Abstract
Background
The high prevalence of delirium among postoperative patients has increased morbidity and mortality. The kind of drug that can effectively reduce the incidence of delirium has become the focus of discussion in recent years. However, a consensus in this respect has yet to be reached.
Methods
Randomized controlled trials (RCTs) were retrieved from the PubMed, Cochrane Library, ClinicalTrials.gov, and Embase databases from their inception through October 12, 2018. We included RCTs of pharmacological prevention for postoperative delirium in adults (at least 18 years), and the Cochrane risk of bias tool was used to evaluate the methodological quality of trials. The primary outcomes were the risk ratios (RRs) of incidence of postoperative delirium, and the secondary outcomes were the RRs of mortality and adverse events in the intervention and control groups.
Results
Thirty-eight trials, which comprised 20302 patients and 18 different drugs, were included in the analysis. Of the 38 studies, 17 were rated as low risk with respect to methodological quality. Dexmedetomidine administration (RR 0.58, 95%CI 0.44-0.76, P<0.01) was associated with a significantly lower incidence of postoperative delirium than the control conditions. However, the findings from the studies with a low risk of bias did not show a significant difference in this beneficial effect (RR 0.64, 95%CI 0.39-1.04, P=0.07). The antipsychotic drugs olanzapine (RR 0.44, 95%CI 0.30- 0.65, P<0.01) and risperidone (RR 0.42, 95%CI 0.19-0.92, P=0.03) had promising effects, but there was a lack of sufficient evidence to obtain a definitive conclusion. The beneficial effect of other drugs, including haloperidol, methylprednisolone, dexamethasone, gabapentin, ketamine, cyproheptadine, donepezil, hypertonic saline, melatonin, nimodipine, ondansetron, pregabalin, rivastigmine, TJ-54, and tryptophan, was not proven on the basis of present evidence.
Conclusion
Among the pharmacological prophylactic measures for postoperative delirium, dexmedetomidine, olanzapine, and risperidone showed higher efficacy than other drugs. However, more high-quality evidence is needed to confirm these results.
1. Introduction
Delirium, a change in neuropsychiatric state from a previous baseline level of mental function, typically involves a set of symptoms such as changes in arousal, cognitive deficits, and perceptual dysfunction, as well as hallucinations and delusions. Delirium itself is not a disease but rather a set of symptoms. Delirium not only is a challenge for medical staff but also has adverse effects on the duration of the hospital stay and mechanical ventilation and the cognitive state, and delirium contributes to increased morbidity and mortality.
Several classes of drugs, such as α2-receptor agonists, atypical antipsychotics, and sleep-regulatory drugs, have received widespread attention for the potential prevention or treatment of delirium [1].
Dexmedetomidine, an agonist of α2-adrenergic receptors in certain parts of the brain, is an anxiolytic, sedative, and modest analgesic [2, 3]. Dexmedetomidine has been promoted for its ability to achieve sedation without risk of respiratory depression (unlike other commonly used sedatives such as midazolam and propofol) and can achieve levels of semiarousable and cooperative sedation. However, the administration of dexmedetomidine has been associated with hypertension and arrhythmia due to peripheral α2-receptor stimulation [4].
Atypical antipsychotics are less likely to cause extrapyramidal side effects, such as body rigidity, bradykinesia, and involuntary tremors, than haloperidol, one of the most widely used typical antipsychotics [5–7]. Although atypical antipsychotics are deemed safer than typical antipsychotics, they still have the potential to induce severe side effects in accordance with their respective side effect profiles, and they more commonly increase the risk of metabolic side effects, such as weight gain and glycemic and lipid imbalances.
Melatonin, a hormone secreted by the pineal gland, is regarded as an important molecular sleep–wake cycle regulator that is used to treat insomnia [8]. Some studies have shown that low or delayed melatonin levels in elderly patients are associated with delirium in intensive care units [9–11]. Several RCTs have been registered and are ongoing to prove the benefits of melatonin in preventing postoperative delirium.
A number of randomized controlled trials (RCTs) have been published focusing on the pharmacological prevention of postoperative delirium. This systematic review was performed to identify recent advances in the pharmaceutical prophylaxis of postoperative delirium and to offer clinicians an updated summary to help make clinical decisions.
2. Materials and Methods
2.1. Retrieval Protocol and Selection Criteria
We searched MEDLINE, Web of Science, the Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, and Embase through October 12, 2018, for RCTs investigating the prevention of postoperative delirium. We also examined the reference lists of the included relevant RCTs and systematic reviews for additional eligible references. Search terms mainly included delirium, confusion, disorientation, surgery, and RCTs.
RCTs that investigated the pharmacological prevention of postoperative delirium were included, with language restricted to English. Patients were adults (at least 18 years of age) and received drugs in the perioperative phase. Studies were excluded if risk ratios (RRs) for analysis were not available or if they investigated the therapeutic effects of the drugs for emergency agitation and anesthesia. The studies in which several drugs were simultaneously used to prevent postoperative delirium were also excluded.
2.2. Data Extraction and Quality Assessment
Data extraction was conducted independently by the 1st and 2nd authors (Liu Y and Liang Y) with a predesigned spreadsheet, and discrepancies were resolved by a 3rd author (Li XJ).
The Cochrane risk of bias tool was used to assess the risk of bias, with items including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and incomplete outcome data [12]. A risk of bias table was created to display the results of the risk assessment.
2.3. Primary and Secondary Results
The primary outcomes were the RRs of the incidence of postoperative delirium between the intervention and control groups after the patients received the drugs, and the secondary outcomes were the RRs of mortality and adverse events. Other results, such as adverse events, side effects, and hospital stays, were also collected for evaluating the safety of the drugs. To maintain consistency between studies with regard to the control groups, only studies using placebo, normal saline, and blank (meaning “no injection”) as control agents were included in the final data analysis.
2.4. Statistical Analysis
All statistical analyses were completed by Stata 13.0 (Stata Corp., College Station, TX). Considering the clinical heterogeneity between studies, the random effects model using the DerSimonian and Laird method was used to merge data. The heterogeneity was evaluated using the I2 statistic, and I2 > 30% indicated the presence of heterogeneity between studies [13]. Subgroup analyses were adopted to identify the effect of different characteristics of the studies on the results. Publication bias was assessed by Egger's asymmetry test and funnel plots [14]. This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [15].
3. Results
3.1. Search Results and Study Characteristics
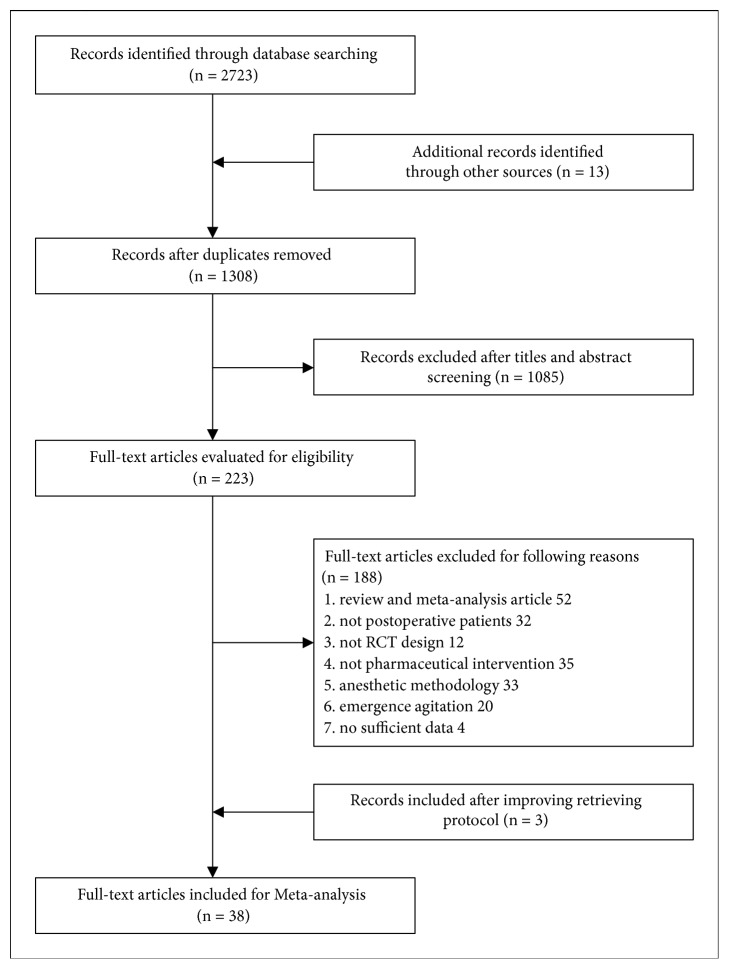
We identified 2723 records, of which 1308 were duplicates (Figure 1). Of the 223 full-text articles reviewed, 38 RCTs were identified as eligible after improving the retrieval protocol [16–53]. Baseline information is listed in Table 1. To maintain consistency between studies, 32 studies involving 19539 patients (including 34 datasets) treated with placebo, normal saline, or blank as controls were included in the final data analysis.
Figure 1.
Flow diagram of the study selection process.
Table 1.
Summary of the baseline characteristics of included trials.
| Study | Country | Dosing Information | Control | Begin Time | Following Time | Type of Surgery | Age (years) |
Male (%) |
Incidence (%) |
N |
|---|---|---|---|---|---|---|---|---|---|---|
| Avidan 2017 [16] |
USA | 0.5 or 1.0 mg/kg ketamine by injection as single dose. | placebo | before surgery | 3d | major surgery | 70 | 62.2 | 19.6 | 672 |
|
| ||||||||||
| Clemmesen 2017 [17] |
Denmark | 125 mg methylprednisolone by injection for once. | saline | before surgery | 3d | hip | 80 | 35.9 | 24.8 | 117 |
|
| ||||||||||
| de Jonghe 2014 [18] |
Netherlands | 3 mg melatonin taken orally for 5 days. | placebo | before surgery | 8d | hip | 84 | 29.9 | 27.5 | 378 |
|
| ||||||||||
| Deiner 2017 [19] |
USA | 0.5 μg/kg/h dexme by infusion continued until 2 hours into recovery. | saline | before surgery | 30d | cardiac | 74 | 48.7 | 15.4 | 390 |
|
| ||||||||||
| Dieleman 2012 [20] |
Netherlands | 1 mg/kg dexamethasone by injection for once. | placebo | during surgery | 30d | cardiac | 66 | 72.5 | 10.4 | 4482 |
|
| ||||||||||
| Dighe 2014 [21] |
Canada | 200 mg gabapentin taken orally tid for 4 days. | placebo | after surgery | NA | knee | 63 | 49.7 | 10.6 | 161 |
|
| ||||||||||
| Djaiani 2016 [22] |
Canada | a bolus of 0.4 μg/kg dexme followed by 0.2-0.7 μg/kg/h infusion for maximum 24 h. | propofol | after surgery | 5d | cardiac | 73 | 75.4 | 24.6 | 183 |
|
| ||||||||||
| Farlinger 2018 [23] |
Canada | 150 mg pregabalin preoperatively and 75 mg bid postoperatively for 7 days. | placebo | before surgery | NA | hip | 60 | 50.9 | 0.6 | 163 |
|
| ||||||||||
| Fukata 2014 [24] |
Japan | 2.5 mg haloperidol for 3 days. | blank | after surgery | 7d | abdominal, orthopedic | 80 | 52.9 | 37.2 | 121 |
|
| ||||||||||
| Gamberini 2009 [25] |
Switzerland | 1.5 mg oral rivastigmine tid for 7 days. | placebo | before surgery | 6d | cardiac | 74 | 68.1 | 31 | 113 |
|
| ||||||||||
| Hudetz 2009 [26] |
USA | 0.5 mg/kg ketamine intravenous bolus for once. | saline | before surgery | 5d | cardiac | 64 | NA | 17.2 | 58 |
|
| ||||||||||
| Kalisvaart 2005 [27] |
Netherlands | 0.5 mg haloperidol tid until 3 days after surgery | saline | before surgery | 14d | hip | 79 | 20.2 | 15.8 | 430 |
|
| ||||||||||
| Kaneko 1999 [28] |
Japan | 5 mg haloperidol intravenous for 5 days | saline | after surgery | 5d | gastrointestinal | 73 | 64.1 | 21.8 | 78 |
|
| ||||||||||
| Larsen 2010 [29] |
USA | 5 mg of orally-disintegrating olanzapine or placebo just before and after surgery. | placebo | before surgery | 8d | joint | 74 | 45.8 | 27.5 | 400 |
|
| ||||||||||
| Lee 2018 [30] |
Korea | 1 μg/kg bolus dexme followed by 0.2-0.7 μg/kg/h infusion during surgery. | saline | before surgery | 5d | laparoscopic | 73 | 44.3 | 17.9 | 318 |
|
| ||||||||||
| Leung 2017 [31] |
USA | 900 mg gabapentin administered preoperatively and for the first 3 postoperative days. | placebo | before surgery | 3d | orthopedic | 73 | 49.6 | 22.4 | 697 |
|
| ||||||||||
| Li.X 2017 [32] |
China | 0.6 μg/kg dexme for 10 minutes followed by 0.4 μg/kg/h during surgery. | placebo | before surgery | 5d | cardiac | 67 | 68.6 | 6.3 | 287 |
|
| ||||||||||
| Li.YN 2017 [33] |
China | 7.5 μg/kg/h nimodipine was injected continually 30minutes before anesthesia induction. | saline | before surgery | 7d | spine | 70 | 40 | 11.7 | 60 |
|
| ||||||||||
| Liu. YZ 2016 [34] |
China | 0.2-0.4 μg/kg/h dexme during surgery | saline | before surgery | 7d | joint | 73 | 48.7 | 29.4 | 197 |
|
| ||||||||||
| Maldonado 2009 [35] |
USA | received one of three postoperative sedation regimens: dexme, propofol and midazolam. | propofol, midazolam | after surgery | 3d | cardiac | 58 | 63.8 | 34.4 | 90 |
|
| ||||||||||
| Mardani 2013 [36] |
Iran | 8 mg of intravenous dexamethasone before surgery and followed by 8 mg q8h for 3 days. | placebo | before surgery | 3d | cardiac | 62 | NA | 18.3 | 93 |
|
| ||||||||||
| Mei 2018 [37] |
China | a bolus of dexme at 0.8-1.0 μg/kg followed by 0.1-0.5 μg/kg/h infusion or propofol. | propofol | before surgery | 7d | hip | 75 | 45.6 | 11.8 | 296 |
|
| ||||||||||
| Mohammadi 2016 [38] |
Iran | 4 mg cyproheptadine tid for 7 days. | placebo | after surgery | 7d | non-cardiac | 60 | 65 | 25 | 40 |
|
| ||||||||||
| Papadopoulos 2014 [39] |
Greece | 8 mg intravenous ondansetron daily for 5 days. | placebo | after surgery | 5d | femoral, hip | 72 | 44.3 | 44.3 | 106 |
|
| ||||||||||
| Park 2014 [40] |
Korea | loading dose of 0.5 μg/kg dexme followed by 0.2-0.8 μg/kg/h or remifentanil 1-2.5 mg/h during ICU. | remifentanil | after surgery | 3d | cardiac | 53 | 55.6 | 16.2 | 142 |
|
| ||||||||||
| Prakanrattana 2007 [41] |
Thailand | 1 mg risperidone sublingually when regained consciousness | placebo | after surgery | 3d | cardiac | 61 | 58.7 | 21.4 | 126 |
|
| ||||||||||
| Priye 2015 [42] |
India | 0.4 μg/kg/h dexme for 12 hours. | saline | after surgery | ICU | cardiac | 43 | 51.6 | 9.4 | 64 |
|
| ||||||||||
| Robinson 2014 [43] |
USA | 1g tryptophan enterally tid for 3 days. | placebo | after surgery | ICU | mixed | 69 | 98 | 38.5 | 301 |
|
| ||||||||||
| Royse 2017 [44] |
Australia | 250 mg methylprednisolone at induction and 250 mg methylprednisolone before surgery. | placebo | during surgery | 3d | cardiac | 74 | 64.1 | 9.2 | 298 |
|
| ||||||||||
| Sampson 2007 [45] |
UK | 5 mg donepezil after surgery and continuing for a further 3 days. | placebo | after surgery | 4d | hip | 69 | 51.5 | 21.2 | 33 |
|
| ||||||||||
| Shehabi 2009 [46] |
Australia | received dexme 0.1–0.7g/kg/h or morphine 10-70g/kg/h to maintain target sedation & analgesia. | morphine | after surgery | 5d | cardiac | 71 | 75.3 | 11.7 | 299 |
|
| ||||||||||
| Sheikh 2018 [47] |
India | 1 μg/kg dexme bolus followed by infusion 0.2–0.6 μg/kg/h or 0.25–1 mg/kg/h propofol | propofol | before surgery | ICU | cardiac | 35 | NA | 13.3 | 60 |
|
| ||||||||||
| Su 2016 [48] |
China | dexme 0.1 μg/kg/h from ICU admission on the day of surgery until 08:00 h on postoperative day 1. | saline | after surgery | 7d | non-cardiac | 74 | 60.4 | 15.9 | 700 |
|
| ||||||||||
| Sugano 2017 [49] |
Japan | 2.5g TJ-54 tid for 7 days prior to surgery and 4 days after surgery, except for the operation day. | blank | before surgery | NA | gastrointestinal, lung | 77 | 64.5 | 8.1 | 186 |
|
| ||||||||||
| Wang 2012 [50] |
China | 0.5 mg haloperidol intravenous bolus followed by infusion at a rate of 0.1 mg/h for 12 hours. | saline | after surgery | 7d | non-cardiac | 74 | 41.1 | 19.3 | 457 |
|
| ||||||||||
| Whitlock 2015 [51] |
Canada | 250 mg methylprednisolone at anaesthetic induction and 250 mg at initiation of cardiopulmonary bypass. | placebo | during surgery | 3d | cardiac | 67 | 60.4 | 7.8 | 7507 |
|
| ||||||||||
| Xin 2017 [52] |
China | 4ml/kg 7.5% hypertonic saline was given before surgery. | saline | before surgery | 3d | hip | 76 | 51.7 | 25 | 120 |
|
| ||||||||||
| Yang 2015 [53] |
China | 0.5 mg/kg dexme was given for 1 hour. | saline | before surgery | 5d | free flap | 50 | 53.2 | 8.9 | 79 |
Note: dexme: dexmedetomidine; N: the number of participants; NA: not available.
3.2. Quality Assessments
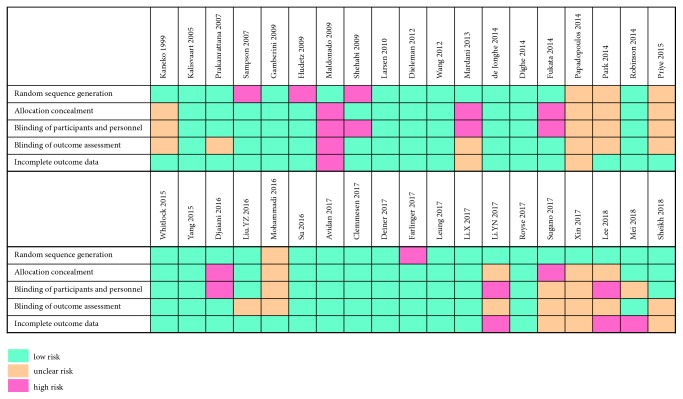
The overall methodological quality of the studies was distributed from low to high (Figure 2). Five items, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and incomplete outcome data, were adequately and unambiguously described in 31 (82%), 25 (66%), 23 (61%), 24 (63%), and 29 (76%) of 38 trials, respectively.
Figure 2.
Summary of risk of bias assessment.
3.3. Prophylactic Efficacy Assessments
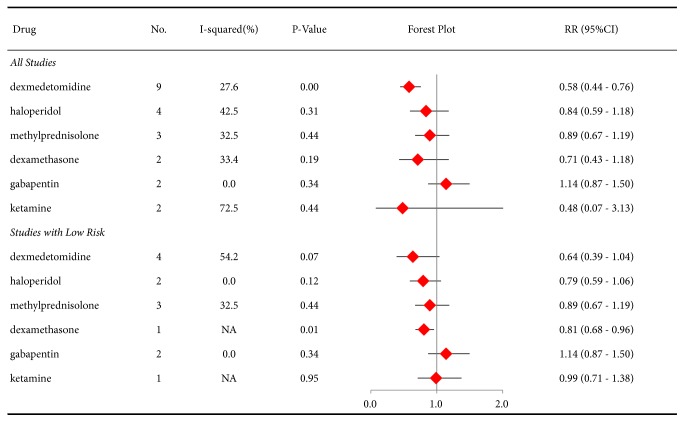
Out of concern about the risk of bias, the RRs for the incidence of postoperative delirium were analyzed at two levels: studies with different levels of bias risk and studies with low risk. First, drugs that were investigated in at least two studies were evaluated, and we found that dexmedetomidine (RR 0.58, 95%CI 0.44-0.76, P<0.01) was associated with the beneficial effect of decreasing the incidence of postoperative delirium, but haloperidol, methylprednisolone, dexamethasone, gabapentin, and ketamine did not display this effect (Figure 3). In contrast, the results of only the studies with low risk showed that dexamethasone (RR 0.81, 95%CI 0.68-0.96, P=0.01) showed a beneficial benefit, while the effects of dexmedetomidine (RR 0.64, 95%CI 0.39-1.04, P=0.07), haloperidol, methylprednisolone, gabapentin, and ketamine were not significantly different from those of controls.
Figure 3.
Forest plot of risk ratios (RRs) for the incidence of postoperative delirium in all studies or studies with a low risk of bias (at least 2 studies for each drug).
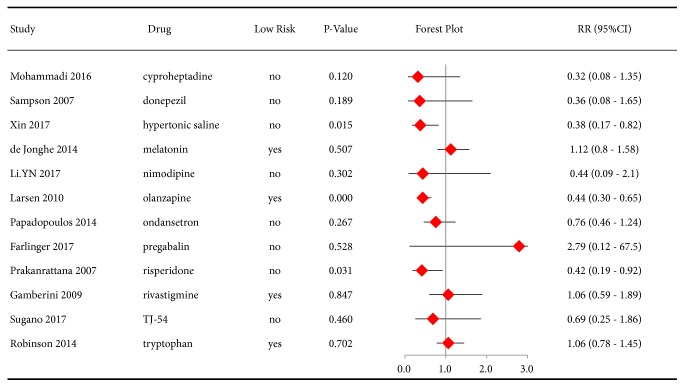
The preventive effect of drugs with 1 eligible study on postoperative delirium was also evaluated. Olanzapine (RR 0.44, 95%CI 0.30-0.65, P<0.01) and risperidone (RR 0.42, 95%CI 0.19-0.92, P=0.03) had protective effects in the prevention of delirium, but cyproheptadine, donepezil, hypertonic saline, melatonin, ondansetron, rivastigmine, TJ-54, and tryptophan did not (Figure 4).
Figure 4.
Forest plot of risk ratios (RRs) for the incidence of postoperative delirium (only one available study for each drug).
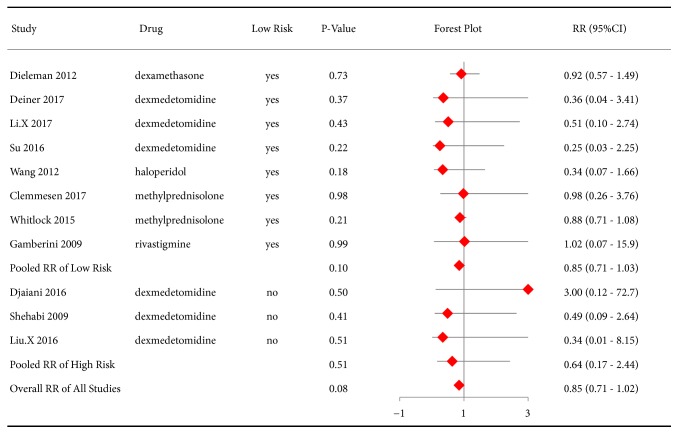
The prophylactic effect of drugs on overall mortality was assessed in our review. The RR from all studies did not show a significant difference between the intervention and control groups (RR 0.85, 95%CI 0.71-1.02, P=0.08). Merging data from the 8 studies with a low risk had a similar result (RR 0.85, 95%CI 0.71-1.03, P=0.10) (Figure 5).
Figure 5.
Forest plot of risk ratios (RRs) for mortality in the included studies.
Adverse events and side effects were also collected to evaluate the balance between the benefits and risks produced by these drugs. We found that dexmedetomidine increased the incidence of bradycardia (RR 1.24, 95%CI 1.01-1.52, P=0.04) and reduced the incidence of tachycardia (RR 0.51, 95%CI 0.32-0.82, P=0.01) and hypertension (RR 0.67, 95%CI 0.52-0.87, P<0.01). Significant differences in adverse events and side effects were not found with the atypical antipsychotics, the acetylcholinesterase inhibitors, ketamine, and the glucocorticoids, as well as for other effects of dexmedetomidine, in part because of insufficient data (Table S2, supplementary materials).
3.4. Subgroup Analysis
We performed the subgroup analysis against only dexmedetomidine because there were 9 datasets, and the other drugs did not have enough data for further analyses. When the datasets were categorized by type of surgery, age, methodological quality, and timing of drug administration, we found that dexmedetomidine had clear protective effects in patients from datasets without cardiac surgery, aged > 65 years, and with insufficient quality. The timing of drug administration, before surgery or after surgery, did not influence postoperative delirium (Table S1, supplementary materials).
3.5. Publication Bias
Egger's test for asymmetry, an indication of publication bias, was performed for all the studies, and P=0.001 indicated significant publication bias among the included studies (Fig. S1, supplementary materials). Nevertheless, the publication bias among studies with low risk did not show a significant difference with P=0.30 (Fig. S2, supplementary materials).
4. Discussion
In this review, we retrieved 38 RCTs from 2723 records investigating the pharmaceutical prevention of postoperative delirium, and 32 of these studies used placebo, saline, or blank as a control. We also systematically evaluated these RCTs of drugs to prevent delirium after surgery, and the overall results showed that α2-adrenergic receptor agonists and atypical antipsychotics could reduce the incidence of postoperative delirium. However, there were no drugs that showed an ability to prevent postoperative delirium based on the evidence from studies with low risk.
Although dexmedetomidine had the advantage of reducing postoperative delirium, the results obtained when all studies, regardless of quality level, were examined were inconsistent with the results obtained when only the high-quality studies were examined. This difference means that a definitive conclusion could not be drawn due to the lack of high-quality evidence. It should be noted that dexmedetomidine has both sedative and analgesic effects, which means that the use of dexmedetomidine can reduce the consumption of other sedative drugs and opioid analgesics, which possibly changes the incidence of delirium in patients and limits our ability to interpret the results [54, 55]. In view of the high risk of delirium with benzodiazepines, dexmedetomidine is deemed to be an alternative to benzodiazepines to achieve the target sedation [56]. High-quality evidence is still needed to determine whether dexmedetomidine can truly reduce the occurrence of delirium when compared with placebo.
Atypical antipsychotics have the risk of serious side effects, such as acute hemorrhagic pancreatitis, status epilepticus, leucopenia, tardive dyskinesia, and neuroleptic malignant syndrome [57]. Although the incidence of these severe adverse events with atypical antipsychotics is lower than that with typical antipsychotics, there is not enough evidence to put atypical antipsychotics into widespread use to prevent delirium in all susceptible patients because of the potential adverse effects [58]. Therefore, the pros and cons of using antipsychotics to prevent and treat delirium need be balanced, and the therapeutic regimens must also be tailored according to the specific situation of individual patients.
This review also has some limitations. First, the relevant information provided by the authors and the evaluation process featured subjectivity, which might lead to a certain degree of deviation from the real situation. Second, some studies had not documented in detail the adverse events and side effects caused by the drugs, which may result in difficulties in weighing the risks and benefits of drug use. Third, although the average age of the patients in 2 of the studies in this review was less than 60 years old, we did not think this difference would influence the results, considering the consistency of the baseline between the intervention and control groups.
Dexmedetomidine and two atypical antipsychotic drugs (olanzapine and risperidone) showed prophylactic effects on postoperative delirium. However, the results of the meta-analysis of all studies on dexmedetomidine were inconsistent with the results from the low-risk studies, and there was not enough evidence to support the use of atypical antipsychotics for preventing delirium. Therefore, we need to carefully understand these results and develop reasonable regimens for delirium prevention according to the specific situation.
Acknowledgments
This research was sponsored by the Sichuan University Spark Project (2018SCUH0031) and the National Natural Science Foundation of China (Grant number 81701880).
Data Availability
All original data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Liu Y and Li XJ were responsible for the conception and design of the study and for the review. Liu Y, Liang Y, and Li XJ extracted and analyzed the data and drafted the manuscript. Kan Y critically revised the manuscript. Yong Liu and Xiao-Jin Li contributed equally to this work.
Supplementary Materials
Table S1: subgroup analyses of studies using dexmedetomidine to prevent postoperative delirium. Table S2: the incidence of adverse events in the intervention and control groups. Figure S1: the funnel plots of the included studies regardless of the risk of bias. Figure S2: the funnel plots of the included studies with low risk.
References
- 1.Gosch M., Nicholas J. A. Pharmacologic prevention of postoperative delirium. Zeitschrift für Gerontologie und Geriatrie. 2014;47(2):105–109. doi: 10.1007/s00391-013-0598-1. [DOI] [PubMed] [Google Scholar]
- 2.Mo Y., Zimmermann A. E. Role of dexmedetomidine for the prevention and treatment of delirium in intensive care unit patients. Annals of Pharmacotherapy. 2013;47(6):869–876. doi: 10.1345/aph.1AR708. [DOI] [PubMed] [Google Scholar]
- 3.Tan J. A., Ho K. M. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Medicine. 2010;36(6):926–939. doi: 10.1007/s00134-010-1877-6. [DOI] [PubMed] [Google Scholar]
- 4.Ebert T. J., Hall J. E., Barney J. A., Uhrich T. D., Colinco M. D. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93(2):382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Leucht S., Corves C., Arbter D., Engel R. R., Li C., Davis J. M. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. The Lancet. 2009;373(9657):31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- 6.Tyrer P., Kendall T. The spurious advance of antipsychotic drug therapy. The Lancet. 2009;373(9657):4–5. doi: 10.1016/S0140-6736(08)61765-1. [DOI] [PubMed] [Google Scholar]
- 7.Orsolini L., Tomasetti C., Valchera A., et al. An update of safety of clinically used atypical antipsychotics. Expert Opinion on Drug Safety. 2016;15(10):1329–1347. doi: 10.1080/14740338.2016.1201475. [DOI] [PubMed] [Google Scholar]
- 8.Chen S., Shi L., Liang F., et al. Exogenous melatonin for delirium prevention: a meta-analysis of randomized controlled trials. Molecular Neurobiology. 2016;53(6):4046–4053. doi: 10.1007/s12035-015-9350-8. [DOI] [PubMed] [Google Scholar]
- 9.Cronin A. J., Keifer J. C., Davies M. F., King T. S., Bixler E. O. Melatonin secretion after surgery. The Lancet. 2000;356(9237):1244–1245. doi: 10.1016/S0140-6736(00)02795-1. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki T., Kuwano H., Kato H., et al. Correlation between serum melatonin circadian rhythm and intensive care unit psychosis after thoracic esophagectomy. Surgery. 2003;133(6):662–668. doi: 10.1067/msy.2003.149. [DOI] [PubMed] [Google Scholar]
- 11.Mistraletti G., Sabbatini G., Taverna M., et al. Pharmacokinetics of orally administered melatonin in critically ill patients. Journal of Pineal Research. 2010;48(2):142–147. doi: 10.1111/j.1600-079X.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 12.Higgins J. P. T., Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. 2011, http://handbook-5-1.cochrane.org.
- 13.Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutton B., Salanti G., Caldwell D. M., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Annals of Internal Medicine. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 16.Avidan M. S., Maybrier H. R., Abdallah A. B., et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. The Lancet. 2017;390(10091):267–275. doi: 10.1016/S0140-6736(17)31467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clemmesen C. G., Lunn T. H., Kristensen M. T., Palm H., Foss N. B. Effect of a single pre-operative 125 mg dose of methylprednisolone on postoperative delirium in hip fracture patients; a randomised, double-blind, placebo-controlled trial. Anaesthesia. 2018;73(11):1353–1360. doi: 10.1111/anae.14406. [DOI] [PubMed] [Google Scholar]
- 18.De Jonghe A., Van Munster B. C., Goslings J. C., et al. Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. Canadian Medical Association Journal. 2014;186(14):E547–E556. doi: 10.1503/cmaj.140495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deiner S., Luo X., Lin H.-M., et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surgery. 2017;152(8):p. e171505. doi: 10.1001/jamasurg.2017.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieleman J. M., Nierich A. P., Rosseel P. M., et al. Intraoperative high-dose dexamethasone for cardiac surgery: A randomized controlled trial. Journal of the American Medical Association. 2012;308(17):1761–1767. doi: 10.1001/jama.2012.14144. [DOI] [PubMed] [Google Scholar]
- 21.Dighe K., Clarke H., McCartney C. J., Wong C. L. Perioperative gabapentin and delirium following total knee arthroplasty: a post-hoc analysis of a double-blind randomized placebo-controlled trial. Canadian Journal of Anesthesia. 2014;61(12):1136–1137. doi: 10.1007/s12630-014-0235-5. [DOI] [PubMed] [Google Scholar]
- 22.Djaiani G., Silverton N., Fedorko L., et al. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology. 2016;124(2):362–368. doi: 10.1097/ALN.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 23.Farlinger C., Clarke H., Wong C. L. Perioperative pregabalin and delirium following total hip arthroplasty: a post hoc analysis of a double-blind randomized placebo-controlled trial. Canadian Journal of Anesthesia. 2018;65(11):1269–1270. doi: 10.1007/s12630-018-1195-y. [DOI] [PubMed] [Google Scholar]
- 24.Fukata S., Kawabata Y., Fujisiro K., et al. Haloperidol prophylaxis does not prevent postoperative delirium in elderly patients: a randomized, open-label prospective trial. Surgery Today. 2014;44(12):2305–2313. doi: 10.1007/s00595-014-0859-7. [DOI] [PubMed] [Google Scholar]
- 25.Gamberini M., Bolliger D., Buse G. A. L., et al. Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery-a randomized controlled trial. Critical Care Medicine. 2009;37(5):1762–1768. doi: 10.1097/CCM.0b013e31819da780. [DOI] [PubMed] [Google Scholar]
- 26.Hudetz J. A., Patterson K. M., Iqbal Z., et al. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia. 2009;23(5):651–657. doi: 10.1053/j.jvca.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 27.Kalisvaart K. J., De Jonghe J. F. M., Bogaards M. J., et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. Journal of the American Geriatrics Society. 2005;53(10):1658–1666. doi: 10.1111/j.1532-5415.2005.53503.x. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko T., Cai J., Ishikura T., Kobayashi M., Naka T., Kaibara N. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta Medica. 1999;42(3):179–184. [Google Scholar]
- 29.Larsen K. A., Kelly S. E., Stern T. A., et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010;51(5):409–418. doi: 10.1016/S0033-3182(10)70723-4. [DOI] [PubMed] [Google Scholar]
- 30.Lee C., Lee C. H., Lee G., Lee M., Hwang J. The effect of the timing and dose of dexmedetomidine on postoperative delirium in elderly patients after laparoscopic major non-cardiac surgery: a double blind randomized controlled study. Journal of Clinical Anesthesia. 2018;47:27–32. doi: 10.1016/j.jclinane.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Leung J. M., Sands L. P., Chen N., et al. Perioperative gabapentin does not reduce postoperative delirium in older surgical patients: a randomized clinical trial. Anesthesiology. 2017;127(4):633–644. doi: 10.1097/ALN.0000000000001804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X., Yang J., Nie X.-L., et al. Impact of dexmedetomidine on the incidence of delirium in elderly patients after cardiac surgery: a randomized controlled trial. PLoS ONE. 2017;12(2):p. e0170757. doi: 10.1371/journal.pone.0170757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y., Zhang Q., Yin C., et al. Effects of nimodipine on postoperative delirium in elderly under general anesthesia: a prospective, randomized, controlled clinical trial. Medicine. 2017;96(19):p. e6849. doi: 10.1097/MD.0000000000006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Ma L., Gao M., Guo W., Ma Y. Dexmedetomidine reduces postoperative delirium after joint replacement in elderly patients with mild cognitive impairment. Aging Clinical and Experimental Research. 2016;28(4):729–736. doi: 10.1007/s40520-015-0492-3. [DOI] [PubMed] [Google Scholar]
- 35.Maldonado J. R., Wysong A., van der Starre P. J. A., Block T., Miller C., Reitz B. A. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50(3):206–217. doi: 10.1176/appi.psy.50.3.206. [DOI] [PubMed] [Google Scholar]
- 36.Mardani D., Bigdelian H. Prophylaxis of dexamethasone protects patients from further post-operative delirium after cardiac surgery: a randomized trial. Journal of Research in Medical Sciences. 2013;18(2):137–143. [PMC free article] [PubMed] [Google Scholar]
- 37.Mei B., Meng G., Xu G., et al. Intraoperative sedation with dexmedetomidine is superior to propofol for elderly patients undergoing hip arthroplasty: a prospective randomized controlled study. The Clinical Journal of Pain. 2018;34(9):811–817. doi: 10.1097/AJP.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 38.Mohammadi M., Ahmadi M., Khalili H., Cheraghchi H., Arbabi M. Cyproheptadine for the prevention of postoperative delirium: a pilot study. Annals of Pharmacotherapy. 2016;50(3):180–187. doi: 10.1177/1060028015624938. [DOI] [PubMed] [Google Scholar]
- 39.Papadopoulos G., Pouangare M., Papathanakos G., Arnaoutoglou E., Petrou A., Tzimas P. The effect of ondansetron on postoperative delirium and cognitive function in aged orthopedic patients. Minerva Anestesiologica. 2014;80(4):444–451. [PubMed] [Google Scholar]
- 40.Park J. B., Bang S. H., Chee H. K., Kim J. S., Lee S. A., Shin J. K. Efficacy and safety of dexmedetomidine for postoperative delirium in adult cardiac surgery on cardiopulmonary bypass. The Korean Journal of Thoracic and Cardiovascular Surgery. 2014;47(3):249–254. doi: 10.5090/kjtcs.2014.47.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prakanrattana U., Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesthesia and Intensive Care. 2007;35(5):714–719. doi: 10.1177/0310057X0703500509. [DOI] [PubMed] [Google Scholar]
- 42.Priye S., Jagannath S., Singh D., Shivaprakash S., Reddy D. P. Dexmedetomidine as an adjunct in postoperative analgesia following cardiac surgery: a randomized, double-blind study. Saudi Journal of Anaesthesia. 2015;9(4):353–358. doi: 10.4103/1658-354X.154715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson T. N., Dunn C. L., Adams J. C., et al. Tryptophan supplementation and postoperative delirium - a randomized controlled trial. Journal of the American Geriatrics Society. 2014;62(9):1764–1771. doi: 10.1111/jgs.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Royse C. F., Saager L., Whitlock R., et al. Impact of methylprednisolone on postoperative quality of recovery and delirium in the steroids in cardiac surgery trial: a randomized, double-blind, placebo-controlled substudy. Anesthesiology. 2017;126(2):223–233. doi: 10.1097/ALN.0000000000001433. [DOI] [PubMed] [Google Scholar]
- 45.Sampson E. L., Raven P. R., Ndhlovu P. N., et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. International Journal of Geriatric Psychiatry. 2007;22(4):343–349. doi: 10.1002/gps.1679. [DOI] [PubMed] [Google Scholar]
- 46.Shehabi Y., Grant P., Wolfenden H., et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to morphine-DEXCOM study) Anesthesiology. 2009;111(5):1075–1084. doi: 10.1097/aln.0b013e3181b6a783. [DOI] [PubMed] [Google Scholar]
- 47.Sheikh T., Dar B., Akhter N., Ahmad N. A comparative study evaluating effects of intravenous sedation by dexmedetomidine and propofol on patient hemodynamics and postoperative outcomes in cardiac surgery. Anesthesia: Essays and Researches. 2018;12(2):555–560. doi: 10.4103/aer.AER_46_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su X., Meng Z.-T., Wu X.-H., et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. The Lancet. 2016;388(10054):1893–1902. doi: 10.1016/S0140-6736(16)30580-3. [DOI] [PubMed] [Google Scholar]
- 49.Sugano N., Aoyama T., Sato T., et al. Randomized phase II study of TJ-54 (Yokukansan) for postoperative delirium in gastrointestinal and lung malignancy patients. Molecular and Clinical Oncology. 2017;7(4):569–573. doi: 10.3892/mco.2017.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W., Li H.-L., Wang D.-X., et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial. Critical Care Medicine. 2012;40(3):731–739. doi: 10.1097/CCM.0b013e3182376e4f. [DOI] [PubMed] [Google Scholar]
- 51.Whitlock R. P., Devereaux P. J., Teoh K. H., et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. The Lancet. 2015;386(10000):1243–1253. doi: 10.1016/s0140-6736(15)00273-1. [DOI] [PubMed] [Google Scholar]
- 52.Xin X., Xin F., Chen X., et al. Hypertonic saline for prevention of delirium in geriatric patients who underwent hip surgery. Journal of Neuroinflammation. 2017;14(1):p. 221. doi: 10.1186/s12974-017-0999-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X., Li Z., Gao C., Liu R. Effect of dexmedetomidine on preventing agitation and delirium after microvascular free flap surgery: a randomized, double-blind, control study. Journal of Oral and Maxillofacial Surgery. 2015;73(6):1065–1072. doi: 10.1016/j.joms.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Aydogan M. S., Korkmaz M. F., Ozgül U., et al. Pain, fentanyl consumption, and delirium in adolescents after scoliosis surgery: dexmedetomidine vs midazolam. Pediatric Anesthesia. 2013;23(5):446–452. doi: 10.1111/pan.12128. [DOI] [PubMed] [Google Scholar]
- 55.de Oliveira G. S., Ahmad S., Fitzgerald P. C., et al. Dose ranging study on the effect of preoperative dexamethasone on postoperative quality of recovery and opioid consumption after ambulatory gynaecological surgery. British Journal of Anaesthesia. 2011;107(3):362–371. doi: 10.1093/bja/aer156. [DOI] [PubMed] [Google Scholar]
- 56.Yang J., Zhou Y., Kang Y., et al. Risk factors of delirium in sequential sedation patients in intensive care units. BioMed Research International. 2017;2017:9. doi: 10.1155/2017/3539872.3539872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peritogiannis V., Stefanou E., Lixouriotis C., Gkogkos C., Rizos D. V. Atypical antipsychotics in the treatment of delirium. Psychiatry and Clinical Neurosciences. 2009;63(5):623–631. doi: 10.1111/j.1440-1819.2009.02002.x. [DOI] [PubMed] [Google Scholar]
- 58.Devlin J. W., Skrobik Y., Gelinas C., et al. Clinical Practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Critical Care Medicine. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: subgroup analyses of studies using dexmedetomidine to prevent postoperative delirium. Table S2: the incidence of adverse events in the intervention and control groups. Figure S1: the funnel plots of the included studies regardless of the risk of bias. Figure S2: the funnel plots of the included studies with low risk.
Data Availability Statement
All original data used to support the findings of this study are available from the corresponding author upon request.