Abstract
Background
Ginsenoside Rf is a ginseng saponin found only in Panax ginseng that affects lipid metabolism. It also has neuroprotective and antiinflammatory properties. We previously showed that Korean Red Ginseng (KRG) inhibited the expression of cyclooxygenase-2 (COX-2) by hypoxia via peroxisome proliferator–activated receptor gamma (PPARγ). The aim of the current study was to evaluate the possibility of ginsenoside Rf as an active ingredient of KRG in the inhibition of hypoxia-induced COX-2 via PPARγ.
Methods
The effects of ginsenoside Rf on the upregulation of COX-2 by hypoxia and its antimigration effects were evaluated in A549 cells. Docking of ginsenoside Rf was performed with the PPARγ structure using Surflex-Dock in Sybyl-X 2.1.1.
Results
PPARγ protein levels and peroxisome proliferator response element promoter activities were promoted by ginsenoside Rf. Inhibition of COX-2 expression by ginsenoside Rf was blocked by the PPARγ-specific inhibitor, T0070907. The PPARγ inhibitor also blocked the ability of ginsenoside Rf to suppress cell migration under hypoxia. The docking simulation results indicate that ginsenoside Rf binds to the active site of PPARγ.
Conclusions
Our results demonstrate that ginsenoside Rf inhibits hypoxia induced-COX-2 expression and cellular migration, which are dependent on PPARγ activation. These results suggest that ginsenoside Rf has an antiinflammatory effect under hypoxic conditions. Moreover, docking analysis of ginsenoside Rf into the active site of PPARγ suggests that the compound binds to PPARγ in a position similar to that of known agonists.
Keywords: COX-2, ginsenoside Rf, hypoxia, PPARγ
1. Introduction
The Panax genus of the Araliaceae family, commonly known as ginseng, is a representative medicinal herb in Asian countries that has been used for more than 2,000 years. Its recent use is not limited to Asia but has been extended to the market of Western countries and is estimated to be worth $2,084 million including ginseng root and its processed products [1]. The global popularity of ginseng consumption indirectly supports the efficacy of ginseng and has scientifically proven its pharmacology [1], [2]. In particular, ginseng reduces inflammation, and both in vitro and in vivo studies have shown that ginseng has antiinflammatory and anticancer effects [3], [4], [5], [6], [7].
Saponins, known as ginsenosides, are widely regarded as the most highly bioactive compounds in ginseng [8]. Single or crude mixtures of saponins have been considered to be responsible for most of the pharmacological effects of ginseng over the years [9]. Based on their chemical structure, ginsenosides are divided into two groups: protopanaxatriol and protopanaxadiol. The protopanaxatriol group includes ginsenosides Rg1, Re, Rf, Rh1, and Rg2. Ginsenoside Rf is a steroid-like compound linked to sugars. The content of ginsenoside Rf was 0.54 ± 0.26 mg/g (Rg1 contains 2.01 ± 0.65 mg/g) in fresh ginseng and 0.78 ± 0.25 mg/g (Rg1 contains 3.34 ± 0.98 mg/g) in red ginseng [10]. The content of ginsenoside Rf in Panax ginseng is shown as follows: roots, 1.8 ± 0.1 mg/g; stems and leaves, 0.3 ± 0.1 mg/g; and fruits, 3.6 ± 1.1 mg/g [11]. Ginsenoside Rf is a ginseng saponin that is only present in Panax ginseng [1]. Although the concentration of ginsenoside Rf is low, it is an important regulator of lipid metabolism with additional neuroprotective, antinociceptive, and antiinflammatory properties [12], [13], [14], [15].
Hypoxia refers to an overall reduction in tissue oxygen, a characteristic of solid tumors, and causes cell metastasis and invasion [16]. The production of cyclooxygenase-2 (COX-2) is increased by diverse factors, such as hypertonicity, lipopolysaccharide, cytokines, and hypoxia [17], [18], [19], [20]. The upregulation of COX-2 increases the risk of metastasis of cancer cells, and the suppression of COX-2 decreases tumor formation and metastasis [21], [22]. Mammary epithelial cells contain peroxisome proliferator–activated receptor gamma (PPARγ), which is important for the formation of breast tumors and is associated with COX-2 [23]. According to these observations, COX-2 inhibition is important to prevent the invasion of cancer cells induced by hypoxia.
PPARγ is a key regulator of adipocytes and is primarily present in adipose tissue. PPARγ is also involved in regulating inflammation by controlling COX-2 expression using the PPAR response element within the COX-2 promoter [24], [25], [26]. However, the effects of PPARγ may vary depending on the cell type, and some studies have indicated that PPARγ activates or inhibits COX-2 via PPARγ-dependent or -independent mechanisms [27], [28], [29].
We reported previously that Korean Red Ginseng (KRG) suppressed COX-2 expression under hypoxia via PPARγ [30]. In this study, we aimed to determine whether ginsenoside Rf is the active constituent of KRG leading to the inhibition of hypoxia-induced COX-2 expression via PPARγ
2. Materials and methods
2.1. Materials
Ginsenoside Rf was supplied by the Korea Ginseng Cooperation (Daejeon, Korea). 17-β-estradiol, dihydrotestosterone, and bicalutamide were purchased from Sigma (St. Louis, MO, USA). T0070907 was purchased from Selleckchem (Houston, TX, USA). ICI 182,780 (ICI) was purchased from ZENECA pharmaceutical (Tocris, UK). Fetal bovine serum and penicillin/streptomycin were purchased from GIBCO Invitrogen (Grand Island, NY, USA). Cell counting kit-8 (CCK-8) was bought from ENZO (Enzo LifeSciences, Lausen, Switzerland). Anti-COX-2 was used from Cayman Chemical (160106), anti-β-actin was used from Sigma (A5441). Anti-sirtuin-1 (SIRT-1), anti-PPARγ, and anti-peroxisome proliferator–activated receptor gamma coactivator-1 alpha were used from Santa Cruz Biotechnology (sc-15404, SC-7196, SC-13067).
2.2. Cell culture and hypoxic conditions
Maintenance of human lung epithelial A549 and human breast cancer MCF-7 cells as described previously [30]. AR-EcoScreen™ cells (gift from Dr, Mitsuru Iida, Hiyoshi Corporation, Japan) were derived from a Chinese Hamster Ovary cell line that is stably transformed with a plasmid containing an androgen response element (ARE) fused to a luciferase gene, and a plasmid encoding the androgen receptor (AR) cDNA sequence [31]. AR-EcoScreen™ cells were maintained in Dulbecco's modified eagle medium/F-12 media containing 10% fetal bovine serum. For the hypoxic condition, A549 cells were incubated at a CO2 level of 5% with 1% O2 balanced with N2 using a hypoxic chamber (Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Transfection and luciferase reporter gene assays
A549 cells were seeded at density of 3 × 104 cells/well. After 1-2 days, plasmids were transiently transfected by using polyethylenimine (Polysciences, Warrington, PA, USA). Luciferase reporter gene assay was performed as described previously [30]. Firefly luciferase reporter construct for PPAR response element (PPRE) was kindly provided from Dr. Ron Evans (The Salk Institute, San Diego California, USA). COX-2 firefly luciferase reporter was kindly provided by Dr. Hiroyasu Inoue (Nara Women's University, Nara, Japan). Estrogen response element (ERE)-Luc, a firefly luciferase reporter construct containing the three copies of vitellogenin estrogen response element, was purchased from Addgene (Cambridge, MA, USA, Addgene plasmid number 11354).
2.4. Western blot analysis
Western blotting was performed as described previously [30]. The membranes were treated with antibodies against COX-2 (1:1000), β-actin (1:5000), SIRT-1 (1:1000), and PPARγ (1:1000).
2.5. Cellular migration assays
The migration assay was performed using transwell (8.0 μm pores transwell, Corning, NY, USA) as reported previously [32]. After incubation under normoxia or hypoxia for 24 h, migrated cells were observed and counted using a light microscope.
2.6. CCK-8 assays
A549 cells were seeded at a density of 5 × 103 cells/well in a 96-well plate. After 24 h, the indicated chemicals were incubated at normoxia or hypoxia for 24 h. CCK-8 assay was performed as described previously [33].
2.7. Preparation of protein structures
Crystal structure of PPARγ (PDB ID: 2ATH) in complex with the agonist BPR1H036 (2-ethanoic acid) was downloaded from RCSB Protein Data Bank [34], [35]. The PPARγ structure was optimized for docking as follows: all water molecules were removed from the crystal structure, and the bound ligand was extracted and then prepared with the protein preparation module of the Sybyl-X 2.1.1 (Certara Inc., Princeton, NJ, USA) using default parameters.
2.8. Docking with Surflex-Dock
Ginsenoside Rf was docked into the prepared PPARγ crystal structure by using Surflex-Dock in Sybyl-X 2.1.1. The program generated the protomol in the active site of PPARγ with a threshold of 0.4 and bloat set of 1 around the embedded BPR1H036 molecule. The residues (SER289, HIS323, HIS449, and TYR473) found to interact with BPR1H036 were considered as crucial residues in the active site of PPARγ. The main setting was 50 solutions for flexible docking of the ligand, and the other parameters accepted the Surflex-Dock Geom default settings. Docking results were analyzed by using Glide program (Schrödinger LLC, NY, USA).
2.9. Statistical analysis
All data were analyzed and expressed as means and standard deviations. The two-tailed, unpaired Student t test was applied using SPSS software (version 23.0; IBM, Armonk, NY, USA).
3. Results
3.1. Ginsenoside Rf induces PPARγ transcriptional activity and its protein expression in A549 cells
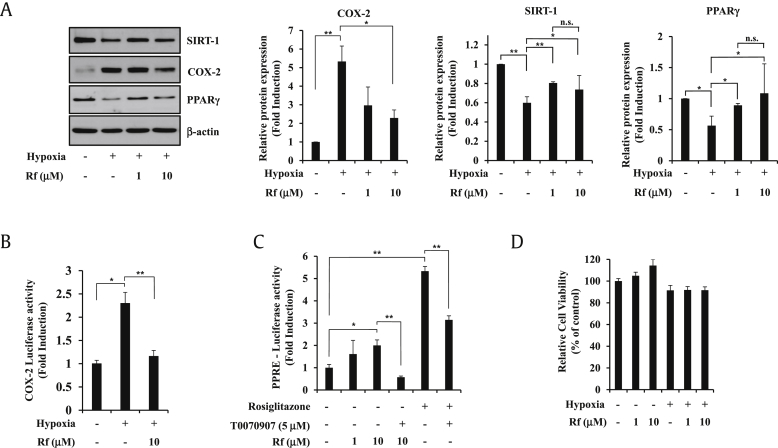
We previously showed that KRG inhibited hypoxia-induced COX-2 activation via PPARγ [32]. In search of the active constituent of KRG that inhibits hypoxia-induced COX-2, ginsenoside Rf was chosen because previous studies suggested that ginsenoside Rf may modulate PPARγ [36]. To assess the effects of ginsenoside Rf on hypoxia-induced COX-2 activation, A549 cells were pretreated with ginsenoside Rf for 1 h and exposed to hypoxic conditions for 24 h. Ginsenoside Rf increased the protein levels of SIRT-1 and PPARγ compared with hypoxia (Fig. 1A). At the same time, 10 μM of ginsenoside Rf efficiently blocked the upregulation of COX-2 transcriptional activity and protein level under hypoxia (Fig. 1A and B). Next, we wanted to examine whether ginsenoside Rf activated PPARγ luciferase reporter activity (Fig. 1C). Ginsenoside Rf significantly activated PPRE luciferase reporter activity. Ginsenoside Rf–induced PPRE luciferase gene activation was blocked by treatment with the PPARγ antagonist, T0070907, indicating that ginsenoside Rf-induced PPRE luciferase gene activation is PPARγ-specific. Cell viability was not affected at concentrations of 1–10 μΜ ginsenoside Rf for 24 h under normoxic or hypoxic conditions (Fig. 1D).
Fig. 1.
Ginsenoside Rf induces PPARγ transcriptional activity and its protein expression. (A) A549 cells were treated with ginsenoside Rf at 1–10 μM for 24 h under hypoxia and analyzed by Western blot. Protein expressions were normalized to β-actin content in each sample. (B) A549 cells were transiently transfected with the COX-2-luciferase reporter gene. The following day, A549 cells were cultured in medium containing vehicle or ginsenoside Rf (10 μM) for 24 h under hypoxia, and luciferase activities were determined. (C) A549 cells were transiently transfected with the PPRE-luciferase reporter gene. The following day, A549 cells were cultured in medium containing vehicle or ginsenoside Rf (1-10 μM) or PPARγ agonist rosiglitazone (1 μM) or PPARγ antagonist T0070907 (5 μM) for 48 h, and luciferase activities were determined. (D) A549 cells were incubated with ginsenoside Rf at 1–10 μM for 24 h under normoxia or hypoxia, and CCK-8 assay was performed. All experiments were repeated at least three times. Values represent the mean ± SD (N = 3). All experiments were repeated at least three times. * represents p < 0.05, ** represents p < 0.01, p > 0.05 is indicated by “n.s.” for not significant. COX-2, cyclooxygenase-2; PPARγ, peroxisome proliferator–activated receptor gamma; PPRE, PPAR response element; CCK-8, cell counting kit-8; SIRT-1, sirtuin-1; SD, standard deviation.
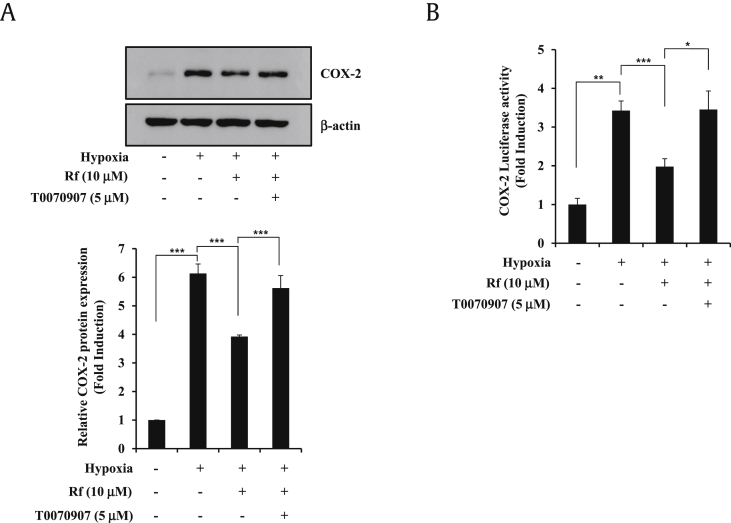
3.2. Ginsenoside Rf inhibits hypoxia-induced COX-2 protein expression and COX-2 transcriptional activity through PPARγ in A549 cells
To determine whether PPRE activation is related to COX-2 inhibition by ginsenoside Rf, COX-2 protein levels were measured using T0070907 (Fig. 2A). The suppression of hypoxia-induced COX-2 protein expression by ginsenoside Rf was blocked by T0070907, indicating that the response involves PPARγ. To further identify the involvement of PPARγ activation, COX-2 promoter activity was measured following treatment with T0070907 (Fig. 2B). These results suggest that the suppression of hypoxia-stimulated COX-2 by ginsenoside Rf in A549 cells is dependent on PPARγ.
Fig. 2.
PPARγ activation is required for the ginsenoside Rf-mediated inhibition of hypoxia-induced COX-2 expression. (A) A549 cells were pretreated with vehicle or ginsenoside Rf (10 μM) and/or T0070907 (5 μM) for 1 h before treatment with hypoxia for 24 h. Total proteins were prepared, and protein levels of COX-2 were determined by Western blot. Proteins expression were normalized to β-actin content in each sample. (B) A549 cells were transiently transfected with the COX-2 luciferase reporter gene. The following day, A549 cells were cultured in medium containing vehicle or ginsenoside Rf (10 μM) and/or PPARγ antagonist T0070907 (5 μM) for 24 h under the hypoxia, and luciferase activities were determined. Values represent the mean ± SD (N = 3). All experiments were repeated at least three times. * represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001. COX-2, cyclooxygenase-2; PPARγ, peroxisome proliferator–activated receptor gamma; SD, standard deviation.
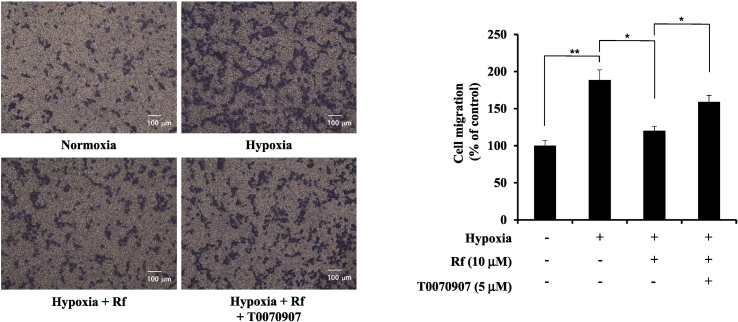
3.3. Ginsenoside Rf repress hypoxia-induced cellular migration in A549 cells
To confirm whether ginsenoside Rf inhibits cellular migration via PPARγ, migration capability was examined using T0070907 in A549 cells. As shown in Fig. 3, ginsenoside Rf inhibited cellular migration under hypoxia. The inhibition of hypoxia-induced cellular migration by ginsenoside Rf was significantly blocked by T0070907. These results suggest that ginsenoside Rf inhibits hypoxia-induced cellular migration via PPARγ.
Fig. 3.
Ginsenoside Rf inhibits hypoxia-induced cellular migration of A549 cells via PPARγ. A549 cells were pretreated with ginsenoside Rf (10 μM) and/or T0070907 (5 μM) treatment for 1 h and incubated under hypoxia for 24 h. Cells that migrated through the membranes were fixed and counted using light microscopy. Bar graph shows relative migrated cells. The cells in the lower side were counted and are graphed below. Scale bar represent 100 μm. Values represent the mean ± SD (N = 3). All experiments were repeated at least three times. * represent p < 0.05, ** represent p < 0.01. PPARγ, peroxisome proliferator–activated receptor gamma; SD, standard deviation.
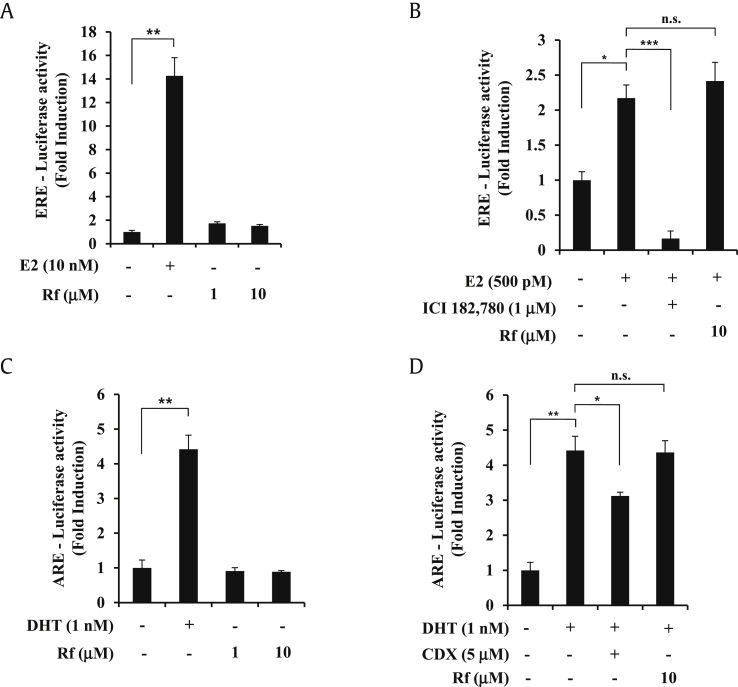
3.4. Ginsenoside Rf has no estrogen receptor or AR transcriptional activity
To determine whether ginsenoside Rf induces other nuclear hormone receptors, estrogen receptor (ER) and AR transcriptional activities were examined. As shown in Fig. 4A, 17-β-estradiol , as a positive control, induced ERE-transcriptional activity at a concentration of 10 nM in MCF-7 cells. However, ginsenoside Rf did not induce ER transcriptional activity. We also analyzed the anti-ERE transcriptional activity of ginsenoside Rf (Fig. 4B). Ginsenoside Rf did not exhibit anti-ER transcriptional activity. Moreover, ginsenoside Rf was examined for ARE transcriptional activity (Fig. 4C). Ginsenoside Rf did not show AR transcriptional activity. Lastly, we checked for anti-ARE transcriptional activity of ginsenoside Rf (Fig. 4D). Ginsenoside Rf did not exhibit anti-AR transcriptional activity. These results suggest that ginsenoside Rf specifically activates PPARγ, not ER or AR.
Fig. 4.
Effects of ginsenoside Rf on estrogen and androgen receptor transcriptional activation. (A) MCF-7 cells were transiently transfected with ERE-luciferase reporter gene. The following day, MCF-7 cells were cultured in medium containing vehicle or ginsenoside Rf (1–10 μM) and/or E2 (10 nM) for 24 h, and luciferase activities were determined. (B) MCF-7 cells were transiently transfected with ERE-luciferase reporter gene. The following day, MCF-7 cells were pretreated with vehicle or ginsenoside Rf (10 μM) or ICI 182,780 (1 μM) for 1 h and then treat E2 (500 pM) during 24 h, and luciferase activities were determined. (C) AR-Ecoscreen cells were stably transfected with AR and ARE-luciferase reporter gene. AR-Ecoscreen cells were cultured in medium containing vehicle or ginsenoside Rf (1–10 μM) or dihydrotestosterone (DHT) (1 nM) for 24 h, and luciferase activities were determined. (D) AR-Ecoscreen cells were pretreated with vehicle or ginsenoside Rf (10 μM) or CDX (5 μM) for 1 h and then treat DHT (1 nM) during 24 h, and luciferase activities were determined. Values represent the mean ± SD (N = 3). All experiments were repeated at least three times. * represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001, p > 0.05 is indicated by “n.s.” for not significant. AR, androgen receptor; CDX, bicalutamide; ERE, estrogen response element; SD, standard deviation.
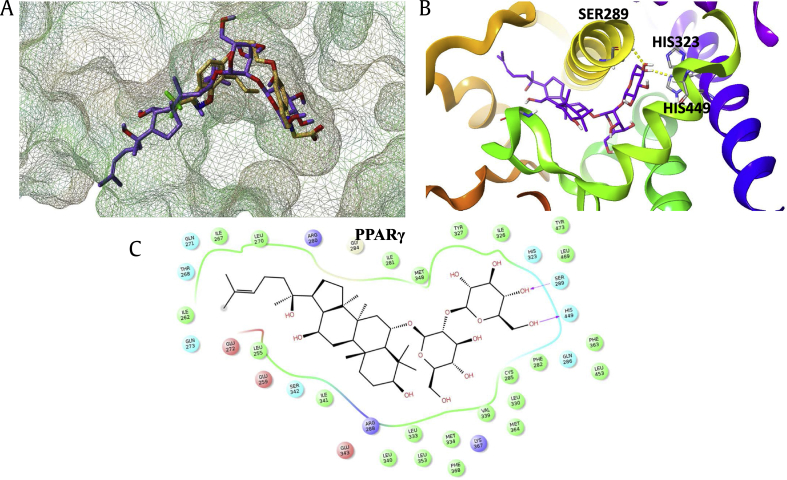
3.5. Docking modeling: ginsenoside Rf fits into the agonist binding site of PPARγ
To investigate whether ginsenoside Rf directly interacts with PPARγ, a molecular docking simulation of the interaction between ginsenoside Rf and PPARγ was performed (Fig. 5). For comparison, the binding pose of ginsenoside Rf was superimposed over the X-ray pose of BPR1H036, a known indole-based PPARγ agonist (Fig. 5B). The overall binding mode of ginsenoside Rf predicted by Surflex-Dock was similar to that of BPR1H036. In the X-ray structure, the carboxylate group in the polar head of BPR1H036 formed a hydrogen bond network with key residues of PPARγ, including SER289, HIS323, HIS449, and TYR473. The glucopyranoside group of ginsenoside Rf also occupied this site, forming two hydrogen bonds with SER289 and HIS449. This hydrogen bond pattern of the acidic polar head group is conserved in most PPARγ agonists, which is believed to be essential for the activity of the ligand. Moreover, the tetracyclic skeleton of ginsenoside Rf exhibited strong hydrophobic interactions with PPARγ, playing an important role in the binding of ginsenoside Rf to the protein. These results indicate that ginsenoside Rf may directly interact with PPARγ; however, further studies are required to confirm the binding mode of ginsenoside Rf to PPARγ.
Fig. 5.
Binding pose of ginsenoside Rf in the active site of PPARγ. (A) Superimposition of the docked pose of ginsenoside Rf and the X-ray pose of reference compound BPR1H036 (PDB id: 2ATH). Ginsenoside Rf is rendered as violet stick, while BPR1H036 is orange stick. The surface of PPARγ is generated by MOLCAD program and depicted as mesh surface. (B) Docking pose of ginsenoside Rf in the active site of PPARγ. Ginsenoside Rf is rendered as violet stick, and the surrounding key residues are rendered as grey stick and labeled. Hydrogen bonds are depicted as dotted yellow lines. (C) 2D view of the interaction between ginsenoside Rf and PPARγ. PPARγ, peroxisome proliferator–activated receptor gamma.
4. Discussion
Many studies have shown that KRG has antioxidant and antiinflammatory effects both in vitro and in vivo [37], [38]. We previously demonstrated that KRG inhibits hypoxia-induced COX-2 activation via PPARγ [30]. In search of the active constituent of KRG that inhibits hypoxia-induced COX-2, we found that ginsenoside Rf activates PPARγ and suppresses hypoxia-induced COX-2 via PPARγ. PPARγ is a member of the nuclear receptor superfamily and is a ligand-dependent transcription factor [39]. PPARγ regulates glucose metabolism, fatty acid storage, and adipocyte differentiation and is a target of antidiabetic drugs [40], [41]. Several studies have shown that the ginsenosides Rg3 and Rh2 and compound K inhibit adipogenesis via the regulation of PPARγ and CCAAT/enhaancer-binding protein alpha expression in 3T3-L1 cells, human primary preadipocytes, and mice [42]. Indeed, it has been suggested that ginsenoside Rf binds directly to the active site of PPARγ [36]. However, these ginsenosides downregulate PPARγ and perilipin protein expression in 3T3-L1 cells, indicating an antiadipogenic effect [42]. It was recently recognized that PPARγ plays a fundamental role in the immune response through its ability to inhibit the expression of inflammatory cytokines and induce the differentiation of immune cells against antiinflammatory phenotypes [24]. Hesperetin, a flavanone from the fruit peel of Citrus aurantium L, exhibits strong antiinflammatory effects through the upregulation of PPARγ [43]. Moreover, fenretinide, a synthetic ligand of PPARγ, induces antiinflammatory activity, although its exact mechanism is not fully understood [44]. Our data are consistent with previous results demonstrating that ginsenoside Rf modulates PPARγ and appears to have distinct functions, such as antiadipogenesis and antiinflammation, according to the cell type. In our study, ginsenoside Rf specifically inhibited COX-2 expression via PPARγ activation under hypoxic conditions. The COX-2 suppression we observed may be because of the sum of the activities of each component and their interactions, as ginsenosides Rb1 and Rg1 elicited some response, albeit weaker than that of ginsenoside Rf. Moreover, the inhibition of COX-2 expression and cellular migration under hypoxia were dependent on PPARγ activation. The docking simulation results indicated that ginsenoside Rf binds to the active site of PPARγ; however, further studies are required to confirm the binding mode.
These findings provide a mechanical explanation for the effects of ginsenoside Rf on metabolic disorders and cancer.
Conflicts of interest
All authors declare that they have no conflicts of interest.
Acknowledgments
This research was supported 2015 grant from the Korean Society of Ginseng to Y.J.L.
References
- 1.Chan T.W., But P.P., Cheng S.W., Kwok I.M., Lau F.W., Xu H.X. Differentiation and authentication of Panax ginseng, Panax quinquefolius, and ginseng products by using HPLC/MS. Anal Chem. 2000;72:1281–1287. doi: 10.1021/ac990819z. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya S.K., Mitra S.K. Anxiolytic activity of Panax ginseng roots: an experimental study. J Ethnopharmacol. 1991;34:87–92. doi: 10.1016/0378-8741(91)90193-h. [DOI] [PubMed] [Google Scholar]
- 3.Jin Y., Kotakadi V.S., Ying L., Hofseth A.B., Cui X., Wood P.A., Windust A., Matesic L.E., Pena E.A., Chiuzan C. American ginseng suppresses inflammation and DNA damage associated with mouse colitis. Carcinogenesis. 2008;29:2351–2359. doi: 10.1093/carcin/bgn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park J.S., Shin J.A., Jung J.S., Hyun J.W., Van Le T.K., Kim D.H., Park E.M., Kim H.S. Anti-inflammatory mechanism of compound K in activated microglia and its neuroprotective effect on experimental stroke in mice. J Pharmacol Exp Ther. 2012;341:59–67. doi: 10.1124/jpet.111.189035. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y., Yang W.S., Yu T., Sung G.H., Park K.W., Yoon K., Son Y.J., Hwang H., Kwak Y.S., Lee C.M. ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean red ginseng water extract. J Ethnopharmacol. 2014;154:218–228. doi: 10.1016/j.jep.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Kim H.J., Kim P., Shin C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J Ginseng Res. 2013;37:8–29. doi: 10.5142/jgr.2013.37.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee B., Sur B., Park J., Kim S.H., Kwon S., Yeom M., Shim I., Lee H., Hahm D.H. Ginsenoside rg3 alleviates lipopolysaccharide-induced learning and memory impairments by anti-inflammatory activity in rats. Biomol Ther (Seoul) 2013;21:381–390. doi: 10.4062/biomolther.2013.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paek K.Y., Chakrabarty D., Hahn E.J. Application of bioreactor systems for large scale production of horticultural and medicinal plants. Plant Cell Tissue Organ Cult. 2005;81:287–300. [Google Scholar]
- 9.Kaku T., Miyata T., Uruno T., Sako I., Kinoshita A. Chemico-pharmacological studies on saponins of Panax ginseng C. A. Meyer. II. Pharmacological part. Arzneimittelforschung. 1975;25:539–547. [PubMed] [Google Scholar]
- 10.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): Histroy, preparation methods, and chemical composition. J Ginseng Res. 2015;39:384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.W., Choi B.R., Kim Y.C., Choi D.J., Lee Y.S., Kim G.S., Baek N.I., Kim S.Y., Lee D.Y. Comprehensive profiling and quantification of ginsenosides in the root, stem, leaf, and berry of Panax ginseng by UPLC-QTOF/MS. Molecules. 2017;22(12):E2147. doi: 10.3390/molecules22122147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn S., Siddiqi M.H., Aceituno V.C., Simu S.Y., Yang D.C. Suppression of MAPKs/NF-kappaB activation induces intestinal anti-inflammatory action of Ginsenoside Rf in HT-29 and RAW264.7 cells. Immunol Invest. 2016;45:439–449. doi: 10.3109/08820139.2016.1168830. [DOI] [PubMed] [Google Scholar]
- 13.Choi K., Kim M., Ryu J., Choi C. Ginsenosides compound K and Rh(2) inhibit tumor necrosis factor-alpha-induced activation of the NF-kappaB and JNK pathways in human astroglial cells. Neurosci Lett. 2007;421:37–41. doi: 10.1016/j.neulet.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Lee H., Gonzalez F.J., Yoon M. Ginsenoside Rf, a component of ginseng, regulates lipoprotein metabolism through peroxisome proliferator-activated receptor alpha. Biochem Biophys Res Commun. 2006;339:196–203. doi: 10.1016/j.bbrc.2005.10.197. [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Wang Q., Yao X.M., Li Y. Induction of CYP3A4 and MDR1 gene expression by baicalin, baicalein, chlorogenic acid, and ginsenoside Rf through constitutive androstane receptor- and pregnane X receptor-mediated pathways. Eur J Pharmacol. 2010;640:46–54. doi: 10.1016/j.ejphar.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Arsenault D., Brochu-Gaudreau K., Charbonneau M., Dubois C.M. HDAC6 deacetylase activity is Required for hypoxia-induced invadopodia formation and cell invasion. Plos One. 2013;8 doi: 10.1371/journal.pone.0055529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredenburgh L.E., Ma J., Perrella M.A. Cyclooxygenase-2 inhibition and hypoxia-induced pulmonary hypertension: effects on pulmonary vascular remodeling and contractility. Trends Cardiovasc Med. 2009;19:31–37. doi: 10.1016/j.tcm.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaidi A., Qualtrough D., Williams A.C., Paraskeva C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683–6691. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- 19.Lee J.J., Natsuizaka M., Ohashi S., Wong G.S., Takaoka M., Michaylira C.Z., Budo D., Tobias J.W., Kanai M., Shirakawa Y. Hypoxia activates the cyclooxygenase-2-prostaglandin E synthase axis. Carcinogenesis. 2010;31:427–434. doi: 10.1093/carcin/bgp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao L., Wu Y., Xu Z., Wang H., Zhao Z., Li Y., Yang P., Wei X. Involvement of COX-2/PGE2 signalling in hypoxia-induced angiogenic response in endothelial cells. J Cell Mol Med. 2012;16:1840–1855. doi: 10.1111/j.1582-4934.2011.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stasinopoulos I., Shah T., Penet M.F., Krishnamachary B., Bhujwalla Z.M. COX-2 in cancer: Gordian knot or Achilles heel? Front Pharmacol. 2013;4:34. doi: 10.3389/fphar.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsujii M., Kawano S., DuBois R.N. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apostoli A.J., Roche J.M., Schneider M.M., SenGupta S.K., Di Lena M.A., Rubino R.E., Peterson N.T., Nicol C.J. Opposing roles for mammary epithelial-specific PPARgamma signaling and activation during breast tumour progression. Mol Cancer. 2015;14:85. doi: 10.1186/s12943-015-0347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyagi S., Gupta P., Saini A.S., Kaushal C., Sharma S. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2:236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazra S., Dubinett S.M. Ciglitazone mediates COX-2 dependent suppression of PGE2 in human non-small cell lung cancer cells. Prostaglandins Leukot Essent Fatty Acids. 2007;77:51–58. doi: 10.1016/j.plefa.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel L., Pass I., Coxon P., Downes C.P., Smith S.A., Macphee C.H. Tumor suppressor and anti-inflammatory actions of PPARgamma agonists are mediated via upregulation of PTEN. Curr Biol. 2001;11:764–768. doi: 10.1016/s0960-9822(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 27.Bren-Mattison Y., Meyer A.M., Van Putten V., Li H., Kuhn K., Stearman R., Weiser-Evans M., Winn R.A., Heasley L.E., Nemenoff R.A. Antitumorigenic effects of peroxisome proliferator-activated receptor-gamma in non-small-cell lung cancer cells are mediated by suppression of cyclooxygenase-2 via inhibition of nuclear factor-kappaB. Mol Pharmacol. 2008;73:709–717. doi: 10.1124/mol.107.042002. [DOI] [PubMed] [Google Scholar]
- 28.Patel K.M., Wright K.L., Whittaker P., Chakravarty P., Watson M.L., Ward S.G. Differential modulation of COX-2 expression in A549 airway epithelial cells by structurally distinct PPAR(gamma) agonists: evidence for disparate functional effects which are independent of NF-(kappa)B and PPAR(gamma) Cell Signal. 2005;17:1098–1110. doi: 10.1016/j.cellsig.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Meade E.A., McIntyre T.M., Zimmerman G.A., Prescott S.M. Peroxisome proliferators enhance cyclooxygenase-2 expression in epithelial cells. J Biol Chem. 1999;274:8328–8334. doi: 10.1074/jbc.274.12.8328. [DOI] [PubMed] [Google Scholar]
- 30.Song H., Lee Y.J. Inhibition of hypoxia-induced cyclooxygenase-2 by Korean Red Ginseng is dependent on peroxisome proliferator-activated receptor gamma. J Ginseng Res. 2017;41:240–246. doi: 10.1016/j.jgr.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satoh K., Ohyama K., Aoki N., Iida M., Nagai F. Study on anti-androgenic effects of bisphenol a diglycidyl ether (BADGE), bisphenol F diglycidyl ether (BFDGE) and their derivatives using cells stably transfected with human androgen receptor, AR-EcoScreen. Food Chem Toxicol. 2004;42:983–993. doi: 10.1016/j.fct.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Park J., Shim M.K., Jin M., Rhyu M.R., Lee Y. Methyl syringate, a TRPA1 agonist represses hypoxia-induced cyclooxygenase-2 in lung cancer cells. Phytomedicine. 2016;23:324–329. doi: 10.1016/j.phymed.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Song H., Park J., Bui P., Choi K., Gye M.C., Hong Y.C., Kim J.H., Lee Y.J. Bisphenol A induces COX-2 through the mitogen-activated protein kinase pathway and is associated with levels of inflammation-related markers in elderly populations. Environ Res. 2017;158:490–498. doi: 10.1016/j.envres.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Mahindroo N., Huang C.F., Peng Y.H., Wang C.C., Liao C.C., Lien T.W., Chittimalla S.K., Huang W.J., Chai C.H., Prakash E. Novel indole-based peroxisome proliferator-activated receptor agonists: design, SAR, structural biology, and biological activities. J Med Chem. 2005;48:8194–8208. doi: 10.1021/jm0506930. [DOI] [PubMed] [Google Scholar]
- 35.Sohn Y.S., Lee Y., Park C., Hwang S., Kim S., Back A., Son M., Suh J.K., Kim H.H., Lee K.W. Pharmacophore identification for peroxisome proliferator-activated Receptor Gamma agonists. Bull Korean Chem Soc. 2011;32:201–207. [Google Scholar]
- 36.Siraj F.M., Natarajan S., Huq M.A., Kim Y.J., Yang D.C. Structural investigation of ginsenoside Rf with PPARgamma major transcriptional factor of adipogenesis and its impact on adipocyte. J Ginseng Res. 2015;39:141–147. doi: 10.1016/j.jgr.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim E.H., Kim I.H., Lee M.J., Thach Nguyen C., Ha J.A., Lee S.C., Choi S., Choi K.T., Pyo S., Rhee D.K. Anti-oxidative stress effect of red ginseng in the brain is mediated by peptidyl arginine deiminase type IV (PADI4) repression via estrogen receptor (ER) β up-regulation. J Ethnopharmacol. 2013;148:474–485. doi: 10.1016/j.jep.2013.04.041. [DOI] [PubMed] [Google Scholar]
- 38.Hong C.E., Lyu S.Y. Anti-inflammatory and anti-oxidative effects of Korean Red Ginseng extract in human keratinocytes. Immune Netw. 2011;11:42–49. doi: 10.4110/in.2011.11.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M., Pascual G., Glass C.K. Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol Cell Biol. 2000;20:4699–4707. doi: 10.1128/mcb.20.13.4699-4707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehmann J.M., Moore L.B., Smith-Oliver T.A., Wilkison W.O., Willson T.M., Kliewer S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 41.Siersbaek R., Nielsen R., Mandrup S. PPARgamma in adipocyte differentiation and metabolism--novel insights from genome-wide studies. FEBS Lett. 2010;584:3242–3249. doi: 10.1016/j.febslet.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L., Virgous C., Si H. Ginseng and obesity: observations and understanding in cultured cells, animals and humans. J Nutr Biochem. 2017;44:1–10. doi: 10.1016/j.jnutbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Chen X., Ding H.W., Li H.D., Huang H.M., Li X.F., Yang Y., Zhang Y.L., Pan X.Y., Huang C., Meng X.M. Hesperetin derivative-14 alleviates inflammation by activating PPAR-gamma in mice with CCl4-induced acute liver injury and LPS-treated RAW264.7 cells. Toxicol Lett. 2017;274:51–63. doi: 10.1016/j.toxlet.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Lin C.H., Lee S.Y., Zhang C.C., Du Y.F., Hung H.C., Wu H.T., Ou H.Y. Fenretinide inhibits macrophage inflammatory mediators and controls hypertension in spontaneously hypertensive rats via the peroxisome proliferator-activated receptor gamma pathway. Drug Des Devel Ther. 2016;10:3591–3597. doi: 10.2147/DDDT.S114879. [DOI] [PMC free article] [PubMed] [Google Scholar]