Abstract
Sensitive measurements of cellular Zn(II) uptake currently rely on quantitating radioactive emissions from cells treated with 65Zn(II). Here, we describe a straightforward and reliable method employing a stable isotope to sensitively monitor Zn(II) uptake by metazoan cells. First, biological media selectively depleted of natural abundance Zn(II) using A12-resin [Richardson, C. E. R. et al. J. Am. Chem. Soc. 2018, 140, 2413] is restored to physiological levels of Zn(II) by addition of a non-natural Zn(II) isotope distribution comprising 70% 70Zn(II). The resulting 70Zn(II)-enriched media facilitates quantitation of Zn(II) uptake using inductively coupled plasma-mass spectrometry (ICP-MS). This sensitive and reliable assay assesses Zn(II)-uptake kinetics at early timepoints and can be used to delineate how chemical and genetic perturbations influence Zn(II) uptake. Further, the use of ICP-MS in a Zn(II)-uptake assay permits simultaneous measurement of multiple metal ion concentrations. We used this capability to show that, across three cell lines, Zn(II) deficiency enhances selectivity for Zn(II) over Cd(II) uptake.
INTRODUCTION
Zn(II) mediates inter- and intra-cellular signaling1-4 and is an obligatory cofactor for members of nearly all enzyme classes.5,6 Loosely bound or ‘mobile’ pools of zinc modulate neurotransmission,3,7,8 particularly in the context of sensory perception,1,3 and Zn(II) fluxes play important roles in diverse biological settings, including immune responses,9 fertilization,10,11 and mammary tissue function.12,13 Establishing mechanisms of Zn(II) function in these and other processes benefits from methods to assay cellular Zn(II) uptake and trafficking.
Small molecule sensors and genetically encoded sensors that exhibit a fluorescent signal upon Zn(II) binding are valuable tools for studying the roles of Zn(II) in tissues and cells.14-18 These sensors, however, are designed to detect mobile Zn(II), which is a labile pool of Zn(II) that comprises only a small fraction of the total Zn(II) in most cell types.14 Consequently, they are not generally useful for quantifying Zn(II) uptake or measuring changes in total cellular Zn(II).
Instead, investigators typically measure cellular assimilation of the radioisotope 65Zn(II), a gamma emitter (t1/2 = 244 d), that can be spiked into cell culture media or a defined media, or administered to a live animal.19-21 Despite the high sensitivity afforded by the use of a radioactive isotope, broad application of 65Zn(II) to monitor Zn(II) uptake is hampered by practical concerns associated with a gamma-emitting radioisotope, including requirements for specialized protective equipment. Furthermore, measurement of 65Zn(II) uptake cannot report on total cellular Zn(II), an important parameter that must then be evaluated in a parallel assay. In contrast, inductively coupled plasma-mass spectrometry (ICP-MS) permits sensitive measurement of total cellular metal content, but routine ICP-MS experiments only provide total metal per unit of cellular material and often cannot readily define Zn(II) uptake rates.
A protocol combining the advantages of both methods – the high signal-to-noise ratio afforded by radioactivity-based assays and the experimental simplicity and capacity to quantitate total Zn(II) provided by ICP-MS – would be valuable. The 70Zn(II) isotope occurs in natural abundance at 0.62%, but it can be sourced at high enrichment (up to 72%) and is readily quantitated using ICP-MS.22-26 Thus, one approach previously applied in whole animal studies and bacterial/yeast experiments is to employ a highly 70Zn(II)-enriched diet or chemically defined microbial growth media followed by ICP-MS to quantitate 70Zn(II) during uptake or excretion studies.22-26 A similar approach to measure Zn(II) uptake could, in principle, be taken for mammalian cell culture. One option is to spike tissue culture media containing natural abundance Zn(II) isotope distributions with 70Zn(II).27 Unfortunately, this strategy suffers from low sensitivity at early time points unless a large quantity of 70Zn(II) is added to the media, elevating total media Zn(II) concentrations to levels that far exceed those in unmodified media. An alternative option would be to deplete the cell culture medium of Zn(II), then add 70Zn(II) back at high enrichment.
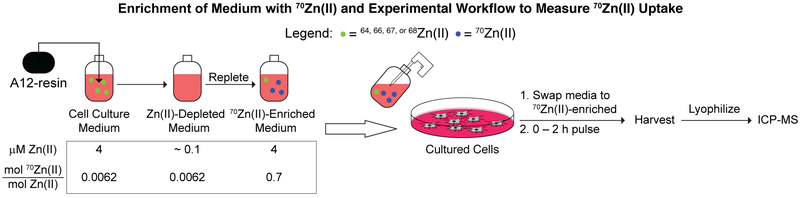
We recently developed the “A12-resin,” comprising the Zn(II)-binding S100A12 protein immobilized on an agarose support.28 A12-resin selectively removes Zn(II) from diverse and complex biological media without perturbing concentrations of other metal ions.29 We anticipated that by treating tissue culture media with A12-resin to remove natural abundance Zn(II), followed by repletion with 70Zn(II) we could enrich 70Zn(II) by greater than 100-fold over its natural abundance in normal media. In order to measure the kinetics of 70Zn(II) uptake, cells could then be ‘pulsed’ with this 70Zn(II)-enriched medium and the intracellular accumulation of the isotope could be measured by ICP-MS as a function of time (Figure 1). Such a protocol would enable ICP-MS-based measurements of Zn(II) uptake by cells cultured in media containing typical Zn(II) concentrations (Figure 1), unlike the 70Zn(II) “spike-in” approach mentioned above.27
Figure 1.
Method to prepare 70Zn-enriched medium and experimental workflow to monitor 70Zn(II) uptake into metazoan cells.
Based on the foregoing premise, we developed and optimized an assay for quantitation of Zn(II) uptake by metazoan cells. To demonstrate the utility of the method, we (i) measured 70Zn(II) uptake kinetics in several cell lines, (ii) assessed the enhancement or attenuation of Zn(II) uptake by a variety of chemical or genetic perturbations, and (iii) investigated whether cell state and lineage alter selectivity for Zn(II) versus Cd(II) uptake. The results are reported here.
MATERIALS AND METHODS
Media Preparation, Cell Lines, and Reagents.
HEK293T, HeLa, HepG2, and HT1080 cells (maintained at fewer than 30 passages) were grown in complete Dulbecco’s Modified Eagle’s Medium (DMEM, Corning) supplemented with 10% fetal bovine serum (FBS), 100 IU penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine in a humidified, 37 °C incubator maintained at 5% CO2(g). DMEM + 10% FBS (DMEM/FBS) was depleted of Zn(II) with A12-resin prepared and applied as previously described.28 70Zn(II)-gluconate was purchased from Millipore Sigma as a solid. In order to prepare 70Zn(II)-enriched media, 70Zn(II)-gluconate (1.0 mg/mL in Milli-Q water) was titrated into Zn(II)-depleted DMEM/FBS and ICP-MS-measured concentrations of 64Zn, 66Zn, 67Zn, 69Zn, and 70Zn were summed until the total Zn concentration in the medium was approximately 250 ppb (~3.8 μM) total Zn(II). Sources of all other reagents are provided in the Supporting Information.
Zn(II) Uptake Assay.
HEK293T cells (~500,000) were plated in a six-well dish. After 2 days, the medium was aspirated and 70Zn(II)-enriched medium (1.0 mL) was added to each appropriate well. Cells were harvested by first washing each well with phosphate-buffered saline (PBS) containing EDTA (100 μM, 2 × 2.0 mL) and subsequently adding Milli-Q water (18.2 mΩ×cm, 1.00 mL) to each well and incubating for 2–4 min. A portion of the resulting cell lysate (0.90 mL) was collected and transferred to an Eppendorf Safe-Lock tube, snap-frozen in N2(l), and then thawed at rt for a total of three freeze-thaw cycles. The insoluble lysate was pelleted (10 min, 10,000 × g), and the soluble protein concentration of each sample was determined by using the Pierce 660 nm Protein Assay in a 96-well plate (200 μL reagent, 14 μL sample, bovine serum albumin standard curve). Samples were then frozen, lyophilized overnight, and the resulting white, flaky solid was taken up in concentrated nitric acid (0.20 mL, ultrapure for trace metal analysis, BDH Aristar) and heated at 70 °C for 1 h. A portion of each sample (142 μL) was then diluted with water (2.00 mL) and a Tb internal standard (Agilent 5190-8590, stock at 10,000 ppb in 10% nitric acid diluted to 100 ppb in Milli-Q water for addition to samples, 2 ppb final concentration), after which the metal content of the samples was assessed by ICP-MS. Time points on the abscissa of plots and described in the text refer to the harvesting times following administration of the 70Zn(II) pulse. The time point 0 h refers to a population of cells harvested immediately after addition of 70Zn(II)-enriched media to parallel samples but never treated with such media.
Metal Competition Assay.
For metal competition experiments, the Zn(II) uptake assay protocol above was followed, with the modification that 50 μM of Cd(II) (aq), Co(II) (aq), Cu(II) (aq), Fe(II) (aq), Mn(II) (aq), Ni(II) (aq), or Zn(II) (aq) (natural abundance isotope distribution) was added from 20 mM stock solutions, as indicated, to 70Zn(II)-enriched media prior to cell treatment. The solution of Fe(II) (aq) solution was prepared by reduction of Fe(III) in 0.1 N HCl with ascorbate (10 equiv) immediately prior to addition to DMEM/FBS.
ICP-MS.
The concentrations of Mg, Ca, Fe, Mn, Co, Ni, Zn isotopes, and Cd were measured by using an Agilent 7900 ICP-MS equipped with an integrated autosampler, in helium mode at the MIT CEHS Bioimaging and Chemical Analysis Core Facility, as previously reported.30 Nitric acid used for ICP-MS sample and standard preparation was ultrapure for trace metals analysis grade (BDH Aristar). Samples for cell pellet ICP-MS were prepared as described above. The ICP-MS instrument was calibrated with dilutions of the Agilent Environmental Calibration Standard Mix (Mg, Ca, Fe: 0–500,000 ppb; Mn, Co, Ni, Zn: 0–5,000 ppb) at 5% nitric acid for cell pellet ICP-MS and at 1.5% nitric acid for cell culture media ICP-MS. Solutions of DMEM/FBS were prepared for ICP-MS measurement by 1:2 dilution of the media with 3% nitric acid (prepared by addition of 22 parts water to 1 part 70% nitric acid) followed by addition of Tb internal standard to 2 ppb (Agilent, described above).
A modification of this protocol was used to measure the 70Zn content of 70Zn(II)-enriched DMEM/FBS. The natural abundance of 70Zn is 0.62%. The commercially available calibration standard used to calibrate the ICP-MS has, at the highest concentration used, 31 ppb 70Zn. Therefore, 70Zn(II)-enriched medium was diluted 1:10 in ultrapure water and then further diluted 1:2 with 3% nitric acid before addition of a 2 ppb Tb internal standard (Agilent, described above) prior to ICP-MS analysis.
In order to calculate the molarities, and the corresponding molar amounts, of metals in samples, a density of 1.00 g/mL was assumed for all solutions. Therefore, 1.00 ppb was taken to be equal to 1.00 μg/L and the molarity of a particular solution equal to the measured concentration of a particular ion in ppb, divided by the molecular weight of the ion, multiplied by 10−6.
Cloning and expression of ZIP4, ZIP8, and ZIP10 into CMV-Sport-6.
Primers to amplify the coding sequence of each gene from pEntr23331 and install an N-terminal KpnI cut site and a C-terminal stop codon and XhoI site (for ZIP8 and ZIP10) or XbaI site (ZIP4) were designed and ordered from Sigma (Table S1). The genes were PCR-amplified by using Q5 polymerase (NEB), and each PCR product was gel-purified. A vector encoding alkaline phosphatase (GE Dharmacon 5754297) in the CMV-Sport 6 backbone was digested in parallel with the above PCR products using the appropriate restriction enzymes, as indicated. Inserts were ligated into the CMV-Sport 6 backbone using T4 ligase (NEB) before transformation into chemically competent DH10B Escherichia coli. Mini-prepped plasmids were sequence-confirmed. Vectors were midi-prepped using an Omega E.Z.N.A. Plasmid Midi Kit. Immediately before transfection, plasmid DNA was further purified with a Cycle Pure Kit (Omega). Subsequently, HEK293T cells plated at a density of 5 × 106 cells per 10-cm dish the previous day were transfected using Lipofectamine 3000 at half the manufacturer-recommended reagent volumes and DNA mass. The following day, transfected cells were plated and treated as described in the Zn(II)-uptake assay protocol. Expression of transfected cDNA constructs was assessed by using qPCR on samples parallel to those harvested for metal analysis.
Quantitative PCR (qPCR).
Cells were washed with PBS and RNA was extracted using the Omega E.Z.N.A. Total RNA Extraction Kit. cDNA was prepared from 1.00 μg of RNA with an Applied Biosystems Reverse Transcriptase cDNA Kit in a thermocycler. Samples were analyzed in a Light Cycler 480 II Real Time PCR Instrument in the MIT BioMicro Center following previously described methods.32 qPCR primer specificity was assessed by agarose gel electrophoresis and melting curve analyses. Primer sequences are listed in Table S1.
Statistical Analyses.
For each experiment, either individual biological replicates are shown along with mean and standard error of the mean (SEM) or solid bars indicating the magnitude of the mean are shown with error bars showing the standard error of the mean (SEM). Statistical significance was assessed by using an unpaired heteroskedastic Student’s t-test in Microsoft Excel. Where appropriate, p-values were adjusted with the Bonferroni correction.33
RESULTS AND DISCUSSION
Zn(II) Uptake Assay Development and Validation
We began by preparing 70Zn(II)-enriched culture medium by replenishing A12-resin-depleted tissue culture media28 with 70Zn(II)-enriched zinc gluconate (Figure 1). This protocol afforded DMEM/FBS that contained approximately 70% 70Zn(II) (Table S2), a >100-fold enrichment of 70Zn relative to the isotope’s natural abundance of 0.62% at a normal (~3.8 μM) concentration of Zn.
Next, we used this 70Zn(II)-enriched media to assay cellular Zn(II) uptake under conditions of normal cell proliferation via the following protocol: (1) Two days after plating cells in 6-well dishes in complete DMEM/FBS medium, the medium was replaced with 70Zn(II)-enriched medium. (2) Cells were incubated at 37 °C and then harvested 15, 30, 60, or 120 min after the media change. At each time point, cell monolayers were washed with 100 μM EDTA in PBS before being taken up in water (18.2 mΩ×cm) directly in the plate. (3) After transferring each cell lysate to a microcentrifuge tube and freeze-thawing, the protein concentration was determined. (4) Samples were then snap-frozen in liquid nitrogen and lyophilized overnight. (5) Lyophilized samples were dissolved in nitric acid and heated at 70 °C for 1 h. (6) Samples were diluted 1:14 into Milli-Q water (18.2 mΩ×cm) containing 2 ppb Tb as an internal standard and the metal concentrations of the resulting samples were measured by ICP-MS (Figure 1).
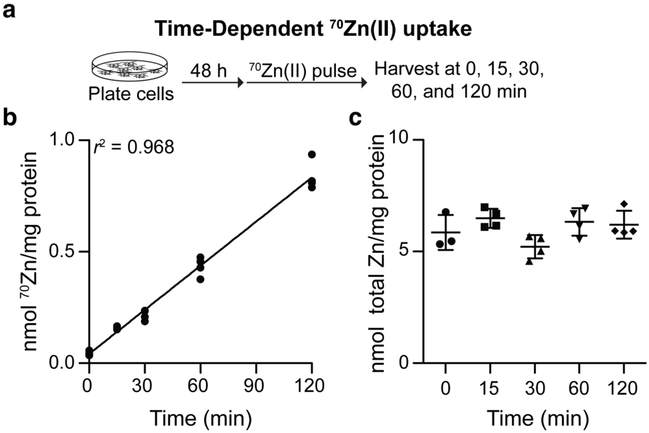
By following this protocol, we observed that cells cultured in 70Zn-enriched media exhibited time-dependent 70Zn(II) uptake (Figures 2a and 2b). Over the course of 2 h, the cells accumulated approximately 0.8 nmol 70Zn(II) per mg-protein, corresponding to an uptake rate of ~ 6 pmol 70Zn(II) per mg-protein per-min. During the 2-h period, the rate of 70Zn(II) uptake was linear and total cellular Zn(II) content per mg-protein did not change (Figure 2c). The observation of linearity highlights the robustness of this assay. At extended time points, if a cell secretes Zn(II) or Zn(II)-containing-proteins, and 70Zn(II) comprises a significant proportion of total cellular Zn(II), significant amounts of 70Zn(II) will be excreted from cells. In this assay, the measurable consequence of such 70Zn(II) secretion would probably be nonlinear cellular accumulation of 70Zn(II). Therefore, the observation of linear uptake (Figure 2b) is consistent with Zn(II) excretion not confounding the results of the assay over this 2-h timecourse.
Figure 2.
(a) Workflow to assess time-dependence of 70Zn(II) uptake. (b) HEK293T cells grown in 70Zn(II)-enriched media exhibited time-dependent 70Zn(II) uptake (N = 3–4) but (c) total Zn per mg-protein did not change over the course of the experiment (N = 3–4, ±SEM).
Notably, the assay was sufficiently sensitive to measure Zn(II) uptake of 150 pmol Zn per mg-protein after 15 min of incubation in 70Zn(II)-enriched media. Previously, to the best of our knowledge, only 65Zn radioactivity-based assays could be applied to measure such small changes in Zn(II) uptake by human cells. We also note that the 70Zn uptake velocity measured in these experiments (6 pmol per mg-protein per-min) was consistent with rates derived from assessing 65Zn(II) uptake in HEK293T cells, which are reported to vary from 2–50 pmol per mg-protein per-min at an extracellular Zn(II) concentration of approximately 4 μM.34-37 This wide range highlights the critical role of the detailed protocol used in a given Zn(II) uptake assay. For instance, detergent-mediated lysis can solubilize five-fold more protein than freeze-thaw cycles in detergent-free buffer,38 the protocol used here. Thus, reported uptake parameters normalized to total protein using different lysis techniques should be expected to differ by as much as a factor of five. Other protocol-specific factors can further broaden the range of measured uptake parameters. As a consequence of such considerations, uptake rate comparisons are best made between experiments conducted with identical protocols.
A key advantage of our protocol over approaches in which 70Zn(II) is “spiked” into media is that it affords high sensitivity. We found that it is possible to measure uptake of 70Zn over the course of 5 h if DMEM/FBS is spiked with 20 ppb 70Zn(II), similar to prior ICP-MS-based protocols (Figure S1).27 However, when we used 70Zn(II)-enriched medium prepared by using the A12-resin as described above, we observed a >10-fold increase in sensitivity compared to spiking DMEM/FBS with 20 ppb 70Zn(II) (Figure S1).
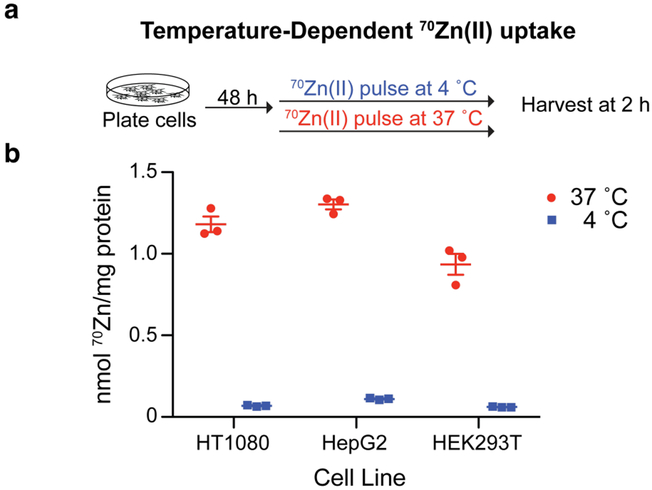
We next used two separate experimental approaches to assess whether this ICP-MS-based Zn(II)-uptake assay selectively measures intracellular Zn(II), as opposed to Zn(II) adhering to cell surfaces or to tissue culture plastic-ware. Previous work with 65Zn revealed that Zn(II) uptake is significantly diminished at low temperatures,39 consistent with the lower activity of recombinant ZIP-family Zn(II) transporters at such temperatures.40 Therefore, in our first approach, we maintained cell monolayers at either 4 or 37 °C throughout a 2-h pulse with 70Zn(II)-enriched media (Figure 3a). Across three cell lines, we observed much more 70Zn(II) uptake in cells maintained at 37 °C compared to those maintained at 4 °C (Figure 3b). In a second experiment, after exposing cells to a 2-h pulse of 70Zn(II)-enriched media, we washed the cell monolayers with PBS containing 100 μM of one of three metal chelators, each of which is known to bind Zn(II) with different affinities and with different associated rate constants (Figures S2a–c),7 or with PBS lacking chelator. Cells were then harvested and prepared for ICP-MS analysis following our standard protocol. None of the chelators altered the subsequent measured amount of 70Zn(II) levels in cell lysates (Figure S2d). Together, these results are consistent with the conclusion that this 70Zn-uptake assay measures intracellular 70Zn(II) levels, not 70Zn(II) bound to cell surfaces or plates.
Figure 3.
(a) Workflow to assess temperature-dependence of 70Zn(II) uptake. (b) HT1080, HepG2, and HEK293T cells grown in 70Zn(II)-enriched media took up significantly more Zn(II) when maintained at 37 °C for 2 h than when maintained at 4 °C (N = 3, ±SEM).
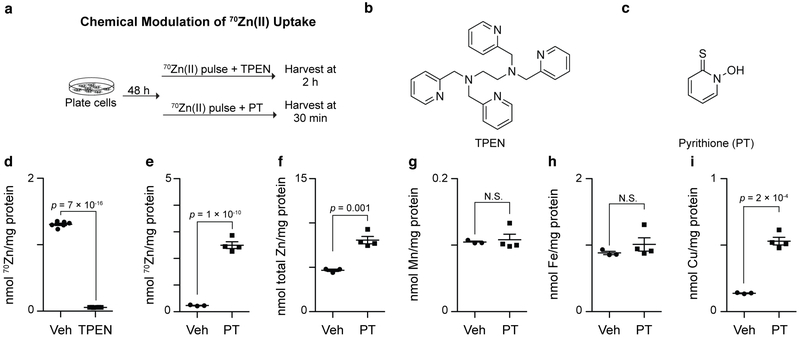
Confident that this assay measures Zn(II) taken up by cells, we next assessed whether it could be used to evaluate the effect of perturbations on Zn(II) uptake mediated by either the metal chelator N,N,N',N'-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine (TPEN) or the ionophore pyrithione. Briefly, HEK293T cells were incubated with 70Zn(II)-enriched medium (3.8 μM total Zn(II)) containing either TPEN (5 μM, forms a 1:1 complex with Zn(II)) or pyrithione (10 μM, forms a 1:2 complex with Zn(II); Figures 4a–c). TPEN significantly diminished cellular Zn(II) uptake (Figure 4d), suggesting that cells are unable to effectively uptake Zn(II)-bound TPEN. In contrast, and as expected based on prior work where pyrithione was used to deliver Zn(II) into cells,41,42 this ionophore significantly increased the 70Zn(II) content of HEK293T cells (Figure 4e). Pyrithione treatment also enhanced total Zn(II) per mg-protein relative to that of cells not treated with pyrithione (Figure 4f), indicating that pyrithione enhances the rate of Zn(II) uptake more than the rate of Zn(II) efflux.
Figure 4.
(a) Workflow to assess effects of TPEN and pyrithione (PT) on 70Zn uptake. (b) Structure of TPEN. (c) Structure of PT. (d) TPEN (5 μM) diminishes 70Zn(II) uptake into HEK293T cells compared to vehicle (Veh) treatment (N = 3, ±SEM). (e) HEK293T cells incubated with 4 μM 70Zn(II) and 10 μM PT take up significantly more 70Zn(II) than cells incubated with 4 μM 70Zn(II) in the absence of PT (N = 3–4 ±SEM). (f–h) HEK293T cells treated with PT take up significantly more Zn (f) and Cu (i) but not Mn (g) or Fe (h) than do vehicle-treated cells (N = 3, ±SEM). p-values indicate Student’s t-test adjusted with the Bonferroni correction for multiple testing. N.S. indicates p > 0.05.
One key advantage of an ICP-MS assay for Zn(II) uptake over a radioactivity-based assay is that the concentrations of other metals can be measured simultaneously with Zn. By analyzing levels of other metal ions, we found that pyrithione treatment did not increase total cellular Mn or Fe levels but, consistent with previous reports,41 it did significantly increase the cellular concentration of Cu (Figures 4g–i, note that the concentration of Cu in DMEM/FBS is approximately 200 nM). This observation highlights that experiments in which cells are treated with high concentrations of Zn(II)-pyrithione should be interpreted with caution, as the reagent can significantly perturb cellular Cu levels.
Collectively, these experiments show that depletion of natural abundance Zn(II) from tissue culture media using the A12-resin followed by repletion with 70Zn(II) yields an assay for Zn(II) uptake by metazoan cells that is sensitive and straightforward to apply. Moreover, while we have performed the assay under normal Zn(II) concentrations (~3.8 μM),28 we note that it could be performed at a range of 70Zn(II) concentrations by repleting 70Zn(II) to different final concentrations, providing additional experimental flexibility. In the following sections, we illustrate the utility of this assay by (i) assessing the effects of Zn(II) transporter overexpression on Zn(II) uptake and (ii) evaluating how Zn(II) deficiency affects the selectivity of Zn(II) vs Cd(II) uptake.
Monitoring How Genetic Perturbations Modulate Zn(II) Uptake Kinetics
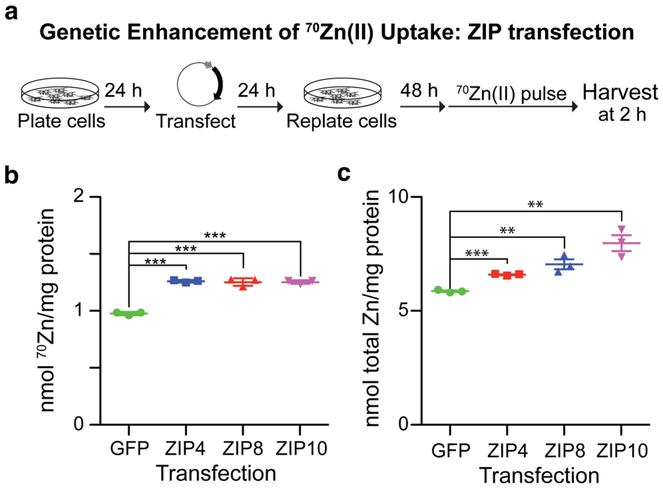
We next employed our 70Zn(II)-uptake assay to examine the effects of Zn(II) transporter overexpression on Zn(II) uptake — an experiment that has previously been performed only with the radioactivity-based 65Zn(II) assay, to the best of our knowledge.21 We selected three cell surface-localized Zn(II) transporters thought to deliver Zn(II) from outside the cell into the cytosol, specifically ZIP4, ZIP8, and ZIP10. Two factors motivated selection of these particular transporters.43 First, uptake through ZIP transporters is one of the main routes by which Zn(II) enters metazoan cells.43 Second, previous work established both ZIP4 and ZIP8 as bona fide Zn(II) transporters.35,44,45 On the other hand, although ZIP10 is known to be transcriptionally upregulated under Zn(II)-deficient conditions,46 to our knowledge the potential impact of human ZIP10 expression on Zn(II) uptake has not been rigorously evaluated to date.
We began by transfecting vectors encoding either SLC39A4/ZIP4, SLC39A8/ZIP8, SLC39A10/ZIP10, or GFP (as a control) into HEK293T cells. qPCR analysis revealed that Z1P4, ZIP8, and ZIP 10 mRNA levels were all enhanced as a consequence of the transfection (Figure S3). Further corroborating successful overexpression of two of the ZIP transporters, levels of transcripts encoding metallothionein 1A, a transcriptionally regulated Zn(II)-buffering protein in mammalian cells,5 were significantly enhanced in cells overexpressing ZIP4 and ZIP8 (Figure S3). Elevated metallothionein transcript levels are consistent with elevated intracellular Zn(II) content.47
Transfected HEK293T cells were next incubated in 70Zn(II)-enriched media for 2 h before ICP-MS analysis of 70Zn(II) content, revealing two key results (Figure 5a). First, across experiments, cells overexpressing any one of the three ZIP transporters accumulated significantly (p < 0.001) more 70Zn during the pulse than did GFP-expressing cells (Figure 5b). This observation is in agreement with previous reports showing that ZIP4 and ZIP8 expression enhances the rate of Zn(II) uptake35,45 and supports the notion that ZIP10, like other human ZIP-family members, transports Zn(II). Second, and consistent with literature reports regarding other ZIPs,21 ZIP overexpression significantly increased (p < 0.01) total Zn(II) per mg-protein relative to cells expressing GFP (Figure 5c). Both observations — increased Zn(II) uptake rate and increased total Zn(II) upon ZIP overexpression —are important to consider given that aberrant ZIP expression is associated with a number of human pathologies.48,49
Figure 5.
(a) Workflow to assess effects of ZIP transporter overexpression on 70Zn(II) uptake. (b) HEK293T cells transfected with ZIP4, ZIP8, or ZIP10 took up significantly more 70Zn(II) over a 2-h period than did cells transfected with GFP (N = 3, ±SEM). (c) HEK293T cells transfected with ZIP4, ZIP8, or ZIP10 contained significantly more total Zn(II) than did cells expressing GFP (N = 3, ±SEM). p-values indicate Student’s t-test adjusted with the Bonferroni correction for multiple testing (*** indicates p < 0.001, ** indicates p < 0.01).
In summary, monitoring 70Zn(II) uptake from 70Zn(II)-enriched media provides an alternative to measuring radioactive 65Zn(II) uptake that can be used to sensitively evaluate how genetic perturbations impact Zn(II) uptake. Application of the assay here provided evidence that ZIP10 is a Zn(II) transporter. We anticipate that, beyond cell-surface Zn(II) transporters, this experimental procedure could be used to assess the effects of diverse genetic and chemical genetic perturbations on cellular Zn(II) uptake. Candidate proteins for future work could include those involved in Zn(II) homeostasis (e.g., metallothioneins and intracellular Zn(II) transporters) or zinc-binding proteins (e.g., zinc finger transcription factors or zinc enzymes).
Metal Competition Experiments
As noted above, one benefit of using ICP-MS to monitor 70Zn(II) uptake is that a single experiment reports on Zn(II) uptake kinetics, total cellular Zn(II) content, and changes in the levels of other metals. Regarding this last point, the ability to monitor multiple metals in a single experiment can be important in many contexts. For instance, this capability is valuable when assessing selectivity of metal uptake. In a typical radioactivity-based experiment to assess uptake selectivity using 65Zn(II), a decrease in 65Zn(II) uptake is commonly taken to indicate competition by other metals for uptake through Zn(II) transporters.21,34,35,39,50,51 However, unless the uptake of other ions is directly measured, possibly by using a second radioisotope,45,52,53 such a conclusion is necessarily uncertain.
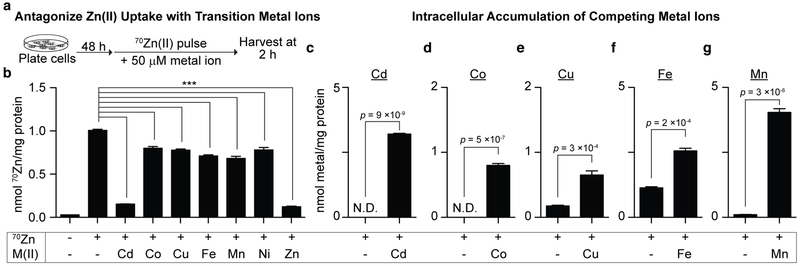
To investigate whether other metals compete with Zn(II) for uptake by HEK293T cells, we prepared 70Zn(II)-repleted DMEM/FBS alone or with a 50 μM (~13 equiv. relative to Zn(II) levels in 70Zn(II)-repleted DMEM/FBS) supplement of another metal ion (Cd(II), Co(II), Cu(II), Fe(II), Mn(II), Ni(II), and natural abundance Zn(II)). We then monitored (i) the effect of other metal ions on 70Zn(II) uptake over a 2-h 70Zn(II) pulse with this medium (Figure 6a) as well as (ii) the intracellular accumulation of each ion.
Figure 6.
(a) Workflow to assess metal selectivity of cellular Zn(II) import machinery. (b) 70Zn(II) import into HEK239T cells was significantly inhibited by 50 μM of several divalent cations (denoted M(II)), but most severely by Cd(II) (N = 3, ±SEM, *** indicates p < 0.001 for each comparison). (c–g) Treatment of HEK293T cells with 50 μM divalent cation also increased total cellular content of the ions (N = 3, ±SEM, N.D. indicates not detected above background). p-values indicate Student’s t-test adjusted with Bonferroni correction for multiple testing.
Consistent with literature reports,21,34,35,39,50,51 each metal ion significantly (p < 0.001) attenuated 70Zn(II) uptake, with the greatest diminution of uptake caused by Cd(II) (Figure 6b). Inhibition of Zn(II) uptake by Cd(II) may be explained by the fact that the closed-shell d10 metal ions Cd(II) and Zn(II) often bind at the same sites in proteins and have similar binding affinities to amino acid residues.54,55 Thus, it is likely that a principal mechanism by which the toxic metal Cd(II) enters cells involves co-opting endogenous Zn(II)-uptake machinery. The inhibition of Zn(II) uptake by Cd(II) taken together with the cellular accumulation of the latter (Figure 6c) is consistent with this hypothesis. Coordinated uptake inhibition and accumulation was not observed for the other metal ions (Figures 6b and 6d–g). For instance, Mn(II) lowers Zn(II) uptake to a lesser extent than does Cd(II), but Mn(II) nonetheless markedly accumulates in HEK293T cells. Taken together, these observations suggest that Mn(II) uptake in this context is not mediated by Zn(II) transport machinery. Overall, monitoring total cellular metal ion levels in these experiments can provide important mechanistic information regarding metal uptake.
The molecular basis for the selectivity of Zn uptake vs Mn, Fe, Co, and Ni uptake is unclear. Selection for Zn over Fe, Mn, and Co may be rationalized based upon the Irving-Williams series,56 which describes the binding preferences of ligands for first-row transition metals in octahedral ligand fields and is related to decreasing ionic radii across the series. For instance, Zn(II) binds ~104-fold more tightly to zinc-finger mimics than does Co(II),57 an ion often used as a proxy for Zn(II) in protein biochemistry.58 However, justification of selection against Cu(II) is challenging because the series predicts greater affinity of ligands for Cu(II) than for Zn(II). We expect that future structural studies of Zn(II) transporters in tandem with assays like the one we report here may allow prediction or explication of Zn(II)-transporter selectivity.
Zn(II) Deficiency Alters the Metal Ion Selectivity of Cellular Zn(II) Uptake Systems
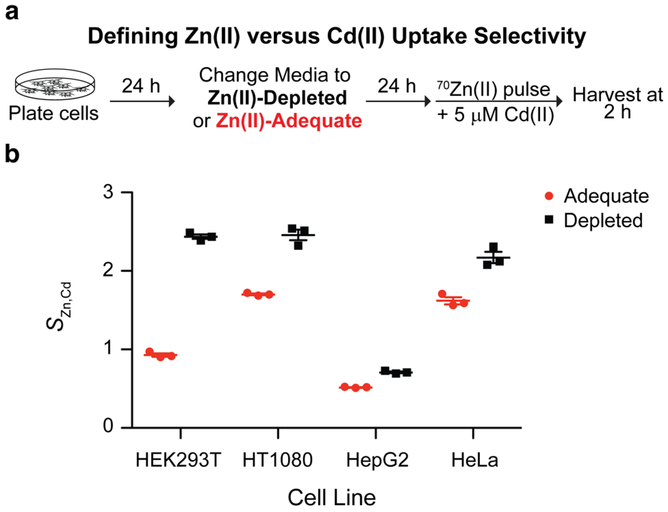
The observation that Cd(II) strongly inhibits Zn(II) uptake (Figure 6b) motivated us to probe how Zn(II) deficiency affects relative Zn(II) and Cd(II) uptake rates. We were inspired by a recent approach using a trace amount of 70Zn(II) in which Cd and 70Zn uptake rates were directly compared.59 The protocol we describe below, however, utilizes a medium with a greater enrichment of 70Zn and therefore has the potential to afford superior signal and resolution over a shorter experimental time window.
In particular, we sought to evaluate how cellular metal-uptake selectivity for Cd(II) over Zn(II) is affected by (i) Zn(II) deficiency or (ii) cell lineage. The former is a question of singular interest because Zn(II) deficiency alters zinc importer expression patterns.46 The latter question arises because different cell types express different zinc transporters and different zinc transporters exhibit different metal selectivities.60 Because the cellular concentrations of both Cd(II) and 70Zn(II) are very low in cells grown in DMEM/FBS, we could readily calculate the selectivity of cellular metal uptake machinery defined as SZn,Cd using Equation 1:
| (1) |
Note that this calculation is specifically made possible by the use of 70Zn(II)-enriched media in these assays. A key benefit of computing the ratio of 70Zn to Cd is that normalization of measured metal content to protein concentration is unnecessary, reducing the number of experimental steps required for the assay.
We began by culturing HEK293T cells in either Zn(II)-depleted or Zn(II)-adequate (media was treated with a control-resin28) media for 24 h (Figure 7a). We then cultured the cells in 70Zn(II)-enriched medium containing 5 μM Cd(II) for 2 h before analyzing cell metal content according to our usual protocol. HEK293T cells starved of Zn(II) exhibited a SZn,Cd value ~3-fold greater than cells cultured under Zn(II)-adequate conditions, indicating that Zn(II)-starved HEK293T cells preferentially uptake Zn(II) over Cd(II) to a greater extent than cells cultured under Zn(II)-adequate conditions (Figure 7b). Cell lines of diverse lineages, including HepG2, HeLa, and HT1080, exhibited this trend after 48 h of growth in Zn(II)-depleted or Zn(II)-adequate medium; however, the magnitude of SZn,Cd was different for each cell line (Figure 7b). For instance, HepG2 cells were markedly less selective for Zn(II) over Cd(II) than any other tested cell line. The identities of transporters that (i) underlie changes in uptake selectivity in the context of Zn(II) deficiency and (ii) account for differences in uptake selectivity of cell lines are presently unknown but merit further investigation.
Figure 7.
(a) Workflow to assess selectivity for Zn(II) over Cd(II). (b) HEK293T, HT1080, HepG2, and HeLa cells displayed enhanced Zn(II) to Cd(II) selectivity when the cells were previously starved of Zn(II) (N = 3, ±SEM).
SUMMARY AND CONCLUSIONS
In summary, we present the design, validation, and implementation of a protocol for measuring Zn(II) uptake by cultured metazoan cells. Depletion of cell culture medium of Zn(II) using the A12-resin, followed by repletion of the medium with 70Zn(II), enables highly sensitive quantitation of cellular Zn(II) uptake from media containing normal Zn(II) concentrations. There are at least three advantages of this approach over other methods to monitor Zn(II) uptake. First, the assay does not rely on a gamma-emitting radioisotope of Zn(II). Second, the assay is designed to measure Zn(II) uptake from cell culture media rather than abiological buffers commonly used in radioactivity assays. Third, because ICP-MS is used as the detection technique, the assay provides simultaneous insight into how total cellular Zn and other metal concentrations change as a consequence of any perturbation of interest.
We envision that the methods described herein will have many applications in the study of metazoan Zn(II) biology. In particular, we anticipate that the 70Zn(II)-uptake assay will be well-suited for discovering small molecules that modulate Zn(II) transport and/or for single-cell ICP-MS experiments to assess heterogeneity in Zn(II)-uptake rates in cell populations. We also expect that organelles or proteins of interest could be purified after a 70Zn(II) pulse and that the abundance of 70Zn in the sample could be quantified to gain insights into cellular Zn(II) trafficking. Finally, our protocol could be modified to study either the secretion of Zn(II) metalloproteins or Zn(II) efflux by first ‘loading’ cells with 70Zn(II) prior to culture in media lacking 70Zn and subsequently assessing 70Zn(II) levels outside the cell.
Supplementary Material
Acknowledgments
FUNDING SOURCES
This work was supported by the 56th Edward Mallinckrodt Jr. Foundation Faculty Scholar Award and a Pilot Grant from the MIT Center for Environmental Health Sciences supported by NIH/NIEHS P30-ES002109 (both to M.D.S.), NIH/NIGMS Grant R01-GM065519 (to S.J.L.), and NIH/NIBIB T32-EB019940 (traineeship to C.E.R.R). This work was also supported in part by the Koch Institute Support (core) Grant P30-CA14051 from the NIH/NCI. The ICP-MS is housed in the MIT Center for Environmental Health Sciences Bioanalytical Core supported under IH/NIEHS P30-ES002109.
REFERENCES
- [1].Anderson CT, Kumar M, Xiong S, and Tzounopoulos T (2017) Cell-specific gain modulation by synaptically released zinc in cortical circuits of audition, eLife 6, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Frederickson CJ, Koh J-Y, and Bush AI (2005) The neurobiology of zinc in health and disease, Nat. Rev. Neurosci. 6, 449–462. [DOI] [PubMed] [Google Scholar]
- [3].Blakemore LJ and Trombley PQ (2017) Zinc as a neuromodulator in the central nervous system with a focus on the olfactory bulb, Front. Cell. Neurosci. 11, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, and Hirano T (2007) Zinc is a novel intracellular second messenger, J. Cell Biol. 177, 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maret W and Li Y (2009) Coordination dynamics of zinc in proteins, Chem. Rev. 109, 4682–4707. [DOI] [PubMed] [Google Scholar]
- [6].Laitaoja M, Valjakka J, and Jänis J (2013) Zinc coordination spheres in protein structures, Inorg. Chem. 52, 10983–10991. [DOI] [PubMed] [Google Scholar]
- [7].Pan E, Zhang XA, Huang Z, Krezel A, Zhao M, Tinberg CE, Lippard SJ, and McNamara JO (2011) Vesicular zinc promotes presynaptic and inhibits postsynaptic long-term potentiation of mossy fiber-ca3 synapse, Neuron 71, 1116–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kalappa BI, Anderson CT, Goldberg JM, Lippard SJ, and Tzounopoulos T (2015) Ampa receptor inhibition by synaptically released zinc, Proc. Natl. Acad. Sci. U. S. A. 112, 15749–15754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wessels I, Maywald M, and Rink L (2017) Zinc as a gatekeeper of immune function, Nutrients 9, 1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Duncan FE, Que EL, Zhang N, Feinberg EC, O'Halloran TV, and Woodruff TK (2016) The zinc spark is an inorganic signature of human egg activation, Sci. Rep. 6, 24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim AM, Vogt S, O'Halloran TV, and Woodruff TK (2010) Zinc availability regulates exit from meiosis in maturing mammalian oocytes, Nat. Chem. Biol. 6, 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee S and Kelleher SL (2016) Molecular regulation of lactation: The complex and requisite roles for zinc, Arch. Biochem. Biophys. 611, 86–92. [DOI] [PubMed] [Google Scholar]
- [13].Lee S, Rivera OC, and Kelleher SL (2017) Zinc transporter 2 interacts with vacuolar ATPase and is required for polarization, vesicle acidification, and secretion in mammary epithelial cells, J. Biol. Chem. 292, 21598–21613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Carter KP, Young AM, and Palmer AE (2014) Fluorescent sensors for measuring metal ions in living systems, Chem. Rev. 114, 4564–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].A., A. S. J., Dierickx P, and Merkx M (2016) Dual readout BRET/FRET sensors for measuring intracellular zinc, ACS Chem. Biol. 11, 2854–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Que EL, Bleher R, Duncan FE, Kong BY, Gleber SC, Vogt S, Chen S, Garwin SA, Bayer AR, Dravid VP, Woodruff TK, and O'Halloran TV (2015) Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks, Nat. Chem. 7, 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Walkup GK, Burdette SC, Lippard SJ, and Tsien RY (2000) A new cell-permeable fluorescent probe for Zn2+, J. Am. Chem. Soc. 23, 5644–5645. [Google Scholar]
- [18].Bourassa D, Elitt CM, McCallum AM, Sumalekshmy S, McRae RL, Morgan MT, Siegel N, Perry JW, Rosenberg PA, and Fahrni CJ (2018) Chromis-1, a ratiometric fluorescent probe optimized for two-photon microscopy reveals dynamic changes in labile Zn(II) in differentiating oligodendrocytes, ACS Sens. 3, 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Heath JC and Liquier-Milward J (1950) The distribution and function of zinc in normal and malignant tissues. I. Uptake and distribution of radioactive zinc, 65Zn, Biochim. Biophys. Acta 5, 404–415. [DOI] [PubMed] [Google Scholar]
- [20].Tupper R, Watts RW, and Wormall A (1951) Studies on red and white blood cells labelled with 65Zn, Biochem. J. 48, xxxvii–xxxviii. [PubMed] [Google Scholar]
- [21].Gaither LA and Eide DJ (2001) The human ZIP1 transporter mediates zinc uptake in human k562 erythroleukemia cells, J. Biol. Chem. 276, 22258–22264. [DOI] [PubMed] [Google Scholar]
- [22].Egan CB, Smith FG, Houk RS, and Serfass RE (1991) Zinc absorption in women: Comparison of intrinsic and extrinsic stable-isotope labels, Am. J. Clin. Nutr. 53, 547–553. [DOI] [PubMed] [Google Scholar]
- [23].Lowe NM, Green A, Rhodes JM, Lombard M, Jalan R, and Jackson MJ (1993) Studies of human zinc kinetics using the stable isotope 70Zn, Clin. Sci. (Lond) 84, 113–117. [DOI] [PubMed] [Google Scholar]
- [24].Tran CD, Miller LV, Krebs NF, Lei S, and Hambidge KM (2004) Zinc absorption as a function of the dose of zinc sulfate in aqueous solution, Am. J. Clin. Nutr. 80, 1570–1573. [DOI] [PubMed] [Google Scholar]
- [25].Ziegler EE, Serfass RE, Nelson SE, Figueroa-Colón R, Edwards BB, Houk RS, and Thompson JJ (1989) Effect of low zinc intake on absorption and excretion of zinc by infants studied with 70Zn as extrinsic tag, J. Nutr. 119, 1647–1653. [DOI] [PubMed] [Google Scholar]
- [26].Nairn BL, Lonergan ZR, Wang J, Braymer JJ, Zhang Y, Calcutt MW, Lisher JP, Gilston BA, Chazin WJ, de Crécy-Lagard V, Giedroc DP, and Skaar EP (2016) The response of acinetobacter baumannii to zinc starvation, Cell Host Microbe 19, 826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bonaventura P, Lamboux A, Albarède F, and Miossec P (2016) A feedback loop between inflammation and Zn uptake, PLoS One 11, e0147146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Richardson CER, Cunden LS, Butty VL, Nolan EM, Lippard SJ, and Shoulders MD (2018) A method for selective depletion of Zn(II) ions from complex biological media and evaluation of cellular consequences of Zn(II) deficiency, J. Am. Chem. Soc 140, 2413–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].A12-resin selectively removes Zn(II) from all tested mammalian cell culture media. However, it is important to test the selectivity of medium depletion with A12 resin when the resin is applied to deplete a new medium.
- [30].Cunden LS, Gaillard A, and Nolan EM (2016) Calcium ions tune the zinc-sequestering properties and antimicrobial activity of human S100A12, Chem. Sci. 7, 1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wiemann S, Pennacchio C, Hu Y, Hunter P, Harbers M, MAmiet A, Bethel G, Busse M, Carninci P, Diekhans M, Dunham I, Hao T, Harper JD, Hayashizaki Y, Heil O, Hennig S, Hotz-Wagenblatt A, Jang W, Jöcker A, Kawai J, Koenig C, Korn B, Lambert C, LeBeau A, Lu S, Maurer J, Moore T, Ohara O, Park J, Rolfs A, Salehi-Ashtiani K, Seimer C, Simmons B, Smith A. v. B., Seteel J, Wagner L, Weaver T, Wellenreuther R, Yang S, Vidal M, Gerhard DS, LaBaer J, Temple G, and Hill DE (2016) The orfeome collaboration: A genome-scale human orf-clone resource, Nat. Methods 13, 191–192. [DOI] [PubMed] [Google Scholar]
- [32].Dewal MB, DiChiara AS, Antonopoulos A, Taylor RJ, Harmon CJ, Haslam SM, Dell A, and Shoulders MD (2015) XBP1s links the unfolded protein response to the molecular architecture of mature N-glycans, Chem. Biol. 22, 1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Krzywinski M and Altman N (2014) Comparing samples–part ii, Nat. Methods 11, 355–356. [Google Scholar]
- [34].Jeong J, Walker JM, Wang F, Park JG, Palmer AE, Giunta C, Rohrbach M, Steinmann B, and Eide DJ (2012) Promotion of vesicular zinc efflux by ZIP13 and its implications for spondylocheiro dysplastic Ehlers-Danlos syndrome, Proc. Natl. Acad. Sci. U. S. A. 109, E3530–E35388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dufner-Beattie J, Wang F, Kuo Y-M, Gitschier J, Eide D, and Andrews GK (2003) The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice, J. Biol. Chem. 278, 33474–33481. [DOI] [PubMed] [Google Scholar]
- [36].Greenough MA, Volitakis I, Li Q-X, Laughton K, Evin G, Ho M, Dalziel AH, Camakaris J, and Bush AI (2011) Presenilins promote the cellular uptake of copper and zinc and maintain copper chaperone of SOD1-dependent copper/zinc superoxide dismutase activity, J. Biol. Chem. 286, 9776–9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang T, Sui D, and Hu J (2016) Structural insights of ZIP4 extracellular domain critical for optimal zinc transport, Nat. Commun. 7, 11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dhabaria A, Cifani P, Reed C, Steen H, and Kentsis A (2015) A high-efficiency cellular extraction system for biological proteomics, J. Proteome Res. 14, 3403–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gaither LA and Eide DJ (2000) Functional expression of the human hZIP2 zinc transporter, J. Biol. Chem. 275, 5560–5564. [DOI] [PubMed] [Google Scholar]
- [40].Lin W, Chai J, Love J, and Fu D (2010) Selective electrodiffusion of zinc ions in a zrt-, irt-like protein, ZIPb, J. Biol. Chem. 285, 39013–39020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Colvin RA, Lai B, Holmes WR, and Lee D (2015) Understanding metal homeostasis in primary cultured neurons. Studies using single neuron subcellular and quantitative metallomics, Metallomics 7, 1111–1123. [DOI] [PubMed] [Google Scholar]
- [42].Carraway RE and Dobner PR (2012) Zinc pyrithione induces erk- and pkc-dependent necrosis distinct from tpen-induced apoptosis in prostate cancer cells, Biochim. Biophys. Acta 1823, 544–557. [DOI] [PubMed] [Google Scholar]
- [43].Jeong J and Eide DJ (2013) The SLC39 family of zinc transporters, Mol. Aspects. Med 34, 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, and Nebert DW (2006) ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: Characterization of transporter properties, Mol. Pharmacol 70, 171–180. [DOI] [PubMed] [Google Scholar]
- [45].Wang C-Y, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B, and Knutson MD (2012) ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading, J. Biol. Chem. 287, 34032–34043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lichten LA, Ryu M-S, Guo L, Embury J, and Cousins RJ (2011) MTF-1-mediated repression of the zinc transporter zip10 is alleviated by zinc restriction, PLoS One 6, e21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bin BH, Hojyo S, Hosaka T, Bhin J, Kano H, Miyai T, Ikeda M, Kimura-Someya T, Shirouzu M, Cho E-G, Fukue K, Kambe T, Ohashi W, Kim K-H, Seo J, Choi D-H, Nam Y-J, Hwang D, Fukunaka A, Fujitani Y, Yokoyama S, Superti-Furga A, Ikegawa S, Lee TR, and Fukada T (2014) Molecular pathogenesis of spondylocheirodysplastic Ehlers-Danlos syndrome caused by mutant ZIP13 proteins, EMBO Mol. Med. 6, 1028–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kim JH, Jeon J, Shin M, Won Y, Lee M, Kwak JS, Lee G, Rhee J, Ryu JH, Chun CH, and Chun JS (2014) Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis, Cell 156, 730–743. [DOI] [PubMed] [Google Scholar]
- [49].Bafaro E, Liu Y, Xu Y, and Dempski RE (2017) The emerging role of zinc transporters in cellular homeostasis and cancer, Signal Transduct. Target. Ther 2, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chowanadisai W, Graham DM, Keen CL, Rucker RB, and Messerli MA (2013) Neurulation and neurite extension require the zinc transporter ZIP12 (SLC39A12), Proc. Natl. Acad. Sci. U. S. A. 110, 9903–9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang F, Kim B-E, Petris MJ, and Eide DJ (2004) The mammalian ZIP5 protein is a zinc transporter that localizes to the basolateral surface of polarized cells, J. Biol. Chem. 279, 51433–51441. [DOI] [PubMed] [Google Scholar]
- [52].Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, and Nebert DW (2008) SLC39A14 gene encodes ZIP14, a metal/bicarbonate symporter: Similarities to the ZIP8 transporter, Mol. Pharmacol. 73, 1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Franz MC, Pujol-Giménez J, Montalbetti N, Fernandez-Tenorio M, DeGrado TR, Niggli E, Romero MF, and Hediger MA (2018) Reassessment of the transport mechanism of the human zinc transporter SLC39A2, Biochemistry 57, 3976–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Maret W and Moulis J-M (2013) The bioinorganic chemistry of cadmium in the context of its toxicity, In Cadmium: From toxicity to essentiality (Sigle A, Sigel H, and Sigel RKO, Eds.), pp 1–26, Springer. [DOI] [PubMed] [Google Scholar]
- [55].Martin RB (1987) A stability ruler for metal ion complexes, J. Chem. Ed. 64, 402. [Google Scholar]
- [56].Irving H and Williams RJP (1953) The stability of transition-metal complexes, J. Chem. Soc. 0, 3192–3210. [Google Scholar]
- [57].Dudev T and Lim C (2003) Principles governing Mg, Ca, and Zn binding and selectivity in proteins, Chem. Rev. 103, 773–788. [DOI] [PubMed] [Google Scholar]
- [58].Maret W and Vallee BL (1993) Cobalt as probe and label of proteins, Methods Enzymol. 226, 52–71. [DOI] [PubMed] [Google Scholar]
- [59].Bonaventura P, Lamboux A, Albarède F, and Miossec P (2017) Regulatory effects of zinc on cadmium-induced cytotoxicity in chronic inflammation, PLoS One 12, e0180879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dempski RE (2012) The cation selectivity of the ZIP transporters, In Metal transporters (Argüello JM, and Lutsenko S, Eds.), pp 221–245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.