Abstract
Background: Poorly differentiated thyroid cancer (PDTC) is a rare but clinically highly significant entity because it accounts for most fatalities from non-anaplastic follicular cell–derived thyroid cancer. Due to the relative rarity of the disease and heterogeneous diagnostic criteria, studies on PDTC have been limited. In light of the evolution of ultra-deep next-generation sequencing technologies and through correlation of clinicopathologic and genomic characteristics of PDTC, an improved understanding of the biology of PDTC has been facilitated. Here, the diagnostic criteria, clinicopathologic characteristics, management, and outcomes in PDTC, as well as genomic drivers in PDTC reported in recent next-generation sequencing studies, are reviewed. In addition, future prospects in improving the outcomes in PDTC patients are reviewed.
Summary: PDTC patients tend to present with adverse clinicopathologic characteristics: older age, male predominance, advanced locoregional disease, and distant metastases. Surgery with clearance of all gross disease can achieve satisfactory locoregional control. However, the majority of PDTC patients die of distant disease. Five-year disease-specific survival for PDTC patients has been reported at 66%. On multivariate analysis, reported predictors of poor survival in PDTC patients have been older age (>45 years), T4a pathological stage, extrathyroidal extension, high mitotic rate, tumor necrosis, and distant metastasis at presentation. BRAFV600E or RAS mutations (27% and 24% of cases, respectively) remain mutually exclusive main drivers in PDTC. TERT promoter mutations represent the most common alteration in PDTC (40%). Mutation in translation initiation factor EIF1AX (11%) and tumor suppressor TP53 (16%) have also been reported in PDTC. High rates of novel mutations (MED12 and RBM10) have been reported in fatal PDTC (15% and 12%, respectively). Chromosome 1q gains represent the most common arm-level alterations in PDTC, and those patients show worse survival rates. Chromosome 22q losses are also found in PDTC and show strong association with RAS mutation.
Conclusions: These new insights into the clinicopathologic and molecular characteristics of PDTC, together with further advancement in ultra-deep sequencing technologies, will be conducive in narrowing the focus in order to develop novel targeted therapies and improve the outcomes in PDTC patients.
Keywords: poorly differentiated, thyroid cancer, presentation, outcomes, next-generation sequencing
Introduction
Poorly differentiated thyroid carcinoma (PDTC) is a rare type of thyroid cancer, with a reported incidence from 2% to 15% of all thyroid cancers (1). The variation in incidence reflects geographic (environmental) influences or differences in histopathological interpretation (2). Its morphology and clinical behavior is generally considered intermediate on a tumor progression model of follicular cell–derived thyroid carcinomas, between differentiated thyroid cancer (DTC) and anaplastic thyroid cancer (ATC). PDTC represents the main cause of morbidity and mortality from non-anaplastic follicular cell–derived thyroid cancer (non-ANA FCDC) and is therefore clinically highly significant. Nevertheless, studies on PDTC have been limited due to its relative rarity and heterogeneity of inclusion criteria. Here, the evolution of PDTC as a separate diagnostic entity, its clinicopathologic characteristics, and its intermediate position on a tumor progression model of FCDCs, as well as management and outcomes in PDTC, are reviewed. Furthermore, new molecular insights into the genomic profile of PDTC and future perspectives in improving the prognosis of PDTC patients are reviewed.
Review
Definition of PDTC
A specific malignant epithelial tumor of the thyroid with strikingly nesting pattern was first described by Langhans in 1907 and named “wuchernde Struma” (proliferative goiter) (3). However, the term “poorly differentiated carcinoma of the thyroid” was introduced half a century later in 1963 by Granner et al. In the following decades, many PDTCs were categorized as conventional follicular carcinomas due to their ability to form follicles. In the early 1980s, Sakamoto et al. (4) and Carcangiu et al. (5) suggested that PDTC represented a separate entity with intermediate prognosis between DTC and ATC, respectively. However, it was not until 2004 that PDTC was introduced as a distinct diagnostic entity by the World Health Organization (WHO) Classification of Endocrine Tumors (6).
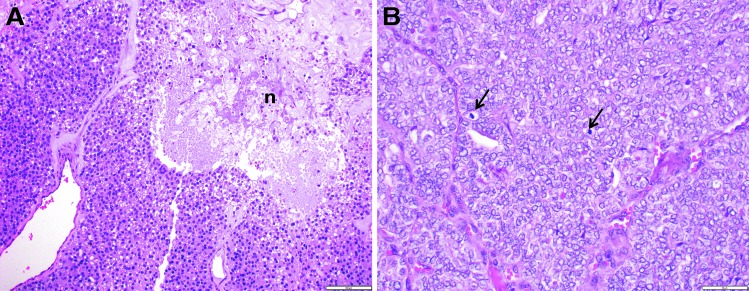
While there was universal agreement on the recognition of PDTC, pathologists differed on its histologic definition. Some relied on a solid/trabecular growth pattern alone (7), while the head and neck pathologists at Memorial Sloan Kettering Cancer Center (MSKCC) used high mitotic rate and/or tumor necrosis to diagnose this tumor (8). In 2006, an international group of pathologist gathered in Turin, Italy, and issued the following PDTC diagnostic criteria (Turin-PDTC): (i) solid/trabecular/insular pattern of growth, (ii) absence of conventional nuclear features of papillary carcinoma, and (iii) at least one of the following features: convoluted nuclei, mitotic activity ≥3/10 high power microscopic fields (HPF), and tumor necrosis (9) (Fig. 1A). The prevalence of solid, trabecular, and insular architecture shows distinctive geographical distribution (1), which may underestimate the diagnosis of PDTC in certain geographical areas. Furthermore, a solid pattern of growth (defined by Nikiforov et al. as solid/trabecular/insular pattern) (10) may not be representative of tumor aggressiveness in PDTC based on studies of solid variant of papillary thyroid carcinoma (PTC) (10). In agreement with this hypothesis, Hiltzik et al. showed that growth patterns (solid vs. follicular/papillary) did not correlate with overall survival (OS) in PDTC using multivariate analysis (8). On the other hand, proliferative grading (i.e., mitotic rate and tumor necrosis) was shown to categorize a more homogenous subset of thyroid carcinoma. Indeed, the most common cause of radioiodine (RAI)-refractory positron emission tomography–positive thyroid cancer was PDTC defined by mitosis and necrosis (11), while the same relationship has not been shown for other definitions of PDTC. The criteria for diagnosis of PDTC based on the proliferative grading proposed by MSKCC (MSKCC-PDTC) are: presence of follicular cell differentiation (on a routine microscopy and/or by immunohistochemistry, e.g., thyroglobulin positivity) with presence of ≥5 mitosis/10 HPF (400 × ) and/or fresh tumor necrosis (8) (Fig. 1B). The study of Gnemmi et al. validated the intermediate prognosis of PDTC diagnosed by MSKCC-PDTC and Turin-PDTC criteria (12). The most current WHO classification (13) adopted the Turin-PDTC criteria, but acknowledged that mitosis and tumor necrosis by themselves can also capture intermediate prognosis thyroid carcinomas. Although PDTC is by definition an intermediate prognosis thyroid carcinoma, the WHO does not recommend calling intermediate prognosis cancer PDTC who fit MSKCC but not Turin criteria (13). However, when MSKCC-PDTC criteria were used to differentiate between PDTC and other histotypes in the study on fatal non-ANA FCDC (14), all patients who died of the disease had at least one of four aggressive features: gross extrathyroidal extension (ETE), extensive vascular invasion, PDTC diagnosis in the primary tumor or the neck, or distant metastasis (DM) at presentation. On the other hand, when Turin-PDTC criteria were used to differentiate between PDTC and other histotypes in fatal non-ANA FCDC (14), some tumors were labeled as a variant of DTC with no other risk factors. This study showed that MSKCC-PDTC criteria can identify high-risk patients with diagnosis of PDTC and no other risk factors, who would otherwise escape high-risk classification. This results in a more aggressive initial treatment and closer follow-up of such patients.
FIG. 1.
Microscopic pictures of poorly differentiated thyroid carcinoma. (A) Solid/nested growth pattern with tumor necrosis, without nuclear features of papillary thyroid carcinoma (PTC). This tumor fulfills both the Turin and Memorial Sloan Kettering Cancer Center (MSKCC) criteria for the entity (n, tumor necrosis; 200 × ). (B) Solid/nested growth pattern with high mitotic rate (arrows indicate two mitotic figures) with nuclear features of PTC. Because of the nuclear appearance, the tumor does not satisfy the Turin criteria. However, it fulfills the MSKCC definition because of high mitotic rate (400 × ).
Clinical characteristics and management of PDTC
PDTC can occur de novo or as a result of progression from DTC. The intermediate position of PDTC between DTC and ATC is reflected in its clinicopathologic features. Indeed, significant progression has been found in tumor size, ETE, lymph node metastasis, and DM between DTC, PDTC, and ATC (15,16). The main clinical characteristics of PTDC and a comparison to DTC (17–22) and ATC (17,23–31) are presented in Table 1.
Table 1.
Main Clinical Characteristics of PDTC Compared to DTC and ATC
| DTC | PDTCa | ATC | |
|---|---|---|---|
| Origin | (i) Follicular thyroid cells (de novo) or (ii) follicular thyroid adenoma progression (follicular thyroid carcinoma) | (i) Follicular thyroid cells (de novo) or (ii) DTC progression | (i) Follicular thyroid cells (de novo) or (ii) DTC/PDTC progression |
| Prevalence | 90% of thyroid cancers (17) | 2–15% of thyroid cancers (1) | 2% of thyroid cancers (17) |
| Diagnostic criteria | Cytology and/or architecture | Turin: cytology, architecture, and proliferative grading (9); MSKCC: proliferative grading (8) | Cytology and/or architecture |
| Clinicopathologic characteristics | Median age 51 years (33), female-to-male ratio 3:1 (33) | Median age 59 years (32), female-to-male ratio 1.6:1 (32) | Median age 58–76 years (23), female-to-male ratio 1.8:1 (23) |
| 21% of patients present with extrathyroidal extension (18) | 69% of patients present with extrathyroidal extension (32) | >90% of patients present with locoregional invasion (24,28) | |
| 40–75% of patients develop metastases in neck lymph nodes (37) | 50–85% of patients develop metastases in neck lymph nodes (1,36) | 20–50% of patients present with distant metastases (24,28) | |
| 10–33% of patients develop distant metastases (38) | |||
| 85% of patients develop distant metastases (36) | |||
| Predictors of poor survival on multivariate analysis | Age (≥55 years) (21) male sex, tumor size, multicentricity, histologic grade and type, extrathyroidal extension, distant metastasis at presentation, and completeness of resection (19) | Age (>45 years) (7), T4a pathological stage (32), extrathyroidal extension (8,39), mitosis and necrosis (7), distant metastasis at presentation (32) | Age (>70 years) (30,31), acute symptoms (31), leukocytosis (31), T4b stage (31), tumor size >5 cm (31), distant metastasis at presentation (27,31), radiation dose <60 Gy (30); survival significantly improved if: age <60 years (27), tm confined to thyroid (27), combined surgery with EBRT (27), radical surgery versus palliative resection (29) |
| Management | Surgery (lobectomy or total thyroidectomy and central or lateral neck dissection if clinically or radiologically suspicious lymph nodes). Adjuvant RAI and thyrotropin suppression therapy. Adjuvant EBRT and systemic therapy for aggressive RAI-refractory DTC. Standardized guidelines for management of DTC involve the risk of death, risk of recurrence, and response to therapy stratification (20). | Surgeryb (total thyroidectomy and central or lateral neck dissection if clinically or radiologically suspicious lymph nodes). Adjuvant treatment benefits inconclusive. Standardized guidelines for management of PDTC are lacking. | Surgery (extended total thyroidectomy and central and lateral neck dissection) and/or radiochemotherapy. Standardized guidelines for management of ATC (25). |
| Outcomes | 10-year disease-specific survival >90% (18). Cause of disease-specific deaths: continuous trend away from local recurrence toward distant disease (22). | Intermediate prognosis between DTC and ATC (Level IV evidence) (1). Five-year overall survival 62–85% (7,32). Five-year disease-specific survival 66% (32,45). Distant disease represents the major cause of death (32). | Median survival five months, median one-year survival 20% (23). Uncontrollable locoregional disease with invasion of vital structures in the neck represents the major cause of death (29). |
| Most common molecular drivers mutations (predominant histological type) | BRAF (classical or tall-cell PTC) (51), RAS (follicular histotypes) (17), RTK fusions (PTC) (53), PAX8/PPARγ fusion (follicular histotypes) (53) | BRAF, RAS, TERT promoter (46) | BRAF, RAS, TERT promoter, TP53, PI3K/AKT, SWI/SNF, HMTs (46,52), CDKN2A (52) |
Initial surgery with clearance of all gross disease important for satisfactory locoregional control in PDTC.
PDTC, poorly differentiated thyroid carcinoma; DTC, differentiated thyroid carcinoma; ATC, anaplastic thyroid carcinoma; RAI, radioactive iodine; EBRT, external beam radiation therapy; PTC, papillary thyroid carcinoma; SWI/SNF, switch/sucrose non-fermentable; HMT, histone methyltransferases.
PDTC (Table 1) generally presents at an older age (median age of 59 years) (32) and with a higher male-to-female ratio (1:1.6) (32) than DTC (median age 51 years and male-to-female ratio of 1:3) (33). Older age and male sex have been reported as adverse prognostic factors in thyroid cancer (34), and their prevalence in PDTC patients indicates aggressive tumor biology. Furthermore, unlike DTC, PDTC tends to present with locally invasive extrathyroidal disease in more than half of patients (32,35). PDTC metastasizes to regional lymph nodes in up to 50–85% of cases (1,36) in comparison to 40–75% lymph node metastasis in DTC (37). However, DM are much more frequent in PDTC, occurring in up to 85% of cases (36), compared to 10–33% of cases in DTC (38).
Similar clinicopathologic characteristics were found to predict poor outcome in PDTC on multivariate analysis as those predictive in DTC. Age (>45 years) (7) and ETE (8,39) have been associated with significantly reduced OS in PDTC. Microscopic presence of necrosis and mitosis (>3/10 HPF) have also been associated with poor outcome in older patients (>45 years) with PDTC (7). A recent study of one of the largest cohorts of PDTC (91 patients) (32) confirmed previous reports and showed that PDTC patients who died of the disease when compared to those who survived were significantly older, presented with larger tumors (>4 cm), ETE, higher stage, and DM. pT4a and M1 stood as significant predictors of worse outcome on multivariate analysis (32).
Unlike DTC, treatment of PDTC has not been standardized due to the rarity of the disease and heterogeneity of inclusion criteria. Therapeutic decisions on PDTC have thus been mainly extrapolated from the treatment experience on DTC. Surgery is the treatment of choice for PDTC, whereby the extent of surgery is determined by preoperative assessment with appropriate imaging studies and intraoperative findings. Total thyroidectomy and clearance of all gross disease can achieve satisfactory locoregional control (five-year locoregional control in up to 81% of PDTC patients) (32). In addition, central and/or lateral neck dissection should be performed if there is clinical or radiological evidence of enlarged lymph nodes. Regarding adjuvant treatment, its indications and effectiveness in PDTC are not clear. When compared to DTC, PDTC does not usually respond as well to the same modalities of adjuvant treatment. RAI avidity of PDTC is variable (8), likely due to variation in tumor heterogeneity and variable admixtures of well and less well differentiated tumor components. Older age and ETE and/or extranodal extension are associated with aggressive biology and loss of RAI avidity in DTC (40). PDTC often presents at an older age and at an advanced stage (16), which are both manifestation of a more aggressive biology and thus can be accompanied by loss of RAI avidity. The role of postoperative external beam radiation therapy (EBRT) in PDTC is equally controversial. EBRT can be beneficial in patients with DTC who are at high risk for locoregional recurrence (41). Similar criteria could be applied to PDTC whereby EBRT has been recommended in PDTC patients with T3 and T4 tumors and in patients with neck node involvement (1). However, no significant survival improvement has been recorded in PDTC patients following EBRT (1,42). Although reports of EBRT benefits on local control of PDTC are inconclusive, it may still be considered in high-risk settings due to a low toxicity profile with intensity-modulated radiotherapy (IMRT). Regarding chemotherapy in PDTC, reports have been scarce. There is level III evidence (nonrandomized trials with contemporaneous controls according to Sackett et al.) (43) with short follow-up that patients with inoperable PDTC who received chemotherapy regimen with or without EBRT became operable or were free of disease (44).
Outcomes in PDTC
In a previous report from the authors' institution, PDTC was the most common cause for disease-specific death (DSS) in fatal non-ANA FCDC (14). PDTC shows a more aggressive course compared to DTC, regardless of focal or diffuse presence of PDTC (7), with a higher propensity for local recurrence (16). Along the progression spectrum of FCDC, there is level IV evidence that the prognosis of PDTC is intermediate between DTC and ATC (1) based on retrospective or cohort studies (according to the classification by Sackett et al.) (43). Indeed, PDTC holds an intermediate position on a progression scale from DTC to ATC in terms of five-year OS, DSS, and disease-free survival (DFS; Table 1) (15). PDTC is not as invariably lethal as ATC and has a 62–85% five-year OS (7,32) and a 66% five-year DSS (32,45). Recent reports show that satisfactory five-year locoregional control can be achieved in PDTC patients (five-year locoregional control in 81% of cases) (32) if all gross disease is cleared at initial surgery. Locoregional disease is the cause of death in only 18% of PDTC patients who die of thyroid cancer (in 21% of PDTC patients with gross ETE at presentation who die of thyroid cancer) (32). This is in contrast with older reports on DTC, where 36–47% of patients died due to uncontrollable locoregional disease (1). However, these data need to be interpreted in the proper context, since some of these patients may have had PDTC that had been inaccurately classified as DTC because PDTC was not diagnosed as a distinct entity until recently. Nevertheless, this improvement in locoregional control in patients with PDTC may reflect more comprehensive initial surgery and successful gross disease clearance achieving a R0 resection. However, distant control in PDTC is low (59% at five years) (32), with the most common metastatic sites being the lung and bone (1,32,36). Moreover, distant disease represents the major cause of death in PDTC, accounting for up to 85% of disease-related deaths (32). This is particularly important, since treatment modalities have not been successful in preventing the systemic spread of the disease, and therefore development of new targeted therapies is necessary in order to improve outcomes.
Molecular characteristics of PDTC in light of next-generation sequencing technologies
In an effort to elucidate the molecular characteristics of PDTC that are responsible for driving disease progression, several groups have published genomic findings in PDTC using next-generation sequencing (NGS) techniques (46–49). The study by Landa et al. (46) using the MSK-IMPACT NGS platform (50) has been the largest and the most comprehensive published study on PDTC to date. It examined the largest PDTC cohort (84 patients), with targeted sequencing of all exons and selected introns of 341 cancer-related genes. Studies by Gerber et al. (47), Nikiforova et al. (49), and Sykorova et al. (48) also examined relatively smaller numbers of PDTC patients with sequencing of select exons and hotspot regions of cancer-related genes (Table 2).
Table 2.
Studies Investigating Genomic Alterations in PDTC Using Next-Generation Sequencing
| No. of PDTC | Diagnostic criteria | Tissue source | DNA sequencing | Coverage depth | |
|---|---|---|---|---|---|
| Landa 2016 (46) | 84 | MSKCC and Turin | FFPE, FFT | Targeted exome sequencing of 341 cancer-related genes | 584 × |
| Gerber 2018 (47) | 23 | MSKCC and Turin | FFPE | Targeted exome sequencing of 48 cancer-related genes | 161 × |
| Nikiforova 2013 (49) | 10 | WHO (2004) | FFPE, FFT | Targeted exome sequencing of 12 cancer-related genes | 3753 × |
| Sykorova 2015 (48) | 3 | Turin | FFT | Targeted exome sequencing of 94 cancer-related genes | 338 × |
MSKCC, Memorial Sloan Kettering Cancer Center; WHO, World Health Organization; FFPE, formalin-fixed paraffin-embedded; FFT, fresh-frozen tissue.
NGS showed molecular-level evidence for the intermediate position of PDTC: mutation burden increased significantly from PTC (51) toward PDTC (46) and ATC (46,52) (median number of mutations: 1, 2, and 6, respectively) (46). In PDTC, the mutation burden showed prognostic value: it was significantly associated with aggressive clinicopathologic features (older age, larger tumors, and DM) and reduced OS (46).
Similar to PTC (51) and ATC (46), the most common driver mutations in PDTC (Table 3) are mutually exclusive mutations of BRAF and RAS. BRAFV600E is less frequent in PTDC (27%) compared to PTC (58%) (51) and ATC (45%) (46), while mutated RAS is more frequent in PTDC (24%) compared to PTC (13%) (51) but occurs with similar frequency in ATC (24–27%) (46,52). BRAF-mutated PDTCs showed significantly higher rates of regional nodal metastases, and RAS-mutated PDTCs showed significantly higher rates of distant metastases (46), consistent with the clinical behavior of BRAF- and RAS-mutated DTCs (53). BRAF-mutated PTCs show high MAPK output (due to unresponsiveness of BRAFV600E monomer to the negative feedback by ERK) and are less differentiated, while RAS-mutated PTCs show attenuated MAPK output (due to maintained negative feedback by ERK) and are highly differentiated (51). These phenomena were preserved in PDTC (46), as reflected in the preserved correlation of BRAF-RAS score (BRS) (51) and thyroid differentiation score (TDS) (51) with BRAF or RAS mutational status in PDTC. However, the correlation of BRS and TDS with BRAF or RAS mutational status was lost in ATC, probably due to accumulation of additional genomic complexity, in agreement with the progression model of thyroid carcinogenesis.
Table 3.
Common Somatic Mutations in PDTC Calculated Based on Combined Data from Four NGS Studiesa
| Mutations | % of tumors | Mutations | % of tumors |
|---|---|---|---|
| RAS-RAF-MAPK pathway | TERT promoter mutation | 40 | |
| BRAFV600E | 27 | Tumor suppressors | |
| RAS | 24 | TP53 | 16 |
| NRAS | 17 | ATM | 8 |
| KRAS | 3 | RB1 | 2 |
| HRAS | 4 | CHEK2 | 2 |
| PI3K-AKT-mTOR pathway | MEN1 | 1 | |
| PTEN | 4 | Other mutations | |
| PIK3CA | 3 | EIF1AX | 11 |
| AKT1 | 2 | ABL1 | 4 |
| AKT3 | 1 | GNAS | 3 |
| STAT pathway | HNF1A | 3 | |
| JAK3 | 3 | RECQL4 | 3 |
| RAS + PI3K pathway | IDH1 | 2 | |
| EGFR | 4 | STK11 | 2 |
| PDGFRA | 2 | TSHR | 2 |
| RAS + PI3K + STAT pathway | Histone methyltransferases | 7 | |
| FLT3 | 4 | SWI/SNF complex | 5 |
| MET | 2 | MMR genes | 2 |
| ALK | 1 | RBM10* | 12 |
| Wnt pathway | MED12* | 15 | |
| APC | 4 | ||
| CDH1 | 3 | ||
| CTNNB1 | 1 |
References (46–49).
Novel alterations in fatal PDTC (% of fatal PDTC).
NGS, next-generation sequencing.
TERT promoter mutations represent the most common alterations in PDTC (Table 3), with a stepwise increase from PTC (9%) (51) to PDTC (40%) and ATC (65–73%) (46,52). TERT promoter mutations were subclonal in PTC and clonal in PDTC and ATC (46), whereby clonality may indicate possible cell immortalization in advanced thyroid cancer. In PDTC, TERT promoter mutations were associated with an aggressive phenotype (significantly more distant metastases) and a trend toward greater mortality (46). In PDTC and ATC, a significant association between TERT promoter mutations and BRAF or RAS mutations was found (46). This is most likely due to de novo binding elements on the mutated TERT promoter for MAPK signaling-activated ETS family of transcription factors (54), causing TERT overexpression and induction of an immortalized phenotype.
The most frequently mutated tumor suppressor gene in PDTC is TP53 (16%; Table 3), albeit with significantly lower prevalence when compared to ATC (65–73%) (46,52). This is also in contrast with earlier reports, where TP53 was the most frequently mutated gene in PDTC (27%) (55). On the other hand, mutation of another tumor suppressor gene, ATM, shows similar prevalence in PDTC (8%) and ATC (9%) (46). Therefore, ATM mutation in PDTC may predict aggressive behavior before progression to ATC. ATM is responsible for cell-cycle checkpoint control and DNA repair, which is in agreement with a significantly higher mutation burden in ATM-mutant PDTC and ATC (46).
Mutations in genes encoding for members of PI3K/AKT/mTOR pathway occur with low frequency in PDTC (Table 3), and the reported prevalence in PDTC is significantly lower compared to ATC (11% vs. 39%) (46). Alterations in switch/sucrose non-fermentable (SWI/SNF) chromatin remodeling complexes and histone methyltransferases (HMTs) also show low prevalence in PDTC compared to ATC (46) (5% and 7% in PDTC and 36% and 24% in ATC, respectively). Due to their accumulation in ATC, PI3K/AKT/mTOR pathway mutations, TERT promoter mutations, TP53 mutations, mutations in SWI/SNF complexes, and HMTs may represent key genetic events distinguishing ATC and PDTC. Other infrequent mutations of pathways and genes that have been reported in PDTC are: JAK-STAT, WNT, DNA mismatch repair (MMR), EGFR, PDGFRA, FLT3, MET, ALK, ABL1, GNAS, HNF1A, RECQL4, IDH1, STK11, and TSHR (Table 3).
Several novel mutations have recently been reported in thyroid cancer. Mutation of translation initiation factor gene EIF1AX occurs with comparable frequencies in PDTC (11%) and in ATC (9%) (46) and rarely in PTC (1%) (51). Strong co-occurrence of mutated EIF1AX and RAS was present in PDTC and ATC (46), which was not found in The Cancer Genome Atlas (TCGA) PTC cohort (51). However, the impact of this co-occurrence is currently unclear. Nevertheless, EIF1AX mutations are predictive of worse survival in PDTC (46) and may therefore represent a useful marker for risk stratification in these tumors. Mutations of Mediator of RNA polymerase II transcription subunit 12 homolog (MED12) have not been found in TCGA PTC study (51). However, they have been reported with high frequency in PDTC patients who died of the disease (15%) (56) compared to ATC (3%) (46). Mutations of RNA Binding Motif Protein 10 (RBM10), which encodes for an RNA binding protein that participates in an alternative pre-mRNA splicing, may represent another novel tumor marker of aggressiveness. It is found in 12% of PDTC patients who died of the disease (56), in 3% of ATC (46), and in only 0.5% of PTC (51).
When fatal PDTC were compared to nonfatal PDTC (46,56), fatal PDTC showed a higher frequency of mutations in TERT promoter, MED12, RBM10, BRAF, HRAS, TP53, ATM, and EIF1AX, further establishing their role in tumor aggressiveness and progression.
Chromosomal rearrangements previously reported in thyroid cancer were found in 14% of PDTC by NGS, while they were absent in ATC (46). Rearrangements in PDTC included RET/PTC, PAX8/PPARγ, and fusions of ALK (46).
Landa et al. also used NGS to analyze the copy number alterations (CNA) in PDTC. 1q gain was one of the most common arm-level CNA in PDTC and was associated with worse survival rates (46). In addition, 22q losses were strongly associated with RAS-mutant PDTC (46). This can be explained by the transcriptional activation of the oncogenic and wild-type RAS by the loss of 22q tumor suppressor gene NF2 (51,57). Higher frequency of 1q gain and 22q loss was further confirmed in the study on fatal non-anaplastic thyroid cancer (FNAT; 1q gain in 26% and arm-level 22 loss in 14% of FNAT) (56). Therefore these CNA may represent important markers of thyroid cancer aggressiveness.
Future prospects
The main challenge in PDTC is the management of distant disease, which often does not respond to current treatment modalities and is the main cause of disease specific death (DSS) (32). Dramatically improved cost efficiency of genomic sequencing over the years has enabled a better insight into the biology of PDTC, which is necessary in order to develop novel targeted therapies. As opposed to sequencing of the most frequently altered regions, or mutational “hot spots,” there is a new rationale in sequencing of tumor samples for the most commonly mutated genes in cancer. They represent “actionable targets,” that is, genes that can either be targeted with drugs or provide clinically relevant information about the disease. The approach of detecting the most common mutations in cancer has been implemented in basket studies, which serve as clinical trials. In basket studies, targeted therapies are tested in patients whose tumors carry particular mutations, regardless of the tumor type or origin. Compared to traditional disease-specific trials, basket studies function as molecular allocation studies (58) and can provide more information on anticancer therapies while including a greater number of patients. Basket studies are particularly useful for rare tumors or tumors with rare mutations (58). Examples are low-prevalence mutations in thyroid cancer, such as rearrangements of RET, NTRK1, NTRK3, or ALK for example. These types of thyroid cancers could be included in trials with selective kinase inhibitors that have shown efficacy in other types of cancer.
So far, research on targeted therapy in thyroid cancer has been mostly focused on tyrosine kinase inhibitors (TKIs), which bind to one or multiple tyrosine receptor kinases (TRKs). The U.S. Food and Drug Administration (FDA) has approved sorafenib and lenvatinib for FCDC in cases of progressive, recurrent, or metastatic disease not responsive to RAI (59). Both sorafenib and lenvatinib act on multiple TRKs and inhibit tumor growth through antiangiogenic and antiproliferative mechanisms. Sorafenib and lenvatinib were approved after seminal multicenter Phase III studies (DECISION and SELECT, respectively) (60,61) that showed significantly improved progression-free survival (PFS) among patients with progressive RAI-refractory DTC. Compared to sorafenib, lenvatinib inhibits additional targets, such as fibroblast growth factor receptors (62), which may be effective in resistant cases. In addition, lenvatinib may also be effective as a second-line treatment in patients previously treated with TKIs (62), since lenvatinib was associated with significantly higher PFS in both naïve patients and those previously treated with TKIs compared to placebo (61). When comparing the percentage of dose reductions and interruptions due to adverse events, no major differences were found between lenvatinib and sorafenib (62). However, a higher number of deaths were recorded for lenvatinib compared to sorafenib use (60,61). This may be attributed to more advanced disease in patients involved in the lenvatinib SELECT versus sorafenib DECISION study (62). In addition, dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) have recently been approved for inoperable locally invasive or metastatic ATC (63). In the future, PDTC patients should be included in clinical trials on ATC, since therapies effective in ATC may also prove effective for aggressive PDTC due to their similar clinical course. The studies on re-differentiation (restoration of RAI uptake) through inhibition of MAPK signaling have also been of particular interest. Selumetinib (a MEK inhibitor) restored RAI uptake successfully in a subset of patients with metastatic RAI-refractory thyroid cancer (64). Dabrafenib (BRAF inhibitor) has achieved similar effects in another study (65). A recent re-differentiation study, conducted on patient-derived tumor tissue from three ATC and two PDTC patients, explored the effects of the histone deacetylase inhibitor panobinostat and the TKIs sorafenib and selumetinib (66). All tissues showed a strong significant overexpression of sodium–iodide symporter (NIS) transcripts after treatment with panobinostat, while only the tissue from one PDTC patient showed an upregulation of NIS after treatment with sorafenib, selumetinib, or panobinostat (66). RAI uptake was subsequently restored in tissues from three patients (two ATC and one PDTC) but not in a PDTC sample, despite transcriptional NIS upregulation after the treatment with any of the three compounds (66). In order to overcome the resistance to TKIs, the paradigm of treatment is shifting toward targeting of multiple pathways simultaneously. In addition, such therapy may also produce a synergistic effect. Indeed, dual inhibition of the MAPK and mTOR pathways resulted in a strong inhibitory synergism in thyroid cancer cell lines, including those from ATC (67). In terms of functional restoration, simultaneous inhibition of MAPK, PI3K/AKT, and histone deacetylase pathways could increase iodide uptake and RAI avidity due to re-expression of genes responsible for iodide incorporation, which could be additionally enhanced with co-treatment with thyrotropin (68). Another treatment strategy that could overcome drug resistance and/or improve drug efficacy is to combine targeted drugs with traditional modes of therapy such as chemotherapy or radiotherapy. An example is the combination of the dual inhibitor of PI3K/mTOR (BEZ235) with paclitaxel, which showed synergistic effects in vitro compared to the effect of single-agent treatment (69).
In addition to TRKs, other drivers of thyroid cancer progression are being considered for targeted therapy. The proteasome inhibitor bortezomib prevents inhibitory-kappa B degradation and thereby blocks constitutive activation of the NF-κB pathway and cell proliferation (17). Furthermore, cancer adaptation to hypoxia and angiogenesis through the transcription factor hypoxia-inducible factor 1-alpha (HIF1α) has also been the subject of targeted therapy research. In addition to hypoxia, PI3K/AKT and MAPK signaling pathways also upregulate HIF1α (17). The TKI cabozantinib may be the most promising in targeting angiogenesis, since it blocks both HIF1α and VEGFR signaling (70). Since it is found in cancer and not in normal tissues, HIF1α may represent a precision medicine paradigm for further research focused on drivers uniquely present in cancer tissues. Other antiangiogenic agents are also being tested, such as microtubule-depolymerizing agent combretastatin A-4 phosphate (CA4P), which acts on tumor vasculature function and induces ischemic necrosis (70). Furthermore, antiangiogenic agents could increase the efficacy of conventional therapies (chemotherapy, radiotherapy, or RAI treatment) (71). Clinical trials on modulators of growth, apoptosis, or other novel targets are currently pending.
In addition, new potential targets are emerging as a result of the comprehensive genomic profiling of advanced thyroid tumors such as PDTC. With the high incidence of TERT promoter mutations, there has been a renewed interest in TERT-based immunotherapy. Mutations of the TERT promoter lead to TERT-protein overexpression, which could increase TERT-antigen presentation by cancer cells and increase susceptibility to T-cell recognition and attack (72). Another actionable alteration is the STRN/ALK rearrangement, which could be targeted by ALK inhibitors such as crizotinib and TAE864 (73). Mutations of MED12 represent a promising novel target due to the significant role of MED12 in cell transcription as part of the kinase module in the mediator complex. So far, sorafenib, a multi tyrosine kinase inhibitor/CDK8 inhibitor, and Senexin A, a CDK8/19 inhibitor (74), have been used to target the kinase module of the mediator complex. Novel therapeutic strategies at the level of transcription may also target mutated RBM10. Promising results have been reported from a Phase I trial of the spliceosome inhibitor E7107 in patients with advanced solid tumors unresponsive to standard therapies (75). Splicing factor inhibitors may thus prove useful in patients with RBM10 mutations.
Continuous evolution of sequencing technologies will address current limitations in our understanding of tumor biology. As opposed to “bulk sequencing methods,” introduction of single-cell sequencing (76) has the potential to overcome the challenges of tumor genetic heterogeneity and aid in targeting the “driver” as well as resistant clones. In addition, detection of subclonal genomic alterations associated with aggressive behavior in otherwise differentiated tumors may become instrumental in the prevention of thyroid cancer progression. Future technological development might facilitate convergence of single-cell sequencing with third-generation single molecule technologies (77) without the need for amplification, which will further improve cost-efficiency and accuracy of sequencing. Indeed, introduction of cost-effective genomic sequencing into routine clinical practice will possibly evolve into dynamic genomic profiling during the course of treatment and follow-up. This will likely become the standard of care as we continue to advance our insights into the biology of the disease.
Summary and Conclusions
PDTC is rare but clinically highly significant, since it accounts for majority of deaths from non-ANA FCDC. Diagnostic criteria based on proliferative grading (8) may be able to detect PDTC more accurately. Patients with PDTC tend to present with clinicopathologic characteristics associated with adverse prognosis in thyroid cancer. Initial surgery with clearance of all gross disease is the hallmark for tumor control and can achieve satisfactory locoregional control. However, DSS is low (66%) (32,45), and treatment failure is caused by distant metastases in the majority of cases (32). Benefits of adjuvant therapy are inconclusive. Adjuvant RAI may be considered in patients with RAI-avid disease and IMRT in patients with gross residual locoregional disease or at high risk of recurrence. Age (>45 years) (7), ETE (8), necrosis and mitosis in patients >45 years (7), and pT4a and M1 (32) have been associated with significantly reduced survival in PDTC patients on multivariate analysis. The most common mutations in PDTC are TERT promoter mutations (40%), and the most commonly mutated tumor suppressor gene is TP53 (16%). However, mutually exclusive mutations of BRAF and RAS continue to be the most common driver mutations in PDTC, similar to DTC and ATC. Furthermore, mutations of EIF1AX, MED12, and RBM10 have been recently reported in PDTC and may be predictive of poor survival. Fatal PDTC compared to nonfatal PDTC (46,56) showed a higher frequency of mutations in the TERT promoter, MED12, RBM10, BRAF, HRAS, TP53, ATM, and EIF1AX. With further advancement of sequencing technologies and correlation of molecular profile with clinicopathologic characteristics, novel precision therapies will continue to develop, with the ultimate goal of reducing morbidity and mortality in PDTC patients.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Sanders EM, Jr, LiVolsi VA, Brierley J, Shin J, Randolph GW. 2007. An evidence-based review of poorly differentiated thyroid cancer. World J Surg 31:934–945 [DOI] [PubMed] [Google Scholar]
- 2. Asioli S, Erickson LA, Righi A, Jin L, Volante M, Jenkins S, Papotti M, Bussolati G, Lloyd RV. 2010. Poorly differentiated carcinoma of the thyroid: validation of the Turin proposal and analysis of IMP3 expression. Mod Pathol 23:1269–1278 [DOI] [PubMed] [Google Scholar]
- 3. Langhans T. 1907. Über die epithelialen Formen der malignen Struma. Virchows Arch 189:69–188 [Google Scholar]
- 4. Sakamoto A, Kasai N, Sugano H. 1983. Poorly differentiated carcinoma of the thyroid. A clinicopathologic entity for a high-risk group of papillary and follicular carcinomas. Cancer 52:1849–1855 [DOI] [PubMed] [Google Scholar]
- 5. Carcangiu ML, Zampi G, Rosai J. 1984. Poorly differentiated (“insular”) thyroid carcinoma. A reinterpretation of Langhans' “wuchernde Struma.” Am J Surg Pathol 8:655–668 [DOI] [PubMed] [Google Scholar]
- 6. DeLellis RA, Lloyd RV, Heitz PU, Eng C (eds) 2004. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Endocrine Organs. IARC Press, Lyon, France [Google Scholar]
- 7. Volante M, Landolfi S, Chiusa L, Palestini N, Motta M, Codegone A, Torchio B, Papotti MG. 2004. Poorly differentiated carcinomas of the thyroid with trabecular, insular, and solid patterns: a clinicopathologic study of 183 patients. Cancer 100:950–957 [DOI] [PubMed] [Google Scholar]
- 8. Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, Shah JP, Singh B, Ghossein RA. 2006. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer 106:1286–1295 [DOI] [PubMed] [Google Scholar]
- 9. Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Katoh R, Lloyd RV, LiVolsi VA, Papotti M, Sobrinho-Simoes M, Bussolati G, Rosai J. 2007. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol 31:1256–1264 [DOI] [PubMed] [Google Scholar]
- 10. Nikiforov YE, Erickson LA, Nikiforova MN, Caudill CM, Lloyd RV. 2001. Solid variant of papillary thyroid carcinoma: incidence, clinical-pathologic characteristics, molecular analysis, and biologic behavior. Am J Surg Pathol 25:1478–1484 [DOI] [PubMed] [Google Scholar]
- 11. Rivera M, Ghossein RA, Schoder H, Gomez D, Larson SM, Tuttle RM. 2008. Histopathologic characterization of radioactive iodine-refractory fluorodeoxyglucose-positron emission tomography-positive thyroid carcinoma. Cancer 113:48–56 [DOI] [PubMed] [Google Scholar]
- 12. Gnemmi V, Renaud F, Do Cao C, Salleron J, Lion G, Wemeau JL, Copin MC, Carnaille B, Leteurtre E, Pattou F, Aubert S. 2014. Poorly differentiated thyroid carcinomas: application of the Turin proposal provides prognostic results similar to those from the assessment of high-grade features. Histopathology 64:263–273 [DOI] [PubMed] [Google Scholar]
- 13. Lloyd RV, Osamura RY, Klöppel G, Rosai J. 2017. WHO Classification of Tumours of Endocrine Organs WHO/IARC Classification of Tumours. IARC Publications, Lyon, France [Google Scholar]
- 14. Xu B, Ibrahimpasic T, Wang L, Sabra MM, Migliacci JC, Tuttle RM, Ganly I, Ghossein R. 2016. Clinicopathologic features of fatal non-anaplastic follicular cell-derived thyroid carcinomas. Thyroid 26:1588–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wreesmann VB, Ghossein RA, Patel SG, Harris CP, Schnaser EA, Shaha AR, Tuttle RM, Shah JP, Rao PH, Singh B. 2002. Genome-wide appraisal of thyroid cancer progression. Am J Pathol 161:1549–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nishida T, Katayama S, Tsujimoto M, Nakamura J, Matsuda H. 1999. Clinicopathological significance of poorly differentiated thyroid carcinoma. Am J Surg Pathol 23:205–211 [DOI] [PubMed] [Google Scholar]
- 17. Xing M. 2013. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 13:184–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nixon IJ, Ganly I, Patel SG, Palmer FL, Whitcher MM, Ghossein R, Michael Tuttle R, Shaha AR, Shah JP. 2012. Changing trends in well differentiated thyroid carcinoma over eight decades. Int J Surg 10:618–623 [DOI] [PubMed] [Google Scholar]
- 19. Dean DS, Hay ID. 2000. Prognostic indicators in differentiated thyroid carcinoma. Cancer Control 7:229–239 [DOI] [PubMed] [Google Scholar]
- 20. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nixon IJ, Wang LY, Migliacci JC, Eskander A, Campbell MJ, Aniss A, Morris L, Vaisman F, Corbo R, Momesso D, Vaisman M, Carvalho A, Learoyd D, Leslie WD, Nason RW, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, Gonen M, Pathak KA, Shen WT, Sywak M, Kowalski L, Freeman J, Perrier N, Shah JP. 2016. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid 26:373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nixon IJ, Ganly I, Palmer FL, Whitcher MM, Patel SG, Tuttle RM, Shaha AR, Shah JP. 2011. Disease-related death in patients who were considered free of macroscopic disease after initial treatment of well-differentiated thyroid carcinoma. Thyroid 21:501–504 [DOI] [PubMed] [Google Scholar]
- 23. Smallridge RC, Copland JA. 2010. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol 22:486–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, van Heerden JA, Goellner JR. 2001. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery 130:1028–1034 [DOI] [PubMed] [Google Scholar]
- 25. Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal MS, Shah MH, Shaha AR, Tuttle RM. 2012. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 22:1104–1139 [DOI] [PubMed] [Google Scholar]
- 26. Sugitani I, Miyauchi A, Sugino K, Okamoto T, Yoshida A, Suzuki S. 2012. Prognostic factors and treatment outcomes for anaplastic thyroid carcinoma: ATC Research Consortium of Japan cohort study of 677 patients. World J Surg 36:1247–1254 [DOI] [PubMed] [Google Scholar]
- 27. Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. 2005. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer 103:1330–1335 [DOI] [PubMed] [Google Scholar]
- 28. O'Neill JP, Shaha AR. 2013. Anaplastic thyroid cancer. Oral Oncol 49:702–706 [DOI] [PubMed] [Google Scholar]
- 29. Ursino S, Fiorica F, Stefanelli A, Pedriali M, Colosimo C, Cocuzza P, Mazzotti V, Taibi R, Cartei F, Greco C. 2014. Anaplastic thyroid cancer: a case report of a long term survival patient and review of literature data. Eur Rev Med Pharmacol Sci 18:1368–1372 [PubMed] [Google Scholar]
- 30. Sherman EJ, Lim SH, Ho AL, Ghossein RA, Fury MG, Shaha AR, Rivera M, Lin O, Wolden S, Lee NY, Pfister DG. 2011. Concurrent doxorubicin and radiotherapy for anaplastic thyroid cancer: a critical re-evaluation including uniform pathologic review. Radiother Oncol 101:425–430 [DOI] [PubMed] [Google Scholar]
- 31. Sugitani I, Onoda N, Ito KI, Suzuki S. 2018. Management of anaplastic thyroid carcinoma: the fruits from the ATC Research Consortium of Japan. J Nippon Med Sch 85:18–27 [DOI] [PubMed] [Google Scholar]
- 32. Ibrahimpasic T, Ghossein R, Carlson DL, Nixon I, Palmer FL, Shaha AR, Patel SG, Tuttle RM, Shah JP, Ganly I. 2014. Outcomes in patients with poorly differentiated thyroid carcinoma. J Clin Endocrinol Metab 99:1245–1252 [DOI] [PubMed] [Google Scholar]
- 33. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. SEER Stat Fact Sheets: Thyroid Cancer. Available at: https://seer.cancer.gov/statfacts/html/thyro.html (accessed July31, 2018)
- 34. Shaha AR, Loree TR, Shah JP. 1994. Intermediate-risk group for differentiated carcinoma of thyroid. Surgery 116:1036–1040; discussion 1040–1031. [PubMed] [Google Scholar]
- 35. Nikiforov YE, Biddinger PW, Thompson LD. 2009. Diagnostic Pathology and Molecular Genetics of the Thyroid. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 36. Chao TC, Lin JD, Chen MF. 2004. Insular carcinoma: infrequent subtype of thyroid cancer with aggressive clinical course. World J Surg 28:393–396 [DOI] [PubMed] [Google Scholar]
- 37. Shaha AR. 1998. Management of the neck in thyroid cancer. Otolaryngol Clin North Am 31:823–831 [DOI] [PubMed] [Google Scholar]
- 38. Shaha AR, Ferlito A, Rinaldo A. 2001. Distant metastases from thyroid and parathyroid cancer. ORL J Otorhinolaryngol Relat Spec 63:243–249 [DOI] [PubMed] [Google Scholar]
- 39. de la Fouchardiere C, Decaussin-Petrucci M, Berthiller J, Descotes F, Lopez J, Lifante JC, Peix JL, Giraudet AL, Delahaye A, Masson S, Bournaud-Salinas C, Borson Chazot F. 2018. Predictive factors of outcome in poorly differentiated thyroid carcinomas. Eur J Cancer 92:40–47 [DOI] [PubMed] [Google Scholar]
- 40. Vassilopoulou-Sellin R, Schultz PN, Haynie TP. 1996. Clinical outcome of patients with papillary thyroid carcinoma who have recurrence after initial radioactive iodine therapy. Cancer 78:493–501 [DOI] [PubMed] [Google Scholar]
- 41. Brierley J, Tsang R, Panzarella T, Bana N. 2005. Prognostic factors and the effect of treatment with radioactive iodine and external beam radiation on patients with differentiated thyroid cancer seen at a single institution over 40 years. Clin Endocrinol (Oxf) 63:418–427 [DOI] [PubMed] [Google Scholar]
- 42. Walczyk A, Kowalska A, Sygut J. 2010. The clinical course of poorly differentiated thyroid carcinoma (insular carcinoma)—own observations. Endokrynol Pol 61:467–473 [PubMed] [Google Scholar]
- 43. Sackett DL. 1989. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest 95:2s–4s [PubMed] [Google Scholar]
- 44. Auersperg M, Us-Krasovec M, Petric G, Pogacnik A, Besic N. 1990. Results of combined modality treatment in poorly differentiated and anaplastic thyroid carcinoma. Wien Klin Wochenschr 102:267–270 [PubMed] [Google Scholar]
- 45. Lee DY, Won JK, Lee SH, Park DJ, Jung KC, Sung MW, Wu HG, Kim KH, Park YJ, Hah JH. 2016. Changes of clinicopathologic characteristics and survival outcomes of anaplastic and poorly differentiated thyroid carcinoma. Thyroid 26:404–413 [DOI] [PubMed] [Google Scholar]
- 46. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. 2016. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 126:1052–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gerber TS, Schad A, Hartmann N, Springer E, Zechner U, Musholt TJ. 2018. Targeted next-generation sequencing of cancer genes in poorly differentiated thyroid cancer. Endocr Connect 7:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sykorova V, Dvorakova S, Vcelak J, Vaclavikova E, Halkova T, Kodetova D, Lastuvka P, Betka J, Vlcek P, Reboun M, Katra R, Bendlova B. 2015. Search for new genetic biomarkers in poorly differentiated and anaplastic thyroid carcinomas using next generation sequencing. Anticancer Res 35:2029–2036 [PubMed] [Google Scholar]
- 49. Nikiforova MN, Wald AI, Roy S, Durso MB, Nikiforov YE. 2013. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab 98:E1852–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, Chandramohan R, Liu ZY, Won HH, Scott SN, Brannon AR, O'Reilly C, Sadowska J, Casanova J, Yannes A, Hechtman JF, Yao J, Song W, Ross DS, Oultache A, Dogan S, Borsu L, Hameed M, Nafa K, Arcila ME, Ladanyi M, Berger MF. 2015. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 17:251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cancer Genome Atlas Research Network 2014. Integrated genomic characterization of papillary thyroid carcinoma. Cell 159:676–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pozdeyev N, Gay LM, Sokol ES, Hartmaier R, Deaver KE, Davis S, French JD, Borre PV, LaBarbera DV, Tan AC, Schweppe RE, Fishbein L, Ross JS, Haugen BR, Bowles DW. 2018. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res 24:3059–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fagin JA, Wells SA., Jr 2016. Biologic and clinical perspectives on thyroid cancer. New Engl J Med 375:1054–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bell RJ, Rube HT, Kreig A, Mancini A, Fouse SD, Nagarajan RP, Choi S, Hong C, He D, Pekmezci M, Wiencke JK, Wrensch MR, Chang SM, Walsh KM, Myong S, Song JS, Costello JF. 2015. Cancer. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science 348:1036–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pita JM, Figueiredo IF, Moura MM, Leite V, Cavaco BM. 2014. Cell cycle deregulation and TP53 and RAS mutations are major events in poorly differentiated and undifferentiated thyroid carcinomas. J Clin Endocrinol Metab 99:E497–507 [DOI] [PubMed] [Google Scholar]
- 56. Ibrahimpasic T, Xu B, Landa I, Dogan S, Middha S, Seshan V, Deraje Vasudeva S, Carlson D, Migliacci J, Knauf JA, Untch BR, Berger MF, Morris LG, Tuttle RM, Chan TA, Fagin JA, Ghossein R, Ganly I. 2017. Genomic alterations in fatal forms of non-anaplastic thyroid cancer: Identification of MED12 and RBM10 as novel thyroid cancer genes associated with tumor virulence. Clin Cancer Res 23:5970–5980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Garcia-Rendueles ME, Ricarte-Filho JC, Untch BR, Landa I, Knauf JA, Voza F, Smith VE, Ganly I, Taylor BS, Persaud Y, Oler G, Fang Y, Jhanwar SC, Viale A, Heguy A, Huberman KH, Giancotti F, Ghossein R, Fagin JA. 2015. NF2 loss promotes oncogenic RAS-induced thyroid cancers via YAP-dependent transactivation of RAS proteins and sensitizes them to MEK inhibition. Cancer Discov 5:1178–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hyman DM, Solit DB, Arcila ME, Cheng DT, Sabbatini P, Baselga J, Berger MF, Ladanyi M. 2015. Precision medicine at Memorial Sloan Kettering Cancer Center: clinical next-generation sequencing enabling next-generation targeted therapy trials. Drug Discov Today 20:1422–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. National Cancer Institute. Drugs Approved for Thyroid Cancer. Available at: https://www.cancer.gov/about-cancer/treatment/drugs/thyroid (accessed July31, 2018)
- 60. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Pena C, Molnar I, Schlumberger MJ, investigators D. 2014. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, Phase 3 trial. Lancet 384:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. 2015. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. New Engl J Med 372:621–630 [DOI] [PubMed] [Google Scholar]
- 62. Lorusso L, Pieruzzi L, Biagini A, Sabini E, Valerio L, Giani C, Passannanti P, Pontillo-Contillo B, Battaglia V, Mazzeo S, Molinaro E, Elisei R. 2016. Lenvatinib and other tyrosine kinase inhibitors for the treatment of radioiodine refractory, advanced, and progressive thyroid cancer. OncoTargets Ther 9:6467–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. U.S. Food and Drug Administration. FDA News Release. FDA approves new uses for two drugs administered together for the treatment of BRAF-positive anaplastic thyroid cancer. Available at: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm606686.htm (accessed September2, 2018)
- 64. Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, Pentlow KS, Zanzonico PB, Haque S, Gavane S, Ghossein RA, Ricarte-Filho JC, Dominguez JM, Shen R, Tuttle RM, Larson SM, Fagin JA. 2013. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. New Engl J Med 368:623–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. 2015. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res 21:1028–1035 [DOI] [PubMed] [Google Scholar]
- 66. Wachter S, Wunderlich A, Roth S, Mintziras I, Maurer E, Hoffmann S, Verburg FA, Fellinger SA, Holzer K, Bartsch DK, Di Fazio P. 2018. Individualised multimodal treatment strategies for anaplastic and poorly differentiated thyroid cancer. J Clin Med 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu D, Xing J, Trink B, Xing M. 2010. BRAF mutation-selective inhibition of thyroid cancer cells by the novel MEK inhibitor RDEA119 and genetic-potentiated synergism with the mTOR inhibitor temsirolimus. Int J Cancer 127:2965–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hou P, Bojdani E, Xing M. 2010. Induction of thyroid gene expression and radioiodine uptake in thyroid cancer cells by targeting major signaling pathways. J Clin Endocrinol Metab 95:820–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lin SF, Huang YY, Lin JD, Chou TC, Hsueh C, Wong RJ. 2012. Utility of a PI3K/mTOR inhibitor (NVP-BEZ235) for thyroid cancer therapy. PLoS One 7:e46726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Viola D, Valerio L, Molinaro E, Agate L, Bottici V, Biagini A, Lorusso L, Cappagli V, Pieruzzi L, Giani C, Sabini E, Passannati P, Puleo L, Matrone A, Pontillo-Contillo B, Battaglia V, Mazzeo S, Vitti P, Elisei R. 2016. Treatment of advanced thyroid cancer with targeted therapies: ten years of experience. Endocr Relat Cancer 23:R185–205 [DOI] [PubMed] [Google Scholar]
- 71. O'Reilly MS. 2006. Radiation combined with antiangiogenic and antivascular agents. Semin Radiat Oncol 16:45–50 [DOI] [PubMed] [Google Scholar]
- 72. Zanetti M. 2016. A second chance for telomerase reverse transcriptase in anticancer immunotherapy. Nat Rev Clin Oncol 14:115–128 [DOI] [PubMed] [Google Scholar]
- 73. Kelly LM, Barila G, Liu P, Evdokimova VN, Trivedi S, Panebianco F, Gandhi M, Carty SE, Hodak SP, Luo J, Dacic S, Yu YP, Nikiforova MN, Ferris RL, Altschuler DL, Nikiforov YE. 2014. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci U S A 111:4233–4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Clark AD, Oldenbroek M, Boyer TG. 2015. Mediator kinase module and human tumorigenesis. Crit Rev Biochem Mol Biol 50:393–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Eskens FA, Ramos FJ, Burger H, O'Brien JP, Piera A, de Jonge MJ, Mizui Y, Wiemer EA, Carreras MJ, Baselga J, Tabernero J. 2013. Phase I pharmacokinetic and pharmacodynamic study of the first-in-class spliceosome inhibitor E7107 in patients with advanced solid tumors. Clin Cancer Res 19:6296–6304 [DOI] [PubMed] [Google Scholar]
- 76. Navin N, Hicks J. 2011. Future medical applications of single-cell sequencing in cancer. Genome Med 3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schadt EE, Turner S, Kasarskis A. 2010. A window into third-generation sequencing. Hum Mol Genet 19:R227–240 [DOI] [PubMed] [Google Scholar]