Abstract
Background
Thromboxane A2 (TXA2) induces platelet aggregation and promotes thrombus formation. Although ginsenoside Ro (G-Ro) from Panax ginseng is known to exhibit a Ca2+-antagonistic antiplatelet effect, whether it inhibits Ca2+-dependent cytosolic phospholipase A2 (cPLA2α) activity to prevent the release of arachidonic acid (AA), a TXA2 precursor, is unknown. In this study, we attempted to identify the mechanism underlying G-Ro-mediated TXA2 inhibition.
Methods
We investigated whether G-Ro attenuates TXA2 production and its associated molecules, such as cyclooxygenase-1 (COX-1), TXA2 synthase (TXAS), cPLA2α, mitogen-activated protein kinases, and AA. To assay COX-1 and TXAS, we used microsomal fraction of platelets.
Results
G-Ro reduced TXA2 production by inhibiting AA release. It acted by decreasing the phosphorylation of cPLA2α, p38-mitogen-activated protein kinase, and c-Jun N-terminal kinase1, rather than by inhibiting COX-1 and TXAS in thrombin-activated human platelets.
Conclusion
G-Ro inhibits AA release to attenuate TXA2 production, which may counteract TXA2-associated thrombosis.
Keywords: Arachidonic acid, Cytosolic phospholipase A2α, Ginsenoside Ro, Mitogen-activated protein kinases, Thromboxane A2
1. Introduction
Platelets are activated via breakdown of phosphatidylinositol 4,5-bisphosphate in the plasma membrane (PM) to inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DG) by phospholipase C (PLC) [1]. IP3 mobilizes Ca2+ from the endoplasmic reticulum to the cytoplasm, and Ca2+ activates Ca2+/calmodulin-dependent protein kinase [2]. Apart from phosphorylating pleckstrin by binding to protein kinase C, DG acts as a donor of arachidonic acid (AA) [3], a precursor of thromboxane A2 (TXA2) [4]. TXA2 is an autacoid produced from AA by the actions of cyclooxygenase-1 (COX-1) and TXA2 synthase (TXAS) and initiates thrombogenesis [5], [6], [7]. Antithrombotic drugs, such as aspirin, imidazole, and indomethacin, block TXA2 production by inhibiting COX-1 or TXAS activity [8].
Mitogen-activated protein kinases (MAPKs), such as extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38-MAPK, are phosphorylated in thrombin-activated human platelets [9], [10], [11]. Phosphorylated p38-MAPK and ERK2 induce TXA2 production [12], [13], [14]. Moreover, the phosphorylation of p38-MAPK is essential for the activation of cytosolic phospholipase A2α (cPLA2α), leading to AA release [14].
Thrombin elevates the intracellular Ca2+ level, leading to the translocation of cPLA2α from the cytosol to the PM. Subsequently, p38-MAPK activates cPLA2α by phosphorylating it at Ser505 [15]. Therefore, it may be beneficial to evaluate the antiplatelet potential of a compound on TXA2 production in relation to phosphorylation of MAPKs.
Roots of Panax (P) ginseng are used in traditional Oriental medicine. In a previous study, we reported that total saponin from Korean Red Ginseng inhibits both COX-1 and TXAS to reduce the production of TXA2 [16]; however, its individual components have not yet been evaluated. Therefore, we evaluated the effects of ginsenoside Ro (G-Ro), an oleanane-type saponin (Fig. 1) in P. ginseng, on the production of TXA2 along with its associated enzymes and signaling molecules.
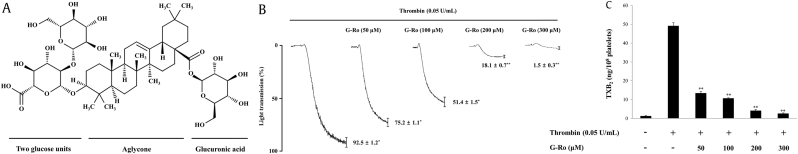
Fig. 1.
Effects of G-Ro on thrombin-induced human platelet aggregation and thromboxane B2 production. (A) Structure of G-Ro. (B) Effect of G-Ro on thrombin-induced human platelet aggregation. (C) Effect of G-Ro on thromboxane B2 production. Platelet aggregation and thromboxane B2 production were carried out as described in “Materials and methods” section. The data are expressed as the mean ± standard deviation (n = 4). ∗p < 0.05 versus the thrombin-stimulated human platelets, ∗∗p < 0.01 versus the thrombin-stimulated human platelets.
TXB2, thromboxane B2.
2. Materials and methods
2.1. Materials
G-Ro was obtained from Ambo Institute (Daejon, Korea). Thrombin was obtained from Chrono-Log Corporation (Havertown, PA, USA). Fura 2-AM was obtained from Invitrogen Molecular Probes (Eugene, OR, USA). Aspirin was obtained from Sigma Chemical Corporation (St. Louis, MO, USA). Thromboxane B2 (TXB2) enzyme immunoassay (EIA) kit, COX-1 fluorescence activity assay kit, ozagrel, and prostaglandin H2 were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Anti-phosphor-cPLA2 (Ser505), anti-phosphor-p38-MAPK, anti-phosphor-JNK (1/2), anti-p38-MAPK, anti-JNK (1/2), anti-COX-1, anti-TXAS, anti-rabbit IgG-horseradish peroxidase conjugate, and lysis buffer were obtained from Cell Signaling Technology (Beverly, MA, USA). PD98059, SB203580, SP600125, and anti-β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Polyvinylidene difluoride membrane and enhanced chemiluminescence solution were purchased from GE Healthcare (Piscataway, NJ, USA). Human AA EIA kit was obtained from Cusabio (Wuhan, Hubei, China).
2.2. Preparation of washed human platelets
Human platelet-rich plasma with acid–citrate–dextrose solution (0.8% citric acid, 2.2% sodium citrate, 2.45% glucose) was procured from Korean Red Cross Blood Center (Changwon, Korea). It was centrifuged for 10 min at 1,300×g to obtain the platelet pellets. The platelets were washed twice using a washing buffer (138 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.36 mM NaH2PO4, 5.5 mM glucose, and 1 mM Na2EDTA, pH 6.5) and resuspended in a suspension buffer (138 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.36 mM NaH2PO4, 0.49 mM MgCl2, 5.5 mM glucose, and 0.25% gelatin, pH 6.9) to a final concentration of 5 × 108 cells/mL. All the aforementioned procedures were performed at 25°C to preserve platelet activity. These experiments were approved (PIRB12-072) by the National Institute for Bioethics Policy Public Institutional Review Board (Seoul, Korea).
2.3. Determination of platelet aggregation
Platelets (108 cells/mL) were preincubated, with or without G-Ro, in a CaCl2 (2 mM) solution for 3 min at 37°C. They were stimulated with thrombin (0.05 U/mL) and allowed to aggregate for 5 min in an aggregometer (Chrono-Log Corporation). Platelet aggregation rate was determined as an increase in light transmission. G-Ro was dissolved in the platelet suspension buffer (pH 6.9), and MAPK inhibitors were dissolved in 0.1% dimethyl sulfoxide.
2.4. Western blot analysis of COX-1 and TXAS, and phosphorylation of p38-MAPK, JNK1/2, and cPLA2α
Platelet aggregation was terminated by adding an equal volume (250 μL) of lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM Na2EDTA, 1 mM ethylene glycol tetraacetic acid (EGTA), 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM ATPase, 1 mM Na3VO4, 1 μg/mL leupeptin, and 1 mM phenylmethanesulfonyl fluoride, pH 7.5). Protein content in the platelet lysate was measured using a bicinchoninic acid protein assay kit (Pierce Biotechnology, IL, USA). COX-1 and TXAS were analyzed by Western blotting after separating equal amounts of total protein (30 μg) in the lysate, microsomal, and cytosol fractions of platelets via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (8%, 1.5 mm). Phosphorylation of p38-MAPK, JNK1/2, and cPLA2α was evaluated by Western blotting after separating 15 μg of total protein by SDS-PAGE (6%, 1.5 mm). A Polyvinylidene difluoride membrane was used for protein transfer. The primary and secondary antibodies were diluted 1:1,000 and 1:10,000, respectively. The membranes were visualized using an enhanced chemiluminescence solution solution. The degrees of phosphorylation were analyzed using the Quantity One 1-D analysis software, Version. 4.5 (Bio-Rad, Hercules, CA, USA).
2.5. Measurement of TXB2
Because TXA2 is unstable and gets converted spontaneously to TXB2, it was quantified by determining the TXB2 content [4]. After platelet aggregation, the reaction was terminated by adding ice-cold EDTA (5 mM) and indomethacin (0.2 mM) to prevent the metabolism of AA to TXA2. The amount of TXB2, a stable metabolite of TXA2, was determined using a TXB2 EIA kit according to the procedure described by the manufacturer.
2.6. Isolation of microsomal fraction
Washed platelets (108 cells/mL), suspended in a buffer (pH 7.4) with 1% protease inhibitor, were sonicated 10 times at 100% sensitivity for 20 s on ice (Bandelin, HD2070, Germany) to obtain the platelet lysate. The microsomal fraction, containing endoplasmic reticulum membrane, was obtained by ultracentrifugation at 105,000×g for 1 h at 4°C [16].
2.7. AA release
The reaction was terminated after platelet aggregation, and the aggregates were centrifuged at 200×g at 4°C for 10 min. AA in the supernatant was quantified using an AA EIA kit (Cusabio), and the absorbance was measured at 450 nm using a Synergy HT multi-mode microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
2.8. COX-1 activity assay
The microsomal fraction of platelets was preincubated with aspirin (500 μM), a positive control, or with various concentrations of G-Ro and other reagents at 37°C for 30 min. COX-1 activity was assayed with a COX-1 fluorescence assay kit (Cayman Chemical Co).
2.9. TXAS activity assay
The microsomal fraction of platelets was preincubated with ozagrel (11 nM, IC50), a positive control, or with various concentrations of G-Ro and other reagents at 37°C for 5 min. The reaction was initiated by adding prostaglandin H2, and the samples were incubated at 37°C for 1 min; the reaction was terminated by adding citric acid (1 M). After neutralization with 1 N NaOH, the amount of TXB2 was determined using a TXB2 EIA kit according to the procedure described by manufacturer.
2.10. Statistical analyses
All experimental results are indicated as the mean ± standard deviation accompanied by the number of trials. Significant differences were determined by analysis of variance followed by the Newman–Keuls multiple comparisons method. All statistical analyses were conducted using the SPSS 21.0.0.0 software (SPSS, Chicago, IL, USA). A p value < 0.05 was considered to be statistically significant.
3. Results
3.1. Effects of G-Ro on platelet aggregation
We used thrombin at a dose of 0.05 U/mL, which induces maximum human platelet aggregation [17] to stimulate the platelets in this study. Thrombin increased platelet aggregation up to 92.5 ± 1.2%. However, G-Ro reduced the thrombin-induced platelet aggregation in a dose-dependent manner (Fig. 1B).
3.2. Effects of G-Ro on TXA2 production
We determined whether G-Ro reduced platelet aggregation by inhibiting TXA2 production (by measuring the TXB2 level). As shown in Fig. 1C, thrombin increased TXB2 level (49.2 ± 1.6 ng/108 platelets), whereas G-Ro dose-dependently (50–300 μM) reduced the TXB2 level that was induced by thrombin; G-Ro (300 μM) inhibited the thrombin-mediated elevation in TXB2 level by 94.9%.
3.3. Effects of G-Ro on activities of COX-1 and TXAS
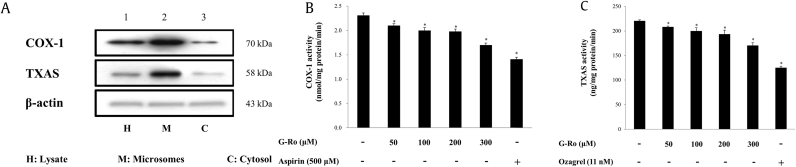
We evaluated the activities of COX-1 (70 kDa) and TXAS (58 kDa) in the microsomal fraction to investigate whether they contributed to the reduction in TXB2 by G-Ro (Fig. 2A, lane 2). COX-1 activity in the absence of G-Ro (negative control) was 2.3 ± 0.1 nmol/mg protein. However, G-Ro dose-dependently (50–300 μM) reduced its activity (Fig. 2B); at 300 μM, COX-1 activity was reduced by 26.4% of that of the negative control. TXAS activity in the absence of G-Ro (negative control) was 220.8 ± 1.8 ng/mg protein/min. However, G-Ro dose-dependently (50–300 μM) reduced its activity (Fig. 2C); at 300 μM, TXAS activity was reduced by 22.9% of that of the negative control. We observed that G-Ro (300 μM) reduced COX-1 (26.4%) and TXAS (22.9%) activities to similar extents.
Fig. 2.
Effects of G-Ro on COX-1 and TXAS activities. (A) Determination of the effects of the enzyme sources on COX-1 and TXAS activities. (B) Determination of the effects of G-Ro on COX-1. (C) Determination of the effects of G-Ro on TXAS activities. Western blot analysis and COX-1 and TXAS activities were determined as described in “Materials and methods” section. The data are expressed as the mean ± standard deviation (n = 4). ∗p < 0.05 versus the thrombin-stimulated human platelets.
COX-1, cyclooxygenase-1; TXAS, thromboxane A2 synthase.
3.4. Effects of G-Ro on cPLA2α phosphorylation and AA release
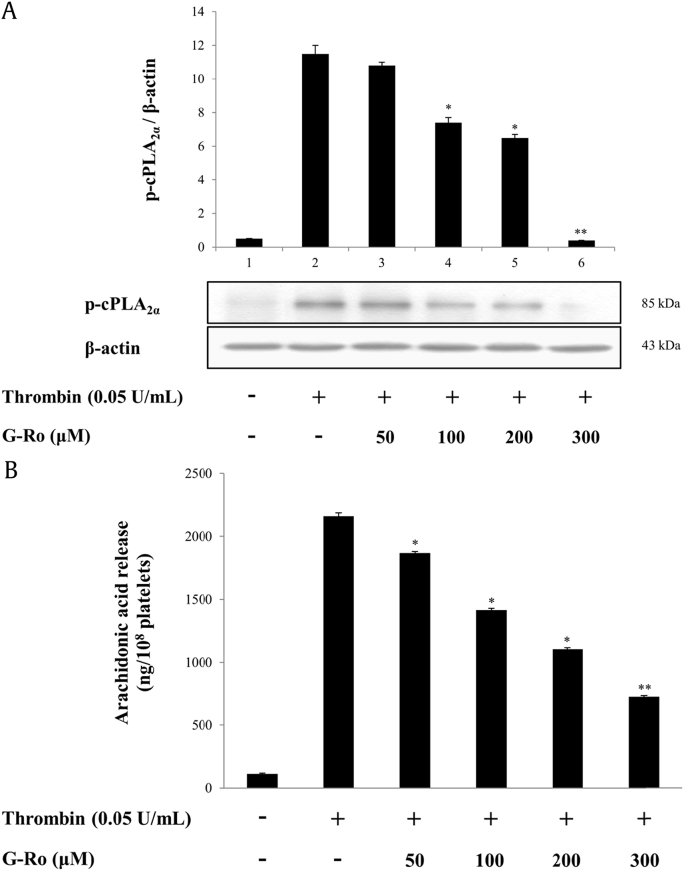
The inhibitory effect of G-Ro (300 μM) on TXB2 production (94.9%, Fig. 1C) was significantly higher than those on COX-1 (26.4%, Fig. 2B) and TXAS (22.9%, Fig. 2C) activities. This suggested that G-Ro might also inhibit AA release, a precursor of TXA2, from PM phospholipids to reduce TXA2 production in thrombin-activated platelets.
Because Ca2+-dependent cPLA2α is activated by phosphorylation [18] and releases AA from PM phospholipids in thrombin-activated human platelets [10], we investigated the effect of G-Ro on the phosphorylation of cPLA2α. As shown in Fig. 3A, G-Ro inhibited the thrombin-mediated phosphorylation of cPLA2α (Ser505) in a dose-dependent manner as it is reported that cPLA2α is activated by phosphorylation of cPLA2α at Ser505 [18], [19]. At 300 μM, G-Ro inhibited the thrombin-induced cPLA2α (Ser505) phosphorylation by 96.5% (Fig. 3A). Moreover, it reduced the thrombin-induced AA release in a dose-dependent manner (Fig. 3B); at 300 μM, it inhibited AA release by 61.1% of that induced by thrombin (2159.2 ± 29.0 ng/108 platelets).
Fig. 3.
Effects of G-Ro on cPLA2α-phosphorylation and AA release. (A) Effects of G-Ro on cPLA2-phosphorylation. (B) Effects of G-Ro on AA release. Western blot and AA release assay were determined as described in “Materials and methods” section. The data are expressed as the mean ± standard deviation (n = 3). ∗p < 0.05 versus the thrombin-stimulated human platelets, ∗∗p < 0.01 versus the thrombin-stimulated human platelets.
AA, arachidonic acid; cPLA2, cytosolic phospholipase A2.
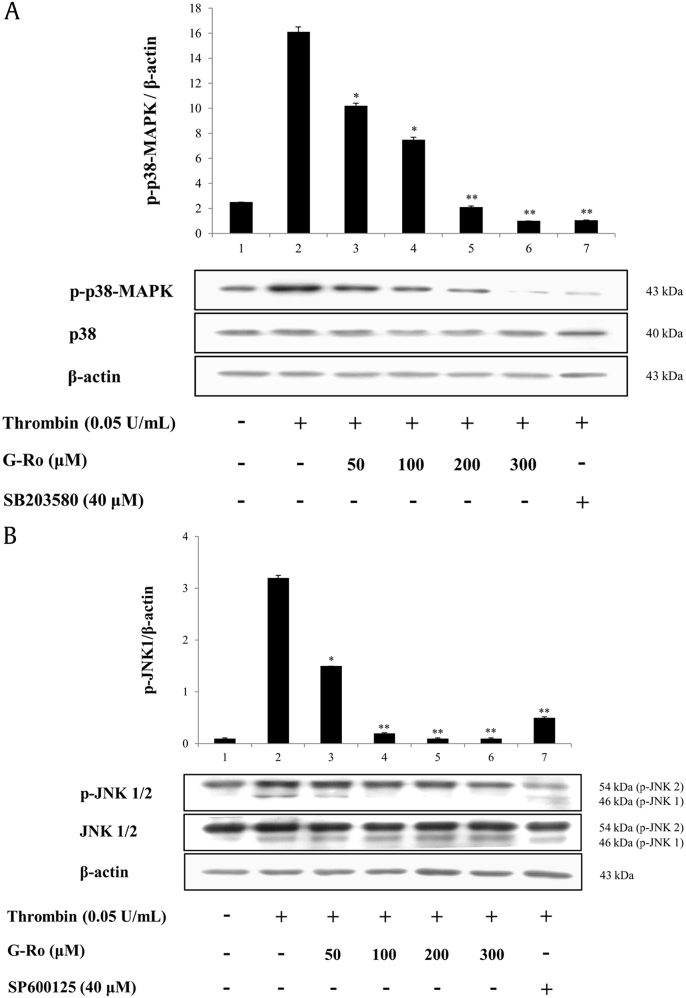
3.5. Effects of G-Ro on the phosphorylation of MAPKs
Platelets contain MAPKs, such as ERK, JNK, and p38-MAPK [20], that phosphorylate Ser505 of cPLA2α [10], [14], [18], [19], [21], [22], [23]. Therefore, we investigated whether G-Ro inhibited the phosphorylation of cPLA2α (Ser505) in thrombin-activated human platelets. Thrombin-mediated p38-MAPK phosphorylation (Fig. 4A, lane 2) was dose-dependently (50–300 μM) inhibited by G-Ro (Fig. 4A, lanes 3–6). Furthermore, the p38-MAPK inhibitor, SB203580, attenuated the thrombin-induced phosphorylation of p38-MAPK (Fig. 4A, lane 7).
Fig. 4.
Effects of G-Ro on the phosphorylation of MAPKs. (A) Effects of G-Ro on the phosphorylation of p38-MAPK. (B) Effects of G-Ro on JNK1/2 phosphorylation. Western blot was determined as described in “Materials and methods” section. The data are expressed as the mean ± standard deviation (n = 3). ∗p < 0.05 versus the thrombin-stimulated human platelets, ∗∗p < 0.01 versus the thrombin-stimulated human platelets.
JNK, Jun N-terminal kinase; MAPK, mitogen-activated protein kinases.
Thrombin phosphorylated JNK1 (46 kDa), but not JNK2 (54 kDa), as shown (Fig. 4B, lane 2). G-Ro attenuated the thrombin-induced phosphorylation of JNK1 in a dose-dependent manner (Fig. 4B, lanes 3–6). The inhibitor of JNK, SP600125, inhibited the phosphorylation of both JNK1 and JNK2 in thrombin-activated human platelets (Fig. 4B, lane 7).
3.6. Effects of MAPK inhibitors on cPLA2α phosphorylation, AA release, and TXA2 production
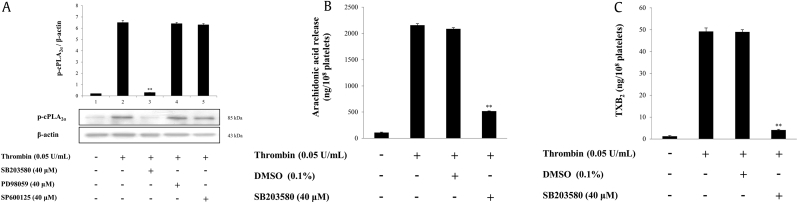
Furthermore, we investigated whether MAPK inhibitors inhibited the phosphorylation of cPLA2α. Thrombin extensively phosphorylated cPLA2α; however, it was inhibited by SB203580 (40 μM). Nevertheless, PD98059 (40 μM) and SP600125 (40 μM) did not influence the thrombin-induced cPLA2α phosphorylation (Fig. 5A). Among the MAPK inhibitors, only SB203580 (40 μM), a p38-MAPK inhibitor, strongly inhibited the thrombin-mediated cPLA2α phosphorylation. This suggested that p38-MAPK induces cPLA2α phosphorylation and may stimulate TXA2 production by promoting AA release. Therefore, we tested this hypothesis using SB203580.
Fig. 5.
Effects of MAPK inhibitors on cPLA2α-phosphorylation, AA release, and TXA2 production. (A) Effects of MAPK inhibitors on cPLA2-phosphorylation. (B) Effects of SB203580 on AA release. (C) Effects of SB203580 on TXA2 production. Western blot, AA release assay, and TXA2 production were determined as described in “Materials and methods” section. The data are expressed as the mean ± standard deviation (n = 3). ∗p < 0.05 versus the thrombin-stimulated human platelets in the presence of 0.1% DMSO, ∗∗p < 0.01 versus the thrombin-stimulated human platelets in the presence of 0.1% DMSO.
AA, arachidonic acid; cPLA2α, cytosolic phospholipase A2α; DMSO, dimethyl sulfoxide; MAPK, mitogen-activated protein kinases; TXB2, thromboxane B2.
We observed that it inhibited the thrombin-induced AA release and TXA2 production by 75.2% and 91.6%, respectively (Figs. 5B, 5C).
4. Discussion
The autacoid TXA2, produced in platelets, constricts blood vessels and initiates thrombogenesis [7], [24], [25]. P. ginseng compounds, such as ginsenoside Rp1 [26], panaxadiol, and panaxatriol saponins [27], [28], [29], inhibit TXA2 production and attenuate platelet aggregation. In this study, we evaluated whether G-Ro inhibits thrombin-induced platelet aggregation by decreasing TXA2 production and investigated the mechanisms underlying the attenuation of AA release. We sought to identify the TXA2 antagonistic potential of G-Ro for development into an antiplatelet agent.
G-Ro inhibited TXA2 production to abolish thrombin-induced platelet aggregation. We determined the activities of COX-1 (70 kDa) and TXAS (58 kDa) in the microsomal fraction, which has the highest activity of cytochrome c reductase (an endoplasmic reticulum marker enzyme) to justify this inhibitory effect [16]. G-Ro reduced the production of TXA2 more than it reduced the activities of COX-1 and TXAS, suggesting that it may also inhibit AA release by cPLA2α and AA utilization by COX-1 and TXAS in thrombin-activated platelets. As expected, G-Ro strongly inhibited both thrombin-induced Ca2+-dependent cPLA2α (Ser505) phosphorylation and AA release. These results verify that the reduction in intracellular Ca2+ level by G-Ro [30] prevents the binding of cPLA2α to its PM substrates, such as phosphatidylcholine (PC), phosphatidylserine (PS), and phosphatidylethanolamine (PE). Accordingly, the Ca2+-antagonistic effects of G-Ro [30] reduce AA release from cPLA2α substrates (PC, PS, and PE) to decrease TXA2 production. Moreover, thrombin-elevated intracellular Ca2+ hydrolyzes the AA bond at position 2 of PS, PC, and PE in the PM of human platelets [31], indicating that the AA, bound at position 2 of glycerophospholipids, is attacked by Ca2+-dependent cPLA2α. Thrombin also activates Ca2+-dependent PLCβ to produce DG and IP3 from phosphatidylinositol 4,5-bisphosphate in the PM. DG is hydrolyzed to AA and glycerol via the DG– and monoacylglycerol–lipase pathway [1]. Accordingly, we cannot rule out G-Ro-mediated inhibition of the PLCβ/DG–lipase/monoacylglycerol–lipase pathway to reduce AA release in thrombin-activated platelets.
In the present study, G-Ro inhibited the activities of both the AA release enzyme (cPLA2α) and AA utilization enzymes (COX-1 and TXAS) to decrease the thrombin-induced TXA2 production. These enzymes are known to be activated by phosphorylated MAPKs [12], [13], [14], [19], [20], [21], [32], [33], [34], [35], [36], [37], [38], [39]. Therefore, we used MAPK inhibitors to investigate whether G-Ro requires inhibition of thrombin-phosphorylated MAPKs for attenuating TXA2 production. SB203580 (a p38-MAPK inhibitor) inhibited the thrombin-induced p38-MAPK phosphorylation, cPLA2α phosphorylation, AA release, and TXA2 production. These results confirm that thrombin-phosphorylated p38-MAPK increases AA release and TXA2 production by promoting cPLA2α phosphorylation.
Similar to SB203580, G-Ro attenuated thrombin-induced p38-MAPK phosphorylation, cPLA2α phosphorylation, AA release, and TXA2 production. Therefore, we can assume that G-Ro inhibits thrombin-induced AA release and TXA2 production by preventing the phosphorylation of both p38-MAPK and cPLA2α. Moreover, G-Ro is reported to inhibit the thrombin-mediated phosphorylation of ERK2 [30] and JNK1. However, G-Ro failed to inhibit AA release through suppression of ERK2- and JNK1-induced cPLA2α phosphorylation in thrombin-activated platelets. Furthermore, both PD98059 (ERK inhibitor) and SP600125 (JNK inhibitor) did not inhibit thrombin-induced cPLA2α phosphorylation. Therefore, other platelet-activating mechanisms, such as Ca2+ influx [9], [40], [41] and COX-1 activation by ERK2 [39] and serotonin release by JNK1 [20], might have led to the suppression of ERK2 and JNK1 by G-Ro. Many compounds of ginseng, such as G-Ro, G-Rp4, Rg3-enriched red ginseng extract, and G-Rp1, inhibit the phosphorylation of MAPKs to attenuate Ca2+ influx and serotonin release in platelets [26], [42], [43].
We previously showed that G-Ro inhibits thrombin-induced Ca2+-dependent platelet-activating reactions, including granule secretion, fibrinogen binding, and fibrin clot retraction, by upregulating the cyclic adenosine monophosphate (cAMP)-dependent phosphorylation of IP3 (Ser1756) and vasodilator-stimulated phosphoprotein (Ser157) [44]. In this study, we observed that G-Ro attenuated thrombin-induced TXA2 production by inhibiting AA release, and this effect was due to the inhibition of Ca2+-dependent cPLA2α phosphorylation by p38-MAPK. In addition, G-Ro abolishes Ca2+-dependent p-selectin expression in thrombin-activated platelets [30]. Because its expression in activated platelets causes leukocytic inflammatory atherosclerosis, G-Ro may counteract inflammation and atherosclerosis [45], [46], [47]. The in vitro and in vivo antiinflammatory activities of G-Ro and Korean Red Ginseng are reported [48], [49], [50].
In conclusion, G-Ro attenuates TXA2 production by inhibiting p38-MAPK-mediated cPLA2α phosphorylation and AA release. It also reduced the activities of microsomal COX-1 and TXAS in thrombin-activated human platelets. Combined with previous reports [30], [44], [48], [49], G-Ro holds significant antiplatelet potential.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This research was supported by the National Research Foundation of Korea Grant funded by the Korean government (Grant no. 2015R1D1A1A09057204)
Contributor Information
Man Hee Rhee, Email: rheemh@knu.ac.kr.
Hwa-Jin Park, Email: mlsjpark@inje.ac.kr.
References
- 1.Berridge M.J., Irvine R.F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa M., Tanaka T., Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980;287:863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- 3.Craig K.L., Harley C.B. Phosphorylation of human pleckstrin on Ser-113 and Ser-117 by protein kinase C. Biochem J. 1996;314:937–942. doi: 10.1042/bj3140937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci USA. 1975;72:2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrignani P., Sciulli M.G., Manarini S., Santini G., Cerletti C., Evangelista V. COX-2 is not involved in thromboxane biosynthesis by activated human platelets. J Physiol Pharmacol. 1999;50:661–667. [PubMed] [Google Scholar]
- 6.Needleman P., Moncada S., Bunting S., Vane J.R., Hamberg M., Samuelsson B. Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature. 1976;261:558–560. doi: 10.1038/261558a0. [DOI] [PubMed] [Google Scholar]
- 7.Jennings L.K. Role of platelets in atherothrombosis. Am J Cardiol. 2009;103:4A–10A. doi: 10.1016/j.amjcard.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Patrono C. Aspirin as an antiplatelet drug. N Engl J Med. 1994;330:1287–1294. doi: 10.1056/NEJM199405053301808. [DOI] [PubMed] [Google Scholar]
- 9.Nadal-Wollbold F., Pawlowski M., Lévy-Toledano S., Berrou E., Rosa J.P., Bryckaert M. Platelet ERK2 activation by thrombin is dependent on calcium and conventional protein kinases C but not Raf-1 or B-Raf. FEBS Lett. 2002;531:475–482. doi: 10.1016/s0014-5793(02)03587-1. [DOI] [PubMed] [Google Scholar]
- 10.Kramer R.M., Roberts E.F., Strifler B.A., Johnstone E.M. Thrombin induces activation of p38 MAP kinase in human platelets. J Biol Chem. 1995;270:27395–27398. doi: 10.1074/jbc.270.46.27395. [DOI] [PubMed] [Google Scholar]
- 11.Bugaud F., Nadal-Wollbold F., Lévy-Toledano S., Rosa J.P., Bryckaert M. Regulation of c-jun-NH2 terminal kinase and extracellular-signal regulated kinase in human platelets. Blood. 1990;94:3800–3805. [PubMed] [Google Scholar]
- 12.Yacoub D., Théorêt J.F., Villeneuve L., Abou-Saleh H., Mourad W., Allen B.G., Merhi Y. Essential role of protein kinase Cδ in platelet signaling, αIIbβ3 activation, and thromboxane A2 release. J Biol Chem. 2006;281:30024–30035. doi: 10.1074/jbc.M604504200. [DOI] [PubMed] [Google Scholar]
- 13.Garcia A., Shankar H., Murugappan S., Kim S., Kunapuli S.P. Regulation and functional consequences of ADP receptor-mediated ERK2 activation in platelets. Biochem J. 2007;404:299–308. doi: 10.1042/BJ20061584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer R.M., Roberts E.F., Um S.L., Börsch-Haubold A.G., Watson S.P., Fisher M.J., Jakubowski J.A. p38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets evidence that proline-directed phosphorylation is not required for mobilization of arachidonic acid by cPLA2. J Biol Chem. 1996;271:27723–27729. doi: 10.1074/jbc.271.44.27723. [DOI] [PubMed] [Google Scholar]
- 15.McNicol A., Shibou T.S. Translocation and phosphorylation of cytosolic phospholipase A2 in activated platelets. Thromb Res. 1998;92:19–26. doi: 10.1016/s0049-3848(98)00097-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee D.H., Cho H.J., Kang H.Y., Rhee M.H., Park H.J. Total saponin from Korean Red Ginseng inhibits thromboxane A2 production associated microsomal enzyme activity in platelets. J Ginseng Res. 2012;36:40–46. doi: 10.5142/jgr.2012.36.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin J.H., Kwon H.W., Cho H.J., Rhee M.H., Park H.J. Inhibitory effects of total saponin from Korean Red Ginseng on [Ca2+]i mobilization through phosphorylation of cyclic adenosine monophosphate-dependent protein kinase catalytic subunit and inositol 1, 4, 5-trisphosphate receptor type I in human platelets. J Ginseng Res. 2015;39:354–364. doi: 10.1016/j.jgr.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo I., Murakami M. Phospholipase A2 enzymes. Prostagl Other Lipid Mediat. 2002;68:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 19.Lin L.L., Wartmann M., Lin A.Y., Knopf J.L., Seth A., Davis R.J. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 20.Adam F., Kauskot A., Rosa J.P., Bryckaert M. Mitogen-activated protein kinases in hemostasis and thrombosis. J Thromb Haemost. 2008;6:2007–2016. doi: 10.1111/j.1538-7836.2008.03169.x. [DOI] [PubMed] [Google Scholar]
- 21.Börsch-Haubold A.G., Pasquet S., Watson S.P. Direct inhibition of cyclooxygenase-1 and-2 by the kinase inhibitors SB 203580 and PD 98059 SB 203580 also inhibits thromboxane synthase. J Biol Chem. 1998;273:28766–28772. doi: 10.1074/jbc.273.44.28766. [DOI] [PubMed] [Google Scholar]
- 22.Hefner Y., Börsch-Haubold A.G., Murakami M., Wilde J.I., Pasquet S., Schieltz D., Cohen P. Serine 727 phosphorylation and activation of cytosolic phospholipase A2 by MNK1-related protein kinases. J Biol Chem. 2000;275:37542–37551. doi: 10.1074/jbc.M003395200. [DOI] [PubMed] [Google Scholar]
- 23.Nemenoff R.A., Winitz S., Qian N.X., Van Putten V., Johnson G.L., Heasley L.E. Phosphorylation and activation of a high molecular weight form of phospholipase A2 by p42 microtubule-associated protein 2 kinase and protein kinase C. J Biol Chem. 1993;268:1960–1964. [PubMed] [Google Scholar]
- 24.Ruggeri Z.M. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 25.FitzGerald G.A. Mechanisms of platelet activation: thromboxane A2 as an amplifying signal for other agonists. Am J Cardiol. 1991;68:11B–15B. doi: 10.1016/0002-9149(91)90379-y. [DOI] [PubMed] [Google Scholar]
- 26.Endale M., Lee W.M., Kamruzzaman S.M., Kim S.D., Park J.Y., Park M.H., Rhee M.H. Ginsenoside-Rp1 inhibits platelet activation and thrombus formation via impaired glycoprotein VI signalling pathway, tyrosine phosphorylation and MAPK activation. Br J Pharmacol. 2012;167:109–127. doi: 10.1111/j.1476-5381.2012.01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park K.M., Rhee M.H., Shin H.J., Song Y.B., Hyun H.C., Park K.H., Kim H.S. Inhibitory effects of Panaxatriol from Panax ginseng C.A. Meyer on phosphoinositide breakdown induced by thrombin in platelets. J Ginseng Res. 2008;32:107–113. [Google Scholar]
- 28.Park K.M., Rhee M.H., Park H.J. Panaxadiol and panaxatriol from Panax ginseng C.A. Meyer inhibit the synthesis of thromboxane A2 in adrenaline-stimulated human platelet aggregation. J Ginseng Res. 1994;18:44–48. [Google Scholar]
- 29.Park H.J., Rhee M.H., Park K.M., Nam K.Y., Park K.H. Panaxadiol from Panax ginseng C.A. Meyer inhibits synthesis of thromboxane A2 in platelet aggregation induced by thrombin. J Ginseng Res. 1993;17:131–134. [Google Scholar]
- 30.Kwon H.W., Shin J.H., Lee D.H., Park H.J. Inhibitory effects of cytosolic Ca2+ concentration by ginsenoside Ro are dependent on phosphorylation of IP3RI and dephosphorylation of ERK in human platelets. Evid-based Compl Alt. 2015 doi: 10.1155/2015/764906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takamura H., Narita H., Park H.J., Tanaka K.I., Matsuura T., Kito M. Differential hydrolysis of phospholipid molecular species during activation of human platelets with thrombin and collagen. J Biol Chem. 1987;262:2262–2269. [PubMed] [Google Scholar]
- 32.Leslie C.C. Regulation of the specific release of arachidonic acid by cytosolic phospholipase A2. Prostagl Leukot Essent Fatty Acids. 2004;70:373–376. doi: 10.1016/j.plefa.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Hsiao G., Lee J.J., Lin K.H., Shen C.H., Fong T.H., Chou D.S., Sheu J.R. Characterization of a novel and potent collagen antagonist, caffeic acid phenethyl ester, in human platelets: in vitro and in vivo studies. Cardiovasc Res. 2007;75:782–792. doi: 10.1016/j.cardiores.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Kim S.D., Lee I.K., Lee W.M., Cho J.Y., Park H.J., Oh J.W., Rhee M.H. The mechanism of anti-platelet activity of davallialactone: involvement of intracellular calcium ions, extracellular signal-regulated kinase 2 and p38 mitogen-activated protein kinase. Eur J Pharmacol. 2008;584:361–367. doi: 10.1016/j.ejphar.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Flevaris P., Li Z., Zhang G., Zheng Y., Liu J., Du X. Two distinct roles of mitogenactivated protein kinases in platelets and a novel Rac1-MAPK-dependent integrin outside-in retractile signaling pathway. Blood. 2009;113:893–901. doi: 10.1182/blood-2008-05-155978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leslie C.C., Gangelhoff T.A., Gelb M.H. Localization and function of cytosolic phospholipase A2 alpha at the Golgi. Biochimie. 2010;92:620–626. doi: 10.1016/j.biochi.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moscardó A., Vallés J., Latorre A., Madrid I., Santos M.T. Reduction of platelet cytosolic phospholipase A2 activity by atorvastatin and simvastatin: biochemical regulatory mechanisms. Thromb Res. 2013;131:e154–159. doi: 10.1016/j.thromres.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Jackson E.C., Ortar G., McNicol A. The effects of an inhibitor of diglyceride lipase on collagen-induced platelet activation. J Pharmacol Exp Ther. 2013;347:582–588. doi: 10.1124/jpet.113.205591. [DOI] [PubMed] [Google Scholar]
- 39.Bi Y., Guo X.K., Zhao H., Gao L., Wang L., Tang J., Feng W.H. Highly pathogenic porcine reproductive and respiratory syndrome virus induces prostaglandin E2 production through cyclooxygenase 1, which is dependent on the ERK1/2-p-C/EBP-β pathway. J Virol. 2014;88:2810–2820. doi: 10.1128/JVI.03205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosado J.A., Sage S.O. Role of the ERK pathway in the activation of store-mediated calcium entry in human platelets. J Biol Chem. 2001;276:15659–15665. doi: 10.1074/jbc.M009218200. [DOI] [PubMed] [Google Scholar]
- 41.Rosado J.A., Sage S.O. The ERK cascade, a new pathway involved in the activation of store-mediated calcium entry in human platelets. Trends Cardiovasc Med. 2002;12:229–234. doi: 10.1016/s1050-1738(02)00161-5. [DOI] [PubMed] [Google Scholar]
- 42.Son Y.M., Jeong D.H., Park H.J., Rhee M.H. The inhibitory activity of ginsenoside Rp4 in adenosine diphosphate-induced platelet aggregation. J Ginseng Res. 2017;41:96–102. doi: 10.1016/j.jgr.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeong D., Irfan M., Kim S.D., Kim S., Oh J.H., Park C.K., Rhee M.H. Ginsenoside Rg3-enriched red ginseng extract inhibits platelet activation and in vivo thrombus formation. J Ginseng Res. 2017;41:548–555. doi: 10.1016/j.jgr.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin J.H., Kwon H.W., Cho H.J., Rhee M.H., Park H.J. Vasodilator-stimulated phosphoprotein-phosphorylation by ginsenoside Ro inhibits fibrinogen binding to αIIb/β 3 in thrombin-induced human platelets. J Ginseng Res. 2016;40:359–365. doi: 10.1016/j.jgr.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Broos K., Feys H.B., De Meyer S.F., Vanhoorelbeke K., Deckmyn H. Platelets at work in primary hemostasis. Blood Rev. 2011;25:155–167. doi: 10.1016/j.blre.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Zarbock A., Polanowska-Grabowska R.K., Ley K. Plateletneutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 2007;21:99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Dav`ı G., Patrono C. Mechanisms of disease: platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 48.Matsuda H., Samukawa K.I., Kubo M. Anti-inflammatory activity of ginsenoside Ro. Planta Med. 1990;56:19–23. doi: 10.1055/s-2006-960875. [DOI] [PubMed] [Google Scholar]
- 49.Kim S., Oh M.H., Kim B.S., Kim W.I., Cho H.S., Park B.Y., Kwon J. Upregulation of heme oxygenase-1 by ginsenoside Ro attenuates lipopolysaccharide-induced inflammation in macrophage cells. J Ginseng Res. 2015;39:365–370. doi: 10.1016/j.jgr.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baek K.S., Yi Y.S., Son Y.J., Yoo S., Sung N.Y., Kim Y., Cho J.Y. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J Ginseng Res. 2016;40:437–444. doi: 10.1016/j.jgr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]