Abstract
We describe a series of EBV-positive circumscribed, ulcerative lesions associated with various types of immunosuppression (IS). The study group (26 patients) comprised 10 males and 16 females, median age 77 years (range 42–101). IS in 9 cases included azathioprine (AZA), methotrexate (MTX) or cyclosporin-A (CyA). 17 patients had age related immunosenescence. Patients presented with isolated sharply circumscribed ulcers involving oropharyngeal mucosa (16), skin (6), and gastrointestinal tract (4). Lesions were histologically characterized by a polymorphous infiltrate and atypical large B-cell blasts often with Hodgkin/Reed-Sternberg (HRS) cell-like morphology. The B-cells showed strong CD30 and EBER positivity, some with reduced CD20 expression, in a background of abundant T-cells. CD15 was positive in 43% of cases (10/23). The pathological features were identical regardless of the anatomical site or cause of IS. PCR revealed 39% (7/18) clonal Ig gene rearrangements with 38% (6/16) and 31% (5/16) clonal and restricted T-cell patterns respectively. 25% of patients (5/20) received standard chemotherapy and/or radiotherapy. 45% (9/20) regressed spontaneously with no treatment and 15% (3/20) were characterized by a relapsing and remitting course. All of the iatrogenic lesions (6/6) with available follow up responded to reduction of IS. All patients achieved complete remission with no disease associated deaths over a median follow up period of 22 months (range 3–72).
We propose EBV positive Mucocutaneous Ulcer (EBVMCU) as a newly recognized clinico-pathological entity with Hodgkin-like features and a self limited, indolent course, generally responding well to conservative management. Association with various forms of IS implies a common pathogenetic mechanism. The localised nature of the disease may be due to a minimal and localized lapse in immunosurveillance over EBV.
Keywords: Epstein Barr virus, Immunosuppression, Muco-cutaneous, Lymphoproliferative
Introduction
In patients with various types of IS, the Epstein Barr virus (EBV) has long been associated with B-cell lymphoproliferative disorders. Following primary infection at an early age EBV persistently infects B-cells of most adults. The virus elicits B-cell transformation and proliferation through complex mechanisms. The products of viral genes upregulate a variety of cellular antigens and expression of genes in B-cells. Key molecular pathways controlling the cell cycle, such as NF-kB, are activated and virus induced cytokines exert paracrine proliferative effects. 28, 38, 70, 35 In physiological circumstances the inherent propensity of EBV to induce B-cell proliferation is counterbalanced by complex immunological interactions that maintain the overall number of EBV infected B-cells in the body at a very low level. 55
Immunosuppression affects various aspects of immune homeostasis and surveillance, thus permitting the emergence of EBV-induced B-cell lymphoproliferative disorders (EBV-LPDs). The spectrum of immunosuppressive aetiologies resulting in EBV-LPDs has expanded to include primary immune deficiency, HIV infection, post transplant setting and other iatrogenic causes such as MTX and TNF-alpha antagonists. They affect reactivity of the immune system to EBV infected cells in a variety of ways. 1, 7, 21, 32, 53, 62
Immunosenescence due to ageing has recently been recognised as a cause of EBV positive diffuse large B-cell lymphoma in elderly patients. 50, 51, 60, 61 In the elderly, the T-cell response appears most profoundly affected, with the accumulation of clonal CD8 positive T-cells with mature, memory cell phenotypes but diminished functionality, due to a markedly restricted epitope specific repertoire. Thus, in advanced age there is a 100 fold decrease of T-cell specificities compared to a young immunocompetent individual. 16, 18, 30, 31, 42, 43, 46–49, 52, 56, 67, 69
The pathological features of EBV-LPDs arising in the setting of immune suppression (IS) are diverse and often shared among different types of immunosuppressive disorders. 1 However, the spectrum differs depending on the aetiology of IS. Polymorphous lesions, best characterised in post-transplant EBV-LPD, occur with all types of IS. In patients receiving MTX therapy there is a higher incidence of classical Hodgkin’s lymphoma (cHL) or Hodgkin-like lesions. A polymorphous form of diffuse large B-cell lymphoma (DLBCL) has been described in the elderly related to decreased immune surveillance and incorporated as a provisional subtype of DLBCL in the 2008 WHO classification. 1, 26, 27, 32, 45, 50, 51, 60, 61 However, overlap exists, and reactive lymphoid hyperplasia associated with EBV also has been described in the context of advanced age. 34–36 Despite better characterisation and improved classification, diagnosis and management of EBV-LPDs is still problematic. Pathological assessment in many instances provides limited prognostic information and management often relies on clinical parameters and empirical decisions. Additional features to aid prognostication and indications for aggressive therapy are still being sought.
In our diagnostic practice we have encountered lymphoproliferative lesions presenting as isolated cutaneous and mucosal ulcers during IS with MTX, AZA, CyA or in elderly patients not receiving immunosuppressive agents. The pathological features of these lesions have not been consistently described in the literature. We provide an analysis of 26 cases with a view to highlighting their distinctive pathological appearances and characteristic clinical behaviour and propose that these lesions constitute a new clinicopathological category. We provide discussion on the possible common pathogenetic mechanisms of EBV-induced mucocutaneous ulcer in different IS settings including old age. Circumstances resulting in localised and self limiting disease are analysed together with the morphological differential diagnosis, prognostic significance and therapeutic implications.
Material and methods
Case selection
The cases were selected from the files of the Laboratory of Pathology of the National Cancer Institute (Bethesda MD, USA) and All Wales Lymphoma Panel (AWLP) (Cardiff, United Kingdom). The source included primary diagnostic material and second opinion referrals from pathology departments across the United States and 17 departments of pathology in Wales over a total period of 15 years. The initial selection included all cases of EBV-LPD with significant expression of EBER on in situ hybridisation exceeding that seen in normal lymphoid tissue. 22 The following were excluded: patients with a previous history of lymphoma; lymphoma entities in which presence of EBER in the neoplastic cells or background infiltrate represents a diagnostic requirement, e.g. lymphomatoid granulomatosis (LyG), DLBCL associated with chronic inflammation, primary effusion lymphoma, plasmablastic lymphoma. Well circumscribed, isolated ulcerating lesions presenting in the skin or on mucosal surfaces were selected from this wider group. For the lesions matching the selection criteria we designated the term “EBV Associated Mucocutaneous Ulcer” (EBVMCU). Adequately fixed and processed tissue material was available together with detailed clinical history.
Histological assessment
The biopsy material was fixed in 10% formalin and embedded in paraffin following routine histological tissue processing. Three to four micron histological sections were stained with H&E for microscopic examination.
Immunohistochemistry
Immunostaining on paraffin sections was performed for CD3, CD4, CD8, CD20, CD30 and CD15. For additional characterisation of the lesional cells a proportion of cases were immunostained for CD45, CD79a, MUM1, PAX5, Oct-2, Bob.1, CD138, LMP1, CD10 and BCL-6. Staining was performed with an automated immunostaining system, either DAKO Autostainer+ (AWLP cases) (Table 1) or Ventana Medical Systems [Tucson, AZ] (NCI cases), with antigen retrieval and antibody dilutions per manufacturer’s recommendation and as previously published. 71
Table 1.
Immunohistochemistry - Primary antibodies (AWLP)
| Antibody | Dilution | Manufacturer |
|---|---|---|
| CD45 | 1/200 | Dako M0701 |
| CD3 | 1/200 | Dako A0452 |
| CD4 | 1/50 | Vision Biosystems NCL-CD4–368 |
| CD8 | 1/100 | DAKO M7103 |
| CD20 | 1/500 | DAKO M0755 |
| CD79a | 1/200 | DAKO M7050 |
| MUM 1 | 1/50 | DAKO M7259 |
| PAX 5 | 1/80 | BD Transduction Laboratories 610863 |
| Oct-2 | 1/500 | Santa Cruz SC-233 |
| Bob.1 | 1/500 | Santa Cruz SC-955 |
| CD138 | 1/100 | DAKO M7228 |
| LMP-1 | 1/50 | DAKO MO897 |
| CD30 | 1/50 | DAKO MO751 |
| CD15 | 1/40 | Becton Dickinson 347420 |
| CD10 | 1/100 | Vision Biosystems NCL-L-CD10–270 |
| BCL6 | 1/25 | DAKO M7211 |
In situ hybridisation
In situ hybridisation for Epstein-Bar Virus Encoded RNA (EBER) was conducted on formalin fixed paraffin sections using a FITC labelled oligonucleotide probe supplied by Ventana on an automated stainer (Ventana-Benchmark). Visualisation was achieved using the ISH iView system with Alk-Phosphatase and NT/BCIP substrate, with Fast red as contrast.
PCR for immunoglobulin (IG) and T-cell receptor (TCR) gene rearrangements
For NIH cases PCR analysis was performed using a routine diagnostic protocol with in house custom made primers. DNA was extracted from formalin fixed paraffin embedded tissue blocks using a standard protocol. For IgH rearrangements DNA amplification by PCR was conducted using Framework Region III primers. 57, 58 Additional semi-nested PCR amplifications were performed using primers to Framework Region II of the immunoglobulin heavy chain gene according to the method of Ramasamy et al. 54 Cases negative for IgH rearrangement were further studied for rearrangements of the IGκ locus using the Biomed II primer set described by van Dongen et al 65 and supplied by InVivoScribe Technologies (IGK Gene Clonality Assay - ABI Fluorescence Detection). These reactions interrogate rearrangements involving the Vκ loci and Jκ (Tube A), the Vκ locus and the κ DE locus (Tube B), and the κ intron RSS locus and the κ DE locus (Tube B). The products were analyzed by capillary electrophoresis on an ABI 3130 Genetic Analyzer, and electropherograms were analyzed using GeneMapper software version 3.7 (ABI).
For TCR gene rearrangements amplification was performed using primers to the TCR gamma chain gene. 40 Two separate reactions were performed for each extract, one with primers V-gamma-101, J-gamma-11 and J-gamma-12 (set 1), and a second with primers V-gamma-101, V-gamma-11 and Jp12 (set 2).
All the amplification reactions were carried out in duplicate. The products were analysed by acrylamide gel or capillary electrophoresis. The results were interpreted in conjunction with a blank (water), polyclonal and monoclonal control.
PCR analysis on the cases from the AWLP files was conducted using the Biomed 2 primers interrogating specific gene segments of the IgH, IGK, Kappa deleting element, TCR-beta and TCR-gamma gene rearrangements. DNA extraction and amplification from formalin fixed paraffin embedded tissue was conducted using a standardised protocol. 14, 65 The tests were carried out in duplicate and the results were visualised by acrylamide gel electrophoresis and by GeneScan. The results were interpreted in conjunction with a blank, polyclonal and monoclonal control.
Results
Patient characteristics
The demographic and clinical information is summarised in table 2. Twenty six cases of EBVMCU were identified from 200 EBV positive B-cell LPDs. The study group comprised 10 male and 16 female patients with a median age of 77 (range 42–101). Nine patients were receiving therapeutic IS for an autoimmune condition (rheumatoid arthritis (5), ulcerative colitis (1), sarcoidosis with myasthenia gravis (1) and systemic lupus erythematosus (1)) or allogeneic stem cell bone marrow transplantation (1). The median age of these patients was 69 (42–80). The IS regimens included standard therapeutic doses of MTX, AZA and CyA. For the remaining 17 patients there was no apparent source of IS. These patients were designated as age related EBV-LPD. The median age was 80 years (range 64–101). The clinical presentation was characterized by slowly developing indurated ulcers which in 16 cases involved oropharyngeal mucosa (buccal mucosa (2), tongue (8), tonsils (5), palate (1)). Four patients each had oesophageal, large bowel (2) and rectal ulcers respectively and 6 had lesions on the skin of the lips, arms and chest. Two patients had concomitant isolated unilateral neck lymphadenopathy (cases 9 and 13) but none had evidence of systemic lymphadenopathy, hepatosplenomegaly or bone marrow involvement. In all patients with oropharyngeal or cutaneous lesions the clinical suspicion leading to biopsy was of a non-haematological malignancy, usually thought to be squamous cell carcinoma. The oesophageal, colonic and rectal lesions were on clinical grounds initially also considered to represent non-lymphoid tumours or inflammatory bowel disease. Upon request for pathology review, the submitted histological diagnoses in 10 cases included classical Hodgkin lymphoma (5), diffuse large B-cell lymphoma (3) CD30 positive cutaneous T-cell lymphoproliferation (1) and EBV positive lymphoproliferative process (1).
Table 2.
Patients - Presentation, treatment and outcome
| Case number | Age | Sex | Summary of presentation | Source of immunosuppression | Site | Treatment | Outcome | Length of follow up (mo) |
|---|---|---|---|---|---|---|---|---|
| 1 | 79 | M | Ulcer right cheek | Old age | Skin | None | SR DNED | 25 |
| 2 | 82 | M | Ulcerated right lower lip | Old age | Lip, Skin | None | SR | NA |
| 3 | 82 | F | Ulcer on lower lip, history of waxing and waning | Old age | Lip | None | SR/RR | NA |
| 4 | 88 | F | Ulcerated mass on base of tongue, waxing and waning | Old age | Tongue | None | SR/RR DNED | 24 |
| 5 | 80 | F | Ulcerated mass in palate | Old age | Palate | Radiotherapy | CR | 60 |
| 6 | 101 | M | Large tonsillar ulcer | Old age | Tonsil | R-CHOP | CR DNED | 12 |
| 7 | 76 | M | Ulcerated mass on base of tongue | Old age | Tongue | None | SR | 12 |
| 8 | 84 | F | Ulcerated lesion on tongue and floor of mouth; | Old age | Tongue | None | SD DNED | 5 |
| 9 | 68 | F | Tonsillar swelling and ipsilateral neck lymphadenopathy | Old age | Tonsil | R-CHOP and Radiotherapy | CR | 24 |
| 10 | 64 | F | Massive ulcerated tongue lesion; previous spontaneously resolving oropharyngeal lesions | Old age | Tongue | Radiotherapy | CR DNED | 15 |
| 11 | 63 | F | Tonsillar ulcer | Old age | Tonsil | NA | NA | NA |
| 12 | 73 | M | Localised ulcerated tonsillar mass and subsequent tongue lesion | Old age | Tonsil | None | SR/RR DNED | 12 |
| 13 | 68 | F | Tongue ulcer; ipslateral neck lymphadenopathy | Old age | Tongue | None | SR | 36 |
| 14 | 75 | F | Ulcerative lesion on left arm | Old age | Skin | NA | NA | NA |
| 15 | 85 | F | Ulcerated mass in pharynx | Old age | Tonsil | Radiotherapy | CR | 3 |
| 16 | 88 | M | Large ulcerated papule on chest | Old age | Skin | None | SR | 3 |
| 17 | 89 | M | Ulcerated lesion on base of tongue | Old age | Tongue | NA | NA | NA |
| 18 | 80 | M | Ulcerated lesion on tongue; rheumatoid arthritis | MTX | Tongue | NA | NA | NA |
| 19 | 69 | F | Colonic mass; History of rheumatoid arthritis | MTX | Colon | NA | NA | NA |
| 20 | 42 | M | Poorly healing mucosal ulcer and maxillary ostemyelitis; History of sarcoidosis and myasthenia gravis | AZA | Oral mucosa | NA | NA | NA |
| 21 | 48 | F | Large tongue ulcer; Severe LE | CYA | Tongue | Reduction of IS | CR | 24 |
| 22 | 78 | M | Anorectal ulcer. Long history of ulcerative colitis | CYA | Rectum | Reduction of IS | CR | 23 |
| 23 | 80 | F | Ulcer on right arm; Long history of rheumatoid arthritis | MTX | Skin | Reduction of IS | CR | 60 |
| 24 | 60 | F | Lip mucosal ulcer penetrating onto skin surface; History of rheumatoid arthritis | MTX | Oral mucosa | Reduction of IS | CR | 72 |
| 25 | 75 | F | Oesophageal ulcer. History of rheumatoid arthritis | AZA | Oesophagus | Reduction of IS | CR | 17 |
| 26 | 64 | F | History of essential thrombocythaemia (2000) and secondary myelodysplasia (2007) treated with reduced intensity sibling stem cell transplant (2007 and 2009). Four months post transplant - diarrhoea suspicious of graft versus host disease. Small shallow ulcer at rectosigmoid junction with loss of background mucosal vascular pattern suspicious of inflammatory bowel disease. | CYA | Colon | Reduction of IS | CR | 6 |
NA-not available; CR-complete remission; DNED-died, no evidence of disease; SR-spontaneous remission; RR-relapsing/remitting, SD-stable disease
There was significant statistical difference between the age means of the age related and iatrogenic IS groups (t-test, pexact = 0.021).
Clinical course
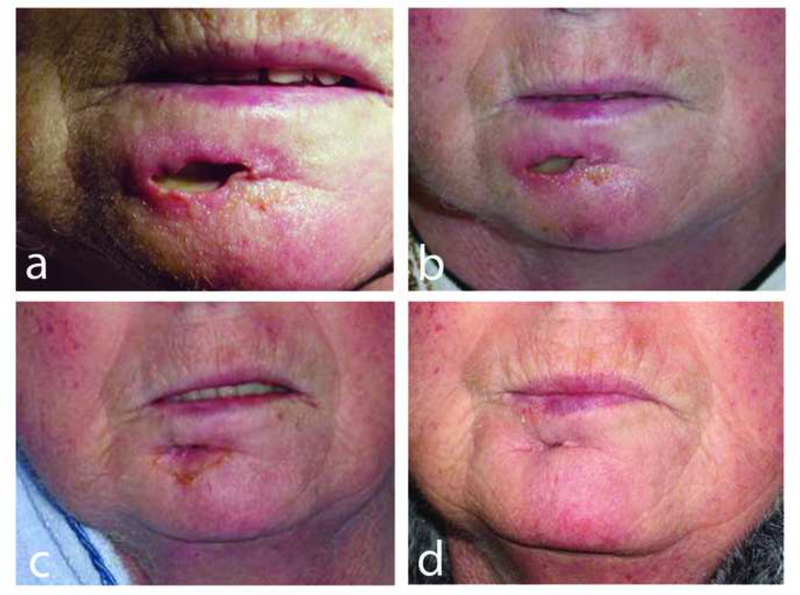
Follow-up information was available for 20 of the 26 patients (table 2). Of the 17 patients with age-related EBVMCU, 3 had multiple episodes of oral mucosal ulceration with spontaneous resolution. Nine patients in this group did not receive any therapy. Of those with available follow up, 1 patient (case 8) was deemed too frail for any treatment. He had stable persistent disease for five months and died of unrelated causes. Following biopsy, there was spontaneous regression of 5 lesions in the age-related group with no further evidence of disease after a median follow up of 18 (3–36) months. Five patients with age-related EBVMCU were actively treated. One received chemotherapy, 2 had combined radiotherapy and chemotherapy while 3 had radiotherapy only. All treated patients remained in complete remission after a median follow-up of 15 months (range 3–60). All 6 patients with iatrogenic IS and available follow up (cases 21–26) stayed in complete remission upon reduction of IS after a median follow up of 23 months (range 6–60) (figure 1 a-d). In all 26 cases there were no disease related deaths after an overall follow up of 22 months (3–72). Six patients died of unrelated causes. (AWLP cases 15, 21–26).
Figure 1.
EBVMCU in a patient treated with methotrexate (MTX) for rheumatoid arthritis (case 23). Spontaneous resolution after withdrawal of MTX. (a) At presentation the ulcer had perforated from the buccal sulcus onto the skin surface of the lower lip. Ulcer healing (b) 2 weeks, (c) 4 weeks and (d) 8 weeks after withdrawal of MTX.
Histology
The morphological features are summarised in table 3. All the lesions showed similar histological features regardless of the nature of IS. These were shallow, sharply circumscribed mucosal or cutaneous ulcers (figure 2a). The underlying infiltrate appeared polymorphous with a mixture of lymphocytes and immunoblasts. While scattered plasma cells, histiocytes and eosinophils were generally present in all cases, in some they were deemed to be particularly prominent (table 3, figure 2b, 2d). Scattered large pleomorphic blasts reminiscent of Hodgkin and Reed-Sternberg (HRS) cells were present in variable numbers in all cases (figure 2c). The lymphocytes in the background were abundant, many with angulated and medium size nuclei. A constant finding was dispersed “plasmacytoid” apoptotic cells (figure 2e). These were variably sized cells with abundant basophilic cytoplasm and apoptotic nuclei frequently showing coarse radial distribution of clumped nuclear chromatin reminiscent of the “cart-wheel” nuclear appearance of plasma cells. In six cases the large lesional cells were seen infiltrating medium-size arteries with associated thrombosis (figure 2f). This was usually accompanied by surrounding necrosis in addition to surface ulceration, which in one case appeared very marked. The base of the lesions was sharply delineated by a rim of small lymphocytes (figure 2g). The adjacent squamous epithelium showed reactive nuclear atypia, often with pseudoepitheliomatous hyperplasia. In the colon and rectum the infiltrate permeated between the intestinal crypts which were otherwise unremarkable (figure 2h).
Table 3.
Summary of histological features
| Dominant histological features (*) | |
| Well circumscribed ulcer | |
| Polymorphous background infiltrate | |
| RS and Hodgkin-like cells | |
| Numerous medium size T-cells | |
| Plasmacytoid apoptotic bodies | |
| Additional prominent histological features | % of cases |
| Plasma cells | 31 |
| Histiocytes | 19 |
| Eosinophils | 8 |
| Angioinvasion | 23 |
| Necrosis | 23 |
Features present in all cases
Figure 2.
Histological features of EBVMCU. (a) Well circumscribed ulcerated mucosal lesion with a band-like infiltrate underlying squamous mucosa. (b) The infiltrate is polymorphous, containing lymphocytes, histiocytes, immunoblasts, Hodgkin-like cells and apoptotic bodies. Intermediate size lymphoid cells with angulated nuclei and pale cytoplasm are present. (c) The lymphoid blasts show marked pleomorphism with Hodgkin and Reed-Sternberg cell features. (d) Eosinophils appear focally prominent. (e) Scattered apoptotic cells with plasmacytoid features are noted. (f) A thrombosed vessel with an aggregate of large cells adherent to and infiltrating the vessel wall. (g) The base of the lesion is rimmed off by a band of small lymphocytes. (h) Rectal lesion containing a polymorphous infiltrate between the crypts. (HE, original magnification: a X 20; g X 40; h X 100; b, f X 200; c, d X 400; e X 600).
Immunohistochemistry
The immunophenotype of the B-cell blasts and cells with the HRS features is summarised in table 4. The pleomorphic HRS cells were of B-cell phenotype (figure 3a). The lesional blasts were unequivocally positive for CD45 in 56% of cases (5/9) and showed no expression of this marker in 11% (1/9). Due to positivity in the background cells expression of CD45 could not be assessed with confidence in 33% of cases (3/9). There was reduced or absent expression of CD20 in 31% of cases (8/26) (figure 3b) and in a small proportion of tested cases the pleomorphic lesional blasts appeared positive for PAX5 and Oct-2 with variable expression of Bob.1 (table 4; figure 3 c-e). These cells were strongly positive for CD30 and in 43% of cases (10/23) co-expressed CD15 (figure 3 g-h). The cells positive for CD20, CD30, Oct-2 and PAX5 varied in size, ranging from small lymphocytes to larger immunoblasts to pleomorphic HRS-like cells. In all tested cases these cells were positive for MUM1 (8/8) (figure 3f) with no expression of CD10 and absent or focal positivity for BCL6. In all but one case tested (8/9) the lesional blasts were positive for LMP1 (figure 4b). Large numbers of T-lymphocytes were highlighted by CD3. Amongst the T-cell infiltrate there were scattered larger cells with immunoblastic features together with a large number of intermediate size cells. CD4 positive T-lymphocytes were more abundant compared to CD8. The T-cells with large and intermediate size morphology tended to be positive for CD8 (figure 3 j,k). In cases in which the base of the lesion was well represented, there was a dense rim of small CD3 positive lymphocytes between the lesion and adjacent soft tissue.
Table 4.
Summary of immunophenotype of EBER-positive B-cell blasts including cells with HRS-like features
| Antibody | Tested | Strong positive | Weak positive | Focal positive | Overall positive | Negative | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| CD45 | 9 | 5 | (55.5) | 0 | (0.0) | 0 | (0.0) | 5 | (55.5) | 1 | (33.3) |
| CD20 | 26 | 18 | (69.2) | 3 | (11.5) | 2 | (7.7) | 23 | (88.5) | 3 | (11.5) |
| CD79a | 12 | 11 | (91.7) | 1 | (8.3) | 0 | (0.0) | 12 | (91.7) | 0 | (0.0) |
| CD30 | 25 | 25 | (100.0) | 0 | (0.0) | 0 | (0.0) | 25 | (100.0) | 0 | (0.0) |
| CD15 | 23 | 4 | (17.4) | 0 | (0.0) | 6 | (26.1) | 10 | (43.5) | 13 | (56.5) |
| PAX5 | 9 | 7 | (77.8) | 2 | (22.2) | 0 | (0.0) | 9 | (100.0) | 0 | (0.0) |
| Oct-2 | 6 | 4 | (66.7) | 2 | (33.3) | 0 | (0.0) | 6 | (100.0) | 0 | (0.0) |
| Bob.1 | 5 | 1 | (20.0) | 3 | (60.0) | 0 | (0.0) | 4 | (80.0) | 1 | (20.0) |
| MUM1 | 8 | 8 | (100.0) | 0 | (0.0) | 0 | (0.0) | 8 | (100.0) | 0 | (0.0) |
| CD10 | 4 | 4 | (100.0) | 0 | (0.0) | 0 | (0.0) | 4 | (100.0) | 0 | (0.0) |
| Bcl6 | 5 | 0 | (0.0) | 0 | (0.0) | 2 | (40.0) | 2 | (40.0) | 3 | (60.0) |
| LMP1 | 9 | 8 | (88.9) | 0 | (0.0) | 0 | (0.0) | 8 | (88.9) | 1 | (11.1) |
Figure 3.
Immunohistochemical features of EBVMCU. (a) CD20 highlights a range of cell sizes including the large pleomorphic blasts. (b) In some cases, the lesional cells showed a reduced level of expression of CD20. (c) PAX5 and (d) Oct-2 are positive in the pleomorphic blasts. (e) Bob.1 is largely negative. (f) There is strong nuclear expression of MUM1. (g) There is abundant expression of CD30 highlighting a range of cell sizes. (h) Few atypical cells show expression of CD15 but in some cases (i) most cells were positive. (j) The background lymphocytic infiltrate is CD3 positive. There is a variation of cell size with occasional intermediate size cells. (k) The infiltrating T-cells are highlighted by CD8. (Original magnification: a-g X 400; h-k X 600).
Figure 4.
Expression of EBV markers in EBVMCU and T-cell PCR. (a) Linear positivity for EBER underlying the ulcerated mucosal squamous epithelium. The epithelium itself is negative. (b) The lesional cells are also positive for LMP1. (c) The EBER positive nuclei are of variable size. (d) Polyacrilamide PCR gel with TCR gamma primers showing multiple, reproducible prominent bands (boxed), highlighting the “restricted” T-cell response. (e) Inset of a genescan plot with the Biomed-2 TCR gamma primers show multiple irregular peaks (arrows) in keeping with the “restricted” T-cell response. (Original magnification: a X 20; b, c X 400).
In situ hybridisation
In situ hybridisation for EBER in all cases showed abundant staining highlighting a range of nuclear sizes, from small lymphocytes to immunoblasts and large pleomorphic cells with HRS cell morphology (figure 4c). Positivity for EBER co-localised with the expression of LMP1, CD20 and PAX5. In cases with identifiable angioinvasion, EBER positive blasts were seen scattered within vessel walls. EBER also highlighted the circumscribed nature of the lesions, displaying a band-like positivity underlying the ulcerated surface (figure 4a). No staining could be seen within the epithelium.
PCR for IG and TCR gene rearrangements
A PCR result could be obtained in 18 cases (table 5). 18 and 16 cases were successfully tested for IG and TCR gene rearrangements respectively. In 3 cases no DNA amplification could be achieved and 5 cases could not be tested. Monoclonal IG rearrangement was found in 7 cases (38.9%). There was monoclonal TCR gene rearrangement in 6 cases (37.6%). A pattern characterised by multiple prominent irregular bands or peaks, on gels or genescan plots respectively, was considered to represent evidence of “restricted” T-cell response (figure 4 d,e). Such a pattern was detected in 5 cases (31.2%). The cases with clonal or restricted T-cell patterns were not confined to any particular IS type and there was no significant difference in the proportions of clonal/restricted TRG between the two groups (Fisher test, two-sided pexact=1.00). A monoclonal or restricted T-cell response was seen in 60% (6/10) of age related cases and in 83.3% (5/6) of those associated with iatrogenic IS. A monoclonal IG rearrangement was accompanied by a restricted T-cell response in 3 cases.
Table 5.
Summary of PCR clonality assessment.*
| IG (n=18) | TCR (n=16) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PC | C | PC | C | R | ||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Age-Related | 7 | (63.6) | 4 | (36.4) | 4 | (40.0) | 4 | (40.0) | 2 | (20.0) |
| Iatrogenic IS | 4 | (57.1) | 3 | (42.9) | 1 | (16.7) | 2 | (33.3) | 3 | (50.0) |
| All cases | 11 | (61.1) | 7 | (38.9) | 5 | (31.2) | 6 | (37.6) | 5 | (31.2) |
PC-polyclonal; C-clonal; R-restricted
Significance of differences in proportions of clonal IGH and clonal/restricted TCR cases across the two groups (Age-related and iatrogenic IS) were examined by Fisher exact test using SAS 9.2® (SAS institute, Cary, NC). There was no significant differences in the proportions of clonal IGH or clonal/restricted TCR cases between both group (two-sided pexact=1.00 for both analyses).
Clonality per institution
AWLP cases: IG - PC (n=3); C (n=4). TCR – PC (n=1); C (n=2); R (n=4)
NIH cases: IG - PC (n=8); C (n=3); TCR – PC (n=4); C (n=4); R (n=1)
Discussion
We describe a series of localised mucosal and cutaneous ulcerative EBV positive LPDs with polymorphous and Hodgkin-like morphology and immunophenotype. This proliferation was associated with advanced age and varied forms of medication-induced IS for autoimmune disorders and bone marrow stem cell transplantation. Despite the presence of highly atypical cells, the clinical course was indolent, without progression to disseminated disease in any patient, and spontaneous remissions were seen in several cases. Thus, the distinctive clinical and pathological features of these lesions warrant special recognition, and separation from other EBV-LPDs. As the nature of the IS in our cases was variable, this implies the possibility of a common pathogenetic mechanism across different types of IS including the post transplant setting, although interestingly, no HIV infection associated cases were observed.
Lesions resembling EBV-positive mucocutaneous ulcer have been reported previously, most often in association with MTX. 3, 25 Most regressed upon withdrawal of the drug and were regarded a consequence of a toxic/metabolic effect rather than EBV related. Microscopically, they were reported to show non-specific ulceration but the majority of reported cases were not histologically assessed. 12, 13, 29 Similar lesions have also been seen with CyA in the post transplant setting, 6 most often affecting the oropharyngeal mucosa. 45 Age-related EBV-LPDs have a high incidence of extranodal presentation involving skin and tonsils in 13% and 8% of cases respectively. However, in the literature published on this topic so far, it is difficult to identify with certainty cases similar to ours. 50, 51 Our study identifies EBVMCU as a distinctive lesion that can occur in a variety of clinical settings associated with defective immune surveillance for EBV, including advanced age in a high proportion of cases.
In our series all cases harboured large atypical blasts, often with HRS cell morphology, in a polymorphous background as may be seen in cHL. The large blasts showed reduced level of expression of CD20 with retained positivity of PAX5 and Oct-2 but with variable expression of Bob.1. While CD30 was uniformly positive, expression of CD15 varied, being co-expressed in 43.5% of cases. The lesional blasts showed a post germinal centre phenotype, generally lacking expression of CD10 and BCL6, with strong positivity for MUM1, as often seen in cHL. However, in most of the tested cases (8/9) the HRS-like cells were CD45 positive.
A combination of clinical, morphological and immunophenotypic parameters is useful in reaching the correct diagnosis. EBV-LPDs frequently harbour prominent immunoblasts, and HRS-like cells have been described in many settings, including acute infectious mononucleosis. However, the phenotype varies, and in infectious mononucleosis and PTLD, the HRS-like cells are CD30 positive but CD15 has been reported negative. 8,10 In EBV-LPDs associated with MTX therapy, the lack of expression of CD15 in CD30 positive HRS-like cells also has been considered a feature against the diagnosis of cHL. 27 Asano et al 2 also have investigated Hodgkin-like features in age-related DLBCL. Indications for a diagnosis of DLBCL rather than cHL in their study included involvement of extranodal sites, prominent necrosis, abundance of cytotoxic T-cells, higher positivity for CD20 and EBNA2, and lack of expression of CD15. In fact, absence of CD15 was used as a major discriminatory factor, in that no case of age-related DLBCL was CD15-positive. 50, 51 Our study indicates that CD15 expression clearly can occur in EBV-positive lesions outside of the diagnosis of cHL, and that one must be cautious in considering the presence or absence of CD15 as an independent determining factor, particularly in lesions involving extranodal sites. cHL seldom involves extranodal sites; in particular, primary mucosal or cutaneous involvement is considered exceptionally rare, if it occurs at all. 2, 8
An additional useful parameter not typical for cHL was the wide range in cell size of the EBV-positive cells as highlighted by CD20, CD30 and EBER. Typically, these included some small lymphocytes, immunoblasts, as well as large HRS-like cells which, unlike in cHL, were CD45 positive.
EBVMCU has distinctive pathological features that may be regarded as hallmarks of the condition. Notable features are the well circumscribed and superficial nature of the lesions with a characteristic architecture in the form of a prominent rim of small T-lymphocytes at the base. The infiltrates contain abundant CD8 positive T-cells and large numbers of EBV-positive plasmacytoid apoptotic cells, both not common features of cHL. Considering apoptosis, it has been proposed that EBV may be able to rescue germinal centre cells from undergoing apoptosis. It can provide the CD40 mediated survival stimulus resulting in the perpetual activation of the NFkB pathway, stimulating proliferation and abrogating apoptosis. Abundant apoptosis is not a prominent feature of EBV-associated cHL and thus, the presence of prominent apoptotic cells in EBVMCU may be a hallmark of retained normal control mechanisms and lack of a transformed phenotype, as seen in EBV-positive lymphomas such as cHL. 64, 83
The HRS-cells of cHL typically lack the transcription factors Oct-2, Bob.1 and PU1, while retaining weak expression of PAX5. 15, 41, 64 Although the number of cases analysed was small, partial loss of expression of Bob.1 seen in our series further resembles the immunophenotype of cHL, and should not by itself prompt a diagnosis of cHL.
Finally, the presence of vasculitic changes in some cases might suggest a differential diagnosis with LyG. The underlying pathogenetic mechanism of vascular change might be similar to LyG. LMP1 appears to stimulate secretion of interferon-gamma-inducible-protein-10 and a monokine induced by INF-gamma facilitating vascular damage and necrosis. 11, 63 Cutaneous or mucosal involvement in the absence of pulmonary presentation would argue against the diagnosis of LyG. 4, 24, 59 In LyG the background infiltrate is composed almost exclusively of T-cells with relatively few EBV positive B-cell blasts while in EBVMCU there was a more polymorphous infiltrate containing lymphocytes, plasma cells, histiocytes and eosinophils. 17
Results of clonality studies of EBVMCU may provide some insight into underlying pathogenesis. The focus of clonality assessment in IS EBV-LPDs has always been on the lesional B-cell population. However, recent studies have shown that defective control of EBV may be related to a diminished T-cell repertoire and an inability to recognize the full range of EBV-epitopes. 5 In addition, it has been shown that clonally-restricted T-cell populations may be detected in the response to EBV 6, and in association with some EBV-driven LPD’s secondary to iatrogenic immunosuppression. 3 Clonal T-cell expansion is a feature of incomplete control of EBV, and is more often found in patients with symptomatic disease. 11
The nature of the T-cell response to EBV has not been extensively studied in age-related EBV LPDs. Seventeen patients from our study group had no evidence of IS other than advanced age beyond 60. With ageing the complex spectrum of regressive changes affecting the immune system are collectively termed immunosenescence. 13, 23, 61, 66 T-cell responses appear most profoundly affected, with the accumulation of clonal CD8 positive T-cells with mature, memory cell phenotypes but diminished functionality. This appears to be a result of a decline in the capacity to generate a pool of new, naïve T-cells, which is compensated for by proliferation within the mature senescent memory cell pool. The result is prevalence of oligoclonal T-cell populations in elderly subjects (“restricted” T-cell response) that are markedly restricted and deficient in their epitope specific repertoire, rendering the host at increased risk of infection. The underlying mechanisms are complex including exhaustion of the T-cell response and diminished capacity to renew the pool of virgin T-cells. 16, 18, 42, 43, 52
In our series monoclonal TCR gene rearrangement was seen in 38% of cases. Evidence of “restricted” T-cell response was noted in another 31% of cases and a monoclonal IG rearrangement was accompanied by a restricted T-cell response in 3 cases. This finding is compatible with a limited T-cell repertoire to EBV in patients with EBVMCU, reflecting deficiency in T-cell control, rather than being a secondary event.
Evidence of clonality as demonstrated by clonal IG gene rearrangement is variably present in EBV-LPDs, and correlates to some extent with the clinical findings. Most cases of PTLD, both polymorphic and monomorphic, are clonal, at least in individual lesions. 33 Most cases of age-related DLBCL in the Japanese cohort also showed evidence of B-cell clonality. 50, 51, 61 In EBVMCU we found PCR evidence of B-cell clonality in 39% (7/18) of cases, with a similar rate of rearrangements in the age related and iatrogenic groups. The lack of demonstrated monoclonality may suggest that these lesions are not true B-cell neoplasms.
A number of untreated cases from our series exhibited either spontaneous regression, or had persistent but limited disease confined to the skin or mucosa. Such clinical behaviour suggests a possibility of partial and minimal reduction in immunosurveillance over EBV. The question arises as to why does such a lapse in immunity present itself in mucosal sites and skin. No specific pathological clues could be identified to explain this phenomenon. One possible explanation may be that in normal circumstances, concentration of EBV infected B-cells appears the highest in the Waldeyer’s ring. 39 The majority of our cases involved oropharyngeal sites; however, such data for other mucosal sites and skin are not available. An additional speculative explanation might be related to minor localised mucosal irritation (e.g. from dentures) or mucocutaneous injury, creating a point of lowered resistance for localised proliferation of EBV infected cells, which is then “walled off” by a brisk T-cell reaction. All the lesions appear very well demarcated from the surrounding structures and encircled by a dense rim of lymphocytes. Chronic inflammation has been implicated as a cause of other EBV-related B-cell proliferations, most often involving chronic pyothorax. 9, 23, 44
Interestingly, EBVMCU was associated with a variety of forms of IS. Immunosuppressive agents in our series included MTX, AZA and CyA. These are used for treatment of a range of idiopathic inflammatory and autoimmune conditions. AZA and CyA are also used in solid organ transplant recipients, although in this patient population the extent of immunosuppression may be more profound than in patients with autoimmune disease. Association of these agents with LPDs has been studied previously. 1, 7, 21, 32, 53, 62, 66,68 Interestingly, a prior report of EBV-associated cHL (or Hodgkin-like lesions) in patients with inflammatory bowel disease identified features very similar to our cases of EBVMCU. Two of the four patients had received AZA. 37
Age may be a confounding feature in the patients in whom EBVMCU was linked to iatrogenic immunodeficiency. Notably, even in this group the median age was 69, in comparison to the “age-related” group in which the median age was 80. We found evidence for clonal and restricted T-cell responses in both these groups, but there was no significant difference in proportions of clonal/restricted TRG across both groups (Fisher test, two-sided pexact=1.00).
It is possible that in a minority of people T-cell incompetence related to the ageing process alone reaches a critical level such that in sites where EBV infected cells are more abundant, the localised EBV driven B-cell LP is established. Effective immunosurveillance may be “on the cusp” and potentially reversible. Thus, in 3 of our patients there was clinical evidence of waxing and waning lesions. However, in a proportion of patients, the additional administration of an immunosuppressive agent might tip the balance towards loss of local immunosurveillance over EBV infected B-cells. MTX directly induces EBV replication by activating viral early promoters. 17 There is also evidence of CyA induced, oxidative stress mediated, direct EBV activation of B-cells. 3, 68 These effects could be responsible for localised increase in the content of EBV infected cells in the anatomical sites where the virus appears most prevalent. Such a scenario might potentially explain why administration of various immunosuppressive medications, with different mechanisms of action, result in the same type of lesion. Significant statistical difference in the mean ages of patients with and without iatrogenic IS respectively confirms the assumption that age alone plays an important aetiological role.
The therapeutic approach in EBV-LPDs depends on a variety of factors. In the post transplant setting prospects for graft survival needs to be considered as well. When possible, reduction of IS is the first management step, but of course is not possible in patients in whom immune senescence is the underlying cause. In the age related cases 21% had a relapsing and remitting character, with spontaneous complete remission in 57% of cases (in 6/14 the outcome is unknown). However, 36% of the age related EBVMCU received aggressive therapy, which might further compromise the host immune system. The overall outcomes of EBVMCU highlight an indolent and self limited condition, arguing for a conservative therapeutic approach, possibly including rituximab therapy or agents capable of enhancing immunological control over EBV. 11, 19, 20
The incidence of EBVMCU is difficult to establish with certainty. As diagnosis of all lymphomas in Wales is made centrally and most atypical LPDs are referred for central review, some estimation can be drawn. With the exception of one case which was an external referral, 6 cases originated from the Welsh population of 3 million over a period of 5 years (2004–2009) at a rate of approximately 1 case per year. However, the spontaneously remitting nature of the EBVMCU is likely to result in empirical conservative management without biopsy in many cases, thus resulting in an underestimated incidence. Literature reports of MTX associated mucosal and skin ulcers which were not accompanied by pathological assessment make this assumption likely. 12, 25, 29
In the most recent WHO classification update EBV-LPD’s are grouped under separate headings depending on underlying aetiology and clinical setting. EBV-positive DLBCL of the elderly is included as a provisional subtype of DLBCL, but is described as a clinically aggressive form of lymphoma with poor outcome in most patients. Therefore, it is important to draw attention to EBVMCU as a more indolent form of EBV-LPD occurring in the same age group, but requiring a different management approach.
In summary, EBV associated Mucocutaneous Ulcer is a new rare pathological entity associated with IS from a variety of origins, suggesting a common pathogenetic mechanism. Typified by an isolated and circumscribed cutaneous and mucosal presentation, it has an indolent behaviour and a self limited clinical course. It may result from a minimal and localized lapse in immunosurveillance over EBV. The high regression rate upon withdrawal of IS and a waxing and waning course in age related cases point to a condition for which conservative management is advised. The morphological and immunophenotypic features to a certain extent resemble cHL, in particular with a high percentage of CD15 positivity. However, correct diagnosis is facilitated by consideration of the clinical history and the observed spectrum in the EBV-positive cells.
Acknowledgements:
We thank Geraint T Williams for his comments, Nick Moran for his contribution with the clinical illustrations and Keith Wilson for provision of clinical information for case 26.
This study was supported by funding from:
The Center for Cancer Research, National Cancer Institute, National Institutes of Health, and the British Division of the International Academy of Pathology and the British Society for Haematology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. Lyon: IARC; 2008. [Google Scholar]
- 2.Asano N, Yamamoto K, Tamaru J, et al. Age-related Epstein-Barr virus (EBV)-associated B-cell lymphoproliferative disorders: comparison with EBV-positive classic Hodgkin lymphoma in elderly patients. Blood. 2009;113:2629–36. [DOI] [PubMed] [Google Scholar]
- 3.Au WY, Ma ES, Choy C, et al. Therapy-related lymphomas in patients with autoimmune diseases after treatment with disease-modifying anti-rheumatic drugs. Am J Hematol. 2006;81:5–11. [DOI] [PubMed] [Google Scholar]
- 4.Beaty MW, Toro J, Sorbara L, et al. Cutaneous lymphomatoid granulomatosis: correlation of clinical and biologic features. Am J Surg Pathol. 2001;25:1111–20. [DOI] [PubMed] [Google Scholar]
- 5.Bharadwaj M, Burrows SR, Burrows JM, et al. Longitudinal dynamics of antigen-specific CD8+ cytotoxic T lymphocytes following primary Epstein-Barr virus infection. Blood. 2001;98:2588–9. [DOI] [PubMed] [Google Scholar]
- 6.Callan MF, Steven N, Krausa P, et al. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996;2:906–11. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Johnston TD, Jeon H, et al. Cyclosporine promotes epstein-barr virus-infected human B-cell transformation assayed by three correlated assay methods. Transplant Proc. 2009;41:366–70. [DOI] [PubMed] [Google Scholar]
- 8.Chetty R, Biddolph S, Gatter K. An immunohistochemical analysis of Reed-Sternberg-like cells in posttransplantation lymphoproliferative disorders: the possible pathogenetic relationship to Reed-Sternberg cells in Hodgkin’s disease and Reed-Sternberg-like cells in non-Hodgkin’s lymphomas and reactive conditions. Hum Pathol. 1997;28:493–8. [DOI] [PubMed] [Google Scholar]
- 9.Cheuk W, Chan AC, Chan JK, et al. Metallic implant-associated lymphoma: a distinct subgroup of large B-cell lymphoma related to pyothorax-associated lymphoma? Am J Surg Pathol. 2005;29:832–6. [DOI] [PubMed] [Google Scholar]
- 10.Childs CC, Parham DM, Berard CW. Infectious mononucleosis. The spectrum of morphologic changes simulating lymphoma in lymph nodes and tonsils. Am J Surg Pathol. 1987;11:122–32. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JI, Kimura H, Nakamura S, et al. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8–9 September 2008. Ann Oncol. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeming GM, Collingwood J, Pemberton MN. Methotrexate and oral ulceration. Br Dent J. 2005;198:83–5. [DOI] [PubMed] [Google Scholar]
- 13.del PJ, Martinez W, Garcia-Silva J, et al. Cutaneous ulceration as a sign of methotrexate toxicity. Eur J Dermatol. 2001;11:450–2. [PubMed] [Google Scholar]
- 14.Droese J, Langerak AW, Groenen PJ, et al. Validation of BIOMED-2 multiplex PCR tubes for detection of TCRB gene rearrangements in T-cell malignancies. Leukemia. 2004;18:1531–8. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Cosio M, Santon A, Martin P, et al. Analysis of transcription factor OCT.1, OCT.2 and BOB.1 expression using tissue arrays in classical Hodgkin’s lymphoma. Mod Pathol. 2004;17:1531–8. [DOI] [PubMed] [Google Scholar]
- 16.Ghia P, Prato G, Stella S, et al. Age-dependent accumulation of monoclonal CD4+CD8+ double positive T lymphocytes in the peripheral blood of the elderly. Br J Haematol. 2007;139:780–90. [DOI] [PubMed] [Google Scholar]
- 17.Guinee D Jr., Jaffe E, Kingma D, et al. Pulmonary lymphomatoid granulomatosis. Evidence for a proliferation of Epstein-Barr virus infected B-lymphocytes with a prominent T-cell component and vasculitis. Am J Surg Pathol. 1994;18:753–64. [DOI] [PubMed] [Google Scholar]
- 18.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70:179–89. [DOI] [PubMed] [Google Scholar]
- 19.Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110:1123–31. [DOI] [PubMed] [Google Scholar]
- 20.Heslop HE. Biology and treatment of epstein-barr virus-associated non-hodgkin lymphomas. Hematology Am Soc Hematol Educ Program. 2005;260–6. [DOI] [PubMed] [Google Scholar]
- 21.Ho S, Clipstone N, Timmermann L, et al. The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol. 1996;80:S40–S45. [DOI] [PubMed] [Google Scholar]
- 22.Hummel M, Anagnostopoulos I, Dallenbach F, et al. EBV infection patterns in Hodgkin’s disease and normal lymphoid tissue: expression and cellular localization of EBV gene products. Br J Haematol. 1992;82:689–94. [DOI] [PubMed] [Google Scholar]
- 23.Iuchi K, Aozasa K, Yamamoto S, et al. Non-Hodgkin’s lymphoma of the pleural cavity developing from long-standing pyothorax. Summary of clinical and pathological findings in thirty-seven cases. Jpn J Clin Oncol. 1989;19:249–57. [PubMed] [Google Scholar]
- 24.Jaffe ES, Wilson WH. Lymphomatoid granulomatosis: pathogenesis, pathology and clinical implications. Cancer Surv. 1997;30:233–48. [PubMed] [Google Scholar]
- 25.Kalantzis A, Marshman Z, Falconer DT, et al. Oral effects of low-dose methotrexate treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:52–62. [DOI] [PubMed] [Google Scholar]
- 26.Kamel OW, van de RM, LeBrun DP, et al. Lymphoid neoplasms in patients with rheumatoid arthritis and dermatomyositis: frequency of Epstein-Barr virus and other features associated with immunosuppression. Hum Pathol. 1994;25:638–43. [DOI] [PubMed] [Google Scholar]
- 27.Kamel OW, Weiss LM, van de Rijn M, et al. Hodgkin’s disease and lymphoproliferations resembling Hodgkin’s disease in patients receiving long-term low-dose methotrexate therapy. Am J Surg Pathol. 1996;20:1279–87. [DOI] [PubMed] [Google Scholar]
- 28.Kanegane H, Wakiguchi H, Kanegane C, et al. Viral interleukin-10 in chronic active Epstein-Barr virus infection. J Infect Dis. 1997;176:254–7. [DOI] [PubMed] [Google Scholar]
- 29.Kazlow DW, Federgrun D, Kurtin S, et al. Cutaneous ulceration caused by methotrexate. J Am Acad Dermatol. 2003;49:S197–S198. [DOI] [PubMed] [Google Scholar]
- 30.Khan N, Hislop A, Gudgeon N, et al. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol. 2004;173:7481–9. [DOI] [PubMed] [Google Scholar]
- 31.Khan N, Shariff N, Cobbold M, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–92. [DOI] [PubMed] [Google Scholar]
- 32.Knowles DM. Immunodeficiency-associated lymphoproliferative disorders. Mod Pathol. 1999;12:200–17. [PubMed] [Google Scholar]
- 33.Knowles DM, Cesarman E, Chadburn A, et al. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood. 1995;85:552–65. [PubMed] [Google Scholar]
- 34.Kojima M, Kashimura M, Itoh H, et al. Epstein-Barr virus-related reactive lymphoproliferative disorders in middle-aged or elderly patients presenting with atypical features. A clinicopathological study of six cases. Pathol Res Pract. 2007;203:587–91. [DOI] [PubMed] [Google Scholar]
- 35.Kojima M, Morita Y, Nakamura N, et al. Plasmacytic hyperplasia in age-related Epstein-Barr virus-associated lymphoproliferative disorders: a report of two cases. Pathol Res Pract. 2008;204:267–72. [DOI] [PubMed] [Google Scholar]
- 36.Kojima M, Sugiura I, Itoh H, et al. Histological varieties of Epstein-Barr virus-related lymph node lesion resembling autoimmune disease-like clinicopathological findings in middle-aged and elderly patients: a study of six cases. Pathol Res Pract. 2006;202:609–15. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S, Fend F, Quintanilla-Martinez L, et al. Epstein-Barr virus-positive primary gastrointestinal Hodgkin’s disease: association with inflammatory bowel disease and immunosuppression. Am J Surg Pathol. 2000;24:66–73. [DOI] [PubMed] [Google Scholar]
- 38.Kutok JL, Wang F. Spectrum of Epstein-Barr virus-associated diseases. Annu Rev Pathol. 2006;1:375–404. [DOI] [PubMed] [Google Scholar]
- 39.Laichalk LL, Hochberg D, Babcock GJ, et al. The dispersal of mucosal memory B cells: evidence from persistent EBV infection. Immunity. 2002;16:745–54. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy KP, Sloane JP, Kabarowski JH, et al. A simplified method of detection of clonal rearrangements of the T-cell receptor-gamma chain gene. Diagn Mol Pathol. 1992;1:173–9. [PubMed] [Google Scholar]
- 41.McCune RC, Syrbu SI, Vasef MA. Expression profiling of transcription factors Pax-5, Oct-1, Oct-2, BOB.1, and PU.1 in Hodgkin’s and non-Hodgkin’s lymphomas: a comparative study using high throughput tissue microarrays. Mod Pathol. 2006;19:1010–8. [DOI] [PubMed] [Google Scholar]
- 42.Messaoudi I, Warner J, Nikolich-Zugich D, et al. Molecular, cellular, and antigen requirements for development of age-associated T cell clonal expansions in vivo. J Immunol. 2006;176:301–8. [DOI] [PubMed] [Google Scholar]
- 43.Nakahara K, Utsunomiya A, Hanada S, et al. Transient appearance of CD3+CD8+ T lymphocytes with monoclonal gene rearrangement of T-cell receptor beta locus. Br J Haematol. 1998;100:411–4. [DOI] [PubMed] [Google Scholar]
- 44.Nakatsuka S, Yao M, Hoshida Y, et al. Pyothorax-associated lymphoma: a review of 106 cases. J Clin Oncol. 2002;20:4255–60. [DOI] [PubMed] [Google Scholar]
- 45.Nalesnik MA, Jaffe R, Starzl TE, et al. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988;133:173–92. [PMC free article] [PubMed] [Google Scholar]
- 46.Olsson J, Wikby A, Johansson B, et al. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000;121:187–201. [DOI] [PubMed] [Google Scholar]
- 47.Ouyang Q, Wagner WM, Walter S, et al. An age-related increase in the number of CD8+ T cells carrying receptors for an immunodominant Epstein-Barr virus (EBV) epitope is counteracted by a decreased frequency of their antigen-specific responsiveness. Mech Ageing Dev. 2003;124:477–85. [DOI] [PubMed] [Google Scholar]
- 48.Ouyang Q, Wagner WM, Wikby A, et al. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J Clin Immunol. 2003;23:247–57. [DOI] [PubMed] [Google Scholar]
- 49.Ouyang Q, Wagner WM, Zheng W, et al. Dysfunctional CMV-specific CD8(+) T cells accumulate in the elderly. Exp Gerontol. 2004;39:607–13. [DOI] [PubMed] [Google Scholar]
- 50.Oyama T, Ichimura K, Suzuki R, et al. Senile EBV+ B-cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am J Surg Pathol. 2003;27:16–26. [DOI] [PubMed] [Google Scholar]
- 51.Oyama T, Yamamoto K, Asano N, et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res. 2007;13:5124–32. [DOI] [PubMed] [Google Scholar]
- 52.Pawelec G, Larbi A. Immunity and ageing in man: Annual Review 2006/2007. Exp Gerontol. 2008;43:34–8. [DOI] [PubMed] [Google Scholar]
- 53.Pereira GM, Miller JF, Shevach EM. Mechanism of action of cyclosporine A in vivo. II. T cell priming in vivo to alloantigen can be mediated by an IL-2-independent cyclosporine Aresistant pathway. J Immunol. 1990;144:2109–16. [PubMed] [Google Scholar]
- 54.Ramasamy I, Brisco M, Morley A. Improved PCR method for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell neoplasms. J Clin Pathol. 1992;45:770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rezk SA, Weiss LM. Epstein-Barr virus-associated lymphoproliferative disorders. Hum Pathol. 2007;38:1293–304. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez-Caballero A, Garcia-Montero AC, Barcena P, et al. Expanded cells in monoclonal TCR-alphabeta+/CD4+/NKa+/CD8−/+dim T-LGL lymphocytosis recognize hCMV antigens. Blood. 2008;112:4609–16. [DOI] [PubMed] [Google Scholar]
- 57.Segal GH, Jorgensen T, Masih AS, et al. Optimal primer selection for clonality assessment by polymerase chain reaction analysis: I. Low grade B-cell lymphoproliferative disorders of nonfollicular center cell type. Hum Pathol. 1994;25:1269–75. [DOI] [PubMed] [Google Scholar]
- 58.Segal GH, Jorgensen T, Scott M, et al. Optimal primer selection for clonality assessment by polymerase chain reaction analysis: II. Follicular lymphomas. Hum Pathol. 1994;25:1276–82. [DOI] [PubMed] [Google Scholar]
- 59.Shanti RM, Torres-Cabala CA, Jaffe ES, et al. Lymphomatoid granulomatosis with involvement of the hard palate: a case report. J Oral Maxillofac Surg. 2008;66:2161–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimoyama Y, Oyama T, Asano N, et al. Senile Epstein-Barr virus-associated B-cell lymphoproliferative disorders: a mini review. J Clin Exp Hematop. 2006;46:1–4. [DOI] [PubMed] [Google Scholar]
- 61.Shimoyama Y, Yamamoto K, Asano N, et al. Age-related Epstein-Barr virus-associated B-cell lymphoproliferative disorders: special references to lymphomas surrounding this newly recognized clinicopathologic disease. Cancer Sci. 2008;99:1085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanner JE, Alfieri C. The Epstein-Barr virus and post-transplant lymphoproliferative disease: interplay of immunosuppression, EBV, and the immune system in disease pathogenesis. Transpl Infect Dis. 2001;3:60–9. [DOI] [PubMed] [Google Scholar]
- 63.Teruya-Feldstein J, Jaffe ES, Burd PR, et al. The role of Mig, the monokine induced by interferon-gamma, and IP-10, the interferon-gamma-inducible protein-10, in tissue necrosis and vascular damage associated with Epstein-Barr virus-positive lymphoproliferative disease. Blood. 1997;90:4099–105. [PubMed] [Google Scholar]
- 64.Ushmorov A, Ritz O, Hummel M, et al. Epigenetic silencing of the immunoglobulin heavychain gene in classical Hodgkin lymphoma-derived cell lines contributes to the loss of immunoglobulin expression. Blood. 2004;104:3326–34. [DOI] [PubMed] [Google Scholar]
- 65.van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98–3936. Leukemia. 2003;17:2257–317. [DOI] [PubMed] [Google Scholar]
- 66.Van BS, Velde SV, De BR, et al. Epstein-Barr virus related lymphoma in inflammatory bowel disease. Acta Gastroenterol Belg. 2008;71:33–5. [PubMed] [Google Scholar]
- 67.Vescovini R, Telera A, Fagnoni FF, et al. Different contribution of EBV and CMV infections in very long-term carriers to age-related alterations of CD8+ T cells. Exp Gerontol. 2004;39:1233–43. [DOI] [PubMed] [Google Scholar]
- 68.Vial T, Choquet-Kastylevsky G, Descotes J. Adverse effects of immunotherapeutics involving the immune system. Toxicology. 2002;174:3–11. [DOI] [PubMed] [Google Scholar]
- 69.Wikby A, Johansson B, Olsson J, et al. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002;37:445–53. [DOI] [PubMed] [Google Scholar]
- 70.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–68. [DOI] [PubMed] [Google Scholar]
- 71.Zu Y, Steinberg SM, Campo E, et al. Validation of tissue microarray immunohistochemistry staining and interpretation in diffuse large B-cell lymphoma. Leuk Lymphoma. 2005;46:693–701. [DOI] [PubMed] [Google Scholar]