Abstract
A specific HPLC-MS/MS (High-Performance Liquid Chromatography with tandem Mass Spectrometry) method was developed and validated for simultaneous determination of several monosaccharides in three kinds Osmanthus fragrans Lour. After extraction, separation, protein removal, pigment removal and hydrolysis, monosaccharides was finally obtained from Osmanthus fragrans Lour. Positive ion mode detection and Multiple Reaction Monitoring (MRM) mode were used for quantitative analysis by PMP pre-column derivatization and Electrospray Ionization (ESI). Analysis and content determination of 6 monosaccharide components in 3 kinds of Osmanthus fragrans Lour. The HPLC separation was achieved on a Shim-pack VP-ODS6022748 (150 L × 2.0) with gradient elution at a flow rate of 0.2 ml/min in a run time of 40 min, and the mobile phase was acetonitrile-5 mmol/L ammonium acetate. PMP derivatization in HPLC-MS/MS can accurately measure Osmanthus fragrans Lour. mannose (Man), ribose (Rib), rhamnose (Rha), galacturonic acid (Gal UA), glucose (Glu), galactose (Gal), xylose (Xyl), fucose (Fuc). The results showed that HPLC-MS/MS pre-column derivatization method was simple and rapid, with small measurement error, but high sensitivity and good repeatability. The analysis of monosaccharide components in polysaccharide components has important practical significance.
Keywords: Osmanthus fragrans Lour., PMP pre-column derivatization, HPLC-MS/MS
1. Introduction
Osmanthus fragrans Lour belongs to the Oleaceae family and is an ornamental plant used as traditional folk medicine in the southern and central parts of China for the treatment of various diseases [1]. It had been used as a medicinal plant in China for thousands of years and had a wide range of biological effects, including inflammation reduction [2], antioxidation [3], aging prevention [3] and melanogenesis inhibition [4]. In addition, mostly bio-active components in the Osmanthus fragrans Lour. extraction are carotenoids and phenolic acids [5]. And there has been extensive study on natural pharmaceutical polysaccharides [6,7]. Beginning in the 1950s till now, hundreds of polysaccharides have been found to be biologically active [8]. Polysaccharides play important biological roles in antiinflammatory, anti-viral, anti-tumor, anti-aging, and immuno-activation processes [9]. Moreover, polysaccharides are a very important class of organic compounds in living organisms. In addition to participating in the structure formation of organisms, they also function as information molecules for energy substances and cell recognition, because they participate in the conversion and metabolism of substances in the body to produce essential amino acids, nucleotides, fatty acids, etc. [10]. Polysaccharide is one of the important components of Osmanthus fragrans Lour., but research on Osmanthus fragrans Lour. is mainly focused on essential oil of Osmanthus fragrans Lour, while the monosaccharide content of Osmanthus fragrans Lour. polysaccharide is rarely reported.
HPLC-MS/MS is widely used in the determination of active ingredients, including traditional Chinese medicines due to its strong specificity and high sensitivity [11–13]. Over the past decades, there has been significant advances in the development of HPLC-MS/MS methods for the analysis of sugars and monosaccharides with better sensitivity and specificity while maintaining the speed and simplicity of implementation. However, due to the low ionization efficiency of monosaccharides, the HPLC-MS/MS method is limited by sensitivity loss, and therefore, derivatization of monosaccharides is indispensable for obtaining highly sensitive detection. Because1-pheny-3-methyl-5-pyrazolone (PMP) pre-column derivatization in high performance liquid chromatography, the product is not stereo isomeric, leading to higher UV detection sensitivity [14–17].
In this study, by collecting data on the characteristic fragment ions of six PMP-derived monosaccharides, we optimized the detection and analysis time, polysaccharide hydrolysis conditions, and pre-column pre-derivative reaction conditions. A new method for the simultaneous determination of six monosaccharides in Osmanthus fragrans Lour. by precolumn PMP derivatization with HPLC-ESI-MS/MS method was established. The method was applied to analyze the monosaccharide content of three different varieties of Osmanthus fragrans Lour., providing a basis for the development and utilization of Osmanthus fragrans Lour. resources.
2. Results
2.1. Mass spectrometry
All analytes were monitored in the positive mode electrospray ionization (ESI) and quantified by the multiple reaction monitoring (MRM) mode. MS parameters were as follows: heating block temperature, 400 °C; DL temperature, 250 °C; electrospray voltage, 3.5 KV; collision gas pressure, 230 KPa; nebulizer gas flow rate, 3.0 L/min; dry gas flow rate, 15.0 L/min. Nitrogen was used in all cases. The analytes were quantified by scanning from m/z 100–1000 at a scan rate of 0.1 s/cycle. Figs. 1 and 2 shows Q3 scan and Product Ion Scan of six monosaccharide standards.
Fig. 1.
Q3 Scan of PMP-labeled monosaccharide standard. a-Man, b-Rib, c-Glu, d-Gal, e-Xyl, f-Fuc
Fig. 2.
Product Ion Scan of PMP-labeled monosaccharide standard. a-Man, b-Rib, c-Glu, d-Gal, e-Xyl, f-Fuc.
2.2. Preparation of standard solutions
An accurately weighed amount (10 mg) of mannose, ribose, glucose, galactose, xylose, fucose was transferred into a 10-ml volumetric flask and dissolved in ammonia solution (5 ml) to produce mixed monosaccharide standard solutions of 2 mg/ml, according to the method for PMP derivatization. Fig. 3 shows PMP derivatization process of glucose standards and MS fragmentation pathway of standards for PMP-labeled glucose derivatives in positive ion mode.
Fig. 3.
PMP derivatization of glucose standards and mass spectrometry fragmentation of PMP-labeled glucose standard derivatives in positive ion mode.
2.3. Linearity
The standard curve consisted of six concentration levels, and each level of solution was prepared and assayed during the following days. The concentrations of standard solution were 2 μg/ml, 200 ng/ml, 100 ng/ml, 50 ng/ml, 20 ng/ml, 2 ng/ml. According to the PMP derivatization of monosaccharides, the samples were transferred to a 1.5 ml autosampler vial for HPLC-MS/MS analysis within 7 days, to record the Chromatographic peak areas of the corresponding concentration. The standard curves were shown in Table 1, The HPLC-MS/MS method showed excellent linearity over the entire concentration range (2–2000 ng/mL) for monosaccharides, with correlation coefficient (R2) >0.9990.
Table 1.
Standard curve of mixed monosaccharide standards.
| Analyte | Regression equation | R2 | Linear range (ng/ml) | LOD (ug/ml) | LOQ (ug/ml) |
|---|---|---|---|---|---|
| Man | Y = 25.693x + 1099 | 0.9996 | 2–2000.0 ng/ml | 0.06 | 0.18 |
| Rib | Y = 50.486x + 13,571 | 0.9992 | 2–2000.0 ng/ml | 0.06 | 0.20 |
| Glu | Y = 25.14x + 2756.7 | 0.9999 | 2–2000.0 ng/ml | 0.17 | 0.50 |
| Gal | Y = 33.63x + 2074.1 | 0.9997 | 2–2000.0 ng/ml | 0.08 | 0.20 |
| Xyl | Y = 75.164x + 20,942 | 0.9992 | 2–2000.0 ng/ml | 0.15 | 0.45 |
| Fuc | Y = 47.213x + 12,139 | 0.9994 | 2–2000.0 ng/ml | 0.20 | 0.60 |
2.4. Precision (n = 6)
The precision of the method was assessed by analyzing the 200 ng/ml of mixed monosaccharide standard solution, according to the PMP derivatization of monosaccharides. The intra-day precision was evaluated by six replicate standard solutions on the same day. After the injection, the chromatographic peak areas were measured, the precision was expressed as relative standard deviation (RSD). The results were: mannose 1.98%, ribose 1.48%, glucose 3.33%, galactose 2.75%, xylose 3.81%, and fucose 0.82%. The precision of the method was evaluated by repeatability studies, and RSD values calculated were lower than 8% indicating a good repeatability of the method%, indicating that the instrument had good precision.
2.5. Stability (n =6)
To facilitate derivatization reaction of the analytes, the stability of the method was assessed by analyzing the 200 ng/ml of mixed monosaccharides standard solutions, samples were placed at 4 °C for 24 h overnight. 2 μl of solutions were injected in sequence at 0,4, 6,10,12,24 h, and continuously injected 6 times, and recorded the peak areas values. The relative standard deviations were calculated and the results were: mannose 1.50%, ribose 3.00%, rhamnose 1.81%, galacturonic acid 3.09%, glucose 1.99%, galactose 4.94%, xylose 0.99%, fucose 4.21%. The RSD is in the range of 0.99%–4.94%, the stability of this method was evaluated with analytical determination repeated at different intervals, revealing that the RSD of the saccharides was <5% within 12 h. The stability of the analytes in this situation is important for accurate quantification.
2.6. Repeatability (n = 6)
The Osmanthus fragrans Lour. polysaccharide hydrolysate was derived, the samples were analyzed by HPLC-MS/MS within 7 days, this operation was repeated six times. and the results were: mannose 2.88%, Ribose 2.72%, rhamnose 2.36%, galacturonic acid 2.77%, glucose 1.80%, galactose 4.58%, xylose 1.02%, fucose 3.86%. It could be seen that the RSD was in the range of 1.02%–4.58%, which demonstrated that the derivative reaction of the sample was very reproducible.
2.7. Recovery (n = 6)
To determine the accuracy of the proposed method, the analytical recoveries were determined after spiking of known amount of monosaccharide standard. The recoveries were evaluated by analyzing samples (low, medium, high concentration), the blank samples of this study were prepared from mixed monosaccharide standard solution. The effects were evaluated by comparing the peak areas of the analytes in the Osmanthus fragrans Lour, polysaccharide hydrolysate samples with those of pure standard solutions. Each monosaccharide RSD, as shown in Table 2.
Table 2.
Recovery rate of each monosaccharide reference substance in Osmanthus fragrans Lour.
| Analyte | Original quantity (mg/g) | Amount added (mg/g) | Measured amount (mg/g) | Recovery rate (%) | The average recovery rate (%) | RSD (%) |
|---|---|---|---|---|---|---|
| Man | 1.3446 | 15.3159 | 16.7829 | 100.80 | 104.53 | 4.28 |
| 0.7180 | 15.3159 | 16.4978 | 103.29 | |||
| 0.6208 | 15.3159 | 17.3907 | 109.49 | |||
| Rib | 1.2725 | 15.5708 | 16.6808 | 98.96 | 101.36 | 4.66 |
| 0.7060 | 15.5708 | 17.3352 | 106.80 | |||
| 0.4550 | 15.5708 | 15.7632 | 98.31 | |||
| Glu | 5.3383 | 16,1272 | 22.4894 | 106.35 | 105.37 | 1.04 |
| 4.6002 | 16.1272 | 21.6465 | 105.57 | |||
| 4.1715 | 16.1272 | 20.9750 | 104.19 | |||
| Gal | 5.0956 | 13.8081 | 18.5045 | 97.11 | 96.81 | 0.67 |
| 4.3390 | 13.8081 | 17.7675 | 97.25 | |||
| 3.9596 | 13.8081 | 17.2250 | 96.07 | |||
| Xyl | 4.2522 | 14.1550 | 18.4164 | 100.06 | 100.87 | 0.71 |
| 4.6142 | 14.1550 | 18.9303 | 101.14 | |||
| 3.8275 | 14.1550 | 18.1834 | 101.42 | |||
| Fuc | 0.5447 | 16.0358 | 16.3473 | 98.55 | 98.63 | 1.14 |
| 0.2258 | 16.0358 | 15.8670 | 97.54 | |||
| 0.0721 | 16.0358 | 16.0757 | 99.79 |
2.8. Sample content determination
Three kinds of Osmanthus fragrans Lour. samples were taken for polysaccharides extraction, hydrolysis (n = 6), and the sample solutions were prepared by PMP derivatization of monosaccharides, the samples were analyzed by HPLC-MS/MS, the peak areas were recorded and the content of each monosaccharide was calculated.
2.9. Data processing
The concentration of monosaccharides in sweet-scented Osmanthus fragrans Lour. polysaccharides:
Among them: Cx is the concentration of each monosaccharide in the polysaccharide sample (ug/ml), Ax is the peak area of the monosaccharide in the polysaccharide sample (mAU.s), a is slope of the standard curve, and b is the intercept of the standard curve.
Monosaccharide content in Osmanthus fragrans Lour. polysaccharide:
Among them: V is the total volume of the polysaccharide sample solution (ml) and Mx is the mass of the samples (mg).
According to the existing study, it can be concluded that all three kinds of Osmanthus fragrans Lour. contain mannose, ribose, glucose, galactose, xylose and fucose. Based on the references [19–23], the retention time (polar order) of each monosaccharide, the mass spectrometry data and the abundance ratio of the pyrolysis fragments in the mass spectrometer, it can be determined that the Osmanthus fragrans Lour. contain rhamnose and galacturonic acid. According to the MRM map (Fig. 4), it can be concluded that rhamnose has large response value in Osmanthus fragrans var. semperflorens. According to the specific content of each monosaccharide in Osmanthus fragrans Lour., it could be seen at a glance that the content of monosaccharides in Osmanthus fragrans Lour. was relatively stable (Figs. 4, 5, 6 and Table 3), with little change in error, and could more accurately reflect monosaccharides content in Osmanthus fragrans Lour. The identified contents in Osmanthus fragrans var. semperflorens are: galactose glucose > xylose > mannose > ribose > fucose; Osmanthus fragrans scv. latifoliu and Osmanthus fragrans var. thunbergii.: galactose > xylose glucose > mannose > ribose > fucose. The ratio of monosaccharide content in Osmanthus fragrans var. semperflorens was mannose: ribose: glucose:galactose:xylose:fucose 0.9:0.7:5.2:5.8:4.8:0.1; the ratio of monosaccharides in Osmanthus fragrans scv. latifoliu was mannose: ribose: Glucose:Galactose:Xylose:Fucose 0.5:0.3:2.3:3.6:3.1:0.2; the ratio of monosaccharide content in Osmanthus fragrans var. thunbergii. was mannose: ribose: glucose: galactose: xylose: fucose was 0.5:0.3:1.9:3.2:3.0:0.2.
Fig. 4.
The SD of the content of each monosaccharide in Osmanthus fragrans var. semperflorens is indicated by error bars (n = 6).
Fig. 5.
The SD of the content of each monosaccharide in Osmanthus fragrans scv. Latifoliu is indicated by error bars (n = 6).
Fig. 6.
The SD of the content of each monosaccharide in Osmanthus fragrans var. thunbergii. is indicated by error bars (n = 6).
Table 3.
SD and RSD of monosaccharides content in three Osmanthus fragrans Lour. samples.
| Analytes |
Osmanthus fragrans var. semperflorens |
Osmanthus fragrans scv. latifoliu |
Osmanthus fragrans var. thunbergii. |
|||
|---|---|---|---|---|---|---|
| SD (%) | RSD (%) | SD (%) | RSD (%) | SD (%) | RSD (%) | |
| Man | 7.34 | 7.89 | 5.10 | 11.45 | 0.305 | 8.632 |
| Rib | 8.10 | 10.99 | 6.66 | 23.57 | 0.279 | 12.476 |
| Glu | 5.93 | 11.51 | 1.51 | 7.13 | 2.223 | 10.794 |
| Gal | 3.87 | 6.87 | 1.91 | 5.58 | 2.845 | 8.391 |
| Xyl | 4.20 | 9.24 | 2.02 | 6.45 | 0.588 | 2.234 |
| Fuc | 0.53 | 27.05 | 1.87 | 10.30 | 0.432 | 8.011 |
2.10. MRM detection chromatogram and pyrolysis analysis of monosaccharide standards
The PMP-derived monosaccharide standards were diluted to a concentration of200 ng/ml and directly subjected to HPLC-MS/MS analysis. Fig. 1 showed the mass spectrum of ion peaks obtained by qualitative analysis of each monosaccharide in positive ion mode, in the preliminary experiment, the response values of monosaccharides in ESI positive ion mode and negative ion mode were compared, it was found that when ammonium acetate (5 mmol/L)-acetonitrile was used as the mobile phase, the response of monosaccharides in negative ion mode was very low. In positive ion mode, the response value reached 106. Considering the ESI positive ion mode, 5 mmol/L of ammonium acetate-acetonitrile was used as the mobile phase for detection and analysis.
By observing Q3 scan and product ion scan, and the main MS parameters of the PMP-labeled monosaccharide standard in positive ion mode (Fig. 1, Table 4), it was found that monosaccharides PMP derivative signal had the strongest excimer ion peaks [M + 2PMP-H2O + H]+, while monosaccharides derivative secondary fragment ion peaks cracked the m/z175[PMP + H] + child ion. It was anticipated that the fragment ions of m/z175 may be the result of breaking a PMP and a monomolecular monosaccharide, with a uniform cleavage pattern. The specific cleavage process is exemplified by a glucose derivative as shown in Fig. 2.
Table 4.
Main MS parameters of monosaccharide standard derivatization products in positive ion mode.
| Analyte | keep time (min) | Molecular weight | Precursorion (m/z) | Production (m/z) | CE/V |
|---|---|---|---|---|---|
| d-Man | 12.172 | 180 | 511.40 [M + H]+ | 175.10 | −35 |
| d-Rib | 15.220 | 150 | 481.36 [M + H]+ | 175.10 | −35 |
| d-Glu | 22.297 | 180 | 511.40 [M + H]+ | 175.10 | −35 |
| d-Gal | 23.968 | 180 | 511.40 [M + H]+ | 175.10 | −35 |
| d-Xyl | 24.775 | 150 | 481.35 [M + H]+ | 175.10 | −35 |
| l-Fuc | 29.090 | 164 | 495.20 [M + H]+ | 175.10 | −35 |
In this experiment, the chromatographic peaks were confirmed by comparing the retention time of the standard monosaccharides, literature data and mass spectrometry fragment information. As shown in Fig. 7, it could be seen from the figure that the chromatographic peaks of the standard derivatives were symmetrical with each other, and there was no tailing phenomenon, indicating that the monosaccharides reacted exclusively with the PMP derivative, with no by-products formed, and that the mixed polysaccharides were well separated. It was anticipated that the PMP derivatization process would greatly change the polarity of the sugars. Because the hydrophobicity of the derivative products were enhanced, it is easier to separate the sugars on the column.
Fig. 7.
Multiple reaction detection chromatogram ofPMP-labeled mixed monosaccharide standard in positive ion mode. 1-mannose, 2-ribose, 3-glucose, 4-galactose, 5-xylose, 6-fucose.
2.11. MRM detection chromatogram and pyrolysis analysis of Osmanthus fragrans Lour. polysaccharide
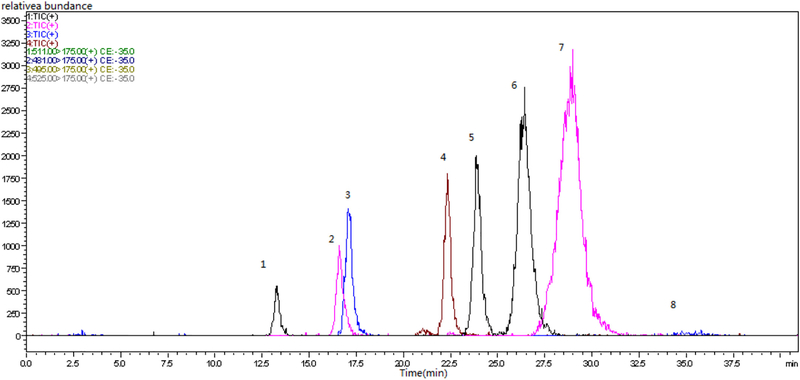
After MRM analysis of the derivatized products of Osmanthus fragrans Lour. polysaccharides by water extraction and acid hydrolysis, there were eight ion peaks derived from the same rule of monosaccharides cleavage (Figs. 8, 9 and 10), which was consistent with the standards. Following the cleavage law of monosaccharides derivative that was optimized for the mass spectrometry conditions, standards for selecting the precursors and daughter ions were optimized for mass spectrometry conditions in MRM mode, as shown in Table 5. The monosaccharides composition of three kinds of Osmanthus fragrans Lour. are mannose, ribose, rhamnose, galacturonic acid, glucose, galactose, xylose, fucose.
Fig. 8.
MRM ofPMP-labeled Osmanthus fragrans var. semperflorens in positive ion mode. 1-mannose, 2-ribose, 3-rhamnose, 4-galacturonic acid, 5-glucose, 6-galactose, 7-xylose, 8-fucose.
Fig. 9.
MRM ofPMP-labeled Osmanthus fragrans scv. latifoliu in positive ion mode. mannose, 2-ribose, 3-rhamnose, 4-galacturonic acid, 5-glucose, 6-galactose, 7-xylose, 8-fucose.
Fig. 10.
MRM of PMP-labeled Osmanthus fragrans var. thunbergii. in positive ion mode. 1-mannose, 2-ribose, 3-rhamnose, 4-galacturonic acid, 5-glucose, 6-galactose, 7-xylose, 8-fucose.
Table 5.
Main mass spectrometry parameters of monosaccharide PMP derivatization products in Osmanthus fragrans Lour. polysaccharides in positive ion mode.
| Monosaccharide | Osmanthus fragrans var. semperflorens keep time (min) | Osmanthus fragrans scv. latifoliu keep time (min) | Osmanthus fragrans var. thunbergii. keep time (min) | Molecular weight | Precursor ion (m/z) | Production (m/z) | CE/V |
|---|---|---|---|---|---|---|---|
| d-Man | 12.568 | 12.996 | 13.282 | 180 | 511.40 [M + H]+ | 175.10 | −35 |
| d-Rib | 15.487 | 16.565 | 16.583 | 150 | 481.36 [M + H]+ | 175.10 | −35 |
| d-Rha | 16.024 | 16.843 | 17.065 | 164 | 495.20 [M + H]+ | 175.10 | −35 |
| GalUA | 20.264 | 21.992 | 21.890 | 194 | 524.18 [M + H]+ | 175.10 | −35 |
| d-Glu | 22.297 | 23.604 | 23.854 | 180 | 511.40 [M + H]+ | 175.10 | −35 |
| d-Gal | 24.235 | 25.898 | 26.425 | 180 | 511.40 [M + H]+ | 175.10 | −35 |
| d-Xyl | 25.581 | 28.039 | 28.979 | 150 | 481.36 [M + H]+ | 175.10 | −35 |
| l-Fuc | 29.410 | 35.053 | 35.794 | 164 | 495.20 [M + H]+ | 175.10 | −35 |
2.12. Selection of pre-treatment methods of osmanthus
The selection of optimal parameters for HPLC and MS played an important role in quantitative analysis. For example, impurities in the samples might inhibit or enhance the ion signal, which affects the precision and repeatability of HPLC-MS/MS. In this study, the time, temperature and hydrochloric acid concentration of the acid hydrolysis in Osmanthus fragrans Lour. polysaccharides were carefully selected: the approximate range of factors affecting hydrolysis was obtained through preliminary experiments, the selected hydrochloric acid concentration of 1 mol/L, 2 mol/L, 3 mol/L, hydrolysis temperature at 80 °C, 90 °C, 100 °C, hydrolysis time of 1 h, 3 h, 5 h, were performed in three-factor and three-level orthogonal experiments. The total sugars peak areas and those of the derivative were analyzed shown in Table 6.
Table 6.
Factors and levels.
| Levels | Hydrochloric acid (mol/L)-A | Temperature (°C)-B | Time (h)-C |
|---|---|---|---|
| 1 | 1 | 80 | 1 |
| 2 | 2 | 90 | 3 |
| 3 | 2.5 | 100 | 5 |
The orthogonal L9 (33) experiment was performed further to investigate the appropriate hydrolytic conditions. The hydrolysis temperature and concentration of HCl were the major factors to affect the release degree, while hydrolysis time was the minor factor. The appropriate hydrolytic conditions of Osmanthus fragrans Lour. polysaccharides were 90 °C, 3 h, 2 mol/L HCl (namely, A2B2C2 in the orthogonal experiment). During the experiment, when the concentration of hydrochloric acid was too high, the phenomenon of sugar carbonization occurred after hydrolysis. When the acid concentration was 1 mol/L, hydrolysis of the polysaccharide was insufficient. As a result, the peak areas of Monosaccharides derivatives decreased when the concentration of HCl is increased.
2.13. Derivative concentration selection
The dosages of the derivative are the key to derivatization. In this study, the effects of different PMP concentrations of 0.1 mol/L, 0.5 mol/L, and 0.7 mol/L on the peak areas of monosaccharide derivatives were investigated. The results were shown in Fig. 10. The approximate PMP concentration range was obtained by pre-experiment, and then the results showed that almost no monosaccharide was detected at a PMP concentration of 0.1 mol/L, and the peak areas were quite small. When the concentration of PMP was too large, the excess PMP in the derivatization process was not completely removed, the signal of PMP was detected to be large, which caused errors in the analysis results by HPLC-MS/MS. A comprehensive selection of 0.5 mol/L was the optimal PMP derivative concentration (Fig. 11).
Fig. 11.
Effect of PMP concentration on derivative results.
2.14. Optimization of separation conditions
Since ribose and xylose are isomeric, the excimer ion peak [M + 2PMP-H2O + H]+ at 481.36 is the same; Mannose, glucose and galactose are isomeric, and the excimer ion peak [M + 2PMP-H2O + H]+ at 511.40 is the same; Rhamnose and fucose are isomeric, and the excimer ion peak [M + 2PMP-H2O + H]+ at 495.20 is the same. The fragment ions [PMP + H]+ at 175.10 of all monosaccharides are also the same, and a single monosaccharide standard for liquid chromatographic separation is required to characterize the retention time of the reference substance to avoid errors. Gradient elution could obtain better separation, improve the detection sensitivity of sugar in mass spectrometry, shorten the separation time and improve the peak shape (Table 7).
Table 7.
Method comparison.
| Method | Composition | Analysis time | LOD | LOQ | References | |
|---|---|---|---|---|---|---|
| Morus nigra Linn polysaccharides | HPLC | Glc, Xyl, Man, Gal, GalUA | 35 min | – | – | [24] |
| Hazelnut polysaccharides | GC–MS | Glu, Fru, Man, Gal, Xyl, Rha, etc. | 32 min | 0.1–1.0 μg/ml | 0.5–5.0 μg/ml | [25] |
| Honey fungus polysaccharides | UPLC-MS | Man, Gal, Xyl, Glu, Fuc, etc. | 7.5 min | 0.01–0.1 u/ml | 0.03–0.3 μg/ml | [26] |
| Sea cucumbers | HPLC-MS | Fucosylated chondroitin sulfate, fucoidan | 14 min | 0.06–0.07 μg/ml | 0.20–0.24 μg/ml | [27] |
| Osmanthus polysaccharides | HPLC-MS | Man, Rib, Glu, Gal, Xyl, Fuc | 30 min | 0.06–0.20 μg/ml | 0.18–0.60 μg/ml | * |
This study.
2.15. Polysaccharides component detection methods
3. Discussion
In this study, the HPLC-MS/MS method was used to simultaneously determine the monosaccharide content of three different varieties of Osmanthus fragrans Lour., the method was simple, precise, highly-selective to achieve accurate quantification of compounds, thus provided scientific basis for sugars analysis.
4. Materials and methods
Osmanthus fragrans var. semperflorens, Osmanthus fragrans var. thunbergii., Osanthus fragrans scv. latifoliu (Guangxi) were purchased from Hubei Jishantang Chinese Herbal Pieces Co.; Ltd., and authenticated by Prof. Hongguo Chen, Hubei University of Science and Technology. Acetonitrile (HPLC grade), methanol (HPLC grade) were obtained from Tianjin Oubokai Chemical Co.; Ltd.. Ammonium acetate (HPLC grade) was obtained from Tianjin Komiou Chemical Reagent Co.; Ltd.. PMP, D-Man (≥99.0%(GC)) were purchased Sinopharm Chemical Reagent Co., Ltd.; D-Glu, D-Gal, D-Xyl (≥99.0%(GC)) were purchased Aladdin Industrial Corporation; D-Rib, L-Fuc (≥99.0%(GC)) were purchased from Cool Chemical Technology Co., Ltd.; Watsons water was from Guangzhou Watsons Food & Beverage Co., Ltd.; And all other reagents were of analytical grade.
The HPLC-MS/MS was a Shimadzu CBM-20A system with an SPD- M30A detector coupled to a mass spectrometer (triple quadrupole analyzer) equipped with an ESI interface and column oven CTO-20 AC type. The HPLC system consisted of a SIL-20 AC autosampler, two LC-20ADXR pumps, and a column oven CTO-20 AC. Separation was performed on a column type Shim-pack VP-ODS6022748 (150 Lx 2.0), the mobile phase consisted of 5 mmol/L aqueous ammonium acetate solution (solvent A) and pure acetonitrile (solvent B). Chromatographic separation was achieved with the following gradient: 0–5 min, 17% B; 5–10 min, 18% B; 10–15 min, 19% B; 15–19 min, 21% B; 19–25 min, 18% B; and 25–40 min, 15% B. The flow rate 0.2 ml/min. 2 μl of each sample were injected. The column was maintained at 30 °C.
The sample (10 g Osmanthus fragrans Lour.) and ethanol absolute (200 ml) were spiked into a 500-ml round-bottomed flask, they were attached to reflux condensation for 1 h in a constant temperature water bath at 80 °C. After filtration was removed, the operation was repeated once again, filtered and dried again. Then, the filter residue and water (400 ml) were placed in a round bottom flask (1000 ml), Crude polysaccharides were extracted by 400 ml water (two times, 90 min each) in constant temperature water bath at 90 °C. After combining the filtrate obtained by suction filtration for two times, the filtrate was concentrated to 150 ml by rotary evaporator. Ethanol absolute (600 ml) was added to the concentrated polysaccharide solution, followed by filtering, precipitating and drying to obtain the crude polysaccharide of Osmanthus fragrans Lour. [18]. the crude polysaccharide was re-dissolved in 50 ml of water, and then added by Sevage reagent (chloroform: n-butanol = 4:1,10 ml) before being shaken vigorously. The digested mixture was centrifuged (3500 r/min, 5 min) and the supernatant was precipitated with ethanol absolute(200 ml). After standing at 4 °C for 12 h, the precipitates were washed with ethanol and acetone, and dried under vacuum, before Osmanthus fragrans Lour. polysaccharide was finally obtained.
The Osmanthus fragrans Lour. polysaccharide(10 mg) was dissolved with 5 ml of 2 mol/L hydrochloric acid solution at 90 °C for 3 h. after the samples were cooled down to room temperature, centrifuged for 5 min, obtained monosaccharide sample hydrolysate solution, monosaccharide hydrolysate was stored at 4 °C for further use.
Osmanthus fragrans Lour. polysaccharide hydrolysate was measured (100 μl), PMP methanolic solution (100 μl, 0.5 mol/L) and ammonia solution (100 μl) were mixed in a 7 ml centrifuge tube, shaken well, the mixture was heated at 70 °C for 30 min, allowed to cool. Subsequently, 10% glacial acetic acid aqueous solution and CHCl3 (1.0 mLeach) were added. The mixture was shaken vigorously, before CHCl3 was removed to extract unincorporated PMP, following a second CHCl3 wash, the mixture was centrifuged for 10 min (8000 r/min). The upper aqueous phase was filtered through a 0.22-um membrane for further analysis.
Acknowledgments
Funding
This work was supported by Supported by Educational Commission of Hubei Province of China (D20142806), the Plan Projects of Science and Technology of Hubei Province of China (2015CFC777).
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- [1].Ouyang XL, Wei LX, Wang HS, Pan YM, Antioxidant activity and phytochemical composition of Osmanthus’ pulps, S. Afr. J. Bot 98 (2015) 162–166. [Google Scholar]
- [2].Huang B, Chen H, Shao L, The ethanol extract of Osmanthus fragrans attenuates Porphyromonas gingivalis lipopolysaccharide-stimulated inflammatory effect through the nuclear factor erythroid 2-related factormediated antioxidant signalling pathway, Arch. Oral Biol 60 (7) (2015) 1030–1038. [DOI] [PubMed] [Google Scholar]
- [3].Xiong L, Mao S, Lu B, Yang J, Zhou F, Hu Y, et al. , Osmanthus fragrans flower extract and acteoside protect against D-galactose-induced aging in an ICR mouse model, J. Med. Food 19 (1) (2016) 54–61. [DOI] [PubMed] [Google Scholar]
- [4].Wu L, Chang L, Chen S, Fan N, Ho J. Annie, Antioxidant activity and melanogenesis inhibitory effect of the acetonic extract of Osmanthus fragrans: a potential natural and functional food flavor additive, LWT Food Sci. Technol 42 (9) (2009) 1513–1519. [Google Scholar]
- [5].Xiong L, Yang J, Jiang Y, Lu B, Hu Y, Zhou F, et al. , Phenolic compounds and antioxidant capacities of 10 common edible flowers from China, J. Food Sci 79 (4) (2014) 517–525. [DOI] [PubMed] [Google Scholar]
- [6].Ji Y, Wang C, Wu T, Ji C, Effect of Sargassum fusiforme polysaccharides on the complex mobility of erythrocytes in tumor-bearing organisms using high performance capillary electrophoresis, Chin.J. Chromatogr 25 (3) (2007) 322–325. [DOI] [PubMed] [Google Scholar]
- [7].Mao WJ, Li BF, Gu QQ, Fang YC, Xing HT, Preliminary studies on the chemical characterization and antihyperlipidemic activity of polysaccharide from the brown alga Sargassum fusiforme, Hydrobiologia 512 (2004) 263–266. [Google Scholar]
- [8].Liu JC, Niu YC, Polysaccharide Pharmacology, People’s Health Publishing, Beijing, 2008. 8–30. [Google Scholar]
- [9].Ding YF, Li LC, Zhao X, et al. , Research progress of polysaccharide, Heilongjiang Med. J 19 (2) (2006) 123–126. [Google Scholar]
- [10].Chen YW, Li YJ, Song ML, et al. , Analysis of monosaccharides composition of maca polysaccharide by PMP pre-column derivatization-HPLC method, Contemp. Chem. Eng 46 (08) (2017) (1513–1516+1520). [Google Scholar]
- [11].Li W, Wu Y, Zheng XR, et al. , Simultaneous determination of seven components in Kuhuang injection by UPLC-MS/MS method, China Pharm. 28 (15) (2017) 2108–2112. [Google Scholar]
- [12].Zh X. Zhao, Wang M, Wang YN, et al. , UPLC-MS/MS method for the determination of five saponins in Xinkeshu capsules, Chin. J. Pharm. Anal 36 (3) (2016) 494–499. [Google Scholar]
- [13].Shi H, Zhang QY, Yang HT, et al. , Simultaneous determination of eight chemical constituents in Jianweixiaoshi tablets by HPLC-MS/MS, Chin. J. Pharm. Anal 35 (9) (2015) 1606–1611. [Google Scholar]
- [14].Honda S, Akao E, Suzuk IS, et al. , High-performance liquid chromatography of reducing carbohydrates as strong ultraviolet-absorbing electrochemically sensitive 1- phenyl-3-methyl-5-pyrazolone derivatives, Anal. Biochem 180 (1989) 351–357. [DOI] [PubMed] [Google Scholar]
- [15].Strydom DJ, Chromatographic of 1-phenyl-3-methy-5-pyrazolone-derivatives neutral, acidic and basic aldoses, J. Chromatogr. A 678 (1994) 17–23. [Google Scholar]
- [16].Fu D, O’Neill RA, Monosaccharide composition analysis of oligosaccharides and glycoprotein by high-performance liquid chromatography, Anal. Biochem 227 (1995) 377–384. [DOI] [PubMed] [Google Scholar]
- [17].Ma DY, Chen J, Li P, et al. , Analysis of monosaccharide composition in polysaccharides by high performance liquid chromatography with precolumn derivatization, Chin. J. Anal. Chem 30 (6) (2002) 702–705. [Google Scholar]
- [18].Li WY, Li P, Cao W, A new method for determination of monosaccharide composition of angelica acidic polysaccharide by high performance liquid chromatography with precolumn derivatization,J. Technol. Eng 15 (10) (2015) 151–154. [Google Scholar]
- [19].Liang WY, Feng WJ, High performance liquid chromatography, Chin. J. Biochem. Pharm 28 (7) (2007) 13–17. [Google Scholar]
- [20].Wang H, Zhao J, Li D, et al. , Comparison of polysaccharides of Haliotis discus hannai and Volutharpa ampullacea perryi by PMP-HPLC-MS(n) analysis upon acid hydrolysis, Carbohydr. Res 415 (2015) 48–53. [DOI] [PubMed] [Google Scholar]
- [21].Zhao H, Chai GF, Wang QH, et al. , Isolation, purification and monosaccharide composition analysis of polysaccharides from semen plantaginis, Chin. J. Exp. Tradit. Med. Formulae 20 (19) (2014) 97–100. [Google Scholar]
- [22].Lü YL, Bu HB, Yang L, et al. , Comparison of polysaccharides from roots, roots and fibrous roots of Aconite, China J. Chin. Mater. Med 36 (9) (2011) 1154–1157. [PubMed] [Google Scholar]
- [23].Zhang YL, Gao J, Miao XY, et al. , Rapid identification of monosaccharide composition in huanglian polysaccharide by HPLC-MS method, World Chin. Med 12 (11) (2017) 2775–2778. [Google Scholar]
- [24].Ma XL, Song FF, Zhang H, et al. , Compositional monosaccharide analysis of Morus nigra Linn by HPLC and HPCE quantitative determination and comparison of polysaccharide from Morus nigra Linn by HPCE and HPLC, Curr. Pharm. Anal 13 (5) (2017) 433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen ND, Cheng Huang Xia QY, et al. , The accumulation of Lycium barbarum polysaccharide and GC-MS analysis of monosaccharide composition, Chin. J. Pharm. Anal 36 (08) (2016) 1349–1356. [Google Scholar]
- [26].Liang YT, Zhou JH, Nan TG, Qian D, et al. , Methodological study and application of 12 kinds of monosaccharide content by pre-column derivatization UPLC-MS/MS, Chin. J. Tradit. Chin. Med (November 3 2018) 1–11. [Google Scholar]
- [27].Zh J. Zhu, Zhu BB, Ch Q. Ai, et al. , Development and application of a HPLC-MS/MS method for quantitation of fucosylated chondroitin sulfate and fucoidan in sea cucumbers, Carbohydr. Res 466 (2018) 11–17. [DOI] [PubMed] [Google Scholar]