin Microbiol Rev 20:391–408. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouton J, Brown DF, Apfalter P, Canton R, Giske CG, Ivanova M, MacGowan AP, Rodloff A, Soussy CJ, Steinbakk M, Kahlmeter G. 2012. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin Microbiol Infect 18:E37–E45. doi: 10.1111/j.1469-0691.2011.03752.x. [DOI] [PubMed] [Google Scholar]
- 22.Dudley MN, Ambrose PG, Bhavnani SM, Craig WA, Ferraro MJ, Jones RN, Antimicrobial Susceptibility Testing Subcommittee of the Clinical Laboratory Standards Institute. 2013. Background and rationale for revised Clinical and Laboratory Standards Institute interpretive criteria (breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa. I. Cephalosporins and aztreonam. Clin Infect Dis 56:1301–1309. doi: 10.1093/cid/cit017. [DOI] [PubMed] [Google Scholar]
- 23.Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, Rank D, Llorens L, Newell P, Pachl J. 2016. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 62:1380–1389. doi: 10.1093/cid/ciw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagenlehner FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GG, Yates K, Gasink LB. 2016. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis 63:754–762. doi: 10.1093/cid/ciw378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmeli Y, Armstrong J, Laud PJ, Newell P, Stone G, Wardman A, Gasink LB. 2016. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis 16:661–673. doi: 10.1016/S1473-3099(16)30004-4. [DOI] [PubMed] [Google Scholar]
- 26.Qin X, Tran BG, Kim MJ, Wang L, Nguyen DA, Chen Q, Song J, Laud PJ, Stone GG, Chow JW. 2017. A randomized, double blind, phase III study comparing the efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem for complicated intra-abdominal infections in hospitalized adults in Asia. Int J Antimicrob Agents 49:579–588. doi: 10.1016/j.ijantimicag.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Torres A, Zhong N, Pachl J, Timsit J-F, Kollef M, Chen Z, Song J, Taylor D, Laud P, Stone G, Chow J. 2018. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis 18:285–295. doi: 10.1016/S1473-3099(17)30747-8. [DOI] [PubMed] [Google Scholar]
- 28.Allergan. 2018. Avycaz (ceftazidime-avibactam) for injection, for intravenous use: prescribing information. Allergan, Madison, NJ: http://www.allergan.com/assets/pdf/avycaz_pi. [Google Scholar]
- 29.Pfizer. 2018. Zavicefta: summary of product characteristics. Pfizer, Groton, CT: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004027/WC500210234.pdf. [Google Scholar]
- 30.Vazquez JA, Gonzalez Patzan LD, Stricklin D, Duttaroy DD, Kreidly Z, Lipka J, Sable C. 2012. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin 28:1921–1931. doi: 10.1185/03007995.2012.748653. [DOI] [PubMed] [Google Scholar]
- 31.Lucasti C, Popescu I, Ramesh MK, Lipka J, Sable C. 2013. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, phase II trial. J Antimicrob Chemother 68:1183–1192. doi: 10.1093/jac/dks523. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Food and Drug Administration. 2014. Guidance for industry expedited programs for serious conditions: drugs and biologics. FDA, Bethesda, MD: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm358301.pdf. [Google Scholar]
- 33.Das S, Nichols W, Li J. 2015. Dose selection of ceftazidime-avibactam in patients with nosocomial pneumonia, including ventilator-associated pneumonia, based on preclinical efficacy, preclinical pharmacokinetic/pharmacodynamic and clinical pharmacokinetic data, abstr P1288. 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Copenhagen, Denmark. [Google Scholar]
- 34.GlaxoSmithKline. 2010. Ceftazidime: summary of product characteristics. GlaxoSmithKline, Brentford, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Fortum_30/WC500098366.pdf. [Google Scholar]
- 35.Hospira. 2018. TAZICEF: ceftazidime injection, powder, for solution. Hospira, Inc, Lake Forest, IL: http://labeling.pfizer.com/ShowLabeling.aspx?id=4386. [Google Scholar]
- 36.Aronson JK. 2016. Cephalosporins, p 197–215. In Aronson JK. (ed), Meyler’s side effects of drugs, 16th ed Elsevier, Oxford, United Kingdom. [Google Scholar]
- 37.Vishwanathan K, Mair S, Gupta A, Atherton J, Clarkson-Jones J, Edeki T, Das S. 2014. Assessment of the mass balance recovery and metabolite profile of avibactam in humans and in vitro drug-drug interaction potential. Drug Metab Dispos 42:932–942. doi: 10.1124/dmd.113.055335. [DOI] [PubMed] [Google Scholar]
- 38.Merdjan H, Rangaraju M, Tarral A. 2015. Safety and pharmacokinetics of single and multiple ascending doses of avibactam alone and in combination with ceftazidime in healthy male volunteers: results of two randomized, placebo-controlled studies. Clin Drug Investig 35:307–317. doi: 10.1007/s40261-015-0283-9. [DOI] [PubMed] [Google Scholar]
- 39.Merdjan H, Tarral A, Das S, Li J. 2017. Phase 1 study assessing the pharmacokinetic profile and safety of avibactam in patients with renal impairment. J Clin Pharmacol 57:211–218. doi: 10.1002/jcph.793. [DOI] [PubMed] [Google Scholar]
- 40.Ambrose P, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clin Infect Dis 44:79–86. doi: 10.1086/510079. [DOI] [PubMed] [Google Scholar]
- 41.European Medicines Agency. 2016. Guideline on the use of pharmacokinetics and pharmacodynamics in the development of antimicrobial medicinal products. Document EMA/CHMP/594085/2015. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500210982.pdf. [Google Scholar]
- 42.Muller A, Punt N, Mouton J. 2013. Optimal exposures of ceftazidime predict the probability of microbiological and clinical outcome in the treatment of nosocomial pneumonia. J Antimicrob Chemother 68:900–906. doi: 10.1093/jac/dks468. [DOI] [PubMed] [Google Scholar]
- 43.MacVane SH, Kuti JL, Nicolau DP. 2014. Clinical pharmacodynamics of antipseudomonal cephalosporins in patients with ventilator-associated pneumonia. Antimicrob Agents Chemother 58:1359–1364. doi: 10.1128/AAC.01463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts JA, Kirkpatrick CM, Lipman J. 2011. Monte Carlo simulations: maximizing antibiotic pharmacokinetic data to optimize clinical practice for critically ill patients. J Antimicrob Chemother 66:227–231. doi: 10.1093/jac/dkq449. [DOI] [PubMed] [Google Scholar]
- 45.Andes D, Craig W. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrob Agents 19:261–268. doi: 10.1016/S0924-8579(02)00022-5. [DOI] [PubMed] [Google Scholar]
- 46.Andes D, Craig W. 2005. Treatment of infections with ESBL-producing organisms: pharmacokinetic and pharmacodynamic considerations. Clin Microbiol Infect 11:10–17. doi: 10.1111/j.1469-0691.2005.01265.x. [DOI] [PubMed] [Google Scholar]
- 47.Frei CR, Wiederhold NP, Burgess DS. 2008. Antimicrobial breakpoints for Gram-negative aerobic bacteria based on pharmacokinetic–pharmacodynamic models with Monte Carlo simulation. J Antimicrob Chemother 61:621–628. doi: 10.1093/jac/dkm536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.EUCAST. 2010. Ceftazidime: rationale for the EUCAST clinical breakpoints, version 1.0. European Committee on Antimicrobial Susceptibility Testing; http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Ceftazidime_Rationale_Document_1.0_2010Nov.pdf. [Google Scholar]
- 49.Clinical Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing. Twenty-eighth informational supplement. CLSI, Wayne, PA. [Google Scholar]
- 50.EUCAST. 2018. EUCAST clinical breakpoint tables v.8.1., valid from 2018-05-15. European Committee on Antimicrobial Susceptibility Testing; http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.1_Breakpoint_Tables.pdf. [Google Scholar]
- 51.Bradford PA, Huband MD, Stone GG. 2018. A systematic approach to the selection of the appropriate avibactam concentration for use with ceftazidime in broth microdilution susceptibility testing. Antimicrob Agents Chemother 62. doi: 10.1128/AAC.00223-00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ambrose P, Bhavnani S, Ellis-Grosse E, Drusano G. 2010. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-associated bacterial pneumonia studies: look before you leap!. Clin Infect Dis 51:S103–S110. doi: 10.1086/653057. [DOI] [PubMed] [Google Scholar]
- 53.Jones RN. 2010. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 51:S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 54.Flamm R, Stone GG, Sader HS, Jones RN, Nichols WW. 2014. Avibactam reverts the ceftazidime MIC90 of European Gram-negative bacterial clinical isolates to the epidemiological cutoff value. J Chemother 26:333–338. doi: 10.1179/1973947813Y.0000000145. [DOI] [PubMed] [Google Scholar]
- 55.Sader H, Castanheira M, Flamm RK, Farrell DJ, Jones RN. 2014. Antimicrobial activity of ceftazidime-avibactam against Gram-negative organisms collected from US medical centers in 2012. Antimicrob Agents Chemother 58:1684–1692. doi: 10.1128/AAC.02429-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levasseur P, Girard A-M, Claudon M, Goossens H, Black MT, Coleman K, Miossec C. 2012. In vitro antibacterial activity of the ceftazidime-avibactam (NXL104) combination against Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother 56:1606–1608. doi: 10.1128/AAC.06064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walkty A, DeCorby M, Lagacé-Wiens P, Karlowsky J, Hoban D, Zhanel G. 2011. In vitro activity of ceftazidime combined with NXL104 versus Pseudomonas aeruginosa isolates obtained from patients in Canadian hospitals (CANWARD 2009 study). Antimicrob Agents Chemother 55:2992–2994. doi: 10.1128/AAC.01696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Zhang F, Zhao C, Wang Z, Nichols WW, Testa R, Li H, Chen H, He W, Wang Q, Wang H. 2014. In vitro activity of ceftazidime-avibactam and aztreonam-avibactam against 372 Gram-negative bacilli collected in 2011 and 2012 from 11 teaching hospitals in China. Antimicrob Agents Chemother 58:1774–1778. doi: 10.1128/AAC.02123-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Castanheira M, Farrell SE, Krause KM, Jones RN, Sader HS. 2014. Contemporary diversity of β-lactamases among Enterobacteriaceae in the nine U.S. census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent β-lactamase groups. Antimicrob Agents Chemother 58:833–838. doi: 10.1128/AAC.01896-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hackel M, Kazmierczak KM, Hoban DJ, Biedenbach DJ, Bouchillon SK, de Jonge BL, Stone GG. 2016. Assessment of the in vitro activity of ceftazidime-avibactam against multidrug-resistant Klebsiella spp. collected in the INFORM global surveillance study, 2012 to 2014. Antimicrob Agents Chemother 60:4677–4683. doi: 10.1128/AAC.02841-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flamm R, Nichols WW, Sader HS, Farrell DJ, Jones RN. 2016. In vitro activity of ceftazidime/avibactam against Gram-negative pathogens isolated from pneumonia in hospitalized patients, including ventilated patients. Int J Antimicrob Agents 47:235–242. doi: 10.1016/j.ijantimicag.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Nichols WW, Newell P, Critchley IA, Riccobene T, Das S. 2018. Avibactam pharmacokinetic/pharmacodynamic targets. Antimicrob Agents Chemother 62. doi: 10.1128/AAC.02446-02417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coleman K, Levasseur P, Girard AM, Borgonovi M, Miossec C, Merdjan H, Drusano G, Shlaes D, Nichols WW. 2014. Activities of ceftazidime and avibactam against beta-lactamase-producing Enterobacteriaceae in a hollow-fiber pharmacodynamic model. Antimicrob Agents Chemother 58:3366–3372. doi: 10.1128/AAC.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berkhout J, Melchers MJ, van Mil AC, Seyedmousavi S, Lagarde CM, Schuck VJ, Nichols WW, Mouton JW. 2016. Pharmacodynamics of ceftazidime and avibactam in neutropenic mice with thigh or lung infection. Antimicrob Agents Chemother 60:368–375. doi: 10.1128/AAC.01269-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.European Medicines Agency. 2016. Zavicefta European public assessment report. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004027/WC500210236.pdf. [Google Scholar]
- 66.Li J, Zhou D, Das S, Lovern M, Wada R, Bellanti F, Riccobene T, Carrothers T, Al-Huniti N. 2015. Population PK modeling and dosing evaluations for ceftazidime-avibactam (CAZ-AVI) in children aged ≥3 months to <18 years receiving systemic antibiotic therapy for suspected or confirmed infection. American Association of Pharmaceutical Scientists Annual Meeting and Exposition, Orlando, FL. [Google Scholar]
- 67.Xiao AJ, Miller BW, Huntington JA, Nicolau DP. 2016. Ceftolozane/tazobactam pharmacokinetic/pharmacodynamic-derived dose justification for phase 3 studies in patients with nosocomial pneumonia. J Clin Pharmacol 56:56–66. doi: 10.1002/jcph.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen R, Qian Q, Sun MR, Qian CY, Zou SL, Wang ML, Wang LY. 2016. Population pharmacokinetics and pharmacodynamics of piperacillin/tazobactam in patients with nosocomial infections. Eur J Drug Metab Pharmacokinet 41:363–372. doi: 10.1007/s13318-015-0276-3. [DOI] [PubMed] [Google Scholar]
- 69.Carlier M, Noe M, De Waele JJ, Stove V, Verstraete AG, Lipman J, Roberts JA. 2013. Population pharmacokinetics and dosing simulations of amoxicillin/clavulanic acid in critically ill patients. J Antimicrob Chemother 68:2600–2608. doi: 10.1093/jac/dkt240. [DOI] [PubMed] [Google Scholar]
- 70.Cerexa. 2014. Ceftazidime-avibactam for injection: briefing document. Anti-Infective Drugs Advisory Committee, FDA, Bethesda, MD: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM425459.pdf. [Google Scholar]
- 71.Welage LS, Schultz RW, Schentag J. 1984. Pharmacokinetics of ceftazidime in patients with renal insufficiency. Antimicrob Agents Chemother 25:201–204. doi: 10.1128/AAC.25.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nicolau D, Siew L, Armstrong J, Li J, Edeki T, Learoyd M, Das S. 2015. Phase 1 study assessing the steady-state concentration of ceftazidime and avibactam in plasma and epithelial lining fluid following two dosing regimens. J Antimicrob Chemother 70:2862–2869. doi: 10.1093/jac/dkv170. [DOI] [PubMed] [Google Scholar]
- 73.Berkhout J, Melchers MJ, van Mil AC, Seyedmousavi S, Lagarde CM, Nichols WW, Mouton JW. 2015. Pharmacokinetics and penetration of ceftazidime and avibactam into epithelial lining fluid in thigh- and lung-infected mice. Antimicrob Agents Chemother 59:2299–2304. doi: 10.1128/AAC.04627-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dimelow R, Wright JG, MacPherson M, Newell P, Das S. 2018. Population pharmacokinetic modelling of ceftazidime and avibactam in the plasma and epithelial lining fluid of healthy volunteers. Drugs R D 18:221–230. doi: 10.1007/s40268-018-0241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neuner EA, Gallagher JC. 2016. Pharmacodynamic and pharmacokinetic considerations in the treatment of critically ill patients infected with carbapenem-resistant Enterobacteriaceae. Virulence doi: 10.1080/21505594.2016.1221021:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL. 2014. Individualized antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Udy AA, Roberts JA, Lipman J. 2013. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med 39:2070–2082. doi: 10.1007/s00134-013-3088-4. [DOI] [PubMed] [Google Scholar]
- 78.Sinnollareddy MG, Roberts MS, Lipman J, Roberts JA. 2012. β-lactam pharmacokinetics and pharmacodynamics in critically ill patients and strategies for dose optimization: a structured review. Clin Exp Pharmacol Physiol 39:489–496. doi: 10.1111/j.1440-1681.2012.05715.x. [DOI] [PubMed] [Google Scholar]
- 79.Bressolle F, de la Coussaye JE, Ayoub R, Fabre D, Gomeni R, Saissi G, Eledjam JJ, Galtier M. 1992. Endotracheal and aerosol administrations of ceftazidime in patients with nosocomial pneumonia: pharmacokinetics and absolute bioavailability. Antimicrob Agents Chemother 36:1404–1411. doi: 10.1128/AAC.36.7.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanes SD, Wood GC, Herring V, Croce MA, Fabian TC, Pritchard E, Boucher BA. 2000. Intermittent and continuous ceftazidime infusion for critically ill trauma patients. Am J Surg 179:436–440. doi: 10.1016/S0002-9610(00)00388-3. [DOI] [PubMed] [Google Scholar]
- 81.Young RJ, Lipman J, Gin T, Gomersall CD, Joynt GM, Oh TE. 1997. Intermittent bolus dosing of ceftazidime in critically ill patients. J Antimicrob Chemother 40:269–273. doi: 10.1093/jac/40.2.269. [DOI] [PubMed] [Google Scholar]
- 82.Ambrose P, Bhavnani SM, Grosse EJE, Drusano GL. 2010. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-associated bacterial pneumonia studies: look before you leap!. Clin Infect Dis 51:S103–S110. doi: 10.1086/653057. [DOI] [PubMed] [Google Scholar]
- 83.Corey GR, Wilcox MH, Talbot GH, Thye D, Friedland D, Baculik T. 2010. CANVAS 1: the first Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 65:iv41–iv51. doi: 10.1093/jac/dkq254. [DOI] [PubMed] [Google Scholar]
- 84.Wilcox MH, Corey GR, Talbot GH, Thye D, Friedland D, Baculik T. 2010. CANVAS 2: the second phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 65:iv53–iv65. doi: 10.1093/jac/dkq255. [DOI] [PubMed] [Google Scholar]
- 85.Fong IW, Tomkins KB. 1984. Penetration of ceftazidime into the cerebrospinal fluid of patients with and without evidence of meningeal inflammation. Antimicrob Agents Chemother 26:115–116. doi: 10.1128/AAC.26.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cottagnoud P, Merdjan H, Acosta F. 2007. Pharmacokinetics of the new β-lactamase inhibitor NXL104 in an experimental rabbit meningitis model: restoration of the bacteriological efficacy of ceftazidime (CAZ) against a class C producing K. pneumoniae, abstr F1-321. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL. [Google Scholar]
- 87.Center for Drug Evaluation and Research. 2017. Antibacterial therapies for patients with an unmet medical need for the treatment of serious bacterial diseases guidance for industry. CDER/FDA, Bethesda, MD: https://www.fda.gov/downloads/Drugs/Guidances/UCM359184.pdf. [Google Scholar]
- 88.Karlowsky JA, Biedenbach DJ, Kazmierczak KM, Stone GG, Sahm DF. 2016. The activity of ceftazidime-avibactam against extended-spectrum and AmpC β-lactamase-producing Enterobacteriaceae collected in the INFORM global surveillance study from 2012 to 2014. Antimicrob Agents Chemother 60:2849–2857. doi: 10.1128/AAC.02286-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nichols WW, Stone GG, Newell P, Broadhurst H, Wardman A, MacPherson M, Yates K, Riccobene T, Critchley IA, Das S. 2018. Ceftazidime-avibactam susceptibility breakpoints against Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 62. doi: 10.1128/AAC.02590-02517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Livermore DM, Warner M, Jamrozy D, Mushtaq S, Nichols WW, Mustafa N, Woodford N. 2015. In vitro selection of ceftazidime-avibactam resistance in Enterobacteriaceae with KPC-3 carbapenemase. Antimicrob Agents Chemother 59:5324–5330. doi: 10.1128/AAC.00678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Livermore DM, Mushtaq S, Doumith M, Jamrozy D, Nichols WW, Woodford N. 2018. Selection of mutants with resistance or diminished susceptibility to ceftazidime/avibactam from ESBL- and AmpC-producing Enterobacteriaceae. J Antimicrob Chemother 73:3336–3345. doi: 10.1093/jac/dky363. [DOI] [PubMed] [Google Scholar]
- 92.Lahiri SD, Walkup GK, Whiteaker JD, Palmer T, McCormack K, Tanudra MA, Nash TJ, Thresher J, Johnstone MR, Hajec L, Livchak S, McLaughlin RE, Alm RA. 2015. Selection and molecular characterization of ceftazidime/avibactam-resistant mutants in Pseudomonas aeruginosa strains containing derepressed AmpC. J Antimicrob Chemother 70:1650–1658. doi: 10.1093/jac/dkv004. [DOI] [PubMed] [Google Scholar]
- 93.Carsenti-Etesse H, Cavallo JD, Roger PM, Ziha-Zarifi I, Plesiat P, Garrabé E, Dellamonica P. 2001. Effect of beta-lactam antibiotics on the in vitro development of resistance in Pseudomonas aeruginosa. Clin Microbiol Infect 7:144–151. doi: 10.1046/j.1469-0691.2001.00225.x. [DOI] [PubMed] [Google Scholar]
- 94.Curtis NA, Eisenstadt RL, Rudd C, White AJ. 1986. Inducible type I beta-lactamases of gram-negative bacteria and resistance to beta-lactam antibiotics. J Antimicrob Chemother 17:51–61. doi: 10.1093/jac/17.1.51. [DOI] [PubMed] [Google Scholar]
- 95.Fung-Tomc J, Huczko E, Pearce M, Kessler RE. 1988. Frequency of in vitro resistance of Pseudomonas aeruginosa to cefepime, ceftazidime, and cefotaxime. Antimicrob Agents Chemother 32:1443–1445. doi: 10.1128/AAC.32.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Queenan AM, Shang W, Bush K, Flamm RK. 2010. Differential selection of single-step AmpC or efflux mutants of Pseudomonas aeruginosa by using cefepime, ceftazidime, or ceftobiprole. Antimicrob Agents Chemother 54:4092–4097. doi: 10.1128/AAC.00060-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61. doi: 10.1128/AAC.02097-02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 63:1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]