Abstract
Background
Glaucoma is a leading cause of irreversible blindness worldwide. In early stages, glaucoma results in progressive loss of peripheral (side) vision; in later stages, it results in loss of central vision leading to blindness. Elevated intraocular pressure (IOP) is the only known modifiable risk factor for glaucoma. Minimally invasive glaucoma surgical (MIGS) techniques, such as ab interno trabecular bypass surgery with iStent (Glaukos Corporation, Laguna Hills, CA, USA), have been introduced as a new treatment modality for glaucoma. However, the effectiveness of MIGS on keeping people 'drop‐free' (i.e. not having to use eye drops to control IOP) and other outcomes is uncertain.
Objectives
To assess the effectiveness and safety of ab interno trabecular bypass surgery with iStent (or iStent inject) for open‐angle glaucoma in comparison to conventional medical, laser, or surgical treatment.
Search methods
Cochrane Eyes and Vision's Information Specialist searched the following databases on 17 August 2018: the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register; 2018, Issue 7), MEDLINE Ovid, Embase Ovid, the ISRCTN registry, ClinicalTrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We applied no date or language restrictions. We searched the reference lists of reports from included studies.
Selection criteria
We included randomized controlled trials (RCTs) that had compared iStent or iStent inject to medical therapy, laser treatment, conventional glaucoma surgery (trabeculectomy), or other MIGS procedures. We included RCTs that had compared iStent or iStent inject in combination with phacoemulsification to phacoemulsification alone.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently screened search results, assessed risk of bias, and extracted data from reports of included RCTs using an electronic data collection form.
Main results
We included seven RCTs (765 eyes of 764 participants; range per study 33 to 239 participants) that evaluated iStent in people with open‐angle glaucoma. We also identified 13 studies that are ongoing or awaiting publications of results. Most participants in the included studies were women (417/764 (55%) participants) and older age (age range: 49 to 89 years). We assessed most trials at unclear or high risk of bias: four trials did not clearly report the method of generating the random sequence or concealing allocation; five were unmasked, open‐label studies, which we assessed at high risk of bias for performance and detection bias. All seven trials were funded by the Glaukos Corporation. We graded the certainty of evidence as very low.
Four RCTs compared iStent in combination with phacoemulsification to phacoemulsification alone. The summary estimate which we derived from two of the four RCTs suggested that participants in the iStent in combination with phacoemulsification group were 1.38 times more likely to be drop‐free between six and 18 months than those in the phacoemulsification alone group (risk ratio (RR) 1.38, 95% confidence interval (CI) 1.18 to 1.63, I2 = 67%). Data from two RCTs also suggested that iStent in combination with phacoemulsification compared to phacoemulsification alone may have offered a small reduction in number of IOP‐lowering drops (mean difference (MD) –0.42 drops, 95% CI –0.60 to –0.23). It is uncertain whether there was any difference in terms of mean reduction in IOP from baseline (no meta‐analysis).
Two RCTs compared treatment with iStent to medical therapy; one of the two trials used the iStent inject. We determined the two trials to be clinically and methodologically heterogeneous and did not conduct a meta‐analysis; however, the investigators of both trials reported that over 90% of participants in the treatment groups were drop‐free compared to no participants in the medical therapy groups at six to 18 months.
One RCT compared treatment with one versus two versus three iStents. There was no difference in terms of participants who were drop‐free at 36 months or less; however, at longer follow‐up (i.e. at 42 months) participants in the one iStent treatment were less likely to be drop‐free than those in the two iStent (RR 0.51, 95% CI 0.34 to 0.75) or three iStent (RR 0.49, 95% CI 0.34 to 0.73) treatment groups. The study did not report the mean change in number of IOP‐lowering drops.
The type and timing of complications reported varied by RCTs. Similar proportions of participants who underwent treatment with iStent in combination with phacoemulsification and who underwent phacoemulsification alone needed secondary glaucoma surgery. None of RCTs reported findings related to quality of life.
Authors' conclusions
There is very low‐quality evidence that treatment with iStent may result in higher proportions of participants who are drop‐free or achieving better IOP control, in the short, medium, or long‐term. Results from the 13 studies with results not yet available may clarify the benefits of treatment of people with iStent. Additionally, future MIGS studies should consider measuring quality of life and outcomes that reflect people's ability to perform vision‐dependent activities.
Plain language summary
iStent for open‐angle glaucoma
What was the aim of this review? The aim of this Cochrane Review was to find out whether the implantation of one or more iStent or iStent inject devices ('iStents'), compared with conventional medical, laser, or surgical treatments, can keep people who have primary open‐angle glaucoma from needing to use glaucoma drops (i.e. keep them 'drop‐free'). The glaucoma drops are used to control the fluid pressure within their eyes (called the intraocular pressure (IOP)). We also looked at average change from baseline in number of glaucoma drops needed to control IOP, average change from baseline (i.e. before treatment) in IOP, and health‐related quality of life as defined by study investigators. We examined all outcomes at short‐term (less than six months), medium‐term (six to ≤ 18 months), long‐term (> 18 months and ≤ 36 months) and greater than 36‐month time points. We collected and analyzed all relevant randomized controlled trials (RCTs; clinical studies where people are randomly put into one of two or more treatment groups) to answer this question and found seven RCTs evaluating iStents.
Key messages There was very low‐quality evidence that treatment with iStents may have resulted in higher proportions of people who were drop‐free at medium‐term time points or who had better control of their IOP. None of the seven RCTs examined how the iStent affected quality of life and reporting on complications was highly variable. At present, clinical practice decisions should be based on provider judgment and patient preferences, given inconsistency in results and risk of bias in relevant studies published to date.
What did we study in this review? Glaucoma is a group of eye diseases that cause irreversible damage to the optic nerve in the eye. If untreated, glaucoma can lead to blindness. Elevated IOP is the only known modifiable risk factor for open‐angle glaucoma, which is the most common form of glaucoma. Conventional first‐choice treatments for open‐angle glaucoma include medical (e.g. glaucoma drops) or laser interventions. Surgery, which has a higher risk profile, is offered when glaucoma progresses despite treatment with medication or laser.
Minimally invasive glaucoma surgical procedures involve implantation of devices such as the iStent. They have been proposed as a safer alternative to standard glaucoma surgeries in people with mild‐to‐moderate forms of open‐angle glaucoma. The iStent creates a 'bypass' between the front chambers of the eye and its natural drainage pathway. This bypass increases the flow of fluids out of the eye, which may decrease IOP and the need to use glaucoma drops to control IOP.
What were the main results of this review? We identified four RCTs that randomized participants to treatment with iStents in combination with cataract surgery (called phacoemulsification) or with phacoemulsification alone. Additionally, we identified two RCTs that randomized participants to treatment with iStents or to medical interventions. We also identified one RCT that randomized participants to treatment with one iStent, with two iStents, or with three iStents. The manufacturer of the iStent provided funding and sponsorship for all the RCTs in this review.
Based on low‐quality evidence, we found that participants who received iStent in combination with cataract surgery were more likely to be drop‐free and may have experienced a modest reduction in number of glaucoma drops used per day to control IOP in the medium term, compared with participants who underwent cataract surgery alone; however, there was no difference in average change from baseline in IOP between the two groups.
Due to substantial heterogeneity, we did not conduct an analysis of the two studies comparing treatment with iStent to medical therapy. Investigators of those two studies reported that no participants in the medical therapy group were drop‐free at 12 months, compared to over 90% in the iStent treatment groups. Data suggested that treatment of people with two or with three iStents may have been more effective than treatment with one iStent in terms of IOP control.
None of the seven studies included in this review provided information on quality of life, and differences in complications or side effects between treatment groups were uncertain, given few reported events and varied effectiveness.
How up to date is the review? We searched for studies published up to 17 August 2018.
Summary of findings
Background
Description of the condition
Glaucoma is a group of diseases characterized by clinical and histopathological manifestations of optic nerve damage that leads to irreversible vision loss (Allingham 2010). Glaucoma is the second leading cause of blindness, affecting approximately 60 million people worldwide (Quigley 2006). One systematic review estimated that the global prevalence of glaucoma in people between 40 and 80 years of age may increase to 76 million by 2020 and to 111.8 million by 2040 (Tham 2014). Open‐angle glaucoma (OAG) is the most common type of glaucoma and accounts for approximately 74% of all cases (Quigley 2006). Women comprise 55% of OAG cases, and OAG disproportionately affects people of African ancestry and older adults (NEI 2015).
OAG is a progressive disease. In early mild‐to‐moderate stages, there are no symptoms (AAO 2015). Due to the 'silent' nature of OAG, people do not usually have any visual problems; there are optic nerve abnormalities consistent with glaucoma, but little to no aberrations in visual fields. In severe stages of glaucoma, people may notice vision loss or blind spots due to significant amounts of irreversible optic nerve damage (AAO 2015). Main signs of glaucoma include atrophied optic nerve and presence of an open angle, both of which can only be seen using specialized instruments.
Many people with OAG also experience elevated intraocular pressure (IOP); however, IOP is not a direct measure of structural or functional glaucomatous optic neuropathy and not all people with glaucoma present with elevated IOP (AAO 2015; Le 2016; Medeiros 2015). Nevertheless, because IOP is the only known modifiable risk factor, treatment for OAG has focused predominantly on lowering IOP (Li 2016; Quigley 2007).
Description of the intervention
Lowering IOP is achieved through medical, laser, and surgical interventions, typically implemented in a step‐wise manner (AAO 2015; Le 2018a; NICE 2009). Since the early 2000s, a series of new treatment modalities, which the US Food and Drug Administration (FDA) refers to as "minimally invasive glaucoma surgical" (MIGS) devices, has emerged. MIGS are ab interno procedures that require minimal to no conjunctival manipulation or scleral dissection, which are readily combined with another intraocular procedure such as cataract extraction by phacoemulsification (cataract surgery). MIGS typically lower IOP to a more modest degree than traditional filtering surgeries (e.g. trabeculectomy or tube shunt implantation); however, MIGS may pose fewer risks than those more invasive surgeries (Caprioli 2015; Francis 2011; Spaeth 2015). While MIGS generally are not used as first‐line therapy for glaucoma at this time, they may reduce the need for medication.
Examples of MIGS interventions include the iStent and iStent Inject, trabectome ab interno trabeculectomy, endoscopic cyclophotocoagulation (ECP), gonioscopy‐assisted transluminal trabeculotomy (GATT), the Hydrus Microstent intracanalicular scaffold, the XEN Gel Stent, and the Innfocus Microshunt. Of these, the first four are currently FDA approved for use in the US; the others are being evaluated in clinical trials.
This Cochrane Review examined the iStent and iStent inject (Glaukos Corporation, Laguna Hills, CA, USA), the former of which was the first MIGS device to have received FDA approval, for people with mild‐to‐moderate OAG.
The iStent is a heparin‐coated non‐ferromagnetic titanium 'L‐shaped' device, 1 mm in length with a head 0.3 mm in height facing the anterior chamber (Glaukos 2016). This MIGS device is preloaded into a single‐use injector and then inserted ab interno through the trabecular meshwork under direct gonioscopic view (Manasses 2016). The iStent creates a permanent opening that directly connects the anterior chamber to Schlemm's canal.
The iStent inject is a second‐generation 'mushroom‐shaped' MIGS device, 360 μm in length with a conical head with maximum width of 230 μm. Like the iStent, the iStent inject is made of heparin‐coated titanium but the conical head contains four evenly spaced outlets that allow fluid to pass from the anterior chamber into Schlemm's canal (Bahler 2012). The injector is preloaded with two iStent inject MIGS devices and is designed to deliver both stents, ab interno, into Schlemm's canal while entering the eye only once (Bahler 2012; Klamann 2015).
How the intervention might work
IOP increases when there is an imbalance between production and outflow of aqueous humor (a clear fluid that provides avascular ocular structures with nutrition). Aqueous humor drains through a complex network of cells and tissue (trabecular meshwork, Schlemm's canal, and collector channels) in an area known as the drainage angle (AAO 2015).
Given that the trabecular meshwork is the primary site of aqueous outflow and that resistance to aqueous humor outflow in this region largely determines IOP (Manasses 2016), bypassing the trabecular meshwork is a viable method to decrease IOP. Ab interno implantation of MIGS devices such as the iStent and iStent inject may increase outflow facility by providing direct access to Schlemm's canal and downstream collector channels via a permanent opening through trabecular meshwork (Francis 2011).
Why it is important to do this review
Most treatments for OAG rely primarily on lowering IOP (AAO 2015; AGIS 2000; EGS 2014), but they all have limitations. Many people with mild‐to‐moderate OAG elect to start with medical treatment (e.g. topical eye drops) as first‐line therapy (AAO 2015; Li 2016; NICE 2009); commercially available eye drops have short durations of effect and adherence is poor (Friedman 2009; Okeke 2009). Conventional surgical procedures to bypass the trabecular meshwork and drainage angle, such as trabeculectomy and tube shunts or valves, are associated with variable frequencies of success and complications (Gedde 2012a; Gedde 2012b; Spaeth 2015). Trabeculectomies fail after about five years in approximately 50% of cases (Gedde 2012a; Gedde 2012b; Kirwan 2013; Lichter 2001). Laser trabeculoplasty (LTP) represents an intermediate intervention between drops and surgery, or can be used as an alternative first line to drops, but its efficacy has been noted to decrease over time and most people ultimately require repeat LTP or surgery (Leahy 2015; Patel 2015; Rolim de Moura 2007; Woo 2015).
MIGS procedures are becoming increasingly common, with their proponents claiming better safety profiles than other glaucoma surgical techniques (Brandao 2013; Larsen 2017). In this review, we specifically examined the evidence for the effectiveness and safety of one type of MIGS device – the iStent and iStent inject – in people with mild‐to‐moderate OAG of any type. This review was undertaken as part of the Cochrane Eyes and Vision MIGS Consortium. The Consortium also reviewed other types of MIGS techniques and devices including the Trabectome (NeoMedix, Tustin, CA, USA), Hydrus Microstent (Ivantis, Irvine, CA, USA) (Otarola 2017), ECP (Endo Optiks, Waltham, MA, USA) (Tóth 2019), and XEN Glaucoma Implant (AqueSys Implant, Aliso Viejo, CA, USA) (King 2018).
Objectives
To assess the effectiveness and safety of ab interno trabecular bypass surgery with iStent (or iStent inject) for open‐angle glaucoma in comparison to conventional medical, laser, or surgical treatment.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs), prepared in any language, irrespective of their publication status.
Types of participants
We included studies of people with mild‐to‐moderate OAG of any type, including primary and secondary OAG. Primary OAG refers to glaucoma that develops due to an unknown cause; secondary OAG develops from a known cause, such as trauma to the eye or ocular inflammatory diseases. In the absence of a universally accepted definition for glaucoma, we permitted studies to use their own criteria to define OAG; however, we excluded studies of participants with angle‐closure glaucoma (where increased IOP occurs because abnormal iris anatomy obstructs aqueous flow to the drainage angle). In addition, we allowed studies that included participants with ocular hypertension (OHT), normal tension glaucoma, or possible OAG (i.e. suspected).
Types of interventions
We included studies that compared iStent or iStent inject (Glaukos Corporation, Laguna Hills, CA, USA) to any of the following:
laser treatment (selective LTP or argon LTP);
other MIGS procedures/techniques;
conventional glaucoma surgery (trabeculectomy);
medical therapy; or
in combination with phacoemulsification compared with phacoemulsification alone. iStent devices are approved in people undergoing phacoemulsification.
Additionally, we conducted stratified analyses based on iStent procedures (e.g. iStent versus iStent inject).
Types of outcome measures
We did not use reporting of particular outcomes as a criterion for including a trial into our systematic review. We, as with other review teams in the Consortium, adapted primary and secondary outcomes from a Cochrane systematic review prepared by Hu and colleagues (Hu 2016).
We evaluated each outcome at a time point in the six to 18 months (medium‐term) time window, in addition to less than six months (short‐term), over 18 but less than or equal to 36 months (long‐term), and over 36 months time windows. We recognized that our primary outcome may not be relevant in RCTs that randomized participants to medical therapy in lieu of an iStent procedure.
Primary outcomes
Proportion of participants who were drop‐free (i.e. not using eye drops) at a time point in each of the time windows.
Secondary outcomes
Mean change in number of IOP‐lowering drops taken per day from baseline to a time point in each of the time windows.
Mean change in IOP, measured using Goldmann applanation tonometry, from baseline to a time point in each of the time windows.
Any health‐related quality of life measures, measured as mean change from baseline or proportion meeting a threshold at a time point in each of the time windows, as defined by the investigators of the included trials.
Adverse outcomes
-
Proportions of participants experiencing intra‐ and postoperative complications at a time point in each of the time windows, including but not restricted to the following:
loss of visual acuity of more than 2 Snellen lines, or more than 0.3 logMAR, according to the method of recording visual acuity; or loss of light perception;
bleeding, as recorded by the investigators;
endophthalmitis, as recorded by the investigators;
IOP spikes, defined as postoperative rise in IOP, measured using Goldmann applanation tonometry, of more than 10 mmHg compared to the previous assessment, including during the first postoperative month;
secondary glaucoma surgery, including laser, as recorded by the investigators of the included trials.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs and controlled clinical trials. We used no restrictions on language or year of publication.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 7; which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 17 August 2018; Appendix 1).
MEDLINE Ovid (1946 to 17 August 2018; Appendix 2).
Embase Ovid (1980 to 17 August 2018; Appendix 3).
ISRCTN registry (www.isrctn.com/editAdvancedSearch; searched 17 August 2018; Appendix 4).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 17 August 2018; Appendix 5).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 17 August 2018; Appendix 6).
US FDA website (www.fda.gov; searched 17 August 2018; Appendix 7).
Searching other resources
We searched the reference lists of included studies for possible studies and the website of the manufacturer for information regarding forthcoming trials (Glaukos 2016).
Data collection and analysis
Selection of studies
Two review authors (JL and TL) worked independently to screen titles and abstracts of all records identified by the search using web‐based review management software (Covidence 2015). We removed duplicates from the search results. The review authors classified each record as either relevant (a 'Yes' vote) or not relevant (a 'No' vote) for full‐text review. The two review authors independently assessed the full‐text copies of all titles and abstracts that they identified as relevant to determine if the reports met the inclusion criteria (an 'Include' vote) or not (an 'Exclude' vote). We did not need to contact the trial authors of any record to clarify details necessary to make a complete assessment of the eligibility. We documented reasons for exclusion for each study assessed as not eligible after review of the full‐text articles. We resolved all discrepancies between review authors by discussion at each stage of the screening process. We then linked multiple reports originating from the same trial.
Data extraction and management
Two review authors (JL and LW) independently extracted data using a web‐based electronic data collection form in SRDR (srdr.ahrq.gov/). We extracted the information as described in Appendix 8, including: study setting, countries where recruitment took place, sample size, study duration and follow‐up time, study design, analysis choice, sources of funding, and potential conflicts of interests; characteristics of the participants (e.g. inclusion/exclusion criteria), underlying disease conditions, and medical history (including IOP at baseline, number of glaucoma medications at baseline, visual acuity, and other vision‐related characteristics); interventions (e.g. iStent or iStent inject) and comparators (e.g. type of laser, drugs, surgery, duration, and timing); outcomes (e.g. domain, specific measurement, specific metric, method of aggregation, and time frame); and quantitative results.
The review authors compared the extracted data and resolved discrepancies by discussion. One review author (JL) completed data entry into Review Manager 5 (Review Manager 2014), and a second review author (LW) verified the data entered.
Assessment of risk of bias in included studies
Two review authors (JL and LW) independently assessed the risk of bias in included studies, following guidance described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). Specific items for consideration included random sequence generation and allocation concealment (selection bias), masking of study personnel (performance bias), masking of outcome assessors (detection bias) for number of IOP‐lowering drops used and for IOP measurement, missing data and intention‐to‐treat analysis (attrition bias), and selective outcome reporting (reporting bias).
We assigned each item as having low risk, high risk, or, if the information provided was insufficient to make an assessment, unclear risk. We documented reasons for those assessments and resolved discrepancies through discussion. We presented the overall assessments as the 'Risk of bias' summary figure (Higgins 2017).
Measures of treatment effect
We used mean difference (MD) as the measure of effect for all continuous outcomes, with 95% confidence intervals (CI). We used risk ratio (RR) with 95% CIs as the measure of effect for all binary and categorical outcomes.
Unit of analysis issues
We assessed whether the included trials included one or both eyes from each participant and whether the trial investigators randomized (and analyzed) at the participant‐level or at the eye‐level. When both eyes were randomized to different treatments, we planned to extract the results that had accounted for the correlation.
Dealing with missing data
Where data on included studies were unclear or missing, we planned to write to the authors and analyze the data using the best information available if we received no response within two weeks. We planned to consider multiple imputation or other imputation approaches for handling missing data if needed. If the quality of the available data prevented any meaningful analysis, we planned to omit the study from quantitative analyses and note this decision in the discussion.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by examining participant characteristics, MIGS techniques and devices, and outcomes, taking into consideration potential risk of bias. We assessed forest plots and examined the I2 statistic and its CI for statistical heterogeneity. Similar to other protocols on MIGS procedures, we considered an I2 statistic greater than 50% as indicative of substantial heterogeneity, suggesting that a meta‐analysis may not be appropriate. However, if all estimates were in the same direction, we pursued a meta‐analysis despite substantial statistical heterogeneity, and we interpreted the findings taking into consideration the heterogeneity.
Assessment of reporting biases
We planned to assess small‐study effects using funnel plots if there were more than 10 trials for each meta‐analysis. We assessed selective reporting as part of the 'Risk of bias' assessment, for example, examining differences between trial registration, protocol, and publication.
Data synthesis
We followed Chapter 9 of theCochrane Handbook for Systematic Reviews of Interventions for data synthesis and analysis (Deeks 2017). We first provided a descriptive, qualitative synthesis of studies and their results. We used fixed‐effect models for all meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We planned to conduct a subgroup analysis by type of iStent (iStent or iStent inject).
Sensitivity analysis
We planned to conduct additional sensitivity analyses to determine the impact of any post hoc decisions made during the review process.
'Summary of findings' tables
We prepared 'Summary of findings' tables using the GRADE approach to assess the certainty of the evidence (GRADEpro GDT 2015). We planned to include following outcomes in the summary.
Proportion of participants who were drop‐free (not using eye drops) at six to 18 months.
Mean change in number of IOP‐lowering drops taken per day from baseline to six to 18 months
Mean change in IOP, measured using Goldmann applanation tonometry, from baseline to six to 18 months.
Health‐related quality of life at six to 18 months.
Intraoperative complications at six to 18 months.
Postoperative complications up to six to 18 months.
Secondary glaucoma surgery, including laser, as recorded by the investigators of the included trials between baseline and six to 18 months.
We summarized findings from two comparison groups: in combination with phacoemulsification compared with phacoemulsification alone and iStent compared to medical therapy. We downgraded the level of certainty of the evidence if the contributing studies were at high or unclear risk of bias for masking of outcome assessors (one level), provided inconsistent estimates (one level) or imprecise estimates (one level) due to small sample size or wide CIs, or may have been subject to publication bias (one level). Guyatt 2011 noted that inclination to downgrade for publication bias should increase when evidence comes from small studies which are "industry sponsored or likely to be industry sponsored (or if the investigators share another conflict of interest)." We also presented results of one trial which compared one iStent with two iStents with three iStents, but did not assess the certainty of the evidence or present a 'Summary of findings' table for this single trial.
Results
Description of studies
Results of the search
The electronic search yielded 362 records (Figure 1). After removal of 90 duplicates, we screened the remaining 272 records and excluded a further 237 records based on information in the title and abstract. We obtained full‐text reports of 35 records for further investigation. We included 19 reports of seven studies (see Characteristics of included studies table) and excluded three reports of three studies (see Characteristics of excluded studies table). We identified 13 ongoing studies that potentially met the inclusion criteria, these studies will be assessed when data become available (see Characteristics of ongoing studies table for further details).
1.

Study flow diagram.
Included studies
Type of studies
We included seven RCTs (Fea 2010; Fea 2014; Fernandez‐Barrientos 2010; Katz 2015; NCT00721968; Samuelson 2011; Vold 2016). Most RCTs were multi‐center trials in which participants were recruited from Armenia (two RCTs); Italy (one RCT); Spain (one RCT); the US (two RCTs); or the previous four countries in addition to Germany and the UK (one RCT) (see Characteristics of included studies table). Six trials began enrollment of participants prior to 2010, and the maximal planned length of follow‐up ranged from one to five years (Fea 2010; Fea 2014; Fernandez‐Barrientos 2010; Katz 2015; NCT00721968; Samuelson 2011). All seven RCTs reported having received support – including financial, non‐study financial, or non‐financial support (e.g. study devices, editorial assistance, or payment of article processing charges) – from the Glaukos Corporation (Laguna Hills, CA, USA), manufacturer of the iStent and iStent inject.
Type of participants
The seven RCTs enrolled 764 participants (765 eyes; range per study: 33 to 239 participants). Most participants were white, female sex (417/764 (55%)), and older age (range: 49 to 89 years). The diagnosis of participants varied between studies: some included people with OHT, pseudoexfoliative glaucoma (PXG), or pigmentary glaucoma (PG) in addition to OAG. Most excluded participants with prior incisional glaucoma surgery, and four trials included only participants with OAG in need of cataract surgery (Fea 2010; Fernandez‐Barrientos 2010; NCT00721968; Samuelson 2011). Four trials reported that participants were washed out of current glaucoma medication (Fea 2014; Fernandez‐Barrientos 2010; Katz 2015; Samuelson 2011); one trial recruited only treatment‐naïve participants (Vold 2016). Table 3 provides a synopsis of the trial‐level eligibility criteria.
1. Eligibility criteria of included studies.
| Study | Diagnosis | Intraocular pressure | Number of glaucoma medication currently taking |
Visual acuity (Snellen; BCVA) |
Prior incisional glaucoma surgery | Prior laser surgery | Washout period |
| Comparison 1: iStent in combination with phacoemulsification vs phacoemulsification alone | |||||||
| Fea 2010 | OAG in need of cataract surgery | > 18 mmHg (medicated) | ≥ 1 | 20/80 or worse | Excluded | NR | NR |
| Fernandez‐Barrientos 2010 | OHT or OAG in need of cataract surgery | > 17 mmHg and < 31 mmHg (medicated); > 21 mmHg and < 36 mmHg (unmedicated) | ≤ 2 | 20/40 or worse | Excluded | Excluded | Yes |
| NCT00721968 | OAG in need of cataract surgery | NR | NR | NR | NR | NR | NR |
| Samuelson 2011 | OAG, PEXG, or PG, in need of cataract surgery | ≤ 24 mmHg (medicated); ≥ 22 mmHg and ≤ 36 mmHg (unmedicated) | ≥ 1 and ≤ 3 | 20/40 or worse | Excluded (except for iridectomy) | Excluded | Yes |
| Comparison 2: iStent or iStent inject vs medical therapy | |||||||
| Fea 2014 | OAG, PEXG, or PG | ≥ 22 mmHg and < 38 mmHg (unmedicated) | 1 | 20/200 or better | Excluded | Includeda | Yes |
| Vold 2016 | OHT, OAG, or PEXG | ≥ 21 mmHg and ≤ 40 mmHg (unmedicated) | 0 | NR | Excluded | Excluded | NA |
| Additional comparison: 1 iStent vs 2 iStents vs 3 iStents | |||||||
| Katz 2015 | OAG, PEXG, or PG; and phakic | ≥ 18 mmHg and ≤ 30 mmHg (medicated); > 22 mmHg and < 38 mmHg (unmedicated) | 2 | 20/200 or better | Excluded | Includeda | Yes |
aAs long as the procedure was not performed within 30 days prior to screening. BCVA: best‐corrected visual acuity; OAG: open‐angle glaucoma; OHT: ocular hypertension; PEXG: pseudoexfoliative glaucoma; PG: pigmentary glaucoma; NA: not available; NR: not reported.
Type of interventions
Four RCTs compared treatment with iStent in combination with phacoemulsification to phacoemulsification alone; specifically: Fea 2010, NCT00721968, and Samuelson 2011 compared the iStent combined with phacoemulsification to phacoemulsification alone, and Fernandez‐Barrientos 2010 compared two iStents combined with phacoemulsification to phacoemulsification alone.
The remaining three RCTs did not use phacoemulsification as a concomitant intervention: Vold 2016 compared two iStents with topical travoprost (Travatan; Alcon, Fort Worth, TX, USA); Fea 2014 compared the iStent inject with fixed combination of latanoprost/timolol (Xalacom; Pfizer, New York, NY, USA); and Katz 2015 compared one iStent with two iStents and with three iStents.
Type of outcomes
Five RCTs reported our primary outcome (proportion of participants who were drop‐free at two years) (Fea 2010; Fea 2014; Katz 2015; Samuelson 2011; Vold 2016). The two RCTs that did not report on our primary outcome (Fernandez‐Barrientos 2010; NCT00721968), along with Samuelson 2011, provided data on the mean change in number of IOP‐lowering drops. Four RCTs reported mean change in IOP postwashout of any glaucoma medications (Fernandez‐Barrientos 2010; Katz 2015; NCT00721968; Samuelson 2011). Proportions of participants experiencing complications were reported variably among the seven RCTs. No RCTs reported quality of life.
Three RCTS described calculating sample sizes, based on the ability to detect a 19.5% difference in proportion of participants with IOP 21 mmHg or less at one year (Samuelson 2011); a difference in IOP of approximately 3 mmHg at 15 months (Fea 2010); or a 0.3 μL/minute/mmHg difference in the outflow facility at one year (Fernandez‐Barrientos 2010).
We summarized clinically important and surgery‐related adverse events of interest in Table 4.
2. Postoperative complications reported by included studies.
| Comparison 1: iStent in combination with phacoemulsification vs phacoemulsification alone | ||
| Fea 2010 | — | — |
| Adverse events | "No postoperative stent‐related adverse events were observed in these eyes [N = 24] through 48 months. IOP was well controlled in both groups throughout the entire follow‐up period; no secondary surgical intervention was required to control IOP." | |
| Fernandez‐Barrientos 2010 | 2 iStents in combination with phacoemulsification at 1 year | Phacoemulsification alone at 1 year |
| Stent malposition | Authors noted that "six of the 34 (18%) implanted stents appeared to be malpositioned" | NA |
| Need for selective trabeculoplasty | 0 | 1/16 |
| NCT00721968 | iStent in combination with phacoemulsification at 1 year | Phacoemulsification alone at 1 year |
| Posterior capsule opacification | 4/27 | 1/17 |
| IOP increase ≥ 10 mmHg vs baseline IOP at any visit | 3/27 | 9/17 |
| Conjunctivitis | 3/27 | 2/17 |
| Corneal abrasion | 2/27 | 1/17 |
| Iritis | 2/27 | 0 |
| Punctate corneal staining | 1/27 | 1/17 |
| Superficial punctate keratitis | 1/27 | 1/17 |
| Blurry vision | 1/27 | 1/17 |
| BCVA loss ≥ 1 line after 3 months postoperative | 0 | 2/17 |
| Eye pain | 0 | 2/17 |
| Retinal detachment | 0 | 1/17 |
| Samuelson 2011 | iStent in combination with phacoemulsification at 2 years | Phacoemulsification alone at 2 years |
| Anticipated early postoperative event (as defined by investigators) | 20/116 | 22/117 |
| Posterior capsule opacification | 7/116 | 12/117 |
| Elevated IOP | 5/116 | 8/117 |
| Stent obstruction | 5/116 | NA |
| Blurry vision or visual disturbance | 4/116 | 8/117 |
| Stent malposition | 3/116 | NA |
| Iritis | 1/116 | 6/117 |
| Conjunctival irritation due to hypotensive medication | 1/116 | 3/117 |
| Disk hemorrhage | 1/116 | 3/117 |
| Comparison 2: iStent (or iStent inject) vs medical therapy | ||
| Fea 2014 | iStent inject at 1 year (94 eyes of 94 participants) | Medical therapy at 1 year (98 eyes of 98 participants) |
| IOP decompensation | 1/94 | 0 |
| Soreness/discomfort | 1/94 | 0 |
| Eye burning | 0 | 1/98 |
| Medical allergy | 0 | 1/98 |
| Secondary glaucoma surgery | 1/94 | NA |
| Vold 2016 | "Safety was favorable in both groups [Two iStents, N = 54; Medical therapy, N = 47; at 36 months). Two complications were reported during stent insertion in the surgery group, both of which were attributed to subject movement during surgery: one of these subjects had hyphema which resolved by day 1 and one subject had a small iridodialysis which resulted in no postoperative ocular sequelae...In the remaining non‐operated subjects, three‐year BCVA was 20/40 or better in 6 eyes (2 in stent group and 4 in med group), 20/100 in 1 eye (stent group), and 20/200 in 6 eyes (3 per group). No other post‐treatment adverse events were reported in either group." | |
| Additional comparison: 1 iStent vs 2 iStents vs 3 iStents | ||
| Katz 2015 | "No complications occurred intraoperatively or perioperatively, including no hypotony, choroidal effusion, hyphema, nor iridodialysis [One iStent, N=38; two iStents, N= 41; three iStents, N=40]. During 42 months of postoperative follow‐up, no device‐related or sight‐threatening adverse events occurred; furthermore, no eyes required additional glaucoma surgery. In this cohort of almost entirely phakic subjects (117 of 119) with mean baseline age between 62 and 69 years, the most common (and expected) adverse event over 3.5 years of follow‐up was progression of preexisting cataract. By Month 42 postoperatively, a total of eight one‐stent eyes, five two‐stent eyes, and seven three‐stent eyes had BCVA loss 1 line due to cataract progression. Of these cases, five one‐stent eyes, two two‐stent eyes, and three three‐stent eyes underwent cataract surgery by Month 42, and their IOP and medication data thereafter were excluded from efficacy analyses; two additional eyes (three‐stent group) had cataract surgery shortly after the Month 42 visit." | |
BCVA: best‐corrected visual acuity; IOP: intraocular pressure; N: number; NA: not available.
Excluded studies
We excluded two studies that were not RCTs. Bacharach 2014 was a subsequent observational extension of Samuelson 2011, where a non‐randomized population of 46 participants were added to the treatment arm. Vlasov 2017 was a retrospective case series review of one versus two iStent implantations in combination with phacoemulsification. We also excluded one study which was withdrawn before enrolling the first participant (NCT03274323).
Ongoing studies
We identified 13 ongoing studies awaiting classification. All 13 are described as RCTs and are recruiting participants from Armenia, Australia, Germany, Japan, Spain, Turkey, and the US. Participants are randomized to treatment with iStent compared to treatment with no iStent (e.g. phacoemulsification alone), to medical therapy (e.g. latanoprost and timolol), to different number of iStents implanted (e.g. one versus two stents), to SLT laser treatment, to the Hydrus Microstent (see Characteristics of ongoing studies table). The studies are funded mostly by manufacturers of the devices.
Risk of bias in included studies
We summarized the risk of bias in the included trials in Figure 2.
2.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Random sequence generation
Three RCTs reported how investigators generated the random allocation sequence: Fea 2010, Fernandez‐Barrientos 2010, and Samuelson 2011 used a computer‐based random generator, a method that we considered to be at low risk of bias. We assessed the remaining four RCTs, which did not report the method of generating the allocation sequence, at unclear risk of bias.
Allocation concealment
No studies described the method used to conceal the allocation sequence. We assessed all seven trials at unclear risk of bias.
Blinding
One RCT reported that the same examiner, "who was masked to the type of surgery performed" performed all postoperative evaluations (Fernandez‐Barrientos 2010). Accordingly, we assessed the risk of bias in blinding of outcome assessments as low for both 'number of IOP‐lowering drops' and 'IOP measurement' outcome domains. Although Fea 2010 noted that "staff members who measured IOP throughout the study" were masked (low risk of bias), it was unclear whether this same staff member also assessed the number of IOP‐lowering drops that participants were taking per day (unclear risk of bias). We assessed the remaining five trials, which investigators described as "not masked" (Fea 2014) or "open‐label" (Katz 2015; NCT00721968; Samuelson 2011; Vold 2016), at high risk of detection bias.
Incomplete outcome data
We considered three RCTs at low risk of bias for incomplete outcome data because there were no missing data on outcomes of our review (Fernandez‐Barrientos 2010; Katz 2015; Samuelson 2011). We assessed two RCTs at high risk of bias for incomplete outcome data: Fea 2010 excluded 3/36 participants (all randomized to the phacoemulsification alone group) from the final analysis and Vold 2016 conducted an available‐case analysis of 73/101 (72%) participants at 36 months. We assessed the remaining two RCTs at unclear risk of bias because the full publication is not yet available (NCT00721968), or the completeness of outcome data varied by time points reported (Fea 2014).
Selective reporting
We considered the risk of selective reporting to be low for five RCTs because outcomes described in the results matched those specified in methods section and in the trial registrations (Fea 2010; Fea 2014; Fernandez‐Barrientos 2010; Katz 2015; Samuelson 2011). We considered the risk of selective reporting was unclear for NCT00721968, because the full publication of trial results is not yet available; and for Vold 2016, because of differences between the primary outcomes specified on ClinicalTrials.gov ("change from screening in mean diurnal IOP (mm Hg) at the Month 12 visit") and in the published trial report (mean IOP up to 36 months; "diurnal measurements of IOP were not performed").
Effects of interventions
Summary of findings for the main comparison. IStent in combination with phacoemulsification compared to phacoemulsification alone for open‐angle glaucoma.
| iStent in combination with phacoemulsification compared to phacoemulsification alone for open‐angle glaucoma | ||||||
| Patient or population: open‐angle glaucoma Setting: – Intervention: iStent in combination with phacoemulsification Comparison: phacoemulsification alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with phacoemulsification alone | Risk with iStent in combination with phacoemulsification | |||||
|
Proportion of participants who were drop‐free Follow‐up: range 6 to ≤ 18 months |
583 per 1000 | 804 per 1000 (688 to 950) | RR 1.38 (1.18 to 1.63) | 239 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Estimate based on data from 2 trials. |
|
Mean change in number of IOP‐lowering drops from baseline Follow‐up: range 6 to ≤ 18 months |
The mean change in number of IOP‐lowering drops from baseline ranged from –1.0 to 0.9 drops | MD 0.42 drops fewer (0.6 fewer to 0.23 fewer) | — | 282 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | In addition, Fernandez‐Barrientos 2010 reported the change in number of IOP‐lowering drops was 0 (SD 0) in the iStent in combination of phacoemulsification treatment group and 0.7 (SD 1) in the phacoemulsification alone group. |
|
Mean change in IOP from baseline Follow‐up: range 6 to ≤ 18 months |
The mean change in IOP from baseline ranged from –8.5 to –1.6 mmHg | MD 1.24 mmHg lower (3.07 lower to 0.58 higher) | — | 284 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,d | — |
| Health‐related quality of life | — | — | — | — | Not reported in any of the 4 studies. | |
| Intraoperative complications | — | — | — | — | Samuelson 2011 reported that "[i]n an eye with intraoperative stent malposition, a second stent was implanted during the same surgery." | |
| Postoperative complications | Based on available data, participants who were randomized to treatment with phacoemulsification in combination with iStent were less likely to experience elevated IOP (or IOP spikes) and loss of vision than those randomized to phacoemulsification alone. | — | 334 (4 RCTs) | — | We did not conduct a meta‐analysis of complications. | |
|
Secondary glaucoma surgery Follow‐up: range 6 to ≤ 18 months |
1 participant randomized to treatment with phacoemulsification in combination with iStent and 1 participant randomized to phacoemulsification alone underwent selective laser trabeculoplasty at 12 months. | — | 290 (3 RCTs) | — | We did not conduct a meta‐analysis of complications. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for high or unclear risk of bias for blinding of outcome assessor. bDowngraded one level for imprecision due to small sample size/wide confidence interval. cDowngraded one level for publication bias due to potential for industry influences. dDowngraded one level for heterogeneity (e.g. I2 > 70%) or inconsistency across trials.
Summary of findings 2. IStent (or iStent inject) compared to medical therapy for open‐angle glaucoma.
| IStent (or iStent inject) compared to medical therapy for open‐angle glaucoma | ||||||
| Patient or population: open‐angle glaucoma Setting: – Intervention: iStent (or iStent inject) Comparison: medical therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with medical therapy | Risk with iStent (or iStent inject) | |||||
|
Proportion of participants who were drop‐free Follow‐up: range 6 to ≤ 18 months |
At 12 months, 0/138 participants randomized to medical therapy were drop‐free at 12 months, while 141/148 (95%) participants randomized to treatment with iStent were drop‐free at 12 months. We did not derive an RR because no events occurred in the control groups of either trial. | — | 286 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | In addition, Vold 2016 noted that 48/54 (88%) participants in the iStent treatment group were drop‐free at 36 months. | |
| Mean change in number of IOP‐lowering drops from baseline | — | — | — | — | — | Not reported in either study. |
|
Mean change in IOP from baseline Follow‐up: range 6 to ≤ 18 months |
The mean change in IOP from baseline was –11.6 mmHg | MD 0.6 mmHg lower (1.28 lower to 0.08 higher) | — | 184 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | Vold 2016 did not report mean change in IOP but did provide mean IOP (without SD) at 6 months (14.2 mmHg), 18 months (13.5 mmHg), and 36 months (14.6 mmHg) in the iStent treatment groups; and at 6 months (13.8 mmHg), 18 months (14.6 mmHg), and 36 months (15.3 mmHg) in the medical therapy group. |
| Health‐related quality of life | — | — | — | — | Not reported in either study. | |
| Intraoperative complications | 1 participant in the iStent treatment group experienced hyphema which resolved by day 1 | — | 101 (1 RCT) | — | We did not conduct a meta‐analysis of complications. | |
| Postoperative complications | Vold 2016 noted that best‐corrected visual acuity was stable between both groups and did not report on any other postoperative complications. Fea 2014 reported that 1 participant in the iStent inject group experienced IOP decompensation with an elevated IOP of 48 mmHg. | — | 286 (2 RCTs) | — | We did not conduct a meta‐analysis of complications. | |
| Secondary glaucoma surgery | Fea 2014 reported that 1 participant needed laser treatment to remove an apparent obstruction | — | 286 (2 RCTs) | — | We did not conduct a meta‐analysis of complications. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for imprecision due to small sample size/wide confidence interval. bDowngraded one level for high or unclear risk of bias for blinding of outcome assessor. cDowngraded one level for publication bias due to potential for industry influences.
Based on the data available, the comparisons that we could make at time of writing this review were: 1. iStent in combination with phacoemulsification versus phacoemulsification alone and 2. iStent (or iStent inject) versus medical therapy. We also summarized findings from one RCT that compared one iStent with two iStents with three iStents. We presented our analyses by comparison, outcome, and duration of follow‐up in the order described.
Comparison 1: iStent in combination with phacoemulsification versus phacoemulsification alone
Four RCTs, which randomized 353 participants with OAG in need of cataract surgery, compared iStent in combination with phacoemulsification versus phacoemulsification alone. Three RCTs implanted one iStent (Fea 2010; NCT00721968; Samuelson 2011); one RCT implanted two iStents (Fernandez‐Barrientos 2010).
Proportion of participants who were drop‐free
Two RCTs reported proportion of participants who were drop‐free in the medium‐term (Figure 3): Fea 2010 at 15 months (RR 2.80, 95% CI 1.18 to 6.64) and Samuelson 2011 at 12 months (RR 1.31, 95% CI 1.11 to 1.54). Although estimates from both studies were consistent in favoring treatment with one iStent in combination with phacoemulsification over phacoemulsification alone, we observed substantial statistical heterogeneity in the meta‐analytical estimate (RR 1.38, 95% CI 1.18 to 1.63; I2 = 67%). We graded the certainty of evidence as very low, downgrading for risk of bias (one level), imprecision (one level), and potential publication bias (one level).
3.
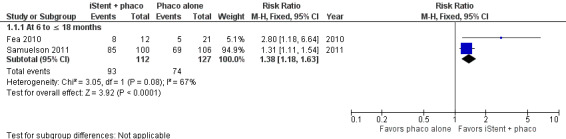
Forest plot of comparison: 1 iStent in combination with phacoemulsification versus phacoemulsification alone, outcome: 1.1 Proportion of participants who were drop‐free.
Mean change in number of intraocular pressure‐lowering drops
Samuelson 2011 observed a greater reduction from baseline in the number of IOP‐lowering drops in the iStent in combination with phacoemulsification group than the phacoemulsification alone group at 12 months (MD –0.40 drops, 95% CI –0.60 to –0.20). NCT00721968 and Fernandez‐Barrientos 2010 reported the mean number (rather than mean change from baseline) of IOP‐lowering drops in each group at medium‐term (Figure 4). Overall, iStent in combination with phacoemulsification reduced the number of IOP‐lowering drops compared with phacoemulsification alone at medium term (MD –0.42, 95% CI –0.60 to –0.23; I2 = 0%). Additionally, Fernandez‐Barrientos 2010 found no statistically significant difference between treatment iStent in combination with phacoemulsification and phacoemulsification alone in the short‐term (MD –0.40, 95% CI –0.82 to 0.02 at 6 months). We graded the certainty of evidence as very low for this outcome, downgrading for risk of bias (one level), imprecision (one level), and potential publication bias (one level).
4.

Forest plot of comparison: 1 iStent in combination with phacoemulsification versus phacoemulsification alone, outcome: 1.2 Mean change in number of intraocular pressure (IOP)‐lowering drops taken per day from baseline.
Mean change in intraocular pressure
Three RCTs reported mean change in IOP at medium‐term (Figure 5): Fea 2010 provided data at 15 months (MD –1.60, 95% CI –3.78 to 0.58); Fernandez‐Barrientos 2010 at 12 months (MD –2.70, 95% CI –4.65 to –0.75); and Samuelson 2011 at 12 months (MD 0.10, 95% CI –0.95 to 1.15). We observed substantial statistical heterogeneity (I2 = 71%) and did not conduct a meta‐analysis; instead, we present the point estimates in a forest plot (Figure 5). We graded the certainty of evidence as very low, downgrading for risk of bias (one level), imprecision (one level), inconsistency (one level) and potential publication bias (one level).
5.

Forest plot of comparison: 1 iStent in combination with phacoemulsification versus phacoemulsification alone, outcome: 1.3 Mean change in IOP.
Of note, Samuelson 2011, in which one iStent was implanted at the time of phacoemulsification in each study eye, reported that although mean reduction in IOP appeared similar in both groups at 12 months, "a substantially higher level of medications was used in the control [phacoemulsification only] group to maintain this similar IOP level." Additionally, at 24 months, the investigators of this RCT observed that mean IOP in the one iStent treatment group was 8.4 mmHg lower than baseline IOP, compared to 7.5 mmHg in the phacoemulsification alone group (Samuelson 2011); no standard deviations (SD) were provided and therefore we could not derive any between‐group estimates.
Health‐related quality of life
No trials reported health‐related quality of life.
Intra‐ and postoperative complications from baseline
Samuelson 2011 reported one intraoperative complication where "[i]n an eye with intraoperative stent malposition, a second stent was implanted during the same surgery."
The reporting of postoperative complications varied by RCT (Table 5).
3. Summary of financial support of included studies.
| Study | Pharmaceutical industry involvement | Other financial support |
| Comparison 1: iStent in combination with phacoemulsification vs phacoemulsification alone | ||
| Fea 2010 | Glaukos Corporation provided funding/support (including study devices) |
Ricerca Finalizaata della Regione Pimonte 2007 |
| Fernandez‐Barrientos 2010 | Glaukos Corporation provided funding/support | — |
| NCT00721968 | Glaukos Corporation provided funding/support | — |
| Samuelson 2011 | Glaukos Corporation provided funding/support (Investigators were consultants to Glaukos for the conduct of this study) |
— |
| Comparison 2: iStents vs medical therapy | ||
| Fea 2014 | Glaukos Corporation provided funding/support (including study devices, editorial assistance, payment of article processing charges, financial support, and non‐study financial support) |
— |
| Vold 2016 | Glaukos Corporation provided funding/support (including non‐financial, financial, and non‐study financial support to some/all authors) |
— |
| Additional comparison: iStent vs 2 iStents vs 3 iStents | ||
| Katz 2015 | Glaukos Corporation provided funding/support (including study devices and non‐financial, financial, and non‐study financial support to some/all authors) |
— |
In Samuelson 2011, one participant in each treatment group experienced best‐corrected visual acuity (BCVA) loss and two participants in each treatment group experienced "subconjunctival hemorrhage" at 12 months. Two participants in the iStent treatment group experienced and elevation of IOP requiring treatment at 12 months compared to one participant in the phacoemulsification alone group. Data comparing number of participants who needed secondary surgical interventions at 24 months in the iStent in combination with phacoemulsification group versus the phacoemulsification groups available for a subset "safety population" that excluded six participants who were terminated from the study before receiving surgery of any type. In this population, one participant treated with phacoemulsification in combination with iStent underwent a secondary glaucoma surgical intervention (trabeculoplasty).
In NCT00721968, participants in the iStent treatment group were less likely to experience IOP spikes at 12 months (RR 0.21, 95% CI 0.07 to 0.67).
In Fernandez‐Barrientos 2010, one participant randomized to phacoemulsification underwent selective LTP at 12 months to manage their glaucoma.
In Fea 2010, none of the 24 participants (10 randomized to iStent in combination with phacoemulsification and 14 randomized to phacoemulsification alone) still under follow‐up at 48 months needed secondary glaucoma surgery.
Comparison 2: iStent (or iStent inject) versus medical therapy
Two RCTs, which randomized 293 participants with OAG, compared treatment with either two iStents (Vold 2016) or the iStent inject (Fea 2014) with medical therapy. Medical therapy consisted of either a fixed combination of latanoprost/timolol (Fea 2014) or topical travoprost (Vold 2016). All participants in Fea 2014 were using one IOP‐lowering medication at recruitment and, "in the opinion of the investigator, required additional IOP lowering." All participants enrolled in Vold 2016 were newly diagnosed and had not "undergone prior treatment of any kind" for their glaucoma. Because the patient population, intervention, and comparison were clinically heterogenous between these two RCTs, we did not conduct a meta‐analysis for any outcomes.
Proportion of participants who were drop‐free
Both RCTs reported proportion of participants who were drop‐free in the medium‐term. As one would expect based on study design, no participants who were randomized to medical therapy in either RCTs were drop‐free at 12 months, compared with 96% (90/94; Fea 2014) and 94% (51/54; Vold 2016) of participants in the iStent treatment groups. Additionally, Vold 2016 observed that at 36 months, no participants in the medical therapy group were drop‐free compared to 88% (48/54) of participants in the iStent treatment group. We did not derive an RR because no events occurred in the control group. We graded the certainty of evidence as very low, downgrading for risk of bias (one level), imprecision (one level), and potential publication bias (one level).
Mean change in number of intraocular pressure‐lowering drops
Vold 2016 specifically reported medications required over and above the travoprost used as a study intervention, while Fea 2014 reported total number of medications required, regardless of response to medical therapy. Thus, neither Fea 2014 nor Vold 2016 provided mean change in number of IOP‐lowering drops that could be analyzed for this review.
Mean change in intraocular pressure
Fea 2014 reported the mean change in IOP in the short‐term (MD 0.10, 95% CI –0.72 to 0.92, at 6 months) and medium‐term (MD –0.60, 95% CI –1.28 to 0.08, at 12 months), comparing iStent inject to medical therapy. Vold 2016 did not report mean change in IOP but provided mean IOP (without SD) at six months (14.2 mmHg in the iStent group versus 13.8 mmHg in the medical therapy group), 18 months (13.5 mmHg in the iStent group versus 14.6 mmHg in the medical therapy group), and 36 months (14.6 mmHg in the iStent group versus 15.3 mmHg in the medical therapy group). We graded the certainty of evidence as very low, downgrading for risk of bias (one level), imprecision (one level), and potential publication bias (one level).
Health‐related quality of life
No studies reported health‐related quality of life.
Intra‐ and postoperative complications from baseline
The reporting of intraoperative and postoperative complications varied by RCT.
Vold 2016 observed one participant with hyphema that resolved by day one and another participant with a small iridodialysis. The investigators also noted that six participants (five in the iStent treatment group and one in the medical therapy group) underwent cataract surgery at 36 months, but they did not report on need for secondary glaucoma surgery.
Fea 2014 reported one participant in the iStent inject treatment group who experienced "IOP decompensation with an elevated IOP (48 mmHg)" that resolved after treatment with medication; and one participant, also in the iStent inject treatment group, who required laser treatment to remove an apparent obstruction.
Additional comparison: one iStent versus two iStents versus three iStents
One three‐arm RCT randomized 119 participants with OAG to treatment with one iStent, two iStents, or three iStents (Katz 2015). The investigators recruited participants from a single center in Armenia and followed them for five years. Results up to 42 months were available.
Proportion of participants who were drop‐free
Comparing treatment with one iStent to treatment with two iStents, there was no difference in terms of participants who were drop‐free at time points in the short‐term (RR 0.94, 95% CI 0.85 to 1.05), medium‐term (RR 0.99, 95% CI 0.85 to 1.15), and long‐term (RR 0.98, 95% CI 0.85 to 1.15) (Figure 6). However, at more than 36 months, participants who were randomized to treatment with one iStent were less likely to be drop‐free than those to treatment with two iStents (RR 0.51, 95% CI 0.34 to 0.75, at 42 months).
6.

Forest plot of comparison: 3 One iStent (without phacoemulsification) versus two iStents (without phacoemulsification), outcome: 3.1 Proportion of participants who were drop‐free.
Comparing treatment with one iStent to treatment with three iStents, there was also no difference in terms of participants who were drop‐free at time points in the short‐term (RR 0.94, 95% CI 0.85 to 1.05), medium‐term (RR 0.97, 95% CI 0.84 to 1.12), and long‐term (RR 0.97, 95% CI 0.83 to 1.12) (Figure 7). At more than 36 months, participants who were randomized to treatment with one iStent were less likely to be drop‐free than those to treatment with three iStents (RR 0.49, 95% CI 0.34 to 0.73, at 42 months).
7.
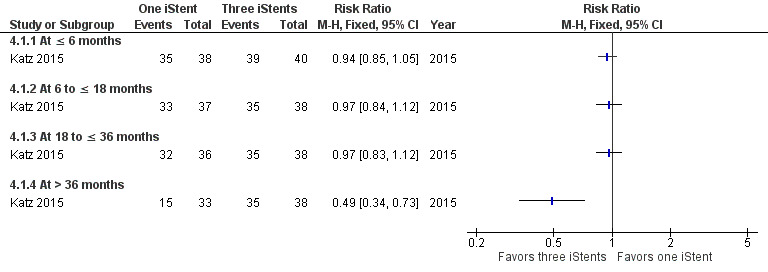
Forest plot of comparison: 4 One iStent (without phacoemulsification) versus three iStents (without phacoemulsification), outcome: 4.1 Proportion of participants who were drop‐free.
Mean change in number of intraocular pressure‐lowering drops
Katz 2015 did not report the mean change in number of IOP‐lowering drops; however, the investigators noted that one participant in the two‐iStent treatment group was prescribed two medications at 18 months. The remaining 13 participants who were using drops at 18 months were on one medication to control their IOP.
Mean change in intraocular pressures
Katz 2015 reported the mean change in IOP at 18 months as –3.94 mmHg in the one‐iStent, –5.99 mmHg in the two‐iStent, and –8.19 mmHg in the three‐iStent treatment groups. No SD were reported; however, investigators provided mean IOP at six, 18, and 42 months with SDs. The investigators reported a statistically significant difference in mean IOP at 18 months, comparing treatment with one iStent to treatment with two iStents (MD 1.80 mmHg, 95% CI 1.17 to 2.43) and to treatment with three iStents (MD 3.50 mmHg, 95% CI 2.88 to 4.12), and no difference in mean IOP comparing treatment with one iStent to treatment with two iStents or to treatment with three iStents at six and 42 months
Health‐related quality of life
Katz 2015 did not report health‐related quality of life.
Intra‐ and postoperative complications from baseline
Katz 2015 noted that "no complications occurred intraoperatively or perioperatively, including no hypotony, choroidal effusion, hypema, nor iridodialysis." By 42 months, eight (21%) participants in the one‐iStent, five (12%) participants in the two‐iStent, and seven (18%) participants in three‐iStent treatment groups had BCVA loss of 1 line or more due to cataract progression. Of these, five participants in the one‐iStent, two participants in the two‐iStent, and three participants in the three‐iStent treatment groups underwent cataract surgery by 42 months.
Discussion
Summary of main results
We identified seven RCTs that compared treatment of people with the iStent (or iStent inject) to treatment with phacoemulsification alone, medical therapy, or different numbers of iStents. We summarized our findings after reviewing the available evidence in the 'Summary of findings' tables for the main comparison section (Table 1; Table 2).
There was considerable variability among the trials with respect to interventions evaluated, outcomes reported, and length of participant follow‐up. The certainty of the evidence was very low.
In comparison 1, we examined four RCTs comparing treatment with iStent in combination with phacoemulsification to phacoemulsification alone. Data from two trials suggest that participants randomized to iStent were 1.38 times more likely to be drop‐free between six and 18 months postsurgery. However, we observed uncertainty in this estimate (95% CI 1.18 to 1.63) and substantial statistical heterogeneity (I2 = 67%).
In comparison 2, we examined RCTs comparing treatment with iStent (without phacoemulsification) to medical therapy. We determined the two studies in this comparison to be clinically and methodologically heterogeneous and did not conduct a meta‐analysis. In both studies, at 12 months, no participants in the medical therapy groups were drop‐free, while over 90% of participants in the iStent groups were drop‐free.
Additionally, we found one RCT that compared participants randomized to treatment with one iStent to treatment with two iStents, and to treatment with three iStents. About 53% of participants in the one iStent treatment group were drop‐free at 42 months compared to over 90% of participants in the two‐iStent treatment group (RR 0.51, 95% CI 0.34 to 0.75) and three‐iStent treatment group (RR 0.49, 95% CI 0.34 to 0.73).
All trials that reported mean change in IOP noted modest to no difference in IOP reduction. No trials reported on health‐related quality of life, and the seven trials reported proportions of participants experiencing complications variably. Therefore, the association between treatment with iStent and quality of life or adverse events could not be estimated reliably from the data provided.
Overall completeness and applicability of evidence
Despite limiting this review to only RCTs, the included studies differed from one another in several regards. Recognizing these differences, we must consider several factors when interpreting the evidence.
Type and number of iStents may matter. Included studies suggested that implantation of two iStents, compared to one iStent, may be associated with greater proportions of participants who are drop‐free and greater mean reduction in IOP in the long‐term (Fernandez‐Barrientos 2010; Katz 2015). However, a dearth of information precludes formal assessments of quality of life and adverse events associated with procedures involving implantation of multiple devices.
Specific racial or ethnic groups may be under‐represented. Most participants randomized were white.
Prior treatments that participants received for glaucoma differed. All trials excluded people with laser glaucoma surgery performed within 30 days of screening and those with any prior incisional glaucoma surgery. Most trials required participants to be on one or more glaucoma medication at time of enrollment, except one trial that recruited treatment‐naïve participants.
All seven trials received support from the Glaukos Corporation. Support included financial support to some/all authors, study devices, payment of article processing charges, and editorial assistance.
Quality of the evidence
The certainty of the evidence was low across comparisons included in this review. Most trials did not report how the random sequence was generated or the method of concealing allocation. There was high or unclear risk of detection bias in most trials because the outcome assessors were not masked. Attrition bias was either at high or unclear risk for four of the seven included trials. Additionally, few meta‐analyses were possible due to considerable clinical, methodological, and statistical heterogeneity in interventions evaluated and length of participant follow‐up. We also downgraded the evidence because only data from small studies sponsored by industry are available (Guyatt 2011). All seven studies were sponsored by the same industry sponsor (Table 5).
Potential biases in the review process
We worked with an information specialist to conduct a highly sensitive search of the literature and searched multiple databases including trial registries. Two review authors independently completed all steps outlined in the Methods section of this review to reduce bias during study selection, 'Risk of bias' assessment, and data extraction.
Agreements and disagreements with other studies or reviews
One Cochrane systematic review of combined surgery versus phacoemulsification alone for eyes with cataract and glaucoma examined interventions including the iStent (Zhang 2015). The authors included three trials of four that were also included in our review (Fea 2010; Fernandez‐Barrientos 2010; Samuelson 2011). The authors reported a summary estimate for mean reduction in IOP at one year (MD –1.37 mmHg, 95% CI –2.76 to 0.03; I2 = 56%), which we did not report due to substantial statistical heterogeneity and qualitative differences in effect estimates. Additionally, the authors extracted values for mean change from the medicated screening IOP rather than change from unmedicated (postwashout) IOP at baseline for one trial (Samuelson 2011).
We also identified two additional systematic reviews involving the iStent (Lavia 2017; Malvankar‐Mehta 2015). Both reviews used inappropriate statistical methods in analyzing their data.
Authors' conclusions
Implications for practice.
We identified very low‐quality evidence comparing treatment of glaucoma using the iStent in combination with phacoemulsification to phacoemulsification alone. In the included trials, there was no difference in terms of keeping participants drop‐free or in terms of mean reduction in intraocular pressure (IOP) from baseline. However, participants who received treatment with iStent in combination with phacoemulsification may have benefited from a modest reduction in number of IOP‐lowering drops used per day, at a time point between six to 18 months, compared to those who underwent phacoemulsification alone. We have previously reported that people with glaucoma identify medication burden as an important outcome of their treatment, but rank other outcomes – such as IOP control and daily functioning – as more important (Le 2018b).
Due to substantial heterogeneity, we did not conduct a meta‐analysis of the two studies comparing treatment with iStent to medical therapy. Investigators of those two studies did report, as expected, that no participants who were randomized to medical therapy were drop‐free at 12 months, compared to over 90% in the iStent treatment groups. Additionally, data from one study suggested that treatment of participants with two or with three iStents may be more effective than treatment with one iStent. None of the seven included studies provided information on quality of life, and differences in adverse events between treatment groups were uncertain, given few reported events and wide confidence intervals of estimates. Evidence from 13 ongoing studies awaiting publication, once available, may clarify the harms and benefits of treating people with iStent devices. At present, clinical practice decisions should be based on provider judgment and patient preferences, given inconsistency in results and risk of bias in relevant studies published to date.
Implications for research.
Given the large and increasing burden of glaucoma and growing interest in minimally invasive glaucoma surgical procedures involving devices such as the iStent, future research should evaluate the effects of these interventions on outcomes that are meaningful both clinically and to patients and regulators (Le 2016). IOP control – both percent lowering and absolute lowering – and medication burden are clinically meaningful outcomes and have been reported in all but two of the included trials: Fernandez‐Barrientos 2010 reported mean IOP but not IOP lowering and NCT00721968 reported absolute IOP change but not percentage change. Safety outcomes are important to all stakeholders, but were only explicitly reported in two of the included trials (Samuelson 2011; Vold 2016).
Our findings show that no trials reported how the iStent affects quality of life. RCTs measuring outcomes that are important to patients can better inform regulatory decision‐making, reimbursements, and other policy changes (Le 2016; Le 2018a). As a first step, research exploring patients' preferences in glaucoma therapy could identify or clarify outcomes that are important to those facing treatment decisions in the glaucoma clinic (Le 2018b; Tarver 2017). For example, in one survey of 274 patients seeking care in an academic glaucoma clinic, we observed that participants identified reduction in number of IOP‐lowering medications as less important to them than maintaining the ability to perform vision‐dependent activities, such as driving during the day or walking outside (Le 2018b). Functional outcome domains such as driving and mobility can be measured as patient‐reported outcomes. In situations where data for clinical effectiveness may be unclear for a treatment, patient‐reported outcomes and outcomes that reflect patients' preferences directly may provide a way to demonstrate the utility that treatment
Acknowledgements
We thank Kay Dickersin, Henry Jampel, Gus Gazzard, Barbara Hawkins, Kuang Hu, Riaz Qureshi, Anupa Shah, and Richard Wormald for their comments and suggestions during title registration and preparation of this review. This protocol was adapted from a Cochrane Review assessing the evidence for another minimally invasive glaucoma surgical (MIGS) procedure (Hu 2016). Cochrane Eyes and Vision (CEV) created and executed the electronic search strategies. We thank Anupa Shah for assisting with the review process. We thank Nitin Anand for peer reviewing the protocol and Jennifer Evans for her comments on the protocol. We are grateful to the following peer reviewers for their time and comments on the review: Henry Jampel, Ian Pitha, and Mark Lesselroth.
We thank the members of the MIGS Consortium for their input in this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Glaucoma, Open‐Angle] explode all trees #2 MeSH descriptor: [Intraocular Pressure] explode all trees #3 MeSH descriptor: [Ocular Hypertension] explode all trees #4 OAG or POAG or IOP or OHT #5 simple near/3 glaucoma* #6 open near/2 angle near/2 glaucoma* #7 chronic near/2 glaucoma* #8 secondary near/2 glaucoma* #9 low near/2 tension near/2 glaucoma* #10 low near/2 pressure near/2 glaucoma* #11 normal near/2 tension near/2 glaucoma* #12 normal near/2 pressure near/2 glaucoma* #13 pigment near/2 glaucoma* #14MeSH descriptor: [Exfoliation Syndrome] this term only #15 exfoliat* near/2 syndrome* #16 exfoliat* near/2 glaucoma* #17 pseudoexfoliat* near/2 syndrome* #18 pseudoexfoliat* near/2 glaucoma* #19 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 #20 MeSH descriptor: [Stents] explode all trees #21 iStent #22 #20 or #21 #23 #19 and #22
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp glaucoma open angle/ 14. exp intraocular pressure/ 15. ocular hypertension/ 16. (OAG or POAG or IOP or OHT).tw. 17. (simple$ adj3 glaucoma$).tw. 18. (open adj2 angle adj2 glaucoma$).tw. 19. (primary adj2 glaucoma$).tw. 20. (chronic adj2 glaucoma$).tw. 21. (secondary adj2 glaucoma$).tw. 22. (low adj2 tension adj2 glaucoma$).tw. 23. (low adj2 pressure adj2 glaucoma$).tw. 24. (normal adj2 tension adj2 glaucoma$).tw. 25. (normal adj2 pressure adj2 glaucoma$).tw. 26. (pigment$ adj2 glaucoma$).tw. 27. exfoliation syndrome/ 28. (exfoliat$ adj2 syndrome$).tw. 29. (exfoliat$ adj2 glaucoma$).tw. 30. (pseudoexfoliat$ adj2 syndrome$).tw. 31. (pseudoexfoliat$ adj2 glaucoma$).tw. 32. or/13‐31 33. exp Stents/ 34. istent.tw. 35. 33 or 34 36. 32 and 35 37. 12 and 36
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase Ovid search strategy
1. exp randomized controlled trial/ 2. exp randomizations/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. open angle glaucoma/ 34. intraocular pressure/ 35. intraocular hypertension/ 36. (OAG or POAG or IOP or OHT).tw. 37. (open adj2 angle adj2 glaucoma$).tw. 38. (primary adj2 glaucoma$).tw. 39. (chronic adj2 glaucoma$).tw. 40. (secondary adj2 glaucoma$).tw. 41. (low adj2 tension adj2 glaucoma$).tw. 42. (low adj2 pressure adj2 glaucoma$).tw. 43. (normal adj2 tension adj2 glaucoma$).tw. 44. (normal adj2 pressure adj2 glaucoma$).tw. 45. (pigment$ adj2 glaucoma$).tw. 46. exfoliation syndrome/ 47. (exfoliat$ adj2 syndrome$).tw. 48. (exfoliat$ adj2 glaucoma$).tw. 49. (pseudoexfoliat$ adj2 syndrome$).tw. 50. (pseudoexfoliat$ adj2 glaucoma$).tw. 51. or/33‐50 52. Stent/ 53. istent.tw. 54. 52 or 53 55. 51 and 54 56. 32 and 55
Appendix 4. ISRCTN search strategy
iStent
Appendix 5. ClinicalTrials.gov search strategy
iStent
Appendix 6. WHO ICTRP search strategy
iStent
Appendix 7. FDA search strategy
istent AND random OR randomly OR randomised OR randomized
Appendix 8. Data on study characteristics
| Mandatory items | Optional items | |
| Methods | ||
| Study design |
|
Number of study arms Method of randomization Exclusions after randomization Losses to follow‐up Number randomized/analyzed Method of masking How were missing data handled? e.g. available case analysis, imputation methods Reported power calculation (Y/N), if yes, sample size and power Unusual study design/issues |
| Eyes Unit of randomization/unit of analysis |
|
|
| Participants | ||
| Country | — | Setting Ethnic group Method of recruitment Participation rate Equivalence of baseline characteristics (Y/N) Diagnostic criteria |
| Total number of participants | This information should be collected for total study population recruited into the study. If these data are reported for the people who were followed up only, please indicate. | |
| Number (%) of men and women | ||
| Average age and age range | ||
| Inclusion criteria | — | |
| Exclusion criteria | — | |
| Interventions | ||
| Intervention (n= ) Comparator (n= ) See MECIR 65 and 70 |
|
iStent or iStent inject surgical parameters, e.g. degrees of meshwork ablated, electrosurgical power Comparator parameters, e.g. dosage of drugs |
| Outcomes | ||
| Primary and secondary outcomes as defined in study reports See MECIR R70 |
Adverse events reported (Y/N) |
Planned/actual length of follow‐up |
| Notes | ||
| Date conducted | Specify dates of recruitment of participants mm/yr to mm/yr | Full study name: (if applicable) Date of publication Reported subgroup analyses (Y/N) Were trial investigators contacted? |
| Sources of funding | — | |
| Declaration of interest See MECIR 69 |
— | |
Data and analyses
Comparison 1. iStent in combination with phacoemulsification versus phacoemulsification alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants who were drop‐free | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 6 to ≤ 18 months | 2 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.18, 1.63] |
| 2 Mean change in number of intraocular pressure (IOP)‐lowering drops taken per day from baseline | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At ≤ 6 months | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.4 [‐0.82, 0.02] |
| 2.2 At 6 to ≤ 18 months | 3 | 315 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐0.60, ‐0.23] |
| 3 Mean change in IOP | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 At ≤ 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At 6 to ≤ 18 months (unmedicated IOP) | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 iStent in combination with phacoemulsification versus phacoemulsification alone, Outcome 1 Proportion of participants who were drop‐free.
1.2. Analysis.
Comparison 1 iStent in combination with phacoemulsification versus phacoemulsification alone, Outcome 2 Mean change in number of intraocular pressure (IOP)‐lowering drops taken per day from baseline.
1.3. Analysis.
Comparison 1 iStent in combination with phacoemulsification versus phacoemulsification alone, Outcome 3 Mean change in IOP.
Comparison 2. iStent (or iStent inject) versus medical therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants who were drop‐free | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 At 6 to ≤18 months | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 125.43 [17.80, 883.89] |
| 1.2 At 18 to ≤ 36 months | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 84.65 [5.36, 1336.23] |
| 2 Mean change in intraocular pressure | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 At ≤ 6 months | 1 | 184 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.72, 0.92] |
| 2.2 At 6 to ≤ 18 months | 1 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.28, 0.08] |
2.1. Analysis.
Comparison 2 iStent (or iStent inject) versus medical therapy, Outcome 1 Proportion of participants who were drop‐free.
2.2. Analysis.
Comparison 2 iStent (or iStent inject) versus medical therapy, Outcome 2 Mean change in intraocular pressure.
Comparison 3. One iStent versus two iStents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants who were drop‐free | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At ≤ 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 6 to ≤ 18 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 18 to ≤ 36 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 At > 36 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean change in intraoperative pressure | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 At 6 to ≤ 18 months | 1 | 69 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
Comparison 3 One iStent versus two iStents, Outcome 1 Proportion of participants who were drop‐free.
3.2. Analysis.
Comparison 3 One iStent versus two iStents, Outcome 2 Mean change in intraoperative pressure.
Comparison 4. One iStent versus three iStents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants who were drop‐free | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At ≤ 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 6 to ≤ 18 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 18 to ≤ 36 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 At > 36 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean change in intraocular pressure | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 At 6 to ≤ 18 months | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
Comparison 4 One iStent versus three iStents, Outcome 1 Proportion of participants who were drop‐free.
4.2. Analysis.
Comparison 4 One iStent versus three iStents, Outcome 2 Mean change in intraocular pressure.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fea 2010.
| Methods |
Study design: parallel‐group RCT Unit of randomization: participant (1 eye per participant) Number randomized: 36 participants; 12 eyes of 12 participants in the 1 iStent in combination with phacoemulsification group and 24 eyes of 24 participants in phacoemulsification alone group Unit of analysis: participant (1 eye per participant) Number analyzed: 33 total; 12 eyes of 12 participants in the 1 iStent in combination with phacoemulsification group and 21 eyes of 21 participants in phacoemulsification alone group Exclusions and losses to follow‐up: 3 participants in the phacoemulsification alone group (1 capsule rupture, 1 did not present at 6 months' follow‐up, 1 died from complications from ankle surgery) Handling of missing data: analysis excluded participants lost to follow‐up |
|
| Participants |
Country: Torino, Italy (single site) Age (mean ± SD): 64.5 ± 3.4 years (range: 60–70 years) in the 1 iStent in combination with phacoemulsification group; 64.9 ± 3.1 years (range: 59–71 years) in the phacoemulsification alone group Gender: 4 men and 8 women in the 1 iStent in combination with phacoemulsification group; 9 men and 15 women in the phacoemulsification alone group Medicated IOP (mean ± SD): 17.9 ± 2.6 mmHg in the 1 iStent in combination with phacoemulsification group; 17.3 ± 3.0 mmHg in the phacoemulsification alone group Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: POAG and cataract |
|
| Interventions |
Intervention 1: 1 iStent in combination with phacoemulsification Intervention 2: phacoemulsification alone Length of follow‐up: 4 years |
|
| Outcomes |
Primary outcome: mean IOP at 1, 2, 3, 6, 9, 12, 15, and 48 months Secondary outcomes: number and type of glaucoma medication; proportion of participants who "do not require ocular hypotensive medication"; mean change in IOP from baseline; mean IOP "after washout of ocular hypotensive agents"; and need for secondary intervention to control IOP at 1, 2, 3, 6, 9, 12, 15, and 48 months |
|
| Notes |
Type of study: published Enrollment start year: 2006 Funding source: study devices provided by Glaukos Corporation, Laguna Hills, CA, USA; supported by Ricerca Finalizzata Della Regione Piemonte 2007 Disclosures of interest: "The author has no financial or proprietary interest in any material or method mentioned." Publication language: English Trial registration: NCT0084718 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators used a computer random number generator. Quote: "Patient randomization was generated with a 2:1 ratio using Stata data analysis and statistical software." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of outcome assessment (detection bias) Number of IOP‐lowering drops | Unclear risk | Unclear who assessed number of IOP‐lowering drops. |
| Blinding of outcome assessment (detection bias) IOP measurement | Low risk | Investigators noted that "[p]atients were masked to their assignment, as were staff members who measured IOP throughout the study." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/36 participants (all randomized to the phacoemulsification alone group) were excluded from the final analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the results matched those specified in methods and in the ClinicalTrials.gov record. |
Fea 2014.
| Methods |
Study design: parallel‐group RCT Unit of randomization: participant (1 eye per participant) Number randomized: 192 participants; 94 eyes of 94 participants in the iStent inject without combined phacoemulsification group and 98 eyes of 98 participants in the medical therapy group Unit of analysis: participant (1 eye per participant) Number analyzed: 192 participants; 94 eyes of 94 participants in the iStent inject without combined phacoemulsification and 98 eyes of 98 participants in the medical therapy group Exclusions and losses to follow‐up: unclear Handling of missing data: not reported |
|
| Participants |
Country: Italy, Spain, Poland, Germany, the UK, and Armenia (multi‐centered) Age (mean ± SD): 64.5 ± 10.3 years in the iStent inject without combined phacoemulsification group; 64.3 ± 9.8 years in the medical therapy group Gender: 37 men and 57 women in the iStent inject without combined phacoemulsification group; 48 men and 50 women in the medical therapy Medicated IOP (mean ± SD): 21.1 ± 1.7 mmHg in the iStent inject without combined phacoemulsification group; 20.7 ± 1.7 mmHg in the medical therapy group Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: POAG, PEXG, or PG |
|
| Interventions |
Intervention 1: iStent inject without combined phacoemulsification Intervention 2: medical therapy, consisting of a fixed combination of latanoprost/timolol (Xalacom; Pfizer, New York, NY, USA) Length of follow‐up: 4 years |
|
| Outcomes |
Primary outcome: mean IOP at 12 months Secondary outcomes: proportion of participants who achieved an IOP reduction ≥ 20% vs baseline unmedicated IOP; proportion of participants who achieved an IOP reduction ≤ 18 mmHg; mean change in IOP from baseline; proportion of participants who achieved an IOP reduction ≥ 30% vs baseline unmedicated IOP; proportion of participants who achieved an IOP reduction ≥ 40% vs baseline unmedicated IOP; proportion of participants who achieved an IOP reduction ≥ 50% vs baseline unmedicated IOP; and proportion of participants taking medication at 12 months Safety outcomes: vertical C:D ratio; eye burning; medication allergy; soreness/discomfort |
|
| Notes |
Type of study: published Enrollment start year: 2009 Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided study devices, sponsorship for performing this study, editorial assistance, and payment of article processing charges. Disclosures of interest: "The authors report no conflicts of interest in this work." Publication language: English Trial registration: NCT00913029 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of outcome assessment (detection bias) Number of IOP‐lowering drops | High risk | Investigators describe a limitation of the study as "not a masked study due to the disparate forms of therapy." |
| Blinding of outcome assessment (detection bias) IOP measurement | High risk | Investigators describe a limitation of the study as "not a masked study due to the disparate forms of therapy." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Completeness of outcome data varied between time points; some participants omitted from analysis at 1 time point returned at the next. |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the results matched those specified in methods and in the ClinicalTrials.gov record. |
Fernandez‐Barrientos 2010.
| Methods |
Study design: parallel‐group RCT Unit of randomization: participant (1 eye per participant) Number randomized: 33 participants; 17 eyes of 17 participants in the 2 iStents in combination with phacoemulsification group and 16 eyes of 16 participants in the phacoemulsification alone group Unit of analysis: participants (1 eye per participant) Number analyzed: 33 participants; 17 eyes of 17 participants in the 2 iStents in combination with phacoemulsification group and 16 eyes of 16 participants in the phacoemulsification alone group Exclusions and losses to follow‐up: 0 for IOP study; intention‐to‐treat analysis |
|
| Participants |
Country: Madrid, Spain (3 sites) Age (mean ± SD): 75.2 ± 7.2 years (range: 63–86 years) in the 2 iStents in combination with phacoemulsification group; 76.7 ± 5.8 years (range: 64–89 years) in phacoemulsification alone group Gender: 6 men and 11 women in the 2 iStents in combination with phacoemulsification group; 9 men and 7 women in the phacoemulsification alone group Medicated IOP: not reported Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: OHT or OAG in need of cataract surgery |
|
| Interventions |
Intervention 1: 2 iStents in combination with phacoemulsification Intervention 2: phacoemulsification alone Study follow‐up: 12 months |
|
| Outcomes | Mean outflow facility rate; anterior chamber volume; anterior chamber distance; anterior chamber angle; aqueous flow; mean IOP; number of ocular hypotensive medication; BCVA; gonioscopic findings; slit lamp biomicroscopy findings; dilated funduscopic exam; and visual field measurements (Octobpus 301 G1 TOP) at 1, 6, and 12 months | |
| Notes |
Type of study: published Enrollment start year: 2006 Funding source: supported by Glaukos Corporation, Laguna Hills, CA, USA Disclosures of interest: authors disclosed having received financial support, consulting, being the recipient of gifts, or a combination of these from the Glaukos Corporation Publication language: English Trial registration: NCT00326066 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators used a "computer‐generated sequence" to assign eligible participants. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of outcome assessment (detection bias) Number of IOP‐lowering drops | Low risk | Investigators noted that all postoperative evaluations "were performed by the same examiner (YFB), who was masked to the type of surgery performed." |
| Blinding of outcome assessment (detection bias) IOP measurement | Low risk | Investigators noted that all postoperative evaluations "were performed by the same examiner (YFB), who was masked to the type of surgery performed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data for IOP; authors reported conducting an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the results matched those specified in methods and in the ClinicalTrials.gov record. |
Katz 2015.
| Methods |
Study design: parallel‐group RCT Unit of randomization: participant (1 eye per participant) Number randomized: 119 participants; 38 eyes of 38 participants in the 1 iStent without phacoemulsification group, 41 eyes of 41 participants in the 2 iStents without phacoemulsification group, and 40 eyes of 40 participants in the 3 iStents without phacoemulsification group Unit of analysis: participants (1 eye per participant) Number analyzed: 119 participants; 38 eyes of 38 participants in the 1 iStent without phacoemulsification group, 41 eyes of 41 participants in the 2 iStents without phacoemulsification group, and 40 eyes of 40 participants in the 3 iStents without phacoemulsification group Exclusions and losses to follow‐up: complete records through 18 months were available for 119 participants |
|
| Participants |
Country: Yerevan, Armenia (single‐site) Age (mean ± SD): 68.1 ± 9.1 years (range: 49–83 years) in the 1 iStent without phacoemulsification group, 67.8 ± 9.3 years (range: 51–83 years) in the 2 iStents without phacoemulsification group, and 60.9 ± 8.1 years (range: 49–85 years) in the 3 iStents without phacoemulsification group Gender: 23 men and 15 women in the 1 iStent without phacoemulsification group, 21 men and 20 women in the 2 iStents without phacoemulsification group, and 17 men and 23 women in the 3 iStents without phacoemulsification group Medicated IOP (mean ± SD): 19.8 ± 1.3 mmHg in the 1 iStent without phacoemulsification group, 20.1 ± 1.6 mmHg in the 2 iStents without phacoemulsification group, and 20.4 ± 1.8 mmHg in the 3 iStents without phacoemulsification group Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: POAG, PEXG, or PG; and phakic |
|
| Interventions |
Intervention 1: 1 iStent without phacoemulsification Intervention 2: 2 iStents without phacoemulsification Intervention 3: 3 iStents without phacoemulsification Study follow‐up: 5 years |
|
| Outcomes |
Primary outcome: "month 12 IOP reduction from baseline unmedicated IOP of ≤20%, without use of topical ocular medications at 12 months and without secondary glaucoma surgical procedures by the 12‐month visit." Secondary outcomes: "month 12 IOP ≤18 mmHg IOP, without use of topical ocular medications at 12 months and without secondary glaucoma surgical procedures by the 12‐month visit. Additional efficacy measures included proportional analyses of month 12 IOP ≤15 mmHg, without use of topical ocular medications at 12 months and without secondary glaucoma surgical procedures by the 12‐month visit, mean IOP and medication usage through 18 months postoperatively, and month 18 unmedicated mean IOP and change in IOP from preoperative values." Safety outcomes: "assessment of BCVA, visual field via perimetry, slit‐lamp evaluation, C:D ratio estimation, corneal thickness via pachymetry, complications, and ocular adverse events" |
|
| Notes |
Type of study: published (longest follow‐up reported: 42 months) Enrollment start year: 2012 Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided study devices, sponsorship for performing this study, data collection, data management, data analysis, and editorial assistance. Disclosures of interest: authors disclosed having received financial support, consulting, being the recipient of gifts, or a combination of these from the Glaukos Corporation Publication language: English Trial registration: NCT01517477 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of outcome assessment (detection bias) Number of IOP‐lowering drops | High risk | Investigators describe the trial as an "open‐label study design, a single‐site study [which] lack[s] of masking to the study‐treatment groups." |
| Blinding of outcome assessment (detection bias) IOP measurement | High risk | Investigators describe the trial as an "open‐label study design, a single‐site study [which] lack[s] of masking to the study‐treatment groups." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete records through 18 months were available for 119 participants. |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the results matched those specified in methods and in the ClinicalTrials.gov record. |
NCT00721968.
| Methods |
Study design: parallel‐group RCT Unit of randomization: participant (1 eye per participant) Number randomized: 44 participants; 27 eyes of 27 participants in the 1 iStent in combination with phacoemulsification group and 17 eyes of 17 participants in phacoemulsification alone group Unit of analysis: participant (1 eye per participant) Number analyzed: 44 participants; 27 eyes of 27 participants in the 1 iStent in combination with phacoemulsification group and 17 eyes of 17 participants in phacoemulsification alone group Exclusions and losses to follow‐up: 2 participants in the 1 iStent in combination with phacoemulsification group lost to follow‐up Handling of missing data: not reported |
|
| Participants |
Country: US (multiple sites) Age: not reported Gender: 7 men and 20 women in the 1 iStent in combination with phacoemulsification group; 7 men and 10 women in the phacoemulsification alone group Medicated IOP: not reported Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: OAG in need of cataract surgery |
|
| Interventions |
Intervention 1: 1 iStent in combination with phacoemulsification Intervention 2: phacoemulsification alone Length of follow‐up: 1 year |
|
| Outcomes |
Primary outcome: proportion of participants with IOP ≤ 18 mmHg without topical hypotensive medications at 12 months Other outcome: number of ocular hypotensive medications by visit and at 12 months |
|
| Notes |
Type of study: study results available on ClinicalTrials.gov Enrollment start year: 2007 Funding source: supported by Glaukos Corporation, Laguna Hills, CA, USA Disclosures of interest: not reported Publication language: English Trial registration: NCT00721968 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of outcome assessment (detection bias) Number of IOP‐lowering drops | High risk | Masking described as "None (Open Label)." |
| Blinding of outcome assessment (detection bias) IOP measurement | High risk | Masking described as "None (Open Label)." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study results available only on ClinicalTrials.gov. |
| Selective reporting (reporting bias) | Unclear risk | Study results available only on ClinicalTrials.gov. |
Samuelson 2011.
| Methods |
Study design: parallel‐group RCT Unit of randomization: participants (1 eye per participant) Number randomized: 240 participants; 117 eyes of 116 participants in the 1 iStent in combination with phacoemulsification group and 123 eyes of 123 participants in phacoemulsification alone group Unit of analysis: participants (1 eye per participant) Number analyzed: 240 participants (intention‐to‐treat); 117 eyes of 116 participants in the 1 iStent in combination with phacoemulsification group and 123 eyes of 123 participants in phacoemulsification alone group Exclusions and losses to follow‐up: 22 eyes/participants; 15 eyes of 15 participants were excluded (6 in the 1 iStent in combination with phacoemulsification group and 9 in the phacoemulsification group) at 12 months, and 7 eyes of 7 participants were lost to follow‐up (5 in the 1 iStent in combination with phacoemulsification group and 2 in phacoemulsification alone group) at 12 months Handling of missing data: intention‐to‐treat; last observation carried forward analysis |
|
| Participants |
Country: US (29 sites) Age (mean ± SD): 74.0 ± 8.0 years (range: 53–88 years) in combined surgery group; 73.0 ± 9.0 years (range: 48–88 years) in cataract surgery alone group Gender: 46 men and 71 women in the 1 iStent in combination with phacoemulsification group; 52 men and 71 women in the phacoemulsification group Medicated IOP (mean ± SD): 18.7 ± 3.3 mmHg in the 1 iStent in combination with phacoemulsification group; 18.0 ± 3.0 mmHg in the phacoemulsification alone group Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: OAG, PEXG, and PG; and in need of cataract surgery |
|
| Interventions |
Intervention 1: 1 iStent in combination with phacoemulsification Intervention 2: phacoemulsification alone Length of follow‐up: 2 years |
|
| Outcomes |
Primary outcome: proportion of participants with IOP ≤ 21 mmHg without ocular hypotensive medication at 12 and 24 months Secondary outcomes: proportion of participants with ≥ 20% reduction in IOP from baseline without medication; mean reduction in IOP; mean number of ocular hypotensive medications at 12 months; mean decrease in medications from screening; and mean IOP at 12 and 24 months Safety outcomes: loss of BCVA of ≥ 1 line ≥ 3 months postoperative; secondary surgical intervention; infection; elevated IOP requiring treatment with oral or intravenous medication or surgical intervention; stent obstruction; corneal thickness; cataract surgery; mean deviation in visual field; frequently reported postoperative ocular complications (≥ 3%); and other complications at 12 and 24 months |
|
| Notes |
Type of study: published Enrollment start year: 2005 Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided funding/support Disclosures of interest: investigators reported receiving financial support from or consulting for Alcon, Allergan, AMO, AqueSys, Glaukos, iScience, Ivantis, Lumenis, Pfizer, QLT, and Santen; all trial investigators were consultants to Glaukos for the conduct of this study; 4 trial investigators are equity owners of Glaukos Publication language: English Trial registration: NCT00323284 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators noted that a "[c]omputer‐generated randomization was performed (PROC PLAN, PC‐SAS, SAS Inc., Cary NC)." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of outcome assessment (detection bias) Number of IOP‐lowering drops | High risk | Quote: "The study was, by design, open‐label, given that there was no way to mask the treatment to the surgeon during the surgical intervention, or to the observer at the time of gonioscopy." |
| Blinding of outcome assessment (detection bias) IOP measurement | High risk | Quote: "The study was, by design, open‐label, given that there was no way to mask the treatment to the surgeon during the surgical intervention, or to the observer at the time of gonioscopy." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Investigators excluded 22/240 (9%) participants from the 12‐month analysis, among whom 11/117 were in the 1 iStent in combination with phacoemulsification and 11/123 were in cataract surgery alone group. |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the results matched those specified in methods and in the ClinicalTrials.gov record. |
Vold 2016.
| Methods |
Study design: parallel‐group RCT Unit of randomization: participant (1 eye per participant) Number randomized: 101 participants; 54 eyes of 54 participants in the 2 iStents without combined phacoemulsification and 47 eyes of 47 participants in medical therapy group Unit of analysis: participant (1 eye per participant) Number analyzed: 73 participants; 39 eyes of 94 participants in the 2 iStents without combined phacoemulsification and 34 eyes of 34 participants in medical therapy group Exclusions and losses to follow‐up: unclear; data not available for 28 participants at 36 months Handling of missing data: available case |
|
| Participants |
Country: Yerevan, Armenia (single site) Age (mean ± SD): 64.5 ± 11.1 years in the 2 iStents without combined phacoemulsification group; 62.0 ± 11.1 years in the medical therapy group Gender: 37 men and 57 women in the 2 iStents without combined phacoemulsification group; 48 men and 50 women in the medical therapy Medicated IOP (mean ± SD): not applicable; enrolled only treatment‐naïve participants Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: POAG, PEXG, or PG |
|
| Interventions |
Intervention 1: 2 iStents without combined phacoemulsification Intervention 2: medical therapy, consisting of topical travoprost (Travatan 0.004%; Alcon, Fort Worth, TX, USA) Length of follow‐up: 3 years |
|
| Outcomes |
Primary outcomes: mean IOP regardless of additional medical therapy; mean IOP in eyes that had not received additional therapy after initial treatment at 12, 24, and 36 months Secondary outcomes: proportion of eyes with postoperative IOP ≤ 18 mmHg without additional medical therapy; proportion of eyes with postoperative IOP ≤ 15 mmHg without additional medical therapy; number of participants for whom medication had been added; at 12, 24, and 36 months Safety outcomes: adverse events and complications; mean deviation in visual field; mean C:D ratio; mean central corneal thickness; proportion of participants with BCVA 20/40 or better, 20/100, and 20/100 separately; at 12, 24, and 36 months; proportion of participants with progression of cataract and proportion of participants with need for cataract surgery at 36 months |
|
| Notes |
Type of study: published Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided funding/support Disclosures of interest: investigators reported receiving non‐financial, financial, and non‐study financial support from Glaukos Publication language: English Trial registration: NCT01443988 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of outcome assessment (detection bias) Number of IOP‐lowering drops | High risk | Investigators described the study as employing an "open‐label unmasked strategy" and "neither subjects nor clinicians were masked to treatment." |
| Blinding of outcome assessment (detection bias) IOP measurement | High risk | Investigators described the study as employing an "open‐label unmasked strategy" and "neither subjects nor clinicians were masked to treatment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Available case analysis of 73/101 (72%) participants at 36 months. |
| Selective reporting (reporting bias) | Unclear risk | Some differences between trial registration and trial report: outcomes modified screening in mean diurnal IOP (mmHg) at the month 12 visit to mean IOP reduction at 3 years; diurnal measurements of IOP were not performed. |
BCVA: best‐corrected visual acuity; C:D: cup‐to‐disk; IOP: intraocular pressure; OAG: open‐angle glaucoma; OHT: ocular hypertension; PEXG: pseudoexfoliative glaucoma; PG: pigmentary glaucoma; POAG: primary open‐angle glaucoma; RCT: randomized controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bacharach 2014 | Not a randomized controlled trial |
| NCT03274323 | Study was withdrawn before enrolling participants |
| Vlasov 2017 | Not a randomized controlled trial |
Characteristics of ongoing studies [ordered by study ID]
EUCTR2009‐018066‐36‐ES.
| Trial name or title | Evaluación aleatorizada prospectiva en abierto del iStent® (GTS400) frente a dos agentes hipotensores oculares en pacientes con glaucoma primario de ángulo abierto – second line study [Open prospective randomized evaluation of iStent® (GTS400) versus two ocular hypotensive agents in patients with primary open‐angle glaucoma] |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Handling of missing data: not reported |
| Participants |
Country: Spain Age: not reported Gender: not reported Inclusion criteria:
Exclusion criteria: not reported (in Spanish) Diagnoses in participants: OAG |
| Interventions |
Intervention 1: iStent Intervention 2: latanoprost; timolol Length of follow‐up: not reported |
| Outcomes |
Primary outcomes: Mean IOP at each visit for the study; mean reduction in IOP; percentage of patients achieving an objective IOP> 18 mmHG; responses to the patient's questionnaire Secondary outcomes: not reported Safety outcomes: not reported |
| Starting date | 11 March 2010 |
| Contact information | No contact details |
| Notes |
Type of study: awaiting classification Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided funding/support Disclosures of interest: not reported Publication language: not reported Trial registration: EudraCT Number 2009‐018066‐36 |
NCT00326079.
| Trial name or title | A study of the Glaukos trabecular micro‐bypass stent in open angle glaucoma subjects 1 stent versus 2 |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Handling of missing data: not reported |
| Participants |
Country: Austria, Germany, Spain, Turkey Age: not reported Gender: not reported Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: OAG |
| Interventions |
Intervention 1: implantation of 1 Glaukos iStent Intervention 2: implantation of 2 Glaukos iStents Length of follow‐up: 2 years |
| Outcomes |
Primary outcomes: efficacy at 2 years Secondary outcomes: IOP measurement from preoperative baseline; incidence of adverse events during the surgical procedure and postoperative period Safety outcomes: (see secondary outcomes) |
| Starting date | August 2004 |
| Contact information | No contact details |
| Notes |
Type of study: awaiting classification – described as unknown on ClinicalTrials.gov Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided funding/support Disclosures of interest: not reported Publication language: not reported Trial registration: NCT00326079 |
NCT01052558.
| Trial name or title | GTS400 stent implantation in conjunction with cataract surgery in subjects with open‐angle glaucoma |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Handling of missing data: not reported |
| Participants |
Country: US Age: not reported Gender: not reported Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: OAG |
| Interventions |
Intervention 1: cataract surgery with subsequent implantation of GTS400 stents Intervention 2: cataract surgery Length of follow‐up: 1 year |
| Outcomes |
Primary outcomes: proportion of participants with 12‐month diurnal IOP ≤ 21 mmHg without use of ocular hypotensive medications for ≥ 4 weeks prior to 12‐month visit Secondary outcomes: not reported Safety outcomes: not reported |
| Starting date | 20 January 2010 |
| Contact information | No contact details |
| Notes |
Type of study: awaiting classification – described as "completed" on ClinicalTrials.gov Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided funding/support Disclosures of interest: not reported Publication language: not reported Trial registration: NCT01052558 |
NCT01252849.
| Trial name or title | Purpose of this study is to evaluate the safety and efficacy of one, two, or three iStents for the reduction of intraocular pressure in open‐angle glaucoma subjects |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Handling of missing data: not reported |
| Participants |
Country: Armenia Age: not reported Gender: not reported Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: POAG |
| Interventions |
Intervention 1: implantation of 1 iStent through a small temporal clear corneal incision Intervention 2: implantation of 2 iStents through a small temporal clear corneal incision Intervention 3: implantation of 3 iStents through a small temporal clear corneal incision Length of follow‐up: 1 year |
| Outcomes |
Primary outcomes: mean diurnal IOP reduction ≥ 20% at month 12 vs baseline at 1 year Secondary outcomes: mean diurnal IOP < 18 mmHg at 1 year Safety outcomes: not reported |
| Starting date | 3 December 2010 |
| Contact information | No contact details |
| Notes |
Type of study: awaiting classification POAG described as "active, not recruiting" on ClinicalTrials.gov Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided funding/support Disclosures of interest: not reported Publication language: not reported Trial registration: NCT01252849 |
NCT01444040.
| Trial name or title | Subjects with open‐angle glaucoma, pseudoexfoliative glaucoma, or ocular hypertension naïve to medical and surgical therapy, treated with two trabecular micro‐bypass stents (iStent inject) or travoprost |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Handling of missing data: not reported |
| Participants |
Country: Armenia Age: not reported Gender: not reported Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: OAG |
| Interventions |
Intervention 1: implantation of 2 iStent inject devices Intervention 2: travoprost drops Length of follow‐up: 5 years |
| Outcomes |
Primary outcomes: change from screening in mean diurnal IOP (mmHg) at 1 year Secondary outcomes: change in mean diurnal IOP vs screening at 2 years; change in screening in time‐wise IOPs at "Various Month 12‐60"; proportion of responders at "Various 12‐60 months." "A responder is defined as a subject with a certain target IOP value or a certain reduction of IOP as compared to screening." Safety outcomes: not reported |
| Starting date | 30 September 2011 |
| Contact information | No contact details |
| Notes |
Type of study: awaiting classification PC‐IOL described as "Active, not recruiting" on ClinicalTrials.gov Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided funding/support Disclosures of interest: not reported Publication language: not reported Trial registration: NCT01444040 |
NCT01444105.
| Trial name or title | Open‐angle glaucoma subjects on one ocular hypotensive medication randomized to treatment with two trabecular micro‐bypass stents or selective laser trabeculoplasty |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Handling of missing data: not reported |
| Participants |
Country: Armenia Age: not reported Gender: not reported Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: OAG |
| Interventions |
Intervention 1: implantation of 2 iStent devices Intervention 2: SLT laser treatment Length of follow‐up: not reported |
| Outcomes |
Primary outcomes: change from baseline in mean diurnal IOP (mmHg) at 1 year Secondary outcomes: not reported Safety outcomes: not reported |
| Starting date | 30 September 2011 |
| Contact information | No contact details |
| Notes |
Type of study: awaiting classification, PC‐IOL described as "active, not recruiting" on ClinicalTrials.gov Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided funding/support Disclosures of interest: not reported Publication language: not reported Trial registration: NCT01444105 |
NCT01455467.
| Trial name or title | Open‐angle glaucoma subjects on one topical hypotensive medication randomized to treatment with one or two trabecular micro‐bypass stents in conjunction with cataract surgery |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Handling of missing data: not reported |
| Participants |
Country: Armenia Age: not reported Gender: not reported Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: OAG |
| Interventions |
Intervention 1: implantation of 1 iStent in conjunction with cataract surgery Intervention 2: implantation of 2 iStent in conjunction with cataract surgery Length of follow‐up: 1 year |
| Outcomes |
Primary outcomes: mean diurnal IOP reduction of ≥ 20% vs baseline mean diurnal IOP at 1 year Secondary outcomes: not reported Safety outcomes: not reported |
| Starting date | 20 October 2011 |
| Contact information | No contact details |
| Notes |
Type of study: awaiting classification, described as "active, not recruiting" on ClinicalTrials.gov Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided funding/support Disclosures of interest: not reported Publication language: not reported Trial registration: NCT01455467 |
NCT01461291.
| Trial name or title | Multicenter study using Glaukos® trabecular micro‐bypass stent model GTS400 using the G2‐M‐IS injector system in conjunction with cataract surgery |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Handling of missing data: not reported |
| Participants |
Country: US Age: not reported Gender: not reported Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: POAG |
| Interventions |
Intervention 1: implantation of 2 GTS400 stents using G2‐M‐IS iStent inject Intervention 2: cataract surgery alone Length of follow‐up: 2 years |
| Outcomes |
Primary outcomes: ≥ 20% reduction in IOP at 2 years Secondary outcomes: diurnal IOP reduction from baseline at 2 years Safety outcomes: not reported |
| Starting date | 28 October 2011 |
| Contact information | No contact details |
| Notes |
Type of study: awaiting classification, described as "active, not recruiting" on ClinicalTrials.gov Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided funding/support Disclosures of interest: not reported Publication language: not reported Trial registration: NCT01461291 |
NCT02023242.
| Trial name or title | Comparing effectiveness of the Hydrus Microstent (TM) to two iStents to lower IOP in phakic eyes (COMPARE) |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Handling of missing data: not reported |
| Participants |
Country: US Age: not reported Gender: not reported Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: OAG |
| Interventions |
Intervention 1: Hydrus Microstent Intervention 2: iStent trabecular micro bypass Length of follow‐up: 2 years |
| Outcomes |
Primary outcomes: difference in proportion of participants unmedicated at 12 months following surgery Secondary outcomes: mean medication use at 12 and 24 months post procedure Safety outcomes: not reported |
| Starting date | 30 December 2013 |
| Contact information | Principal Investigator: Julian Garcia Feijoo, Professor of Medicine, Madrid, Spain |
| Notes |
Type of study: awaiting classification, described as "completed" on ClinicalTrials.gov Funding source: Ivantis, Inc Disclosures of interest: not reported Publication language: not reported Trial registration: NCT02023242 |
NCT02024464.
| Trial name or title | Comparing Hydrus Microstent(TM) to the iStent for lowering IOP in glaucoma patients undergoing cataract surgery |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Handling of missing data: not reported |
| Participants |
Country: US Age: not reported Gender: not reported Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: POAG, PXG, PDG |
| Interventions |
Intervention 1: Hydrus Microstent Intervention 2: iStent trabecular micro bypass Length of follow‐up: 2 years |
| Outcomes |
Primary outcomes: IOP at 2 years Secondary outcomes: proportion of participants requiring supplemental medication and loss of BCVA at 2 years Safety outcomes: not reported |
| Starting date | 31 December 2013 |
| Contact information | Principal Investigator: Iqbal K Ahmed, MD, Mississauga, ON, Canada |
| Notes |
Type of study: awaiting classification, described as "active, not recruiting" on ClinicalTrials.gov Funding source: Ivantis, Inc Disclosures of interest: not reported Publication language: not reported Trial registration: NCT02024464 |
NCT02327312.
| Trial name or title | Multicenter investigation of trabecular micro‐bypass stents vs. laser trabeculoplasty |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Handling of missing data: not reported |
| Participants |
Country: US Age: not reported Gender: not reported Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: POAG |
| Interventions |
Intervention 1: 2 trabecular meshwork micro‐bypass stents into the study eye Intervention 2: laser trabeculoplasty Length of follow‐up: 2 years |
| Outcomes |
Primary outcomes: IOP reduction up to 2 years Secondary outcomes: % IOP reduction up to 2 years Safety outcomes: not reported |
| Starting date | 30 December 2014 |
| Contact information | Jeff Wells jwells@glaukos.com |
| Notes |
Type of study: awaiting classification, described as "recruiting" on ClinicalTrials.gov Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided funding/support Disclosures of interest: not available Publication language: not available Trial registration: NCT02327312 |
NCT03106181.
| Trial name or title | A comparison of cataract surgery alone and cataract surgery with iStent |
| Methods |
Study design: parallel‐group RCT Unit of randomization: unclear Number randomized: not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Handling of missing data: not reported |
| Participants |
Country: Australia Age: not reported Gender: not reported Inclusion criteria:
Exclusion criteria:
Diagnoses in participants: OAG |
| Interventions |
Intervention 1: iStent inject Intervention 2: phacoemulsification Length of follow‐up: 2 years |
| Outcomes |
Primary outcomes: IOP reduction up to 2 years Secondary outcomes: number of glaucoma medications; participant treatment satisfaction up to 2 years Safety outcomes: not reported |
| Starting date | 10 April 2017 |
| Contact information | Jennifer C Fan Gaskin, FRANZCO drjfan@gmail.com |
| Notes |
Type of study: awaiting classification, described as "recruiting" on ClinicalTrials.gov Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided funding/support Disclosures of interest: not reported Publication language: not reported Trial registration: NCT03106181 |
UMIN000027734.
| Trial name or title | A randomized study of efficacy of trabecular micro bypass stent with cataract surgery for open angle glaucoma |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Handling of missing data: not reported |
| Participants |
Country: Japan Age: not reported Gender: not reported Inclusion criteria: not reported Exclusion criteria: not reported Diagnoses in participants: OAG |
| Interventions |
Intervention 1: iStent during cataract surgeries Intervention 2: no iStent during cataract surgeries Length of follow‐up: 1 year |
| Outcomes |
Primary outcomes: IOP post cataract operation at 1, 2, 3, 6, and 12 months Secondary outcomes: not reported Safety outcomes: not reported |
| Starting date | 12 June 2017 |
| Contact information | Yoshiaki Kiuchi Ykiuchi@hiroshma‐u.ac.jp |
| Notes |
Type of study: awaiting classification Funding source: Glaukos Corporation, Laguna Hills, CA, USA provided funding/support Disclosures of interest: not reported Publication language: not reported Trial registration: UMIN000027734 |
AC‐IOL: anterior chamber intraocular lens; ALT: alanine aminotransferase; BCVA: best‐corrected visual acuity; IOP: intraocular pressure; OAG: open‐angle glaucoma; OHT: ocular hypertension; PC‐IOL: posterior chamber intraocular lens; PDG: pigmentary dispersion glaucoma; PEXG: pseudoexfoliative glaucoma; PG: pigmentary glaucoma; POAG: primary open‐angle glaucoma; RCT: randomized controlled trial; SD: standard deviation; SLT: selective laser trabeculoplasty.
Differences between protocol and review
The protocol for this review did not specify time windows for the outcomes analyzed. Working with other author teams of the MIGS Consortium, we clarified time windows of interest as short‐term (six months or less), medium‐term (six to 18 months or less), long‐term (greater than 18 months but less than or equal to 36 months), and over 36 months. When conducting meta‐analyses, we used fixed‐effect models in lieu random‐effects models due to small numbers of randomized controlled trials included. Given that the iStent devices were approved in people undergoing cataract surgery (phacoemulsification), we regarded combined phacoemulsification and MIGS procedure versus phacoemulsification alone as a separate comparison instead of a subgroup analysis.
Contributions of authors
JL, AB, and TL designed and wrote the review. JL, LW, and TL screened studies for inclusion. JL and LW extracted data from studies. JL, AB, LW, and TL drafted the review and will be responsible for updates.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Eye Institute (NEI), National Institutes of Health (NIH), USA.
Cochrane Eyes and Vision US Project (and JL during his time with the Project) is supported by cooperative agreement UG1EY020522, NEI, NIH
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
National Institute of Aging (NIA), National Institutes of Health (NIH), USA.
JL is supported by the Epidemiology and Biostatistics of Aging Training Program, Grant Number T32 AG000247 from the NIA, NIH.
Declarations of interest
JL: This Cochrane Review was prepared while Dr. Le was a doctoral candidate at the Johns Hopkins Bloomberg School of Public Health. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government. AB: none. LW: none. TL: none.
New
References
References to studies included in this review
Fea 2010 {published data only}
- Fea A, Lacroix G, Ghennadian S, Brogliatti B, Grignolo F. A prospective, randomized, double blind 15‐month pilot study on the efficacy of trabecular stent (iStent) implantation with combined phacoemulsification. American Academy of Ophthalmology 2008:214. [Google Scholar]
- Fea AM. Phacoemulsification versus phacoemulsification with micro‐bypass stent implantation in primary open‐angle glaucoma: randomized double‐masked clinical trial. Journal of Cataract and Refractive Surgery 2010;36(3):407‐12. [DOI] [PubMed] [Google Scholar]
- Fea AM, Consolandi G, Zola M, Pignata G, Cannizzo P, Lavia C, et al. Micro‐bypass implantation for primary open‐angle glaucoma combined with phacoemulsification: 4‐year follow‐up. Journal of Ophthalmology 2015;2015:795357. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00847158. A clinical trial of phacoemulsification versus phacoemulsification and the iStent implantation in POAG patients. clinicaltrials.gov/ct2/show/NCT00847158 (first received 9 February 2009).
Fea 2014 {published data only}
- Fea AM, Belda JI, Rekas M, Junemann A, Chang L, Pablo L, et al. Prospective unmasked randomized evaluation of the iStent inject ((R)) versus two ocular hypotensive agents in patients with primary open‐angle glaucoma. Clinical Ophthalmology 2014;8:875‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00913029. Evaluation of the iStent versus two ocular hypotensive agents in patients with primary open‐angle glaucoma (POAG) (SL). clinicaltrials.gov/ct2/show/study/NCT00913029 (first received 3 June 2009).
Fernandez‐Barrientos 2010 {published data only}
- Fernandez‐Barrientos Y, Garcia‐Feijoo J, Martinez‐de‐la‐Casa JM, Pablo LE, Fernandez‐Perez C, Garcia Sanchez J. Fluorophotometric study of the effect of the Glaukos trabecular microbypass stent on aqueous humor dynamics. Investigative Ophthalmology and Visual Science 2010;51(7):3327‐32. [DOI] [PubMed] [Google Scholar]
- NCT00326066. A study of the iStent in combo with cataract surgery in newly diagnosed open angle glaucoma or OH patients. clinicaltrials.gov/ct2/show/NCT00326066 (first received 13 May 2006).
Katz 2015 {published data only}
- Katz LJ, Erb C, Carceller GA, Fea AM, Voskanyan L, Wells JM, et al. Prospective, randomized study of one, two, or three trabecular bypass stents in open‐angle glaucoma subjects on topical hypotensive medication. Clinical Ophthalmology 2015;9:2313‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LJ, Erb C, Carceller Guillamet A, Fea AM, Voskanyan L, Giamporcaro JE, et al. Long‐term titrated IOP control with one, two, or three trabecular micro‐bypass stents in open‐angle glaucoma subjects on topical hypotensive medication: 42‐month outcomes. Clinical Ophthalmology 2018;12:255‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01517477. One, two, or three iStents for the reduction of intraocular pressure in open‐angle glaucoma subjects. clinicaltrials.gov/ct2/show/NCT01517477 (first received 25 January 2012).
NCT00721968 {unpublished data only}
- NCT00721968. Safety and efficacy of the GTS400 stent in conjunction with cataract. clinicaltrials.gov/ct2/show/NCT00721968 (first received 6 March 2014).
Samuelson 2011 {published data only}
- Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro‐bypass stent implantation in patients with mild‐to‐moderate open‐angle glaucoma and cataract: two‐year follow‐up. Journal of Cataract and Refractive Surgery 2012;38(8):1339‐45. [DOI] [PubMed] [Google Scholar]
- NCT00323284. A study of the trabecular micro‐bypass stent in combination with cataract surgery in subjects with open angle glaucoma. clinicaltrials.gov/ct2/show/NCT00323284 (first received 4 October 2012).
- Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro‐bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology 2011;118(3):459‐67. [DOI] [PubMed] [Google Scholar]
Vold 2016 {published data only}
- NCT01443988. Subjects with open‐angle glaucoma, pseudoexfoliative glaucoma, or ocular hypertension naïve to medical and surgical therapy, treated with two trabecular micro‐bypass stents (iStent) or travoprost. clinicaltrials.gov/ct2/show/NCT01443988 (first received 30 September 2011).
- Vold SD, Voskanyan L, Tetz M, Auffarth G, Masood I, Au L, et al. Erratum to: newly diagnosed primary open‐angle glaucoma randomized to 2 trabecular bypass stents or prostaglandin: outcomes through 36 months. Ophthalmology and Therapy 2016; Vol. 5, issue 2:173. [DOI] [PMC free article] [PubMed]
- Vold SD, Voskanyan L, Tetz M, Auffarth G, Masood I, Au L, et al. Newly diagnosed primary open‐angle glaucoma randomized to 2 trabecular bypass stents or prostaglandin: outcomes through 36 months. Ophthalmology and Therapy 2016;5(2):161‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bacharach 2014 {published data only}
NCT03274323 {published data only}
- NCT03274323. Trabeculectomy versus 2‐iStent and prostaglandin study (TIPS). clinicaltrials.gov/ct2/show/NCT03274323 (first received 6 September 2017).
Vlasov 2017 {published data only}
- Vlasov A, Kim WI. The efficacy of two trabecular bypass stents compared to one in the management of open‐angle glaucoma. Military Medicine 2017;182(S1):222‐5. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
EUCTR2009‐018066‐36‐ES {published data only}
- EUCTR2009‐018066‐36‐ES. Open prospective randomized evaluation of iStent® (GTS400) versus two ocular hypotensive agents in patients with primary open‐angle glaucoma (Google translation) [Evaluación aleatorizada prospectiva en abierto del iStent® (GTS400) frente a dos agentes hipotensores oculares en pacientes con glaucoma primario de ángulo abierto – second line study]. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009‐018066‐36‐ES (first received 11 March 2010).
NCT00326079 {published data only}
- NCT00326079. A study of the Glaukos trabecular micro‐bypass stent in open angle glaucoma subjects 1 stent versus 2. clinicaltrials.gov/ct2/show/study/NCT00326079 (first received 16 May 2016).
NCT01052558 {published data only}
- NCT01052558. GTS400 stent implantation in conjunction with cataract surgery in subjects with open‐angle glaucoma. clinicaltrials.gov/ct2/show/NCT01052558 (first received 20 January 2010).
NCT01252849 {published data only}
- NCT01252849. Purpose of this study is to evaluate the safety and efficacy of one, two, or three iStents for the reduction of intraocular pressure in open‐angle glaucoma subjects. clinicaltrials.gov/ct2/show/NCT01252849 (first received 3 December 2010).
NCT01444040 {published data only}
- NCT01444040. Subjects with open‐angle glaucoma, pseudoexfoliative glaucoma, or ocular hypertension naïve to medical and surgical therapy, treated with two trabecular micro‐bypass stents (iStent inject) or travoprost. clinicaltrials.gov/ct2/show/NCT01444040 (first received 30 September 2011).
NCT01444105 {published data only}
- NCT01444105. Open‐angle glaucoma subjects on one ocular hypotensive medication randomized to treatment with two trabecular micro‐bypass stents or selective laser trabeculoplasty. clinicaltrials.gov/ct2/show/NCT01444105 (first received 30 September 2011).
NCT01455467 {published data only}
- NCT01455467. Open‐angle glaucoma subjects on one topical hypotensive medication randomized to treatment with one or two trabecular micro‐bypass stents in conjunction with cataract surgery. clinicaltrials.gov/ct2/show/NCT01455467 (first received 20 October 2011).
NCT01461291 {published data only}
- NCT01461291. Multicenter study using Glaukos® trabecular micro‐bypass stent model GTS400 using the G2‐M‐IS injector system in conjunction with cataract surgery. clinicaltrials.gov/ct2/show/NCT01461291 (first received 28 October 2011).
NCT02023242 {published data only}
- NCT02023242. Comparing effectiveness of the Hydrus Microstent (TM) to two iStents to lower IOP in phakic eyes (COMPARE) [A prospective, multicenter, randomized comparison of the Hydrus to the iStent® for lowering intraocular pressure in primary open angle glaucoma]. clinicaltrials.gov/ct2/show/study/NCT02023242 (first received 30 December 2013).
NCT02024464 {published data only}
- NCT02024464. Comparing Hydrus Microstent(TM) to the iStent for lowering IOP in glaucoma patients undergoing cataract surgery. clinicaltrials.gov/ct2/show/NCT02024464 (first received 31 December 2013).
NCT02327312 {published data only}
- NCT02327312. Multicenter investigation of trabecular micro‐bypass stents vs. laser trabeculoplasty. clinicaltrials.gov/ct2/show/NCT02327312 (first received 30 December 2014).
NCT03106181 {published data only}
- NCT03106181. A comparison of cataract surgery alone and cataract surgery with iStent. clinicaltrials.gov/ct2/show/NCT03106181 (first received 10 April 2017).
UMIN000027734 {published data only}
- UMIN000027734. A randomized study of efficacy of trabecular micro bypass stent with cataract surgery for open angle glaucoma. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000031775 first received 6 June 2017.
Additional references
AAO 2015
- Prum BE Jr, Rosenberg LF, Gedde SJ, Mansberger SL, Stein JD, Moroi SE, et al. Primary open‐angle glaucoma preferred practice pattern® guidelines. Ophthalmology 2016;123(1):P41‐P111. [DOI] [PubMed] [Google Scholar]
AGIS 2000
- AGIS investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. American Journal of Ophthalmology 2000;130(4):429‐40. [DOI] [PubMed] [Google Scholar]
Allingham 2010
- Allingham RR, Damji KF, Freedman SF, Moroi SE, Rhee DJ, Shields MB. Shields Textbook of Glaucoma. 6th Edition. Philadelphia (PA): Wolters Kluwer Health/Lippincott Williams & Wilkins, 2010. [Google Scholar]
Bahler 2012
- Bahler CK, Hann CR, Fjield T, Haffner D, Heitzmann H, Fautsch MP. Second‐generation trabecular meshwork bypass stent (iStent inject) increases outflow facility in cultured human anterior segments. American Journal of Ophthalmology 2012;153(6):1206‐13. [DOI] [PubMed] [Google Scholar]
Brandao 2013
- Brandao LM, Grieshaber MC. Update on minimally invasive glaucoma surgery (MIGS) and new implants. Journal of Ophthalmology 2013;2013:705915. [DOI: 10.1155/2013/705915] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caprioli 2015
- Caprioli J, Kim JH, Friedman DS, Kiang T, Moster MR, Parrish RK 2nd, et al. Special commentary: supporting innovation for safe and effective minimally invasive glaucoma surgery: summary of a joint meeting of the American Glaucoma Society and the Food and Drug Administration, Washington, DC, February 26, 2014. Ophthalmology 2015;122(9):1795‐801. [DOI] [PubMed] [Google Scholar]
Covidence 2015 [Computer program]
- Veritas Health Innovation. Covidence. Version accessed prior to 25 April 2017. Melbourne: Veritas Health Innovation, 2015.
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from handbook.cochrane.org.
EGS 2014
- European Glaucoma Society. Terminology and Guidelines for Glaucoma. 4th Edition. Savona: PubliComm, 2014. [Google Scholar]
Francis 2011
- Francis BA, Singh K, Lin SC, Hodapp E, Jampel HD, Samples JR, et al. Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology 2011;118(7):1466‐80. [DOI] [PubMed] [Google Scholar]
Friedman 2009
- Friedman DS, Okeke CO, Jampel HD, Ying GS, Plyler RJ, Jiang Y, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology 2009;116(6):1097‐105. [DOI] [PubMed] [Google Scholar]
Gedde 2012a
- Gedde SJ, Singh K, Schiffman JC, Feuer WJ. The Tube Versus Trabeculectomy study: interpretation of results and application to clinical practice. Current Opinion in Ophthalmology 2012;23(2):118‐26. [DOI] [PubMed] [Google Scholar]
Gedde 2012b
- Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow‐up. American Journal of Ophthalmology 2012;153(5):804‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Glaukos 2016
- Glaukos Corporation. The iStent procedure. www.glaukos.com/istent (accessed on 27 June 2016).
GRADEpro GDT 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed prior to 14 January 2019. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Guyatt 2011
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence – publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hu 2016
- Hu K, Gazzard G, Bunce C, Wormald R. Ab interno trabecular bypass surgery with Trabectome for open angle glaucoma. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD011693.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2018
- King AJ, Shah A, Nikita E, Hu K, Mulvaney CA, Stead R, Azuara‐Blanco A. Subconjunctival draining minimally‐invasive glaucoma devices for medically uncontrolled glaucoma. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD012742] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirwan 2013
- Kirwan JF, Lockwood AJ, Shah P, Macleod A, Broadway DC, King AJ, et al. Trabeculectomy in the 21st century: a multicenter analysis. Ophthalmology 2013;120(12):2532‐9. [DOI] [PubMed] [Google Scholar]
Klamann 2015
- Klamann MK, Gonnermann J, Pahlitzsch M, Maier AK, Joussen AM, Torun N, et al. iStent inject in phakic open angle glaucoma. Graefe's Archive for Clinical and Experimental Ophthalmology 2015;253(6):941‐7. [DOI] [PubMed] [Google Scholar]
Larsen 2017
Lavia 2017
- Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally‐invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta‐analysis. PloS One 2017;12(8):e0183142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Le 2016
- Le JT, Viswanathan S, Tarver ME, Eydelman M, Li T. Assessment of the incorporation of patient‐centric outcomes in studies of minimally invasive glaucoma surgical devices. JAMA Ophthalmology 2016;134(9):1054‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Le 2018a
- Le JT, Rouse B, Gazzard G. Iridotomy to slow progression of visual field loss in angle‐closure glaucoma. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD012270.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Le 2018b
- Le JT, Bicket, AK Tarver ME, Eydelman M, Li T. Identifying and prioritizing outcomes that matter to patients considering minimally invasive glaucoma surgeries (MIGS) and other treatments for open‐angle glaucoma. American Glaucoma Society Meeting; 2018 Mar 1‐4; New York City (NY). 2018.
Leahy 2015
- Leahy KE, White AJ. Selective laser trabeculoplasty: current perspectives. Clinical Ophthalmology 2015;9:833‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2016
- Li T, Lindsley K, Rouse B, Hong H, Shi Q, Friedman DS, et al. Comparative effectiveness of first‐line medications for primary open‐angle glaucoma: a systematic review and network meta‐analysis. Ophthalmology 2016;123(1):129‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lichter 2001
- Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001;108(11):1943‐53. [DOI] [PubMed] [Google Scholar]
Malvankar‐Mehta 2015
- Malvankar‐Mehta MS, Iordanous Y, Chen YN, Wang WW, Patel SS, Costella J, et al. iStent with phacoemulsification versus phacoemulsification alone for patients with glaucoma and cataract: a meta‐analysis. PloS One 2015;10(7):e0131770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manasses 2016
- Manasses DT, Au L. The new era of glaucoma micro‐stent surgery. Ophthalmology and Therapy 2016;5(2):135‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Medeiros 2015
- Medeiros FA. Biomarkers and surrogate endpoints in glaucoma clinical trials. British Journal of Ophthalmology 2015;99(5):599‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
NEI 2015
- National Eye Institute. Glaucoma, open‐angle. nei.nih.gov/eyedata/glaucoma (accessed 27 October 2015).
NICE 2009
- National Institute for Health and Care Excellence. Glaucoma: diagnosis and management of chronic open angle glaucoma and ocular hypertension. www.nice.org.uk/guidance/CG85/chapter/1‐Guidance#treatment‐for‐people‐with‐coag (accessed 5 June 2017).
Okeke 2009
- Okeke CO, Quigley HA, Jampel HD, Ying GS, Plyler RJ, Jiang Y, et al. Adherence with topical glaucoma medication monitored electronically. The Travatan Dosing Aid study. Ophthalmology 2009;116(2):191‐9. [DOI] [PubMed] [Google Scholar]
Otarola 2017
- Otarola F, Hu K, Gazzard G, Bunce C. Ab interno trabecular bypass surgery with Schlemm´s Canal Microstent (Hydrus) for open angle glaucoma. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD012740] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2015
- Patel V, Hawy E, Waisbourd M, Zangalli C, Shapiro DM, Gupta L, et al. Long‐term outcomes in patients initially responsive to selective laser trabeculoplasty. International Journal of Ophthalmology 2015;8(5):960‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quigley 2006
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology 2006;90(3):262‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quigley 2007
- Quigley HA, Friedman DS, Hahn SR. Evaluation of practice patterns for the care of open‐angle glaucoma compared with claims data: the Glaucoma Adherence and Persistency Study. Ophthalmology 2007;114(9):1599‐606. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rolim de Moura 2007
- Rolim de Moura C, Paranhos A Jr, Wormald R. Laser trabeculoplasty for open angle glaucoma. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003919.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spaeth 2015
- Spaeth GL, Cvintal V, Figueiredo A. Is there a need for new surgical procedures for glaucoma? yes!. Open Ophthalmology Journal 2015;9:101‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tarver 2017
- Tarver M, Eyldelman M. Incorporating patients' perspectives. glaucomatoday.com/2017/04/incorporating‐patients‐perspectives (accessed 15 April 2018).
Tham 2014
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta‐analysis. Ophthalmology 2014;121(11):2081‐90. [DOI] [PubMed] [Google Scholar]
Tóth 2019
- Tóth M, Shah A, Hu K, Bunce C, Gazzard G. Endoscopic cyclophotocoagulation (ECP) for open angle glaucoma and primary angle closure. Cochrane Database of Systematic Reviews 2019, Issue 2. [DOI: 10.1002/14651858.CD012741] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woo 2015
- Woo DM, Healey PR, Graham SL, Goldberg I. Intraocular pressure‐lowering medications and long‐term outcomes of selective laser trabeculoplasty. Clinical and Experimental Ophthalmology 2015;43(4):320‐7. [DOI] [PubMed] [Google Scholar]
Zhang 2015
- Zhang ML, Hirunyachote P, Jampel H. Combined surgery versus cataract surgery alone for eyes with cataract and glaucoma. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD008671.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


