Abstract
Background
Gintonin is a ginseng-derived exogenous ligand of the G protein-coupled lysophosphatidic acid (LPA) receptor. We previously reported that gintonin stimulates gliotransmitter release in primary cortical astrocytes. Astrocytes play key roles in the functions of neurovascular systems. Although vascular endothelial growth factor (VEGF) is known to influence the normal growth and maintenance of cranial blood vessels and the nervous system, there is little information about the effect of gintonin on VEGF regulation in primary astrocytes, under normal and hypoxic conditions.
Methods
Using primary cortical astrocytes of mice, the effects of gintonin on the release, expression, and distribution of VEGF were examined. We further investigated whether the gintonin-mediated VEGF release protects astrocytes from hypoxia.
Results
Gintonin administration stimulated the release and expression of VEGF from astrocytes in a concentration- and time-dependent manner. The gintonin-mediated increase in the release of VEGF was inhibited by the LPA1/3 receptor antagonist, Ki16425; phospholipase C inhibitor, U73122; inositol 1,4,5-triphosphate receptor antagonist, 2-APB; and intracellular Ca2+ chelator, BAPTA. Hypoxia further stimulated astrocytic VEGF release. Gintonin treatment stimulated additional VEGF release and restored cell viability that had decreased due to hypoxia, via the VEGF receptor pathway. Altogether, the regulation of VEGF release and expression and astrocytic protection mediated by gintonin under hypoxia are achieved via the LPA receptor–VEGF signaling pathways.
Conclusion
The present study shows that the gintonin-mediated regulation of VEGF in cortical astrocytes might be neuroprotective against hypoxic insults and could explain the molecular basis of the beneficial effects of ginseng on the central nervous system.
Keywords: Astrocytes, Gintonin, Hypoxia, Lysophosphatidic acid receptor, Vascular endothelial growth factor
1. Introduction
Lysophosphatidic acid (LPA) is a metabolic intermediate primarily produced by phospholipases in plants, and by autotoxin and phospholipases in animals [1]. Although LPAs are considered as minor phospholipids in plant systems, they serve as lipid-derived growth factors and/or local ligands that activate the G protein-coupled LPA receptors of animals [2]. The activation of G protein-coupled LPA receptors is additionally associated with cranial development and cognitive functions in adults, as well as with angiogenesis, embryo implantation, spermatogenesis, and wound healing [2]. Astrocytes are the most abundant cells in the brain and play important roles in the regulation of neurovascular functions that are necessary for brain activity, by forming a blood-brain barrier, providing nutritional support to the neurons, and forming tripartite synapses with neurons [3], [4]. Although the role of the LPA receptors in regulating the interactions between the neurons and astrocytes of the brain is not well understood, recent studies have shown that astrocytes express LPA receptors in abundance [5], [6]. Additional studies have reported that the treatment of astrocytes with LPA induces neuronal differentiation and axonal outgrowth in neurons, indicating that the LPA-mediated release of neurotrophic factors indirectly affects neuronal activity via the LPA receptor-mediated signaling pathways [7]. However, no reports to date have described the LPA receptor-mediated activity of neurotrophic factors in relation to the neurons and blood vessels in the brain, despite the fact that astrocytes are known to closely interact with the neurons and the endothelial cells of blood vessels in the brain.
Ginseng is a prehistorical herbal medicine with diverse physiological and pharmacological effects. Recently, we found that gintonin, derived from ginseng, contains exogenous ligands of the LPA receptor that activate the LPA receptor system in animals [8]. Gintonin contains several subtypes of LPAs such as LPA C18:2, LPA C18:1, and LPA C16:0 and other bioactive lipids such as lysophospholipids and phosphatidic acids as well as ginseng proteins [9]. Especially, gintonin causes a transient release of Ca2+ from cytosolic Ca2+ storage in cells expressing LPA1–LPA6 receptor subtypes, indicating that gintonin is a kind of ginseng-derived GTP-binding protein-coupled LPA receptor ligand [8]. In addition, activations of LPA receptors by gintonin and the following [Ca2+]i transients are coupled to the regulation of cytosolic Ca2+-dependent ion channels and receptors [10]. Further studies showed that gintonin induced-[Ca2+]i transients are further associated with interneuron communications in the hippocampus, stimulating the release of gliotransmitters and neurotransmitters, enhancing long-term potentiation/memory, and encouraging neurogenesis [11], [12], [13]. These reports therefore raise the possibility that gintonin contains lipid-derived growth factors, and gintonin-mediated [Ca2+]i transients are associated with the activity of the LPA receptor that plays an important role in both intra- and inter-neuronal communications. However, there is nearly no information on whether the gintonin-mediated activation of Guanosine phosphate (GTP)-binding protein-coupled Lysophosphatidic acid (LPA) receptors in mouse cortical astrocytes regulates neurotrophic factors such as vascular endothelial growth factor (VEGF), which is currently known to play important roles in the growth and maintenance of blood vessels and neurons in the brain.
In this report, we checked the effects of gintonin on VEGF release, using cultures of murine cortical astrocytes in normal and hypoxic conditions. Here, we report that gintonin induces VEGF release via signal transduction pathways mediated by the LPA receptor located in the cell membrane. In addition, treatment with gintonin also increased the release of VEGF under hypoxic conditions and restored cell viability and proliferation that had decreased due to hypoxia, via the VEGF receptor pathway. We have further discussed the physiological and pharmacological roles of the gintonin-mediated regulation of VEGF in the nervous system.
2. Materials and methods
2.1. Materials
Gintonin was prepared from Panax ginseng according to the methods previously described [14]. Gintonin was dissolved in deionized water and diluted with the medium before use. Dulbecco's Minimum Essential Medium (DMEM), fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Invitrogen (Camarillo, CA, USA). LPA (1-oleoyl-2-hydroxy-sn-Glycero-3-phosphate, 857130P) was purchased from Avanti Polar Lipids, Inc. (AL, USA). Other reagents were purchased from Sigma-Aldrich (St Louis, MO, USA).
2.2. Primary cultures of murine cortical astrocytes
Primary cultures of astrocytes were prepared from the cerebral cortices of 1-day-old postnatal Institute of Cancer Research/ KOATEC, Pyeongtaek-si, Gyeonggi-do, Korea (ICR) mice, according to the method described by Shano et al. The cells were seeded in culture plates coated with poly-L-lysine hydrobromide (100 μg/ml; Sigma-Aldrich) and grown in Dulbecco Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. When the cells reached confluence in 5 to 7 days, they were harvested with 0.05% trypsin/EDTA solution (Life technologies, Carlsbad, CA), seeded in culture plates previously coated with poly-L-lysine hydrobromide (100 μg/ml; Sigma-Aldrich), and re-grown in DMEM supplemented with 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin, for performing experiments.
2.3. Determining VEGF expression in primary astrocytes with immunoblotting
Cell lysates, containing 50 μg of protein, were electrophoresed using a 12% sodium dodecyl sulfate polyacrylamide gel and transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% Bovine Serum Albumin (BSA) in TTBS (20 mM Tris, pH 7.6, 137 mM NaCl, and 0.1% Tween 20) for 1 h and then incubated overnight at 4°C with rabbit anti-VEGF primary antibody (1:500; Santa Cruz). The membranes were then washed several times with TTBS at 5 min intervals and then incubated with anti-rabbit horseradish peroxidase–conjugated secondary antibodies (1:1000; Millipore) for 2 h at room temperature. The chemiluminescence of the protein bands was subsequently visualized using an ECL kit (Abfrontier, Seoul, Korea).
2.4. Measurement of VEGF release
For measuring the release of VEGF using experimental assays, the astrocytes were seeded on 6-well plates at a density of 1 × 106 cells per well and treated with DMEM in the presence or absence of gintonin at 37°C. The level of VEGF in the culture medium was measured using a mouse VEGF ELISA kit (Invitrogen, Waltham, MA, USA), according to the manufacturer's instructions.
2.5. Immunocytochemistry experiments for estimating VEGF expression in primary astrocytes
After the indicated treatments, the cultured astrocytes were fixed on cover slips for 20 min at room temperature and subsequently washed with phosphate buffered saline (PBS). The cells were permeabilized for 30 min with 0.4% Triton X-100 in 3% BSA PBS. After washing with PBS, the cells were incubated for 2 h with rabbit anti-VEGF antibody (1:500; Santa Cruz) in 1% BSA PBS. The cells were then washed several times with PBS and incubated for 2 h at 37°C with Fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG antibodies (1:1000; Millipore) in the absence of light. After finally washing in PBS, the cover slips were mounted on the slides with VECTASHIELD medium (Vector Laboratories, Inc. Burlingame, CA, USA) and allowed to sit overnight at 4°C. The images of the slides were captured using a confocal microscope (Olympus FV1000, Tokyo, Japan).
2.6. Induction of hypoxia
The astrocytes were exposed to a hypoxic environment using the BD GasPak™ EZ anaerobic system [15]. Following exposure to hypoxic conditions for 0, 0.5, 1, 2, or 12 h, the cells were washed. For re-oxygenation, the culture medium was replaced with fresh culture medium, and then the cells were treated with 0–0.3 μg/mL gintonin for 0, 0.5, 2, or 8 h under normal culture conditions, depending on the experimental protocol.
2.7. Data analyses
We first obtained the peak degree of increase in VEGF release using VEGF ELISA kit with various concentrations of gintonin and plotted and obtained concentration–response curves on the release of VEGF by gintonin. Origin program (OriginLab, Northampton, MA, USA) was used to fit the data to the Hill plot: y/ymax = [A]nH/([A]nH + [EC50]nH), where y is the peak at a given concentration of gintonin, ymax is the maximal peak in the absence of gintonin, EC50 is the concentration of gintonin producing a half-maximal effect, [A] is the concentration of gintonin, and nH is the Hill coefficient. All the values are represented as the mean ± the standard error of the mean.
3. Results
3.1. Effect of gintonin, ginsenosides Rb1 and Rg1, and LPA on VEGF release in primary cultures of murine cortical astrocytes, and investigation of associated membrane signaling pathways for VEGF release
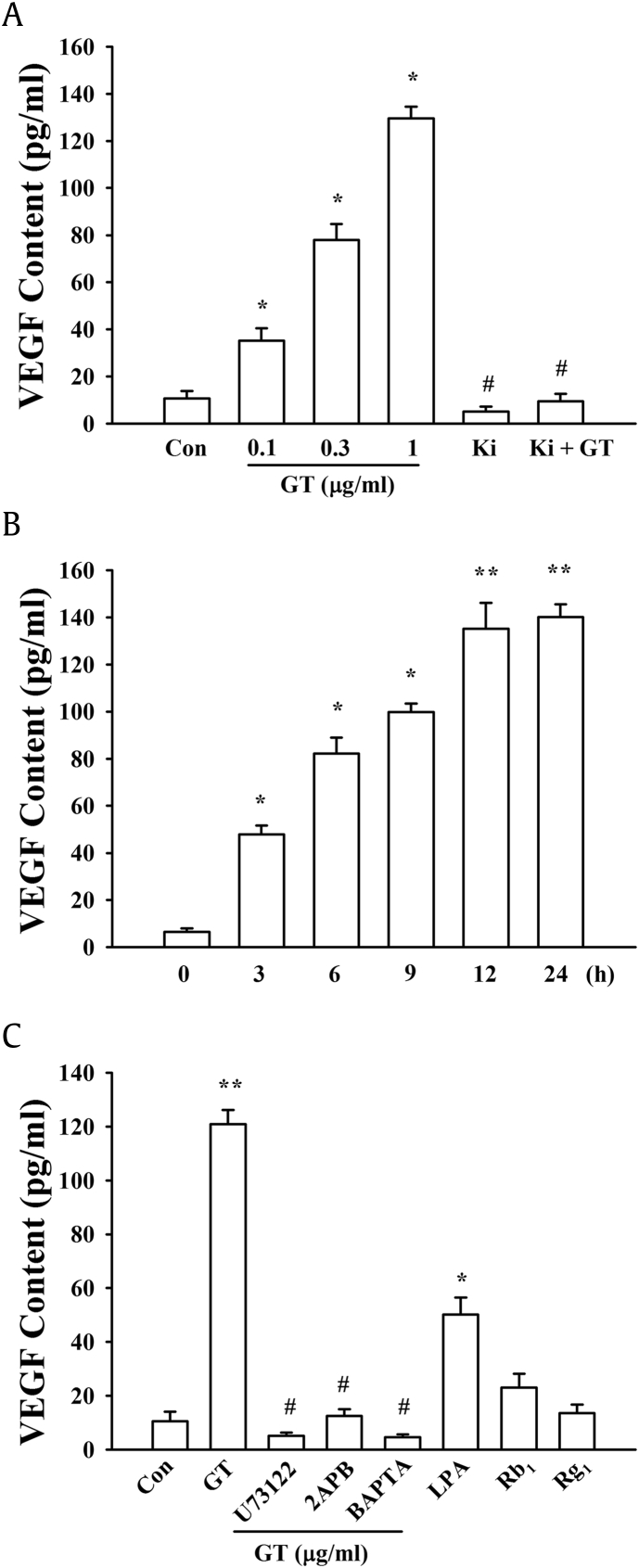
Astrocytes express LPA receptor subtypes [5], [6]. Astrocytes are the major brain cells that come in contact with the blood vessels that supply blood to the brain by forming the blood–brain barrier, which protects the brain from the external environment. Since astrocytes also affect blood vessel formation by releasing VEGF [16], we first checked the effects of gintonin on the VEGF release by astrocytes. As shown in Fig. 1A, the treatment of astrocytes with gintonin at doses of 0.1 to 1 μg/mL for 12 h induced the release of VEGF in a concentration- and time-dependent manner (Figs. 1A, 1B). Although the LPA1/3 receptor antagonist, Ki16425, alone had no effects on VEGF release, it was antagonistic to the gintonin-mediated increase in VEGF expression (Fig. 1A). In time-course experiments, the gintonin-mediated increase in VEGF release was maximal at 12 h following gintonin treatment (Fig. 1B). Treatment with LPA C18:1 (1 μM) also induced the release of VEGF (Fig. 1C). The LPA1/3 receptor antagonist (Ki16425), the inositol 1,4,5-triphosphate receptor antagonist (2-APB), and the intracellular calcium chelator (BAPTA-AM) also antagonized the gintonin-mediated increase in VEGF release (Fig. 1C). Ginsenosides Rb1 and Rg1 had no effect on the release of VEGF in the cultures of mouse cortical astrocytes (Fig. 1C). The present results showed that gintonin, but not the ginsenosides, evokes the release of Ca2+ from the intracellular stores to induce the release of VEGF by the astrocytes by activating the signal transduction pathway mediated by the LPA receptor signaling pathways including phospholipase C and IP3 receptor.
Fig. 1.
The effect of gintonin (GT) on the VEGF content of the media containing primary astrocytes. (A) Concentration-dependent stimulation of VEGF release by GT. Histograms generated from the results of treatment of primary astrocytes with 0.1–1 μg/mL GT (∗p < 0.01), compared to those of the untreated control (Con). The effect on the VEGF content after treatment with the LPA1/3 receptor antagonist (Ki16425, 10 μM) alone or a combination of Ki16425 (10 μM) and GT (0.3 μg/mL) (#p < 0.01), compared to that after treatment with 1 μg/mL GT. All the data are represented as the mean ± S.E.M. (n = 4–5). (B) Time-course of the effect of GT on VEGF release. Histograms showing the secretion of VEGF over time, following treatment with 1 μg/mL GT (∗p < 0.01) compared to that of the control (0 h). ∗∗p < 0.005 compared to control (Con). The data are represented as the mean ± S.E.M. (n = 4–5). (C) Bar graphs representing the inhibition of the signal transduction pathway mediating the GT-induced VEGF release. The LPA1/3 receptor antagonist (Ki16425, 10 μM), the PLC inhibitor (U73122, 5 μM), IP3 receptor antagonist (2-APB, 100 μM), and the intracellular Ca2+ chelator (BAPTA-AM, 50 μM) were separately added to the culture before treatment with GT (1 μg/mL). For comparison, the cells were also treated with LPA (1 μM) and ginsenosides Rb1 or Rg1 (30 μM each). ∗p < 0.01, ∗∗p < 0.005, compared to control; #p < 0.01, compared to treatment with GT (1 μg/mL). The data are represented as the mean ± S.E.M. (n = 4–5). The concentration of VEGF in each sample was determined using a VEGF assay kit, as described in “Materials and methods Section”.
LPA, lysophosphatidic acid; S.E.M., standard error of the mean; VEGF, vascular endothelial growth factor.
3.2. Effects of gintonin on the expression of VEGF in cortical astrocytes
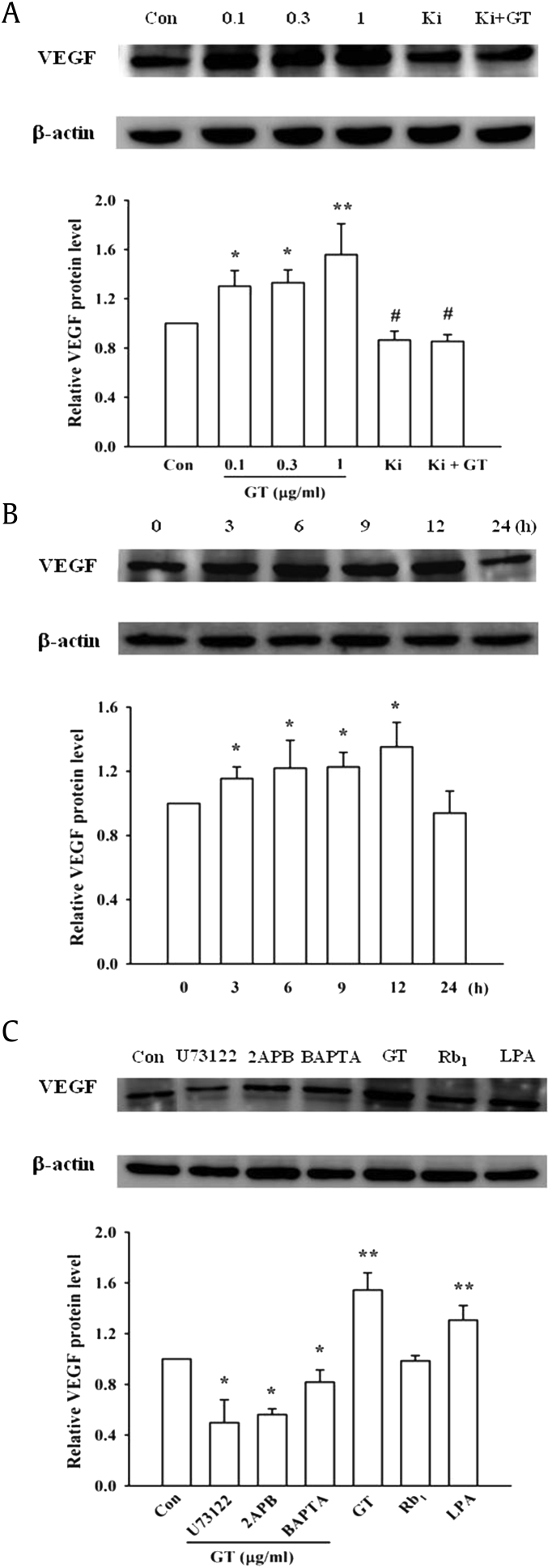
We subsequently quantified the gintonin-mediated VEGF expression level using immunoblotting. As shown in Fig. 2A, gintonin at a concentration of 1 μg/mL increased VEGF expression in a concentration-dependent manner by two-folds, compared to the control setup that was treated with saline. In time-dependent experiments, gintonin increased the expression of VEGF maximally at 12 h after treatment, which decreased to near basal levels after 24 h (Fig. 2B). Treatment with 1 μM LPA C18:1 also induced the expression of VEGF (Fig. 2A). The intracellular calcium chelator, BAPTA-AM, and the LPA1/3 receptor antagonist, Ki16425, also antagonized the gintonin-mediated increase in VEGF expression (Fig. 2C). These results indicate that gintonin induces an increase of VEGF expression level in primary astrocytes in a manner that is dependent on calcium and signaling pathways mediated by the LPA receptor.
Fig. 2.
Western blots showing the expression of VEGF in extracts of primary astrocyte cultures following treatment with gintonin (GT) or inhibitors of signal transduction. (A) The expression of VEGF protein in primary astrocytes following treatment with various concentrations of GT or in the presence of Ki16425 (10 μM). Histograms indicating the levels of VEGF protein expressed in the astrocytes, which were quantified by their density in western blotting experiments. (B) Time course of VEGF protein expression in primary astrocytes treated with 1 μg/mL GT. (C) GT-mediated VEGF protein expression in cells treated with inhibitors of the signal transduction pathway. The concentrations of the inhibitors were the same as those in Fig. 1C. ∗p < 0.01, compared to the control (Con); #p < 0.05, compared to treatment with 1 μg/mL GT. All the data were normalized to the levels of the β-actin protein. The data are represented as the mean ± S.E.M. (n = 3–4). **p < 0.001, compared to the control (Con).
LPA, lysophosphatidic acid; S.E.M., standard error of the mean; VEGF, vascular endothelial growth factor.
3.3. Effects of gintonin on the distribution of VEGF in astrocytes
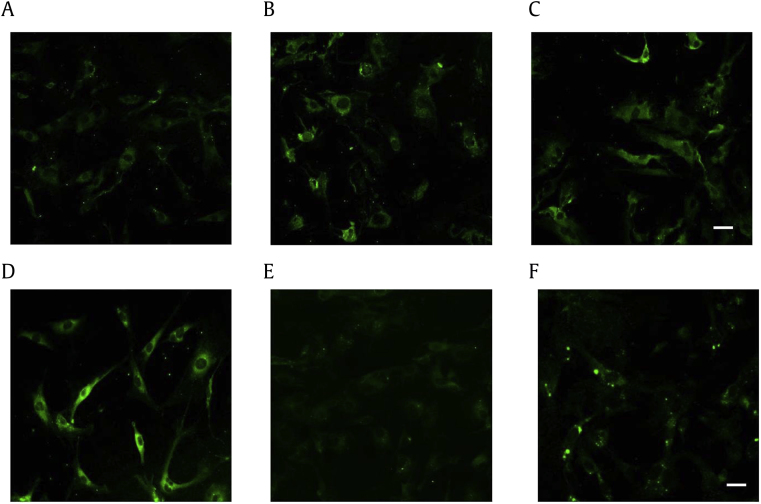
We also examined the effects of gintonin on the distribution of VEGF in the astrocytes. As shown in Fig. 3, the treatment of astrocytes with gintonin at doses of 0.1 to 1 μg/mL for 12 h increased the immunostaining of VEGF expression in the cytosol in a concentration-dependent manner (Fig. 3, arrows). Interestingly, at a low concentration, gintonin primarily increased the expression of VEGF in the cell body; however, at higher concentrations, gintonin changed the distribution of VEGF in the cell body to the cell processes (Figs. 3B, 3D, arrows). The LPA1/3 receptor antagonist, Ki16425, alone had no effects on VEGF expression but inhibited the gintonin-mediated increase in VEGF expression, indicating that the gintonin-induced expression of VEGF is mediated by the LPA receptors (Fig. 3F).
Fig. 3.
An illustration representing the concentration-dependent effects of various concentrations of gintonin (GT) and the LPA1/3 receptor antagonist (Ki16425) on VEGF expression in primary astrocytes. Images captured by confocal laser scanning microscopy, showing the FITC signal. (A) Untreated primary astrocytes. (B–F) Treated primary astrocytes. The astrocytes were treated with (B) 0.1 μg/mL GT; (C) 0.3 μg/mL GT; (D) 1 μg/mL GT; (E) the LPA1/3 receptor antagonist (Ki16425) alone; and (F) 0.3 μg/mL GT and Ki16425. Scale bar: 40 μm.
LPA, lysophosphatidic acid; VEGF, vascular endothelial growth factor.
3.4. Effects of gintonin on VEGF release and astrocytic viability under hypoxic condition
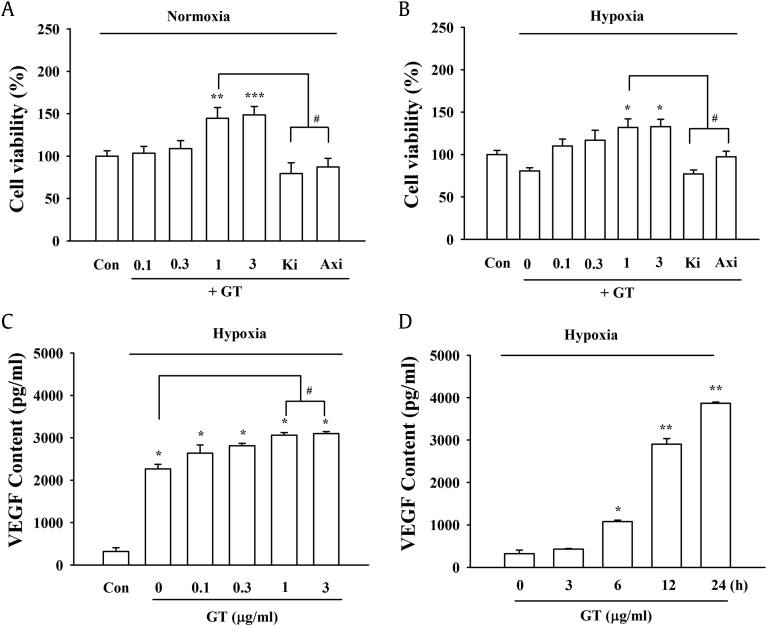
It is well-known that VEGF plays an important role in angiogenesis during hypoxia [17]. Recent studies showed that VEGF has additional nonangiogenic roles in the nervous system. Krum et al (2002) and Mani et al (2005) demonstrated that VEGF increases the proliferation of astrocytes in neocortical explants [18], [19]. In this study, we examined whether the gintonin-mediated release of VEGF is linked to the proliferation of astrocytes. As shown in Fig. 4A, the treatment of astrocytes with gintonin increased cell proliferation; however, the effects of gintonin were inhibited by Ki16425 as well as by the VEGF receptor antagonist, axitinib, raising the possibility that the gintonin-mediated astrocytic proliferation depends on the LPA receptor and VEGF receptor. In addition, we examined the effects of gintonin on cell viability and VEGF release during hypoxia, since VEGF is known to play an important role in cell viability during hypoxic insults. As shown in Fig. 4B, although hypoxia itself significantly reduced cell viability, the treatment of hypoxic astrocytes with gintonin restored cell viability in a concentration-dependent and a LPA receptor- and VEGF receptor-dependent manner (Fig. 4). We subsequently measured the VEGF released from the astrocytes during hypoxia in the absence or presence of gintonin. Consistent with a previous study, hypoxia itself greatly increased the VEGF release in the astrocytes (Fig. 4C) [20], treatment with gintonin further significantly increased the release of VEGF during hypoxia in a concentration- and time-dependent manner (Figs 4C, 4D). This indicated that the restoration of astrocyte viability and proliferation mediated by gintonin during hypoxia could contribute to the regulation of the LPA receptor–mediated VEGF release.
Fig. 4.
Effects of gintonin (GT) on cell viability and VEGF content in the media containing primary astrocytes under normoxic and hypoxic conditions. (A) The cells were treated with the indicated concentrations of GT for 12 h under normal conditions. (B) The cells were treated with the indicated concentrations of GT for 12 h under hypoxia. Astrocyte viability was estimated with the XTT assay. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005 of control vs. Ki16425 (10 μM) + GT (0.3 μg/mL) or GT + Axitinib (Axi); (#p < 0.01) compared to GT (1 μg/mL) treatment. The data are represented as the mean ± S.E.M. (n = 4–5). (C) Concentration-dependent stimulation of VEGF release by GT under hypoxia. Histograms obtained after the primary astrocytes were treated with 0.1–1 μg/mL GT (∗p < 0.001) compared to the untreated control in normoxia (Con) and to the untreated control in hypoxia (#p < 0.01). The data are represented as the mean ± S.E.M. (n = 4–5). (D) Time-course showing the effect of GT on VEGF release under hypoxia. Histograms showing the time course of VEGF secretion following treatment with 1 μg/mL GT (∗p < 0.005, ∗∗p < 0.001) compared to the control (0 h). The data are represented as the mean ± S.E.M. (n = 4–5).
VEGF, vascular endothelial growth factor; S.E.M., standard error of the mean.
4. Discussion
In our previous studies, we reported that acute treatment with gintonin induces [Ca2+]i transients in neuronal and nonneuronal cells via the activation of LPA receptors and regulates intracellular calcium-dependent ion channels and receptors [9]. In addition, gintonin-mediated cytosolic Ca2+ elevations are associated with the release of gliotransmitters, neurotransmitters, Adenosine triphosphate (ATP), glutamate, and norepinephrine for intercellular communication in astrocytes and neuronal cells [9], [11]. Gintonin activates N-methyl-D-aspartate (NMDA) receptor channel currents and enhances synaptic transmission in hippocampal slices [21], [22]. Although acute treatment with gintonin modulates the release of gliotransmitters and neurotransmitters and enhances synaptic transmission [13], [21], relatively little is known about the effects of gintonin on the release and expression of neurotrophic factors. In the present study, we examined the effects of gintonin on the release and expression of VEGF in primary cultures of cortical astrocytes.
The present results showed that gintonin, and not the ginsenosides Rb1 and Rg1, induced the release and expression of VEGF in primary cultures of cortical astrocytes, although previous reports showed that ginseng extracts and ginsenosides stimulate angiogenesis in blood vascular systems by regulating VEGF expression [23], [24]. These results indicate that gintonin, and not ginsenosides, regulates the release and expression of VEGF in the brain. The gintonin-mediated increase in VEGF release is achieved through an LPA receptor–mediated signal transduction pathway (Fig. 1). Second, gintonin also restored the hypoxia-induced damages to cell viability by increasing the release of VEGF; however, the gintonin-induced VEGF release and cell proliferation under hypoxia was attenuated by a VEGF antagonist, showing that the regulation of VEGF by gintonin might be crucial for recovering from hypoxic insults. In our previous studies, we also showed that gintonin increases the formation of VEGF in the endothelial cells of human umbilical vein [25]. Thus, combining the results of the present and previous studies, the fact that gintonin regulates the release and expression of VEGF in neural and nonneuronal cells is further substantiated.
VEGF is a secretary protein, essential for the growth of blood vessels in peripheral systems, for the development of blood vessels throughout the animal body including human, and for numerous functions of the nervous system in adults [26]. In neurovascular systems, hypoxia damages the endothelium of the vascular systems in the brain, which interferes with the normal flow of blood and subsequently leads to ischemic conditions in the brain, as shown in Fig. 5 [27]. It is known that hypoxia itself induces the expression of VEGF in astrocytes [20]. The hypoxia-induced VEGF expression possibly contributes to ischemic tolerance and has neuroprotective effects [28], [31]. VEGF thus exhibits both angiogenesis-dependent and angiogenesis-independent functions that maintain homeostasis in the brain and its blood vessels [16], [17], [29]. In this study we observed that cortical astrocytes treated with gintonin exhibited increases in the release and expression of VEGF, via the signaling pathways mediated by the LPA receptor. Additionally, during hypoxia, the gintonin-induced VEGF release and increase of cell viability via the LPA receptor might also contribute to neuroprotection during hypoxia in the central nervous system (Fig. 4).
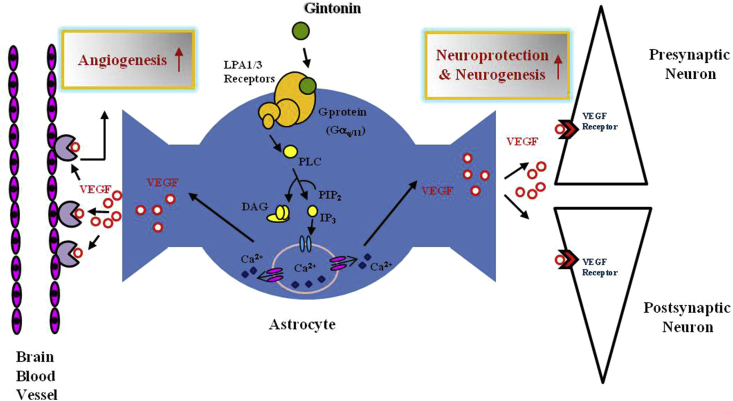
Fig. 5.
A schematic illustration of astrocytic VEGF actions in nervous system released by gintonin. Gintonin stimulates VEGF from cortical astrocytes via LPA1/3 receptors signaling pathways. The released VEGF from astrocytes might exert important actions on both brain blood vessels and neurons. Thus, gintonin-mediated VEGF release might play the dual roles for neurovascular protections by inducing angiogenesis on blood vessel and by stimulating neurogenesis and neuroprotection under abnormal condition such as hypoxia. LPA, lysophosphatidic acid; VEGF, vascular endothelial growth factor; PLC, Phospholipase C; DAG, diacylglycerol).
Gintonin is a unique preparation of ginseng, used as a herbal medicine for its various biological effects on both nervous and nonnervous systems, which are mediated through LPA receptors. The results of this study provide further evidences of the roles of gintonin in regulating the release and production of VEGF in astrocytes during normal conditions and in hypoxia. Astrocytes have dual neurovascular connections and play important roles in regulating the activities of neurons and brain microvessels, including those of the blood–brain barrier. On one side, the astrocytes surround the endothelial cells of the blood vessels in the brain, forming the blood–brain barrier that signals the endothelial cells. On the other side, the astrocytes form tripartite synapses that regulate diverse neuronal functions by releasing gliotransmitters [3] (Fig. 5). Previous studies have reported that acute treatment with gintonin stimulates the release of ATP and glutamate [12]. In this study, we showed that the treatment of astrocytes with gintonin is associated with the release and expression of VEGF from the astrocytes in vitro. The extent of the dual effects of gintonin depends on its concentration and the duration of exposure. Long-term treatments with gintonin induce the release of VEGF, which may affect neuronal survival and the maintenance of microvessels in neurovascular systems, under abnormal condition such as hypoxia (Fig. 5). The observation that gintonin regulates the release and expression of VEGF in astrocytes, together with the previous reports, that gintonin treatment increases Brain-derived neurotrophic factor (BDNF) expression and pCREB phosphorylation in hippocampal neurons, may be linked to the beneficial effects of gintonin in the central nervous system [22], [30].
In conclusion, this study shows that the LPA receptor activation by gintonin on cortical astrocytes is linked to the regulation of VEGF release and expression. The gintonin-mediated regulation of VEGF was further observed to be coupled to the neuroprotective effects of gintonin on cortical astrocytes during hypoxia. Finally, we further suggest that the regulation of VEGF release and expression mediated by gintonin may provide a molecular basis for the pharmacological effects of ginseng on the nervous system.
Conflicts of interest
All authors have no conflict of interest to declare.
Acknowledgments
This work was supported by the Konkuk University in 2018.
References
- 1.Pagès C., Simon M.F., Valet P., Saulnier-Blache J.S. Lysophosphatidic acid synthesis and release. Prostagl Other Lipid Mediat. 2001;64:1–10. doi: 10.1016/s0090-6980(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 2.Yung Y.C., Stoddard N.C., Mirendil H., Chun J. Lysophosphatidic acid signaling in the nervous system. Neuron. 2015;85:669–682. doi: 10.1016/j.neuron.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araque A., Parpura V., Sanzgiri R.P., Haydon P.G. Tripartite synapses: glia, the unacknowledged partner. Trends in Neuroscience. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 4.Halassa M.M., Fellin T., Haydon P.G. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Shano S., Moriyama R., Chun J., Fukushima N. Lysophosphatidic acid stimulates astrocyte proliferation through LPA1. Neurochem Int. 2008;52:216–220. doi: 10.1016/j.neuint.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabuchi S., Kume K., Aihara M., Shimizu T. Expression of lysophosphatidic acid receptor in rat astrocytes: mitogenic effect and expression of neurotrophic genes. Neurochem Res. 2000;25:573–582. doi: 10.1023/a:1007542532395. [DOI] [PubMed] [Google Scholar]
- 7.Spohr T.C., Dezonne R.S., Rehen S.K., Gomes F.C. Astrocytes treated by lysophosphatidic acid induce axonal outgrowth of cortical progenitors through extracellular matrix protein and epidermal growth factor signaling pathway. J Neurochem. 2011;119:113–123. doi: 10.1111/j.1471-4159.2011.07421.x. [DOI] [PubMed] [Google Scholar]
- 8.Hwang S.H., Shin T.J., Choi S.H., Cho H.J., Lee B.H., Pyo M.K., Lee J.H., Kang J., Kim H.J., Park C.W. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012;33:151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi S.H., Jung S.W., Lee B.H., Kim H.J., Hwang S.H., Kim H.K., Nah S.Y. Ginseng pharmacology: a new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front Pharmacol. 2015;6:245. doi: 10.3389/fphar.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J.H., Choi S.H., Lee B.H., Hwang S.H., Kim H.J., Rhee J., Chung C., Nah S.Y. Activation of lysophosphatidic acid receptor by gintonin inhibits Kv1.2 channel activity: involvement of tyrosine kinase and receptor protein tyrosine phosphatase alpha. Neurosci Lett. 2013;548:143–148. doi: 10.1016/j.neulet.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 11.Hwang S.H., Lee B.H., Choi S.H., Kim H.J., Jung S.W., Kim H.S., Shin H.C., Park H.J., Park K.H., Lee M.K. Gintonin, a novel ginseng-derived lysophosphatidic acid receptor ligand, stimulates neurotransmitter release. Neurosci Lett. 2015;584:356–361. doi: 10.1016/j.neulet.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Kim H., Lee B.H., Choi S.H., Kim H.J., Jung S.W., Hwang S.H., Rhim H., Kim H.C., Cho I.H., Nah S.Y. Gintonin stimulates gliotransmitter release in cortical primary astrocytes. Neurosci Lett. 2015;603:19–24. doi: 10.1016/j.neulet.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Park H., Kim S., Rhee J., Kim H.J., Han J.S., Nah S.Y., Chung C. Synaptic enhancement induced by gintonin via lysophosphatidic acid receptor activation in central synapses. J Neurophysiol. 2015;113:1493–1500. doi: 10.1152/jn.00667.2014. [DOI] [PubMed] [Google Scholar]
- 14.Pyo M.K., Choi S.H., Hwang S.H., Shin T.J., Lee B.H., Lee S.M., Lim Y., Kim D., Nah S.Y. Novel glycoproteins from ginseng. J Ginseng Res. 2011;35:92–103. [Google Scholar]
- 15.Kelleher J.A., Chan P.H., Chan T.Y., Gregory G.A. Modification of hypoxia-induced injury in cultured rat astrocytes by high levels of glucose. Stroke. 1993;24:855–863. doi: 10.1161/01.str.24.6.855. [DOI] [PubMed] [Google Scholar]
- 16.Zachary I. Neuroprotective role of vascular endothelial growth factor: signalling mechanisms, biological function, and therapeutic potential. Neurosignals. 2005;14:207–221. doi: 10.1159/000088637. [DOI] [PubMed] [Google Scholar]
- 17.Rosenstein J.M., Krum J.M., Ruhrberg C. VEGF in the nervous system. Organogenesis. 2010;6:107–114. doi: 10.4161/org.6.2.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krum J.M., Mani N., Rosenstein J.M. Angiogenic and astroglial responses to vascular endothelial growth factor administration in adult rat brain. Neuroscience. 2002;110:589–604. doi: 10.1016/s0306-4522(01)00615-7. [DOI] [PubMed] [Google Scholar]
- 19.Mani N., Khaibullina A., Krum J.M., Rosenstein J.M. Astrocyte growth effects of vascular endothelial growth factor (VEGF) application to perinatal neocortical explants: receptor mediation and signal transduction pathways. Exp Neurol. 2005;192:394–406. doi: 10.1016/j.expneurol.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Ijichi A., Sakuma S., Tofilon P.J. Hypoxia-induced vascular endothelial growth factor expression in normal rat astrocyte cultures. Glia. 1995;14:87–93. doi: 10.1002/glia.440140203. [DOI] [PubMed] [Google Scholar]
- 21.Shin T.J., Kim H.J., Kwon B.J., Choi S.H., Kim H.B., Hwang S.H., Lee B.H., Lee S.M., Zukin R.S., Park J.H. Gintonin, a ginseng-derived novel ingredient, evokes long-term potentiation through N-methyl-D-aspartic acid receptor activation: involvement of LPA receptors. Mol Cells. 2012;34:563–572. doi: 10.1007/s10059-012-0254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S., Kim M.S., Park K., Kim H.J., Jung S.W., Nah S.Y., Han J.S., Chung C. Hippocampus-dependent cognitive enhancement induced by systemic gintonin administration. J Ginseng Res. 2016;40:55–61. doi: 10.1016/j.jgr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung K.W., Pon Y.L., Wong R.N., Wong A.S. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J Biol Chem. 2006;281:36280–36288. doi: 10.1074/jbc.M606698200. [DOI] [PubMed] [Google Scholar]
- 24.Yang N., Chen P., Tao Z., Zhou N., Gong X., Xu Z., Zhang M., Zhang D., Chen B., Tao Z. Beneficial effects of ginsenoside-Rg1 on ischemia-induced angiogenesis in diabetic mice. Acta Biochim Biophys Sin. 2012;44:999–1005. doi: 10.1093/abbs/gms092. [DOI] [PubMed] [Google Scholar]
- 25.Hwang S.H., Lee B.H., Choi S.H., Kim H.J., Won K.J., Lee H.M., Rhim H., Kim H.C., Nah S.Y. Effects of gintonin on the proliferation, migration, and tube formation of human umbilical-vein endothelial cells: involvement of lysophosphatidic-acid receptors and vascular-endothelial-growth-factor signaling. J Ginseng Res. 2016;42:35–41. doi: 10.1016/j.jgr.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senger D.R., Galli S.J., Dvorak A.M., Perruzzi C.A., Harvey V.S., Dvorak H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 27.Muche A., Bürger S., Arendt T., Schliebs R. Hypoxic stress, brain vascular system, and β-amyloid: a primary cell culture study. Nutr Neurosci. 2015;18:1–11. doi: 10.1179/1476830513Z.000000000112. [DOI] [PubMed] [Google Scholar]
- 28.Holmes K., Roberts O.L., Thomas A.M., Cross M.J. Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition. Cellular Signaling. 2007;19:2003–2012. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Sondell M., Lundborg G., Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang S.H., Shin E.J., Shin T.J., Lee B.H., Choi S.H., Kang J., Kim H.J., Kwon S.H., Jang C.G., Lee J.H. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates Alzheimer's disease-related neuropathies: involvement of non-amyloidogenic processing. J Alzheimers Dis. 2012;31:207–223. doi: 10.3233/JAD-2012-120439. [DOI] [PubMed] [Google Scholar]
- 31.Sinor A.D., Irvin S.M., Cobbs C.S., Chen J., Graham S.H., Greenberg D.A. Hypoxic induction of vascular endothelial growth factor (VEGF) protein in astroglial cultures. Brain Res. 1998;812:289–291. doi: 10.1016/s0006-8993(98)00976-7. [DOI] [PubMed] [Google Scholar]