Abstract
Depression is a syndrome of stress- and emotion-dysregulation, involving compromised structural integrity of frontal-limbic networks. Meta-analytic evidence indicates that volumetric reductions in the hippocampus, anterior cingulate cortex, prefrontal cortex, striatum, and amygdala, as well as compromised white matter integrity are frequently observed in depressed adults. Exercise has shown promise as an effective treatment for depression, but few studies have attempted to characterize or identify the neural mechanisms of these effects. In this review, we examined the overlap between structural brain abnormalities in depression and the effects of exercise on brain structure in adults, to highlight possible neural mechanisms that may mediate the positive effects of exercise on depressive symptoms. The prefrontal cortex, anterior cingulate cortex, hippocampus, and corpus callosum emerged as structural neural markers that may serve as targets for exercise-based treatments for depression. These findings highlight the need for randomized exercise interventions to test these proposed neurobiological mechanisms of exercise on depression.
Keywords: Exercise, Depression, Gray Matter, White Matter, MRI
Introduction
Depression is a significant global public health concern; it is the leading cause of disability worldwide and is currently estimated to affect 350 million people (1). Depression is characterized by significant impairment in social and occupational functioning, and the majority of depressed individuals have recurrent episodes (~50%) (2) and/or chronic depression (~20%) (3). Exercise has recently shown promise as an effective non-pharmaceutical treatment for depression (4). A recent Cochrane Review and meta-analysis of 35 randomized controlled trials (N= 1356) found that exercise was moderately effective at reducing depressive symptoms relative to a control condition in depressed adults (Standardized mean difference ((SMD) = −0.62 (95% CI: −0.81 to - 0.42)) (4). Subgroup analyses indicated that there was no evidence for a difference in the effectiveness of exercise relative to psychotherapy (7 trials) and pharmacotherapy (4 trials) in treating depression. A sensitivity analysis (8 studies, N = 377) suggested that exercise has a small long-term effect on depressive symptoms post-treatment (SMD −0.33, 95% CI −0.63 to −0.03). In sum, meta-analytic evidence suggests that exercise is a promising treatment for depression in adults, showing effects that are comparable to other first-line treatments for depression (4).
Despite the antidepressant effects of exercise, we have a limited understanding of the underlying neural mechanisms by which exercise alleviates depression. Converging evidence suggests that exercise and antidepressant medication may alleviate depression through common neuromolecular mechanisms (5, 6), including increased expression of neurotrophic factors (i.e., BDNF) (5, 6), increased availability of serotonin and norepinephrine (7), regulation of HPA-axis activity (8), and reduced systemic inflammatory signaling (9) (See (10) for Review). These processes influence the development of new neurons, increase synaptic connections between neurons, and increase cerebral vasculature (11, 12). Considering that exercise and antidepressant medication may exert effects on depression through overlapping molecular pathways, it is possible that they also influence overlapping neural systems. Antidepressant treatment may increase the volume of the hippocampus, anterior cingulate, and orbitofrontal cortex, increase white matter integrity, and induce changes in functional dynamics of frontal-limbic neural networks in depressed adults (13). While there have been few studies examining effects of exercise on neural systems in depressed individuals, we may predict that exercise leads to similar neural changes as antidepressant medication.
The goal of this review is to describe structural brain abnormalities in depression that may also be influenced by exercise. Much of the exercise literature we review is from studies of older adults, because the effects of exercise on brain structure have been most studied in the context of aging. We expect that this review may improve our understanding of the biological pathways involved in both the pathophysiology of depression and the anti-depressant effects of exercise.
Study Selection
Literature search was conducted using PubMed, Google Scholar, and references from included studies and review articles from inception until September 2016. To search for meta-analyses examining structural abnormalities in depression, the following strategy was used: (“depress*”) AND (“MRI”) AND (“meta-analysis”) AND (“brain volume”) OR (“brain structure”) OR (“white matter”). To search for cross-sectional, longitudinal, and intervention studies examining exercise effects on brain structure, the following strategy was used: (“exercise” OR “PA” OR “CRF”) AND (“brain volume”) OR (“brain structure”) OR (“white matter”).
Eligibility of articles was determined by reviewing titles and abstracts. All included studies were published in English. The inclusion criteria for the depression literature were 1) meta-analysis, 2) inclusion of clinically depressed patients (diagnosis of MDD), and 3) use of structural MRI data. The inclusion criteria for the exercise studies were 1) original studies (epidemiological, cross-sectional, and intervention studies), 2) use of structural MRI data, 3) evaluation of the effect of physical activity (PA), cardiorespiratory fitness (CRF), or exercise on regional volumetric assessments, and 4) gray matter volume in regions commonly linked with depression: hippocampus, anterior cingulate cortex, prefrontal cortex, striatum, and amygdala. Meta-analyses from the depression literature were not reviewed if they combined participants with depression and other psychiatric diagnoses (i.e., bipolar disorder) into one group. Articles from the exercise literature were excluded if they included patients with cognitive comorbidities (i.e., dementia).
Structural abnormalities in depression that may be influenced by exercise
Regional gray matter abnormalities have been have identified in acutely depressed adults relative to age-matched non-psychiatric control subjects in numerous meta-analytic studies (14–28); the most reliable regional abnormalities identified through structural MRI studies include the bilateral hippocampus, anterior cingulate cortex, regions within the prefrontal cortex, striatum, and amygdala. The literature of neuroimaging studies examining structural abnormalities in depression is vast and has been reviewed extensively; therefore, only meta-analytic studies and reviews will be described here.
1.1. Hippocampus
The hippocampus is one of the most studied brain regions in the context of depression. The hippocampus plays an important role in stress regulation, as it exerts inhibitory control over HPA-axis activity, and is also more broadly involved in cognitive and affective processing via its widespread connections with other limbic and prefrontal regions (29). Reduced hippocampal volume has been consistently shown to be about 5% smaller in depression (15–17, 19, 21–23, 25)}. Interestingly, these meta-analyses indicate that reductions in hippocampal volume are present throughout the lifespan (26), not explained by comorbid psychiatric comorbidities (19), and are not solely a consequence of medication-effects (25). A recent meta-analysis (N= 1728 depressed; N=7199 control subjects) (16) confirmed that depression was associated with smaller hippocampal volumes with larger reductions for those with an early age of onset (< 21 years). Given that early onset depression increases the risk for recurrent depressive episodes, it is possible that volumetric reductions in the hippocampus may persist even after remission and may increase vulnerability for further volumetric reductions during subsequent episodes.
There may be several factors that moderate the association between depression and hippocampal volume. For example, in the context of chronic stress dysregulation, lower hippocampal volume may represent a risk marker, rather than a consequence, of depression (15, 30, 31). However, some meta-analyses suggest that hippocampal differences exist only in the context of chronic or recurrent depression (16, 23), or among individuals experiencing their first depressive episode (15). This may indicate that the studies included in these meta-analyses may represent different subgroups of depressed individuals that modify the effects of depression on hippocampal volume. For instance, variability in depression severity, age-of-onset of the first depressive episode, Alzheimer’s disease pathology in older adults, or lifestyle factors (e.g., physical activity) may influence hippocampal volumetric reductions in depressed individuals. Nonetheless, reductions in hippocampal volume are a robust structural marker observed in depression.
1.2. Exercise effects on Hippocampal Volume
The association between fitness or exercise and hippocampal volume is a highly replicated finding (See Table 2). In cognitively and psychiatrically healthy older adults, Erickson et al. (32) found that higher CRF was associated with larger hippocampal volumes. CRF refers to an individual’s aerobic capacity, and is commonly used in this literature as a proxy for physical activity habits over a prolonged period of time (also see (33, 34). Another cross-sectional study in older adults found that high levels of exercise engagement may mitigate the cumulative adverse effects of lifetime stress on hippocampal volume in late life (35). Unfortunately, studies examining these associations in mid-life and young adulthood are rare (36). Similar effects have been found longitudinally. For example, in one study, self-reported physical activity (PA), measured by how many blocks an individual walks on average per week were predictive of greater hippocampal volume nine years later (37).
Table 2.
PA, Fitness, and Exercise associations with gray matter regions that have also shown volumetric reductions in depression.
| Brain Region | Study | Sample Size | Mean age (SD) | % female | PA/Fitness Measure | Significant Findings |
|---|---|---|---|---|---|---|
| Hippocampus | Cross-sectional | |||||
| Bugg et al. (2012) | 19 | 68.4 (2.7) | 74 | Fitness: adjusted Vo2 Peak | ↑ HC volume | |
| Erickson et al. (2009) | 165 | 66.55 (5.6) | 65.7 | Fitness: Vo2 Peak | ↑ HC volume | |
| Head et al. (2012) | 91 | 72.5 (8.1) | 48.9 | PA: self-report; exercise engagement in the last 10 yrs | No main effect of exercise; exercise moderated the negative effect of stress on HC volume in older adults. | |
| Pereira et al. (2007) | 11 | 33 (21–45) | 82 | Fitness: Vo2 Max; PA: 3-months of aerobic exercise | 3-months of aerobic exercise: ↑ CBV in dentate gyrus of the hippocampus. ↑ Regional CBV correlated with Δ fitness. | |
| Szabo et al. (2011) | 158 | 66.48 (5.69) | 66.4 | Fitness: Vo2 Peak; PA: PASE self-report | ↑ Fitness associated with ↑ HC volume and in turn, ↑ HC volume associated with ↑ spatial memory; Self-reported PA was not associated with HC volume. | |
| Whiteman et al. (2015) | 33 | 21.1 (2.8) | 60.6 | Fitness: Vo2 Max | ↑ Fitness associated with ↑ EC volume | |
| Longitudinal | ||||||
| Erickson et al. (2010) | 299 | T2: 78 (3.65) | 60.6 | PA: self-report; total # blocks walked over a week | ↑ PA levels predicted ↑ HC volume 9 years later | |
| Randomized Controlled Trial | ||||||
| Erickson et al.(2011) | 120 | 66.55 (5.7) | 66.7 | Fitness: Vo2 Max PA: 12-month PA intervention 3x/wk | 12-months of aerobic exercise: ↑ HC volume by 2%; Balancing and toning group: ↓ HC volume | |
| Kleemeyer et al. 2015 | 52 | 66 (4.36) | 61.5 | Fitness: Vo2Max PA: 6-month PA intervention 3x/wk | Δ Fitness across both HI and LI exercise groups associated with ↓ MD in HC, a proxy for tissue density: ↓ MD in the HC related to ↑ HC volume. | |
| Maas et al. (2015) | 40 | 68.4 (4.3) | 55 | Fitness: Vo2VAT (ventilary threshold); PA: 3-month PA intervention 3x/wk | A Fitness associated with ↑ HC perfusion and ↑ HC head volume; OA < YA | |
| Niemann et al. (2015) | 91 | 68.65 (3.54) | 73.3 | Aerobic fitness: Vo2Peak; Metabolic fitness: grip strength and Vo2peak; Motor fitness: speed, flexibility, feet tapping, balancing on one leg, backwards beam walk; | 12-months of Aerobic OR Coordinative training: ↑ HC volume. | |
| Prefrontal Cortex/Anterior Cingulate Cortex | Cross-sectional | |||||
| Colcombe et al. (2003)a | 55 | 66.5 (5.3) | 55.6 | Fitness: VO2 | No main effect of fitness on PFC and ACC volume; ↑ Fitness associated with ↑ PFC and ACC volume in regions undergoing age-related atrophy (age x fitness) | |
| Erickson et al. (2007)a | 54 | 69.6 (58–80) | 100 | Fitness: VO2 Peak | ↑ fitness associated with ↑ PFC and subgenual ACC volume; ↑ Fitness levels also ↑ beneficial effects of short-duration HRT and ↓ risks associated with longer duration of HRT for GM volume | |
| Floel et al. (2010)a | 75 | 60.5 (6.9) | 62.7 | Fitness: 1) progressive exercise test on cycle ergometer 2) lactate step test; PA: self-report; average total energy expenditure by PA per week | ↑ PA in daily life associated with ↑ PFC and ACC volume | |
| Gordon et al. (2008)a | YA=20 OA=40 | YA: 22.5 (2.1) OA: 71.5 (4.7) | 57.5 | Fitness: VO2 Max | ↑ Fitness levels were associated with ↑ PFC and ACC volume; ↑ education associated with ↑ WMV in PFC | |
| Tian et al. (2014b) | 164 | 82.9 (2.6) | 57.1 | Fitness: 400 meter walk time | ↑ Fitness levels associated with ↑ tissue density in the HC and EC | |
| Weinstein et al. (2012) | 142 | 66.6 (5.6) | 64.1 | Fitness: V02 Max | ↑ Fitness levels associated with ↑ dIPFC volume, and in turn, ↑ dIPFC volume associated with ↑ executive function. | |
| Bugg & Head (2012) | 52 | 69.0 (6.7) | 71.2 | PA: self-report; exercise engagement over the last 10 years | ↑ PA associated with ↑ dorsal PFC volume | |
| Longitudinal | ||||||
| Erickson et al. (2010) | 299 | T2:78 (3.65) | 60.9 | PA: self-report; total # blocks walked over a week | ↑ PA predicted ↑ dIPFC, vIPFC, PMA, and SMA volume 9 years later | |
| Rovio et al. (2010)b | 75 | T1: 50.21 (5.3) | 53.7 | PA: self-report; active (≥2x/week) and non-active (≤1x/week) | ↑ PA in midlife predicted ↑ L dorsal PFC volume 20 years later | |
| Tian et al. (2014a)a | 276 | T1: 72.9 (2.7) | 58.7 | PA: self-report questionnaire-kcal per week calculated and then divided into 3 groups | Most active group (‘exercise-active’) showed ↓ MD in CC 9 years later relative to the least active group (‘sedentary’) | |
| Tian et al. (2016) | 146 | T2: 69.6 (7.9) | 41.8 | Fitness: Vo2 Peak | Midlife CRF did not predict PFC volume in late-life, but progression in ↓ PFC volume in late-life predicted ↓ fitness 13 years later | |
| Randomized Controlled Trial | ||||||
| Colcombe et al. (2006)a | 79 | 66.5 (60–79) | 55 | Fitness: Vo2 max; PA: Experimental group: 40 min. Walking 3x/week; Control group: 60 min. stretching and toning 3x/week | 6-months of aerobic exercise: ↑ PFC and ACC volume; Stretching and toning: no change in GM volume | |
| Ruscheweyh et al. (2011)a | 62 | 60.2 (6.6) | 66.1 | Fitness: progressive exercise test on cycle ergometer; PA: Walking and Gymnastics groups: 3 times/week for 50 min; Total PA: self-report measure collected at baseline and follow-up | No group effects observed; ↑ self-reported PA over 6-months, regardless of intensity, associated with ↑ PFC and ACC volume | |
| Striatum | Cross-Sectional | |||||
| Verstynen et al. (2012) | 179 | 66.6 (5.6) | 60.9 | Fitness: Vo2max | ↑ Fitness levels associated with ↑ striatal volume; ↑ caudate volume associated with ↑ executive function. | |
| Intervention | ||||||
| Erickson et al. (2011) | 120 | 66.55 (5.7) | 66.7 | Fitness: Vo2 Max PA: 12-month PA intervention 3x/wk | 12-months aerobic exercise: ↑ HC volume but not caudate volume. | |
Associations with ACC
Several randomized exercise interventions have detected increases in hippocampal volume (36, 37) or other markers of hippocampal morphology (38, 39) in healthy older adults. For instance, Erickson et al. (36) showed that 12-months of moderate intensity aerobic exercise (brisk walking) 3 times/week (N= 60) resulted in a ~2% increase in hippocampal volume (40). Regional specificity of these effects was also observed, such that volumetric increases were specific to the anterior hippocampus with little effect in the posterior region. The anterior region has been linked to emotional and motivational functioning (41) and neurogenesis (42). In addition, exercise-induced improvements in CRF (~7.8%) positively correlated with increases in hippocampal volume. These findings support the capacity for aerobic PA to induce volumetric increases in the hippocampus in older adults. Another intervention in younger adults (mean age= 33.7; SD= 11.9) using a cross-over design found that 6 weeks of aerobic training for 5 days a week, 30 minutes each day, was sufficient to increase hippocampal volume (43). They also found that hippocampal volume returned to baseline after 6 weeks of inactivity. These findings indicate that maintaining aerobic activity is important to retain exercise-induced volumetric changes.
Other randomized exercise trials have reported stability in hippocampal volume after 3- and 6-month interventions, but increases in other markers of hippocampal morphology, such as cerebral blood volume and tissue density (38, 39). For instance, Maas et al. (38) examined the effects of a 3-month aerobic exercise intervention on cerebral blood volume and hippocampal volume in older adults (N= 40). They found that change in CRF (~10.4%) was related to change in cerebral blood volume (also see (44)). Similar to Erickson et al. (36), change in CRF was related to increased volume of the hippocampal head. These data point to vascular plasticity as a potential mechanism for exercise effects on hippocampal volume. Another randomized trial in older adults (N= 52) examined tissue density of the hippocampus and reported that 6-months of aerobic exercise increased regional tissue density (39).
To date, one study has tested the effects of aerobic exercise on hippocampal morphology in depressed adults. In a 12-week randomized controlled trial (45), no changes were reported in hippocampal volume between the exercise group (N=41) and the active control group (N=38). However, these findings must be interpreted with caution due to poor exercise adherence (mean= 30%).
Collectively, evidence from randomized controlled trials examining exercise-effects on hippocampal volume is promising. Inconsistencies between types and duration of exercise preclude a clear understanding of the mechanisms underlying exercise-induced increases in hippocampal volume. Nonetheless, the current literature points to the promise of moderate-intensity aerobic exercise as a non-pharmaceutical strategy to increase hippocampal volume.
2.1. Prefrontal Cortex/Anterior Cingulate Cortex
Meta-analytic reviews have documented volumetric reductions in several prefrontal cortical (PFC) regions in depressed individuals relative to healthy control subjects, namely in the anterior cingulate cortex (ACC) (17–20, 22, 24, 46), orbitofrontal cortex (OFC) (18, 19, 21, 22), dorsolateral PFC (dlPFC) (17, 25), and dorsomedial PFC (dmPFC) (17, 24). The ACC is a medial prefrontal cortical structure. The dorsal ACC has been implicated in higher-level executive and motor functions, the subgenual ACC in emotional and interoceptive processing, and the pregenual ACC integrates cognitive and emotional information (47). The subgenual ACC is a key region implicated in depression (48). Several meta-analyses have found that volumetric reduction in the ACC is the largest effect size for any structural difference in depression (Cohen’s d = −0.77 p = 0.006) (18, 22).
Similar to results on the hippocampus, there have been inconsistencies regarding clinical moderators of volumetric reductions in the ACC. Medication may influence ACC volume (18), but see (25). Some evidence suggests that the effects may be most pronounced in the subgenual region of the ACC, which is important for affective processing (18, 49). Similar to findings of the hippocampus, abnormalities in the ACC may precede the onset of depression and may persist after remission from a depressive episode, suggesting that volume reductions in the ACC may represent a neurodevelopmental biomarker of risk for depression (49).
Volumetric reductions in other prefrontal regions have also been observed in depressed individuals relative to healthy controls. Reduced volume of the OFC, which is involved in emotion and reward processing, is consistently observed in depression. Several meta-analyses reported volumetric reductions in the OFC (18, 19, 21, 22). Some evidence suggests volumetric reductions in the dlPFC and dmPFC may be linked to depression (17, 24, 25). The dlPFC and dmPFC are both implicated in executive control, and are involved in monitoring performance and adjusting behavior, respectively (50). Volume reductions in dlPFC were reported in a meta-analysis of studies only including unmedicated depressed individuals, suggesting that these volume reductions are not an artifact of antidepressant treatment (25). Two meta-analyses of volumetric differences in individuals with late-life depression relative to healthy controls also reported volumetric reductions in prefrontal regions, including the OFC, medial PFC, and subcallosal cingulate cortex (26) (27). In sum, converging evidence of reduced volume in several PFC regions suggests that structural abnormalities in the PFC may be a neural signature of depression.
2.2. Exercise Effects on PFC and ACC
Cross-sectional evidence supports a positive association between CRF, PA, and PFC volume (See Table 4). These regions include dorsal (51, 52), ventral (53), and lateral PFC (52, 54, 55), as well as ACC (53–55). Several studies found evidence of a positive association between CRF and subgenual ACC volume (53, 55) as well as PFC volume even after accounting for age-related atrophy (51, 54, 55). At least one study found that higher CRF offsets age-related atrophy of the PFC (56). One longitudinal study (N= 75) found greater self-reported PA in midlife to be associated with greater PFC volume in late life (57). Greater self-reported PA also predicted greater PFC volume nine years later (37). These findings suggest potential long-term benefits of PA for PFC volume.
Two 6-month randomized controlled interventions found PA-related changes in PFC and ACC volume. Colcombe et al. (58) found that a 6-month brisk walking intervention resulted in increased volume of PFC and ACC. Another 6-month randomized trial (N= 62) also reported increases in PFC and ACC volume (59). Interestingly, they found that change in self-reported PA from pre- to post-intervention was positively associated with increased PFC and ACC volume. Findings from both studies suggest that increased PA for at least 6 months in older adults may lead to increases in PFC and ACC volume. Given that volumetric reductions in PFC and ACC constitute a central component of both depression and executive impairment, which typically co-occur in late-life depression (LLD), these results suggest that PA may modify neural abnormalities relevant for LLD.
3.1. Striatum
Volumetric reductions in the striatum in depression have been documented in several meta-analytic studies (18, 21, 22, 46). The striatum is a subcortical structure consisting of the caudate and putamen subnuclei, as well as the nucleus accumbens, and is divided into functionally distinct regions involved in executive function, affective processing, motivation, and motor functions (60). Segments of the striatum are critically involved in affective and reward processing; however, neuroimaging studies examining volumetric reductions in the striatum in depression have not examined sub-regions within the striatum involved in mood and motivation.
Similar to other regions discussed in this review, there may be several moderators of volumetric abnormalities in the striatum. One meta-analysis suggested that volumetric reductions in the striatum may distinguish individuals with unipolar depression from those with bipolar disorder (22). Another found that age moderates striatal volume in depression, such that the reduction in volume is greater in late-life rather than mid-life (18). Interestingly, a meta-analysis examining structural abnormalities in un-medicated depressed individuals relative to healthy controls did not report striatal abnormalities (25). Evidence of volumetric reductions in the striatum in depression was not supported by recent whole-brain meta-analyses. This may partially be explained by moderating effects of age and clinical factors described above.
3.2. Effects of PA and CRF on Striatum
PA and CRF associations with the striatum are limited, with only one cross-sectional report supporting a positive association. In healthy older adults, a link was observed between higher CRF levels and volume in the caudate (dorsal) and nucleus accumbens (ventral), but not the putamen (61). Given that the ventral striatum shows structural and functional alterations in depression and parts of the dorsal striatum are implicated in mood and motivation, these findings, suggest that CRF and PA may relate to volume of striatal nuclei that may have particular relevance to depression. One intervention study in older adults showing exercise-related increases in hippocampal volume found no effects of the intervention on caudate nucleus volume (36).
5.1. White Matter
Reduced microstructural integrity of white matter (FA) has been linked to depression in several meta-analyses, with inconsistencies regarding tract- or regional specificity of the effects (62–64). Two meta-analyses (62, 63) found reduced FA in the corpus callosum, one reported reduced FA in the left superior longitudinal fasiculus (62), and one reported widespread reductions in FA in the prefrontal cortex (dlPFC), as well as tracts connecting PFC regions to subcortical, temporal, parietal, and occipital regions in depression (63). One meta-analysis in LLD (N= 15) found lower FA in the uncinate fasiculus among depressed older adults, a tract that facilitates communication between inferior frontal (i.e., OFC) and limbic regions (i.e., amygdala, hippocampus) (65). These results suggest diffuse reductions in structural connectivity in depression.
5.2. Exercise Effects on White Matter Integrity
Twelve cross-sectional studies have examined the association between PA (N=3) or CRF (N=9) and FA in cognitively healthy adults. Eleven out of twelve studies found significant associations between PA or CRF and FA. The majority of these studies were in older adults (N= 11). Among studies in healthy older adults, CRF or PA was consistently associated with FA in the corpus callosum (N=4), the cingulum (N= 6), and superior longitudinal fasiculus (N=3) (SLF) (66–68). The corpus callosum is critical for interhemispheric communication, and the cingulum facilitates communication between sub-regions of the cingulate cortex, as well as between ACC and other limbic regions. Given the importance of the ACC in depression, associations between CRF and the cingulum may have profound implications for depressed individuals.
One cross-sectional study examined the association between CRF and FA in two samples of older adults (N= 113; N=154) (67). This study confirmed earlier findings of positive CRF associations with FA in the cingulum (68–72) and corpus callosum (72–74), and also showed CRF associations with FA in other WM tracts. Additionally, FA in regions within these WM tracts partially mediated the link between CRF and spatial working memory, an executive function that is often impaired in depressed individuals (75).
Three studies have examined CRF or PA associations with FA in younger samples (70, 74, 76) (See Table 5). They found that CRF associations with FA in some tracts were similar across high fit older and younger adults, whereas CRF associations with FA in other tracts were only significant in older adults (74). Another study examined the association between PA and FA of projections from the hippocampus, and did not find a link between PA and FA. Key limitations within this literature include a large number of studies with small sample sizes, and some studies including samples having vascular conditions.
A longitudinal study examined the association between self-reported PA and global FA 3-years later, in a sample of older adults (N=691)(77) and found that PA, but not leisure time activity, was associated with global FA after 3 years, after accounting for age, sex, IQ, and socioeconomic status. However, this effect did not remain after covarying for vascular disease burden (cardiovascular disease, stroke, and hypertension), pointing to the important role of vascular factors in WM integrity.
One randomized controlled trial in healthy older adults examined changes in FA after a 1-year exercise intervention (78). There were no group differences observed in FA between the training groups. However, within the aerobic exercise group, change in CRF was positively associated with change in FA in the prefrontal and temporal lobes. Another 6-month trial compared the effects of an aerobic exercise intervention (cycling) on FA in healthy young adults and individuals with schizophrenia. They found that regardless of psychiatric diagnosis, the aerobic exercise intervention increased FA in the corpus callosum, SLF, and corticospinal tract (79).
This review was limited to exercise effects on white matter microstructure due to its overlap with the meta-analytic literature in depression-related white matter abnormalities. However, evidence also suggests exercise may lead to macro-structural changes in white matter, including reductions in white matter lesions and increases in white matter volume (See (80) for a detailed review). For instance, results from the Look – AHEAD trial suggests that a 10-year lifestyle intervention involving physical activity prevents the development of white matter lesions in adults with Type II diabetes, which often clusters with depressive symptoms (81). Further, given the high prevalence of white matter lesions in LLD, this may be an important mechanism through which exercise may influence depression in late-life (82).
Discussion
Meta-analyses of depressed adults have identified volumetric reductions in the hippocampus, ACC, PFC, striatum, and reduced microstructural integrity of white matter in the rostral corpus callosum and inferior parietal segments of the SLF (See Table 4). In contrast, higher CRF and PA have been linked to greater gray matter volume in the hippocampus, PFC, and ACC in cross-sectional and longitudinal studies in healthy adults. Experimental evidence from randomized trials also supports exercise-induced increases in hippocampal, PFC, and ACC volume. Yet, not all regions implicated in depression appear to be positively affected by PA (i.e., striatum). White matter structure may also be affected by CRF and PA in the corpus callosum and the cingulum, although the paucity of intervention studies and heterogeneity of study designs prevent firm conclusions.
Broader Implications
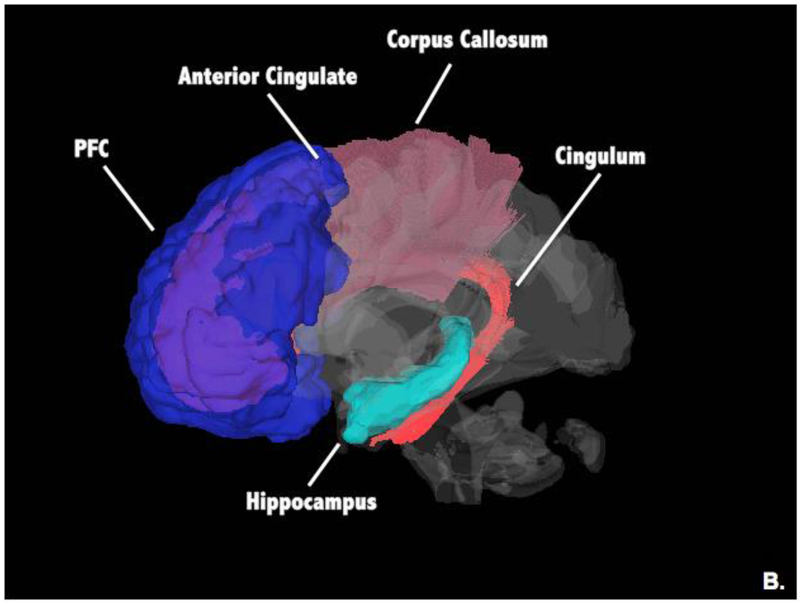
This review identified several regions that show consistent volumetric reductions in depression. Yet, many of these regions also show structural plasticity in response to exercise or in relation to higher levels of fitness. These regions include the hippocampus, ACC, and the PFC (See Figure 1). We speculate that it is partially through these neural volumetric pathways that exercise exerts its anti-depressant effects. Specific mechanisms by which exercise leads to these regional volumetric changes are still uncertain, but could include a number of downstream effects of exercise on cell proliferation, creation of new vasculature, expression of neurotransmitters, and changes in HPA-axis activity (83, 84). As we have seen, exercise and antidepressant medication not only trigger similar neuromolecular changes, but also have overlapping regional effects on brain structure (13). This overlap supports the possibility that volumetric increases in these regions mediate exercise-related reductions in depression (See Figure 1).
Figure 1.
Overall Exercise associations with Gray Matter and White Matter; these regions and tracts may be targeted in exercise-based treatments for depression.
Exercise may also influence white matter connectivity. The most consistent exercise associations have been shown in the genu and body of the corpus callosum, suggesting that exercise may broadly improve deficits in interhemispheric communication (See Figure 1). Further, exercise has been associated with improved integrity of the cingulum, a key tract involved in ACC-hippocampal communication (See Figure 1).
Given that the majority of evidence of exercise on brain structure is based on older adults, these effects may be particularly relevant for the treatment of LLD. Exercise may be a particularly favorable treatment for depressed older adults because its neural benefits may be heightened in this population undergoing age-related atrophy that is exacerbated by LLD-related neuropathology (i.e. white matter lesions). Specifically exercise may reduce atrophy, reduce white matter lesion burden, and improve cognitive function in those with LLD. Moreover, there is a greater need for non-pharmaceutical treatments for depression in late-life, due to limited efficacy of pharmaceutical treatments in this population. Finally, older adults, among other populations, tend to be less physically fit, and thus may show greater changes in fitness after starting to exercise. These potentially robust changes in fitness may translate to greater neural benefits.
Depressed individuals are often less active, suggesting that incentives or motivational factors for initiating and maintaining exercise must be considered (i.e., structured programs in groups). Also, depressed individuals may perceive enrolling in a psychotherapy treatment trial that may be augmented with exercise as more feasible and rewarding than initiating an independent exercise program. A recent meta-analysis examining moderators of the antidepressant effects of exercise found that various constellations of biological, clinical, psychological, and social factors can influence exercise effects on depression, including the presence of somatic symptoms, comorbid anxiety, self-esteem, social support, as well as circulating levels of neurotrophic factors (BDNF) and inflammatory markers (TNFα) (85). Specifically, BDNF may be a key mediator of exercise effects on structural brain markers of depression, given its role in both the etiology of depression and exercise-induced increases in hippocampal volume (11, 12).” Animal studies support the hypothesis that exercise relieves depressive symptoms by acting through BDNF pathways, although this has yet to be fully tested (5, 6).
Limitations
Several limitations should be considered here. Most importantly, the studies examining associations between exercise and regional gray matter volume and white matter integrity were not tested in depressed individuals. Therefore, they do not provide direct evidence of a mechanism, but suggest possible mechanisms through which exercise alleviates depression. Another notable limitation is the exclusion of studies examining brain function. We chose here to focus on volumetric and morphology studies because there is little overlap in the functional neuroimaging paradigms used in the depression and exercise literatures. Additionally, the number of studies and age groups examined differs greatly between the exercise and depression literatures. The exercise neuroimaging literature also includes a limited number of randomized interventions, precluding definitive conclusions about exercise-induced changes in brain structure. Finally, given that depression is a heterogeneous syndrome linked to several etiological factors that may influence these brain abnormalities, it remains unclear the extent to which these abnormalities would improve with various treatments, including exercise.
Future Directions and clinical implications
This review highlights a need to test whether changes to brain structure mediate the antidepressant properties of exercise, and the extent to which these effects may be age-dependent. Further, moderators should also be explored, such as genetic factors, environmental stress, medical illness burden, antidepressant medication use, and severity and duration of depressive episodes. Also, given emerging evidence that structural brain abnormalities may serve as a biomarker of risk (86–88), PA may have important clinical and neuroprotective effects on the risk for depression (89). In fact, individuals at risk for depression may prefer lifestyle modification to taking medication or psychotherapy. Therefore, future preventive interventions could explore neural mechanisms underlying the effects of PA on risk for depression. Finally, this field would benefit from a multi-level characterization of exercise on depression, cutting across molecular pathways, neural systems, and clinical symptoms in depressed individuals.
Conclusion
Exercise is a viable non-pharmaceutical treatment for depression. The benefits of exercise may also persist beyond the end of treatment, unlike antidepressant medication (4, 90). It is critical for future studies to test whether the brain regions identified in this review may be neurobiological markers of depression that may serve as targets for exercise-based treatments for depression.
Table 1.
Comparison of Short-term Effectiveness of Various Treatments for Depression
| Treatment Modality | Meta-Analysis | Effect Size | Pros | Cons |
|---|---|---|---|---|
| Cognitive Behavioral Therapy (CBT) | (91) | 0.71 (0.62–0.79) | Evidence-based Treatments: CBT, BA, and IPT
|
Requires:
|
| Behavioral Activation (BA) | (92) | 0.74 (0.31–1.17) | ||
| Interpersonal Psychotherapy (IPT) | (93) | 0.63 (0.36–0.90) | ||
| Pharmacotherapy | (94) | 0.49 (0.32–0.67) | Common classes: SSRIs, SNRIs
|
|
| Exercise | (4) | 0.62 (0.42–0.81) |
|
|
Table 3.
PA, Fitness, and Exercise Associations with White Matter Microstructure
| Study | Sample Size | Mean age (SD) | % female | PA/Fitness Measure | WMM Measure | Significant Findings |
|---|---|---|---|---|---|---|
| Young Adults | ||||||
| Cross-Sectional | ||||||
| Bracht et al. (2016) | 33 | 25.5 (4.2) | 57.6 | PA: Actigraphy over 72-hours | FA, myelin water fraction | ↑ myelin water fraction in R PHC CING; no association with FA |
| Hayes et al. (2015) | YA: 32 OA: 27 | YA: 21.1 (3.1) OA: 63.4 (6.4) | 54.2 | Fitness: Vo2 peak | FA | High-fit OA & YA: ↑ SPL, CR, superior parietal WM; High-fit OA only: ↑ body and genu of CC, precuneus, and SFG |
| Marks et al. (2007) | YA: 13 OA: 15 | YA: 24 (3) OA: 69.6 (4.7) | - | Fitness: non-exercise estimated Vo2 Max | FA | ↑ CING and UF |
| Intervention | ||||||
| Svatkova et al. (2015) | 81 (48 HC and 33 SZ) | 29.2 (18–48) | 35 | PA: aerobic exercise (cycling) 2 times per week for 6-months | FA | Exercise group > OT group (both SZ and HC): FA in LSLF, LCST, LIFOF, CC, FM, ATR |
| Older Adults | ||||||
| Cross-Sectional | ||||||
| Burzynska et al. (2015) | 88 | 65 (4) | 68 | Fitness: Vo2 Peak | FA | Light PA: ↑ temporal lobe; sedentary behavior: ↓ L PHC |
| Gons et al. (2013) | 440 | 65.18 (8.9) | 45.7 | PA: self-report questionnaire | FA, MD, RD, AD | PA: ↓ MD.RD, and AD in diffuse WM tracts; no sig. associations between PA and FA |
| Hayes et al. (2015) | YA: 32 OA: 27 | YA: 21.1 (3.1) OA: 63.4 (6.4) | 54.2 | Fitness: Vo2 peak | FA | High-fit OA & YA: ↑ SPL, CR, superior parietal WM; High-fit OA only: ↑ body and genu of CC, precuneus, and SFG |
| Johnson et al. (2012) | 26 | 65.79 (2.8) | 53.8 | Fitness: Vo2 Peak | FA, AD, RD, MD | ↑ FA CC; ↓ RD CC |
| Liu et al. (2012) | 15 | - | 46.7 | Fitness: Vo2 | FA | ↑ arcuate fasiculus and SLF |
| Marks et al. (2011) | 15 | 66 (6) | 46.7 | Fitness: Vo2 Peak | MD | ↑ L middle CING, explaining 28% of variance. |
| Marks et al. (2007) | YA: 13 OA: 15 | YA: 24 (3) OA: 69.6 (4.7) | - | Fitness: non-exercise estimated Vo2 Max | FA | ↑ CING and UF |
| Oberlin et al. (2015) | Exp 1:113 Exp 2:154 | 66.06 (.47) | Exp 1: 63.7 Exp 2: 68.8 | Fitness: Vo2 Max | FA | ↑ ACR, AIC, FX, CING, and CC across two samples. Fitness associations with WMM statistically mediated the relationship between fitness levels and spatial working memory performance. |
| Tian et al. (2014a) | 276 | 72.9 (2.7) | 58.7 | PA: self-report questionnaire-kcal per week calculated and then divided into 3 groups | FA, MD | No significant results |
| Tian et al. (2014b) | 164 | 82.9 (2.6) | 57.1 | Fitness: 400 meter walk time | FA | ↑ CING |
| Tseng et al. (2013a) | 20 | 73.5 (4.9) | 25 | Fitness: Vo2 Max | FA | ↑ R SCR, bilateral SLF, R IFOF (IFO), and LILF |
| Longitudinal | ||||||
| Gow et al. (2012) | 691 | 70 | 47.3 | PA: self-report questionnaire-6 point scale | FA, MD | No significant results; ↑ global FA, but became nonsignificant after covarying for vascular disease burden. |
| Intervention | ||||||
| Voss et al. (2013) | 70 | 64.9 (4.5) | 64.3 | Fitness: Vo2 max; PA: 12-month intervention divided into 1) brisk walking 2)balance and toning 3x/wk | FA, AD, RD | No group differences observed; Δfitness: ↑ FA in frontal and temporal lobe ROIs |
Funding Acknowledgment:
SG was supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 2014192810. KIE was supported by research grants (R01 DK095172, P30 AG024827, P30 MH90333). Partial support also received from the UPMC Endowment in geriatric psychiatry (CFR and HA). MAB was supported by research grants (P50 AG005133, P30 MH090333).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. World Suicide Prevention Day. 2012.
- 2.Burcusa SL, Iacono WG. Risk for recurrence in depression. Clinical psychology review. 2007. December;27(8):959–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holzel L, Harter M, Reese C, Kriston L. Risk factors for chronic depression--a systematic review. Journal of affective disorders. 2011. March;129(1–3):1–13. [DOI] [PubMed] [Google Scholar]
- 4.Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. The Cochrane database of systematic reviews. 2013;9:CD004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101(2):305–12. [DOI] [PubMed] [Google Scholar]
- 6.Garza AA, Ha TG, Garcia C, Chen MJ, Russo-Neustadt AA. Exercise, antidepressant treatment, and BDNF mRNA expression in the aging brain. Pharmacology, biochemistry, and behavior. 2004. February;77(2):209–20. [DOI] [PubMed] [Google Scholar]
- 7.Lin TW, Kuo YM. Exercise benefits brain function: the monoamine connection. Brain sciences. 2013;3(1):39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopresti AL, Hood SD, Drummond PD. A review of lifestyle factors that contribute to important pathways associated with major depression: diet, sleep and exercise. Journal of affective disorders. 2013. May 15;148(1):12–27. [DOI] [PubMed] [Google Scholar]
- 9.Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators of inflammation. 2008;2008:109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ernst C, Olson AK, Pinel JP, Lam RW, Christie BR. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? Journal of psychiatry & neuroscience: JPN. 2006. March;31(2):84–92. [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiology of aging. 2014. September;35 Suppl 2:S20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends in cognitive sciences. 2013. October;17(10):525–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh MK, Gotlib IH. The neuroscience of depression: implications for assessment and intervention. Behaviour research and therapy. 2014. November;62:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnone D, McKie S, Elliott R, et al. State-dependent changes in hippocampal grey matter in depression. Molecular psychiatry. 2013. December;18(12):1265–72. [DOI] [PubMed] [Google Scholar]
- 15.Cole J, Costafreda SG, McGuffin P, Fu CH. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. Journal of affective disorders. 2011. November;134(1–3):483–7. [DOI] [PubMed] [Google Scholar]
- 16.Schmaal L, Veltman DJ, van Erp TG, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Molecular psychiatry. 2015. June 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. Journal of affective disorders. 2012. April;138(1–2):9–18. [DOI] [PubMed] [Google Scholar]
- 18.Bora E, Harrison BJ, Davey CG, Yucel M, Pantelis C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychological medicine. 2012. April;42(4):671–81. [DOI] [PubMed] [Google Scholar]
- 19.Du MY, Wu QZ, Yue Q, et al. Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2012. January 10;36(1):11–6. [DOI] [PubMed] [Google Scholar]
- 20.Lai CH. Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry research. 2013. January 30;211(1):37–46. [DOI] [PubMed] [Google Scholar]
- 21.Kempton MJ, Salvador Z, Munafo MR, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Archives of general psychiatry. 2011. July;68(7):675–90. [DOI] [PubMed] [Google Scholar]
- 22.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human brain mapping. 2009. November;30(11):3719–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. Journal of psychiatry & neuroscience: JPN. 2009. January;34(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- 24.Sacher J, Neumann J, Funfstuck T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. Journal of affective disorders. 2012. October;140(2):142–8. [DOI] [PubMed] [Google Scholar]
- 25.Zhao YJ, Du MY, Huang XQ, et al. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychological medicine. 2014. October;44(14):2927–37. [DOI] [PubMed] [Google Scholar]
- 26.Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2013. February;21(2):184–95. [DOI] [PubMed] [Google Scholar]
- 27.Du M, Liu J, Chen Z, et al. Brain grey matter volume alterations in late-life depression. Journal of psychiatry & neuroscience: JPN. 2014. November;39(6):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Li L, Wu M, et al. Brain gray matter alterations in first episodes of depression: A meta-analysis of whole-brain studies. Neuroscience and biobehavioral reviews. 2016. January;60:43–50. [DOI] [PubMed] [Google Scholar]
- 29.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological psychiatry. 2006. June 15;59(12):1116–27. [DOI] [PubMed] [Google Scholar]
- 30.Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk of depression. Archives of general psychiatry. 2010. March;67(3):270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biological psychiatry. 2010. February 15;67(4):357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erickson KI, Prakash RS, Voss MW, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009. October;19(10):1030–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bugg JM, Shah K, Villareal DT, Head D. Cognitive and neural correlates of aerobic fitness in obese older adults. Experimental aging research. 2012;38(2):131–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabo AN, McAuley E, Erickson KI, et al. Cardiorespiratory fitness, hippocampal volume, and frequency of forgetting in older adults. Neuropsychology. 2011. September;25(5):545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Head D, Singh T, Bugg JM. The moderating role of exercise on stress-related effects on the hippocampus and memory in later adulthood. Neuropsychology. 2012. March;26(2):133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011. February 15;108(7):3017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niemann C, Godde B, Voelcker-Rehage C. Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Frontiers in aging neuroscience. 2014;6:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maass A, Duzel S, Goerke M, et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Molecular psychiatry. 2015. May;20(5):585–93. [DOI] [PubMed] [Google Scholar]
- 39.Kleemeyer MM, Kuhn S, Prindle J, et al. Changes in fitness are associated with changes in hippocampal microstructure and hippocampal volume among older adults. NeuroImage. 2015. November 14. [DOI] [PubMed] [Google Scholar]
- 40.Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral cortex. 2005. November;15(11):1676–89. [DOI] [PubMed] [Google Scholar]
- 41.Kheirbek MA, Hen R. Dorsal vs ventral hippocampal neurogenesis: implications for cognition and mood. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011. January;36(1):373–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998. May 1;18(9):3206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas AG, Dennis A, Rawlings NB, et al. Multi-modal characterization of rapid anterior hippocampal volume increase associated with aerobic exercise. NeuroImage. 2016. May 01;131:162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2007. March 27;104(13):5638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krogh J, Rostrup E, Thomsen C, Elfving B, Videbech P, Nordentoft M. The effect of exercise on hippocampal volume and neurotrophines in patients with major depression--a randomized clinical trial. Journal of affective disorders. 2014. August;165:24–30. [DOI] [PubMed] [Google Scholar]
- 46.Arnone D, McIntosh AM, Ebmeier KP, Munafo MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2012. January;22(1):1–16. [DOI] [PubMed] [Google Scholar]
- 47.Gasquoine PG. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neuroscience and biobehavioral reviews. 2013. March;37(3):340–8. [DOI] [PubMed] [Google Scholar]
- 48.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS spectrums. 2008. August;13(8):663–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain structure & function. 2008. September;213(1–2):93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taren AA, Venkatraman V, Huettel SA. A parallel functional topography between medial and lateral prefrontal cortex: evidence and implications for cognitive control. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011. March 30;31(13):5026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiology of aging. 2011. March;32(3):506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weinstein AM, Voss MW, Prakash RS, et al. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain, behavior, and immunity. 2012. July;26(5):811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erickson KI, Colcombe SJ, Elavsky S, et al. Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiology of aging. 2007. February;28(2):179–85. [DOI] [PubMed] [Google Scholar]
- 54.Floel A, Ruscheweyh R, Kruger K, et al. Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? NeuroImage. 2010. February 1;49(3):2756–63. [DOI] [PubMed] [Google Scholar]
- 55.Gordon BA, Rykhlevskaia EI, Brumback CR, et al. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008. September;45(5):825–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colcombe SJ, Erickson KI, Raz N, et al. Aerobic fitness reduces brain tissue loss in aging humans. The journals of gerontology Series A, Biological sciences and medical sciences. 2003. February;58(2):176–80. [DOI] [PubMed] [Google Scholar]
- 57.Rovio S, Spulber G, Nieminen LJ, et al. The effect of midlife physical activity on structural brain changes in the elderly. Neurobiology of aging. 2010. November;31(11):1927–36. [DOI] [PubMed] [Google Scholar]
- 58.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. The journals of gerontology Series A, Biological sciences and medical sciences. 2006. November;61(11):1166–70. [DOI] [PubMed] [Google Scholar]
- 59.Ruscheweyh R, Willemer C, Kruger K, et al. Physical activity and memory functions: an interventional study. Neurobiology of aging. 2011. July;32(7):1304–19. [DOI] [PubMed] [Google Scholar]
- 60.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual review of neuroscience. 1986;9:357–81. [DOI] [PubMed] [Google Scholar]
- 61.Verstynen TD, Lynch B, Miller DL, et al. Caudate Nucleus Volume Mediates the Link between Cardiorespiratory Fitness and Cognitive Flexibility in Older Adults. Journal of aging research. 2012;2012:939285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wise T, Radua J, Nortje G, Cleare AJ, Young AH, Arnone D. Voxel-Based Meta-Analytical Evidence of Structural Disconnectivity in Major Depression and Bipolar Disorder. Biological psychiatry. 2015. March 12. [DOI] [PubMed] [Google Scholar]
- 63.Liao Y, Huang X, Wu Q, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. Journal of psychiatry & neuroscience: JPN. 2013. January;38(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy ML, Frodl T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biology of mood & anxiety disorders. 2011;1(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wen MC, Steffens DC, Chen MK, Zainal NH. Diffusion tensor imaging studies in late-life depression: systematic review and meta-analysis. International journal of geriatric psychiatry. 2014. December;29(12):1173–84. [DOI] [PubMed] [Google Scholar]
- 66.Liu Z, Farzinfar M, Katz L, et al. Automated Voxel-Wise Brain DTI Analysis of Fitness and Aging. The Open Medical Imaging Journal. 2012;6:80–8. [Google Scholar]
- 67.Oberlin LE, Verstynen TD, Burzynska AZ, et al. White matter microstructure mediates the relationship between cardiorespiratory fitness and spatial working memory in older adults. NeuroImage. 2015. October 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tseng BY, Gundapuneedi T, Khan MA, et al. White matter integrity in physically fit older adults. NeuroImage. 2013. November 15;82:510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marks BL, Katz LM, Styner M, Smith JK. Aerobic fitness and obesity: relationship to cerebral white matter integrity in the brain of active and sedentary older adults. British journal of sports medicine. 2011. December;45(15):1208–15. [DOI] [PubMed] [Google Scholar]
- 70.Marks BL, Madden DJ, Bucur B, et al. Role of aerobic fitness and aging on cerebral white matter integrity. Annals of the New York Academy of Sciences. 2007. February;1097:171–4. [DOI] [PubMed] [Google Scholar]
- 71.Tian Q, Erickson KI, Simonsick EM, et al. Physical activity predicts microstructural integrity in memory-related networks in very old adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2014. October;69(10):1284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gons RA, Tuladhar AM, de Laat KF, et al. Physical activity is related to the structural integrity of cerebral white matter. Neurology. 2013. September 10;81(11):971–6. [DOI] [PubMed] [Google Scholar]
- 73.Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. NeuroImage. 2012. January 16;59(2):1514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hayes SM, Salat DH, Forman DE, Sperling RA, Verfaellie M. Cardiorespiratory fitness is associated with white matter integrity in aging. Ann Clin Transl Neurol. 2015. June;2(6):688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychological bulletin. 2013. January;139(1):81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bracht T, Jones DK, Bells S, Walther S, Drakesmith M, Linden D. Myelination of the right parahippocampal cingulum is associated with physical activity in young healthy adults. Brain structure & function. 2016. January 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gow AJ, Bastin ME, Munoz Maniega S, et al. Neuroprotective lifestyles and the aging brain: activity, atrophy, and white matter integrity. Neurology. 2012. October 23;79(17):1802–8. [DOI] [PubMed] [Google Scholar]
- 78.Voss MW, Heo S, Prakash RS, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Human brain mapping. 2013. November;34(11):2972–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Svatkova A, Mandl RC, Scheewe TW, Cahn W, Kahn RS, Hulshoff Pol HE. Physical Exercise Keeps the Brain Connected: Biking Increases White Matter Integrity in Patients With Schizophrenia and Healthy Controls. Schizophrenia bulletin. 2015. July;41(4):869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sexton CE, Betts JF, Demnitz N, Dawes H, Ebmeier KP, Johansen-Berg H. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. NeuroImage. 2016. May 01;131:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Espeland MA, Erickson K, Neiberg RH, et al. Brain and White Matter Hyperintensity Volumes After 10 Years of Random Assignment to Lifestyle Intervention. Diabetes care. 2016. May;39(5):764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Molecular psychiatry. 2013. September;18(9):963–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nature neuroscience. 2007. September;10(9):1110–5. [DOI] [PubMed] [Google Scholar]
- 84.Mahar I, Bambico FR, Mechawar N, Nobrega JN. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neuroscience and biobehavioral reviews. 2014. January;38:173–92. [DOI] [PubMed] [Google Scholar]
- 85.Schuch FB, Dunn AL, Kanitz AC, Delevatti RS, Fleck MP. Moderators of response in exercise treatment for depression: A systematic review. Journal of affective disorders. 2016. January 20;195:40–9. [DOI] [PubMed] [Google Scholar]
- 86.Spalletta G, Piras F, Caltagirone C, Fagioli S. Hippocampal multimodal structural changes and subclinical depression in healthy individuals. Journal of affective disorders. 2014. January;152:105–12. [DOI] [PubMed] [Google Scholar]
- 87.Whittle S, Lichter R, Dennison M, et al. Structural brain development and depression onset during adolescence: a prospective longitudinal study. The American journal of psychiatry. 2014. May;171(5):564–71. [DOI] [PubMed] [Google Scholar]
- 88.Lener MS, Kundu P, Wong E, et al. Cortical abnormalities and association with symptom dimensions across the depressive spectrum. Journal of affective disorders. 2016. January 15;190:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Prev Med. 2008. May;46(5):397–411. [DOI] [PubMed] [Google Scholar]
- 90.Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosomatic medicine. 2000. Sep-Oct;62(5):633–8. [DOI] [PubMed] [Google Scholar]
- 91.Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson KS. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Canadian journal of psychiatry Revue canadienne de psychiatrie. 2013. July;58(7):376–85. [DOI] [PubMed] [Google Scholar]
- 92.Mazzucchelli T, Kane R, Rees C. Behavioral Activation Treatments for Depression in Adults: A Meta-analysis and Review. Clin Psychol-Sci Pr. 2009. December;16(4):383–411. [Google Scholar]
- 93.Cuijpers P, Geraedts AS, van Oppen P, Andersson G, Markowitz JC, van Straten A. Interpersonal psychotherapy for depression: a meta-analysis. The American journal of psychiatry. 2011. June;168(6):581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arroll B, Elley CR, Fishman T, et al. Antidepressants versus placebo for depression in primary care. The Cochrane database of systematic reviews. 2009(3):CD007954. [DOI] [PMC free article] [PubMed] [Google Scholar]