Abstract
Background
Sex separation of mosquitoes at different stages is currently being attempted to ensure the successful release of male mosquitoes in novel vector control approaches. Mechanical and behavioral techniques have been tried most frequently.
Methods
Batches of (n = 300) Aedes aegypti and Ae. albopictus pupae were used for standard sieving (using sieves with 1.12, 1.25, 1.40 and 1.60 mm mesh sizes) and the Fay-Morlan glass plate separation methods. Male and female separation by each method was calculated. For behavioral separation, a spiked blood meal with different concentrations (0, 2, 4, 6, 8 and 10 ppm) of ivermectin and spinosad (spinosyn, 12% w/v), were provided to a batch (n = 300) of adult Ae. aegypti and Ae. albopictus (1:1 sex ratio) followed by observation of mortality. An additional “double feeding method” involved provision of a further blood meal after 24 h, with the same concentrations of ivermectin and spinosad as the initial feeding, followed by a 48-h observation of mortality. All experiments were repeated five times.
Results
In the standard sieving method, the percentage of males and females separated at different pore sizes differed significantly (P < 0.05). The majority of the male pupae were collected in the 1.12 mm pore sized sieve for both Ae. aegypti (73%) and Ae. albopictus (69%) while females were retained mainly in the sieve with the pore size of 1.25 mm. In the Fay-Morlan glass plate separation, 99.0% of the Ae. aegypti and 99.2% of the Ae. albopictus introduced male pupae could be separated, but with female contaminations of 16 and 12%, respectively. Provision of a blood meal spiked with 8 ppm of ivermectin under the “double feeding” was identified as the most effective way of achieving 100% female elimination for both Aedes species.
Conclusions
With 100% separation, use of a spiked blood meal is a more effective method of sex separation than the mechanical methods. Application of the spiked blood meal approach as a second separation level for sexes, after applying the Fay-Morlan glass plate method, could achieve 100% sex separation of sexes whilst allowing a reduction in the amount of toxicants required.
Keywords: Aedes, Sex separation, Ivermectin, Spinosad, Fay-Morlan glass plate method
Background
Mosquitoes are considered the most dangerous vectors of human diseases in the world [1]. Aedes aegypti and Aedes albopictus (Diptera: Culicidae) are highly anthropophilic mosquitoes [2, 3] and in Sri Lanka have been identified as the primary and secondary vectors of dengue, respectively, which is considered the most important national arboviral disease and a global public health problem [3]. Recently, these vectors have gained a new importance, since they are responsible for the transmission of chikungunya and Zika [4, 5].
In Sri Lanka, clinical dengue-like illness has been recorded since the beginning of this century [6]. At present, the disease comes in epidemic form at 2- to 3-year intervals involving high morbidity [7]. Sri Lanka recently experienced its worst epidemic of dengue, with a total of 186,101 suspected dengue cases in 2017, the highest number ever recorded, while 51,591 suspected dengue cases were reported in 2018 [8].
A major problem is the absence of effective drugs and vaccines for dengue. As a result, the main focus is to reduce the vector densities [9]. In view of the drawbacks associated with conventional mosquito control measures, such as development of insecticide resistance, attempts to develop novel strategies are required for use as part of an integrated vector control strategy [10]. Examples of such novel strategies are the sterile insect technique (SIT) [11] and incompatible insect technique (IIT) [12, 13].
The SIT is a species-specific method that can be used against a range of insect pests and vectors. There is an increasing demand for exploration of the potential applicability of SIT in area-wide integrated vector management (AW-IVM) in many countries [13]. Sterility of male insects can be accomplished with ionizing irradiation and SIT focuses on the release of sufficient sterile males to induce sterility in the wild females which, over time, causes the target population to decline [11]. Through repeated releases of sterile male insects, SIT has the ability to suppress the existing vector populations [14].
Use of endosymbiotic Wolbachia is a method to induce sterility in Aedes mosquitoes, and is referred to as an incompatible insect technique [15–20]. Wolbachia are maternally inherited alphaproteobacteria commonly found in arthropods [21]. These bacteria naturally infect some medically important mosquito species such as Culex pipiens [22–24] and Ae. albopictus [16]. In addition, Wolbachia infections can be performed artificially through microinjection under laboratory conditions. Several mosquito species including Ae. albopictus [25–27] and Ae. aegypti [28, 29] are currently being infected artificially. In mosquitoes, Wolbachia induce a form of embryonic death called cytoplasmic incompatibility (CI) [30, 31] due to sperm-egg incompatibility, when Wolbachia infected males mate with either uninfected females or females infected with an incompatible Wolbachia strain.
When a mosquito release programme is considered, mass production of mosquitoes is essential. Elimination or separation of females is one of the major issues to be addressed, since females can act as disease vectors by transmitting the infection to humans and mosquito offspring. Therefore, release of females into the environment in such programmes may increase the transmission potential of the target disease. Hence, an efficient sex-separation or female elimination strategy is required for successful implementation of such approaches. Future options may become available using transgenic mosquitoes, but the inherent characteristics of the target species also provide possibilities for separation, at least until more efficient methods can be developed.
Differences in intrinsic size, behavior and development rate between females and males are often present and useful for sexing [13]. The mostly frequently attempted methods broadly include genetic sex separation techniques, molecular methods, and mechanical and behavioral methods. Mechanical and behavioral tools for sexing may be quicker to develop than genetic methods, in which isolation of sex-specific markers may be a challenging, labor-intensive and serendipitous process [13].
For all blood-feeding mosquitoes, sex separation could be achieved at the adult stage via behavioral separation by spiking blood with insecticides or other mosquito toxins. Ivermectin is a macrocyclic lactone extract of the Streptomyces avarnitalis bacteria. It directly affects the nervous system and muscle function in invertebrates including insects, by inhibiting neurotransmission at glutamate-gated chloride channels. Hence, ivermectin could be used for female elimination in mass-rearing facilities that produce male mosquitoes to be used for IIT and SIT [32].
Spinosad is a naturally-derived insecticide, comprising of a mixture of two neurotoxic macrolide compounds: spinosyn A and D, produced by the fermentation of the Gram-positive soil-dwelling actinomycete Saccharopolyspora spinosa. Spinosad acts on the postsynaptic nicotinic acetylcholine and GABA receptors, resulting in tremors, paralysis and death when ingested by the insect [33].
Even though evaluation of sex separation methods is a pre-requisite in both SIT and IIT approaches, no attempt has been made in Sri Lanka to evaluate the efficacy of these methods to be used in the separation of sexes in vector mosquitoes. Therefore, a proper evaluation should be conducted of sex separation of Aedes vectors under mass rearing conditions, prior to the application of this technique in the country.
The present study focused on evaluating the efficacy of behavioral (via a spiked blood meal treated with ivermectin and spinosad) and mechanical (sieving and Fay-Morlan glass plate separator) sex separation methods in eliminating female Ae. aegypti and Ae. albopictus mosquitoes under laboratory conditions, with the intention of future use for the SIT and IIT approaches.
Methods
Mosquito rearing
The Ae. aegypti and Ae. albopictus strains used in this experiment originated from eggs collected from the Narangodapaluwa Public Health Inspector (PHI) division, located within the Gampaha District of Sri Lanka in 2015. Both colonies have been kept for four generations at the insectary of the Molecular Medicine Unit, Faculty of Medicine, University of Kelaniya, Sri Lanka, where the experiments were carried out. Adults were maintained under standard laboratory conditions [26 ± 1 °C, 75–80% relative humidity (RH) and a photoperiod of 12:12 h (L:D) in 30 × 30 × 30 cm cages] with 10% sucrose solution provided ad libitum. Females were given a blood meal of cattle origin using the Hemotek (PS-6 System, Discovery Workshops, Accrington, UK) artificial membrane feeder.
The larvae were fed with a mixture made of 9.0 g of bovine liver powder, 3.5 g of brewer’s yeast and 12.5 g of tuna meal mixed with 100 ml of distilled water [34]. The larval diet was added once a day in to the tray (25 × 25 × 7 cm3), containing 750 L1 larvae in 1000 ml of de-ionized water according to the following regimes: day 1, 1.5 ml (0.50 g/larva); day 2, 1.55 ml (0.52 g/larva); day 3, 1.6 ml (0.53 g/larva); day 4, 1.65 ml (0.55 g/larva); day 5, 1.7 ml (0.57 g/larva).
Evaluation of mechanical sex separation methods
Standard metal sieving
A set of Retsch Test Sieves (Glenammer Engineering Ltd, Ayr, Ayrshire) with 1.60, 1.40, 1.25 and 1.12 mm pore sizes were used for the mechanical sex separation. The sieves were precisely positioned over a sluiceway keeping the sieve with the largest pore size on top (Fig. 1). A batch of 300 Ae. aegypti pupae (32 h after the onset of pupation) was introduced in to the topmost sieve plate and allowed to pass through by providing a water flow according to the standard operating procedures, following the principle of wet sieving used in ecological monitoring [35].
Fig. 1.

Setup of the standard metal sieving method for pupal separation
Separated pupae in each sieve were placed in appropriately-labeled separate plastic cups and were reared to the adult stage. Adult male and female mosquitoes were identified and enumerated after emergence. Dead pupae were identified as male and female under a light microscope according to the differences in the genital lobe shape [36]. The experiment was repeated five times to ensure the accuracy of the findings. The same procedure was followed for Ae. albopictus.
Fay-Morlan method
Batches of 300 Ae. aegypti or Ae. albopictus pupae (32 h after onset of pupation) in aquatic culture medium, were introduced into the Fay-Morlan glass plate separator (M5412, John W. Hock Company, Gainesville, USA). The distance between the glass plates was adjusted by using knobs on the apparatus, to yield separate layers of pupae based on their body size. Separated pupae were flushed into separate trays using a mild water flow after loosening the glass plates [37]. The isolated pupae were placed in plastic cups and were reared to the adult stage. Male and female adult Ae. aegypti mosquitoes were separated from the collection via visual observation using achromatic magnification lenses (×10). Dead pupae were also counted and sorted to gender using the differences in the genital lobe shape (at the end of the pupal abdominal segments just below the paddles) under a light microscope [36]. The experiment was repeated for five times to ensure the accuracy of the findings.
Behavioural sex separation
Spiking blood meals with ivermectin and spinosad at different concentrations
Blood meals (defrosted bovine blood treated with EDTA) were prepared in 50 ml centrifuge tubes containing various concentrations (2, 4, 6, 8 and 10 ppm) of ivermectin [1% w/v injection (BP. Vet), Star Laboratory Pvt Ltd, Lahore, Pakistan] and spinosad (spinosyn A + spinosyn D, 12% w/v preparation; Dow AgroSciences LLC, Indianapolis, USA). The blood was warmed in a water bath and subsequently poured into a Hemotek device (PS-6 System, Discovery Workshops) that maintained the temperature of the blood at 35 ± 1 °C and was placed on the top of 30 × 30 × 30 cm cages containing 300 mosquitoes (1:1 sex ratio) for a period of 2 h for both Ae. aegypti and Ae. albopictus. Six-day-old adult mosquitoes of both species were used for the experiments. Dead mosquitoes were enumerated at 6 h intervals for 3 days, after the initiation of the experimental trials. Mosquitoes in all cages were provided with 10% sucrose solution ad libitum. The experiment was repeated 5 times at each concentration of mosquito toxicants for both Ae. aegypti and Ae. albopictus. The most effective concentration of toxicants in eliminating females identified in this experiment was selected to be used for the next experiment, described below.
Feeding of the most effective toxin concentration initially (0 h) and at 24 h intervals
The 8 ppm concentration, which caused 100% mortality for both species, was selected for further trials. Six-day-old Ae. aegypti or Ae. albopictus colonies (a batch of 300 mosquitoes with a 1:1 sex ratio) were fed with 8 ppm ivermectin and spinosad separately, initially and at 24 h intervals, with a 2 h feeding duration. The experiment was repeated 5 times for each mosquito species.
Mixed feeding of two mosquito toxicants with the same concentration
The experiment was carried out with cattle blood containing a combined mixture of ivermectin and spinosad in a 1:1 ratio at different concentrations (2 ppm: 2 ppm; 4 ppm: 4 ppm; 6 ppm: 6 ppm; 8 ppm: 8 ppm). These mixtures of spiked blood were provided to 6-day-old Ae. aegypti or Ae. albopictus colonies (with each batch of 300 having a 1:1 sex ratio) initially and then at 24 h intervals with a 2 h feeding duration. The experiment was repeated 5 times.
Statistical analysis and interpretation of data
Blood-feeding rates of female mosquitoes were calculated for each treatment as the percentage of the number of blood-fed females out of the total number of females in each cage. Mortality rates were calculated for males and females separately as a percentage of the number of dead males or females out of total number of males and females (blood-fed) in each cage. One-way analysis of variance (ANOVA) followed by Tukey’s post-hoc pairwise comparison tests were used to investigate the difference in feeding and mortality rates of males and females at different concentrations of ivermectin and spinosad. Probit analysis was performed to determine the toxicities of ivermectin and spinosad, and to calculate the LD50, LD95 and LD99 values for both chemicals separately.
Male and female pupae in each band of the Fay-Morlan glass plate experiment were analyzed using a Chi-square test of independence to evaluate the efficiency of sex separation and also to analyze whether the percentage of male and female pupae separated at different pore sizes (1.12, 1.25, 1.40 and 1.60 mm) differed in the standard sieving method.
Results
Standard sieving method
The majority of the male pupae were retained in the 1.12 mm pore sized sieve in both Ae. aegypti (73%, n = 438) and Ae. albopictus (69%, n = 483) followed by pore sizes < 1.12 and 1.25 mm. On the other hand, the majority of Ae. aegypti (63%, n = 441) and Ae. albopictus females (66%, n = 396) were found at the sieve with a pore size of 1.25 mm followed by 1.12, < 1.12 and 1.40 mm (Table 1). The distribution of sexes captured at different pore sizes differed significantly for both Ae. aegypti (χ2 = 58.38, df = 4, P < 0.001) and Ae. albopictus (χ2 = 41.34, df = 4, P < 0.001).
Table 1.
Percentages of male and female pupae (mean ± standard error) of Ae. aegypti and Ae. albopictus, separated at different pore sizes by the standard sieving method
| Species (N = 1500) | Percentage of male and female pupae retained | |||
|---|---|---|---|---|
| Pore size, mm | Percentage of retained pupae | Pupal mortality (%) | ||
| Male pupae in sieve (%) | Female pupae in sieve (%) | |||
| Ae. aegypti (M: 600; F: 900) | < 1.12 | 26 ± 2.5 (n = 156) | 5 ± 1.0 (n = 35) | 0 |
| 1.12 | 73 ± 3.5 (n = 438) | 28 ± 2.5 (n = 196) | 4 ± 0.5 (n = 60) | |
| 1.25 | 1 ± 0.2 (n = 6) | 63 ± 3.6 (n = 441) | 3 ± 0.8 (n = 45) | |
| 1.4 | 0 | 4 ± 0.8 (n = 28) | 0 | |
| 1.6 | 0 | 0 | 0 | |
| Ae. albopictus (M: 700; F: 800) | < 1.12 | 24 ± 2.2 (n = 168) | 4 ± 0.5 (n = 24) | 0 |
| 1.12 | 69 ± 3.5 (n = 483) | 27 ± 2.6 (n = 162) | 5 ± 0.4 (n = 75) | |
| 1.25 | 7 ± 1.0 (n = 49) | 66 ± 4.0 (n = 396) | 4 ± 0.4 (n = 60) | |
| 1.4 | 0 | 3 ± 0.4 (n = 18) | 0 | |
| 1.6 | 0 | 0 | 0 | |
Note: Values are mean ± SE, with the number of pupae in parenthesis. The male and female proportions in the total sample are stated in the first column
Abbreviations: F, female; M, male
Fay-Morlan glass plate separation method
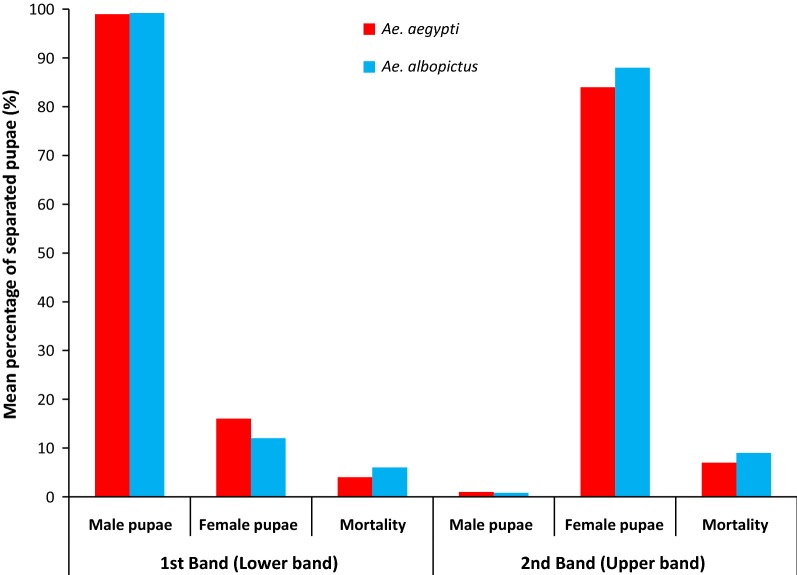
Two separated rows of pupae were observed in the Fay-Morlan glass plate separator as upper and lower bands (Fig. 2). Adult rearing results of the Ae. aegypti pupae separated in the lower band indicated that 99.0% (n = 643) of male pupae were captured in the lower band with 16.0% (n = 136) of females as contamination (Fig. 2). The upper band of the Fay-Morlan glass plate separator captured the remaining 1% of males (n = 7) and 84% of females (n = 714). The mortality of pupae was 4% and 7% in each band, respectively (Fig. 2). For Ae. albopictus, 99.2% (n = 714) of male pupae were found in the lower band with 12.0% (n = 94) of females. In the upper band, all remaining female (88.0%, n = 686) and male (0.8%, n = 6) pupae were retained (Fig. 3). Mortality observed was 6% and 9% in the lower and upper bands for Ae. albopictus, respectively (Fig. 3). The percentage sex separation of Ae. aegypti (χ2 = 22.57, df = 1, P < 0.001) and Ae. albopictus (χ2 = 18.59, df = 1, P < 0.001) separated at each band differed significantly.
Fig. 2.

Pupal separation by the Fay-Morlan glass plate separator showing the lower and upper bands
Fig. 3.
Mean percentages of separated male and female pupae of Ae. aegypti and Ae. albopictus by the Fay-Morlan glass plate separator
Spiking blood meals with different concentrations of ivermectin and spinosad
Feeding rates of both Ae. aegypti and Ae. albopictus decreased gradually with the increasing concentrations of ivermectin and spinosad (one-way ANOVA: F(5,24) = 3.617, P = 0.014 and F(5,24) = 3.373, P = 0.019, respectively) as indicated in Table 2. The female mortality varied among different concentrations of each toxicant for both Ae. aegypti (F(5,24) = 3.291, P = 0.021) and Ae. albopictus (F(5,24) = 3.001, P = 0.030). As indicated in Table 2, the minimum ivermectin concentration delivered within 48 h that achieved 100% female mortality (n = 750) for both species was 8 ppm.
Table 2.
Percentage feeding and cumulative mortality rates (mean ± standard error) of Ae. aegypti and Ae. albopictus with single feeding of ivermectin and spinosad
| Species/toxicant | Concentration (ppm) | Fed females (%) | Percentage mortality (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||||||
| Male | Female | Male | Female | Male | Female | |||
| Ae. albopictus | ||||||||
| Ivermectin | 0 (Control) | 98.0 ± 1.23 (n = 735) |
0.7 ± 0.54 (n = 5) | 1.4 ± 0.98 (n = 10) |
2.0 ± 1.50 (n = 15) |
5.4 ± 1.89 (n = 40) |
3.3 ± 1.38 (n = 25) |
7.5 ± 2.35 (n = 55) |
| 2 | 98.7 ± 1.46 (n = 740) |
0.7 ± 0.40 (n = 5) |
11.5 ± 9.35 (n = 85) |
2.0 ± 1.50 (n = 15) |
35 ± 5.36 (n = 259) |
4.0 ± 2.32 (n = 30) |
49.3 ± 11.25 (n = 364) |
|
| 4 | 100.0 ± 0.2 (n = 750) |
0 ± 0 (n = 0) |
36.0 ± 12.35 (n = 270) |
1.3 ± 0.87 (n = 10) |
65.3 ± 10.32 (n = 490) |
5.3 ± 1.52 (n = 40) |
89.3 ± 9.23 (n = 670) |
|
| 6 | 98.7 ± 0.98 (n = 740) |
0.7 ± 0.54 (n = 8) | 41.2 ± 8.36 (n = 305) |
2.7 ± 2.54 (n = 20) |
87.2 ± 13.26 (n = 645) |
4.0 ± 1.35 (n = 30) |
95.9 ± 2.54 (n = 709) |
|
| 8 | 94.5 ± 1.54 (n = 709) |
1.3 ± 0.87 (n = 10) |
63.9 ± 8.52 (n = 453) |
3.3 ± 1.23 (n = 25) |
100.0 ± 2.50 (n = 709) |
4.7 ± 1.87 (n = 35) |
100.0 ± 0.00 (n = 709) |
|
| 10 | 64.7 ± 1.78 (n = 484) |
1.3 ± 0.87 (n = 10) |
91.8 ± 7.56 (n = 444) |
3.3 ± 1.23 (n = 25) |
100.0 ± 1.40 (n = 484) |
5.7 ± 2.35 (n = 43) |
100.0 ± 0.00 (n = 484) |
|
| Spinosad | 0 (Control) | 98.0 ± 0.87 (n = 735) |
0.7 ± 0.54 (n = 5) |
1.4 ± 0.84 (n = 10) |
2.0 ± 1.23 (n = 15) |
5.4 ± 1.57 (n = 40) |
3.3 ± 1.85 (n = 25) |
7.5 ± 2.31 (n = 55) |
| 2 | 97.3 ± 1.45 (n = 730) |
2.0 ± 0.4 (n = 15) |
7.5 ± 0.55 (n = 55) |
1.3 ± 0.87 (n = 10) |
43.2 ± 10.23 (n = 315) |
2.7 ± 0.98 (n = 20) |
66.4 ± 5.36 (n = 64) |
|
| 4 | 98.7 ± 1.95 (n = 740) |
2.0 ± 1.50 (n = 15) |
15.5 ± 2.68 (n = 113) |
2.0 ± 1.50 (n = 15) |
59.5 ± 12.32 (n = 440) |
5.3 ± 1.98 (n = 40) |
84.5 ± 6.37 (n = 634) |
|
| 6 | 97.3 ± 0.79 (n = 730) |
3.0 ± 20 (n = 22) |
41.8 ± 5.62 (n = 305) |
2.7 ± 0.85 (n = 20) |
76.7 ± 15.36 (n = 560) |
3.3 ± 1.28 (n = 25) |
95.2 ± 5.21 (n = 695) |
|
| 8 | 98.0 ± 1.54 (n = 735) |
0.7 ± 0.54 (n = 5) |
53.7 ± 10.68 (n = 395) |
1.3 ± 0.87 (n = 10) |
86.4 ± 13.25 (n = 635) |
2.7 ± 0.97 (n = 20) |
100.0 ± 0.00 (n = 735) |
|
| 10 | 79.4 ± 1.23 (n = 595) |
2.0 ± 1.50 (n = 15) |
85.5 ± 9.34 (n = 642) |
3.3 ± 0.98 (n = 25) |
100.0 ± 1.00 (n = 595) |
4.0 ± 2.54 (n = 30) |
100.0 ± 0.00 (n = 595) |
|
| Ae. aegypti | ||||||||
| Ivermectin | 0 (Control) | 100.0 ± 0.4 (n = 750) |
1.3 ± 0.87 (n = 10) |
2.0 ± 0.76 (n = 15) |
1.3 ± 0.87 (n = 10) |
4.7 ± 1.58 (n = 35) |
3.3 ± 1.26 (n = 25) |
6.0 ± 2.32 (n = 45) |
| 2 | 100.0 ± 0.25 (n = 750) |
0.7 ± 0.54 (n = 5) |
14.0 ± 5.68 (n = 105) |
2.0 ± 0.95 (n = 15) |
42.0 ± 5.36 (n = 315) |
4.0 ± 1.28 (n = 30) |
59.3 ± 5.36 (n = 445) |
|
| 4 | 98.0 ± 1.45 (n = 735) |
0.7 ± 0.54 (n = 5) |
23.1 ± 6.95 (n = 170) |
1.3 ± 0.83 (n = 10) |
65.3 ± 9.35 (n = 480) |
5.3 ± 2.35 (n = 40) |
91.8 ± 6.34 (n = 674) |
|
| 6 | 100.0 ± 0.8 (n = 750) |
0.7 ± 0.54 (n = 5) |
30.0 ± 7.36 (n = 225) |
2.0 ± 0.85 (n = 15) |
88.0 ± 12.35 (n = 660) |
4.0 ± 1.78 (n = 30) |
98.0 ± 2.34 (n = 735) |
|
| 8 | 91.7 ± 11.27 (n = 686) |
0.7 ± 0.54 (n = 5) |
66.2 ± 5.62 (n = 454) |
1.3 ± 0.95 (n = 10) |
100.0 ± 2.40 (n = 686) |
4.7 ± 1.24 (n = 35) |
100.0 ± 0.00 (n = 686) |
|
| 10 | 50.7 ± 9.31 (n = 380) |
1.3 ± 0.87 (n = 10) |
100.0 ± 1.5 (n = 380) |
3.3 ± 0.97 (n = 25) |
100.0 ± 2.00 (n = 380) |
6.0 ± 2.35 (n = 45) |
100.0 ± 0.00 (n = 380) |
|
| Spinosad | 0 (Control) | 99.3 ± 1.34 (n = 745) |
1.3 ± 0.87 (n = 10) |
4.2 ± 2.32 (n = 31) |
1.3 ± 1.23 (n = 10) |
4.8 ± 1.26 (n = 36) |
3.3 ± 1.58 (n = 25) |
6.0 ± 1.68 (n = 48) |
| 2 | 97.4 ± 0.66 (n = 730) |
0.7 ± 0.54 (n = 5) |
15.9 ± 2.67 (n = 120) |
1.3 ± 1.23 (n = 10) |
44.9 ± 9.35 (n = 328) |
4.0 ± 2.31 (n = 30) |
57.2 ± 9.35 (n = 418) |
|
| 4 | 98.0 ± 1.23 (n = 735) |
0.7 ± 0.54 (n = 5) |
24.6 ± 5.36 (n = 181) |
1.3 ± 0.97 (n = 10) |
62.1 ± 5.39 (n = 456) |
6.2 ± 1.20 (n = 47) |
92.3 ± 5.31 (n = 678) |
|
| 6 | 97.9 ± 1.08 (n = 734) |
0 ± 0 (n = 0) |
26.8 ± 3.68 (n = 198) |
2.0 ± 0.97 (n = 15) |
77.4 ± 8.36 (n = 76) |
4.0 ± 2.35 (n = 30) |
94.1 ± 8.64 (n = 691) |
|
| 8 | 97.3 ± 2.34 (n = 729) |
0.7 ± 0.54 (n = 5) |
74.2 ± 5.68 (n = 541) |
1.3 ± 1.28 (n = 10) |
100.0 ± 1.0 (n = 729) |
5.3 ± 3.21 (n = 40) |
100.0 ± 0.00 (n = 729) |
|
| 10 | 54.3 ± 8.35 (n = 407) |
1.3 ± 0.87 (n = 10) |
100.0 ± 1.20 (n = 407) |
3.3 ± 1.23 (n = 25) |
100.0 ± 0.6 (n = 407) |
6.0 ± 1.54 (n = 45) |
100.0 ± 0.00 (n = 407) |
|
Note: Values are mean ± SE, with the number of pupae in parenthesis. A sample size of 1500 mosquitoes from both species were tested for two toxicants, separately at each concentration. The percentage mortality of females was calculated based on the number of blood-fed females at each concentration
For spinosad, 100% mortality (n = 750) was observed at 10 ppm for Ae. albopictus within 48 h, whereas 100% (n = 750) mortality in Ae. aegypti was observed at 8 ppm 48 h after the initial blood meal. Feeding rates of Ae. aegypti and Ae. albopictus females at 10 ppm were significantly lower than that of 8 ppm for both toxicants (F(5,24) = 3.211, P = 0.023 and F(5,24) = 6.721, P < 0.001, respectively). Therefore, the 8 ppm concentration of ivermectin and spinosad could be suggested as the best concentration for sex separation as a single application. The male mortality of Ae. aegypti and Ae. albopictus in control and experimental settings was not significantly different for either ivermectin or spinosad (P > 0.05).
Probit analysis and lethal dosage (LD) calculations for ivermectin and spinosad
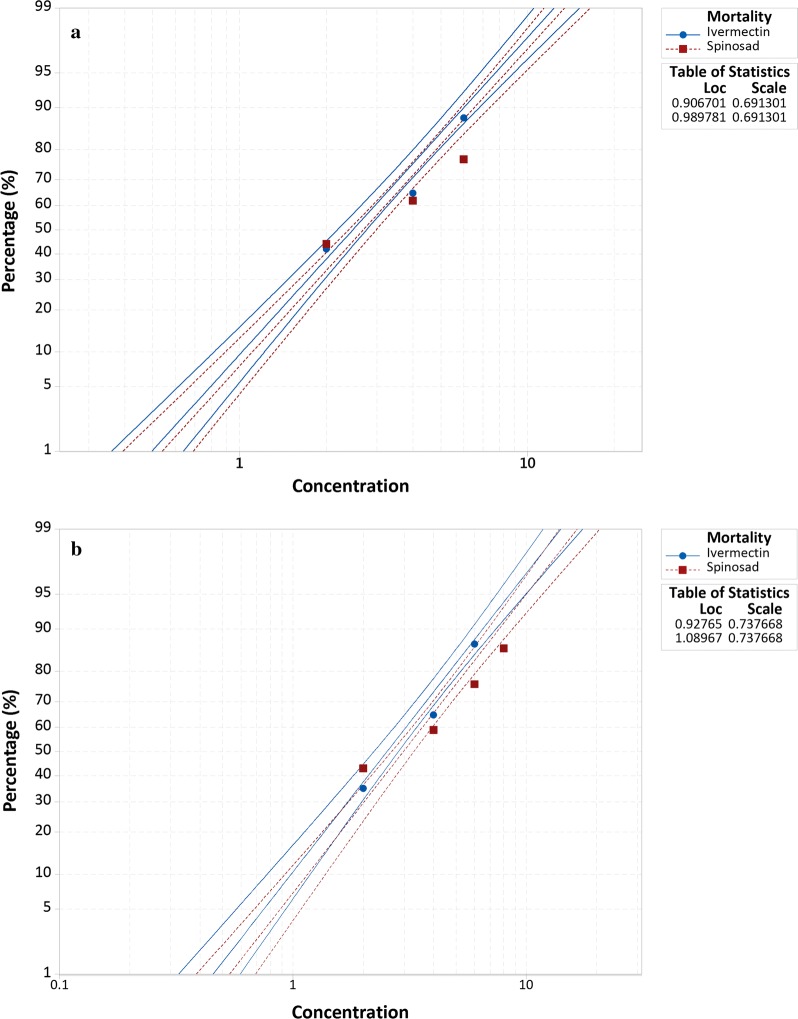
For both Ae. aegypti and Ae. albopictus, ivermectin produced a relatively steeper slope in the mortality than spinosad within 48 h, as denoted by the Probit analysis (Fig. 4). This suggests that the toxic effect of ivermectin on Ae. aegypti and Ae. albopictusis is relatively higher than that of spinosad (Fig. 4). Using a single, rather than multiple, feeding approach, the calculated LD values for ivermectin and spinosad from 48 h mortality of each species also showed relatively lower LD values for ivermectin than spinosad (Table 3).
Fig. 4.
Probit analysis for 24 h mortality of (a) Ae. aegypti and (b) Ae. albopictus after providing one spiked blood meal with ivermectin and spinosad
Table 3.
Lethal dose (LD) values (LD ± standard error, SE) for ivermectin and spinosad at 48 h for Ae. aegypti and Ae. albopictus
| Lethal dose | Ae. aegypti | Ae. albopictus | ||
|---|---|---|---|---|
| Spinosad (ppm) | Ivermectin (ppm) | Spinosad (ppm) | Ivermectin (ppm) | |
| LD50 | 2.61 ± 0.18 | 2.55 ± 0.15 | 2.72 ± 0.21 | 2.76 ± 0.14 |
| LD95 | 8.82 ± 0.79 | 7.36 ± 0.57 | 11.99 ± 1.43 | 7.31 ± 0.52 |
| LD99 | 14.59 ± 1.92 | 11.41 ± 1.27 | 22.15 ± 3.94 | 10.95 ± 1.11 |
Note: Values are mean ± SE, calculated from the Probit analysis
Feeding of the effective toxin concentration (8 ppm) at 24- and 48-h intervals
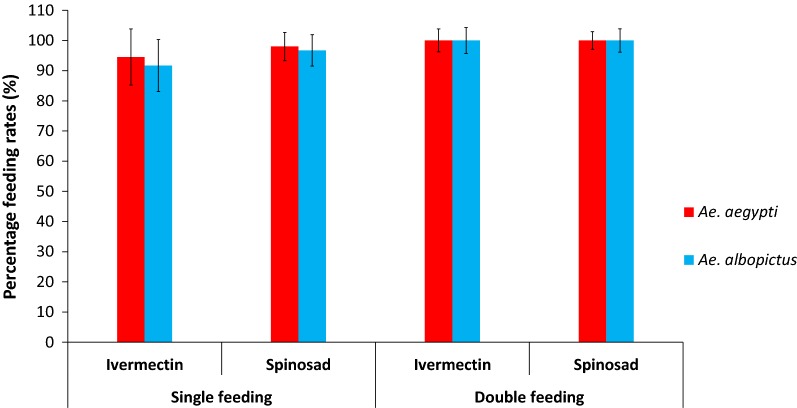
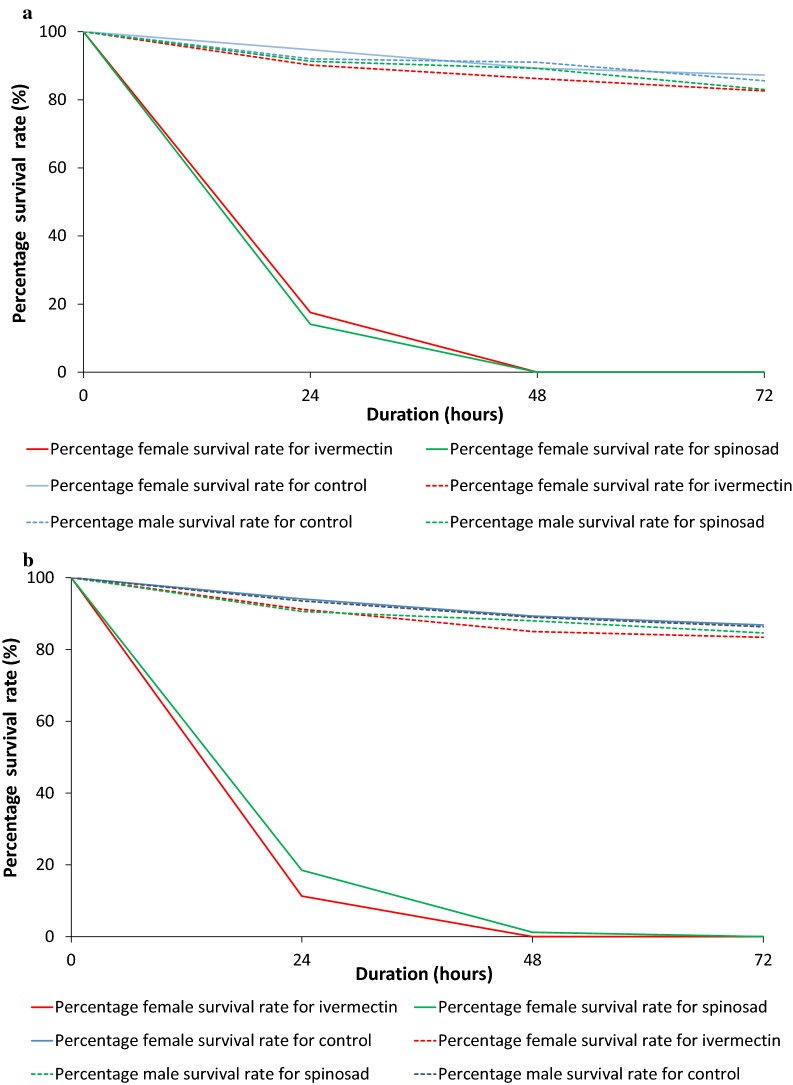
The feeding rates of Ae. aegypti and Ae. albopictus were less than 100% at the initial feeding; however, at 24 h, all females of both species were engorged with the blood meal. The maximum feeding rate of the females of both species was observed after providing two blood meals initially followed by another at 24 h duration (Fig. 5). Both ivermectin and spinosad showed a 100% elimination of Ae. aegypti within 48 h (Fig. 6). Aedes albopictus showed 100% mortality within 24–48 h after feeding with ivermectin and spinosad (Fig. 6). Male mortality of Ae. aegypti and Ae. albopictus at 48 h in the experiment did not differ significantly from controls (P > 0.05).
Fig. 5.
Blood-feeding rates of female Ae. aegypti and Ae. albopictus for spiked blood with 8 ppm concentration of ivermectin and spinosad, separately initially (single feeding) and after 24 h since the initial blood meal (double feeding)
Fig. 6.
Survival rates of (a) Ae. aegypti and (b) Ae. albopictus females using an 8 ppm concentration of ivermectin and spinosad under double feeding
Mixed feeding of two mosquito toxicants with same concentrations
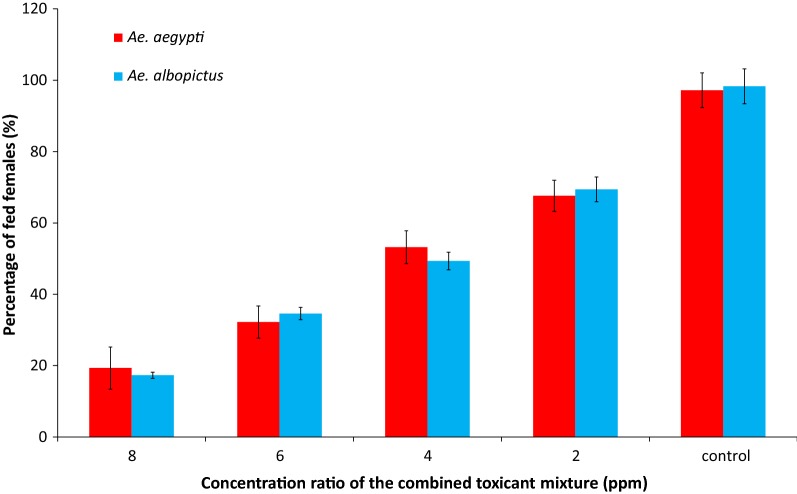
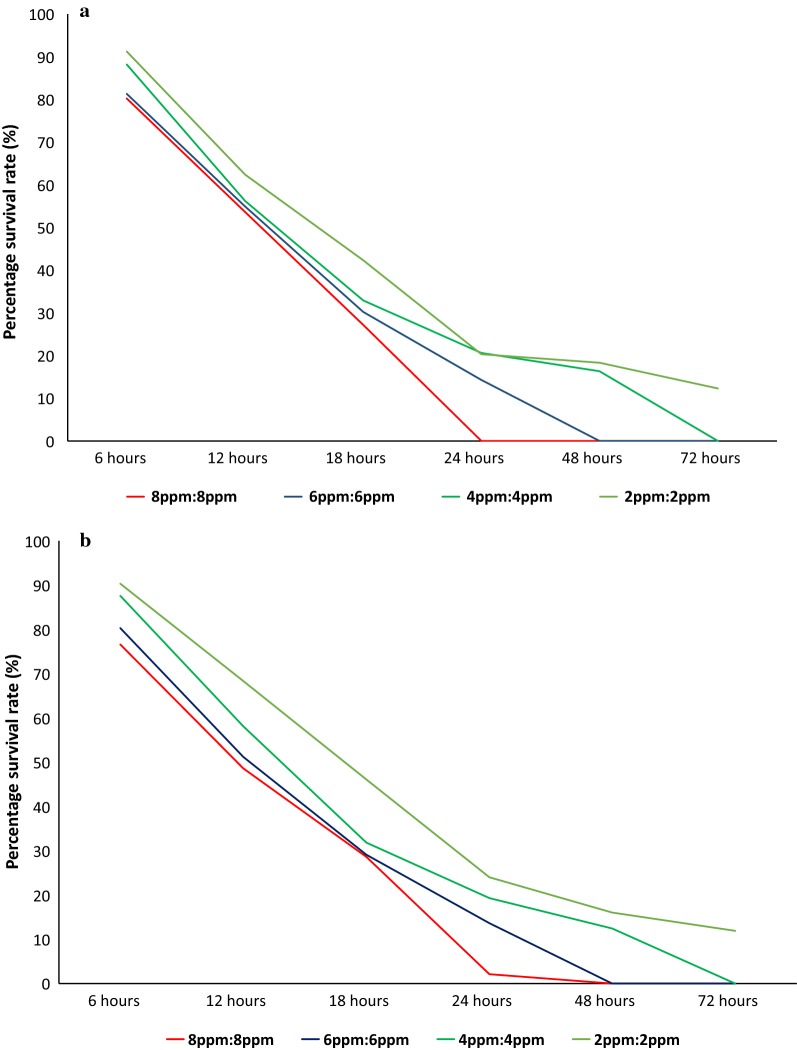
Feeding rates of both Ae. aegypti and Ae. albopictus showed a negative correlation with the concentrations of both toxicants (Fig. 7). A 100% feeding rate was observed in Ae. aegypti in the control treatment, while the blood-feeding rates of mosquitoes varied significantly among different concentration mixtures in both Ae. aegypti (one-way ANOVA, F(4,21) = 3.112, P = 0.037) and Ae. albopictus (F(4,21) = 3.425, P = 0.026). The spiked blood meal containing a 1:1 mixture of ivermectin and spinosad (each at 8 ppm concentration) produced 100% female mortality for Ae. aegypti after 24 h of initial feeding (Fig. 8). However, 100% female mortality was achieved in Ae. albopictus nearly 40 h after the initial blood meal contaminated with 8 ppm of the toxicant mixture (Fig. 8).
Fig. 7.
Blood-feeding rates of Ae. aegypti and Ae. albopictus females with a combined toxicant mixture containing ivermectin and spinosad with equal concentrations
Fig. 8.
Survival rates of Ae. aegypti (a) and Ae. albopictus (b) females using an equal concentration mixture of ivermectin and spinosad after providing two spiked blood meals, an initial meal at 24 h and a second at 48 h
Discussion
To develop efficient sex separation methods for SIT and IIT programmes, it is essential to evaluate the efficacy and applicability of different approaches such as the use of basic genetic/molecular tools as well as available mechanical and behavioral methods. Genetic sexing strains (GSS) mostly rely on genes conferring insecticide resistance, with the male being heterozygous for resistance due to Y chromosome translocation, while females are homozygous susceptible. However, these strains may have some stability issues [13, 38, 39]. Therefore, more attention has focused on separation of insects through basic mechanical and behavioral methods.
On average, male mosquitoes develop to the pupal stage more rapidly than females, a phenomenon called protandry. This has been exploited for Aedes and Culex mosquitoes [40] for sex separation, and could also be considered for other species for which methods are not yet available. Most pupae that emerge on the first day are males, with a relatively smaller body size than the females, more of which emerge on the second or third day. Therefore, it is possible to separate sexes at the pupal stage due to the size dimorphism between male and female pupae, since females are generally larger. A number of methods such as (i) sieving using grids of regularly spaced nylon wires [35], (ii) using the glass plate sex separation system [37, 41] and (iii) using the McCray adjustable slit separation system [42], have already exploited the size dimorphism between males and females in sex separation of mosquito pupae. The present study evaluated the sieving and glass plate methods. Among these methods the sex separation efficiencies of the first and second mechanical methods were evaluated due to their easy applicability, low capital and maintenance costs [35, 41, 42].
According to the results of the standard sieving method, a pore size of 1.12 mm was identified as the best out of the five sieve sizes tested to separate male pupae in the laboratory colonies of Ae. aegypti and Ae. albopictus. Mortality of separated pupae was observed only at 1.12 and 1.25 mm pore sizes. This mortality could occur due to mechanical damage when the pupae pass through the metal pores. However, there were no significant differences observed in the mortality rates of pupae at different sieve pore sizes. It is important to state that this method alone cannot achieve full separation of sexes, since only 73% and 69% male separation occurred at the 1.12 mm pore size for Ae. aegypti and Ae. albopictus, respectively. Therefore, we concluded that this method cannot be considered as a promising method to be used for sex separation of Aedes mosquitoes.
A better separation of sexes was detected from the Fay-Morlan glass plate method compared to the sieving method. Approximately 99% of males were identified in the lower band from this method. Therefore, it is suggested that this glass plate separation method can provide a good separation of sexes for mass rearing colonies. This method is easier and faster than a behavioral sex separation method. However, 16% contamination of females is still not negligible, because this will represent a considerable number of females, if tens of thousands of mosquitoes are released during a mass-release programme. Hence, an additional mechanism to achieve 100% sex separation is essential in SIT and IIT approaches.
Sex separation could be achieved at the adult stage by spiking blood with insecticides (malathion, dieldrin) or other mosquito toxins (ivermectin, spinosad) as a behavioural sex separation approach. A study conducted for Anopheles arabiensis females with spiked blood of 7.5 ppm ivermectin and spinosad solutions resulted complete elimination of females within 48 and 72 h, respectively, after the first blood-feeding [43]. Use of toxins that have little or no contact or vapor toxicity, such as ivermectin and spinosad, is effective for such applications [13, 43].
According to the present experiment, a 100% mortality rate for fed Aedes females of both species was observed at 8 and 10 ppm for ivermectin and spinosad, respectively, within 48 h of the first blood meal. However, a considerable proportion of female mosquitoes were reluctant to feed on spiked blood contaminated with 10 ppm of ivermectin and spinosad compared to lower concentrations. This could be due to elevation of the vapour concentration with increasing concentrations of toxicants, inducing rejection of the blood meal by the female mosquitoes [13]. Therefore, another blood meal was given 24 h after the first blood meal treated with 8 ppm ivermectin and spinosad, separately, that resulted in 100% female mortality for both toxins.
The present study was conducted in cages with high mosquito densities and regular blood-feeding that caused elevated mortality rates in insectary rearing. However, survival of females may be improved by provision of resting sites and additional sugar sources [43]. It is also important to motivate as many of the females to take a blood meal as quickly as possible. This can be achieved by: removing the sugar source 12 h before offering the blood-meal, using fresh blood, and timing the blood-feeding to preferred times of the day. In some mosquito species, virgin females sometimes do not feed on blood, but later take a blood meal once inseminated. Therefore, in application of this method for mass rearing, the female mortality rate could be increased by prolonging blood-feeding for several hours or feeding multiple times per day [43].
Both ivermectin and spinosad induced low levels of cumulative male mortality rates (< 6%) in this experiment. However, mortality did not differ from controls and some mortality in males was expected due to the damage and stress caused while aspirating relatively small and easily damaged male mosquitoes into test cages. Furthermore, removal of the sugar for a longer period may have increased mortality.
It was observed that when both toxicants are used at a 1:1 concentration mixture, effectiveness was comparatively low compared to individual use of toxicants. Therefore, we recommend ivermectin alone as the more effective preparation for behavioral sex separation than spinosad, since LD values of ivermectin are less than for spinosad for both species. According to a previous study, females of Culex tritaeniorhynchus was more susceptible to aqueous sucrose baits with spinosad than ivermectin [32]. A previous study on Ae. aegypti indicated that the adult survival after ingestion of ivermectin in a blood meal varied between strains. Interestingly, studies have suggested that ivermectin can inhibit nuclear import thereby inhibiting dengue viral replication, which might increase usefulness of ivermectin over spinosad [44, 45].
Relatively low operational cost, easy applicability, less time, limited labor and space requirements remain the major factors favoring the use of mechanical sex separation methods. Meanwhile, high capital cost (for purchasing of the equipment), relatively low efficiencies of sex separation with high female contamination rates, and a notable degree of male mortality are the major limitations. When the spiked blood meal approach for sexing is considered, elimination of 100% of females (through the provision of two consecutive blood meals, initially and after 24 h) is a major advantage. On the other hand, keeping females until blood-feeding age in a mass-rearing facility will significantly increase the space, labor and costs compared to rearing of only males until adulthood (especially in terms of feeding), which is the main difficulty in the spiked blood meal approach. In addition, a large amount of the toxicant will be required, which could be relatively expensive and obtaining blood to provide far more feeds than required for routine stock maintenance could be difficult due to various practical and ethical issues [46]. Furthermore, use of toxicants might cause undesirable impacts on both the mosquitoes and the technical staff involved in mass rearing, and this would need careful assessment before full-scale application.
In addition, the reliability of the method may not remain constant at 100%, as it is dependent on behavioral attributes, which can never be controlled completely and may change over time or in response to other external factors. However, the present study has yielded promising results by showing a 100% mortality of females fed with spiked blood contaminated with 8 ppm concentration of either of the tested toxins under double feeding method. Therefore, it is suggested that if the spiked blood meal approach is used as the second screening for sexes after applying the Fay-Morlan glass plate method, a 100% separation of sexes might be achieved reliably with reduced costs, since the female contamination by Fay-Morlan mechanical separation processes was only 16%. This contamination could be effectively eliminated through a spiked blood meal with ivermectin, since the present study indicated a 100% feeding rate of females and thereby 100% female elimination. Hence, this study suggests a combined approach of Fay-Morlan glass plate separation method followed by behavioral separation of remaining females be feeding a blood meal spiked with relatively low concentration ivermectin may achieve total elimination of female mosquitoes and production of males alone in mass releases for SIT and IIT applications.
Conclusions
Neither of the mechanical sex separation methods, standard sieving and the Fay-Morlan glass plate separation, were able to produce 100% sex separation of Ae. aegypti and Ae. albopictus at the pupal stage. Providing an initial spiked blood meal of ivermectin (8 ppm) along with an additional blood meal (24 h later) was capable of eliminating females of both species within 24 to 48 h. Based on these findings, we suggest that the use of a spiked blood meal approach as a secondary screening after applying mechanical glass plate separation method may be capable of producing 100% separation of sexes.
Acknowledgements
This study was supported by the International Atomic Energy research contact no. 17959 as part of the Coordinated Research Project “Exploring genetic, molecular, mechanical and behavioural methods of sex separation in mosquitoes” and National Research Council Funded Dengue Mega Grant (NRC TO 14-04), Sri Lanka.
Funding
Research activities were supported by the International Atomic Energy Agency (IAEA CRP 17959) and National Research Council Funded Dengue Mega Grant (NRC TO 14-04), Sri Lanka.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Authors’ contributions
NG designed and supervised the research and wrote the manuscript. TR conducted laboratory experiments and reviewed the manuscript. LU conducted laboratory experiments and statistical analysis and wrote the manuscript. AW assisted laboratory experiments and reviewed the manuscript. JRLG and WA performed the overall supervision of the research and reviewed the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Ethical clearance for the study was obtained from Ethical Review Committee (ERC) of the Faculty of Medicine, University of Kelaniya, Sri Lanka.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- MOH
Medical Officer of Health
- PHI
Public Health Inspector
- ANOVA
one-way analysis of variance
- AW-IVM
area-wide integrated vector management
- GSS
genetical sex separation
- IAEA
International Atomic Energy Agency
- IIT
incompatible insect techniques
- LD
lethal dosage
- RH
relative humidity
- RIDL
release of insects with dominant lethality
- SIT
sterile insect technique
- WHO
World Health Organization
Contributor Information
Nayana Gunathilaka, Email: n.gunathilaka@kln.ac.lk.
Tharaka Ranathunge, Email: tharaka.ranathunge@gmail.com.
Lahiru Udayanga, Email: udayangaln@gmail.com.
Asha Wijegunawardena, Email: asha@kln.ac.lk.
Jeremie Roger Lionel Gilles, Email: jeremie.gilles@gmail.com.
Wimaladharma Abeyewickreme, Email: wabeyewickreme@yahoo.com.
References
- 1.WHO . World malaria report 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- 2.Christophers SR. Aedes aegypti (L.), the yellow fever mosquito. Its life history, bionomics and structure. London: Cambridge University Press; 1960. [Google Scholar]
- 3.Soares FA, Silva JC, Oliveira JBBDS, Abreu FVSD. Study of oviposition behaviour of Aedes aegypti in two neighborhoods under the influence of semi-arid climate in the municipality of Salinas, State of Minas Gerais, Brazil. Rev Patol Trop. 2015;44:77–88. doi: 10.5216/rpt.v44i1.34817. [DOI] [Google Scholar]
- 4.Nadeeka PVJ, Gunathilaka PADHN, Amarasinghe LD. Geographic, economic and socio-cultural factors which defining the risk of dengue transmission in Kelaniya, Sri Lanka. J Exp Biol Agric Sci. 2014;2:158–164. [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). Surveillance and control of Aedes aegypti and Aedes albopictus in the United States; 2017. http://www.cdc.gov/chikungunya/resources/vector-control.html. Accessed 28 Dec 2017.
- 6.Sirisena PDNN, Noordeen F. Evolution of dengue in Sri Lanka, changes in the virus, vector, and climate. Int J Infect Dis. 2014;19:6–12. doi: 10.1016/j.ijid.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Kanakaratne N, Wahala MPB, Messer WB, Tissera HA, Shahani A, Abeysinghe N, et al. Severe dengue epidemics in Sri Lanka, 2003–2006. Emerg Infect Dis. 2009;5:192–199. doi: 10.3201/eid1502.080926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epidemiology Unit, Ministry of Health, Sri Lanka. Dengue surveillance trends; 2018. http://www.epid.gov.lk/web/index.php?option=com_casesanddeaths&Itemid=448&lang=en#. Accessed 2 Mar 2019.
- 9.WHO . Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 10.McGraw EA, O’Neill SL. Beyond insecticides: new thinking on an ancient problem. Nat Rev Microbiol. 2013;11:181–193. doi: 10.1038/nrmicro2968. [DOI] [PubMed] [Google Scholar]
- 11.Lees RS, Gilles JRL, Hendrichs J, Vreysen MJB, Bourtzis K. Back to the future: the sterile insect technique against mosquito disease vectors. Curr Opin Insect Sci. 2015;10:156–162. doi: 10.1016/j.cois.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Bourtzis K, Lees RS, Hendrichs J, Vreysen MJB. More than one rabbit out of the hat: radiation, transgenic and symbiont-based approaches for sustainable management of mosquito and tsetse fly populations. Acta Trop. 2016;157:115–130. doi: 10.1016/j.actatropica.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Gilles JRL, Schetelig MF, Scolari F, Marec F, Capurroe ML, Franz G, Bourtzis K. Towards mosquito sterile insect technique programmes: exploring genetic, molecular, mechanical and behavioural methods of sex separation in mosquitoes. Acta Trop. 2014;132(Suppl.):S178–S187. doi: 10.1016/j.actatropica.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Dyck VA, Hendrichs J, Robinson AS. Sterile insect technique: principles and practice in area-wide integrated pest management. Dordrecht: Springer; 2005. [Google Scholar]
- 15.Brelsfoard CL, Sechan Y, Dobson SL. Interspecific hybridization yields strategy for South Pacific filariasis vector elimination. PLoS Negl Trop Dis. 2008;2:e129. doi: 10.1371/journal.pntd.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambers EW, Hapairai L, Peel BA, Bossin H, Dobson SL. Male mating competitiveness of a Wolbachia-introgressed Aedes polynesiensis strain under semi-field conditions. PLoS Negl Trop Dis. 2011;5:e1271. doi: 10.1371/journal.pntd.0001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atyame CM, Pasteur N, Dumas E, Tortosa P, Tantely M, Pocquet N, et al. Cytoplasmic incompatibility as a means of controlling Culex pipiens quinquefasciatus mosquito in the islands of the south-western Indian Ocean. PLoS Negl Trop Dis. 2011;5:e1440. doi: 10.1371/journal.pntd.0001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moretti R, Calvitti M. Male mating performance and cytoplasmic incompatibility in a wPip Wolbachia trans-infected line of Aedes albopictus (Stegomyia albopicta) Med Vet Entomol. 2012;274:377–386. doi: 10.1111/j.1365-2915.2012.01061.x. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor L, Plichart C, Cheong Sang A, Brelsfoard CL, Bossin HC, Dobson SL. Open release of male mosquitoes infected with a Wolbachia biopesticide: field performance and infection containment. PLoS Negl Trop Dis. 2012;6:e1797. doi: 10.1371/journal.pntd.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atyame CM, Cattel J, Lebon C, Flores O, Dehecq JS, Weill M, et al. Wolbachia-based population control strategy targeting Culex quinquefasciatus mosquitoes proves efficient under semi-field conditions. PLoS One. 2015;10:e0119288. doi: 10.1371/journal.pone.0119288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 22.Rasgon JL, Scott TW. Wolbachia and cytoplasmic incompatibility in the California Culex pipiens mosquito species complex: parameter estimates and infection dynamics in natural populations. Genetics. 2003;165:2029–2038. doi: 10.1093/genetics/165.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duron O, Fort P, Weill M. Hypervariable prophage WO sequences describe an unexpected high number of Wolbachia variants in the mosquito Culex pipiens. Proc R Soc Lond Ser B. 2006;273:495–502. doi: 10.1098/rspb.2005.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atyame CM, Delsuc F, Pasteur N, Weill M, Duron O. Diversification of Wolbachia endosymbiont in the Culex pipiens mosquito. Mol Biol Evol. 2011;28:761–2772. doi: 10.1093/molbev/msr083. [DOI] [PubMed] [Google Scholar]
- 25.Calvitti M, Moretti R, Lampazzi E, Bellini R, Dobson SL. Characterization of a new Aedes albopictus (Diptera: Culicidae)-Wolbachia pipientis (Rickettsiales: Rickettsiaceae) symbiotic association generated by artificial transfer of the wPip strain from Culex pipiens (Diptera: Culicidae) J Med Entomol. 2010;47:179–187. doi: 10.1603/me09140. [DOI] [PubMed] [Google Scholar]
- 26.Fu Y, Gavotte L, Mercer DR, Dobson SL. Artificial triple Wolbachia infection in Aedes albopictus yields a new pattern of unidirectional cytoplasmic incompatibility. Appl Environ Microbiol. 2010;76:5887–5891. doi: 10.1128/AEM.00218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blagrove MS, Arias-Goeta C, Failloux AB, Sinkins SP. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc Natl Acad Sci USA. 2012;109:255–260. doi: 10.1073/pnas.1112021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu GJ, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 29.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann AA. Entomology: incompatible mosquitoes. Nature. 2005;436:189. doi: 10.1038/436189a. [DOI] [PubMed] [Google Scholar]
- 31.Bourtzis K, Dobson SL, Xi Z, Rasgon JL, Calvitti M, Moreira LA, et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 2014;132:150–163. doi: 10.1016/j.actatropica.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Bockarie MJ, Hii JL, Alexander ND, Bockarie F, Dagoro H, Kazura JW, Alpers MP. Mass treatment with ivermectin for filariasis control in Papua New Guinea: impact on mosquito survival. Med Vet Entomol. 1999;13:120–123. doi: 10.1046/j.1365-2915.1999.00159.x. [DOI] [PubMed] [Google Scholar]
- 33.Perez CM, Marina CF, Bond JG, Rojas JC, Valle J, Williams T. Spinosad, a naturally derived insecticide, for control of Aedes aegypti (Diptera: Culicidae): efficacy, persistence, and elicited oviposition response. J Med Entomol. 2007;44:631–638. doi: 10.1093/jmedent/44.4.631. [DOI] [PubMed] [Google Scholar]
- 34.Puggioli A, Balestrino F, Damiens D, Lees RS, Soliban SM, Dindo O, et al. Efficiency of three diets for larval development in mass rearing Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2013;50:819–825. doi: 10.1603/ME13011. [DOI] [PubMed] [Google Scholar]
- 35.Sharma VP, Patterson RS, Ford HR. A device for the rapid separation of male and female mosquito pupae. Bull World Health Organ. 1972;47:429–432. [PMC free article] [PubMed] [Google Scholar]
- 36.Jones JC. A simple Method for sexing living Anopheles larvae (Diptera, Culicidae) Ann Entomol Soc Am. 1957;50:104–106. doi: 10.1093/aesa/50.1.104. [DOI] [Google Scholar]
- 37.Fay RM, Morlan HB. A mechanical device for separating the developmental stages, sexes and species of mosquitoes. Mosq News. 1959;19:144–147. [Google Scholar]
- 38.Yamada H, Benedict MQ, Malcolm CA, Oliva CF, Soliban SM, Gilles JR. Genetic sex separation of the malaria vector, Anopheles arabiensis, by exposing eggs to dieldrin. Malar J. 2012;11:208. doi: 10.1186/1475-2875-11-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada H, Marc JB, Vreysen MJB, Bourtzis K, Tschirk W, Chadee DD, Gilles JRL. The Anopheles arabiensis genetic sexing strain ANO IPCL1 and its application potential for the sterile insect technique in integrated vector management programmes. Acta Trop. 2015;142:138–144. doi: 10.1016/j.actatropica.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Gerberg EJ, Hopkins TM, Gentry JW. Mass rearing of Culex pipiens L. Mosq News. 1969;29:382–385. [Google Scholar]
- 41.Focks DA. An improved separator for the developmental stages, sexes, and species of mosquitoes (Diptera: Culicidae) J Med Entomol. 1980;17:567–568. doi: 10.1093/jmedent/17.6.567. [DOI] [PubMed] [Google Scholar]
- 42.McCray EM., Jr A mechanical device for the rapid sexing of Aedes aegypti pupae. J Econ Entomol. 1961;54:819. doi: 10.1093/jee/54.4.819. [DOI] [Google Scholar]
- 43.Yamada H, Soliban SM, Vreysen MJB, Chadee DD, Gilles JRL. Eliminating female Anopheles arabiensis by spiking blood meals with toxicants as a sex separation method in the context of the sterile insect technique. Parasites Vectors. 2013;6:197. doi: 10.1186/1756-3305-6-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deus KM, Saavedra-Rodriguez K, Butters MP, Black WC, 4th, Foy BD. The effect of ivermectin in seven strains of Aedes aegypti (Diptera: Culicidae) including a genetically diverse laboratory strain and three permethrin resistant strains. J Med Entomol. 2012;49:356–363. doi: 10.1603/ME11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitehorn J, Thi LV, Dui LT, Simmons CP. Aedes aegypti (L.) survival after exposure to Ivermectin. Southeast Asian J Trop Med Public Health. 2013;44:179–181. [PMC free article] [PubMed] [Google Scholar]
- 46.Gunathilaka N, Ranathunge T, Udayanga L, Abeyewickreme W. Efficacy of blood sources and artificial blood feeding methods in rearing of Aedes aegypti (Diptera; Culicidae) for sterile insect technique and incompatible insect technique approaches in Sri Lanka. Biomed Res Int. 2017;2017:3196924. doi: 10.1155/2017/3196924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.