Abstract
Background
Congenital cytomegalovirus (CMV) infection is a major cause of sensorineural hearing loss. By law, newborns in Connecticut who fail newborn hearing screening are tested for infection with CMV. This targeted screening is controversial, because most children with congenital CMV infection are asymptomatic, and CMV-related hearing loss can have a delayed onset. Our hospital uses a saliva polymerase chain reaction (PCR) assay (confirmed by a urine PCR assay) to detect CMV. Here, we report the results of the first year of our screening program.
Methods
We reviewed the medical records of newborns in the Yale New Haven Health System who failed the newborn hearing screening test between January 1 and December 31, 2016.
Results
Of 10964 newborns, 171 failed newborn hearing screening, and 3 of these newborns had positive saliva CMV PCR test results. Of these 3 newborns, 2 had positive results on the confirmatory test (for 1 of them the confirmatory test was not performed until the infant was 10 weeks old), and 1 had a negative result on the confirmatory test. Three additional newborns with congenital CMV infection were tested because of clinical indications (1 for ventriculomegaly on prenatal ultrasound and 2 for CMV infection of the mother). Results of audiology follow-up were available for 149 (87.1%) of the 171 newborns who failed newborn hearing screening; 127 (85.2%) had normal results.
Conclusion
Our targeted screening program for congenital CMV infection had a low yield. Consideration should be given to other strategies for identifying children at risk of hearing loss as a result of congenital CMV infection.
Keywords: congenital CMV screening, failed newborn hearing screen, newborns
Cytomegalovirus (CMV) is the most common cause of congenital infection and a major cause of both sensorineural hearing loss and cognitive deficits in children [1, 2]. Although some newborns with congenital CMV infection present with microcephaly, thrombocytopenia, and/or hepatosplenomegaly, most are asymptomatic [3, 4]. Both symptomatic and otherwise asymptomatic infected newborns can develop progressive or fluctuating hearing loss that can either be present at birth or develop later in childhood [5–7]. Antiviral treatment, the efficacy of which has been studied only in infants with symptomatic congenital CMV, can prevent additional deterioration of hearing and might improve developmental outcomes [8–12].
Universal screening for congenital CMV infection is controversial because most infected newborns are asymptomatic and will not develop hearing loss [13]. Targeted screening programs that screen only newborns who have failed the newborn hearing screen seek to identify otherwise asymptomatic newborns with CMV-related hearing loss who would otherwise go undiagnosed [14, 15]. Results of 1 recent study suggest that screening for hearing in the immediate newborn period can miss many infants with congenital CMV infection who will later develop CMV-related hearing loss [16].
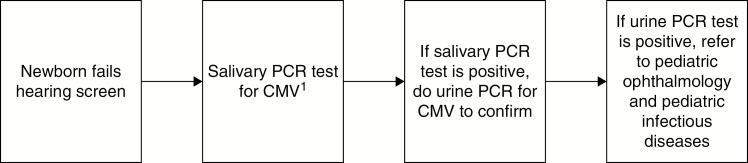
In 2015, the state of Connecticut introduced a legal mandate for targeted newborn screening for congenital CMV infection [17]. We developed a protocol (Figure 1) for all children born in the Yale-New Haven Health System (YNHHS), which includes ~11000 deliveries annually. The YNHHS hospitals serve a diverse population in Connecticut. Of infants born in YNHHS hospitals in 2014, 54% were white or Caucasian, 20% were of an “other” race/ethnicity (80% of whom were Hispanic or Latino), 16% were black or African American, 4% were Asian, and 6% were of unknown race/ethnicity [18]. Of the 4 hospitals that were included, some first used an otoacoustic emissions (OAE) test followed by assessment with an auditory brainstem response (ABR) test for those who failed the initial test. Other hospitals first used an ABR test followed by a repeat ABR test for those who failed the initial test. Both protocols were deemed appropriate for normal newborns by the Joint Committee on Infant Hearing [19].
Figure 1.
Targeted newborn screening protocol for cytomegalovirus at Yale-New Haven Health System. Abbreviations: CMV, cytomegalovirus; PCR, polymerase chain reaction.
We estimated that our targeted screening program would lead to the detection of 4 to 9 otherwise asymptomatic newborns with CMV-related hearing loss each year on the basis of the following data: 0.58% of newborns in developed countries have congenital CMV infection [20, 21]; at birth, 10% to 15% of CMV-infected newborns are symptomatic; and approximately one-third of symptomatic newborns and 7% to 15% of otherwise asymptomatic infected newborns develop sensorineural hearing loss [5, 20]. Here, we report the results from the first year of our targeted newborn screening program for congenital CMV infection.
METHODS
We reviewed the electronic medical records of all children delivered between January 1 and December 31, 2016, at 4 hospitals in the YNHHS (the York Street and St. Raphael Campuses of Yale-New Haven Hospital, Bridgeport Hospital, and Greenwich Hospital) who failed newborn hearing screening at 24 hours of life or who tested positive for CMV (through a saliva or urine polymerase chain reaction [PCR] assay) within 3 weeks of birth. Failed hearing screening was defined as either unilateral or bilateral failure of the OAE or ABR test that was not followed by a normal test result before hospital discharge. This study was approved by the institutional review board of Yale University.
RESULTS
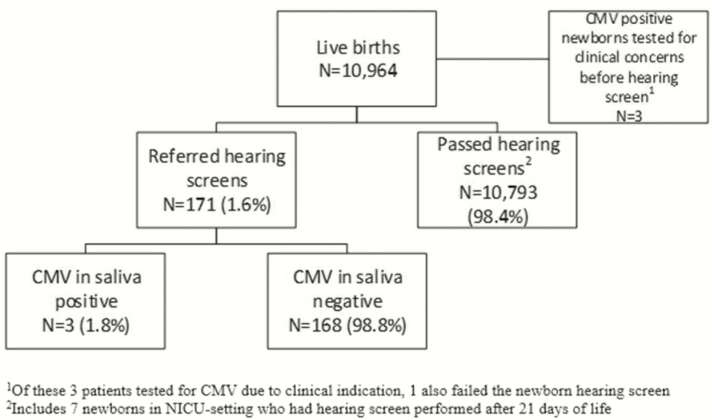
A total of 10964 live births occurred at YNHHS hospitals in 2016. Characteristics of the 171 (1.6%) newborns who failed newborn hearing screening are shown in Table 1, and in Figure 2, we show the results of the CMV screening program. All 171 of the newborns who failed newborn hearing screening were tested for CMV infection with a PCR assay of saliva, and 3 (1.8%) had a positive result. Of these 3 infants, 1 was confirmed as having congenital CMV infection, 1 had a CMV-positive PCR result from urine obtained at 10 weeks of age (which was too late to be certain whether the infection was congenital or acquired), and 1 had a false-positive result. In addition, 3 newborns who were tested shortly after birth because of clinical indications were found to have congenital CMV infection. Details of the newborns with positive CMV results are shown in Table 2.
Table 1.
Characteristics of Newborns Screened for Cytomegalovirus After Failed Newborn Hearing Screening Test (N = 171)
| Characteristic | N | % |
|---|---|---|
| Male sex | 96 | 56.1 |
| Racea | ||
| White | 68 | 39.8 |
| Other | 53 | 31.0 |
| Black | 37 | 21.6 |
| Unknown | 10 | 5.8 |
| Asian | 3 | 1.8 |
| Ethnicitya | ||
| Non-Hispanic | 116 | 67.8 |
| Hispanic | 46 | 26.9 |
| Unknown | 9 | 5.3 |
| Mother’s age <35 years | 132 | 77.2 |
| Vaginal delivery | 143 | 83.6 |
| Term gestationb | 159 | 93.5 |
| Birth weight percentilec | ||
| <10th | 22 | 12.9 |
| 10th–90th | 128 | 74.9 |
| >90th | 21 | 12.3 |
| Head circumference percentilec | ||
| <10th | 40 | 23.4 |
| 10th–90th | 112 | 65.5 |
| >90th | 19 | 11.1 |
| Newborn hearing screening test result | ||
| Unilateral fail | 94 | 55.0 |
| Bilateral fail | 77 | 45.0 |
| Sample collected for CMV test | ||
| Saliva and urine | 95 | 55.6 |
| Saliva only | 74 | 43.3 |
| Urine only | 2 | 1.2 |
| Audiology evaluation completed | 149 | 87.1 |
| Results of audiology evaluation | ||
| No hearing loss | 127 | 85.2 |
| Unilateral hearing loss | 12 | 8.1 |
| Bilateral hearing loss | 10 | 6.7 |
Abbreviation: CMV, cytomegalovirus.
aRace and ethnicity listed are as documented in the electronic medical record.
bGestational age was calculated from last menstrual period; term gestation was defined as ≥37 weeks.
cFor birth weight and head circumference percentiles, the World Health Organization growth curves were used if gestational age was ≥37 weeks, and the Fenton growth curves were used if gestational age was <37 weeks.
Figure 2.
Results of the targeted cytomegalovirus screening program. Abbreviations: CMV, cytomegalovirus; PICU, pediatric intensive care unit.
Table 2.
Newborns With Positive Cytomegalovirus Test Results (N = 6)
| Patient | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Reason for CMV testing | Failed hearing screen | Failed hearing screen | Failed hearing screen | Mother with prenatal CMV infection | Ventriculomegaly on prenatal US | Twin of newborn E |
| Sex | Female | Female | Female | Female | Male | Female |
| Race/ethnicity | White/Non-Hispanic | Black/Non-Hispanic | White/Non-Hispanic | Other/Hispanic | White/Non-Hispanic | White/Non-Hispanic |
| Delivery | Vaginal | Vaginal | Vaginal | Vaginal | Cesarean | Vaginal |
| Gestational age (weeks, days) | 39, 6 | 40, 1 | 39, 0 | 40, 5 | 38, 4 | 38, 4 |
| BW (%)a | 3.2 | 97.2 | 4.9 | 92.4 | 1.3 | 0.6 |
| Head circumference (%)a | 2.2 | 63.6 | 0.2 | 54.1 | 12.8 | 40.3 |
| Newborn hearing screen result | Unilateral fail | Unilateral fail | Unilateral fail | Normal | Bilateral fail | Normal |
| Confirmatory audiology test result | Bilateral hearing loss | Normal | Normal | Unknownb | Bilateral hearing loss | Normal |
| CMV test results | Saliva+, urine+ | Saliva+, urine−c | Saliva+, urine+ at 10 weeks | Saliva+, urine+ | Saliva not tested, urine+ |
Saliva not tested, urine+ |
| Additional workup | Bilateral subependymal cysts | None | None | Normal | Thrombocytopenia | Thrombocytopenia, choroid plexus cysts |
| Treatment with valganciclovir | Yes | No | No | No | Yes | Yes |
Abbreviations: +, positive; −, negative; BW, birth weight; CMV, cytomegalovirus; US, ultrasound.
aFor birth weight and head circumference percentiles, the WHO growth curves were used if gestational age was ≥37 weeks, and the Fenton growth curves were used if gestational age was <37 weeks.
bResults of outpatient audiology evaluation were not documented for this patient.
cThe saliva polymerase chain reaction (PCR) assay result was weakly positive, and the urine PCR assay result was negative.
Of the 171 newborns who failed hearing screening, collection of a urine specimen before discharge was documented for 97 (56.7%). Of these 97 specimens, 87 (89.7%) were discarded appropriately without being tested for CMV infection, 8 (8.2%) were tested in error because the saliva test results for CMV were negative, and 2 (2.1%) had a positive result for CMV.
Of 171 newborns who failed hearing screening, 149 (87.1%) were reevaluated by an audiologist before they were 3 months of age. Of these 149 newborns, 127 (85.1%) had normal hearing, whereas 22 (14.9%) had some degree of hearing loss from a variety of different causes. Of the 171 newborns who failed the hearing screening test, communication of the hearing screen results to the outpatient pediatrician was documented in the electronic medical record for 109 (63.7%).
DISCUSSION
During the first year, only 1 case of hearing loss related to congenital CMV infection was detected as a result of the targeted newborn screening program for congenital CMV infection at YNHHS. Given the relatively high incidence of congenital CMV infection in industrialized nations, we identified a lower number of affected newborns than we had expected or than has been reported in the literature. From an Italian study, Barbi et al [22] reported that CMV DNA was detected in blood spots from 10% of the newborns with hearing loss. In a 2008 study, Stehel et al [14] found that 6% of newborns with hearing loss tested positive for CMV. In the large prospective Congenital Cytomegalovirus Infection and Hearing Multicenter Screening Study (CHIMES), investigators found that targeted CMV screening captured approximately 57% of infants with hearing loss attributable to CMV [16]. Targeted newborn screening for congenital CMV infection can miss newborns who develop delayed-onset CMV-related hearing loss, which might partly explain our findings. Recent studies from Fowler et al. [16] also found that targeted screening can miss many cases of CMV-related hearing loss present in the newborn period. This finding might be a result of either poor sensitivity of newborn hearing screening or progressive CMV-related hearing loss that is not present at birth.
Of the newborns who failed their hearing screening test at YNHHS, 1 (0.6%) was found to have congenital CMV infection, and a second child (who had a positive urine assay result at 10 weeks of age) might have had congenital infection. This incidence is lower than that reported in Utah, the first state to introduce targeted newborn screening for congenital CMV infection, where 6% of newborns who failed their hearing screening test were found to have congenital CMV infection [22]. This difference might be partly attributable to either demographic or regional differences between the 2 populations or differences in the screening protocols. Our study population was from 1 region (south central) of Connecticut; regional differences in the incidence of CMV might exist within the state. Although the YNHHS tested all newborns who failed newborn hearing screening for congenital CMV infection, in Utah, newborns were tested for CMV infection only after they failed both the newborn hearing screenings (OAE and ABR) and an outpatient ABR before they were 21 days old [23]. The percentage of newborns tested for CMV was higher in our study (1.6%) than in the Utah study (0.7%) [23]. This finding might be a result of the higher specificity of the outpatient hearing screening test in the Utah study, which suggests that a higher proportion of the newborns who were tested for congenital CMV truly had abnormal hearing.
The identification of CMV-positive newborns could have been affected by the sensitivity of the PCR assay for CMV. However, it is unlikely that the small number of newborns who we identified was a result of poor sensitivity of our assay. The sensitivity of our assay is excellent and can detect ≥200 copies of virus per milliliter (and usually even fewer) in assays of blood and was one of the best-performing assays in 2 multicenter studies [24, 25]. Of the newborns who failed their newborn hearing screen, 100% were tested for CMV with a salivary PCR assay. The addition of CMV screening to the established newborn hearing screening workflow likely contributed to our high screening rate. Attempts were made to collect a urine specimen from newborns who failed the hearing screen, but many infants were discharged before that specimen was obtained. One newborn with a positive salivary PCR result was discharged before a urine specimen could be collected; although the private pediatrician was notified promptly, there was a delay before the urine specimen that confirmed CMV infection was obtained, which led to uncertainty about the diagnosis. This scenario demonstrates the importance of collecting a urine specimen before discharge from all newborns who fail their hearing screen. Because urine collection with sterile bags can be time-consuming, uncomfortable for newborns (especially for those who were circumcised recently), and can create additional stress for new parents, alternative methods of urine collection should be considered. For example, 1 study found that urine collected from cotton balls placed in a newborn’s diaper and tested by a PCR assay for CMV had results similar to those of urine collected from sterile bags [26].
In summary, our large hospital health system successfully implemented targeted screening for congenital CMV infection, but the proportion of newborns found to have congenital CMV infection because of the screening program was very low—only 0.009% or 0.018%, depending on how 1 child’s case is classified. Consideration should be given to other strategies for identifying children at risk of hearing loss as a result of congenital CMV infection.
Notes
Author contributions. E. V. and J. L. conceptualized and designed the study, carried out the initial analyses, drafted the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted. E. D. S. worked on revised analyses and reviewed, revised, and approved the final manuscript as submitted.
Disclaimer. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by Ruth L. Kirschstein National Research Service award 4T35HL007649-30 from the National Heart, Lung, and Blood Institute and by Clinical and Translational Science Award (CTSA) grant UL1 TR000142 from the National Center for Advancing Translational Science (NCATS), NIH, and the NIH Roadmap for Medical Research.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Morton CC, Nance WE. Newborn hearing screening—a silent revolution. N Engl J Med 2006; 354:2151–64. [DOI] [PubMed] [Google Scholar]
- 2. Fowler KB, Boppana SB. Congenital cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol 2006; 35:226–31. [DOI] [PubMed] [Google Scholar]
- 3. Boppana SB, Pass RF, Britt WJ et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J 1992; 11:93–9. [DOI] [PubMed] [Google Scholar]
- 4. Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis 2013; 57(Suppl 4):S178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fowler KB. Congenital cytomegalovirus infection: audiologic outcome. Clin Infect Dis 2013; 57(Suppl 4):S182–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fowler KB, McCollister FP, Dahle AJ et al. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr 1997; 130:624–30. [DOI] [PubMed] [Google Scholar]
- 7. Dahle AJ, Fowler KB, Wright JD et al. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol 2000; 11:283–90. [PubMed] [Google Scholar]
- 8. Kimberlin DW, Jester PM, Sánchez PJ et al. ; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med 2015; 372:933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kimberlin DW, Lin CY, Sánchez PJ et al. ; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr 2003; 143:16–25. [DOI] [PubMed] [Google Scholar]
- 10. Oliver SE, Cloud GA, Sánchez PJ et al. ; National Institute of Allergy, Infectious Diseases Collaborative Antiviral Study Group Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J Clin Virol 2009; 46(Suppl 4):S22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kimberlin DW, Acosta EP, Sánchez PJ et al. ; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. J Infect Dis 2008; 197:836–45. [DOI] [PubMed] [Google Scholar]
- 12. Amir J, Wolf DG, Levy I. Treatment of symptomatic congenital cytomegalovirus infection with intravenous ganciclovir followed by long-term oral valganciclovir. Eur J Pediatr 2010; 169:1061–7. [DOI] [PubMed] [Google Scholar]
- 13. Cannon MJ, Griffiths PD, Aston V, Rawlinson WD. Universal newborn screening for congenital CMV infection: what is the evidence of potential benefit?Rev Med Virol 2014; 24:291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stehel EK, Shoup AG, Owen KE et al. Newborn hearing screening and detection of congenital cytomegalovirus infection. Pediatrics 2008; 121:970–5. [DOI] [PubMed] [Google Scholar]
- 15. Grosse SD, Dollard SC, Kimberlin DW. Screening for congenital cytomegalovirus after newborn hearing screening: what comes next?Pediatrics 2017; 139:e20163837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fowler KB, McCollister FP, Sabo DL et al. A targeted approach for congenital cytomegalovirus screening within newborn hearing screening. Pediatrics 2017; 139:e20162128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. State of Connecticut. An act concerning cytomegalovirus Available at: https://www.cga.ct.gov/2015/act/pa/pdf/2015PA-00010-R00HB-05525-PA.pdf. Accessed June 1, 2017.
- 18. Klausner R, Shapiro ED, Elder RW et al. Evaluation of a screening program to detect critical congenital heart defects in newborns. Hosp Pediatr 2017; 7:214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. American Academy of Pediatrics, Joint Committee on Infant Hearing. Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics 2007; 120:898–921. [DOI] [PubMed] [Google Scholar]
- 20. Goderis J, De Leenheer E, Smets K et al. Hearing loss and congenital CMV infection: a systematic review. Pediatrics 2014; 134:972–82. [DOI] [PubMed] [Google Scholar]
- 21. Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007; 17:253–76. [DOI] [PubMed] [Google Scholar]
- 22. Barbi M, Binda S, Caroppo S et al. A wider role for congenital cytomegalovirus infection in sensorineural hearing loss. Pediatr Infect Dis J 2003; 22:39–42. [DOI] [PubMed] [Google Scholar]
- 23. Diener ML, Zick CD, McVicar SB et al. Outcomes from a hearing-targeted cytomegalovirus screening program. Pediatrics 2017; 139:e20160789. [DOI] [PubMed] [Google Scholar]
- 24. Pang XL, Fox JD, Fenton JM et al. ; American Society of Transplantation Infectious Diseases Community of Practice; Canadian Society of Transplantation Interlaboratory comparison of cytomegalovirus viral load assays. Am J Transplant 2009; 9:258–68. [DOI] [PubMed] [Google Scholar]
- 25. Hirsch HH, Lautenschlager I, Pinsky BA et al. An international multicenter performance analysis of cytomegalovirus load tests. Clin Infect Dis 2013; 56:367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ross SA, Ahmed A, Palmer AL et al. ; National Institute on Deafness and Other Communication Disorders CHIMES Study Urine collection method for the diagnosis of congenital cytomegalovirus infection. Pediatr Infect Dis J 2015; 34:903–5. [DOI] [PMC free article] [PubMed] [Google Scholar]