Abstract
Reports of myocarditis and pericarditis following smallpox vaccination in adults suggested a need to assess inflammatory cardiac disease risk among adults who receive live viral vaccinations. From 1996 through 2007, among 416,629 vaccinated adults in the Vaccine Safety Datalink, we identified one probable pericarditis case and no cases of myocarditis in the 42 days following a live viral vaccination. Our self-controlled risk interval analysis found that, based on one case identified during the risk interval and 10 cases during the control interval, there is no increased risk of myopericarditis in the 42 days following vaccination (IRR, 0.57; 95% CI, 0.07, 4.51). Our study suggests that the occurrence of myopericarditis following live viral vaccination is rare with an estimated incidence of 0.24 per 100,000 vaccinated, which is not higher than the background rate and is much lower than the incidence rates reported following smallpox vaccination.
Keywords: Myocarditis, Pericarditis, Vaccination adverse effects
1. Introduction
Reports of cardiac complications – including myocarditis, pericarditis, and arrhythmias – following smallpox vaccination, have dated back to the 1950s in the United States, although a total of only six such cases were reported prior to 2003 [1–4]. This changed when, from December 2002 through December 2003, a campaign was held to vaccinate 540,824 U.S. military personnel against smallpox using DryVax vaccine. During this campaign, 67 cases of myocarditis or pericarditis were reported with an average period of 10 days between vaccination and symptom onset [5].
Although cardiovascular complications due to vaccination are rare, these reports of myocarditis and pericarditis following smallpox vaccination in adults have raised the issue of assessing inflammatory cardiac disease risk following other live viral vaccinations in adults. We sought to determine the risk of myocarditis and pericarditis following the administration of non-smallpox, live viral vaccinations among adults.
2. Methods
We identified a retrospective cohort of adults 18 years and older who received a measles-mumps-rubella vaccine (MMR), varicella vaccine (VZV), oral polio vaccine (OPV), or yellow fever vaccine (YFV) between January 1, 1996 and December 31, 2007 at four integrated healthcare delivery organizations: Kaiser Permanente Northwest, Kaiser Permanente Washington (formerly Group Health), Northern California Kaiser Permanente, and Southern California Kaiser Permanente. These organizations collaborate with the Centers for Disease Control and Prevention (CDC) as the Vaccine Safety Datalink (VSD) to conduct population-based research on vaccine safety [6,7]. The cohort was restricted to patients with at least 6 months of health plan enrollment prior to their first live viral vaccination. We then identified cohort members who had a diagnosis of myocarditis or pericarditis within the study period at any point following vaccination.
Acute myocarditis was identified with the following ICD-9 codes: 422.0 (acute myocarditis in diseases classified elsewhere); 422.90 (acute myocarditis NOS); 422.91 (idiopathic myocarditis); 422.99 (other and unspecified acute myocarditis); and, 429.0 (myocarditis unspecified). Acute pericarditis was identified with ICD-9 code 420.x. We identified the earliest diagnosis during the study period and cases diagnosed prior to 1996 were excluded. We also collected information about comorbid conditions diagnosed prior to the myocarditis or pericarditis diagnosis date, including diabetes, hyperlipidemia, hypertension, coronary insufficiency, prior myocardial infarction, chronic ischemic heart disease, coronary artery disease, and connective tissue disorder.
During medical record abstraction, we confirmed that the cardiac event diagnosis was recorded in the medical record and the earliest diagnosis occurred during the study period. We excluded diagnoses that were miscoded or considered to be a ‘rule-out’ diagnosis, could not be confirmed because of a lack of information in the medical record, or occurred prior to 1996. We also collected information about cardiac symptoms, co-morbidities, and diagnostic tests (e.g., echocardiograms, chest x-rays, cardiac enzymes). A cardiology nurse and physician then further classified cases with validated diagnoses as definite, probable or possible according to surveillance case definitions for myocarditis and pericarditis developed by the U.S. Department of Defense and CDC for their smallpox vaccine program [8–10]. We defined definite pericarditis cases as those with at least two of the following criteria: presence of chest pain made worse by lying down and relieved by sitting up or leaning forward; pericardial rub or auscultatory sign with 1–3 components per beat; electrocardiogram with ST elevations, or PR depressions without reciprocal ST depressions; presence of abnormal collection of pericardial fluid on echocardiogram; or, histopathologic evidence of pericardial inflammation. Persons with elevated cardiac enzymes or evidence of myocardial inflammation plus at least two of the following criteria were considered definite myocarditis cases: presence of dyspnea, palpitations, or chest pain; electrocardiogram (ECG) abnormalities; or evidence of focal or diffuse depressed left ventricular function identified by an imaging study.
We conducted a self-controlled risk interval analysis using conditional Poisson regression. Our analysis included possible, probable, and definite cases who were vaccinated during the study period, and we used a composite outcome of myopericarditis consistent with many of the previously published smallpox vaccine studies. We defined the risk interval as the 42-day window following YFV, VZV, OPV, or MMR vaccination; the control interval was defined as the time period 85 to 365 days following vaccination. We censored a ‘wash out’ period of 43 to 84 days after vaccination. We selected the 42-day exposure window based on prior smallpox vaccination investigations that suggested these cardiac events developed soon after vaccination at a mean onset time of 10 days [11]. We calculated an incidence rate ratio (IRR) and 95% confidence interval (CI), which compared the incidence of myopericarditis in the risk interval to incidence in the control interval.
This study was approved by the Institutional Review Boards at all participating sites.
3. Results
We identified 416,629 adults who received at least one live viral vaccine between 1996 and 2007 and met eligibility criteria (Fig. 1). Two hundred ninety-seven thousand doses of measles, mumps, or rubella-containing vaccine, 87,295 doses of VZV, 76,606 doses of YFV, and 35,291 doses of OPV were administered to this cohort during the study period. Of the 416,629 eligible vaccinated adults, 120 had a coded pericarditis diagnosis and 32 had a coded myocarditis diagnosis at any time following vaccination. After review of these potential cases, 54 (45%) had a validated pericarditis diagnosis and 18 (56%) had a validated myocarditis diagnosis. The remaining 80 potential cases were excluded because they had miscoded or rule-out diagnoses, lacked sufficient information in the medical record to confirm the diagnosis, or were diagnosed prior to 1996. Twelve (67%) of the myocarditis diagnoses occurred among 18–29-year-olds, whereas pericarditis was more evenly distributed across the age strata. Both diagnoses were more common in males than females (myocarditis: 79% male; pericarditis: 69% male). Hypertension (n = 16, 30%)and hyperlipidemia (n = 11, 20%) were common comorbid diagnoses in pericarditis cases. After adjudication, only 5 patients were classified as definite pericarditis and only 3 were classified as definite myocarditis cases according to the DoD/CDC definitions.
Fig. 1.
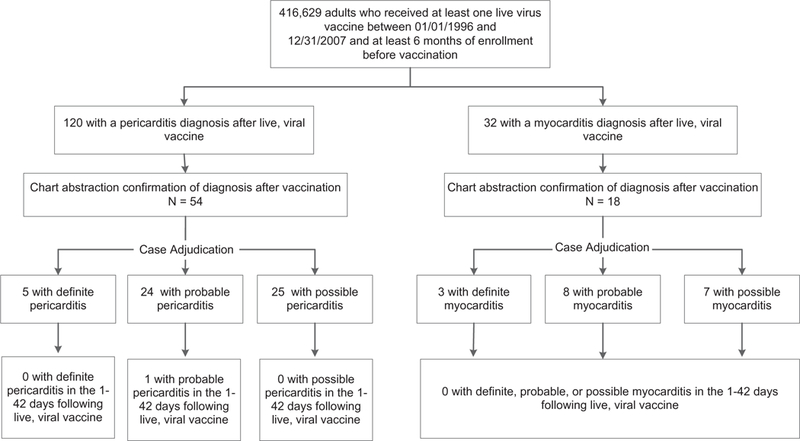
Identification and validation of myocarditis and pericarditis cases following live viral vaccination.
One probable pericarditis case occurred in the 42 days following vaccination; no myocarditis cases and no definite or possible pericarditis cases occurred in the 42-day period. The one probable pericarditis case occurred 36 days after YFV. This patient also received tetanus-diphtheria (Td) and meningococcal vaccines on the same day as YFV and inactivated polio, hepatitis A, and influenza vaccines in the four days after YFV. Thus, the incidence rate of diagnosed myopericarditis in the 42 days following vaccination with VZV, YFV, OPV, or MMR was 0.24 per 100,000 vaccinated. The remaining 71 definite, probable, and possible cases occurred at least 43 days after vaccination and were relatively evenly distributed over the course of the maximum of 10 years of follow-up after vaccination (Fig. 2). Only 10 of these cases occurred in the 85 to 365 days following vaccination control window. Based on the one case identified during the risk interval and 10 cases that occurred during the control interval, we found no increased risk of myopericarditis in the 42 days following vaccination (IRR, 0.57; 95% CI, 0.07, 4.51).
Fig. 2.
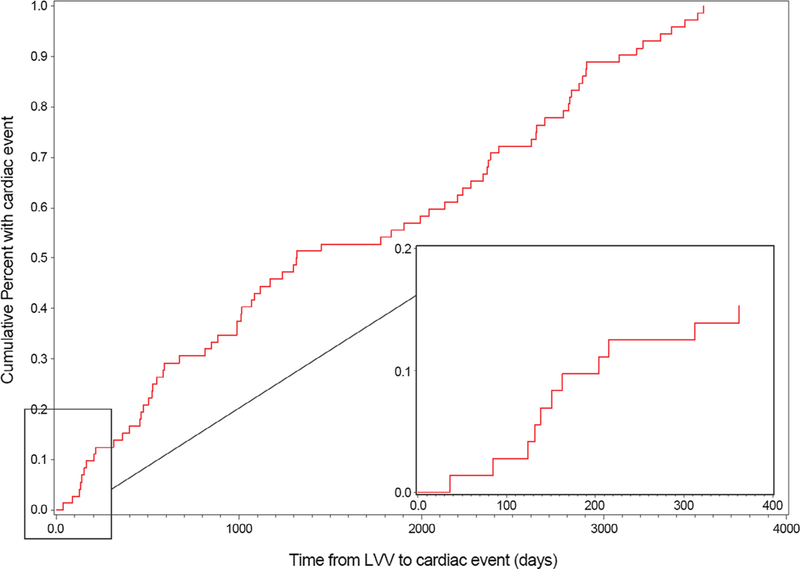
Distribution of myocarditis (n = 18) and pericarditis (n = 54) cases over time since live viral vaccination.
4. Discussion
There are limited published data about cardiac events after non-smallpox vaccinations. This study evaluated myopericarditis risk after non-smallpox, live viral vaccination in a population-based cohort. Seven cases of pericarditis after hepatitis B and influenza vaccine were reported in 1980–90s [12,13]. In 2000, a case of myopericarditis in a 31-year old man was reported 2 days after diphtheria, tetanus and polio vaccination [14]. Additional research reported no detectable increase in the risk of myocardial infarction or stroke following influenza, tetanus, or pneumococcal vaccinations [15,16].
In the absence of additional incidence studies of non-smallpox vaccinations, we can only compare our rates to those from published smallpox vaccination studies [5,17]. Among U.S. military personnel, the observed incidence of myopericarditis that came to medical attention over a 30-day observation window following smallpox vaccination was 12.3 cases per 100,000 [5]. In turn, Engler et al. prospectively followed a cohort of 1081 healthy military personnel to identify cardiac symptoms, biomarkers (specifically troponin T), and ECG changes, and identified four cases of probable myocarditis and one case of suspected pericarditis in the 30 days after smallpox vaccination, resulting in a higher incidence of 463 cases per 100,000 [17]. While our study does not speak to risk related to smallpox vaccination, our results provide evidence for the lack of an association between other commonly-administered live viral vaccines and these cardiac outcomes.
Myocarditis and pericarditis are inflammatory processes with variable and unpredictable clinical presentations and course. Thus, diagnosis of these conditions is often difficult. Considering this, we acknowledge the potential for underestimation of the true occurrence of myocarditis and pericarditis, as patients may not present in the healthcare setting for these conditions or the diagnosis may not be accurately reflected in coded data. For example, the Engler et al. study reported a higher incidence of myopericarditis after vaccination through active follow up of vaccinated participants; the authors note that 3 of the 5 cases they observed would not have sought medical care for their symptoms outside of the study protocol [15]. Reducing the underestimation and misclassification of cases would require further enhancement of VSD active surveillance, an effort that would not be feasible, especially given the population size that would be needed to detect rare outcomes such as these.
Our study focused on non-smallpox, live viral vaccines recommended for previously unvaccinated adults, those without documented immunity, or those at increased risk for exposure or transmission, such as healthcare personnel or international travelers. Although a live attenuated influenza vaccine was licensed in 2003 for use in adults, we did not include it in our analysis since use of the vaccine in adults during most of the study period was limited in the VSD. A herpes zoster vaccine was licensed and recommended for adults 60 years of age and older in 2006, but also was not included in this analysis; a separate VSD study reported no increased risk of myocarditis or pericarditis in the 1–42 days following zoster vaccination [18].
We found that the rate of myocarditis and pericarditis in the 42 days following VZV, YFV, OPV, or MMR vaccination was very low and was not statistically different than the rate of these events during unexposed, control intervals. These findings confirm that cardiovascular complications related to commonly-administered, live viral vaccination are rare in adults.
Acknowledgements
The authors thank Mindy Gramberg, RN and Stephen Fortmann, MD for their assistance with case adjudication.
Financial support for this study was provided in full by the Centers for Disease Control and Prevention (200–2002-00732), through America’s Health Insurance Plans. The manuscript was reviewed and approved through the clearance process of the Centers for Disease Control and Prevention prior to submission. The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention or America’s Health Insurance Plans.
References
- [1].Mead J. Serum transaminase and electrocardiographic findings after smallpox vaccination: case report. J Am Geriatr Soc 1966;14(7):754–6. [DOI] [PubMed] [Google Scholar]
- [2].Phillips M, Robinowitz M, Higgins JR, Boran KJ, Reed T, Virmani R. Sudden cardiac death in Air Force recruits. A 20-year review. JAMA 1986;256 (19):2696–9. [PubMed] [Google Scholar]
- [3].Cangemi VF. Acute pericarditis after smallpox vaccination. N Engl J Med 1958;258(25):1257–9. [DOI] [PubMed] [Google Scholar]
- [4].Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968. N Engl J Med 1969;281(22):1201–8. [DOI] [PubMed] [Google Scholar]
- [5].Eckart RE, Love SS, Atwood JE, Arness MK, Cassimatis DC, Campbell CL, et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol 2004;44(1):201–5. [DOI] [PubMed] [Google Scholar]
- [6].Baggs J, Gee J, Lewis E, Fowler G, Benson P, Lieu T, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics 2011;127 (Suppl. 1):S45–53. [DOI] [PubMed] [Google Scholar]
- [7].McNeil MM, Gee J, Weintraub ES, Belongia EA, Lee GM, Glanz JM, et al. The Vaccine Safety Datalink: successes and challenges monitoring vaccine safety. Vaccine 2014;32(42):5390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Update: cardiac-related events during the civilian smallpox vaccination program–United States, 2003. MMWR Morb Mortal Wkly Rep 2003;52 (21):492–6. [PubMed] [Google Scholar]
- [9].Cardiac adverse events following smallpox vaccination–United States, 2003. MMWR Morb Mortal Wkly Rep 2003;52(12):248–50. [PubMed] [Google Scholar]
- [10].Morgan J, Roper MH, Sperling L, Schieber RA, Heffelfinger JD, Casey CG, et al. Myocarditis, pericarditis, and dilated cardiomyopathy after smallpox vaccination among civilians in the United States, January-October 2003. Clin Infect Dis 2008;46(Suppl. 3):S242–50. [DOI] [PubMed] [Google Scholar]
- [11].Halsell JS, Riddle JR, Atwood JE, Gardner P, Shope R, Poland GA, et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA 2003;289(24):3283–9. [DOI] [PubMed] [Google Scholar]
- [12].Amsel SG, Hanukoglu A, Fried D, Wolyvovics M. Myocarditis after triple immunisation. Arch Dis Child 1986;61(4):403–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].de MA, Luwaert R, Chaudron JM: Symptomatic pericarditis after influenza vaccination: report of two cases. Chest 2000;117(6):1803–5. [DOI] [PubMed] [Google Scholar]
- [14].Boccara F, Benhaiem-Sigaux N, Cohen A. Acute myopericarditis after diphtheria, tetanus, and polio vaccination. Chest 2001;120(2):671–2. [DOI] [PubMed] [Google Scholar]
- [15].Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med 2004;351(25):2611–8. [DOI] [PubMed] [Google Scholar]
- [16].Tseng HF, Slezak JM, Quinn VP, Sy LS, Van den Eeden SK, Jacobsen SJ. Pneumococcal vaccination and risk of acute myocardial infarction and stroke in men. JAMA 2010;303(17):1699–706. [DOI] [PubMed] [Google Scholar]
- [17].Engler RJ, Nelson MR, Collins LC Jr, Spooner C, Hemann BA, Gibbs BT, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One 2015;10(3):e0118283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tseng HF, Liu A, Sy L, Marcy SM, Fireman B, Weintraub E, et al. Jacobsen SJ, for the Vaccine Safety Datalink (VSD) Team. Safety of zoster vaccine in adults from a large managed-care cohort: a Vaccine Safety Datalink study. J Intern Med 2012;271:510–20. [DOI] [PubMed] [Google Scholar]


