Abstract
Background
Infants born prematurely are at risk of a deficiency in ω‐6 and ω‐3 long‐chain polyunsaturated fatty acids (LC‐PUFAs) arachidonic acid (AA) and docosahexaenoic acid (DHA). We investigated how fatty acids from breast milk and parenteral lipid emulsions shape serum LC‐PUFA profiles in extremely preterm infants during early perinatal life.
Methods
Ninety infants born < 28 weeks gestational age were randomized to receive parenteral lipids with or without the ω‐3 LC‐PUFAs eicosapentaenoic acid (EPA) and DHA (SMOFlipid: Fresenius Kabi, Uppsala, Sweden, or Clinoleic: Baxter Medical AB, Kista, Sweden, respectively). The fatty acid composition of infant serum phospholipids was determined from birth to postmenstrual age 40 weeks, and in mother's milk total lipids on postnatal day 7. Enteral and parenteral intake of LC‐PUFAs was correlated with levels in infant serum.
Results
Infants administered parenteral ω‐3 LC‐PUFAs received 4.4 and 19.3 times more DHA and EPA, respectively, over the first 2 weeks of life. Parenteral EPA but not DHA correlated with levels in infant serum. We found linear relationships between dietary EPA and DHA and infant serum levels in the Clinoleic (Baxter Medical AB) group. The volume of administered SMOFlipid (Fresenius Kabi) was inversely correlated with serum AA, whereas Clinoleic (Baxter Medical AB) inversely correlated with serum EPA and DHA.
Conclusions
There appears to be no or low correlation between the amount of DHA administered parenterally and levels measured in serum. Whether this observation reflects serum phospholipid fraction only or truly represents the amount of accreted DHA needs to be investigated. None of the parenteral lipid emulsions satisfactorily maintained high levels of both ω‐6 and ω‐3 LC‐PUFAs in infant serum.
Keywords: arachidonic acid, docosahexaenoic acid (DHA), extremely preterm, human milk, long‐chain polyunsaturated fatty acids (LC‐PUFA), parenteral nutrition
Clinical Relevancy Statement
Infants born extremely preterm are deprived of the full last trimester in utero. Thus, they are also deprived of the placental transfer of long‐chain polyunsaturated fatty acids (LC‐PUFAs), which are essential for normal development of the central nervous system. These infants are dependent on an exogenous supply of LC‐PUFAs from enteral or parenteral sources in early perinatal life. However, the most effective LC‐PUFA supplementation strategy in terms of mode of delivery, dose, and duration remains to be determined.
Here, we show that ω‐3 and ω‐6 LC‐PUFAs administered via breast milk or parenteral lipid emulsion differ considerably in their ability to increase neonate serum levels in infants born before 28 weeks gestation.
Introduction
In the interim between birth and the time when infants born extremely preterm reach full enteral feeding (150–180 mL kg−1 d−1), human milk must be complemented with parenteral lipid emulsions to meet the infant's requirements for energy and essential fatty acids (FAs). Parenteral lipid emulsions used in pediatric care are based on plant oils containing the essential FAs linoleic acid (LA, 18:2n‐6) and α‐linolenic acid (ALA; 18:3n‐3) of the ω‐6 (n‐6) and ω‐3 (n‐3) families in various ratios.1 These emulsions may also be fortified with a fraction of oils derived from fish to provide the infant long‐chain polyunsaturated fatty acids (LC‐PUFAs), such as docosahexaenoic acid (DHA, 22:6n‐3) and its precursor eicosapentaenoic acid (EPA, 20:5n‐3). LC‐PUFAs are essential for proper development and functioning of the central nervous system. An inadequate LC‐PUFA supply during the perinatal period following moderately to extremely preterm birth has been associated with long‐term effects on cognition and visual acuity.2, 3, 4 Growing evidence indicates that extremely preterm infants under standard care receive insufficient amounts of LC‐PUFAs5; however, no consensus exists regarding the best practice for LC‐PUFA supplementation to neonates in terms of lipid composition, dosage, duration of treatment, or mode of delivery.
DHA and arachidonic acid (AA; 20:4n‐6) are highly enriched in brain gray matter and the retina, where they are integral components of cellular membranes. These and other LC‐PUFAs serve as precursors of potent signaling molecules, including prostaglandins, thromboxanes, leukotrienes, and resolvins, with well‐documented roles in inflammation and immune functions.6 DHA and AA deficiency in neonates has been associated with an altered growth rate, manifestations of allergy, reduced intellectual development, and increased risk of several morbidities commonly associated with prematurity.5, 7 Preterm infants are capable, to a certain extent, of de novo synthesis of DHA and AA from their shorter‐chain precursors ALA and LA, respectively.8 However, based on estimations from intrauterine accretion rates, the conversion is not sufficient to supply the infant with appropriate amounts of these FAs.9 As such, infant blood LC‐PUFA status is determined by the exogenous supply, endogenous synthesis, peripheral accretion, and extent of catabolic consumption.
Numerous studies have addressed how enteral supplementation of LC‐PUFAs affects the levels in infant blood and long‐term outcomes.10, 11, 12, 13, 14 A strong correlation between the amount of DHA supplemented enterally and infant blood levels has been reported.15 Many studies have also addressed the effect of parenteral supplementation of LC‐PUFAs.16 Comparably less attention has been paid to determining the quantitative contribution of these 2 modes of LC‐PUFA delivery (ie, enteral supply via milk and parenteral supply via intravenous lipid emulsions) and how these together shape an infant's FA levels in the blood.
The aim of the present study was to investigate how milk FAs in combination with 2 different parenteral lipid solutions, 1 exclusively containing plant‐derived oils (Clinoleic; Baxter Medical AB, Kista, Sweden) and 1 containing 15% fish oil with n‐3 LC‐PUFAs (SMOFlipid; Fresenius Kabi, Uppsala, Sweden), affect FA fractions in infant serum during the first 2 weeks after birth.
Materials and Methods
Study Design
This investigation is a part of the Donna Mega study, a randomized monocentric controlled clinical trial aiming to determine the effect of parental lipid emulsions Clinoleic (Baxter Medical AB) and SMOFlipid (Fresenius Kabi) on infant morbidities and growth. Women delivering < 28 weeks gestation who were admitted to the neonatal care unit at Sahlgrenska University Hospital in Gothenburg, Sweden, between April 4, 2013, and September 22, 2015, were eligible for inclusion in the study. Seventy‐eight mothers giving birth to 90 infants prematurely were included in the study (Figure 1). Twelve infants died during the study period, leaving 78 infants and 75 mothers to complete the study. Infants with major malformations were excluded. The study protocol can be found at clinicaltrials.gov (Clinical trial NCT 02760472). The details of the cohort were described previously.17
Figure 1.
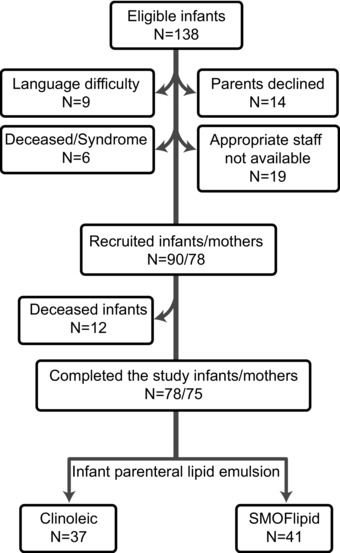
Patient enrollment flowchart.
Sampling and FA Analysis
Mother's milk was collected on postnatal day 7 and the FA composition determined. The results from this analysis are published elsewhere.18 Frozen, anonymous donor milk samples from 6 mothers were obtained from Modersmjölkcentralen at Queen Silvia Children's Hospital. Total lipids were extracted from milk and FA methyl esters (FAMEs) prepared and analyzed by gas chromatography‐mass spectrometry (GC‐MS).18 In accordance with previous studies, no effect of pasteurization on the FA composition was noted.19, 20 Venous blood samples were collected from the infants at birth (cord blood), on postnatal days 1, 7, 14, and 28, and postmenstrual age (PMA) weeks 32, 36, and 40. Phospholipids were extracted from blood serum and FAMEs analyzed by GC‐MS.17
Enteral and Parenteral Nutrition Strategy
Infants were randomized to receive 1 of 2 lipid emulsions: Clinoleic (Baxter Medical AB) based on olive oil and soy oil, or SMOFlipid (Fresenius Kabi) based on soybean oil, medium‐chain triglycerides, olive oil, and 15% fish oil containing n‐3 LC‐PUFAs. The randomization procedure and nutrition strategy were described previously.17
The LC‐PUFA content of lipid emulsions was analyzed by GC‐MS.17 The LA and ALA content was 40.3 and 4.0 mg mL−1 in Clinoleic (Baxter Medical AB), and 56.1 and 7.6 mg mL−1 in SMOFlipid (Fresenius Kabi), respectively. The AA content was 0.20 mg mL−1 in Clinoleic (Baxter Medical AB) and 0.54 mg mL−1 in SMOFlipid (Fresenius Kabi). The EPA and DHA concentrations in SMOFlipid (Baxter Medical AB) were 5.64 mg mL−1 and 5.65 mg mL−1, respectively. As expected, Clinoleic (Baxter Medical AB) did not contain any detectable amounts of EPA or DHA.
Enteral and Parenteral Intake of FAs
The FA concentrations determined in mother's milk and averages from donor milk (n = 6) were used to calculate the total enteral intake of AA, DHA, and EPA during the first 2 weeks of infant life. When calculating the total FA intake from milk, a total fat concentration of 3.6% was assumed for both the mother's own milk and the donor milk over the first 2 weeks of lactation. Milk from mothers delivering preterm has been reported to have a higher fat content than full‐term deliveries, and fat content changes during the lactation period.21, 22, 23, 24 In addition, we assumed equal uptake of FAs from pasteurized and nonpasteurized donor milk, although the absorption of FAs from pasteurized milk may be reduced due to denaturation of milk lipases.25 All milk fat was calculated to be comprised only of FAs. However, other lipid components, such as the triacylglycerol‐, phospholipid‐, and cholesterol ester backbones, make up a minor part of the total fat content.26 Finally, all FAs were assumed to be fully absorbed, although the true bioavailability in preterm infants is reported to be in the range of 85%–100% depending on FA species.27 Birth weight was used when normalizing milk and parenteral lipid emulsion intake to mg per kg body weight during the first 2 weeks of life.
Statistical Analysis
SPSS 24.0 (IBM Corp., Armonk, NY) was used for all statistical analyses. P values < 0.05 were considered significant. Nonparametric Spearman rank order test was applied for correlation analyses, and Mann‐Whitney U test was applied to compare treatment groups. Infants deceased before 40 weeks PMA were not included in the correlation analyses of serum FAs and milk FAs or enteral/parenteral intake. Multiple linear regression analyses were validated using common practices. The parameters used to describe models from the multiple linear regression analyses were: β, slope of the specified independent variable in the model; P‐value, significance of the specified independent variable in the model; and model R2, goodness‐of‐fit of the model.
Ethics
The study was approved by the Regional Ethical Board, Gothenburg (Dnr 303–11; Clinical trial NCT 02760472). Informed signed consent was obtained from all participating parents/guardians.
Results
Study Population
Of the 78 infants who completed the study, 37 were randomized to receive Clinoleic (Baxter Medical AB) and 41 to receive SMOFlipid (Fresenius Kabi) parenteral lipids (Figure 1). Detailed clinical characteristics of the infants can be found elsewhere.17 The total volume of parenteral lipid solution and milk administered during the first 2 weeks of life is reported in Table 1. Intake of mother's milk, donor milk, and parenteral lipids did not differ between treatment groups during this time. The proportions of the 21 most abundant FAs in the phospholipid fraction from cord blood and infant serum collected at postnatal age (PNA) 1, 7, 14, and 28 days; and PMA 32, 36, and 40 weeks were analyzed by GC‐MS17 (Figure S1).
Table 1.
Intake of Human Milk and Lipids During First 2 Weeks of Life
| Clinoleic (n = 37) | SMOFlipid (n = 41) | Clinoleica vs SMOFlipidb | |||||
|---|---|---|---|---|---|---|---|
| P‐Value | P‐Value | ||||||
| Week 1 | Week 2 | Week 1 | Week 2 | Week 1 | Week 2 | ||
| Enteral | Mother's milk (mL) | 48.7 (34.6–64) | 125.1 (89.6–161.1) | 39.8 (25.9–56.5) | 106.6 (74.2–140.9) | 0.062 | 0.260 |
| Donor milk (mL) | 8.3 (4.2–16) | 0 (0–3.3) | 6.4 (4.1–17.5) | 0 (0–0) | 0.334 | 0.812 | |
| Total fat (g·kg−1·d−1)c | 2.2 (1.7–2.8) | 4.8 (3.3–6.2) | 1.7 (1.1–2.5) | 4.3 (3.3–5.3) | 0.098 | 0.360 | |
| LA (mg·kg−1·d−1) | 198.6 (138.8–230.4) | 408.1 (286.7–533.6) | 163.3 (94.2–242.3) | 392.5 (256.7–499.9) | 0.303 | 0.572 | |
| ALA (mg·kg−1·d−1) | 24.9 (14.7–33.4) | 52.2 (32.7–70.9) | 23.4 (11.9–31.6) | 49.9 (30.7–65.1) | 0.375 | 0.552 | |
| AA (mg·kg−1·d−1) | 8.5 (5.5–11.8) | 19.3 (10.7–25.1) | 6.8 (4.9–8.9) | 16.4 (12.8–21.9) | 0.214 | 0.444 | |
| EPA (mg·kg−1·d−1) | 1.3 (0.8–2.1) | 2.2 (1.4–3.9) | 1.1 (0.6–1.8) | 2.6 (1.4–3.6) | 0.457 | 0.924 | |
| DHA (mg·kg−1·d−1) | 8.1 (3.8–11.4) | 17.6 (8.8–25.4) | 7.1 (3.8–10.3) | 15.1 (11–24.6) | 0.668 | 0.956 | |
| Parenteral | Total fat (g·kg−1·d−1) | 1.7 (1.2–2.1) | 0.7 (0–1.9) | 1.8 (1.5–2.1) | 1.6 (0.3–2.2) | 0.552 | 0.149 |
| LA (mg·kg−1·d−1) | 345.2 (250.7–424.8) | 150.8 (0–389.4) | 516.8 (428.9–596.1) | 448.6 (78.4–618.1) | 0.000 | 0.008 | |
| ALA (mg·kg−1·d−1) | 34.7 (25.2–42.7) | 15.1 (0–39.1) | 69.8 (57.9–80.5) | 60.6 (10.6–83.5) | 0.000 | 0.001 | |
| AA (mg·kg−1·d−1) | 1.8 (1.3–2.2) | 0.8 (0–2) | 5 (4.1–5.8) | 4.3 (0.8–6) | 0.000 | 0.000 | |
| EPA (mg·kg−1·d−1) | 0 (0–0) | 0 (0–0) | 52 (43.1–59.9) | 45.1 (7.9–62.1) | – | – | |
| DHA (mg·kg−1·d−1) | 0 (0–0) | 0 (0–0) | 52.1 (43.2–60.1) | 45.2 (7.9–62.3) | – | – | |
Data are presented as median (25th–75th percentile). P‐values were determined using Mann‐Whitney U‐test.
Clinoleic: Baxter Medical AB, Kista, Sweden.
SMOFlipid: Fresenius Kabi, Uppsala, Sweden.
Total fat concentration of 3.6% was assumed for both the mother's milk and donor milk.
AA, arachidonic acid; ALA, α‐linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid.
Enterally Supplied FAs Strongly Correlate with Infant Serum Levels
To determine the association between FA levels in mother's milk and infant serum, correlation analyses were performed between FA fractions of milk collected on postnatal day 7 and infant serum phospholipid FAs from birth up to PMA 40 weeks (Table 2). We only consider correlations to be significant if they are found at 2 or more consecutive time points because we expected the influence of milk FA on infants’ serum to persist over time. Using this criterion, significant correlations were evident for margaric acid (17:0), ALA, eicosadienoic acid (20:2n‐6), AA, EPA, and DHA. These observations indicate that intake of mother's milk impacts phospholipid FA composition in infant serum, particularly the LC‐PUFAs.
Table 2.
Correlation Between Fatty Acids in Breast Milk Collected Postnatal Day 7 and Cord Blood or Infant Serum
| PNA | PNA | PNA | PNA | PMA | PMA | PMA | ||
|---|---|---|---|---|---|---|---|---|
| CB | 1 Day | 7 Days | 14 Days | 28 Days | 32 Weeks | 36 Weeks | 40 Weeks | |
| (n = 37) | (n = 76) | (n = 77) | (n = 76) | (n = 72) | (n = 73) | (n = 62) | (n = 61) | |
| 17:0 (margaric acid) | 0.361 (0.001) | 0.366 (0.001) | 0.34 (0.006) | |||||
| 20:2n‐6 (eicosadienoic acid) | −0.317 (0.005) | −0.327 (0.005) | −0.328 (0.005) | |||||
| 20:4n‐6 (arachidonic acid) | 0.313 (0.007) | 0.334 (0.004) | ||||||
| 20:5n‐3 (eicosapentaenoic acid) | 0.264 (0.020) | 0.330 (0.004) | ||||||
| 22:6n‐3 (docosahexaenoic acid) | 0.469 (0.00002) | 0.301 (0.008) | 0.301 (0.010) | 0.226 (0.054) | 0.348 (0.006) |
Spearman rank correlation coefficient (r) and significance (P‐value) are shown.
CB, cord blood; D, day(s); n, number of infants; PMA, postmenstrual age; PNA, postnatal age.
Total Intake of DHA During the First 2 Weeks of Life and Relation to Serum Levels
Within 14 days of life, 62% of the infants (48/78) had ceased receiving parenteral lipids (Figure 2A), and full enteral feeding was achieved (> 150 mL kg−1 d−1; Figure 2B). The total amount of DHA provided via milk during this time period correlated with infant serum levels at PNA 14 days in the Clinoleic (Baxter Medical AB) group (r = 0.507, P = 0.001) but not in the SMOFlipid (Fresenius Kabi) group (Figures 3A and 3B). The effect of milk DHA on the molar fraction of DHA in infant serum remained significant in the Clinoleic (Baxter Medical AB) group after adjusting for gestational age in a multiple linear regression model (Enteral DHA (mg), β = 0.002, P = 0.031; Gestational age (weeks), β = 0.109, P = 0.038, model R2 = 0.347). In the SMOFlipid (Fresenius Kabi) group, parenterally administered DHA did not correlate with infant serum levels (Figure 3C). Finally, the combined DHA administered via milk and parenteral lipids did not correlate with levels in serum from infants receiving SMOFlipid (Fresenius Kabi; Figure 3D). Because Clinoleic (Baxter Medical AB) does not contain DHA, only the contribution from milk DHA is shown in Figure 3D for this patient group.
Figure 2.
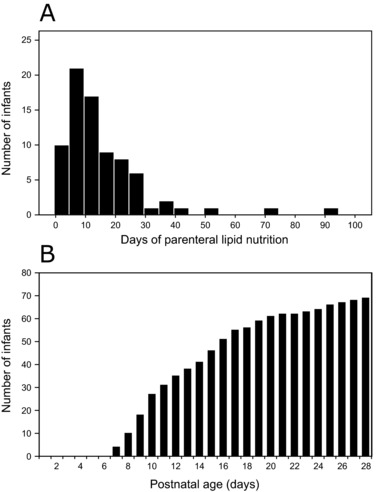
(A) Histogram showing the number of days infants received parenteral lipid nutrition. (B) Total number of infants receiving full enteral feeding (> 150 mL kg−1 d−1).
Figure 3.
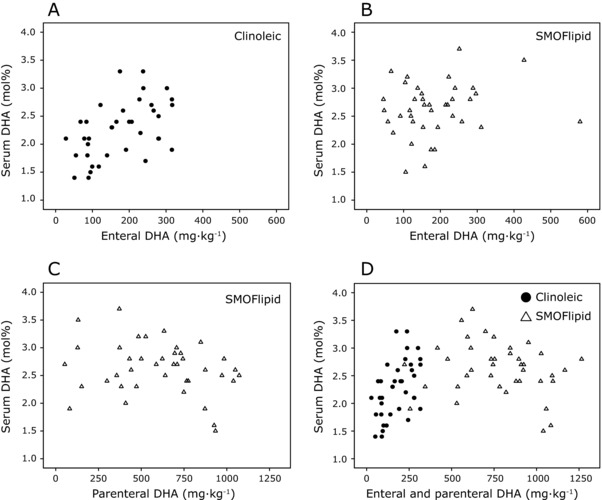
Relationship between total intake of DHA and infant serum level on postnatal day 14. (A) Total DHA administered enterally (mother's milk and donor milk) vs infant serum in the Clinoleic (Baxter Medical AB, Kista, Sweden) group and (B) SMOFlipid (Fresenius Kabi, Uppsala, Sweden) group. (C) Total DHA administered parenterally vs infant serum levels in the SMOFlipid group (Fresenius Kabi). (D) Total DHA administered enterally and parenterally vs infant serum levels in the Clinoleic (Baxter Medical AB) and SMOFlipid (Baxter Medical AB) groups. DHA, docosahexaenoic acid.
Total Intake of EPA and AA During the First 2 Weeks of Life and Relation to Serum Levels
Milk EPA administered to infants the first 2 weeks correlated with serum levels of EPA at PNA 14 days in the Clinoleic (Baxter Medical AB) group (r = 0.541, P = 0.001) but not in the SMOFlipid (Fresenius Kabi) group (Figures 4A and 4B). Conversely, parenterally administered EPA correlated with serum levels in the SMOFlipid (Fresenius Kabi) group (Figure 4C; r = 0.341, P = 0.031). We found an inverse association between the volume of parenterally administered Clinoleic (Baxter Medical AB) over the first 2 weeks of life and infant serum EPA levels (Figure 4D; r = −0.381, P = 0.020).
Figure 4.
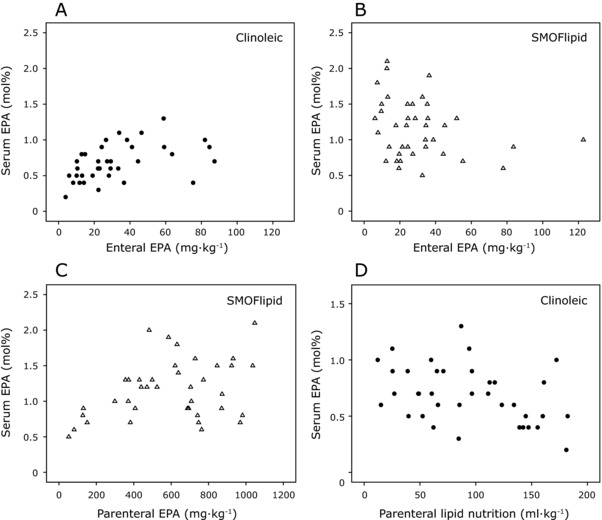
Relationship between total intake of EPA and infant serum levels on postnatal day 14. (A) Total EPA administered enterally (mother's milk and donor milk) vs infant serum levels in the Clinoleic (Baxter Medical AB, Kista, Sweden) group (B) and in the SMOFlipid (Fresenius Kabi, Uppsala, Sweden) group. (C) Total EPA administered parenterally vs serum levels in the SMOFlipid (Fresenius Kabi) group. (D) Total volume of administered parenteral lipid emulsion vs infant serum EPA levels in the Clinoleic (Baxter Medical AB) group. EPA, eicosapentaenoic acid.
Milk AA and total AA administered via milk and parenteral lipids exhibited weak positive correlations with infant serum levels in the SMOFlipid (Fresenius Kabi) group at PNA 14 days (r = 0.387, P = 0.015 and r = 0.357, P = 0.026, respectively). The total volume of parenterally administered SMOFlipid (Fresenius Kabi) over the first 2 weeks was negatively associated with infant serum AA (Figure 5; r = −0.338, P = 0.033). No significant correlation was found between administered parenteral and/or enteral AA and infant serum levels in the Clinoleic (Baxter Medical AB) group.
Figure 5.
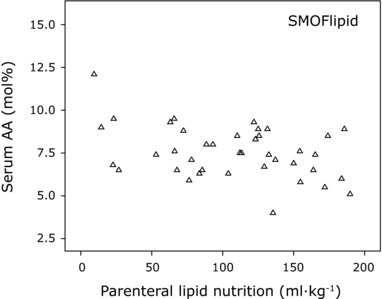
Relationship between total intake of parenteral lipid emulsion and infant serum AA levels on postnatal day 14. Total volume of administered parenteral lipid emulsion vs infant serum AA levels in the SMOFlipid (Fresenius Kabi, Uppsala, Sweden) group. AA, arachidonic acid.
Discussion
LC‐PUFAs are actively transferred across the placenta at an increasing rate as pregnancy progresses, coinciding with high fetal accretion of LC‐PUFAs, which peak during the third trimester when substantial organ development occurs. The intrauterine accretion rate during the last trimester has been estimated to be 212 and 45 mg kg−1 d−1 for AA and DHA, respectively.9 The current daily recommended intake to sustain normal growth and development after preterm birth is 385–1540 mg LA, > 50 mg AA, 50–60 mg DHA, and ≤ 20 mg EPA per kg of body weight.28 In this study, the intake of FAs was estimated by measurements of the relative concentration of FAs in mother´s own milk and donor milk; based on previously reported data,29 we assumed a fat content of 3.6% to calculate infants’ enteral intake of FAs. We also determined the FA content in Clinoleic (Baxter Medical AB) and SMOFlipid (Fresenius Kabi) to calculate parenteral FA intake. On average, infants enrolled in this study received the recommended dose of LA during their first week of life, when the contribution from both milk and parenteral lipids was included. LA intake was also sufficient to meet the recommendation during the second week, when only FAs from milk were considered (Table 1). Both treatment groups received approximately half the recommended dose of AA, and only infants on SMOFlipid (Fresenius Kabi) received the recommended dose of DHA in the second week. As parenteral lipids were the main source of DHA in the SMOFlipid (Fresenius Kabi) group, these infants were also at risk of developing DHA deficiency once parenteral supplementation ceased, which occurred a median 12 days after birth. The proportion of LC‐PUFAs in mother's milk decreases during the lactation period; DHA and AA concentrations are reduced to almost half during the first month after delivery.18, 30 The rapid decrease in milk LC‐PUFAs implicates a reduced relative intake of these FAs by the infant over time if not supplemented. Thus, LC‐PUFA deficiency in preterm infants may not only persist during the first months of life but also may escalate, with potentially life‐long consequences.
LC‐PUFA supplementation may be administered either enterally, directly as an oil, or by fortifying milk and/or through parenteral lipid emulsions during the early perinatal period. Parenteral lipid emulsions containing fish oil rich in EPA and DHA have been shown to be well‐tolerated by preterm infants and to increase blood levels of DHA and/or EPA compared with emulsions lacking these FAs.31, 32 However, the potential benefits of parenteral lipid solutions containing fish oil, such as SMOFlipid (Fresenius Kabi) and Omegaven (Fresenius Kabi, Uppsala, Sweden), in neonates have been questioned.28 One concern is the high EPA content, which may increase circulating EPA levels far above what is physiological, with unknown consequences for infant health.17 We have previously reported that infants on SMOFlipid (Fresenius Kabi) exceeded the recommended intake of EPA by >100%, concomitant with a doubling of serum EPA levels at PNA 14 days compared with the levels at birth.17 The second concern regarding the use of fish oils is that they have a high ratio of EPA + DHA to AA (about 20:1 mol%). As previously shown in this17 and other33 cohorts, the use of fish oils in parenteral solutions may further reduce the already low AA levels in infant serum. We found a significant negative correlation between the amount of SMOFlipid (Fresenius Kabi) administered and serum AA levels. We interpret this as high parenteral EPA and DHA having a negative effect on serum AA levels. Association between intake of Clinoleic and EPA is shown in Figure 4D. There was a similar association between intake of Clinoleic and DHA but this data is not shown. As expected, infants receiving the highest amounts of parenteral lipids were also the ones with the lowest milk intake. Thus, the smallest and most vulnerable neonates suffered the most from receiving parenteral lipids devoid of n‐3 LC‐PUFAs in terms of maintaining DHA status.
We assessed the levels of circulating FAs in infant serum phospholipids as a surrogate for the actual tissue levels. In a recent comprehensive animal study, enteral formula feeding was compared with the administration of parenteral lipid emulsion.34 In this study, preterm piglets were fed formula or 1 of 3 parenteral lipid emulsions (Intralipid (Fresenius Kabi, Uppsala, Sweden), Omegaven, or SMOFlipid [Fresenius Kabi]) for 14 days. After treatment, the FA profiles of total lipid extracts were determined in the liver, brain, and plasma; DHA and EPA were enriched in all body fractions from SMOFlipid‐fed (Fresenius Kabi) piglets compared with formula‐fed piglets or those receiving Intralipid (a fully plant‐based emulsion analogous to Clinoleic [Baxter Medical AB]). Furthermore, the AA fraction was greater in piglets receiving Intralipid compared with those receiving SMOFlipid (Fresenius Kabi). These results agree with data presented here on the effect of Clinoleic (Baxter Medical AB) and SMOFlipid (Fresenius Kabi) on serum LC‐PUFA status and suggest that circulating FA levels are a good predictor of FAs accreted into organs.
Fourteen days postnatally, there were strong correlations between the amount of EPA and DHA given via milk and the levels measured in serum among infants receiving Clinoleic (Baxter Medical AB). Although these correlations were highly significant, the correlation coefficients were rather low; only 50%–60% of the variance in serum EPA and DHA could be explained by dietary intake according to our models. Several factors that were not considered here may contribute to this, including the actual fat content of the milk, level of endogenous synthesis, intestinal absorption efficiency, and metabolic state.
Surprisingly, we found no correlation between infant serum DHA and DHA administered parenterally and/or enterally in the SMOFlipid (Fresenius Kabi) group. As we previously reported,17 serum DHA concentration at PNA 14 days was significantly higher in infants receiving SMOFlipid (Fresenius Kabi). However, the increase was rather modest: median (25th–75th percentile) 2.7 (2.4–2.9) mol% for SMOFlipid (Fresenius Kabi) compared with 2.3 (1.9–2.7) mol% for Clinoleic (Baxter Medical AB; Figure S1). The FA analysis used in this study does not discriminate the origin of the serum DHA from enteral or parenteral sources. The fact that the enteral intake of DHA did not correlate with serum levels in the SMOFlipid (Fresenius Kabi) group indicates that the lipid emulsion modulates the incorporation of dietary DHA into phospholipids. Further studies are needed to determine how the 2 modes of nutrition supply interact with each other and to elucidate the quantitative contribution to serum phospholipid levels.
The best effect of DHA supplementation in terms of increasing infant serum levels using SMOFlipid (Fresenius Kabi) was found with a dose of 500–750 mg kg−1 during the first 2 weeks, corresponding to 35–55 mg kg−1 d−1. Together with the contribution from milk, the total DHA intake for these infants was 45–70 mg kg−1 d−1 (ie, in the recommended range for supplementation).28 In the DHA intake and measurement of neural development (DIAMOND) trial, where full‐term infants were given formula containing 0%, 0.32%, 0.64%, or 0.96% DHA (while keeping AA constant), a curvilinear relationship between formula DHA and blood DHA determined at ages 4 and 12 months was found10; increasing formula DHA from 0.64%–0.96% only marginally increased blood DHA. One explanation for the lack of a linear increase in serum DHA with increased intake of SMOFlipid (Fresenius Kabi) may be that a level of saturation of the serum phospholipids is reached. In the DIAMOND study, such saturation was first observed when red blood cell DHA doubled in the treated group vs untreated.10 In our cohort, the average serum DHA level was 18% lower on day 14 compared with day 1, making it unlikely that DHA saturation is the full explanation for our findings.
Dietary LC‐PUFAs are absorbed by enterocytes, assembled into triglycerides, and then packaged into lipoproteins particles (chylomicrons) that may enter the lymphatic system. The triglyceride‐rich particles of parenteral lipid emulsions are comparable in size to endogenously synthesized chylomicrons, and both types of particles are coated by a monolayer of phospholipids. However, lipid emulsions particles differs from chylomicrons in that they contain a higher proportion of phospholipids, they lack cholesteryl esters, and upon infusion they do not contain the apolipoproteins that confer specificity on the lipoprotein particles.35 Nevertheless, the catabolic fate of lipid emulsions in the blood was previously thought to be analogous to that of chylomicrons.36 More recent data indicate that there are differences in the particle blood clearance rate and clearance mechanism, as well as tissue targeting between lipid emulsions and chylomicrons.37 In addition, the FA composition of the lipid emulsion particles impact clearance rate and tissue targeting.38, 39 The biochemical differences between chylomicrons and lipid emulsion particles could possibly explain the differences in efficiency to convert DHA administered enterally or parenterally into serum phospholipids.
To the best of our knowledge, this is only the second study investigating the combined effect of enteral and parenteral lipids on blood FA levels in extremely preterm infants. De Rooy et al determined the cumulative intake of LC‐PUFAs from milk, formula, and parenteral sources over the first 6 weeks of life for infants born < 28 weeks gestational age.40 The infants received 1.2 g kg−1 less DHA and 7.7 g kg−1 less AA than normally provided in utero during the corresponding period. Enteral and parenteral FA intake during week 1 and 2 were comparable in the study by De Rooy et al and the Clinoleic (Baxter Medical AB) group in this study. Thus, infants on Clinoleic (Baxter Medical AB) in the present cohort may suffer from a similar deprivation of AA and DHA during their first weeks of life.
Conclusions
Our results suggest that it is difficult to increase serum fractions of the LC‐PUFAs AA and DHA in extremely preterm infants through parenteral lipid supplementation. High intake of fish oil‐containing lipid emulsion rich in n‐3 LC‐PUFAs (SMOFlipid; Fresenius Kabi) resulted in reduction of serum AA, whereas high intake of a plant oil‐only emulsion (Clinoleic; Fresenius Kabi) decreased serum EPA and DHA levels. There is a need for further development of parenteral lipid emulsions for use in neonates to obtain an FA composition that results in appropriate serum lipid levels that meet the needs of the preterm infant.
Supporting information
Figure S1. Fatty acid fractions in cord blood (CB) and infant serum. Data are presented as median (25th‐75th percentile). Figure S1 is available online at http:/pen.sagepub.com.
Acknowledgment
The authors thank all participating families, the study team led by Carola Pfeiffer‐Mosesson, Anne Rosenqvist, and Pia Lundgren for help retrieving the data for the study, and Berit Holmberg for preparation of the FAMEs esters.
Statement of Authorship
A. Hellström, C. Löfqvist, and A. K. Nilsson contributed to the conception of the research; A. K. Nilsson, C. Löfqvist, S. Najm, G. Hellgren, K. Sävman, M. X. Andersson, L. E. H. Smith, and A. Hellström contributed to the design of the research; M. X. Andersson, A. Hellström, S. Najm, C. Löfqvist, and A. K. Nilsson contributed to the data acquisition and analysis; A. K. Nilsson, C. Löfqvist, S. Najm, G. Hellgren, K. Sävman, M. X. Andersson, L. E. H. Smith, and A. Hellström contributed to the interpretation of the data. A. K. Nilsson drafted the manuscript. A. K. Nilsson, C. Löfqvist, S. Najm, G. Hellgren, K. Sävman, M. X. Andersson, L. E. H. Smith, and A. Hellström critically revised the manuscript. A. K. Nilsson, C. Löfqvist, S. Najm, G. Hellgren, K. Sävman, M. X. Andersson, L. E. H. Smith, and A. Hellström agree to be fully accountable for ensuring the integrity and accuracy of the work. All authors read and approved the final manuscript.
Financial Disclosure: This study was supported by grants provided by the Swedish Research Council (DNR# 2015‐00810), Gothenburg County Council (ALFGBG‐426531), and long‐term support by De Blindas Vänner.
Conflicts of interest: None declared.
References
- 1. Driscoll DF, Bistrian BR, Demmelmair H, Koletzko B. Pharmaceutical and clinical aspects of parenteral lipid emulsions in neonatology. Clin Nutr 2008;27:497‐503. [DOI] [PubMed] [Google Scholar]
- 2. Qawasmi A, Landeros‐Weisenberger A, Bloch MH. Meta‐analysis of LCPUFA supplementation of infant formula and visual acuity. Pediatrics 2013;131:e262‐e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fang PC, Kuo HK, Huang CB, Ko TY, Chen CC, Chung MY. The effect of supplementation of docosahexaenoic acid and arachidonic acid on visual acuity and neurodevelopment in larger preterm infants. Chang Gung Med J 2005;28:708‐715. [PubMed] [Google Scholar]
- 4. Wang Q, Cui Q, Yan C. The effect of supplementation of long‐chain polyunsaturated fatty acids during lactation on neurodevelopmental outcomes of preterm infant from infancy to school age: a systematic review and meta‐analysis. Pediatr Neurol 2016;59:54‐61.e1. [DOI] [PubMed] [Google Scholar]
- 5. Lapillonne A. Enteral and parenteral lipid requirements of preterm infants. World Rev Nutr Diet 2014;110:82‐98. [DOI] [PubMed] [Google Scholar]
- 6. Calder PC. n‐3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 2006;83:1505s‐1519s. [DOI] [PubMed] [Google Scholar]
- 7. Lapillonne A, Moltu SJ. Long‐chain polyunsaturated fatty acids and clinical outcomes of preterm infants. Ann Nutr Metab 2016;69(Suppl 1):35‐44. [DOI] [PubMed] [Google Scholar]
- 8. Carnielli VP, Simonato M, Verlato G, et al. Synthesis of long‐chain polyunsaturated fatty acids in preterm newborns fed formula with long‐chain polyunsaturated fatty acids. Am J Clin Nutr 2007;86:1323‐1330. [DOI] [PubMed] [Google Scholar]
- 9. Lapillonne A, Jensen CL. Reevaluation of the DHA requirement for the premature infant. Prostaglandins Leukot Essent Fatty Acids 2009;81:143‐150. [DOI] [PubMed] [Google Scholar]
- 10. Colombo J, Jill Shaddy D, Kerling EH, Gustafson KM, Carlson SE. Docosahexaenoic acid (DHA) and arachidonic acid (ARA) balance in developmental outcomes. Prostaglandins Leukot Essent Fatty Acids 2017;121:52‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lapillonne A, Groh‐Wargo S, Gonzalez CH, Uauy R. Lipid needs of preterm infants: updated recommendations. J Pediatr 2013;162: S37‐S47. [DOI] [PubMed] [Google Scholar]
- 12. Baack ML, Puumala SE, Messier SE, Pritchett DK, Harris WS. Daily enteral DHA supplementation alleviates deficiency in premature infants. Lipids 2016;51:423‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molloy CS, Stokes S, Makrides M, Collins CT, Anderson PJ, Doyle LW. Long‐term effect of high‐dose supplementation with DHA on visual function at school age in children born at <33 wk gestational age: results from a follow‐up of a randomized controlled trial. Am J Clin Nutr 2016;103:268‐275. [DOI] [PubMed] [Google Scholar]
- 14. Berseth CL, Harris CL, Wampler JL, Hoffman DR, Diersen‐Schade DA. Liquid human milk fortifier significantly improves docosahexaenoic and arachidonic acid status in preterm infants. Prostaglandins Leukot Essent Fatty Acids 2014;91:97‐103. [DOI] [PubMed] [Google Scholar]
- 15. Collins CT, Sullivan TR, McPhee AJ, Stark MJ, Makrides M, Gibson RA. A dose response randomised controlled trial of docosahexaenoic acid (DHA) in preterm infants. Prostaglandins Leukot Essent Fatty Acids 2015;99:1‐6. [DOI] [PubMed] [Google Scholar]
- 16. Kapoor V, Glover R, Malviya MN. Alternative lipid emulsions versus pure soy oil‐based lipid emulsions for parenterally fed preterm infants. Cochrane Database Syst Rev 2015; 12:CD009172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Najm S, Löfqvist C, Hellgren G, et al. Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: a randomized controlled trial. Clin Nutr ESPEN 2017;20:17‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nilsson AK, Löfqvist C, Najm S, Hellgren G, Sävman K, Andersson MX, et al. Long‐chain polyunsaturated fatty acids decline rapidly in milk from mothers delivering extremely preterm indicating the need for supplementation. Acta Paediatr 2018. 10.1111/apa.14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baack ML, Norris AW, Yao J, Colaizy T. Long‐chain polyunsaturated fatty acid levels in US donor human milk: meeting the needs of premature infants? J Perinatol 2012;32:598‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henderson TR, Fay TN, Hamosh M. Effect of pasteurization on long chain polyunsaturated fatty acid levels and enzyme activities of human milk. J Pediatr 1998;132:876‐878. [DOI] [PubMed] [Google Scholar]
- 21. Molto‐Puigmarti C, Castellote AI, Carbonell‐Estrany X, Lopez‐Sabater MC. Differences in fat content and fatty acid proportions among colostrum, transitional, and mature milk from women delivering very preterm, preterm, and term infants. Clin Nutr 2011;30:116‐123. [DOI] [PubMed] [Google Scholar]
- 22. Bitman J, Wood L, Hamosh M, Hamosh P, Mehta NR. Comparison of the lipid composition of breast milk from mothers of term and preterm infants. Am J Clin Nutr 1983;38:300‐312. [DOI] [PubMed] [Google Scholar]
- 23. Bauer J, Gerss J. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clin Nutr 2011;30:215‐220. [DOI] [PubMed] [Google Scholar]
- 24. Guerra E, Downey E, O'Mahony JA, et al. Influence of duration of gestation on fatty acid profiles of human milk. Eur J Lipid Sci Technol 2016;118:1775‐1787. [Google Scholar]
- 25. Andersson Y, Sävman K, Bläckberg L, Hernell O. Pasteurization of mother's own milk reduces fat absorption and growth in preterm infants. Acta Paediatrica 2007;96:1445‐1449. [DOI] [PubMed] [Google Scholar]
- 26. Delplanque B, Gibson R, Koletzko B, Lapillonne A, Strandvik B. Lipid quality in infant nutrition: current knowledge and future opportunities. J Pediatr Gastroenterol Nutr 2015;61:8‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin CR, Cheesman A, Brown J, Makda M, Kutner AJ, DaSilva D, et al. Factors determining optimal fatty acid absorption in preterm infants. J Pediatr Gastroenterol Nutr 2016;62:130‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robinson DT, Martin CR. Fatty acid requirements for the preterm infant. Semin Fetal Neonatal Med 2017;22:8‐14. [DOI] [PubMed] [Google Scholar]
- 29. Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013;60:49‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Genzel‐Boroviczeny O, Wahle J, Koletzko B. Fatty acid composition of human milk during the 1st month after term and preterm delivery. Eur J Pediatr 1997;156:142‐147. [DOI] [PubMed] [Google Scholar]
- 31. Deshpande G, Simmer K, Deshmukh M, Mori TA, Croft KD, Kristensen J. Fish oil (SMOFlipid) and olive oil lipid (Clinoleic) in very preterm neonates. J Pediatr Gastroenterol Nutr 2014;58:177‐182. [DOI] [PubMed] [Google Scholar]
- 32. Rayyan M, Devlieger H, Jochum F, Allegaert K. Short‐term use of parenteral nutrition with a lipid emulsion containing a mixture of soybean oil, olive oil, medium‐chain triglycerides, and fish oil: a randomized double‐blind study in preterm infants. JPEN J Parenter Enteral Nutr 2012;36:81s‐94s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao Y, Wu Y, Pei J, Chen Z, Wang Q, Xiang B. Safety and efficacy of parenteral fish oil‐containing lipid emulsions in premature neonates. J Pediatr Gastroenterol Nutr 2015;60:708‐716. [DOI] [PubMed] [Google Scholar]
- 34. Guthrie G, Kulkarni M, Vlaardingerbroek H, et al. Multi‐omic profiles of hepatic metabolism in TPN‐fed preterm pigs administered new generation lipid emulsions. J Lipid Res 2016;57:1696‐1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hippalgaonkar K, Majumdar S, Kansara V. Injectable lipid emulsions‐advancements, opportunities and challenges. AAPS PharmSciTech 2010;11:1526‐1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carpentier YA. Intravascular metabolism of fat emulsions: the Arvid Wretlind Lecture, ESPEN 1988. Clin Nutr 1989;8:115‐125. [DOI] [PubMed] [Google Scholar]
- 37. Hultin M, Carneheim C, Rosenqvist K, Olivecrona T. Intravenous lipid emulsions: removal mechanisms as compared to chylomicrons. J Lipid Res 1995;36:2174‐2184. [PubMed] [Google Scholar]
- 38. Simoens CM, Deckelbaum RJ, Massaut JJ, Carpentier YA. Inclusion of 10% fish oil in mixed medium‐chain triacylglycerol‐long‐chain triacylglycerol emulsions increases plasma triacylglycerol clearance and induces rapid eicosapentaenoic acid (20:5n‐3) incorporation into blood cell phospholipids. Am J Clin Nutr 2008;88:282‐288. [DOI] [PubMed] [Google Scholar]
- 39. Qi K, Seo T, Jiang Z, Carpentier YA, Deckelbaum RJ. Triglycerides in fish oil affect the blood clearance of lipid emulsions containing long‐ and medium‐chain triglycerides in mice. J Nutr 2006;136:2766‐2772. [DOI] [PubMed] [Google Scholar]
- 40. De Rooy L, Hamdallah H, Dyall SC. Extremely preterm infants receiving standard care receive very low levels of arachidonic and docosahexaenoic acids. Clin Nutr 2016;36:1593‐1600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Fatty acid fractions in cord blood (CB) and infant serum. Data are presented as median (25th‐75th percentile). Figure S1 is available online at http:/pen.sagepub.com.


