Abstract
Background
Colorectal cancer (CRC) originating from the right-sided or left-sided colon is distinct clinicopathological entity. The KRAS status and its prognostic value in CRC remain controversial. This study aimed to investigate the association of KRAS status with clinicopathological features and prognostic value in CRC.
Methods
178 colon cancer and 145 rectal cancer patients were enrolled. KRAS mutation test was performed on paraffin-embedded tumor samples using PCR methods. The colon cancer was divided into right-sided colon cancer (RCC) and left-sided colon cancer (LCC). Studies that reported the association of KRAS mutation with CRC clinical features and prognosis in databases were searched prior to 2018. The data of the present study was combined with the data of published studies using meta-analysis methods.
Results
No significant difference between colon cancer and rectal cancer regarding the KRAS status. The KRAS mutation was much frequent in RCC than in LCC (p = 0.010). 17 studies with 11,385 colon cancer patients were selected, the pooled results of our data and previous published data showed that KRAS mutation was more frequent in RCC compared with in LCC (p < 0.01); KRAS mutation was not associated with the prognosis in RCC patient; however, KRAS mutation indicated a poor prognosis in LCC patients compared with KRAS wild type (p < 0.01).
Conclusion
KRAS status has no difference between colon cancer and rectal cancer. KRAS mutation was more frequent in RCC than in LCC, and associated with a poor prognosis in LCC patients, but not in RCC patients.
Keywords: Colorectal cancer, KRAS mutation, Tumor location, Prognosis
Background
Colorectal cancer (CRC) is the third most common malignancy globally, accounting for approximately 10.0% of all new cancer cases [1]. CRC can be divided into colon cancer and rectal cancer base on their primary tumor location within the colon and rectum. The colon cancer can further be classified into right-sided colon cancer (RCC) and left-sided colon cancer (LCC) divided at the site of splenic flexure of colon. In recent years, a growing evidences revealed that there were significant differences between RCC and LCC with regard to the clinical findings, pathology, genetic mutations and survival time [2]. Thus, the location of tumor is an important factor that affects the prognosis of CRC.
Knowledge has shown that CRC tumorigenesis was characterized by the accumulation of genetic mutations, and Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation was an early event in tumorigenesis [3]. KRAS mutation occur in approximately 30 to 50% of CRC, and 90% of mutation occur in codon 12 or 13 [4–6]. At present, anti-epidermal growth factor receptor (EGFR) antibody has been showed to be an effective therapy in the treatment of CRC patients. However, patients with KRAS mutation are unlikely to benefit from anti-EGFR therapy [7, 8], thus the KRAS status is used as an important biomarker for the selection of suitable patients. To date, many studies reported the clinicopathological features of CRC, and some studies further analyzed the KRAS status in RCC and LCC. However, the results of KRAS status in RCC and LCC remained inconsistent [9–11]. Here, we reported our results of clinicopathological features and KRAS status in Chinese patients with CRC, and further compared the KRAS status in RCC and LCC.
Although some studies showed that the tumor location and KRAS status could affect the effectiveness of patients treated with cetuximab [12, 13], the association between KRAS mutation and patients’ survival remained controversial, as some reports have failed to show any prognostic value of KRAS [14–17]. For example, there was a study found that KRAS mutations were not associated with risk of death in the overall patients of CRC, but LCC patients harboring KRAS mutation have a greater risk of death [18]. These results suggested that the prognostic value of KRAS status in CRC patients might also depending on the location of primary tumor; however, due to the limited of reports, this result still need to be further validated. Therefore, in this study, we combined the published data to further explore the prognostic value of KRAS status in both RCC and LCC.
Methods
Patients and data extraction
This study was retrospective design, patients who diagnosed with CRC and undergoing radical surgery in the Affiliated Tumor Hospital of Guangxi Medical University from January 2015 to January 2018 were included. The inclusion criteria were: CRC was confirmed by historical biopsy; Patients with inflammatory bowel disease or a known history of familial adenomatous polyposis were excluded. Patients with unknown KRAS status or receiving anti-EGFR agents in the perioperative period were also excluded. Detailed information was obtained on patients’ age, gender, histological differentiation, location of primary tumor, tumor infiltration, nodal status, distant metastasis, primary tumor American Joint Committee on Cancer (AJCC) stage. The location of primary tumor was determined by pathologic and operative reports. The right colon includes the cecum, ascending colon, liver flexure, and transverse colon, and the left colon includes the splenic flexure, descending colon and sigmoid colon. This study was approval by the Ethics Committee of Affiliated Tumor Hospital of Guangxi Medical University, written informed consent was obtained from each patient.
DNA extraction from FFPE specimens
FFPE tumor blocks were selected by the surgical pathologist for clinical testing. Tissue was deparaffinized using xylene, ethanol washes, and acetone dehydration, and after cell lysis and proteinase K treatment, the DNA was extracted using the Puregene DNA Isolation or QIAquick PCR purification kit (QIAGEN, Inc. Valencia, CA).
KRAS mutational analysis and sequencing
Mutations in KRAS codons 12 and 13 in exon 2 were detected using amplification refractory mutation system (ARMS)-PCR methods. KRAS mutation status was assessed with Human KRAS Gene 7 Mutations Fluorescence Polymerase Chain Reaction Diagnostic Kit (Amoy Diagnostics Co. Ltd., Xiamen, China) on the AgilentStratagene M × 3000P QPCR System (Agilent Technologies, Santa Clara, CA), according to the manufacturers’ instructions. The 7 most common KRAS mutations (p.G12D, p.G12 V, p.G12A, p.G12C, p.G12S, p.G12R, and p.G13D) in CRCs were detected. The reaction conditions included 1 cycle at 95 °C for 5 min; 15 cycles at 95 °C for 25 s, 64 °C for 20 s, 72 °C for 20 s; and a final 31 cycles at 93 °C for 25 s, 64 °C for 20 s, 72 °C for 20 s. Amplicons were detected using capillary electrophoresis on an ABI 3130xl Genetic Analyzer (Applied Biosystems/Life Technologies, Grand Island, NY) and analyzed using GeneMapper Software (Applied Biosystems/Life Technologies, Grand Island, NY).
Search strategy for articles
Because the association of KRAS status with clinicopathological features and prognostic value in CRC might depend on the primary tumor location, we retrieval articles that analyzing the KRAS status and the prognostic value in RCC and LCC prior to April 2018 by searching the following electronic databases, PubMed, Cochrane Library, Web of Science, EBSCO and Chinese National Knowledge Infrastructure (CNKI). The following search terms were employed: “colon cancer”, “KRAS”, “left-side” or “right-side,” “prognosis”. Included articles were limited to human studies but not limited by language. The first author, year of publication, study location, number of KRAS status in LCC and RCC patients, hazard ratio (HR) and the corresponding 95%CI of prognostic value of KRAS status in LCC and RCC were extracted.
Statistical analysis
Demographic and clinicopathological characteristics of the patients were stratified according to primary tumor location and KRAS mutation status. Continuous variables were presented as mean ± standard deviation, and compared using a Student’s t-test. Summary statistics for the patients were presented as totals for categorical variables. The differences between wild-type KRAS (wt- KRAS) and mutant-type KRAS (mt-KRAS) in each group were assessed by the χ2 test. The analyses were performed using R software version 3.4.3.
The meta-analysis of KRAS status in RCC and LCC, and the prognostic value of KRAS status in RCC and LCC was performed using Stata 11.2 software (Stata Corp, College Station, TX) with 2-tailed p-values. The pooled odds ratio (OR) with the corresponding 95% CI were used to estimate then KRAS status in RCC and LCC. The pooled HR with the corresponding 95% CI was used to assess the prognostic value of KRAS status in RCC and LCC. The p-value < 0.05 was considered statistically significant.
Results
Clinicopathological characteristics of CRC patients
There were 178 colon cancer and 145 rectal cancer patients finally enrolled in this study. The mean age of colon cancer and rectal cancer was (57.62 ± 12.67) years and (59.57 ± 11.89) years, respectively. No significant different between colon cancer and rectal cancer in the KRAS status (p = 0.393). Most of the colon cancer and rectal cancer was moderate differentiation, but no significant difference between colon cancer and rectal cancer (p = 0.099). There was no obvious difference in nodal status, distant metastases, AJCC stage between colon cancer and rectal cancer (p > 0.05). However, the number of advanced tumor infiltration patients (T3 + 4 stage) of colon cancer was greatly larger than that of rectal cancer (p = 0.018). Detail of included CRC patients was listed in Table 1.
Table 1.
Characteristics of colon and rectal cancer patients
| Colon (n = 178) | Rectal (n = 145) | t/χ2 value | P value | |
|---|---|---|---|---|
| Gender | 0.209 | 0.648 | ||
| Male | 116 | 98 | ||
| Female | 62 | 47 | ||
| Mean age | 57.62 ± 12.67 | 59.57 ± 11.89 | 1.359 | 0.175 |
| KRAS status | 0.730 | 0.393 | ||
| Mutation | 62 | 44 | ||
| Wild-type | 116 | 101 | ||
| Differentiation | 4.617 | 0.099 | ||
| Poor | 34 | 22 | ||
| Moderate | 137 | 122 | ||
| High | 7 | 1 | ||
| Tumor infiltration | 10.064 | 0.018 | ||
| T1 | 2 | 8 | ||
| T2 | 19 | 27 | ||
| T3 | 32 | 21 | ||
| T4 | 125 | 89 | ||
| Nodal status | 2.503 | 0.286 | ||
| N0 | 62 | 63 | ||
| N1 | 57 | 40 | ||
| N2 | 59 | 42 | ||
| Distant metastases | 3.388 | 0.066 | ||
| M0 | 113 | 106 | ||
| M1 | 65 | 39 | ||
| AJCC stage | 5.631 | 0.131 | ||
| I | 13 | 20 | ||
| II | 41 | 36 | ||
| III | 58 | 49 | ||
| IV | 66 | 40 | ||
Clinicopathological characteristics of colon cancer in different status of KRAS
Of the 178 colon cancer patients, 62 occur KRAS mutation, the remained116 were KRAS wild-type. We divided these patients based on the status of KRAS, and found that no obvious differences in patients’ gender, age, histological differentiation, nodal status, distant metastases, AJCC stage (p > 0.05). However, significant difference was found between RCC and LCC regarding the KRAS status, with KRAS mutation in RCC was 46.4% (32/69), and in LCC was 27.5% (30/109), p = 0.010. Table 2 showed the detail of the characteristics in different status of KRAS.
Table 2.
Characteristics of colon cancer in different KRAS status
| Mutation (n = 62) | Wild-type (n = 116) | t/χ2 value | P value | |
|---|---|---|---|---|
| Gender | 0.630 | 0.427 | ||
| Male | 38 | 78 | ||
| Female | 24 | 38 | ||
| Mean age | 56.63 ± 12.15 | 58.15 ± 12.94 | 0.761 | 0.448 |
| Differentiation | 0.218 | 0.897 | ||
| Poor | 12 | 22 | ||
| Moderate | 47 | 90 | ||
| High | 3 | 4 | ||
| Tumor infiltration | 4.227 | 0.238 | ||
| T1 | 1 | 1 | ||
| T2 | 5 | 14 | ||
| T3 | 7 | 25 | ||
| T4 | 49 | 76 | ||
| Nodal status | 0.434 | 0.805 | ||
| N0 | 22 | 40 | ||
| N1 | 18 | 39 | ||
| N2 | 22 | 37 | ||
| Distant metastases | 2.029 | 0.154 | ||
| M0 | 35 | 78 | ||
| M1 | 27 | 38 | ||
| AJCC stage | 2.698 | 0.441 | ||
| I | 4 | 9 | ||
| II | 12 | 29 | ||
| III | 18 | 40 | ||
| IV | 28 | 38 | ||
| Location | 6.617 | 0.010 | ||
| RCC | 32 | 37 | ||
| LCC | 30 | 79 | ||
Characteristics of included studies
Seventeen studies [9–13, 18–29] with 11,385 colon cancer patients were included in this study based on the included criteria. Among them, sixteen studies [9–13, 19–29] with 5, 835 patients provided the data of KRAS status in colon cancer, with 3961 LCC patients and 1874 RCC patients, respectively. Four studies [11, 18, 23, 28] with 6697 patients provided the survival data of KRAS status in colon cancer, with 3670 LCC patients and 3027 RCC patients, respectively. Table 3 presented the detail of the characteristics of included studies. A flow chart of the article selection process was shown in Fig. 1.
Table 3.
Characteristics of included studies
| Author | Year/country | Study design | LCC | RCC | LCC | RCC | ||
|---|---|---|---|---|---|---|---|---|
| wt | mut | wt | mut | HR(95%CI) | HR(95%CI) | |||
| Chiu JW | 2018/Canada | P | 65 | 46 | 36 | 37 | – | – |
| Hou Y | 2018/China | R | 196 | 117 | 62 | 86 | – | – |
| Natsume S | 2018/Japan | R | 327 | 97 | 98 | 53 | 0.58 (0.37–0.86) | – |
| Charlton ME | 2017/USA | R | 2849 | 2701 | 1.18 (1.05–1.3) | 0.93 (0.83–1.03) | ||
| Gao XH | 2017/China | P | 42 | 30 | 27 | 16 | – | – |
| Kim ST | 2017/Korea | R | 115 | 9 | 25 | 4 | – | – |
| Chang YY | 2016/China | P | 425 | 221 | 221 | 187 | – | – |
| Sasaki K | 2016/USA | R | 201 | 96 | 65 | 64 | 1.81 (1.11–2.96) | 1.03 (0.51–2.08) |
| Sun P | 2016/China | R | 60 | 48 | 44 | 40 | – | – |
| Kodaz H | 2015/Turkey | R | 80 | 72 | 13 | 12 | – | – |
| Ye JX | 2015/China | R | 100 | 50 | 94 | 67 | – | – |
| Tong JH | 2014/China | R | 680 | 461 | 156 | 209 | – | – |
| von Einem JC | 2014/Germany | P | 68 | 32 | 27 | 19 | 1.3 (0.68–2.34) | 0.63 (0.43–0.92) |
| Cushman- Vokoun AM | 2013/USA | R | 13 | 16 | 21 | 15 | – | – |
| Zhu XL | 2012/China | R | 79 | 45 | 59 | 47 | – | – |
| Abubaker J | 2009/Saudi Arabia | R | 79 | 62 | 26 | 18 | – | – |
| Bleeker WA | 2000/Netherlands | R | 26 | 3 | 16 | 10 | – | – |
LCC left-side colon cancer, RCC right-side colon cancer, wt KRAS wild type, mut KRAS mutant type, HR hazard ratio, CI confidence interval, P prospective, R retrospective
Fig. 1.
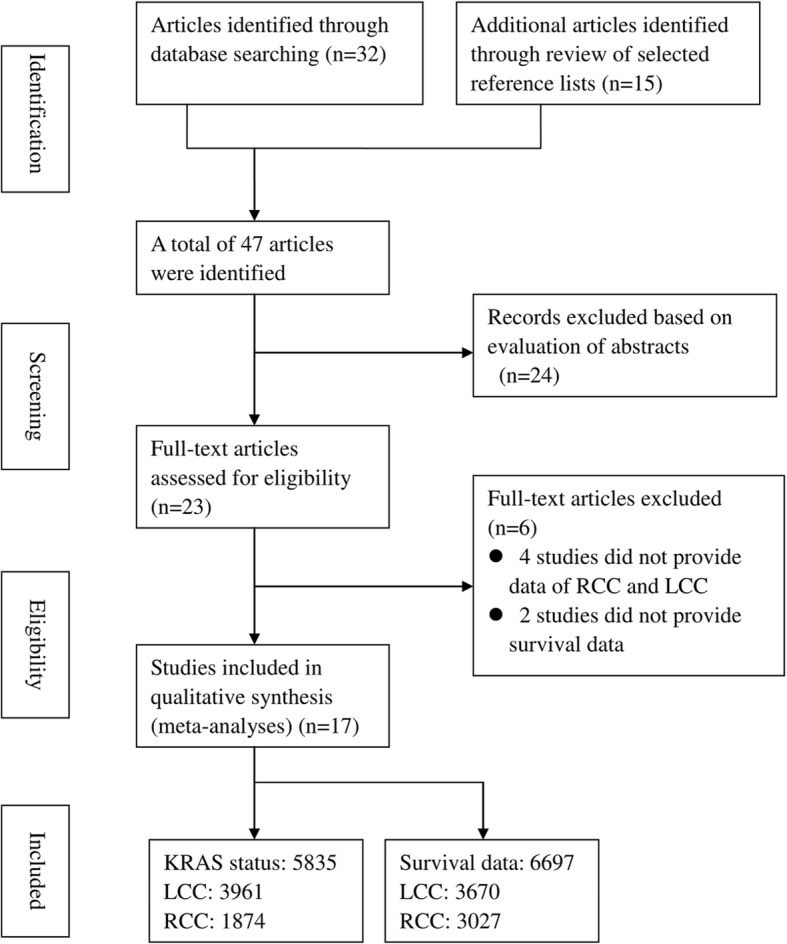
Flow diagram of the study selection process
Pooled results of KRAS status in RCC and LCC
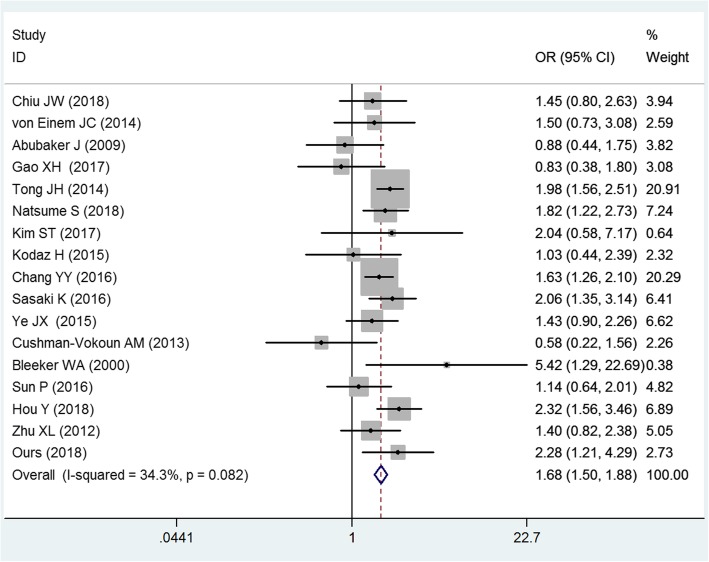
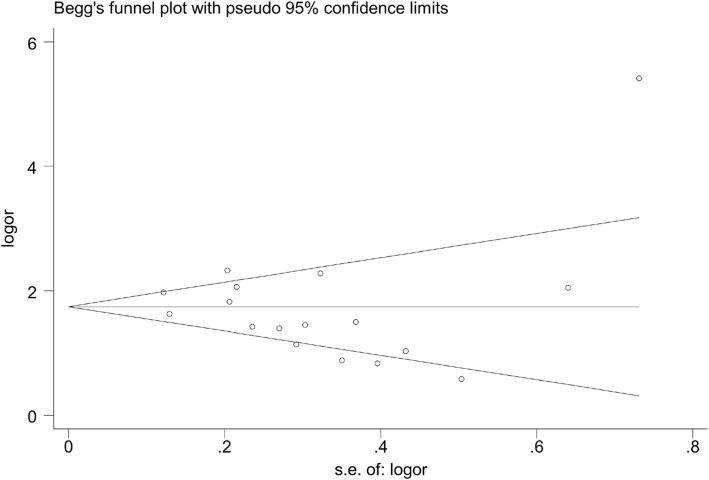
To clarify the KRAS status in RCC and LCC, we combined the data from sixteen studies that provided the KRAS status in RCC and LCC with our data. The pooled results showed that KRAS mutation was much more frequent in RCC compared with LCC (OR = 1.68, 95%CI = 1.50–1.88, p < 0.01), and no significant heterogeneity across the studies (I2 = 34.3%, p = 0.082). See Fig. 2. There was little publication bias across the studies (Begg’s Test = 0.343; Egger’s test = 0.575). See Fig. 3.
Fig. 2.
Forest plot of the KRAS mutation between right-sided colon cancer and left-sided colon cancer. The squares and horizontal lines correspond to the study-specific OR and 95% CI. The diamond represents the summary OR and 95% CI. The diamond locates to the right of vertical line means the KRAS mutation was much more frequent in RCC compared with in LCC (OR < 1)
Fig. 3.
Begg’s funnel plot for publication bias test
Pooled results of prognostic value of KRAS status in RCC and LCC
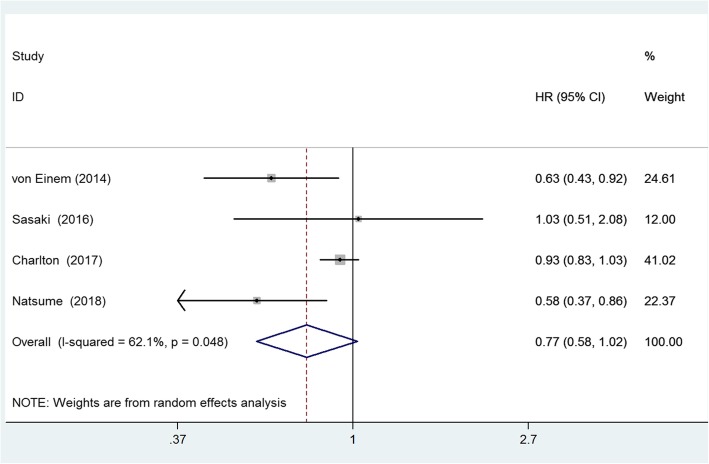
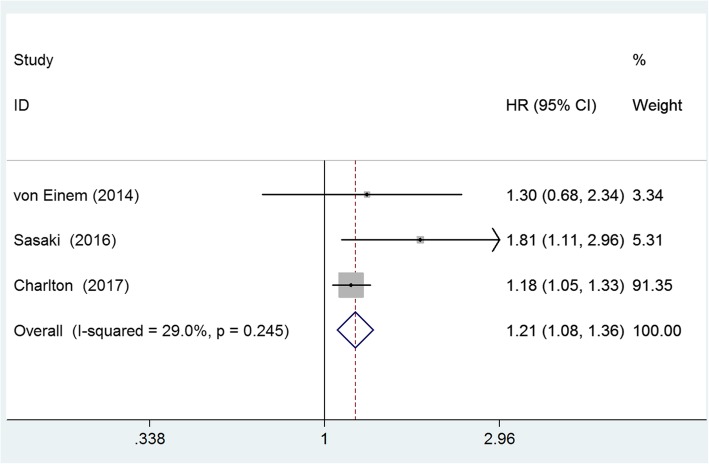
To estimate the difference prognostic value of KRAS status in RCC and LCC, we combined the data from four studies that provided the data of overall survival (OS) of RCC and LCC patients with different KRAS status. All the studies included metastatic CRC cases, and patients receiving chemotherapy and/or radiotherapy after surgical resection. The multivariate analysis was performed by adjusting the confounding prognostic factors in each study. The pooled results showed that RCC patients with KRAS mutation has no significant different OS compared with patients with KRAS wild type (HR = 0.77, 95%CI = 0.58–1.02, p = 0.073; I2 = 62.1%), see Fig. 4; however, LCC patients with KRAS mutation has a shorter OS than patients with KRAS wild type (HR = 1.21, 95%CI = 1.08–1.36, p < 0.01; I2 = 29.0%), see Fig. 5.
Fig. 4.
Forest plot of the KRAS mutation in the prediction of right-sided colon cancer patients. The squares and horizontal lines correspond to the study-specific HR and 95% CI. The diamond represents the summary HR and 95% CI. The diamond locates to the left but touches the vertical line means the no significant difference between patients with and without KRAS mutation regarding the OS
Fig. 5.
Forest plot of the KRAS mutation in the prediction of left-sided colon cancer patients. The squares and horizontal lines correspond to the study-specific HR and 95% CI. The diamond represents the summary HR and 95% CI. The diamond locates to the right of vertical line means the LCC patients with KRAS mutation has a shorter OS than patients with KRAS wild type (HR > 1)
Discussion
In this study, by analyzing the clinicopathological features of 178 colon cancers and 145 rectal cancers, we failed to find the difference between colon cancer and rectal cancer regarding the KRAS status. By dividing the CRC based on the KRAS status, we did not observe the difference between KRAS mutation and wild type regarding the clinicopathological features, but found that RCC harboring more KRAS mutation compared with LCC (46.4% vs. 37.5%). We next combined the data of KRAS status in RCC and LCC, by pooling the data of sixteen studies and our data, we found that KRAS mutation was much more frequent in RCC than in LCC. In addition, by pooling the data of four studies, we found an obvious difference of OS in RCC and LCC regarding the KRAS status, that is, LCC patients with KRAS mutation has a shorter OS than with KRAS wild type, while RCC patients with KRAS mutation has no significant different OS compared with patients with KRAS wild type. These results indicated that both tumor location and KRAS status play important roles in the prognosis of CRC patients.
Knowledge has shown that the right and left sides of the colon have different embryologic origins. Tumor that origins from the two sites of the colon has different molecular carcinogenic characters, including KRAS, BRAF mutations and microsatellite instability (MSI) [12, 30, 31]. KRAS has been confirmed as a proto-oncogene which induces tumorigenesis in several cancers. In CRC, KRAS mutations status and tumor location are associated with targeted therapy effectiveness. In this study, KRAS status has no obvious difference in colon cancer or rectal cancer, but showed significant difference in RCC and LCC, which was in consistent with Natsume et al. [11] and Tong et al. [27] reports, but in contrast to Cushman-Vokoun et al. [10] report. Then the following meta-analysis with larger patients further verified the different KRAS status in RCC and LCC, indicating that the KRAS mutation was more frequent in RCC than LCC.
Since the effect of anti-EGFR therapy on CRC is associated with KRAS status, many studies have estimated the prognostic value of KRAS status in CRC patients [8–10], and some studies showed that mutation of KRAS indicated a poor prognosis of CRC patients, but there were also some reports have failed to show the similar result [14–17, 19], thus, the current conclusions regarding the prognostic value of KRAS status remain inconclusive. Because the distinct genetic alteration between RCC and LCC, both of the location of tumor and KRAS status are proposed to influence the prognostic value CRC. As Sasaki et al. [23] pointed that, KRAS mutation in RCC was not associated with the prognosis of CRC, while KRAS mutation in LCC indicated a poor prognosis of CRC patients. However, this result was based on a relative small sample size; thus, the robustness of the conclusion was undermined. In this study, by combining the data from four studies with 6697 patients, we found that LCC patients with KRAS mutation has a poor prognosis, but RCC patients with KRAS mutation did not show the similar results, which can partly explain the inconsistent results of the prognostic value of KRAS status in CRC patients. And these results further verified that both the KRAS status and location of tumor could affect the treatment effectiveness and prognosis in CRC patients.
Although this study showed the different KRAS status in RCC and LCC, and found the prognostic value of KRAS mutation was depending on the location of tumor, there were several limitations should be considered when interpreting the results. First, due to lack of the survival data in our center, we did not combine our data with published studies, thus, the sample size of the analysis for the prognostic value of KRAS status was relative small. Second, due to the limited studies available, we did not divide the patients based on the their ethnicity, so we did not know whether various ethnicity could affect the prognostic value of KRAS status in CRC, since evidence has shown that there were many differences in CRC between Asian and Caucasian ethnicity [32, 33]. Third, the present study only included the data of mutation of KRAS codons 12 and 13 in exon 2, other mutations, such as NRS and BRAF mutation, were not included. Although these type of mutations were fewer compared with the KRAS mutation, the lack of data of other mutations might lead to selected bias in the analysis. Fourth, the pooling analysis included all stages of CRC patients without stratified them into different stage, that is, early stage or advanced stage of CRC, hence the data very heterogeneous and would reduce the robustness of the results. Fifth, some of the included studies were retrospective design, which may lead selected bias and undermine the robustness of the results. Therefore, future research should be conducted to address the aforementioned limitations.
Conclusion
This study demonstrated that no significant difference of KRAS status between colon cancer and rectal cancer. KRAS mutation was much more frequent in RCC compared with LCC, and LCC patients with KRAS mutation has a poor prognosis compared with KRAS wild type, but RCC patients did not show the similar effect.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation (No. 81260315) and Guangxi Medicine and Scientific Research Projects (No.Z20180626; Z20180627; Z20180613). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviation
- CRC
Colorectal cancer
- EGFR
Epidermal growth factor receptor
- HR
Hazard ratio
- KRAS
Kirsten rat sarcoma viral oncogene homolog
- LCC
Left-sided colon cancer
- OR
Odds ratio
- RCC
Right-sided colon cancer
Authors’ contributions
Study concept and design: XMZ and HBL; Collection and assembly of data: XMZ and LJL; Performed the experiment: XMZ and CZM; Data analysis and interpretation: HBL, CZM and XMZ, LKZ; Manuscript writing and review: All authors. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approval by the Ethics Committee of Affiliated Tumor Hospital of Guangxi Medical University, written informed consent was obtained from each patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ming-zhi Xie, Email: 1292552295@qq.com.
Ji-lin Li, Email: 455620024@qq.com.
Zheng-min Cai, Email: lamp518@qq.com.
Ke-zhi Li, Email: 49783925@qq.com.
Bang-li Hu, Phone: +86 0771 5310593, Email: hubangli@gxmu.edu.cn.
References
- 1.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Stintzing S, Tejpar S, Gibbs P, Thiebach L, Lenz HJ. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur J Cancer. 2017;84:69–80. doi: 10.1016/j.ejca.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Tumorigenesis VVE. RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418(6901):934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 4.Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, et al. Kirsten ras mutations in patients with colorectal cancer: the 'RASCAL II' study. Br J Cancer. 2001;85(5):692–696. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nosho K, Irahara N, Shima K, Kure S, Kirkner GJ, Schernhammer ES, et al. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS One. 2008;3(11):e3698. doi: 10.1371/journal.pone.0003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 7.De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304(16):1812–1820. doi: 10.1001/jama.2010.1535. [DOI] [PubMed] [Google Scholar]
- 8.Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 9.Chiu JW, Krzyzanowska MK, Serra S, Knox JJ, Dhani NC, Mackay H, et al. Molecular profiling of patients with advanced colorectal Cancer: Princess Margaret Cancer Centre experience. Clin Colorectal Cancer. 2018;17(1):73–79. doi: 10.1016/j.clcc.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Cushman-Vokoun AM, Stover DG, Zhao Z, Koehler EA, Berlin JD, Vnencak-Jones CL. Clinical utility of KRAS and BRAF mutations in a cohort of patients with colorectal neoplasms submitted for microsatellite instability testing. Clin Colorectal Cancer. 2013;12(3):168–178. doi: 10.1016/j.clcc.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Natsume S, Yamaguchi T, Takao M, Iijima T, Wakaume R, Takahashi K, et al. Clinicopathological and molecular differences between right-sided and left-sided colorectal cancer in Japanese patients. Jpn J Clin Oncol. 2018;48(7):609–618. doi: 10.1093/jjco/hyy069. [DOI] [PubMed] [Google Scholar]
- 12.Bleeker WA, Hayes VM, Karrenbeld A, Hofstra RM, Hermans J, Buys CC, et al. Impact of KRAS and TP53 mutations on survival in patients with left- and right-sided Dukes’ C colon cancer. Am J Gastroenterol. 2000;95(10):2953–2957. doi: 10.1111/j.1572-0241.2000.02327.x. [DOI] [PubMed] [Google Scholar]
- 13.Gao XH, Yu GY, Gong HF, Liu LJ, Xu Y, Hao LQ, et al. Differences of protein expression profiles, KRAS and BRAF mutation, and prognosis in right-sided colon, left-sided colon and rectal cancer. Sci Rep. 2017;7(1):7882. doi: 10.1038/s41598-017-08413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrowsky H, Sturm I, Graubitz O, Kooby DA, Staib-Sebler E, Gog C, et al. Relevance of Ki-67 antigen expression and K-ras mutation in colorectal liver metastases. Eur J Surg Oncol. 2001;27(1):80–87. doi: 10.1053/ejso.2000.1029. [DOI] [PubMed] [Google Scholar]
- 15.Rose JS, Serna DS, Martin LK, Li X, Weatherby LM, Abdel-Misih S, et al. Influence of KRAS mutation status in metachronous and synchronous metastatic colorectal adenocarcinoma. Cancer. 2012;118(24):6243–6252. doi: 10.1002/cncr.27666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng HW, Huang YC, Lin JK, Chen WS, Lin TC, Jiang JK, et al. BRAF mutation is a prognostic biomarker for colorectal liver metastasectomy. J Surg Oncol. 2012;106(2):123–129. doi: 10.1002/jso.23063. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Heo JS, Lee J, Lee JY, Lee MY, Lim SH, et al. The impact of KRAS mutations on prognosis in surgically resected colorectal cancer patients with liver and lung metastases: a retrospective analysis. BMC Cancer. 2016;16:120. doi: 10.1186/s12885-016-2141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlton ME, Kahl AR, Greenbaum AA, Karlitz JJ, Lin C, Lynch CF, et al. KRAS testing, tumor location, and survival in patients with stage IV colorectal Cancer: SEER 2010-2013. J Natl Compr Cancer Netw. 2017;15(12):1484–1493. doi: 10.6004/jnccn.2017.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang YY, Lin PC, Lin HH, Lin JK, Chen WS, Jiang JK, et al. Mutation spectra of RAS gene family in colorectal cancer. Am J Surg. 2016;212(3):537–44 e3. doi: 10.1016/j.amjsurg.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Hou Y, Li SB, Shi XQ, Fu GH, Wu J. Retrospective analysis of KRAS, NRAS and BRAF gene mutations and clinicopathological features in patients with colorectal cancer. J Shanghai Jiaotong Uni: Med Sci. 2018;38(1):4–9. [Google Scholar]
- 21.Kim ST, Lee SJ, Lee J, Park SH, Park JO, Lim HY, et al. The impact of microsatellite instability status and sidedness of the primary tumor on the effect of Cetuximab-containing chemotherapy in patients with metastatic colorectal Cancer. J Cancer. 2017;8(14):2809–2815. doi: 10.7150/jca.18286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kodaz H, Hacibekiroglu I, Erdogan B, Turkmen E, Tozkir H, Albayrak D, et al. Association between specific KRAS mutations and the clinicopathological characteristics of colorectal tumors. Mol Clin Oncol. 2015;3(1):179–184. doi: 10.3892/mco.2014.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki K, Margonis GA, Wilson A, Kim Y, Buettner S, Andreatos N, et al. Prognostic implication of KRAS status after hepatectomy for colorectal liver metastases varies according to primary colorectal tumor location. Ann Surg Oncol. 2016;23(11):3736–3743. doi: 10.1245/s10434-016-5361-6. [DOI] [PubMed] [Google Scholar]
- 24.Sun P, Wan JY, Wu LH, Xiao Y. Relationship between KRAS/NRAS/BRAF gene mutations and clinical pathological characteristics in colorectal cancer. Chin J General Surg. 2016;31(1):50–54. [Google Scholar]
- 25.Ye JX, Liu Y, Qin Y, Zhong HH, Yi WN, Shi XY. KRAS and BRAF gene mutations and DNA mismatch repair status in Chinese colorectal carcinoma patients. World J Gastroenterol. 2015;21(5):1595–1605. doi: 10.3748/wjg.v21.i5.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abubaker J, Bavi P, Al-Haqawi W, Sultana M, Al-Harbi S, Al-Sanea N, et al. Prognostic significance of alterations in KRAS isoforms KRAS-4A/4B and KRAS mutations in colorectal carcinoma. J Pathol. 2009;219(4):435–445. doi: 10.1002/path.2625. [DOI] [PubMed] [Google Scholar]
- 27.Tong JH, Lung RW, Sin FM, Law PP, Kang W, Chan AW, et al. Characterization of rare transforming KRAS mutations in sporadic colorectal cancer. Cancer Biol Ther. 2014;15(6):768–776. doi: 10.4161/cbt.28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Einem JC, Heinemann V, von Weikersthal LF, Vehling-Kaiser U, Stauch M, Hass HG, et al. Left-sided primary tumors are associated with favorable prognosis in patients with KRAS codon 12/13 wild-type metastatic colorectal cancer treated with cetuximab plus chemotherapy: an analysis of the AIO KRK-0104 trial. J Cancer Res Clin Oncol. 2014;140(9):1607–1614. doi: 10.1007/s00432-014-1678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu XL, Cai X, Zhang L, Yang F. KRAS and BRAF gene mutations in correlation with clinicopathologic features of colorectal carcinoma in Chinese. Chin J Pathol. 2012;41(9):584–589. doi: 10.3760/cma.j.issn.0529-5807.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Nawa T, Kato J, Kawamoto H, Okada H, Yamamoto H, Kohno H, et al. Differences between right- and left-sided colon cancer in patient characteristics, cancer morphology and histology. J Gastroenterol Hepatol. 2008;23(3):418–423. doi: 10.1111/j.1440-1746.2007.04923.x. [DOI] [PubMed] [Google Scholar]
- 31.Hutchins G, Southward K, Handley K, Magill L, Beaumont C, Stahlschmidt J, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29(10):1261–1270. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
- 32.Simon MS, Thomson CA, Pettijohn E, Kato I, Rodabough RJ, Lane D, et al. Racial differences in colorectal cancer incidence and mortality in the Women's Health Initiative. Cancer Epidemiol Biomark Prev. 2011;20(7):1368–1378. doi: 10.1158/1055-9965.EPI-11-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virk R, Gill S, Yoshida E, Radley S, Salh B. Racial differences in the incidence of colorectal cancer. Can J Gastroenterol. 2010;24(1):47–51. doi: 10.1155/2010/565613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.