Abstract
Background
Living at a high plateau in a very hostile environment and low oxygen levels often leads to the development of high-altitude polycythemia (HAPC) and gastric mucosal lesions caused by high-level reactive oxygen species (ROS). Hypoxia-inducible factor-1A (HIF-1A) helps maintain oxygen homeostasis by promoting the transcription of various genes and can be affected by ROS levels. To evaluate the molecular mechanism by which HAPC causes the gastric mucosal lesions, the expression of HIF-1A was measured in Tibetans with HAPC and in healthy subjects. Ultrastructural, histopathological, and immunohistochemical analyses were performed in the gastric tissues of both groups, and the expression of HIF-1A in the gastric mucosa was detected using qPCR and Western Blot.
Results
The microvessel density and average diameter of gastric mucosal vessels were significantly greater in the HAPC patients than in the healthy subjects (p < 0.05). The number of red blood cells in the gastric mucosa was also significantly higher in the HAPC group than in the healthy subjects (p < 0.05). In addition, the density of the mitochondrial vacuoles and endoplasmic reticulum and pathological apoptosis were significantly increased in the gastric cells from HAPC patients compared to those from the healthy subjects. The levels of ROS and HIF-1A in the gastric mucosa were increased in HAPC patients compared to those in controls (p < 0.05).
Conclusions
An increased level of HIF-1A was associated with HAPC development in the stomach of Tibetans living at a high altitude. ROS upregulated the levels of HIF-1A. Thus, ROS-mediated HIF-1A signaling transduction may be the mechanism associated with HAPC-induced gastric lesions.
1. Background
Living at a high plateau with a very hostile environment and low oxygen levels [1] can result in a series of physiological effects on the structure of gastric tissues, and physiological functions that are associated with high-plateau disease and major health problems may be impaired [2, 3]. The gastrointestinal tract is commonly affected and has greater sensitivity to hypoxia. Long-term and high-altitude hypoxia has varying effects on the digestive system [4].
Red blood cells play a central role in normal physiological processes in human tissues and organs by delivering oxygen and removing waste. The concentration of red blood cells should be maintained at a certain level [5]. Erythropoietin (EPO) is necessary for the growth and survival of erythroid progenitor cells and production of red blood cells. The EPO level is regulated in response to oxygen tension [6, 7].
Hypoxia-inducible factor-1 (HIF-1) is a transcription factor, consisting of α and β heterodimer subunits, and is coded by the HIF-1A gene. HIF-1A acts as the main regulator of homeostasis in hypoxic cells by activating the transcription of various genes, including those involved in energy metabolism [8, 9], angiogenesis [10, 11], and apoptosis [12, 13], as well as other genes involved in increasing oxygen delivery or promoting metabolism under hypoxic conditions [14, 15]. Therefore, HIF-1A plays an important role in the pathophysiology of ischemic diseases [16, 17]. HIF-1A is activated in hypoxia and promotes the transcriptional activation of downstream genes, including EPO [18, 19], leading to the increase in red blood cell production.
The mechanism underlying hypoxia-induced morphological changes in the microstructure of gastric tissues and the role of HIF-1A in the pathogenesis of high-altitude polycythemia (HAPC) in Tibetans remain unclear. Therefore, we investigated the effects of a high-altitude hypoxic environment on physiological function in human gastrointestinal mucosa and evaluated the molecular mechanisms associated with HIF-1A in the pathogenesis of HAPC.
2. Methods
2.1. Participants
All protocols were approved by the Human Research Ethics Committee of People's Hospital of Tibet Autonomous Region (Approval No. 20150108x2, Lhasa City, People's Republic of China). Written informed consent was obtained from all participants. The studies were performed according to the Guidelines of the Declaration of Helsinki and Ethical Principles for Medical Research Involving Human Subjects.
The severity of mucosal lesions was classified according to HE staining of gastric tissues and red blood cell aggregation: (1) controls had healthy gastric tissues without red blood cell aggregation or leakage, and or seepage in gastric tissues; (2) HAPC patients had mucosal lesions with oozing blood red cell clustering (clusters of > 3 erythrocytes). The categories were determined according to the number of red blood cells and vessels, degree of red blood cell aggregation, and the pathological findings. In July 2013, 24 HAPC patients who lived on the Tibetan Plateau at an altitude of 3600 m to 4800 m, were assigned to the HAPC patient group. Complications in the HAPC patients were assessed with gastroscopy. In addition, 21 healthy Tibetans from the same altitudes were assigned to the control group. All subjects were from Lhasa, Nagqu, Shannan, or Rigaze in Tibet. All subjects were indigenous and had lived in the same locations for more than 30 years. Every subject was matched with a control based on sex, place of residence, age, body mass index (BMI), and life habits among others. As BMI and daily caloric intake are major contributors to gastric injury [20], so these components were carefully measured. All patients were male native Tibetans from Lhasa with age ranging from 40 to 45 years. All patients provided written informed consent before tissue sampling.
Peripheral venous blood was obtained and oxygen saturation was detected using pulse oximetry. Inclusion criteria for the study were HAPC diagnosed according to the definition given by 2004 Qinghai International High Altitude Medicine Conference, including hemoglobin (Hb) concentration > 21 g/dL (males) and > 19 g/dL (females) [21]. Exclusion criteria were chronic pulmonary disorders, such as emphysema, bronchitis, bronchiectasis, alveolar fibrosis, lung cancer, and other serious pulmonary diseases; serious respiratory diseases or secondary polycythemia due to hypoxemia associated with documented chronic disease; intestinal tract obstruction; and history of intestinal bleeding. The diagnosis of chronic gastritis was based on the guidelines developed by the Chinese consensus on chronic gastritis [22]. Histopathological diagnosis was performed according to the gastritis assessment staging system [23].
2.2. Endoscopic Observation
The upper gastrointestinal tracts of all participants were observed using endoscopy [24]. HAPC was examined after ingestion of 10-mL 2% lidocaine HCl viscous solution (Jiangsu Jichuan Pharmaceutical Co., Ltd., China). All subjects were observed under a gastroscope (OLYMPUS GIF-260, Olympus, Japan) by a professional endoscopist. The same light source and endoscopy lamps were used during the whole experiment. The color of the upper gastrointestinal tracts was observed under an endoscope and color changes were photographed. Meanwhile, mucosal biopsy was taken from all participants.
2.3. Histological Analysis
Mucosal sampling was performed in the Digestive Endoscopy Department, People's Hospital of the Tibet Autonomous Region (Tibet, China). We analyzed 45 gastric antrum biopsy specimens, of which 24 were from HAPC patients and 21 from controls with normal mucosa. The gastric mucosal samples obtained with forceps biopsy were flash-frozen in liquid nitrogen immediately and stored at -80°C until use. Gastric mucosal tissues were fixed in formalin and embedded in paraffin for histological analyses. Five-μm sections were stained with H&E (Bi Yuntian, China). Blood vessels and red blood cells in microvessels were assessed with a 400× magnification, and the diameter of gastric mucosal vessels was measured. The preserved samples were prefixed in 3% glutaraldehyde, followed by 1% osmium tetroxide fixation, gradual dehydration in gradient acetone, Epon812 embedding, optical positioning of semithin sections, slicing of ultrathin sections, and double staining with both uranyl acetate and citrate lead. Finally, the sections were photographed with a transmission electron microscope (Hitachi H-600IV, Japan). The results were evaluated by 2 researchers in a blinded fashion. The mucosa was considered destroyed if one of the following was apparent: discontinuous surface, dilated glands, hemorrhage, or superficial cell damage [25].
2.4. Immunohistochemical Analysis
Gastric mucosal cells were cultured in hydrogen peroxide solution for 15 min, and 100 μL of normal serum was added, with further culturing for 10 min. Anti-HIF-1A antibody (rabbit anti-human, 1:200 dilution, ProteintechTM, ABCAM, USA) was added, and the cells were incubated overnight (16 h) at 4°C. The mixture was washed 3 times with PBS. Horseradish peroxidase goat anti-rabbit secondary antibody (goat anti-rabbit, 1: 200, ProteintechTM, ZSGB-BIO, China) was added and incubated for 30 min. Finally, the mixture was washed 3 times. The staining was developed with a DAB color kit (ZSGB-BIO, China). Astrocytes were stained with hematoxylin and whole-mounted. The expression and distribution of HIF-1A were observed in the tissue.
2.5. RNA Extraction
Gastric mucosal samples from 24 HAPC patients and 21 controls were pooled for each genotype. Each pool was placed in 3-mL of Trizol (Shanghai Sangon Co., China) and grounded with a glass homogenizer. After extraction with chloroform, RNA was precipitated with isopropanol. The final mixtures were placed into TE buffer (Tris-HCl buffer, pH 8.0, containing 1.0 mM EDTA) and digested with DNase; RNA quality was evaluated at 260/280 nm using a spectrophotometer (NanoDrop 1000, NanoDrop Technologies, Wilmington, DE, USA).
2.6. Real-Time qRT-PCR
Chip results were reevaluated using real-time qRT-PCR for the differentially expressed HIF-1A gene, with the β-actin gene as a control. Total RNA from gastric mucosal samples was purified with a Trizol reagent kit (Life Technologies, Maryland, CA, USA). Five-μg of RNA was reverse-transcribed with the One-Step PrimeScript cDNA Synthesis Kit (Takara, Dalian, China). Platinum TaqDNA polymerase was purchased from ABI (USA). The primers were designed as follows: HIF-lA, forward primer, 5′-ACCCTCTGATTTAGCATGTAG-3′, reverse primer, 5′-GTAGGTTTCTGCTGCCTTGT-3′; Probe, 5′-FAM- CTGGTCAGCTGTGGTAATCC-TAMRA-3′; β-actin, forward primer, 5′- GAAGATCAAGATCATTGCTCCT-3′, reverse primer, 5′- TACTCCTGCTTGCTGATCCA-3′; probe, 5′-FAM-CTGTCCACCTTCCAGCAGA-TAMRA-3′; PCR was repeated three times using SYBR Green PCR Master Mix and the reactions were performed in 7500 Fast real-time PCR detection system (ABI Biosystems, Foster City CA, USA) under the following amplification conditions: 94°C for 2 min, 45 cycles at 94°C for 20 s, 53°C for 30 s, and 60°C for 45 s. Relative expression value was calculated with the 2-∆∆T method.
2.7. Western Blot Analysis of HIF-1A
Glandular epithelial cells of gastric mucosa were extracted and the protein levels of HIF-1A were analyzed using Western Blot. The total protein was obtained using cocktail after the cells were lysed for 3 times by freezing and thawing [26]. Supernatant protein was separated using SDS-PAGE and transferred to a PVDF membrane, and blocked by 2.5% nonfat dry milk for half an hour. The membrane was subsequently incubated with HIF-1A antibodies (diluted 1:1000) overnight at 4°C. An ABC peroxidase and peroxidase substrate kits were used to examine the primary antibodies. Using X-ray film exposure and photographs, expression of HIF-1A was measured using Quantity One with β-actin as an internal control.
2.8. Terminal Deoxynucleotidyl Transferase-Mediated Deoxyuridine Triphosphate Nick-End Labelling (TUNEL) Analysis for Apoptosis
The slides of mucosal issues were treated with 10 μg/ml proteinase K (Sigma, St Louis, MO, USA) for 15 min at RT and washed for 2 min with ddH2O. Endogenous peroxidases were deactivated by 2% of hydrogen peroxide for 5 min. The sections were rinsed with ddH2O, and immersed in TDT buffer [26]. TDT (200 U/mL; Takara, Tokyo, TUNEL assay for apoptosis of gastric mucosa) and biotinylated dUTP (0.01 mM BMY) in TDT buffer were then added to the sections, and incubated in a humid atmosphere at 37°C for 1 h. The reaction was stopped by using TB buffer (0.3 M NaCl, 0.04 M sodium citrate) for 20 min. The sections were rinsed with ddH2O, covered with 10% BSA for 10 min, then rinsed in ddH2O, and immersed in PBS for 5 min. The sections were incubated with horseradish peroxidase (HRP) (Sigma) for 50 min, then washed with 20 μM PBS and 150 mM NaCl, and treated with 0.02% DAB. After being rinsed in ddH2O, the slides were stained with hematoxylin for half an hour at 37°C. Dehydration was performed according to a previous report [26]. The tissue sections were then mounted in Permount (Fisher Scientific, Pittsburgh, PA, USA). Apoptotic cells identified by the TUNEL assay were quantitated under brightfield microscopy by a single observer. Cell number was counted under high power using 6 different views from each specimen. The POD apoptosis detection kit (Roche, USA) was used in accordance with instructions. Paraffin sections of gastric mucosa were TUNEL stained and maximized at 400× under a microscope.
2.9. Assay of Intracellular ROS
Intracellular ROS levels were determined using the dichloro-dihydro-fluorescein diacetate (DCFH-DA) method according to a previous study [27]. Isolated gastric mucosal cells were incubated with DCFH-DA in PBS buffer at 37°C for 30 min. Subsequently, the buffer was removed and the samples were washed 3 times using the same buffer. The fluorescence absorbing values were measured by using a fluorescence plate reader. Excitation wavelength was 485 nm and emission wavelength was 535 nm. Relative ROS values were compared between the patients and healthy subjects.
2.10. Statistical Analysis
All results were presented as mean ± SD. SPSS11.5 statistical software was used for analysis of variance. A chi-square test was used for number analysis and a Student's t-test was used for normally distributed data analysis after testing for normality by means of Kolmogorov Smirnov test in the present study. One-way analysis of variance (ANOVA) was used to compare variables between two groups. The post hoc test was the Newman-Keuls test. Sample power was calculated by using STATA 10 (StataCorp, College Station, TX, USA). The level of significance is set a priori if p < 0.05.
3. Results
3.1. Baseline Participant Characteristics
Baseline and physical characteristics of the study population were shown in Tables 1 and 2. The statistical difference was insignificant for living altitude, age, smoking, drinking, BMI, or duration at different altitudes between HAPC subjects and controls (p > 0.05, Table 1). Hb, EPO, ROS and diastolic pressures in the HAPC group were higher than in the control group (p < 0.01). In contrast, oxygen saturation was lower than in a control group (p < 0.01). No significant difference in other parameters was found between the control and experimental groups (Table 2).
Table 1.
The baseline characters of participants.
| Characteristic | Control | HAPC | t-value | p-Value |
|---|---|---|---|---|
| Age (years) | 42.5 ± 2.5 | 42.3 ± 2.3 | 0.3645 | 0.3819 |
| Smoking (no/yes) | 4/10 | 3/8 | 0.1254 | 0.2465 |
| Drinking (no/yes) | 5/9 | 4/7 | 0.2487 | 0.4526 |
| BMI | 32.4± 3.5 | 33.5 ± 2.8 | 0.2241 | 0.3708 |
| Living at altitude (meters) | 4557 ± 668 | 4523 ± 623 | 0.8509 21. | 0.2168 |
| Time on high plateau (years) | 34.4 ± 4.2 | 35.6 ± 5.1 | 0.2324 | 0.3682 |
Note: BMI, body mass index. There is a significant difference if p < 0.05.
Table 2.
Basic physiological indices in participants.
| Characteristic | Control | HAPC | t-value | p-Value |
|---|---|---|---|---|
| Hb (g/dl) | 21.54 ± 2.34 | 22.85 ± 2.17 | 2.3452 | 0.0126 |
| EPO (ng/ml) | 1.53 ± 0.12 | 1.78 ± 0.14 | 5.6541 | 0.0168 |
| ROS (fluorescence intensity) | 521.4 ± 20.6 | 651.2 ± 30.9 | 5.8801 | 0.0012 |
| Systolic pressure (mmHg) | 112.4 ± 11.6 | 121.1 ± 14.5 | 2.9083 | 0.0748 |
| Diastolic pressure (mmHg) | 74.2 ± 8.8 | 88.2 ± 10.1 | 4.1258 | 0.0225 |
| Blood oxygen saturation (%) | 90.2 ± 2.3 | 84.1 ± 2.8 | 5.4618 | 0.0064 |
| Heart rate (time/mini) | 78.1 ± 4.2 | 79.6 ± 5.1 | 2.0786 | 0.1342 |
Note: there is a significant difference if p < 0.05.
3.2. Endoscopic Upper Gastrointestinal Tract Examination
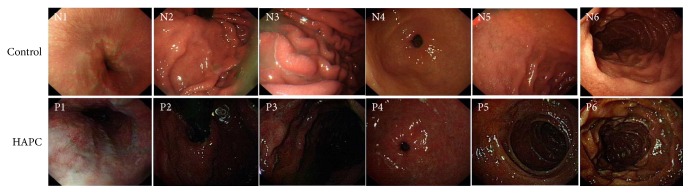
The HAPC group was found to have mucosal hyperemia and edema with a darker color in the upper gastrointestinal tract, including the esophagus (N1/P1), gastric fundus (N2/P2), gastric body (N3/P3), gastric antrum (N4/P4), duodenal bulb (N5/P5), and descending duodenum (N6/P6) (87.5% versus 4.8%, p<0.001) (Figure 1). Moreover, in the HAPC group, the esophageal mucosa became thinner and redder and fine vascular nets could be found under the mucosa as cord-like and branchlike structures. Furthermore, the veins became wider in the observed area. In addition, deepened pink of the esophageal mucosa destroyed the boundary that could be distinguished between esophageal and stomach mucosa.
Figure 1.
Endoscopic findings of an upper gastrointestinal (upper GI) series. N1/P1, esophagus; N2/P2, cardia; N3/P3, gastric fundus; N4/P4, antrum stomach body; N5/P5, duodenal bulb; and N6/P6, descending portion. N stands for healthy subjects and P presents for polycythemia ones.
In the HAPC group, gastric mucosa was dark red, or red-purple, and diffuse hyperemia and edema as well as significant changes of congestion. In the duodenal bulb and descending parts of upper gastrointestinal tract in the HAPC group, the mucosa was browner and brownish red when comparing with controls. In addition, the villi in the duodenal bulb became enlarged with hyperemia and swelling (Figure 2).
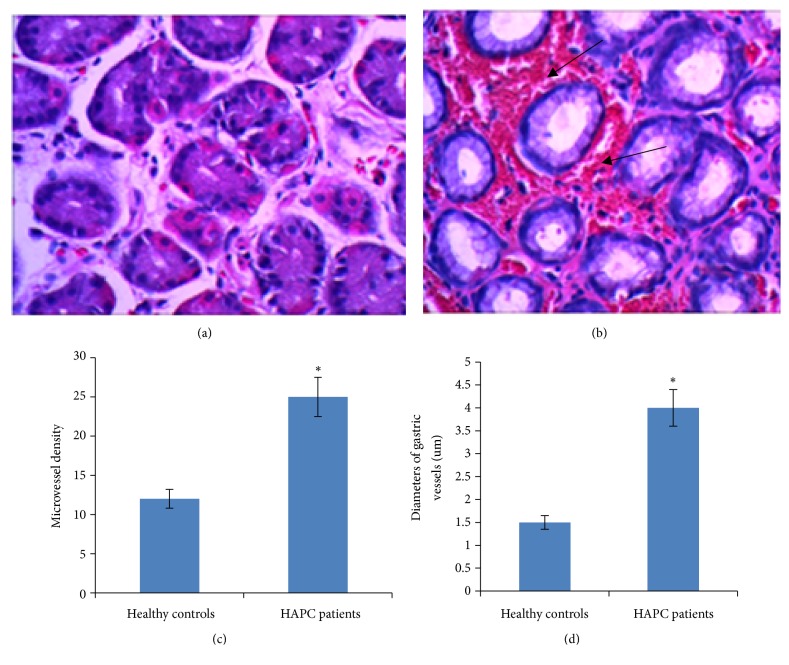
Figure 2.
HE staining comparison for gastric biopsy between HAPC patients and healthy controls. (a) For the gastric biopsy from healthy controls, the duct is in normal structure with suitable amounts of blood vessels and red blood cells; (b) for the gastric biopsy from HAPC patients, the duct is destroyed. A large number of red blood cells were gathered in gastric vessels. Parietal cells are overproliferated in gastric biopsies. The number of gastric cells is decreased while the diameter of gastric mucosal vessels is increased compared with healthy controls. (c) Microvessel density (MVD). (d) Average diameter of gastric mucosal vessels. ∗p < 0.05 via controls.
3.3. H&E Staining of Gastric Biopsy Specimens from HAPC Patients and Healthy Controls
In gastric biopsy specimens from the controls, the ducts appeared normal with appropriate numbers of blood vessels and red blood cells (Figure 2(a)). In gastric biopsy specimens from HAPC patients, the ducts were destroyed. A large amount of red blood cells was aggregated in gastric vessels. Parietal cells showed overproliferation in gastric biopsies. The amounts of gastric cells were reduced while the diameters of gastric mucosal vessels were increased when comparing with the controls (Figures 2(a) and 2(b)).
3.4. Number of Gastric Vessels
Statistical analysis showed that gastric microvessel density (MVD) was significantly greater in HAPC patients than in healthy controls (24.33 ± 4.58 and 12.00 ± 3.03) (Table 3). There were significant differences between the two groups (P <0.05) (Figure 2(c)).
Table 3.
Characters of gastric mucosal vessels.
| Number of vessels | Average diameter of vessels | The number of erythrocyte (400×) | |||||
|---|---|---|---|---|---|---|---|
| Groups | Slices | Average | SD | Average | SD | 28.71 | 11.71 |
| Healthy controls | 21 | 12.16 | 2.64 | 11.73 | 2.80 | 154.63 | 46.24 |
| HAPC patients | 24 | 25.78 | 3.60 | 33.60 | 9.80 | 28.71 | 11.71 |
3.5. Average Diameter of Gastric Mucosal Vessels
Statistical analysis showed that the average diameter of gastric mucosal vessels in the stomach of HAPC patients was significantly greater than in healthy controls (3.90 ± 1.31 and 1.63 ± 0.56). There were significant differences between the two groups (p < 0.05) (Figure 2(d), Table 3).
3.6. Number of Erythrocytes in Gastric Mucosa
Statistical analysis showed that the number of erythrocytes in gastric mucosa of HAPC patients was significantly higher than in healthy controls (149.58 ± 69.63 and 29.95 ± 16.79). There was a significant difference between the two groups (p < 0.05) (Table 3). The erythrocytes were not only distributed in blood vessels, but also in tissue spaces, suggesting that high altitude increases the number of gastric vessels and causes stomach injury.
3.7. Microstructure of Gastric Mucosa
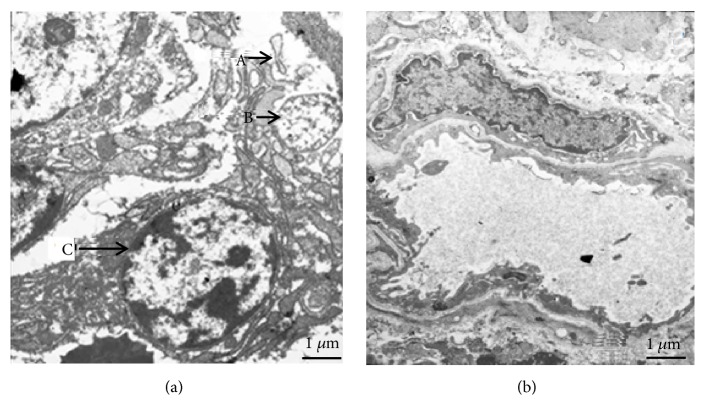
Transmission electron microscopy (TEM) showed normal gastric microstructure in healthy controls while significant changes were observed in HAPC patients. The endoplasmic reticulum was increased while mitochondria became swollen and degenerated vacuoles were found in HAPC patients (Figure 3). TEM revealed various degrees of expansion of the endoplasmic reticulum, as well as mitochondrial swelling in epithelial cells of the gastric mucosa in the HAPC group. In addition, some epithelial cells disappeared and demonstrated development of vascular degeneration. Some of the cell nuclei were damaged and lymphocytic infiltration was observed. In contrast, in the control group, the epithelial cell structure of the gastric mucosa appeared normal (90.9% versus 8.3%, p < 0.001) (Figure 3).
Figure 3.
Ultrastructure of the gastric mucosa in the high-altitude polycythemia group double staining of both uranyl acetate and lead citrate ×10 000. Transmission electron microscopy of epithelial cells of the gastric mucosa in high-altitude polycythemia group (a), swelling of endoplasmic reticulum (A), mitochondria edema (B), and lymphocytic infiltration (C); control group (b), the epithelial cell structure of the gastric mucosa appeared normal.
3.8. Immunohistochemical Analysis of HIF-1A
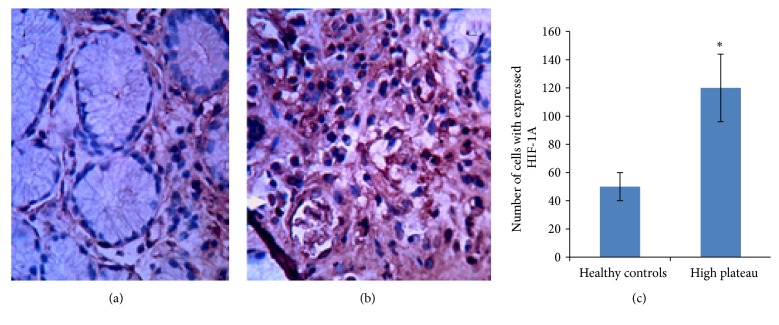
Immunohistochemistry showed that the levels of HIF-1A in gastric mucosa of HAPC patients were significantly higher than those from healthy controls. There were significant differences between the two groups (p < 0.05) (Figure 4).
Figure 4.
Immunohistochemistrial analysis for the expression of HIF-1A. (a) Normal gastric mucosa from healthy controls. (b) Gastric mucosa from HAPC patients. (c) ∗P <0.05 via controls.
3.9. The Effects of HAPC on the mRNA Levels of HIF-1A
qPCR analysis showed that the mRNA levels of HIF-1A in gastric mucosa of HAPC patients were significantly higher than in healthy controls. There were significant differences between the two groups (p < 0.05) (Figure 5(a)).
Figure 5.
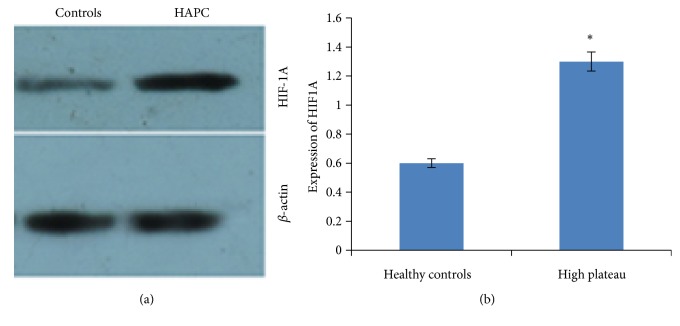
The expression of HIF-1A. (a) qPCR analysis for the mRNA levels of HIF-1A. ∗P <0.05 via controls. (b) Western Blot analysis of the protein levels of HIF-1A. (a) The comparison for the protein levels of HIF-1A between HAPC patients and healthy controls. (b) ∗p <0.05 via controls.
3.10. Effects of HAPC on the Protein Levels of HIF-1A
Western Blot analysis showed that the protein levels of HIF-1A in gastric mucosa of HAPC patients were significantly higher than in healthy controls. There were significant differences between the two groups (p < 0.05) (Figure 5(b)).
3.11. TUNEL Analysis
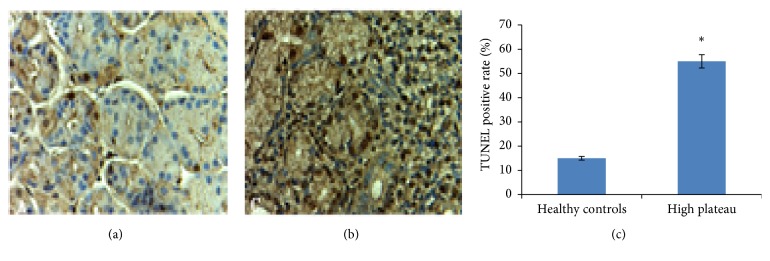
TUNEL analysis showed that apoptosis in gastric mucosa was significantly greater in HAPC patients than in healthy controls. There were significant differences between the two groups (p < 0.05) (Figures 6(a) and 5(b)).
Figure 6.
TUNEL analysis of levels of HIF-1A in gastric mucosa. (a) The levels of HIF-1A in gastric mucosa from healthy controls. (b) The levels of HIF-1A in gastric mucosa from HAPC patients. (c) The comparison for apoptotic gastric cells between HAPC patients and healthy controls. ∗p <0.05 via controls.
3.12. Apoptotic Analysis
Analysis showed that the levels of apoptotic gastric cells in the stomach of HAPC patients were significantly greater than in healthy controls. There were significant differences between the two groups (p < 0.05) (Figure 6(c)).
3.13. Intercellular ROS Concentration
ROS levels were significantly higher (to 220%) in the cells from healthy subjects (p < 0.05, Figure 7). The results were consistent with previous reports: ROS are important risk factors for polycythemia at high altitude.
Figure 7.
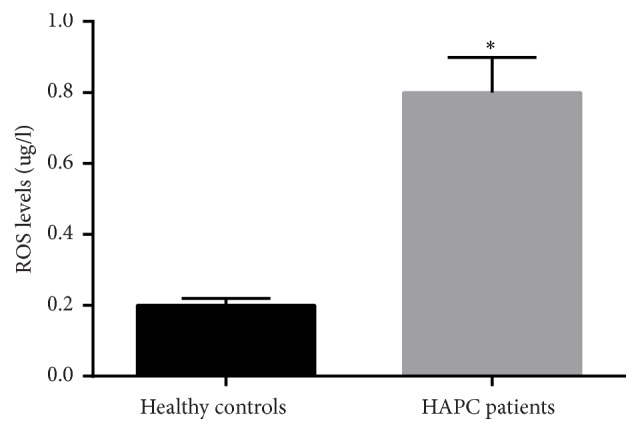
ROS generation in different cells from between HAPC patients and healthy controls. All the data were represented as the mean values ± SD. ∗p < 0.05 via a healthy subject.
4. Discussion
Classic high-altitude physiological responses include hyperventilation [28] and polycythemia [29, 30]. The gastrointestinal tract is more sensitive to hypoxia [31]. Thus, hypoxia can have varying degrees of influence on the gastric mucosa and polycythemia is the main pathology of HAPC-induced disorders in most Tibetans [30, 32]. Our results showed a significant increase in the number of red blood cells, gastric vessels, and diameters of gastric mucosal vessels in HAPC patients when compared with healthy controls. Furthermore, more red blood cells were distributed in gastric tissue not only at vascular levels but also in tissue spaces. The number of vacuoles was increased in gastric mucosal cells. Meanwhile, the number of mitochondria and the endoplasmic reticulum were also increased. Moreover, there was a significant increase in apoptosis of gastric mucosal cells. These pathologic changes show the effect of high-altitude hypoxia on the human gastrointestinal mucosal barrier, leading to adverse effects on physiological, and immune function, among others.
Comparing with our reported work [26, 33], the population size was increased from 6 and 24 to 45 subjects in the present study. Furthermore, the color of brown lesions under the mucosa in upper gastrointestinal tract was much darker than before reported in HAPC patients (Figure 1). Present findings showed that there were 21 cases (87.5%) and 1 case (4.8%) with darker brown color under the gastrointestinal mucosa in the HAPC and control groups (p<0.001), respectively. The color-based biomarker detection can be used for the diagnosis of HAPC but the data could not be obtained from previous work with smaller population.
Hypoxia induces HIF-mediated gene expression [34]. Hypoxia promotes caspase-3 activation [35] and will increase the apoptosis of gastric mucosal cells. Hypoxic states can be manipulated if oxygen is increased [36]. The increase in partial pressure of oxygen (ppO2) and normobaric air [37] can suppress the expression of caspase-3 [38] and will inhibit the apoptosis of gastric mucosal cells.
Production of HIF is the body's response to hypoxia and important in adaptation [39–42]. HIF production has an obvious impact on the expression of other genes. qPCR, Western Blot and immunohistochemical analyses showed that the levels of HIF-1A in the gastric mucosa of HAPC patients were significantly higher than in healthy controls. There were significant differences between the two groups (p < 0.05) (Figures 5 and 6).
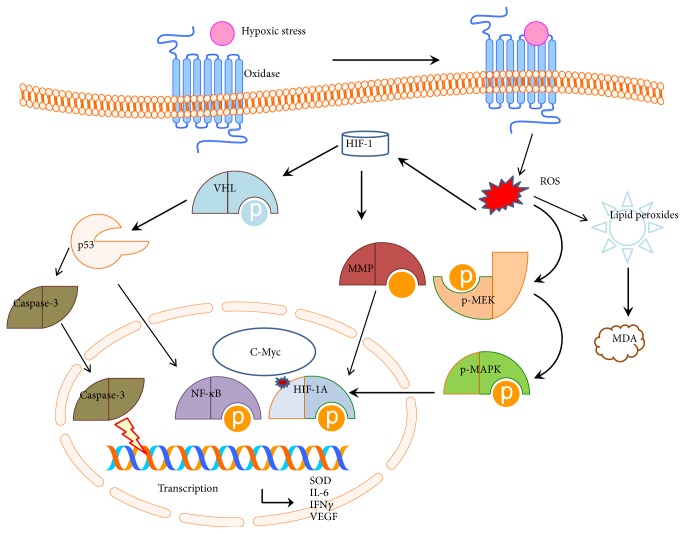
High attitude hypoxic conditions will significantly increase ROS [43, 44] (Figure 8). The increased ROS act as signal transduction messengers that induce stabilization of HIF-1A, which is important in maintaining normal physiologic responses under hypoxic conditions [45]. HAPC is closely associated with angiogenesis [46], which will affect the development of gastric mucosal lesions. The c-Myc/HIF-1A signaling axis (Figure 8) can reduce lesion-stimulated angiogenesis [47]. An association between Von Hippel Lindau (VHL) gene and the HIF pathway has been reported in some human disorders [48]. HIF-1 inhibits mitochondrial biogenesis and O2 consumption in cells lacking the VHL gene by inhibiting c-Myc activity [49] (Figure 8). The loss of VHL protein will result in impaired p14/ARF function and suppression of p53 expression [50]. p53 and caspase-3 are the main biomarkers in apoptotic-signaling pathways [51] and can be affected by HIF-1A (Figure 8). All these molecules will affect the expression of SOD, IL-6, IFN and VEGF and result in changes of antioxidant, anti-inflammatory and antiapoptosis in gastric cells (Figure 8). HIF-1A can cause ischemia-reperfusion-induced gut mucosal injury. HIF-1A responses in gastric mucosa may be potentially maladaptive or damage gastric barrier function and result in other organ injury according to previous report [52]. All results have suggested that high-level expression of HIF may lead to HAPC-induced gastric mucosa lesion and is an important ROS-mediated signaling pathway.
Figure 8.
The schematic diagram of ROS-medicate HIF-1 signaling pathway.
There were some limitations in the present work. No experiment was performed to determine whether ROS actually exacerbated gastric mucosal lesions or to determine a role for HIF-1A. Our work showed an increase of both in the gastric mucosa but not the role they play. Further work is needed to address this issue.
5. Conclusions
In sum, MVD, the diameters of gastric mucosal vessels, and the number of apoptotic cells were significantly greater in HAPC patients than in healthy controls. This study also suggested a molecular mechanism for the pathogenesis of HAPC. The levels of HIF-1A in gastric mucosa of HAPC patients were significantly higher than in healthy controls (p < 0.05). The results provide a basis for pathogenesis of gastrointestinal mucosal lesions in HAPC patients.
Acknowledgments
This study was supported by a grant from the National “Twelfth Five-Year” Plan for Science & Technology Support of China (2013BAI05B04), the Natural Science Foundation of Guangdong Province, China (2014A030313679), and the Key Project for Natural Science Foundation in Tibet Autonomous Region, China (2012). Bo Liu, Dan Wang, and Fei Wang are thanked for technical assistance.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
All procedures were approved by the Ethics Committee at People's Hospital of Tibet Autonomous Region (Approval no. 20150108x2, Lhasa, China).
Consent
The samples were collected from all subjects with the owner's consent.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Kang Li collected all subjects, designed and coordinated the experiments, and drafted the manuscript. Chaohui He designed, coordinated the experiments, collected samples selection, and analyzed the data. Kang Li carried out the clinical study. Chaohui He designed the experiments, supervised and conceived the experiment, and prepared the manuscript. All authors read and approved the final manuscript.
References
- 1.Roberts A. D., Clark S. A., Townsend N. E., Anderson M. E., Gore C. J., Hahn A. G. Changes in performance, maximal oxygen uptake and maximal accumulated oxygen deficit after 5, 10 and 15 days of live high: Train low altitude exposure. European Journal of Applied Physiology. 2003;88(4-5):390–395. doi: 10.1007/s00421-002-0720-3. [DOI] [PubMed] [Google Scholar]
- 2.Li S., Wang Y., Huang X., Cao J., Yang D. Diffuse alveolar hemorrhage from systemic lupus erythematosus misdiagnosed as high altitude pulmonary edema. High Altitude Medicine & Biology. 2015;16(1):67–70. doi: 10.1089/ham.2014.1094. [DOI] [PubMed] [Google Scholar]
- 3.Luo Y., Wang Y., Lu H., Gao Y. ‘Ome’ on the range: update on high-altitude acclimatization/adaptation and disease. Molecular BioSystems. 2014;10(11):2748–2755. doi: 10.1039/C4MB00119B. [DOI] [PubMed] [Google Scholar]
- 4.Xu C., Sun R., Qiao X., et al. Effect of Vitamin e supplementation on intestinal barrier function in rats exposed to high altitude hypoxia environment. Korean Journal of Physiology & Pharmacology. 2014;18(4):313–320. doi: 10.4196/kjpp.2014.18.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs R. A., Lundby C., Robach P., Gassmann M. Red blood cell volume and the capacity for exercise at moderate to high altitude. Sports Medicine. 2012;42(8):643–663. doi: 10.1007/BF03262286. [DOI] [PubMed] [Google Scholar]
- 6.Balestra C., Germonpré P. Increasing EPO using the normobaric oxygen paradox: A 'not so simple' task. Acta Physiologica. 2011;203(2):287–288. doi: 10.1111/j.1748-1716.2011.02282.x. [DOI] [PubMed] [Google Scholar]
- 7.Debevec T., Keramidas M. E., Norman B., et al. Acute short-term hyperoxia followed by mild hypoxia does not increase EPO production: resolving the normobaric oxygen paradox. European Journal of Applied Physiology. 2012;112(3):1059–1065. doi: 10.1007/s00421-011-2060-7. [DOI] [PubMed] [Google Scholar]
- 8.Dai Q., Yin Q., Wei L., et al. Oroxylin A regulates glucose metabolism in response to hypoxic stress with the involvement of Hypoxia-inducible factor-1 in human hepatoma HepG2 cells. Molecular Carcinogenesis. 2015 doi: 10.1002/mc.22369. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura H., Makino Y., Okamoto K., et al. TCR engagement increases hypoxia-inducible factor-1α protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. The Journal of Immunology. 2005;174(12):7592–7599. doi: 10.4049/jimmunol.174.12.7592. [DOI] [PubMed] [Google Scholar]
- 10.Bae W., Choi J., Kim J., Jeong J. Cinnamic aldehyde suppresses hypoxia-induced angiogenesis via inhibition of hypoxia-inducible factor-1α expression during tumor progression. Biochemical Pharmacology. 2015;98(1):41–50. doi: 10.1016/j.bcp.2015.08.095. [DOI] [PubMed] [Google Scholar]
- 11.Zimna A., Kurpisz M. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: applications and therapies. BioMed Research International. 2015;2015:13. doi: 10.1155/2015/549412.549412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X., Li J., Wu D., Bu X., Qiao Y. Hypoxia promotes apoptosis of neuronal cells through hypoxia-inducible factor-1α-microRNA-204-B-cell lymphoma-2 pathway. Experimental Biology and Medicine. 2015;241(2):177–183. doi: 10.1177/1535370215600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu G., Xue M., Wang H., Xiang C. Hypoxia-inducible factor-1α antagonizes the hypoxia-mediated osteoblast cell viability reduction by inhibiting apoptosis. Experimental and Therapeutic Medicine. 2015;9(5):1801–1806. doi: 10.3892/etm.2015.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahimi-Horn M. C., Giuliano S., Saland E., et al. Knockout of Vdac1 activates hypoxia-inducible factor through reactive oxygen species generation and induces tumor growth by promoting metabolic reprogramming and inflammation. Cancer & Metabolism. 2015;3:p. 8. doi: 10.1186/s40170-015-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menendez M. T., Teygong C., Wade K., Florimond C., Blader I. J. siRNA screening identifies the host hexokinase 2 (HK2) gene as an important hypoxia-inducible transcription factor 1 (HIF-1) target gene in Toxoplasma gondii-infected cells. mBio. 2015;6(3):p. e00462. doi: 10.1128/mBio.00462-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li D. F., Tian J., Guo X., et al. Induction of microRNA-24 by HIF-1 protects against ischemic injury in rat cardiomyocytes. Physiological Research. 2012;61(6):555–565. doi: 10.33549/physiolres.932270. [DOI] [PubMed] [Google Scholar]
- 17.Abe Y., Uchinami H., Kudoh K., et al. Liver epithelial cells proliferate under hypoxia and protect the liver from ischemic injury via expression of HIF-1 alpha target genes. Surgery. 2012;152(5):869–878. doi: 10.1016/j.surg.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benderro G. F., Sun X., Kuang Y., Lamanna J. C. Decreased VEGF expression and microvascular density, but increased HIF-1 and 2alpha accumulation and EPO expression in chronic moderate hyperoxia in the mouse brain. Brain Research. 2012;1471:46–55. doi: 10.1016/j.brainres.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C., Shi Y., Han Z. Suppression of the dual-specificity phosphatase MKP-1 enhances HIF-1 trans-activation and increases expression of EPO. Biochemical and Biophysical Research Communications. 2003;312(3):780–786. doi: 10.1016/j.bbrc.2003.10.186. [DOI] [PubMed] [Google Scholar]
- 20.Singh N., Deb R., Kashyap P. C., et al. Body mass index and per capita income influence duodenal ulcer healing and H. pylori eradication whilst dietary factors play no part. Tropical Gastroenterology. 2008;29(1):26–31. [PubMed] [Google Scholar]
- 21.León-Velarde F., Maggiorini M., Reeves J. T., et al. Consensus statement on chronic and subacute high altitude diseases. High Altitude Medicine & Biology. 2005;6(2):147–157. doi: 10.1089/ham.2005.6.147. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y. Q., Lang Q. B., Li G. G. Study on diagnostic standard for dampness syndrome in patients with chronic gastritis. Chinese Journal of Integrated Traditional and Western Medicine. 2005;25(11):975–979. [PubMed] [Google Scholar]
- 23.Fang J. Y., Liu W. Z., Shi Y., Ge Z. Z., Xiao S. D. Consensus on chronic gastritis in China - second national consensus meeting on chronic gastritis (14–16 september 2006 Shanghai, China) Journal of Digestive Diseases. 2007;8(2):107–119. doi: 10.1111/j.1443-9573.2007.00295.x. [DOI] [PubMed] [Google Scholar]
- 24.Manninen P., Karvonen A.-L., Laukkarinen J., Aitola P., Huhtala H., Collin P. Colorectal cancer and cholangiocarcinoma in patients with primary sclerosing cholangitis and inflammatory bowel disease. Scandinavian Journal of Gastroenterology. 2015;50(4):423–428. doi: 10.3109/00365521.2014.946085. [DOI] [PubMed] [Google Scholar]
- 25.D’Argenio G., Mazzone G., Tuccillo C., et al. Apple polyphenol extracts prevent aspirin-induced damage to the rat gastric mucosa. British Journal of Nutrition. 2008;100(6):1228–1236. doi: 10.1017/S0007114508988747. [DOI] [PubMed] [Google Scholar]
- 26.Li K., Gesang L., He C. Mechanism of apoptosis involved in gastric mucosal lesions in Tibetans with high-altitude polycythemia. Experimental and Therapeutic Medicine. 2017;14(4):3780–3787. doi: 10.3892/etm.2017.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song J.-L., Choi J.-H., Seo J.-H., Kil J.-H., Park K.-Y. Antioxidative effects of fermented sesame sauce against hydrogen peroxide-induced oxidative damage in LLC-PK1 porcine renal tubule cells. Nutrition Research and Practice. 2014;8(2):138–145. doi: 10.4162/nrp.2014.8.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saul G. D., Lukina W. J., Brakebush S. C., Wilmot D. E., Tammelin B. R. Voluntary hyperventilation into a simple mixing chamber relieves high altitude hypoxia. Aviation, Space, and Environmental Medicine. 2002;73(4):404–407. [PubMed] [Google Scholar]
- 29.Julian C. G., Gonzales M., Rodriguez A., et al. Perinatal hypoxia increases susceptibility to high-altitude polycythemia and attendant pulmonary vascular dysfunction. American Journal of Physiology-Heart and Circulatory Physiology. 2015;309(4):H565–H573. doi: 10.1152/ajpheart.00296.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J., Yang Y.-Z., Tang F., et al. CYP17A1 and CYP2E1 variants associated with high altitude polycythemia in Tibetans at the Qinghai-Tibetan Plateau. Gene. 2015;566(2):257–263. doi: 10.1016/j.gene.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 31.Nefti O., Grongnet J. F., David J. C. Overexpression of αB crystallin in the gastrointestinal tract of the newborn piglet after hypoxia. Shock. 2005;24(5):455–461. doi: 10.1097/01.shk.0000183396.06143.36. [DOI] [PubMed] [Google Scholar]
- 32.Xu J., Yang Y. Z., Tang F., et al. EPAS1 gene polymorphisms are associated with high altitude polycythemia in tibetans at the qinghai-tibetan plateau. Wilderness & Environmental Medicine. 2015;26(3):288–294. doi: 10.1016/j.wem.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Li K., Gesang L., Dan Z., Gusang L., Dawa C., Nie Y. Transcriptome reveals 1400-fold upregulation of APOA4-APOC3 and 1100-fold downregulation of GIF in the patients with polycythemia-induced gastric injury. PLoS ONE. 2015;10(10):p. e0140534. doi: 10.1371/journal.pone.0140534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cimino F., Balestra C., Germonpré P., et al. Pulsed high oxygen induces a hypoxic-like response in human umbilical endothelial cells and in humans. Journal of Applied Physiology. 2012;113(11):1684–1689. doi: 10.1152/japplphysiol.00922.2012. [DOI] [PubMed] [Google Scholar]
- 35.Han S. H., Kim M., Park K., Kim T.-H., Seol D.-W. Blockade of processing/activation of caspase-3 by hypoxia. Biochemical and Biophysical Research Communications. 2008;375(4):684–688. doi: 10.1016/j.bbrc.2008.08.091. [DOI] [PubMed] [Google Scholar]
- 36.Rocco M., D'Itri L., De Bels D., Corazza F., Balestra C. The ‘normobaric oxygen paradox’: a new tool for the anesthetist? Minerva Anestesiologica. 2014;80(3):366–372. [PubMed] [Google Scholar]
- 37.Kiboub F. Z., Balestra C., Loennechen Ø., Eftedal I. Hemoglobin and erythropoietin after commercial saturation diving. Frontiers in Physiology. 2018;9:p. 1176. doi: 10.3389/fphys.2018.01176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S., Peng H., Rowat A., et al. The effect of concentration and duration of normobaric oxygen in reducing caspase-3 and -9 expression in a rat-model of focal cerebral ischaemia. Brain Research. 2015;1618:205–211. doi: 10.1016/j.brainres.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 39.Pereira E. R., Frudd K., Awad W., Hendershot L. M. Endoplasmic reticulum (ER) stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 (HIF-1) transcriptional activity on targets like vascular endothelial growth factor (VEGF) The Journal of Biological Chemistry. 2014;289(6):3352–3364. doi: 10.1074/jbc.m113.507194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ousset M., Bouquet F., Fallone F., et al. Loss of ATM positively regulates the expression of hypoxia inducible factor 1 (HIF-1) through oxidative stress: role in the physiopathology of the disease. Cell Cycle. 2010;9(14):2814–2822. doi: 10.4161/cc.9.14.12248. [DOI] [PubMed] [Google Scholar]
- 41.Jusman S. W., Halim A., Wanandi S. I., et al. Expression of hypoxia-inducible factor-1alpha (HIF-1alpha) related to oxidative stress in liver of rat-induced by systemic chronic normobaric hypoxia. Acta Medica Indonesiana. 2010;42(1):17–23. [PubMed] [Google Scholar]
- 42.Heise K., Estevez M. S., Puntarulo S., et al. Effects of seasonal and latitudinal cold on oxidative stress parameters and activation of hypoxia inducible factor (HIF-1) in zoarcid fish. Journal of Comparative Physiology B. 2007;177(7):765–777. doi: 10.1007/s00360-007-0173-4. [DOI] [PubMed] [Google Scholar]
- 43.Hosain M. Z., Mori T., Kishimura A., Katayama Y. Synergy between phenotypic modulation and ROS neutralization in reduction of inflammatory response of hypoxic microglia by using phosphatidylserine and antioxidant containing liposomes. Journal of Biomaterials Science, Polymer Edition. 2016;27(3):290–302. doi: 10.1080/09205063.2015.1125565. [DOI] [PubMed] [Google Scholar]
- 44.Ren H., Li X., Cheng G., et al. The effects of ROS in prostatic stromal cells under hypoxic environment. The Aging Male. 2015;18(2):84–88. doi: 10.3109/13685538.2015.1018159. [DOI] [PubMed] [Google Scholar]
- 45.Saito Y., Ishii K.-A., Aita Y., et al. Loss of SDHB elevates catecholamine synthesis and secretion depending on ROS production and HIF stabilization. Neurochemical Research. 2016;41(4):696–706. doi: 10.1007/s11064-015-1738-3. [DOI] [PubMed] [Google Scholar]
- 46.Murphy P., Ahmed N., Hassan H. T. Increased serum levels of vascular endothelial growth factor correlate with splenomegaly in polycythemia vera. Leukemia Research. 2002;26(11):1007–1010. doi: 10.1016/S0145-2126(02)00053-X. [DOI] [PubMed] [Google Scholar]
- 47.Fu R., Chen Y., Wang X.-P., et al. Wogonin inhibits multiple myeloma-stimulated angiogenesis via c-Myc/VHL/HIF-1a signaling axis. Oncotarget . 2016;7(5):5715–5727. doi: 10.18632/oncotarget.6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parsanejad M., Zhang Y., Qu D., et al. Regulation of the VHL/HIF-1 pathway by DJ-1. The Journal of Neuroscience. 2014;34(23):8043–8050. doi: 10.1523/jneurosci.1244-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H., Gao P., Fukuda R., et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11(5):407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Jung Y.-S., Lee S.-J., Lee S.-H., et al. Loss of VHL promotes progerin expression, leading to impaired p14/ARF function and suppression of p53 activity. Cell Cycle. 2013;12(14):2277–2290. doi: 10.4161/cc.25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dolka I., Krol M., Sapierzynski R. Evaluation of apoptosis-associated protein (Bcl-2, Bax, cleaved caspase-3 and p53) expression in canine mammary tumors: an immunohistochemical and prognostic study. Research in Veterinary Science. 2016;105:124–133. doi: 10.1016/j.rvsc.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Feinman R., Deitch E. A., Watkins A. C., et al. HIF-1 mediates pathogenic inflammatory responses to intestinal ischemia-reperfusion injury. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2010;299(4):G833–G843. doi: 10.1152/ajpgi.00065.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.