Abstract
Purpose
The purpose of this study is to provide an example of the Medical Research Council's guidelines for evaluating complex health care interventions in the context of smartphone-connected listening devices in adults with hearing loss.
Method
Twenty existing hearing aid users trialed 1 of the following smartphone-connected listening devices: made-for-smartphone hearing aids, a personal sound amplification product, and a smartphone “hearing aid” application used with either wireless or wired earphones. Following 2 weeks of use in their everyday lives, participants completed self-report outcome measures.
Results
Relative to conventional hearing aids, self-reported use, benefit, and satisfaction were higher, and residual disability was lower for made-for-smartphone hearing aids. The converse was found for the other smartphone-connected listening devices trialed. Similarly, overall usability was judged to be “above average” for the made-for-smartphone hearing aids, but “below average” for the remaining devices.
Conclusions
This developmental work, guided by the Medical Research Council's framework, lays the foundation for feasibility and pilot studies, leading to high-quality research assessing the effectiveness of smartphone-connected listening devices. This future evidence is necessary to guide health care commissioners and policymakers when considering new service delivery models for adults living with hearing loss.
Hearing aids improve hearing-specific, health-related quality of life, general health-related quality of life, and listening abilities in adults with mild–moderate hearing loss (Ferguson et al., 2017). Despite being effective, hearing aids are not taken up by the majority of individuals who would benefit from using them (Chien & Lin, 2012; Davis, Smith, Ferguson, Stephens, & Gianopoulos, 2007; Gopinath et al., 2011). For patients who do obtain hearing aids, estimates of nonuse vary from 3% to 24% (Ferguson et al., 2017). Self-management of hearing loss is important because both suboptimal use and nonuse of hearing aids result in continued communication difficulties, which can lead to social isolation and reduced quality of life for both the individual and their frequent communication partners (Barker, Leighton, & Ferguson, 2017; Kamil & Lin, 2015; Vas, Akeroyd, & Hall, 2017). Untreated hearing loss is also associated with an increased risk of developing other health care conditions, including depression and anxiety (Ciorba, Bianchini, Pelucchi, & Pastore, 2012).
One reason why people fitted with hearing aids do not use them is because they continue to experience difficulties when listening to and understanding speech, particularly in noisy situations (McCormack & Fortnum, 2013). Typically, hearing aids must be programmed and adjusted by a trained audiologist using special equipment. Patients themselves can make either limited or no changes to their hearing aid programs to address their individual needs and preferences. More recently, advances in technology have led to a rapid increase in the availability of smartphone-connected listening devices that require limited or no input from a trained audiologist in terms of device programming and adjustment. Smartphone-connected listening devices can connect wirelessly via Bluetooth to smartphone technologies, enabling the user to conveniently personalize and adjust his or her hearing device programs (e.g., gain, frequency response) in any listening situation via a smartphone application (or app). There is a range of smartphone-connected listening devices currently available, including made-for-smartphone hearing aids, personal sound amplification products (PSAPs), and smartphone “hearing aid” apps. Made-for-smartphone hearing aids are prescribed to the individual's hearing loss and must be programmed by an audiologist, whereas PSAPs are a type of direct-to-consumer (or over-the-counter [OTC]) listening device that are not fitted to the individual's audiologic prescription. Smartphone hearing aid apps enable smartphones to perform like a conventional hearing aid when used with either wireless or wired earphones and can also be adjusted by the user.
It is imperative that alternative service delivery models are identified to increase the likelihood that individuals will successfully manage their hearing loss. Indeed, smartphone-connected listening devices present an opportunity to improve both accessibility and affordability of hearing health care for adults. In the case of PSAPs and smartphone hearing aid apps, these devices can be low cost and purchased directly by the user. To date, evidence suggests that premium-priced PSAPs and smartphone hearing aid apps are equally effective as conventional hearing aids in terms of improving speech-in-noise perception under controlled laboratory conditions (Amlani, Taylor, Levy, & Robbins, 2013; Reed, Betz, Kendig, Korczak, & Lin, 2017; Sacco et al., 2016). A recent qualitative study examining made-for-smartphone hearing aids has further demonstrated that smartphone connectivity can increase opportunities for patients to participate more fully in their everyday lives (Ng, Phelan, Leonard, & Galster, 2017). Nevertheless, there is a lack of high-quality evidence (i.e., randomized controlled trials [RCTs]) demonstrating whether smartphone-connected listening devices are an effective intervention for adults living with hearing loss (Maidment, Barker, Xia, & Ferguson, 2016, 2018).
To address the need for high-quality evidence in this area, we are using the United Kingdom's Medical Research Council (MRC) guidelines for developing and evaluating complex health care interventions (Campbell et al., 2000; MRC, 2006). These guidelines are being increasingly applied in hearing research (Ferguson, Brandreth, Leighton, Brassington, & Wharrad, 2016) and are primarily intended to help researchers identify and adopt the most appropriate methods to provide the highest quality evidence. The MRC (2006) guidelines specify four distinct stages in the evaluation process (see Table 1). Progression from one stage to the next is not always linear, but can also be iterative (Campbell et al., 2000). In Stage 1, existing evidence is identified, ideally through the completion of a systematic review. This stage can also include developmental studies involving both quantitative and qualitative methodologies to provide important insights into how health care interventions operate, such as barriers to delivery. Stage 2 focuses on feasibility and pilot studies that address any uncertainties and determine whether the trial can be done. The findings from Stages 1 and 2 can then be used to inform and refine the design of the clinical effectiveness trial at Stage 3 to ensure that the intervention can be delivered effectively. Finally, Stage 4 incorporates dissemination and implementation (i.e., getting evidence into practice), as well as monitoring and long-term follow-up, to ascertain the generalizability of intervention effectiveness.
Table 1.
The four distinct stages of the United Kingdom's Medical Research Council guidelines for developing and evaluating complex health care interventions.
| Stage | Description |
|---|---|
| 1. Development | Identify existing evidence (i.e., systematic review) |
| Mixed-methods study to identify how health care interventions operate | |
| 2. Feasibility and piloting | Address any uncertainties (e.g., recruitment and retention rates) |
| Determine whether the main trial can be done/delivered | |
| 3. Full-scale evaluation | Assess clinical effectiveness of health care interventions |
| 4. Implementation | Dissemination and getting research into practice |
| Monitoring and long-term follow-up |
Note. In this research note, we provide an example of a mixed-method study as part of the development stage, shown in bold text.
In view of the MRC (2006) guidelines, this research note presents an example of how to undertake a developmental study after the completion of a systematic review. Namely, following our systematic review (Maidment et al., 2016, 2018), we have assessed the usability of smartphone-connected listening devices when used by adults with hearing loss in their everyday lives. The aims were to identify potential barriers and facilitators to delivery by (a) measuring self-reported use, residual disability, benefit, satisfaction, and usability of smartphone-connected listening devices, and (b) comparing these outcomes with conventional hearing aids. In this research note, quantitative data from our developmental study are reported. These data supplement preliminary qualitative analysis that has also been undertaken (Maidment & Ferguson, 2017).
Method
Participants
Twenty existing hearing aid users (seven female; 13 male), with a mean age of 62.25 years (SD = 11.59), were recruited via e-mail from the United Kingdom's National Institute for Health Research Nottingham Biomedical Research Centre participant database. All participants used conventional hearing aids obtained from the publicly funded National Health Service. Mean self-reported duration of hearing loss was 16.41 years (SD = 13.96). Mean better-ear average across octave frequencies (0.25 to 4 kHz) was 30.49 dB HL (SD = 17.51).
Interventions
Made-for-smartphone hearing aids. Behind-the-ear Halo i110 hearing aids (Starkey Hearing Technologies) were individually programmed using the InspireX 2016.2 fitting software (National Acoustic Laboratories Non-Linear 2 algorithm) and fitted with either custom earmolds or open-fit slim tubes depending on the participant's hearing thresholds (Starkey Hearing Technologies, 2016). The Halo connected wirelessly to the participant's smartphone via Bluetooth and could be controlled using the TruLink smartphone app.
PSAP. In-the-ear AMP Personal Amplifiers (Starkey Hearing Technologies) were programmed using the AMP smartphone app. In accordance with manufacturer guidance, participants wore foam-padded, over-the-ear headphones during fitting. The personal amplifiers were adjusted using dual-tone, multifrequency signals generated by the AMP app. One of three preset starting points, corresponding to mild, mild–moderate, or moderate sloping hearing loss, was first selected based on the participant's audiogram. Participants then listened to the media available within the app (adult female speech, adult male speech, restaurant conversation, and music). Participants made adjustments to low-frequency gain, high-frequency gain, overall gain, and/or output, based on their preferences if necessary.
Smartphone hearing aid app with wireless earphones. The Petralex hearing aid smartphone app (http://petralex.pro/) was trialed, as it is available on both the Apple and Google Play (i.e., Android) app stores. The Petralex app includes an audiometric test for adjustment and personalization purposes only. Participants in the wireless earphones group were provided Bragi Dash earphones (Bragi, 2015), which pair with the user's smartphone via Bluetooth and include additional functionalities, such as health monitoring (e.g., heart rate) and activity tracking (e.g., step count).
Smartphone hearing aid app with wired earphones. This was identical to the wireless earphones group, with the exception that participants were instructed to use the hearing aid app with wired earphones.
Self-Reported Outcome Measures
The Glasgow Hearing Aid Difference Profile (GHADP; Gatehouse, 1999) assessed use (“What proportion of time do you use your hearing aid?”) and residual disability (“With your hearing aid, how much difficulty do you have?”) with “current” aids (Part I), as well as use and residual disability with the “new” aids, and difference in benefit (“How much does your new hearing aid help you compared to your previous one?”) and satisfaction (“How satisfied are you with your new hearing aid compared to your previous one?”) between “previous” and new aids (Part II). In this study, “current/previous” aids referred to participants' existing conventional hearing aids, whereas new aids referred to the assigned smartphone-connected listening device. Each domain was measured on a 5-point scale; and the mean score across predefined situations (listening to the television with other family or friends when the volume is adjusted to suit other people; having a conversation with one other person when there is no background noise; carrying on a conversation in a busy street or shop; having a conversation with several people in a group) and up to four user-defined situations in which it is important for the respondent to be able to hear as well as possible was converted into a percentage score. Higher percentage scores were indicative of greater use, residual disability (i.e., poorer), benefit, and satisfaction.
The System Usability Scale (SUS; Brooke, 1996) is a 10-item questionnaire that assessed the overall usability of the smartphone-connected listening device trialed. Each item was measured on a 5-point Likert scale, ranging from strongly disagree to strongly agree. Scores for each item ranged from 0 to 4. A composite score, ranging from 0 to 100, was obtained by multiplying the sum of all item scores by 2.5. A score greater than or equal to 68 is considered “above average,” and anything less than 68 is “below average” (Sauro, 2011).
Study Design and Procedure
Participants attended a 1-hr session at the National Institute for Health Research Nottingham Biomedical Research Centre, where they first completed Part I of the GHADP before being fitted with a smartphone-connected listening device. An equal number of participants (n = 5) were assigned to one of the four listening device groups (made-for-smartphone hearing aids, PSAP, smartphone app and wireless earphones, smartphone app and wired earphones). Six participants owned Android smartphones that were not compatible with the made-for-smartphone hearing aids trialed. For this reason, we randomly assigned participants to a listening device that was compatible with their smartphone. Demographic information for each smartphone-connected listening device group is provided in Table 2. During this session, participants also downloaded the accompanying smartphone app and/or paired the device via Bluetooth with their smartphone where appropriate.
Table 2.
Demographic information for participants in each smartphone-connected listening device group.
| Demographic | Made-for-smartphone hearing aids | Personal sound amplification products | Smartphone app with wireless earphones | Smartphone app with wired earphones | |
|---|---|---|---|---|---|
| Gender | Female | 2 | 2 | 0 | 2 |
| Male | 3 | 3 | 5 | 3 | |
| Age (years) | Mdn | 62.00 | 67.00 | 63.50 | 64.00 |
| IQR | 10.50 | 13.50 | 10.50 | 32.50 | |
| Hearing loss duration (years) | Mdn | 12.00 | 30.00 | 4.00 | 10.00 |
| IQR | 29.92 | 25.00 | 8.88 | 16.04 | |
| Better-ear average0.25 –4 kHz (dB HL) | Mdn | 40.83 | 37.50 | 31.92 | 24.17 |
| IQR | 40.42 | 85.50 | 11.17 | 32.33 | |
| GHADP use (%; Part I, old aid) | Mdn | 45.00 | 100.00 | 60.00 | 67.86 |
| IQR | 64.59 | 12.50 | 60.00 | 82.15 | |
| GHADP residual disability (%; Part I, old aid) | Mdn | 45.83 | 31.25 | 36.46 | 37.50 |
| IQR | 5.63 | 12.50 | 23.37 | 7.59 | |
Note. Part I of the GHADP (Gatehouse, 1999) assessed self-reported use (“What proportion of time do you use your hearing aid?”) and residual disability (“With your hearing aid, how much difficulty do you have?”) with participants' existing conventional hearing aids. Higher percentage scores are indicative of greater use/residual disability. GHADP = Glasgow Hearing Aid Difference Profile.
As the primary aim was to assess the use of the smartphone-connected listening device away from the laboratory (i.e., in everyday life), participants trialed the assigned device for a period of 2 weeks. If participants experienced any difficulties, they were advised to read the brochures provided, consult the manufacturer's website, or contact the research team via e-mail or telephone. Following 2 weeks of use, participants attended a second session, where they completed Part II of the GHADP and SUS. In addition, a 1-hr semistructured interview was completed (for preliminary results, see Maidment & Ferguson, 2017). The study was approved by the Faculty of Medicine and Health Sciences Research Ethics Committee, University of Nottingham, United Kingdom.
Analysis of Outcome Measures
In accordance with the MRC (2006) guidelines, developmental studies do not rigorously assess the effectiveness of an intervention (i.e., they do not compare the benefits of one health care intervention to another), as this is undertaken by the future RCT. As such, it is not necessary, or appropriate, to power developmental studies to detect statistically significant differences between interventions. In this study, therefore, descriptive (as opposed to inferential) statistics are reported for each outcome measure.
Results
GHADP
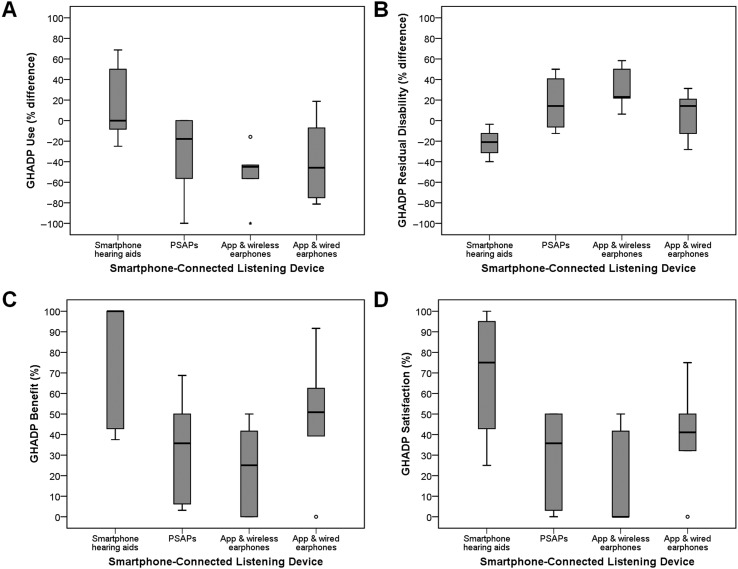
To compare self-reported use and residual disability between conventional hearing aids and smartphone-connected listening devices, difference scores were calculated; use and residual disability scores for each smartphone-connected listening device (Part II) were subtracted from use and residual disability scores reported for existing hearing aids (Part I). As shown in Figure 1A, following the 2-week trial, self-reported use was highest for the made-for-smartphone hearing aids relative to conventional hearing aids (Mdn = 0.00%, interquartile range [IQR] = 76.04). It should be noted that, although there was no change in the median, the upper quartile was 50.00% (maximum value = 68.75%). The converse pattern was observed for all other smartphone-connected listening devices, suggesting poorer use compared with their conventional hearing aids. Similarly, in comparison to conventional hearing aids, residual disability scores (see Figure 1B) were lower (i.e., better) for the made-for-smartphone hearing aids (Mdn = −20.83%, IQR = 27.59) and higher (i.e., poorer) for the other smartphone-connected listening devices.
Figure 1.
Boxplots for each Glasgow Hearing Aid Difference Profile (GHADP; Gatehouse, 1999) subscale across both predefined and user-defined situations. A. Use for each smartphone-connected listening device (Part II) minus use for existing hearing aids (Part I); B. Residual disability for each smartphone-connected listening device (Part II) minus residual disability for existing hearing aids (Part I); C. Difference in benefit between existing hearing aids and each smartphone-connected listening device; D. Difference in satisfaction between existing hearing aids and each smartphone-connected listening device. Higher percentage scores are indicative of greater use, residual disability (i.e., poorer), benefit, and satisfaction. PSAPs = personal sound amplification products.
In terms of the difference in benefit between conventional hearing aids and the smartphone-connected listening devices (see Figure 1C), a similar pattern of scores was seen. Scores for the made-for-smartphone hearing aids were highest (i.e., much better than existing hearing aids; Mdn = 100.00%, IQR = 59.82) and lowest for the smartphone hearing aid app used with wireless earphones (i.e., much worse than existing hearing aids; Mdn = 25.00%, IQR = 45.83). The same pattern of results was also shown for the difference in satisfaction (see Figure 1D). Scores were highest for the made-for-smartphone hearing aids (i.e., more satisfied with smartphone-connected listening device than existing hearing aids; Mdn = 75.00%, IQR = 63.57) and lowest for the smartphone hearing aid app used with wireless earphones (i.e., much less satisfied with smartphone-connected listening device than existing hearing aids; Mdn = 0.00%, IQR = 45.83).
SUS
Overall usability scores are shown in Figure 2. The only smartphone-connected listening device with an SUS score greater than or equal to 68 (i.e., above average) was the made-for-smartphone hearing aids (Mdn = 72.61, IQR = 30.00). Scores less than 68 (i.e., below average) were reported, in descending order, for the smartphone hearing aid app used with wired earphones (Mdn = 62.50, IQR = 22.50), PSAP (Mdn = 47.50, IQR = 26.25), and smartphone hearing aid app used with wireless earphones (Mdn = 40.00, IQR = 36.25).
Figure 2.
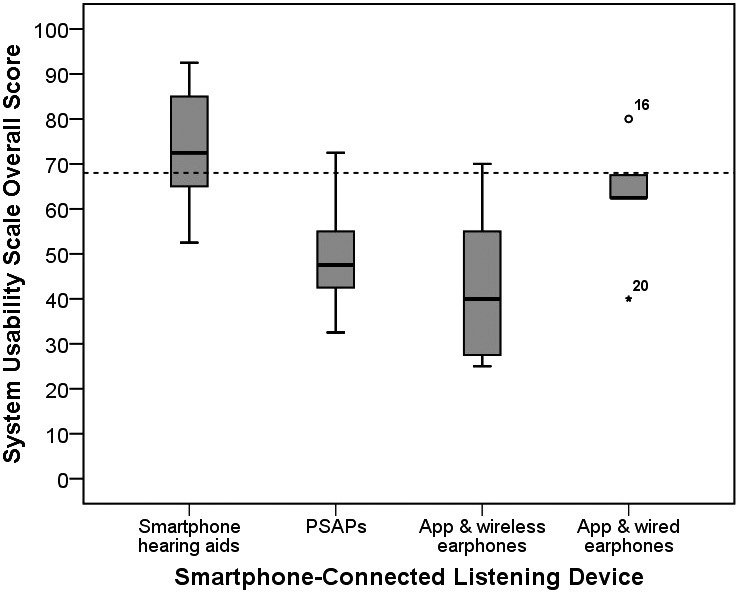
Boxplots showing overall System Usability Scale (Brooke, 1996) scores for each smartphone-connected listening device group. Dashed line denotes a score of greater than or equal to 68, which is considered above average (Sauro, 2011). PSAPs = personal sound amplification products.
Discussion
The current developmental study aimed to provide novel insights into the potential barriers and facilitators affecting the use of smartphone-connected listening devices when used by existing hearing aid users in their everyday lives. This work was undertaken in accordance with the MRC's (2006) guidelines for developing complex health care interventions (see Table 1), which stipulate that, in addition to identifying existing evidence, developmental studies should be undertaken to identify how complex interventions operate, informing the robust design of future clinical effectiveness trials. Overall, we found that, in comparison to conventional hearing aids, self-reported use, benefit, satisfaction, and usability were rated higher for made-for-smartphone hearing aids. By comparison, although all outcomes were lower for the remaining smartphone-connected listening devices, the smartphone hearing aid app with wired earphones was rated consistently higher relative to both the PSAP and smartphone hearing aid app with wireless earphones.
This developmental study demonstrates the utility of applying the MRC's (2006) guidelines by identifying key differences between smartphone-connected listening devices in terms of use, benefit, satisfaction, and usability. Moreover, these results highlight a number of considerations that should be addressed in the design of a future RCT. Firstly, higher outcomes for the made-for-smartphone hearing aids may have arisen because, relative to the other devices trialed, they were specifically programmed to compensate for individual's hearing loss. In addition, the made-for-smartphone hearing aids were more technologically advanced compared with participants' existing conventional hearing aids. While equivalent outcomes have been shown for “basic” and “advanced” hearing aids (Cox, Johnson, & Xu, 2016; Johnson, Xu, & Cox, 2016), a future trial in this area should assess technologically equivalent listening devices, whereby additional smartphone functionalities are enabled versus disabled to determine the incremental benefit they provide. Secondly, identifying how usability can be enhanced for smartphone-connected listening devices that scored below average (i.e., PSAP, smartphone hearing aid app used with wired or wireless earphones) would be important before proceeding to an RCT, as this may also improve reported use, residual disability, benefit, and satisfaction. It has been suggested that adults living with hearing loss may require additional information and support to successfully use listening devices that require limited or no input from a trained hearing health care professional in terms of fitting and/or fine-tuning (Keidser & Convery, 2018). Supplementary information and support could, therefore, be incorporated in the design of a future effectiveness trial, potentially improving the likelihood that participants will successfully use the smartphone-connected listening device to manage their hearing loss.
Thirdly, we did not screen for potential confounding factors, such as cognitive abilities (e.g., working memory capacity), which could account for differences between groups. In relation, differences between groups in terms of self-reported use and residual disability of participants' existing conventional hearing aids could also have biased the outcomes of the study. As a result, to control for potential biases, future studies should match groups on these variables. Fourthly, we also opted to sample existing hearing aid users to allow for a comparison between participants' existing conventional hearing aids and smartphone-connected listening devices. Prior experience with hearing aids would likely have affected participants' views concerning the usability of the smartphone-connected listening device trialed. Indeed, McLellan, Muddimer, and Peres (2012) found that usability scores are typically higher for experienced users relative to individuals with limited or no experience of a product. Consequently, a future trial could include both hearing aid users and nonusers, given that differing results might be expected from people living with hearing loss who have yet to use any form of amplification. Finally, smartphone-connected listening devices were trialed by participants in their everyday lives for a period of 2 weeks. The opportunity to use each listening device over a longer period should be considered in a future trial, as this may alter participants' initial views regarding usability (see McLellan et al., 2012).
In accordance with the MRC's (2006) guidelines, the next stage of this research would be to incorporate these considerations into the design of a full-scale evaluation, leading to feasibility and pilot studies to determine whether a trial assessing smartphone-connected listening devices can be done. Feasibility studies can be used to estimate a number of parameters necessary for the robust design of the RCT, such as identifying an appropriate primary outcome measure, determining the required study sample size, and assessing the willingness of clinicians to randomize and the willingness of adults with hearing loss to be randomized to different groups. A feasibility study can also provide estimates of follow-up, response, and compliance rates, as well as determine the time needed to recruit participants and collect data. This then leads to a pilot study, which is considered a miniature version of the trial, assessing whether all processes (e.g., recruitment, randomization, intervention delivery) work in combination. Together, feasibility and pilot studies can ensure that the future RCT is both viable and cost effective (i.e., represents a good use of the available resources; Campbell et al., 2000).
It should be noted that, in the United Kingdom, the provision of hearing health care is free, potentially limiting the generalizability of findings of a future RCT to other health care systems that incur high out-of-pocket costs to the individual. Although cost has been identified as a potential barrier for hearing aid adoption in the United States (Grundfast & Liu, 2017), it has been counterargued that cost is not the primary impediment (Valente & Amlani, 2017). On this basis, the planned RCT should also aim to evaluate alternative service delivery models that have the potential to address both accessibility and affordability to hearing health care for adults. This work is timely given changes in U.S. legislation concerning the Over-the-Counter Hearing Aid Act of 2017. A recently published randomized, double-blind, placebo-controlled clinical trial has shown that, in comparison to hearing aids programmed by an audiologist, preprogrammed hearing aids (i.e., OTC service delivery model where the consumer decides) result in similar effect sizes for measures of speech recognition and hearing aid benefit (Humes et al., 2017). However, the percentage of individuals who would have been likely to purchase hearing aids posttrial, as well as their self-reported satisfaction scores, was lower for the OTC delivery model. We propose that smartphone-connected listening devices could complement OTC service delivery models, whereby users could continue to adjust their preprogrammed hearing aids to meet their individual hearing and communication needs/preferences, potentially improving satisfaction. On this basis, a future trial could also identify the combined benefits of preprogrammed and smartphone-connected listening devices.
Summary and Conclusions
The current article provides an example of a developmental study, guided by the MRC's (2006) framework, assessing outcomes from a range of smartphone-connected listening devices when used by existing hearing aid users in their everyday lives. This developmental work can be used to inform the design of future high-quality research in this area, assessing the effectiveness of smartphone-connected listening devices. In the longer term, such research evidence would have the potential to guide commissioners and policymakers when considering new service delivery models that could benefit people living with hearing loss. With high-quality evidence, we anticipate that innovations in smartphone technologies could transform hearing health care service delivery in the future.
Acknowledgments
This research was funded by the National Institute for Health Research Nottingham Biomedical Research Centre. Portions of this research note were presented at the 3rd International Internet & Audiology Meeting, Louisville, KY, July 2017, which was funded by National Institute on Deafness and Other Communication Disorders (NIDCD) Grant 1R13DC016547 and the Oticon Foundation. The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the United Kingdom's Department of Health and Social Care. The authors would like to thank Starkey Hearing Technologies, United Kingdom, for providing devices for the purposes of the research and Dr. Anne Olson for her comments on a draft of this research note.
Funding Statement
This research was funded by the National Institute for Health Research Nottingham Biomedical Research Centre. Portions of this research note were presented at the 3rd International Internet & Audiology Meeting, Louisville, KY, July 2017, funded by National Institute on Deafness and Other Communication Disorders Grant 1R13DC016547, awarded to Dr. Jill Preminger (Principal Investigator), and the Oticon Foundation.
References
- Amlani A. M., Taylor B., Levy C., & Robbins R. (2013). Utility of smartphone-based hearing aid applications as a substitute to traditional hearing aids. The Hearing Review, 20(13), 16–18. [Google Scholar]
- Barker A. B., Leighton P., & Ferguson M. A. (2017). Coping together with hearing loss: A qualitative meta-synthesis of the psychosocial experiences of people with hearing loss and their communication partners. International Journal of Audiology, 56(5), 297–305. [DOI] [PubMed] [Google Scholar]
- Bragi. (2015). The Dash. Retrieved from https://support.bragi.com/hc/en-us/categories/200470531-The-Dash
- Brooke J. (1996). SUS: A “quick and dirty” usability scale. In Jordan P. W., Thomas B., Weerdmeester B. A., & McClelland I. L. (Eds.), Usability evaluation in industry (pp. 189–194). London, United Kingdom: Taylor & Francis. [Google Scholar]
- Campbell M., Fitzpatrick R., Haines A., Kinmonth A. L., Sandercock P., Spiegelhalter D., & Tyrer P. (2000). Framework for design and evaluation of complex interventions to improve health. British Medical Journal, 321, 694–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien W., & Lin F. R. (2012). Prevalence of hearing aid use among older adults in the United States. Archives of Internal Medicine, 172(3), 292–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciorba A., Bianchini C., Pelucchi S., & Pastore A. (2012). The impact of hearing loss on the quality of life of elderly adults. Clinical Interventions in Aging, 7, 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. M., Johnson J. A., & Xu J. (2016). Impact of hearing aid technology on outcomes in daily life. I: The patients' perspective. Ear and Hearing, 37(4), e224–e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A., Smith P., Ferguson M., Stephens D., & Gianopoulos I. (2007). Acceptability, benefit and costs of early screening for hearing disability: A study of potential screening tests and models. Health Technology Assessment, 11(42), 1–294. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Brandreth M., Leighton P., Brassington W., & Wharrad H. (2016). A randomized controlled trial to evaluate the benefits of a multimedia educational programme for first-time hearing aid users. Ear and Hearing, 37(2), 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Kitterick P., Chong L., Edmondson-Jones M., Barker F., & Hoare D. (2017). Hearing aids for mild and moderate hearing loss in adults. Cochrane Database of Systematic Reviews, CD012023(9), 1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatehouse S. (1999). Glasgow Hearing Aid Benefit Profile: Derivation and validation of client-centred outcome measures for hearing aid services. Journal of the American Academy of Audiology, 10(2), 80–103. [Google Scholar]
- Gopinath B., Schneider J., Hartley D., Teber E., McMahon C. M., Leeder S. R., & Mitchell P. (2011). Incidence and predictors of hearing aid use and ownership among older adults with hearing loss. Annals of Epidemiology, 21(7), 497–506. [DOI] [PubMed] [Google Scholar]
- Grundfast K., & Liu S. (2017). What otolaryngologists need to know about hearing aids. JAMA Otolaryngology–Head & Neck Surgery, 143(2), 109–110. [DOI] [PubMed] [Google Scholar]
- Humes L. E., Rogers S. E., Quigley T. M., Main A. K., Kinney D. L., & Herring C. (2017). The effects of service-delivery model and purchase price on hearing-aid outcomes in older adults: A randomized double-blind placebo-controlled clinical trial. American Journal of Audiology, 26, 53–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. A., Xu J., & Cox R. M. (2016). Impact of hearing aid technology on outcomes in daily life. II: Speech understanding and listening effort. Ear and Hearing, 37(5), 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamil R. J., & Lin F. R. (2015). The effects of hearing impairment in older adults on communication partners: A systematic review. Journal of the American Academy of Audiology, 26(2), 155–182. [DOI] [PubMed] [Google Scholar]
- Keidser G., & Convery E. (2018). Outcomes with a self-fitting hearing aid. Trends in Hearing, 22, 1–12. https://doi.org/10.1177/2331216518768958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidment D. W., Barker A. B., Xia J., & Ferguson M. A. (2016). The effectiveness of alternative listening devices to conventional hearing aids for adults with hearing loss: A systematic review protocol. BMJ Open, 6, e011683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidment D. W., Barker A. B., Xia J., & Ferguson M. A. (2018). A systematic review and meta-analysis assessing the effectiveness of alternative listening devices to conventional hearing aids in adults with hearing loss. International Journal of Audiology. ISSN 1708-8186 (In Press). [DOI] [PubMed] [Google Scholar]
- Maidment D. W., & Ferguson M. A. (2017). Improving hearing aid take-up, use and adherence: Are smartphones the answer? Innovations, 7(3), 26–32. Retrieved from https://starkeypro.com/innovations/Volume7-Issue3/index.html?page=26 [Google Scholar]
- McCormack A., & Fortnum H. (2013). Why do people fitted with hearing aids not wear them? International Journal of Audiology, 52(5), 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan S., Muddimer A., & Peres S. C. (2012). The effect of experience on system suability scale ratings. Journal of Usability Studies, 7(2), 56–67. [Google Scholar]
- Medical Research Council. (2006). Developing and evaluating complex interventions: Following considerable development in the field since 2006, MRC and NIHR have jointly commissioned an update of this guidance to be published in 2019. Retrieved from https://www.mrc.ac.uk/documents/pdf/complex-interventions-guidance/
- Ng S. L., Phelan S., Leonard M., & Galster J. A. (2017). A qualitative case study of smartphone-connected hearing aids: Influences on patients, clinicians, and patient–clinician interactions. Journal of the American Academy of Audiology, 28(6), 506–521. [DOI] [PubMed] [Google Scholar]
- Reed N. S., Betz J., Kendig N., Korczak M., & Lin F. R. (2017). Personal sound amplification products vs a conventional hearing aid for speech understanding in noise. Journal of the American Medical Association, 318(1), 89–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco G., Gonfrier S., Teboul B., Gahide I., Prate F., Demory-Zory M., … Guevara N. (2016). Clinical evaluation of an over-the-counter hearing aid (TEO First) in elderly patients suffering of mild to moderate hearing loss. BMC Geriatrics, 16, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauro J. (2011). Measuring usability with the System Usability Scale (SUS). Retrieved from https://measuringu.com/sus/
- Starkey Hearing Technologies. (2016). InspireX 2016.2. Retrieved from http://www.starkeyhearingtechnologies.com/inspirehelp/aah/index.htm#t=Inspire_Overview%2FInspire_Overview.htm
- Valente M., & Amlani A. A. (2017). Cost as a barrier for hearing aid adoption. JAMA Otolaryngology–Head & Neck Surgery, 143(7), 647–648. [DOI] [PubMed] [Google Scholar]
- Vas V., Akeroyd M. A., & Hall D. A. (2017). A data-driven synthesis of research evidence for domains of hearing loss, as reported by adults with hearing loss and their communication partners. Trends in Hearing, 21, 1–25. https://doi.org/10.1177/2331216517734088 [DOI] [PMC free article] [PubMed] [Google Scholar]