Abstract
Antimicrobial resistance (AMR) has emerged as a major threat to public health estimated to cause 10 million deaths annually by 2050. India carries one of the largest burdens of drug-resistant pathogens worldwide. NDM-1 reported in 2008, rapidly spread to other countries was named after India's capital. India is one of the largest consumers of antibiotics worldwide, and antibiotic sale is increasing rapidly. AMR develops when microbes develop mechanisms to evade the action of antimicrobials. The factors that contribute to AMR include irrational and overuse of antibiotics. In India, various actions have been taken including setting up of a National Task Force on AMR Containment (2010), “Chennai Declaration” by a consortium of the Indian Medical Societies (2012), Setting of Indian Council of Medical Research national surveillance network of laboratories, “Redline” campaign for educating public and National Action Plan on AMR 2017. There is a need integrating AMR education in medical education. India needs to start the subspecialty of infectious diseases and strengthen laboratory services. Every hospital needs to have an AMR policy including infection control, improvement in hygiene, and sanitation and antibiotic use. An element of research needs to be integrated into the AMR policy and encouragement of the pharmaceutical industry to develop “superbug antibiotics.” Unless AMR is addressed effectively the gains made in health are likely to be lost.
Keywords: Antibiotic, antimicrobial resistance, global action plan, India, National Action Plan, National Health Policy, New Delhi metallo-β-lactamase, public health problem
THE GLOBAL PROBLEM
Antimicrobial resistance (AMR) occurs when microbes (i.e., bacteria, viruses, fungi, and parasites) develop mechanisms to evade antimicrobials (i.e., antibiotics, antivirals, antifungals, and antiparasitics) rendering them ineffective. A recent report from the WHO found that due to AMR, our armamentarium of effective antimicrobials is declining rapidly.[1]
Just like all living organisms, microbes are evolving for survival. AMR has existed even before the first antibiotic was discovered. AMR commonly develops due to selective pressure applied by antibiotic use, through genetic mutations or acquisition of genetic material through plasmid transfer from a resistant bacterium.[2]
AMR has been identified as a global health threat with serious health, political, and economic implications.[3] The progress made in modern medicine is under serious threat because of the emergence of AMR. Annual deaths due to AMR are anticipated to rise to 10 million worldwide by 2050.[3] This public health problem is receiving growing attention globally. Several countries are facing the emergence of bacteria that are completely resistant to available antibiotics and countries are preparing country-specific action plan for AMR based on the global action plan of the WHO.[4]
THE PROBLEM IN INDIA
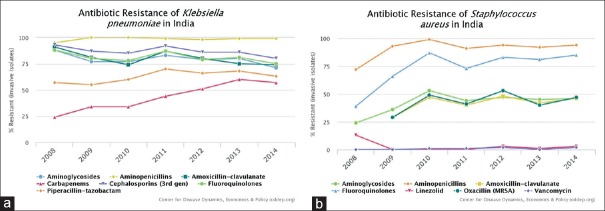
India carries one of the largest burdens of drug-resistant pathogens worldwide, including the highest burden of multidrug-resistant tuberculosis,[5] alarmingly high resistance among Gram-negative and Gram-positive bacteria [Figure 1][6] even to newer antimicrobials such as carbapenems and faropenem since its introduction in 2010.[7] Regional studies report high AMR among pathogens such as Salmonella typhi, Shigella, Pseudomonas, and Acinetobacter.[8] Annually, more than 50,000 newborns are estimated to die from sepsis due to pathogens resistant to first-line antibiotics.[9] While exact population burden estimates are not available, neonates and elderly are thought to be worse affected. Two million deaths are projected to occur in India due to AMR by the year 2050.[10] It is no surprise that emergence of enzyme New Delhi metallo-β-lactamase (NDM-1), named after the national capital of India, in 2008 rapidly spread to other countries.[11] The present article reviews the progress in addressing AMR in India after 10 years of the emergence of NDM-1.
Figure 1.
(a and b) Resistance patterns of Klebsiella pneumoniae (left) and Staphylococcus aureus (right) isolates in India (Figure generated by Center for Disease Dynamics using blood and cerebrospinal fluid isolates from inpatients collected by a private laboratory network in India with 5700 collection centers nationwide)
Infectious diseases remain a leading cause of mortality in India. Bacterial sepsis, acute respiratory illness, and acute diarrheal diseases are leading killers of children under 5 years of age. India is one of the largest consumers of antibiotics worldwide,[12] and antibiotic sales continue to increase rapidly.[13] Despite the decline in communicable diseases, antibiotic use continues to increase [Figure 2].[6]
Figure 2.
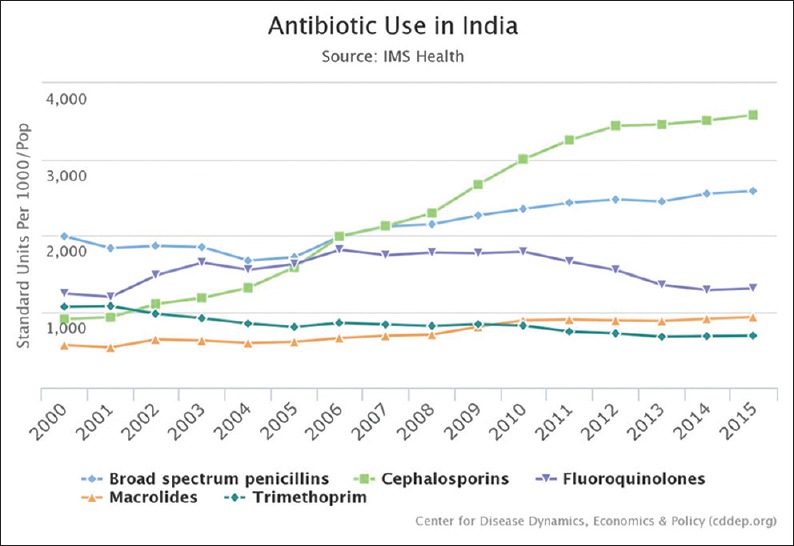
Use data from Center for disease dynamics aggregated from two antibiotic consumption databases
The high burden of AMR in India is driven by multiple factors. Antibiotic over-prescription is driven by a poor understanding of its dangers and contribution to AMR on the part of the provider as well as patients who lack knowledge regarding appropriate antibiotic use.[14] In addition, pharmacists who are often the first point of care, dispense antibiotics without a physician prescription, offer alternative antibiotics even when patients present with a prescription. Within the hospitals lack of monitoring of antibiotic use is one of the major factors driving the spread of resistance.[15] Health system factors are also at fault. Doctors routinely receive compensation from pharmaceutical companies in exchange for antibiotic prescriptions.[16] Alarming rates of resistance have been reported in animal isolates of human pathogens,[8] but the evidence is insufficient to make national estimates.
Available data indicate to rising rates of AMR, across multiple pathogens of clinical importance, in the country. In 2008, about 29% of isolates of Staphylococcus aureus were methicillin resistant, and by 2014, this had risen to 47%. In contrast, in countries with effective antibiotic stewardship and/or infection prevention and control programs, the proportion of methicillin-resistant S. aureus isolates have been decreasing.[17]
Economic considerations
India spends only 4.7% of its total Gross Domestic Product on health, with government share only one-fourth (1.15%)[18] of it, makes the task massive. One study found the median cost of treatment of a resistant bacterial infection to be more than a year wages of a rural worker.[19]
Poor public health infrastructure, a high burden of disease, and unregulated sales of antibiotics have contributed to a rapid rise in resistant infections in India. This has a huge socioeconomic impact due to deaths and increased costs due to prolonged stay in hospital, additional and repeated laboratory investigations, and loss of work for treating resistant bacterial infections. There are no proper documented estimates on the economic impacts of AMR in India. It can be assumed that due to the high incidence of malaria, tuberculosis, and HIV in India and lack of regulations on the use of antibiotics in humans and the production of food-producing animals, the impacts of AMR on the Indian economy could be huge.[20]
ACTIONS TAKEN SO FAR
Over the past 8 years, national commitment to address AMR has steadily increased. The momentum can be seen from the increased research focus, where more than 50% (2184) of the 4220 articles indexed in PubMed for “AMR India” and 68 of the 77 articles resulting from “antibiotic stewardship India” searches were published in the past 5 years. The national situation was very well aligned to Kingdon's three-stream policy window model[21] that led to major policy changes in India. In the model, for a policy change to occur, three “streams” must flow together: one – problem (in need of attention), two – policy (available solutions), and three – politics (recognition of the problem by politicians). National and international attention was brought to the issue with the emergence of NDM-1; global examples of successful strategies were available to address the problem.
The first major step toward tackling this problem was taken in the form of a National Task Force on AMR Containment in 2010 followed by the adoption of National Policy for Containment of AMR, the Jaipur Declaration and the inclusion of antimicrobial containment in the 12th 5-year plan in 2011.[22] However, this policy made little progress due to difficulties in implementation.
Further progress was made with the active involvement of the Indian Council of Medical Research (ICMR), and the adoption of the “Chennai Declaration” at the second annual conference of the Clinical Infectious Disease Society at Chennai on August 24, 2012.[23] This was the first-ever meeting of medical societies in India on this issue. The declaration provided a roadmap to tackle the challenges of AMR from an Indian perspective.[24,25] The declaration has had an unprecedented impact at national and international arena. ICMR established a national surveillance network of laboratories at tertiary medical academic centers.
The political commitment came from the Prime Minister himself. Public education came to the forefront with prime minister directly addressing the nation in his 2016 radio address highlighting the issue of antibiotic resistance and the launch of the “Red Line” campaign. A red line on the antibiotic packaging is aimed to draw public attention to the dangers of its misuse and has been lauded internationally.[26]
The Government recently adopted a National Action Plan (NAP) on AMR in 2017.[27] The strategic objectives of NAP-AMR are aligned with the global action plan based on national needs and priorities. In addition to the five priorities of Global Action Plan on AMR, India has a sixth priority dealing with India's leadership on AMR. Six strategic priorities have been identified under the NAP-AMR: (i) improving awareness and understanding of AMR through effective communication, education, and training; (ii) strengthening knowledge and evidence through surveillance; (iii) reducing the incidence of infection through effective infection prevention and control; (iv) optimizing the use of antimicrobial agents in health, animals, and food; (v) promoting investments for AMR activities, research, and innovations; and (vi) strengthening India's leadership on AMR.[27]
CHALLENGES FOR CONTROL OF AMR AND POSSIBLE STRATEGIES
To prevent over the counter sales of important antibiotics, the Central Drugs Standard Control Organization implemented Schedule H1 in India starting March 1, 2014. The H1 list includes 24 antibiotics, such as third-generation and fourth-generation cephalosporins, carbapenems, antituberculosis drugs, and newer fluoroquinolones. Antibiotics have previously been listed under Schedule H, which contained drugs that could be sold only with a valid prescription. Drugs included in Schedule H1 can only be sold with the prescription of a registered medical practitioner and require that that pharmacist maintained a separate register with the patient's name, contact details of the prescribing doctor, and the name and dispensed quantity of the drug. The register has to be retained for at least 3 years and is subject to audit by the government.[28]
While the significant progress has been made, major challenges remain, including the implementation of the most recent National Health Policy (2017)[18] which includes education to the general public and professionals. Many examples exist from around the world that have been successful in integrating AMR in education early.[29,30] A stark missing component is the total lack of training in infectious disease other than tuberculosis. There is no mention in the policy about the mechanism of bringing practitioners of alternative medicine who also prescribe antibiotics into the fold.
Another missing component is empowering the public to ask questions regarding the appropriateness of treatment when they receive antibiotics from a prescriber or a chemist. Many successful media campaigns have harnessed the celebrity power in India, for example, polio vaccination and tuberculosis screening. A new celebrity champion for AMR would be a good start.
ICMR's surveillance network currently only includes tertiary medical centers. The data from these centers are unlikely to be representative of the problem in the community. This component can be made feasible only if a broader strategy for strengthening of laboratory services at a multisectoral level and integration of data is undertaken.
Hospital infection control and prevention go hand in hand with any strategy to tackle AMR. The health policy makes this a priority for India. There is currently no reporting system for hospital-acquired infections in India. While a 2013 study of 20 hospitals found that 75% of hospitals surveyed had written infection control policy,[17] studies looking at implementation are lacking. Creating a public demand would drive hospitals to adopt effective infection control practices.
The regulation of antimicrobial sale is the most difficult challenge for the NAP-AMR 2017. The incentives given by pharmaceutical companies to doctors for prescription of branded drugs is a major issue in India, although being addressed now with the increased push to use generic drugs nationwide. A large component of antibiotics are not prescription based and beyond the purview of any regulation. Newer antibiotics can be made available in a highly restrictive manner as was seen with the government's restriction on bedaquiline.[31] Further studies to distinguish appropriate and inappropriate antibiotic use that bypasses the doctor is needed.
A search for postgraduate training in infectious diseases other than tuberculosis yields no results on the Medical Council of India website. This is a major barrier to implementation of hospital-based antibiotic stewardship programs that have had success in tackling the AMR issue worldwide. Successful stewardship models India are in their infancy,[32] and a strategy for rapid scale-up is necessary.
Poor sanitation remains a challenge that allows for the environmental spread of AMR. Poorly filtered effluent from hospitals, pharmaceutical production allows for easy bacterial mixing creating a setup for transfer of resistance-conferring mechanisms. The human consumption of such contaminated water allows for easy transmission as was demonstrated in the isolation of NDM-1 in drinking water and seepage samples in New Delhi.[33]
The lack of newer antibiotics is a worldwide problem and is certainly a challenge for India. The Indian Pharmaceutical Industry has established itself as one with low production costs and high volume. Only a few pharmaceutical companies are focusing on “superbug antibiotics,” including the CARB-X funded Bugworks.[34] The exclusion of a strategy to incentivize the development of such drugs from the national policy is detrimental.
A lack of research and evidence compounds the problem.[35] Table 1 provides several knowledge and research gaps identified by the authors in NAP–AMR 2017. Data sharing mechanism, not included in the policy, are needed to ensure that these results are available to guide policymakers and clinicians in a timely manner.
Table 1.
Priorities under 2017 National Action Plan on Antimicrobial Resistance and relevant research gaps
| Strategic priority (in 2017 plan) | Knowledge and research gaps |
|---|---|
| Improve awareness and understanding of AMR through effective education and training | KAP studies among medical professionals, general public, pharmaceutical industry etc.Effective educational strategies across sections and training levels |
| Strengthen knowledge and evidence through surveillance | Appropriate sampling procedures for surveillance - where, who, and how many labsoratories/samples.Understanding impact of environmental residues of antibiotics |
| Reduce the incidence of infection through effective infection prevention and control | Effective strategies and interventions in the Indian context Current knowledge and practices in the community and health-care settings Current of hand hygiene practices in community and health-care settings |
| Optimize the use of antimicrobials in health, animals and food | Effective regulatory mechanisms for the market Incentives for chemists and unauthorized dispensers Appropriate versus inappropriate antibiotic use unrelated to physician prescriptions Effective antibiotic stewardship in India Knowledge and awareness among general public |
| Promote investments for AMR activities, research and innovations | Cost-effectiveness of interventions Implementation strategy for roll-out of newer antibiotics |
| Strengthen India’s commitment and collaborations on AMR at international, national and subnational levels | Comprehensive stakeholder analysis Integration of parallel drug resistance containment efforts Needs assessment at subnational and local level |
Source: Original. AMR: Antimicrobial resistance, KAP: Knowledge, attitude and practice
Tackling AMR is an ethical obligation and an ethical analysis of proposed interventions is necessary. Majority of health-care provided in India is through the unregulated private sector. Limited access and long wait-times to see specialist allopathic physicians drive patients to seek alternative medicine practitioners or ask chemist shops for direct medical advice or self-prescription or use leftover antibiotics at home.
In summary, tackling the AMR challenge in India requires significant efforts from all stakeholders involved. This issue has been slow to gain momentum but is now dominating the national conscience. Despite the adoption of a national policy and significant activities already underway, progress is limited by a lack of clear implementation strategy and research gaps.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization. Antibacterial Agents in Clinical Development – An Analysis of the Antibacterial Clinical Development Pipeline, Including Mycobacterium Tuberculosis. World Health Organization. 2017. [Last accessed on 2018 Apr 06]. Available from: http://www.who.int/medicines/areas/rational_use/antibacterial_agents_clinical_development/en/
- 2.Lipsitch M, Samore MH. Antimicrobial use and antimicrobial resistance: A population perspective. Emerg Infect Dis. 2002;8:347–54. doi: 10.3201/eid0804.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Review on Antimicrobial Resistance. 2014. [Last accessed on 2018 Apr 06]. Available from: https://www.amr.review.org/sites/default/files/AMR%20Review%20Paper%20.%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf .
- 4.World Health Organization. Country Progress in the Implementation of the Global Action Plan on Antimicrobial Resistance: WHO, FAO and OIE Global Tripartite Database. World Health Organization. [Last accessed on 2018 Apr 12]. Available from: http://www.who.int/antimicrobial-resistance/global-action-plan/database/en/
- 5.TB India 2017: Revised National Tuberculosis Program Annual Status Report. New Delhi, India: Directorate General of Health Services, Ministry of Health and Family Welfare; 2017. Central TB Division. [Google Scholar]
- 6.Center for Disease Dynamics Economics and Policy. Resistance Map: India. [Last accessed on 2018 Jan 19]. Available from: https://www.resistancemap.cddep.org/CountryPage.php?country=India .
- 7.Gandra S, Klein EY, Pant S, Malhotra-Kumar S, Laxminarayan R. Faropenem consumption is increasing in India. Clin Infect Dis. 2016;62:1050–2. doi: 10.1093/cid/ciw055. [DOI] [PubMed] [Google Scholar]
- 8.Kakkar M, Walia K, Vong S, Chatterjee P, Sharma A. Antibiotic resistance and its containment in India. BMJ. 2017;358:j2687. doi: 10.1136/bmj.j2687. [DOI] [PubMed] [Google Scholar]
- 9.Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13:1057–98. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 10.State of the World’s Antibiotics. Washington, DC: Center for Disease Dynamics, Economics & Policy; 2015. [Last accessed on 2018 Jan 20]. Center for Disease Dynamics, Economics & Policy 2015. Available from: https://www.cddep.org/wp-content/uploads/2017/06/swa_edits_9.16.pdf . [Google Scholar]
- 11.Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791–8. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, et al. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14:742–50. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 13.McGettigan P, Roderick P, Kadam A, Pollock AM. Access, watch, and reserve antibiotics in India: Challenges for WHO stewardship. Lancet Glob Health. 2017;5:e1075–6. doi: 10.1016/S2214-109X(17)30365-0. [DOI] [PubMed] [Google Scholar]
- 14.Kumar SG, Adithan C, Harish BN, Sujatha S, Roy G, Malini A. Antimicrobial resistance in India: A review. J Nat Sci Biol Med. 2013;4:286–91. doi: 10.4103/0976-9668.116970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker AK, Brown K, Ahsan M, Sengupta S, Safdar N. What drives inappropriate antibiotic dispensing? A mixed-methods study of pharmacy employee perspectives in Haryana, India. BMJ Open. 2017;7:e013190. doi: 10.1136/bmjopen-2016-013190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee R. Can India stop drug companies giving gifts to doctors? BMJ. 2013;346:f2635. doi: 10.1136/bmj.f2635. [DOI] [PubMed] [Google Scholar]
- 17.Walia K, Ohri VC, Mathai D. Antimicrobial Stewardship Programme of ICMR. Antimicrobial stewardship programme (AMSP) practices in India. Indian J Med Res. 2015;142:130–8. doi: 10.4103/0971-5916.164228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Health and Family Welfare. National Health Policy. 2017. [Last accessed on 2018 Apr 13]. Available from: http://www. 164.100.158.44/showfile.php?lid=4275 .
- 19.Chandy SJ, Naik GS, Balaji V, Jeyaseelan V, Thomas K, Lundborg CS, et al. High cost burden and health consequences of antibiotic resistance: The price to pay. J Infect Dev Ctries. 2014;8:1096–102. doi: 10.3855/jidc.4745. [DOI] [PubMed] [Google Scholar]
- 20. [Last accessed on 2018 Jul 10]. Available from: https://www.downtoearth.org.in/news/antimicrobial-resistance-to-cost-global-economy-up-to-us-210-trillion-47948 .
- 21.Kingdon JW. Agendas, Alternatives, and Public Policies. New York: Longman; 1995. [Google Scholar]
- 22.Directorate General of Health Services. Nirman Bhawan, New Delhi: Ministry of Health & Family Welfare; 2011. [Last accessed on 2018 Jul 10]. National Policy for Containment of Antimicrobial Resistance in India. Available from: https://www.mohfw.gov.in/sites/default/files/3203490350abpolicy%20%281%29.pdf . [Google Scholar]
- 23.Ghafur A, Mathai D, Muruganathan A, Jayalal JA, Kant R, Chaudhary D, et al. “The Chennai Declaration” recommendations of “A roadmap to tackle the challenge of antimicrobial resistance”—A joint meeting of medical societies of India. Indian J Cancer. 2012;49 doi: 10.4103/0019-509X.104065. DOI:10.4103/0019-509X.104065. [DOI] [PubMed] [Google Scholar]
- 24.Ghafur A, Mathai D, Muruganathan A, Jayalal JA, Kant R, Chaudhary D, et al. The Chennai declaration: A roadmap to tackle the challenge of antimicrobial resistance. Indian J Cancer. 2013;50:71–3. doi: 10.4103/0019-509X.104065. [DOI] [PubMed] [Google Scholar]
- 25.Voss A, Ghafur A. “The Chennai declaration” – Indian doctors' fight against antimicrobial resistance. Antimicrob Resist Infect Control. 2013;2:7. doi: 10.1186/2047-2994-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.India Lauded for Red Line Campaign on Antibiotics – The Hindu. [Last accessed on 2018 Jan 20]. Available from: http://www.thehindu.com/news/national/india-lauded-for-red-line-campaign-on-antibiotics/article8622474.ece .
- 27.Ministry of Health and Family Welfare, Government of India. National Action Plan on Antimicrobial Resistance (NAP AMR), 2017-2021. India: Ministry of Health and Family Welfare, Government of India; 2017. [Google Scholar]
- 28.The Gazette of India. G.S.R. 588(E) 2013. Aug 30, [Last accessed on 2019 Jan 14]. Available from: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=MTM0NA==
- 29.McNulty CA, Bowen J, Gelb D, Charlett A. “The bug investigators”: Assessment of a school teaching resource to improve hygiene and prudent use of antibiotics. Health Educ. 2007;107:10–26. [Google Scholar]
- 30.McNulty CA, Lecky DM, Farrell D, Kostkova P, Adriaenssens N, Koprivová Herotová T, et al. Overview of e-bug: An antibiotic and hygiene educational resource for schools. J Antimicrob Chemother. 2011;66(Suppl 5):v3–12. doi: 10.1093/jac/dkr119. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan V. 'India's Refusal to Scale up Bedaquiline is Really the World's Problem. ’The Hindu. 2017. Jan 08, [Last accessed on 2018 Jan 18]. Available from: http://www.thehindu.com/sci-tech/health/%E2%80%98India%E2%80%99s-refusal-to-scale-up-bedaquiline-is-really-the-world%E2%80%9a9s-problem%E2%80%99/article17005742.ece .
- 32.Singh S, Menon VP, Mohamed ZU, Kumar A, Nampoothiri V, Sudhir S, et al. Implementation of antibiotic stewardship: A South Indian experience. [Last accessed on 2019 Jan 15];Open Forum Infectious Diseases. 2017 4(Suppl 1):S267–8. Available from: https://www.researchgate.net/publication/328817281_Implementation_and_impact_of_an_Antimicrobial_Stewardship_Program_at_a_tertiary_care_centre_in_South_India . [Google Scholar]
- 33.Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: An environmental point prevalence study. Lancet Infect Dis. 2011;11:355–62. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 34.The Indian Companies Taking on Superbugs. BBC News. 2017. Nov 22, [Last accessed on 2018 Jan 20]. Available from: http://www.bbc.com/news/world-asia-india-42063013 .
- 35.Das B, Chaudhuri S, Srivastava R, Nair GB, Ramamurthy T. Fostering research into antimicrobial resistance in India. BMJ. 2017;358:j3535. doi: 10.1136/bmj.j3535. [DOI] [PMC free article] [PubMed] [Google Scholar]