Abstract
Background: The thyroid hormone triiodothyronine (T3) is critical for vertebrate development and affects the function of many adult tissues and organs. Its genomic effects are mediated by thyroid hormone nuclear receptors (TRs) present in all vertebrates. The discovery of patients with resistance to thyroid hormone (RTHβ) >50 years ago and subsequent identification of genetic mutations in only the THRB gene in these patients suggest that mutations in the THRA gene may have different pathological manifestations in humans. Indeed, the recent discovery of a number of human patients carrying heterozygous mutations in the THRA gene (RTHα) revealed a distinct phenotype that was not observed in RTH patients with THRB gene mutations (RTHβ). That is, RTHα patients have constipation, implicating intestinal defects caused by THRA gene mutations.
Methods: To determine how TRα1 mutations affect the intestine, this study analyzed a mutant mouse expressing a strong dominantly negative TRα1 mutant (denoted TRα1PV; Thra1PV mice). This mutant mouse faithfully reproduces RTHα phenotypes observed in patients.
Results: In adult Thra1PV/+ mice, constipation was observed just like in patients with TRα mutations. Importantly, significant intestinal defects were discovered, including shorter villi and increased differentiated cells in the crypt, accompanied by reduced stem-cell proliferation in the intestine.
Conclusions: The findings suggest that further analysis of this mouse model should help to reveal the molecular and physiological defects in the intestine caused by TRα mutations and to determine the underlying mechanisms.
Keywords: thyroid hormone receptor, adult organ-specific stem cell, cell proliferation and differentiation, small intestine, resistance to thyroid hormone
Introduction
The thyroid hormone (TH) triiodothyronine (T3) has long been known to be critical for human development and adult life. The most obvious and earliest known abnormalities of human body and behavior associated with T3 deficiency are goiter (a lump in the neck due to thyroid gland enlargement) and cretinism (a form of mental deficiency together with defects in skeletal growth) (1). These developmental abnormalities can often be treated or prevented with exogenous T3 starting soon after birth (1). In addition to its developmental roles, T3 is also important for normal function of various organs. T3 deficiency leads to a reduced metabolic rate (2), and abnormal TH levels are associated with a number of cardiovascular symptoms (3).
The genomic effects of T3 are mostly mediated by the three major T3-binding nuclear receptors (TRs): TRα1, TRβ1, and TRβ2. TRs are transcription factors that can form heterodimers with 9-cis retinoic acid receptors (RXRs) to bind to DNA and regulate target gene transcription (4–14). For genes whose transcription is induced by T3, TR/RXR heterodimers bind to T3 response elements (TREs) in chromatin and recruit histone-deacetylase containing co-repressor complexes to repress their expression in the absence of T3. When T3 is present, the liganded TR/RXR heterodimers release the co-repressor complexes and recruit co-activator complexes to the target genes to cause local chromatin remodeling, including the loss of nucleosomes and various histone modifications, and to facilitate gene activation. Given the roles of T3 in human development and the critical roles of TRs in mediating T3 signaling, mutations in TRs can have detrimental effects in humans. Indeed, it was discovered more than half a century ago that the syndrome of resistance to thyroid hormone (RTHβ) is linked to genetic changes in patients (15–18). The classic signs and symptoms include goiter and tachycardia, and the characteristic biochemical constellation is defined by elevated peripheral hormones (thyroxine [T4] and T3) with an inappropriately non-suppressed and elevated thyrotropin (15–19). Interestingly, all such RTHβ patients have mutations in only the THRB gene, and most of the mutations have reduced or lost T3 binding to the receptors (17,18). These findings suggest that mutations in TRα1 could have distinct effects in humans, which may largely reflect the fact that the THRA and THRB genes have different developmental and tissue-specific expression profiles (19,20).
In recent years, a number of patients with TRα1 mutations have been discovered (21–24). The mutations in the THRA gene lead to reduced or lost T3 binding to receptors, thus making the patient resistant to T3 in target organs (RTHα). These patients have very different phenotypes compared to RTHβ patients (17,21–24). Of particular interest is that such patients have constipation, suggesting intestinal defects due to the THRA gene mutations (21,22). While the exact defects in the intestine remain unclear, several lines of evidence support a role of T3 signaling in the intestinal development and physiology. First, T3 is critical for normal physiological functions of most, if not all, organs in the adult vertebrates and T3 levels regulates metabolic rate (3,25,26). Second, T3 affects stem-cell function and regeneration in different tissues (27–31). Third, altered T3 levels are associated with intestinal abnormalities and diseases, including inflammatory bowel diseases (IBD), such as ulcerative colitis and Crohn's disease (32). Fourth, studies in mice have shown that T3 deficiency or TRα knockout leads to abnormal intestinal morphology (33–37). Finally, in thyroid patients with either hypothyroidism or hyperthyroidism, it is common to have gastrointestinal manifestations, such as altered motility, autoimmune gastritis, or esophageal compression (38). Further, hyperthyroidism can cause frequent bowel movements, diarrhea, nausea, and vomiting, while hypothyroidism results in overall decreased metabolic functions accompanied by slow intestinal motility and constipation (39). These resemble the observed effects in human patients with TRα mutations.
To investigate the effects of TRα1 mutations on the intestine, this study analyzed the intestine of the Thra1PV/+ mouse, a mouse model of RTHα (40). In this model, a knock-in mutation (TRα1PV) was introduced into the endogenous mouse Thra gene to produce a potent dominant negative mutant TR, TRα1PV (40). The TRα1PV has a C-terminal mutated sequence (398-PPFVLGSVRGLD- 409) (40) similar to the truncated C-terminal sequence in two RTHα patients (398-PPTLPRGL -405) (41). Previous studies have shown that Thra1PV/+ mice exhibit many of the phenotypic features of RTHα found in patients, including growth retardation and delayed bone development, but with very mild abnormal thyroid function tests (40,42,43). This model has been used to test T4 as a potential treatment modality to mitigate the bone abnormalities in RTHα patients (44), indicating that the Thra1PV/+ mouse is a valid model to study the effects of TRα1 mutations in intestine abnormalities in patients. This study shows that adult Thra1PV/+ mice also exhibit constipation, mimicking the phenotypic manifestations of human patients with THRA mutations. Importantly, the analyses revealed that Thra1PV/+ mice intestine had shorter villi and increased differentiated Paneth and goblet cells in the crypts, accompanied by reduced cell proliferation in the crypts. Further analysis of the intestine of this mouse model should help the physiological defects in RTHα patients and how TRα1 mutations could lead to intestinal defects to be better understood.
Methods
Animals
Mice harboring a heterozygous mutated Thra1PV gene (Thra1PV/+ mice) were generated, as previously described (40). All mice were maintained in accordance with the National Institutes of Health animal facility guidelines for laboratory animal research. All animal care and treatment was done as approved by National Cancer Institute Animal Care and Use Committee.
Weight measurements for the small intestine, colon, and body
After euthanization, wild-type and Thra1PV/+ mice were weighed, and the small intestine and colon were dissected and weighed with the content inside. Between 5 and 33 animals per genotype were used for these and other analyses, as described below.
Size measurements for the small intestine, intestinal crypts, villi, and body
The body length was measured on euthanized Thra1PV/+ mice from the tip of the mouth to the base of the tail. The length of the dissected small intestine was measured from the beginning of the duodenum to the end of the ileum. The length of the villus was measured from the mouth of the crypt to the tip of the villus on hematoxylin and eosin (H&E)-stained intestinal cross-sections.
Immunohistochemistry
The intestine was removed from age-matched littermates of wild-type and Thra1PV/+ mice. The isolated intestine was flushed with ice-cold 1 × phosphate-buffered saline (PBS) and fixed in 4% formaldehyde (and, if needed, stored at 4°C), followed by embedding in paraffin and then cutting into 5 μm sections.
For H&E staining, the 5 μm sections were stained with H&E following the manufacturer's protocol (Sigma–Aldrich) and analyzed under a bright-field microscope.
For immunofluorescent staining of proliferating cells, an antibody against Ki67 (G-protein coupled receptor 67) was used, which is present during all active phases of the cell cycle (G1, S, G2, and mitosis) but is absent in resting (quiescent) cells (G0). Paraffin sections (5 μm) were deparaffinized in xylene and rehydrated in a series of different concentrations of ethanol. Antigen retrieval was performed by boiling in an antigen retrieve buffer (1 mM Tris, 1 mM EDTA, and 0.05% Tween-20) for three minutes at 125°C followed by washing the slides under running water and rinsing them in 1 × TBS-Tween (Tris-buffered saline +0.1% Tween-20) for five minutes. After incubation in the blocking buffer (10% normal goat serum in PBS) for one hour at room temperature, anti-Ki67 antibody (diluted 1:400; Abcam) was added, and the slides were incubated at 4°C overnight. The slides were then washed in 1× TBS-Tween and subsequently incubated with a fluorescence-labeled secondary antibody (Millipore) for one hour at room temperature, washed three times with 1× TBS-Tween, and covered with DAPI-containing mounting medium to counterstain the DNA. The fluorescent pictures for different colors and different sections were taken under the same settings. The Ki67 fluorescent pictures were analyzed using Fiji ImageJ at the same setting to count the positive cell numbers.
Detection of apoptosis
Apoptosis was characterized with terminal deoxynuclotidyltransferase-mediated dUTP nick-end labeling (TUNEL; Roche) following the manufacturer's protocol. Briefly, the TUNEL in situ Cell Death Detection Kit was used on 5 μm paraffin sections of the intestine. Sections were dewaxed with xylene and rehydrated with ethanol. Antigen retrieval was performed by microwaving the sections (700 W; 1.5 minutes) in sodium citrate buffer (pH 6.0) followed by rinsing in PBS. The sections were incubated for 30 minutes in 0.1 M Tris-HCL, pH 7.5, containing 1.5% bovine serum albumin and 20% normal bovine serum, washed in PBS, and incubated with TUNEL reaction mixture at 37°C for one hour. After removing the TUNEL reaction mixture, sections were washed in PBS several times and counterstained with DAPI. The fluorescent pictures for different colors and different sections were taken under the same settings and then analyzed using Fiji ImageJ at the same setting to count the positive cell numbers.
Lysozyme staining for Paneth cells
Paraffin sections of the intestine were incubated with anti-lysozyme antibody (diluted 1:1000; Abcam) for one hour at room temperature, followed by washing steps with PBS, as described above for immunohistochemistry. The HRP/DAB (ABC) Detection kit (Abcam) was used to detect the signals by following the manufacturer's protocol. The brown Paneth cells in the crypt were then counted visually.
Alcian blue staining for goblet cells
Paraffin sections of the intestine were stained with an Alcian Blue Kit (Abcam) following the manufacturer's protocol. The blue goblet cells in the crypt and villus were counted visually or analyzed by using Fiji ImageJ to count the positive cell numbers.
In situ hybridization
In situ hybridization with a RNAscope 2.5 HD Reagent Kit-Brown (322371; Advanced Cell Diagnostics) was performed on 5 μm formalin-fixed, paraffin-embedded sections according to the manufacturer's instructions. The RNAscope probes used were LGR5 (NM_010195.2, target region 2165–3082, cat no. 312171), the negative control probe DapB (EF191515, region 414–862, cat no. 310043), and the positive control probe Ppib (NM_011149.2, target region 98–856, cat no. 313911). The LGR5 signal in crypt was quantified using Fiji ImageJ.
Statistical analysis
All statistical analyses and the graphs were performed and generated using GraphPad Prism v5.0 (GraphPad Software). Student's t-test was used to examine the differences between groups, and a p-value of <0.05 was considered statistically significant. For the analysis of intestinal cross-sections, individual cross-sections instead of individual animals were used as samples for the Student's t-test. All data are expressed as the mean ± standard error of the mean.
Results
Thra1PV/+ mice exhibit a gastrointestinal phenotype mimicking patients with mutations of the THRA gene
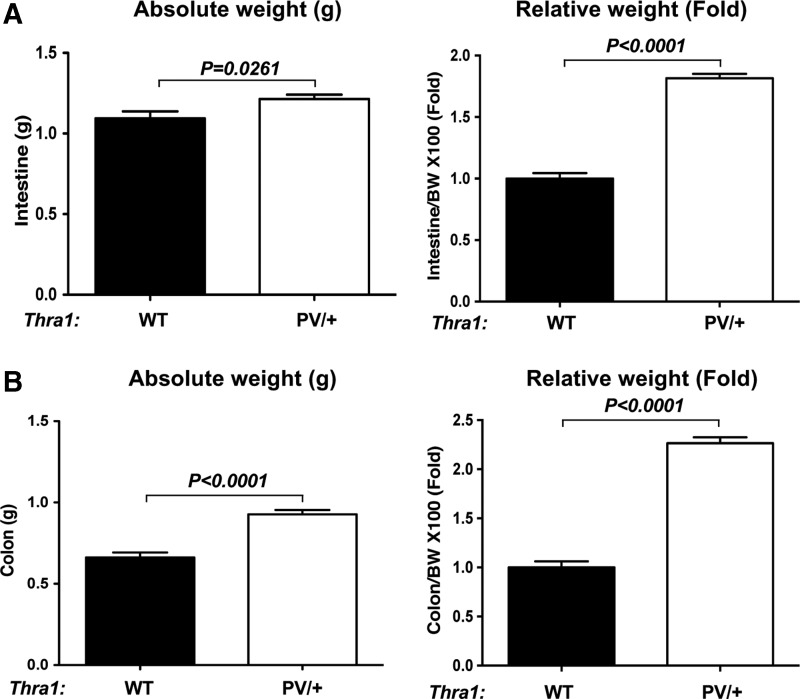
To determine whether adult Thra1PV/+ mice could serve as a model to study the gastrointestinal defects in RTHα patients, the intestinal phenotype of Thra1PV/+ mice was studied. First, the total weights of the small intestine and colon of adult wild-type and Thra1PV/+ mice were measured. The small intestine and colon of mutant mice were heavier than those of wild-type mice by ∼10% and ∼40%, respectively (Fig. 1A and B, two left panels). Since Thra1PV/+ mice are dwarfed (40), the small intestine and colon weights were normalized by the body weight. The two panels on the right in Figure 1 show more pronounced effects due to the mutation, that is, a weight increase of 1.8- and 2.3-fold, respectively, in the small intestine and colon after normalization with the respective body weight. In addition, there was only a relatively small (25%) increase in the normalized length of the mutant intestine (see below), and the mutant mice were lighter and had a smaller body size (data not shown). Thus, there was a net increase in the weight of the small intestine and colon, even if normalized against the length. These results indicate more contents had accumulated in the intestine and colon of Thra1PV/+ mice than in those of the wild-type mice. These findings suggest that similar to RTHα patients, adult Thra1PV/+ mice also have constipation.
FIG. 1.
Thra1PV/+ mice have constipation, mimicking resistance to thyroid hormone (RTHα) patients. The total weights in grams of the small intestine (A) and colon (B) of wild-type (WT) and Thra1PV mice were measured and plotted directly or after normalizing against the total body weight (BW). Note that there is more content retention (i.e., they are heavier) in both the small intestine and colon of the mutant mice, indicative of constipation. Mouse numbers: Thra1PV/+ mice, n = 33; WT mice, n = 12.
TRα1 mutation is associated with defects in the small intestine
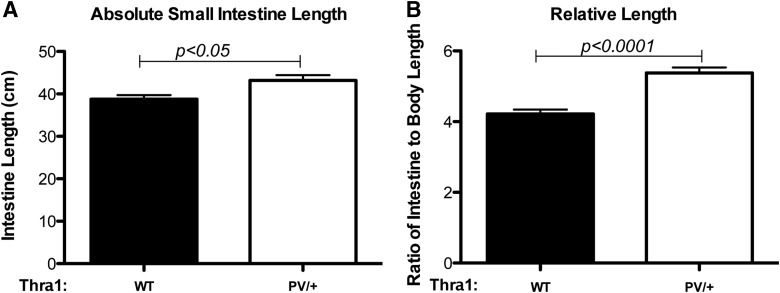
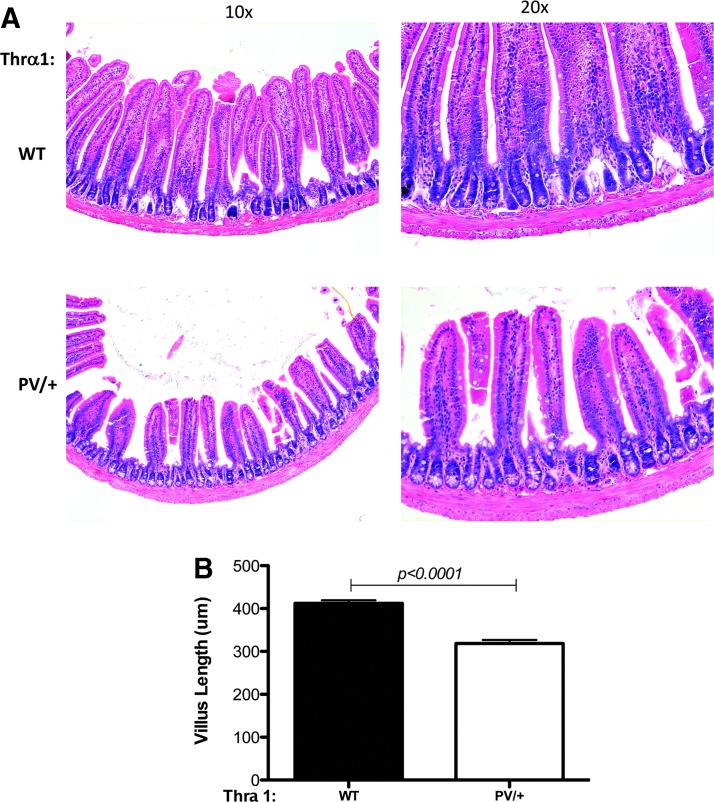
To characterize the changes in the adult intestine associated with TRα1 mutations further, the study focused on the intestine, where stem cell–mediated self-renewal has been well characterized. The total length of the small intestine was measured. As shown in Figure 2A, the total absolute length of the intestine shows a small but significant increase in Thra1PV/+ mice compared to that in wild-type siblings. Upon normalization with the body length, a more prominent increase (25%) was observed in the Thra1PV/+ mice (Fig. 2B). When the cross-section of the small intestine was analyzed, it was observed that the intestine in the mutant mice had apparently normal morphology compared to that in wild-type mice (Fig. 3A). In addition, no significant changes in the crypt width or length were observed. However, the length of the villi was reduced in the Thra1PV/+ mice by ∼20% (Fig. 3B), indicating that TRα1PV results in a decreased growth of the villi. In addition, in the large intestine or colon, which is devoid of villi, there was also a reduction in the length of the crypts in Thra1PV/+ mice (Supplementary Fig. S1).
FIG. 2.
Thra1PV/+ mice have longer small intestine relative to body length. The total length of the small intestine and body (from the tip of the head to the base of the tail after maximally stretching the mouse and scruff of the neck). The total intestine length (A) or the ratio of the intestine to body length (B) was plotted. Note that there is a significant increase in the ratio in the mutant mice. Mouse numbers: Thra1PV/+ mice, n = 8; WT mice, n = 8.
FIG. 3.
Reduction in the length of intestinal villus in Thra1PV/+ mice. Hematoxylin and eosin (H&E)-stained jejunum sections for WT and Thra1PV/+ mice (A). (B) The average length of the villus is reduced in the mutant animals. Mouse numbers: Thra1PV/+ mice, n = 5; WT mice, n = 5. Similar results were obtained with another batch of animals. There is also an increase in crypt width, although not statistically significant in another batch of animals (data not shown).
TRα1 mutation results in reduced cell proliferation in adult intestinal crypts
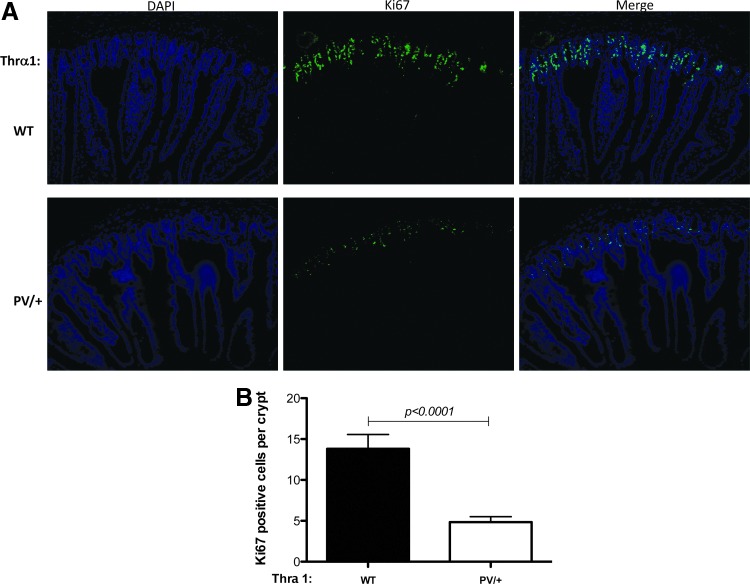
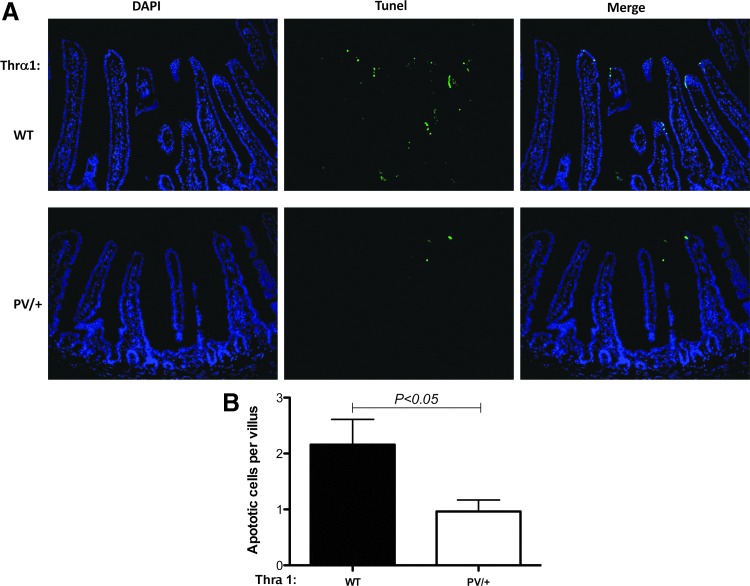
As the epithelium of the small intestine in adult mice undergoes constant self-renewal throughout adult life via cell proliferation in the crypts and cell death mainly in the villi, next the study investigated whether cell proliferation and cell death in the small intestine were affected by TRα1 mutations in adult mice. First, cell proliferation was analyzed by using immunofluorescence staining for Ki67, a marker for cell proliferation, on jejunum sections of wild-type and Thra1PV/+ mice. As shown in Figure 4A, Ki67 intensities were lower in the jejunum sections of Thra1PV/+ mice than in those of wild-type mice (middle lower panel vs. middle upper panel). Quantification indicates that more than a twofold reduction was observed in Ki67-positive cell numbers in the intestinal crypts of Thra1PV/+ mice compared to that of wild-type mice (Fig. 4B). A similar reduction in cell proliferation was also observed in the crypts of the large intestine of Thra1PV/+ mice (Supplementary Fig. S2). In addition, when cell death was analyzed by TUNEL assay on jejunum sections of wild-type and Thra1PV/+ mouse intestine, it was observed that the Thra1PV/+ mice have more than a twofold reduction in apoptotic cells in the intestinal villi (Fig. 5). Thus, the heterozygous mutation of the TRα1 affected both crypt cell proliferation and epithelial apoptosis in the adult mouse intestine.
FIG. 4.
Reduced cell proliferation in the intestinal crypts of Thra1PV/+ mice. (A) Immunofluorescent staining for Ki67 was performed on jejunum sections of WT and Thra1PV/+ mouse intestine. (B) Quantified data of Ki67-positive cells in the crypt showing reduced cell proliferation in the Thra1PV/+ mice. Mouse numbers: Thra1PV/+ mice, n = 5; WT mice, n = 5. Similar results were obtained in another batch of Thra1PV/+ mice and WT siblings.
FIG. 5.
Decreased cell death in the small intestinal epithelium of Thra1PV/+ mice. (A) Immunofluorescence staining of sections after terminal deoxynuclotidyltransferase-mediated dUTP nick-end labeling assay performed on jejunum sections of WT and Thra1PV/+ mouse intestine. (B) Quantification of apoptotic cells in the epithelium. Mouse numbers: Thra1PV/+ mice, n = 5; WT mice, n = 5. Similar results were obtained in another batch of Thra1PV/+ mice and WT siblings.
TRα1 mutation alters cell differentiation in the intestine
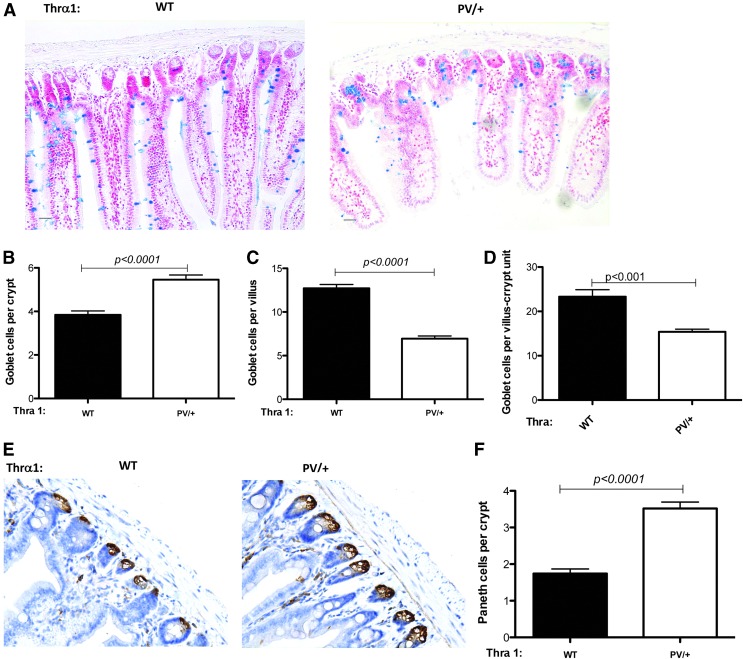
There are three major types of differentiated epithelial cells in the intestine: absorptive epithelial cells in the villus, goblet cells present in both villus and crypts, and Paneth cells in the crypts. In addition, the crypts harbor the adult stem cells, as well as the so-called transit amplifying cells, which rapidly proliferate to replace the dying differentiated epithelial cells mostly at the tip of the villus, thus maintaining intestinal epithelial homeostasis. To investigate why there are fewer proliferating cells despite the apparent normal crypt morphology, the study analyzed the abundance of two differentiated epithelial cells in the crypt: goblet cells and Paneth cells. Alcian blue staining for goblet cells in the jejunum sections revealed that the Thra1PV/+ mouse intestine has a significantly increased number of goblet cells in the crypt (Fig. 6A and B) but a reduced number of goblet cells (Fig. 6A and C) in the villus compared to the wild-type intestine. Since the villus is much larger than the crypt, not surprisingly the total number of goblet cells per villus–crypt unit (as measured by counting the total number of goblet cells in the jejunum sections and then dividing the total by the number of villi present in the sections) was reduced in the mutant animals (Fig. 6D). In the large intestine, which is devoid of any villi, a reduced number of goblet cells was observed in the crypts (Supplementary Fig. S3). Thus, the TRα1 mutation causes a reduction in goblet cells in the intestinal epithelium. In addition, immunohistochemical staining of lysozyme, a marker for Paneth cells in the crypt, showed that in the jejunum, there was a twofold increase in the number of Paneth cells in the crypt of Thra1PV/+ mice compared to the wild-type mice (Fig. 6E and F). Thus, despite of the apparent normal morphology of the crypt, the crypt had very different cell compositions between the wild-type and Thra1PV/+ mice. The data indicate that there is an increase in the differentiated cells and a corresponding reduction in the proliferating cells in the crypt of Thra1PV/+ mice.
FIG. 6.
Altered stem-cell differentiation in the small intestine of Thra1PV/+ mice. (A) Alcian blue staining for goblet cells was performed on jejunum sections of WT and Thra1PV/+ mouse intestine. (B–D) Quantification shows that the number of goblet cells is increased in the crypt (B) but decreased in the villus (C). (D) The total number of goblet cells per the villus–crypt unit (including the surround crypts) is also reduced (measured by counting the total number of goblet cells in the jejunum sections and then dividing the total by the number of villi present in the sections). (E) Immunohistochemical staining for lysozyme, a marker for Paneth cells in the crypt, was performed on jejunum sections of WT and Thra1PV/+ mouse intestine. (F) Quantification of the Paneth cell number in crypt reveals an increase in the mutant mouse intestine. Mouse numbers: Thra1PV/+mice, n = 5; WT mice, n = 5. Similar results were obtained with another batch of animals.
TRα1 mutation reduces stem cells in the intestine
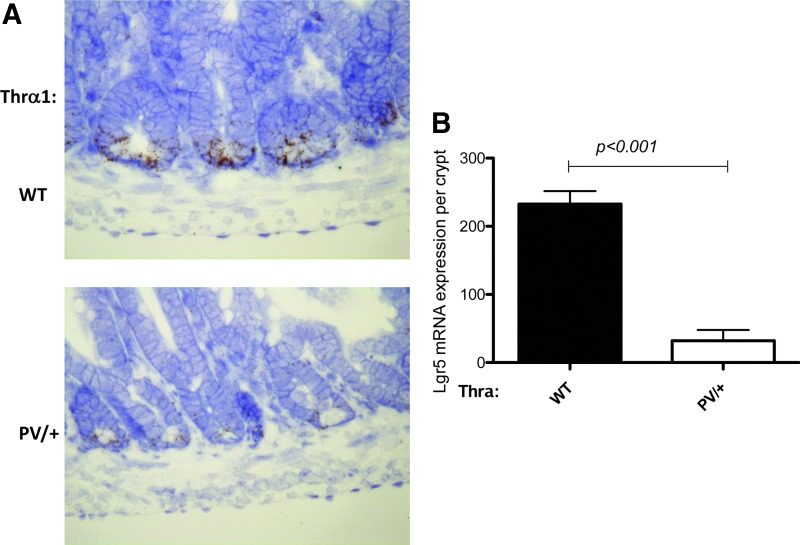
To investigate directly if the mutation affects intestinal stem cells, the expression of the well known adult intestinal stem-cell marker LGR5 was analyzed by in situ hybridization. As shown in Figure 7, LGR5-positive cells were detected, expectedly, near the crypt base in both wild-type and mutant intestines. Importantly, the LGR5 signal was drastically reduced in the Thra1PV/+ mice compared to wild-type animals, suggesting fewer adult stem cells in the mutant intestine.
FIG. 7.
Reduced Lgr5 expression in the intestinal crypts of Thra1PV/+ mice. (A) In situ hybridization for Lgr5 was performed on jejunum sections of WT and Thra1PV/+ mouse intestine. (B) Quantified data of Lgr5 expression in the crypt showing a reduced level in Thra1PV/+ mice. Mouse numbers: Thra1PV/+ mice, n = 3; WT mice, n = 4. Similar results were obtained in another batch of Thra1PV/+ mice and WT siblings.
Discussion
T3 is critical for human development and the function of many adult organs. Patients with TRα1 mutations represent the first genetic evidence for a role of T3 signaling in the function of the human intestine (21–24). On the other hand, it remains unknown whether TRα1 mutations cause intestinal abnormalities in humans other than constipation. Analyses of the intestine of Thra1PV/+ mice in the present study suggest that TRα1 mutations can alter intestinal epithelial cell differentiation and adult stem-cell levels.
Morphologically, the studies indicate that TRα1 mutations are associated with a longer small intestine relative to body size in the mouse. More importantly, Thra1PV/+ mice, mimicking RTHα patients, have drastically reduced cell proliferation in the crypt, indicative of reduced adult stem-cell number. Interestingly, epithelial cell death on the villus was also reduced, which helps to explain the relatively minor alterations in the crypt–villus structure, with only a relatively small reduction in villus length, despite the drastic reduction in stem-cell proliferation in the crypt. On the other hand, at the cellular level, the mutant mice have significantly more differentiated cells (Paneth and goblet cells) but fewer LGR5-positive stem cells in the crypt, leading to reduced cell proliferation. In addition, there are also fewer goblet cells in the villus. It remains unknown if the altered levels of the differentiated cells in the villus and the crypt affect intestinal physiology and function.
While further studies are needed to determine how the TRα1 mutation leads to the defects in the mature intestine and if there are additional alterations in the mature intestine, it is interesting to note that the first patient with a TRα1 mutation was a child who had severe constipation after weaning at seven months (21). This suggests that the TRα1 mutation may cause intestinal defects in human patients during intestinal maturation before and/or around birth when T3 levels are high. This observation and the present findings are consistent with earlier studies showing an important role of T3 signaling in the development of the vertebrate adult intestine. As indicated in the Introduction, altered T3 levels are associated with human intestinal abnormalities and diseases, such as inflammatory bowel diseases (32,38,39). In addition, T3 deficiency or TRα knockout in mice leads to abnormal intestinal morphology and a decrease in stem-cell proliferation in the adult (33–37), bearing similarities to what was observed here. Furthermore, increasing lines of evidence suggest that T3 controls the formation of the intestine during postembryonic development, a period around birth in mammals, and that this control is evolutionally conserved (45–47). One of the best-studied systems for adult intestinal development is perhaps the metamorphosis of amphibian Xenopus laevis (45,48). During this period, the simple tubular structure of the tadpole intestine is drastically remodeled to a complex adult intestine with a multiply folded intestinal epithelium surrounded by thick layers of connective tissue and muscles (48). This involves almost complete degeneration of the tadpole epithelium through apoptosis and de novo development of the adult intestinal stem cells in a T3-dependent process. It has been shown that T3 induces some larval epithelial cells to dedifferentiate to form the adult intestinal stem cells. A similar process may also take place in other vertebrates such as zebrafish and mice (45–47,49). In particular, in the mouse, the intestine at birth lacks any crypts, although villi are present. During the first three weeks after birth, the crypts are formed as the intestine matures with a distinct gene expression pattern and functional status compared to neonatal intestine as the T3 level rises, peaking around two to three weeks after birth (49–53). The resulting mature intestine has stem cells located in the crypts, while the villi contain the different types of differentiated epithelial and other cells. This suggests that the formation of adult intestinal stem cells takes place during this postembryonic developmental period and more importantly is regulated by T3 (49–52). Thus, it is very likely that in the mutant TRα mouse model, the defects that were observed in the adult intestine are likely due to the effect of TRα mutation on intestinal development.
Clearly, there are many important and interesting questions that need to be addressed. First, what is the mechanism for the observed defects in the adult intestine in the Thra1PV/+ mice? The adult phenotype could be due to the effect of the mutation on the homeostasis of the intestinal epithelium. Alternatively, the TRα1 mutation may affect normal development of the intestine as discussed above. Second, what causes the observed constipation in either human patients or adult mice? Possibilities include (i) reduced or altered epithelial absorption, (ii) mis-regulation of intestinal motility by the central nervous system, and (iii) defects in muscle function that affect intestinal motility. Given the broad expression profile of TRα in different tissues, one or more of these defects may exist in human patients or Thra1PV/+ mice. Third, despite the relatively normal intestinal villus–crypt structure, the reduced proliferation in the crypt and apoptosis on the villus suggest a slower replacement of the epithelial cells in the intestine. Thus, it is very likely TRα1 mutations will lead to reduced repair/regeneration but allow the maintenance of relatively normal intestinal morphology in humans. This would suggest that such patients will be prone to intestinal damage-related diseases. Additionally, it will also be interesting to determine if the altered levels of the differentiated cells in the villus and crypt caused by the TRα mutations affect intestinal physiology and function. Further analyses of this mouse model should provide valuable information toward prevention and treatment of intestinal disorders in TRα RTH patients.
Supplementary Material
Acknowledgments
We would like to thank Nga Luu for help. This work was supported in part by the Intramural Research Programs of National Institute of Child Health and Human Development and National Cancer Institutes, National Institutes of Health.
Author Disclosure Statement
The authors have nothing to disclose.
Supplementary Material
References
- 1. Hetzel BS 1989 The Story of Iodine Deficiency: An International Challenge in Nutrition. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 2. Guernsey DL, Edelman IS. 1983. Regulation of thermogenesis by thyroid hormones. In: Oppenheimer J, Samuels H. (eds) Molecular Basis of Thyroid Hormone Action. Academic Press, New York, NY, pp 293–324 [Google Scholar]
- 3. Franklyn JA, Gammage MD. 1996. Thyroid disease: effects on cardiovascular function. Trends Endocrinol Metab 7:50–54 [DOI] [PubMed] [Google Scholar]
- 4. Yen PM. 2001. Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- 5. Buchholz DR, Paul BD, Fu L, Shi YB. 2006. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol 145:1–19 [DOI] [PubMed] [Google Scholar]
- 6. Shi YB, Matsuura K, Fujimoto K, Wen L, Fu L. 2012. Thyroid hormone receptor actions on transcription in amphibia: the roles of histone modification and chromatin disruption. Cell Biosci 2:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsia SC, Wang H, Shi YB. 2001. Involvement of chromatin and histone acetylation in the regulation of HIV-LTR by thyroid hormone receptor. Cell Res 11:8–16 [DOI] [PubMed] [Google Scholar]
- 8. Wong J, Shi YB. 1995. Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J Biol Chem 270:18479–18483 [DOI] [PubMed] [Google Scholar]
- 9. Wong J, Shi YB, Wolffe AP. 1995. A role for nucleosome assembly in both silencing and activation of the Xenopus TR beta A gene by the thyroid hormone receptor. Genes Dev 9:2696–2711 [DOI] [PubMed] [Google Scholar]
- 10. Burke LJ, Baniahmad A. 2000. Co-repressors 2000. FASEB J 14:1876–1888 [DOI] [PubMed] [Google Scholar]
- 11. Jones PL, Shi Y-B. 2003 N-CoR-HDAC corepressor complexes: roles in transcriptional regulation by nuclear hormone receptors. In: Workman JL. (ed) Current Topics in Microbiology and Immunology: Protein Complexes that Modify Chromatin. Vol. 274. Springer-Verlag, Berlin, Germany, pp 237–268 [DOI] [PubMed] [Google Scholar]
- 12. Zhang J, Lazar MA. 2000. The mechanism of action of thyroid hormones. Annu Rev Physiol 62:439–466 [DOI] [PubMed] [Google Scholar]
- 13. Jones PL, Sachs LM, Rouse N, Wade PA, Shi YB. 2001. Multiple N-CoR complexes contain distinct histone deacetylases. J Biol Chem 276:8807–8811 [DOI] [PubMed] [Google Scholar]
- 14. McKenna NJ, O'Malley BW. 2001. Nuclear receptors, coregulators, ligands, and selective receptor modulators: making sense of the patchwork quilt. Ann N Y Acad Sci 949:3–5 [DOI] [PubMed] [Google Scholar]
- 15. Refetoff S, DeGroot LJ, Benard B, DeWind LT. 1972. Studies of a sibship with apparent hereditary resistance to the intracellular action of thyroid hormone. Metabolism 21:723–756 [DOI] [PubMed] [Google Scholar]
- 16. Refetoff S, DeWind LT, DeGroot LJ. 1967. Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab 27:279–294 [DOI] [PubMed] [Google Scholar]
- 17. Refetoff S, Weiss RE, Usala SJ. 1993. The syndromes of resistance to thyroid hormone. Endocrinol. Reviews 14:348–399 [DOI] [PubMed] [Google Scholar]
- 18. Collingwood TN, Adams M, Tone Y, Chatterjee VK. 1994. Spectrum of transcriptional, dimerization, and dominant negative properties of twenty different mutant thyroid hormone beta-receptors in thyroid hormone resistance syndrome. Mol Endocrinol 8:1262–1277 [DOI] [PubMed] [Google Scholar]
- 19. Porterfield SP, Hendrich CE. 1993. The role of thyroid hormones in prenatal and neonatal neurological development—current perspectives. Endocr Rev 14:94–106 [DOI] [PubMed] [Google Scholar]
- 20. Hsu JH, Brent GA. 1998. Thyroid hormone receptor gene knockouts. Trend Endocrinol Metab 9:103–112 [DOI] [PubMed] [Google Scholar]
- 21. Bochukova E, Schoenmakers N, Agostini M, Schoenmakers E, Rajanayagam O, Keogh JM, Henning E, Reinemund J, Gevers E, Sarri M, Downes K, Offiah A, Albanese A, Halsall D, Schwabe JW, Bain M, Lindley K, Muntoni F, Khadem FV, Dattani M, Farooqi IS, Gurnell M, Chatterjee K. 2012. A mutation in the thyroid hormone receptor alpha gene. N Engl J Med 366:243–249 [DOI] [PubMed] [Google Scholar]
- 22. Moran C, Chatterjee K. 2015. Resistance to thyroid hormone due to defective thyroid receptor alpha. Best Pract Res Clin Endocrinol Metab 29:647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Mullem A, van Heerebeek R, Chrysis D, Visser E, Medici M, Andrikoula M, Tsatsoulis A, Peeters R, Visser TJ. 2012. Clinical phenotype and mutant TRalpha1. N Engl J Med 366:1451–1453 [DOI] [PubMed] [Google Scholar]
- 24. van Mullem AA, Visser TJ, Peeters RP. 2014. Clinical consequences of mutations in thyroid hormone receptor-alpha1. Eur Thyroid J 3:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silva JE. 1995. Thyroid hormone control of thermogenesis and energy balance. Thyroid 5:481–492 [DOI] [PubMed] [Google Scholar]
- 26. Freake HC, Oppenheimer JH. 1995. Thermogenesis and thyroid function. Annu Rev Nutr 15:263–291 [DOI] [PubMed] [Google Scholar]
- 27. Dentice M, Marsili A, Ambrosio R, Guardiola O, Sibilio A, Paik JH, Minchiotti G, DePinho RA, Fenzi G, Larsen PR, Salvatore D. 2010. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J Clin Invest 120:4021–4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dentice M, Ambrosio R, Damiano V, Sibilio A, Luongo C, Guardiola O, Yennek S, Zordan P, Minchiotti G, Colao A, Marsili A, Brunelli S, Del Vecchio L, Larsen PR, Tajbakhsh S, Salvatore D. 2014. Intracellular inactivation of thyroid hormone is a survival mechanism for muscle stem cell proliferation and lineage progression. Cell Metab 20:1038–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Milanesi A, Lee J W, Kim NH, Liu YY, Yang A, Sedrakyan S, Kahng A, Cervantes V, Tripuraneni N, Cheng SY, Perin L, Brent GA. 2016. Thyroid hormone receptor alpha plays an essential role in male skeletal muscle myoblast proliferation, differentiation, and response to injury. Endocrinology 157:4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lemkine GF, Raj A, Alfama G, Turque N, Hassani Z, Alegria-Prevot O, Samarut J, Levi G, Demeneix BA. 2005. Adult neural stem cell cycling in vivo requires thyroid hormone and its alpha receptor. FASEB J 19:863–865 [DOI] [PubMed] [Google Scholar]
- 31. Lopez-Juarez A, Remaud S, Hassani Z, Jolivet P, Pierre Simons J, Sontag T, Yoshikawa K, Price J, Morvan-Dubois G, Demeneix BA. 2012. Thyroid hormone signaling acts as a neurogenic switch by repressing Sox2 in the adult neural stem cell niche. Cell Stem Cell 10:531–543 [DOI] [PubMed] [Google Scholar]
- 32. Yakut M, Üstün Y, Kabacan G, Soykan I. 2011. Thyroid disorders in patients with inflammatory bowel diseases. Int J Clin Med 2:89–92 [Google Scholar]
- 33. Plateroti M, Gauthier K, Domon-Dell C, Freund JN, Samarut J, Chassande O. 2001. Functional interference between thyroid hormone receptor alpha (TRalpha) and natural truncated TRDeltaalpha isoforms in the control of intestine development. Mol Cell Biol 21:4761–4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Flamant F, Poguet AL, Plateroti M, Chassande O, Gauthier K, Streichenberger N, Mansouri A, Samarut J. 2002. Congenital hypothyroid Pax8(−/−) mutant mice can be rescued by inactivating the TRalpha gene. Mol Endocrinol 16:24–32 [DOI] [PubMed] [Google Scholar]
- 35. Kress E, Rezza A, Nadjar J, Samarut J, Plateroti M. 2009. The frizzled-related sFRP2 gene is a target of thyroid hormone receptor alpha1 and activates beta-catenin signaling in mouse intestine. J Biol Chem 284:1234–1241 [DOI] [PubMed] [Google Scholar]
- 36. Plateroti M, Chassande O, Fraichard A, Gauthier K, Freund J N, Samarut J, Kedinger M. 1999. Involvement of T3Ralpha- and beta-receptor subtypes in mediation of T3 functions during postnatal murine intestinal development. Gastroenterology 116:1367–1378 [DOI] [PubMed] [Google Scholar]
- 37. Plateroti M, Kress E, Mori JI, Samarut J. 2006. Thyroid hormone receptor alpha1 directly controls transcription of the beta-catenin gene in intestinal epithelial cells. Mol Cell Biol 26:3204–3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ebert C. 2010. The thyroid and the gut. J Clin Gastroenterol 44:402–406 [DOI] [PubMed] [Google Scholar]
- 39. Maser C, Toset A, Roman S. 2006. Gastrointestinal manifestations of endocrine disease. World J Gastroenterol 12:3174–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaneshige M, Suzuki H, Kaneshige K, Cheng J, Wimbrow H, Barlow C, Willingham MC, Cheng S-Y. 2001. A targeted dominant negative mutation of the thyroid hormone alpha1 receptor causes increased mortality, infertility, and dwarfism in mice. PNAS 98:15095–15100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Mullem AA, Chrysis D, Eythimiadou A, Chroni E, Tsatsoulis A, de Rijke YB, Visser W E, Visser TJ, Peeters RP. 2013. Clinical phenotype of a new type of thyroid hormone resistance caused by a mutation of the TRalpha1 receptor: consequences of LT4 treatment. J Clin Endocrinol Metab 98:3029–3038 [DOI] [PubMed] [Google Scholar]
- 42. Han CR, Park S, Cheng SY. 2017. NCOR1 modulates erythroid disorders caused by mutations of thyroid hormone receptor alpha1. Sci Rep 7:18080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park S, Han CR, Park JW, Zhao L, Zhu X, Willingham M, Bodine DM, Cheng SY. 2017. Defective erythropoiesis caused by mutations of the thyroid hormone receptor alpha gene. PLoS Genet 13:e1006991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bassett JH, Boyde A, Zikmund T, Evans H, Croucher PI, Zhu X, Park JW, Cheng SY, Williams GR. 2014. Thyroid hormone receptor alpha mutation causes a severe and thyroxine-resistant skeletal dysplasia in female mice. Endocrinology 155:3699–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun G, Fu L, Shi Y-B. 2014. Epigenetic regulation of thyroid hormone-induced adult intestinal stem cell development during anuran metamorphosis. Cell Biosci 4:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun G, Roediger J, Shi YB. 2016. Thyroid hormone regulation of adult intestinal stem cells: implications on intestinal development and homeostasis. Rev Endocr Metab Disord 17:559–569 [DOI] [PubMed] [Google Scholar]
- 47. Ishizuya-Oka A, Shi YB. 2011. Evolutionary insights into postembryonic development of adult intestinal stem cells. Cell Biosci 1:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shi Y-B, Ishizuya-Oka A. 1996. Biphasic intestinal development in amphibians: embryogensis and remodeling during metamorphosis. Curr Top Dev Biol 32:205–235 [DOI] [PubMed] [Google Scholar]
- 49. Matsuda H, Shi YB. 2010. An essential and evolutionarily conserved role of protein arginine methyltransferase 1 for adult intestinal stem cells during postembryonic development. Stem Cells 28:2073–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sun G, Shi Y-B. 2012. Thyroid hormone regulation of adult intestinal stem cell development: mechanisms and evolutionary conservations. Int J Biol Sci 8:1217–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Muncan V, Heijmans J, Krasinski SD, Buller NV, Wildenberg ME, Meisner S, Radonjic M, Stapleton KA, Lamers WH, Biemond I, van den Bergh Weerman MA, O'Carroll D, Hardwick J C, Hommes DW, van den Brink GR. 2011. Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat Commun 2:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Harper J, Mould A, Andrews RM, Bikoff EK, Robertson EJ. 2011. The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc Natl Acad Sci U S A 108:10585–10590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Friedrichsen S, Christ S, Heuer H, Schäfer MKH, Mansouri A, Bauer K, Visser TJ. 2003. Regulation of iodothyronine deiodinases in the Pax8−/− mouse model of congenital hypothyroidism. Endocrinology 144:777–784 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.