Abstract
Background:
Burden of metabolic syndrome (MS) is rising. There were many previous studies conducted in India on MS, yet it is less studied in Puducherry which has embraced modern culture and lifestyle. Hence, we aimed to study the prevalence and predictors of MS.
Materials and Methods:
A cross-sectional study was undertaken on a representative sample of 489 adults of age 30 years and above over the period of 18 months. MS was defined according to the International Diabetes Federation (IDF) criteria. Data on sociodemography, lifestyle characteristics, and biochemical parameters were collected by a well-trained health professional using standard methods. Generalized linear models with Poisson distribution and log link function were used to calculate the adjusted prevalence ratio (PR).
Results:
The prevalence of MS was 39.7% (95% confidence interval [CI]: 35.3–44.1) among the study participants. The most commonly deranged component of MS was central obesity (63.6%). Increasing age, upper socioeconomic status, low fruit intake, physical inactivity, use of refined sunflower oil (PR: 1.40, 95% CI: 1.07–1.83) for cooking, and high perceived stress (PR: 1.77, 95% CI: 1.32–2.37) were found to be associated with MS.
Conclusion:
The prevalence of MS in Puducherry was high as per the IDF criteria. Usage of refined sunflower oil for cooking and perceived stress was independently associated with an increased risk of MS along with other routinely studied risk factors.
Keywords: Cross-sectional study, metabolic syndrome, prevalence, risk factors, stress
INTRODUCTION
The metabolic syndrome (MS) is defined by a cluster of factors, namely central obesity, glucose intolerance, hyperinsulinemia, low-high-density lipoprotein (HDL) cholesterol, high triglycerides (TG), and systemic hypertension.[1] In India, recent rise in MS has been attributed to the shift in lifestyles both in the urban and in the rural areas.[2] MS has the potential to double the risk of cardiovascular disease, which is one of the major leading causes of adult death.[3] Thus, early identification of MS and understanding its risk factors is crucial for context-specific interventions. Due to lack of internationally agreed criteria for diagnosing MS, the past studies reported varied prevalence. World Health Organization (1998), European Group for the study of Insulin Resistance (1999), United States third report of the National Cholesterol Education Program, Adult Treatment Panel (ATP) (2001), ATPIII revised criterion (2005), and the International Diabetes Federation (IDF 2005) have proposed various definitions.[4] Among these, the latest IDF definition provides an accessible, platinum standard diagnostic tool suitable for the use in community-based epidemiological studies. The Union Territory of Puducherry is a cultural melting pot for French, Tamil, Telugu, Malayalam, and Kannada. It is a famous tourist destination known for the cheap and easy availability of liquor. Its urban and rural life is influenced by the modern lifestyle. Considering the limited number of studies on MS in this culture, the present study was done to estimate the burden of MS using IDF criteria and identifying its determinants among the rural adult population of Puducherry.
MATERIALS AND METHODS
Study setting
The study was carried out at 13 villages comprising of 8400 households under the service area of Primary Health Centre (PHC) situated at Thirubhuvanai, Puducherry. The PHC provides 24-h services, and it runs a special clinic for noncommunicable diseases (NCDs) on 2 days in 1 week. There were around 1450 patients registered and availing service in NCD clinic.
Study design and population
It was a community-based cross-sectional study conducted among adults (≥30 years) of both genders residing in the service area of PHC. People who were mentally retarded, seriously ill, pregnant, and lactating mothers were excluded from the study. It was carried out between January 2016 and August 2017.
Sample size and sampling
The sample size was calculated using the formula, N = (Z1-α/2)2 p (1-p)/d2, where P is the prevalence of MS as 30% from the previous study,[5] and d is relative precision of 20% with anticipated nonresponse of 20%. To adjust for the effect of cluster random sampling, design effect of 1.8 was adopted and it was calculated using the formula 1+ (k-1) ρ, where k is the cluster size (40) and ρ the intercluster correlation (0.02). Probability proportional to size method was used to select the clusters from villages of this PHC.
Study tool and study variables
Pretested structured questionnaire was used to collect information on sociodemography, diet, physical activity, perceived stress, tobacco and alcohol use, anthropometry, blood pressure, and lipid profile. The questionnaire was administered by trained medical social workers, and blood samples were collected by the trained medical interns under supervision.
Dr. B. G. Prasad's socioeconomic status (SES) scale for the year 2016 was used. The fruits and vegetables consumed per day were classified into normal and low consumption based on the National Nutrition Guidelines.[6] Physical activity and stress were measured using the International Physical Activity Questionnaire[7] and the perceived stress scale, respectively.[8] Tobacco and alcohol usage was ascertained based on standard definitions.[9] Height, waist circumference, and weight were measured using nonstretchable inch tape and bathroom weighing scale, respectively, as per the standard protocol,[10] and blood pressure was measured using a digital sphygmomanometer (OMRON HEM-7111). Fasting lipids (TG and HDL) and fasting blood sugar (FBS) were measured using a portable digital lipid analyzer (SD LipidoCare, SD Biosensor, Inc.). The new IDF definition was used to diagnose MS.[4] SD Lipidocare was validated on 10% of study sample for the present study against the measurements of venous FBS, TG, and HDL using the gold standard auto-analyzer (Chemwell Automated Chemistry Analyser, Awareness Technology, USA).
Procedure for data collection
Institute Ethics Committee clearance was obtained before initiating the study (Code No: 61/2015). Participants with increased waist circumference were assessed for the eligibility to carry out blood investigation. Among the eligible participants, FBS and fasting lipid profile were measured using validated SD LipidoCare. The results were informed to the study participants. Whenever the values were abnormal, they were instructed to consult a physician for further management.
Data entry and analysis
Data were entered into EpiData Manager software (version 4.2, EpiData Association, Odense, Denmark). IBM SPSS Statistics, version 24 (IBM Corp., Armonk, NY, USA) was used for data analysis. The intraclass correlation coefficient (ICC) estimates and the indices of diagnostic accuracy were calculated for the instrument. The prevalence of MS was calculated with its 95% confidence interval (CI). Unadjusted prevalence ratios (PRs) and their 95% CI were calculated. Multivariate generalized linear models with Poisson distribution and log link function were done to calculate adjusted PRs.
RESULTS
Majority of the participants (36.6%) belong to the age group of 45–59 years, 52.8% of them were females, 73.8% were married, and 29% belong to lower SES. Most of the participants (95.6%) were Hindu by religion. Refined sunflower oil was used for cooking by 71% of them. Rest of them consumed palm, groundnut, gingelly, coconut, olive, and mixture of oils. For the ease of analysis, they all were clubbed under others category. Majority of them were not consuming the recommended daily amount of fruits and vegetables. Low level of physical activity was found in 47.5% of them, and high perceived stress was found in 20.8% of them. Current use of any form of tobacco and alcoholic beverages was 16% and 10.2%, respectively [Table 1].
Table 1.
Sociodemographic details and the risk factor profiles and their association (unadjusted and adjusted analyses) with metabolic syndrome of study participants (n=489)
| Study variables | Total, n (%)@ | MS seen, n (%)# | Unadjusted PR (95% CI) | P | Adjusted PR (95% CI) | P |
|---|---|---|---|---|---|---|
| MS | 489 (100) | 194 (39.7) | - | - | - | - |
| Age (years) | ||||||
| 30-44 | 171 (35) | 48 (28.1) | 1 (reference) | NA | 1 (reference) | NA |
| 45-59 | 179 (36.6) | 87 (48.6) | 1.73 (1.30-2.30) | <0.001* | 1.69 (1.26-2.27) | <0.001* |
| ≥60 | 139 (28.4) | 59 (42.4) | 1.51 (1.11-2.06) | 0.008* | 1.58 (1.13-2.20) | 0.006* |
| Marital status^ | ||||||
| Single | 37 (7.6) | 9 (24.3) | 1 (reference) | NA | 1 (reference) | NA |
| Married | 361 (73.8) | 142 (39.3) | 1.62 (0.90-2.90) | 0.07 | 1.41 (0.79-2.49) | 0.23 |
| Others | 81 (16.6) | 35 (43.2) | 1.78 (0.95-3.30) | 0.06 | 1.34 (0.72-2.49) | 0.34 |
| SES^ | ||||||
| Upper | 19 (3.9) | 11 (57.9) | 1.71 (1.1-2.68) | 0.04* | 1.72 (1.09-2.69) | 0.02* |
| Upper-middle | 65 (13.3) | 27 (41.5) | 1.23 (0.85-1.78) | 0.28 | 1.34 (0.95-1.91) | 0.09 |
| Middle | 93 (19) | 42 (45.2) | 1.34 (0.97-1.84) | 0.08 | 1.32 (0.94-1.84) | 0.10 |
| Lower-middle | 151 (30.9) | 58 (38.4) | 1.14 (0.84-1.54) | 0.41 | 1.14 (0.85-1.53) | 0.35 |
| Lower | 142 (29) | 48 (33.8) | 1 (reference) | NA | 1 (reference) | NA |
| Oil type | ||||||
| Refined Sunflower oil | 347 (71.0) | 147 (42.4) | 1.28 (0.98-1.67) | 0.06 | 1.40 (1.07-1.83) | 0.01* |
| Others | 142 (29.0) | 47 (33.1) | 1 (reference) | NA | 1 (reference) | NA |
| Physical activity | ||||||
| High | 100 (20.4) | 27 (27.0) | 1 (reference) | NA | 1 (reference) | NA |
| Medium | 157 (32.1) | 68 (43.3) | 1.60 (1.11-2.32) | 0.008* | 1.56 (1.07-2.26) | 0.02* |
| Low | 232 (47.5) | 99 (42.7) | 1.58 (1.11-2.25) | 0.007* | 1.44 (1.00-2.06) | 0.05* |
| Perceived stress | ||||||
| Low | 174 (35.6) | 52 (29.9) | 1 (reference) | NA | 1 (reference) | NA |
| Medium | 213 (43.6) | 84 (39.4) | 1.32 (1.05-1.75) | 0.05* | 1.33 (0.98-1.78) | 0.06 |
| High | 102 (20.8) | 58 (56.9) | 1.90 (1.43-2.53) | <0.001* | 1.77 (1.32-2.37) | <0.001* |
| Fruit intake per day^ (one portion=100 G) | ||||||
| Normal (≥1) | 89 (18.2) | 26 (29.2) | 1 (reference) | NA | 1 (reference) | NA |
| Low (<1) | 396 (81.0) | 166 (41.9) | 1.43 (1.02-2.02) | 0.03* | 1.49 (1.05-2.10) | 0.02* |
| Vegetable intake per day^ (one portion=100 G) | ||||||
| Normal (≥3) | 81 (16.6) | 28 (34.6) | 1 (reference) | NA | ||
| Low (<3) | 405 (82.8) | 165 (40.7) | 1.18 (0.85-1.63) | 0.30 | ||
| Tobacco usage | ||||||
| Never | 388 (79.3) | 155 (39.9) | 1 (reference) | NA | ||
| Past | 23 (4.7) | 10 (43.5) | 1.09 (0.67-1.76) | 0.73 | ||
| Current | 78 (16) | 29 (37.2) | 0.93 (0.68-1.27) | 0.64 | ||
| Alcohol usage | ||||||
| Never | 414 (84.9) | 160 (38.6) | 1 (reference) | NA | ||
| Past | 24 (4.9) | 11 (45.8) | 1.19 (0.76-1.86) | 0.48 | ||
| Current | 50 (10.2) | 23 (46) | 1.19 (0.86-1.65) | 0.31 | ||
*Statistically significant (P≤0.05), @Column percentage, ^The totals do not tally as the analysis was done after excluding the missing values, #Row percentage. PR: Prevalence ratio, CI: Confidence interval, NA: Not applicable, MS: Metabolic syndrome, SES: Socioeconomic status
There was statistically significant good reliability (ICC: 0.75–0.9) for HDL and excellent reliability (ICC >0.9) for TG and FBS values measured by using SD LipidoCare and auto-analyzer. The sensitivity, specificity, and accuracy of SD LipidoCare were 92.9 (95% CI: 80.7–97.7), 90.5 (95% CI: 0.78–0.96), and 91.4 (95% CI: 80.7–0.97), respectively.
The prevalence of MS was found to be 39.7% (95% CI: 35.3–44.1) among the study participants. The most commonly deranged component of MS was central obesity found in 63.6% of the participants. Figure 1 provides the details of derangement of other components.
Figure 1.
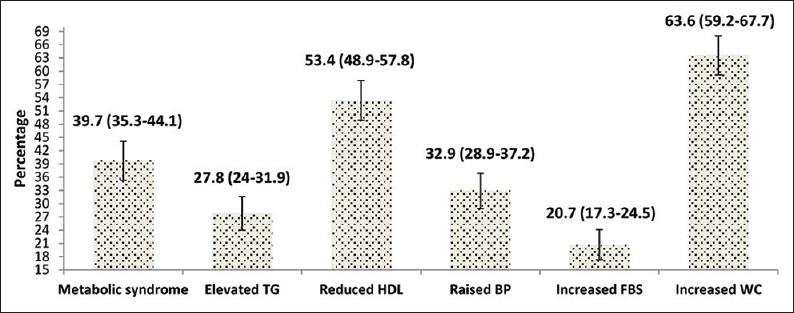
Prevalence of metabolic syndrome as per the International Diabetes Federation criteria and derangement in its individual components included in the International Diabetes Federation criteria (n = 489). Note: Figures within parenthesis indicate the 95% confidence interval
In univariate analysis, age more than 44 years, female, higher SES, Muslim religion, refined sunflower oil usage for cooking, less consumption of fruits and vegetables, physical inactivity, perceived high stress, tobacco, and alcohol consumption were identified as risk factors to develop MS. Univariate analysis showed that females were found to have higher chance (PR – 1.06, 95% CI: 0.85–1.32) and Christians the lower chance (PR – 0.86, 95% CI: 0.17–4.28) to have MS; however, this was not statistically significant. Of the other factors, age, upper SES, low fruit intake, physical inactivity, and perceived stress were significantly associated with MS [Table 1].
Multivariate regression analysis showed that age group of 45–59 years and ≥60 years, upper SES, refined sunflower oil used for cooking, low fruit intake, medium physical activity, and high perceived stress were independently associated with MS [Table 1].
DISCUSSION
We found that 39.7% rural adults (40.7% among females and 38.5% among males) had MS based on modified IDF criterion. The prevalence of MS has been reported to vary widely from 7.9% to 39% among various studies conducted across different developing countries in the world.[11,12,13] In India, it ranged from 9.2% to 43.2% as reported in the previous studies,[5,14,15,16] with a minimum prevalence of 9.2% and the maximum of 36%.[5,14] The variations in prevalence could be attributed to the adoption of different criteria for MS, study settings, laboratory techniques, and risk factors. Notably, in this study, the prevalence of MS was the highest among the previously reported.
In addition to the routine risk factors reported in the previous research,[5,11,12,13,14,15,16] we found that the use of refined oil for cooking and perceived stress was the significant risk factors for MS. These factors were not routinely studied earlier. Stress activates the hypothalamic–pituitary–adrenal axis and the sympathetic–adrenomedullary axis resulting in the cellular level inflammation.[17] Stress when persists longer gives rise to allostatic load, which results in various NCDs, including MS.[18] Unadjusted and adjusted analyses were done in the current study that showed the association of perceived stress with MS. The dose–response relationship was also demonstrated clearly, and the findings were significant [Table 1].
Dietary factors, especially intake of fat, have been linked with the initiation of proinflammatory changes resulting in insulin resistance and MS.[19] The present study captured that the use of refined sunflower oil was independently associated with MS. The probable explanation could be the high linoleic acid/alpha–linolenic acid ratio in sunflower oil and the process of refinement. This finding was consistent with that of a previous study done at Chennai.[20]
Over time, the public awareness about NCDs was raised; however, it failed to be translated into their reduction.[21,22] Hence, science-based behavior-focused communications have to be designed based on nonhealth-related emotional drivers. Creation of more external enabling environment and context-specific behavior change communication strategies are the solutions.[23] Yoga is considered as the way of life which teaches physical poses, breathing exercises, and meditation. A systematic review showed yoga can be preliminarily considered as a safe and effective intervention for reducing waist circumference and blood pressure in patients of MS who were not adhering to conventional forms of exercise.[24]
Strengths and limitations
The present study has several strengths. First, to our knowledge, it was the first community-based study on MS in rural Puducherry; second, it was carried out in a randomly selected relatively large sample; hence, results can be fairly extrapolated; third, we assessed the rarely studied risk factors of MS, including stress; and finally, the multivariate regression analysis was used to find their independent risks. However, being a cross-sectional study, it is difficult to establish the temporal association.
CONCLUSIONS AND RECOMMENDATIONS
The prevalence of MS was found to be high. To reduce the burden, the focus should be given on promotion of healthy lifestyle among youth and early screening for risk factors such as obesity, lack of physical activity, and stress. Context-specific cost-effective and feasible interventions to reduce identified risk factors are the need of the hour.
Financial support and sponsorship
We sincerely thank the management of Sri Manakula Vinayagar Medical College and Hospital for providing the financial support under intramural funding.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge the Medical Officer of Primary Health Centre (Thirubhuvanai), medical interns and social workers for their support.
REFERENCES
- 1.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Misra A, Misra R, Wijesuriya M, Banerjee D. The metabolic syndrome in South Asians: Continuing escalation and possible solutions. Indian J Med Res. 2007;125:345–54. [PubMed] [Google Scholar]
- 3.Olijhoek JK, van der Graaf Y, Banga JD, Algra A, Rabelink TJ, Visseren FL, et al. The metabolic syndrome is associated with advanced vascular damage in patients with coronary heart disease, stroke, peripheral arterial disease or abdominal aortic aneurysm. Eur Heart J. 2004;25:342–8. doi: 10.1016/j.ehj.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 5.Selvaraj I, Gopalakrishnan S, Logaraj M. Prevalence of metabolic syndrome among rural women in a Primary Health Centre area in Tamil Nadu. Indian J Public Health. 2012;56:314–7. doi: 10.4103/0019-557X.106423. [DOI] [PubMed] [Google Scholar]
- 6.National Institute of Nutrition. Dietary Guidelines for Indians: A Manual. 2011. [Last accessed on 2017 May 28]. Available from: http://www.ninindia.org/dietaryguidelinesforninwebsite.pdf .
- 7.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 9.World Health Organisation. Global Tobacco Surveillance System (GTSS), Global Adult Tobacco Survey (GATS): Indicator Guidelines: Definition and Syntax. World Health Organisation. 2009. [Last accessed on 2017 Dec 09]. Available from: http://www.who.int/tobacco/surveillance/en_tfi_gats_indicator_guidelines.pdf .
- 10.Centre for Disease Control. National Health and Nutrition Examination Survey (NHANES): Anthropometry Procedures Manual. Centre for Disease Control. 2007. [Last accessed on 2016 Dec 31]. Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf .
- 11.Yu S, Guo X, Yang H, Zheng L, Sun Y. An update on the prevalence of metabolic syndrome and its associated factors in rural Northeast China. BMC Public Health. 2014;14:877. doi: 10.1186/1471-2458-14-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreira GC, Cipullo JP, Ciorlia LA, Cesarino CB, Vilela-Martin JF. Prevalence of metabolic syndrome: Association with risk factors and cardiovascular complications in an urban population. PLoS One. 2014;9:e105056. doi: 10.1371/journal.pone.0105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhowmik B, Afsana F, Siddiquee T, Munir SB, Sheikh F, Wright E, et al. Comparison of the prevalence of metabolic syndrome and its association with diabetes and cardiovascular disease in the rural population of Bangladesh using the modified national cholesterol education program expert panel Adult Treatment Panel III and International Diabetes Federation definitions. J Diabetes Investig. 2015;6:280–8. doi: 10.1111/jdi.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pathania D, Bunger R, Bunger E, Mishra P, Arora A. An epidemiological study of metabolic syndrome in a rural area of Ambala district, Haryana. J Family Community Med. 2014;21:130–3. doi: 10.4103/2230-8229.134774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deepa M, Farooq S, Datta M, Deepa R, Mohan V. Prevalence of metabolic syndrome using WHO, ATPIII and IDF definitions in Asian Indians: The Chennai urban rural epidemiology study (CURES-34) Diabetes Metab Res Rev. 2007;23:127–34. doi: 10.1002/dmrr.658. [DOI] [PubMed] [Google Scholar]
- 16.Prasad DS, Kabir Z, Dash AK, Das BC. Prevalence and risk factors for metabolic syndrome in Asian Indians: A community study from urban Eastern India. J Cardiovasc Dis Res. 2012;3:204–11. doi: 10.4103/0975-3583.98895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes ME, Ekkekakis P, Eisenmann JC. The physical activity, stress and metabolic syndrome triangle: A guide to unfamiliar territory for the obesity researcher. Obes Rev. 2010;11:492–507. doi: 10.1111/j.1467-789X.2009.00680.x. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein DS, McEwen B. Allostasis, homeostats, and the nature of stress. Stress. 2002;5:55–8. doi: 10.1080/102538902900012345. [DOI] [PubMed] [Google Scholar]
- 19.Cai D, Liu T. Inflammatory cause of metabolic syndrome via brain stress and NF-κB. Aging (Albany NY) 2012;4:98–115. doi: 10.18632/aging.100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakshmipriya N, Gayathri R, Praseena K, Vijayalakshmi P, Geetha G, Sudha V, et al. Type of vegetable oils used in cooking and risk of metabolic syndrome among Asian Indians. Int J Food Sci Nutr. 2013;64:131–9. doi: 10.3109/09637486.2012.728197. [DOI] [PubMed] [Google Scholar]
- 21.Stroebele N, Müller-Riemenschneider F, Nolte CH, Müller-Nordhorn J, Bockelbrink A, Willich SN, et al. Knowledge of risk factors, and warning signs of stroke: A systematic review from a gender perspective. Int J Stroke. 2011;6:60–6. doi: 10.1111/j.1747-4949.2010.00540.x. [DOI] [PubMed] [Google Scholar]
- 22.Devi P, Rao M, Sigamani A, Faruqui A, Jose M, Gupta R, et al. Prevalence, risk factors and awareness of hypertension in India: A systematic review. J Hum Hypertens. 2013;27:281–7. doi: 10.1038/jhh.2012.33. [DOI] [PubMed] [Google Scholar]
- 23.Kreuter MW, Lukwago SN, Bucholtz RD, Clark EM, Sanders-Thompson V. Achieving cultural appropriateness in health promotion programs: Targeted and tailored approaches. Health Educ Behav. 2003;30:133–46. doi: 10.1177/1090198102251021. [DOI] [PubMed] [Google Scholar]
- 24.Cramer H, Langhorst J, Dobos G, Lauche R. Yoga for metabolic syndrome: A systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23:1982–93. doi: 10.1177/2047487316665729. [DOI] [PubMed] [Google Scholar]


