Abstract
Background
Several trials evaluated the role of intensive regimens, made of triplet chemotherapies plus bevacizumab, as first-line treatment for patients with metastatic colorectal cancer (mCRC). We previously reported, in a Phase II prospective study, the efficacy and the tolerability of FIrB/FOx regimen, reporting interesting results in terms of received dose intensities (rDIs) and safety.
Methods
We reported a retrospective update of 85 patients treated with FIrB/FOx, an intensive regimen of 5-fluorouracil, bevacizumab, and weekly alternate irinotecan and oxaliplatin, to confirm its feasibility in “real life”. Subgroup analyses were performed, particularly among patients treated with standard and modified FIrB/FOx (based on age, performance status, and/or comorbidities).
Results
Overall, 3-month objective response rate (ORR) and 6-month ORR were 75.9% and 55.3%, respectively. Median progression-free survival (PFS) and median overall survival (OS) were 14.4 and 34.9 months, respectively. Among the patients treated with standard and modified regimens, 3-month ORR, PFS, and OS were 75.8% and 76% (P=1.0000), 14.4 and 14.4 months (P=0.8589), and 37.8 and 26.6 months (P=0.7746), respectively. Among the K/NRAS wild-type and K/NRAS mutant patients, 3-month ORR, PFS, and OS were 95.2% and 74.5% (P=0.0526), 15.3 and 14.4 months (P=0.8753), and 37.8 and 51.4 months (P=0.8527), respectively. The rDIs were ≥80% of full doses both in the standard and in the modified regimens subgroups. Cumulative G3/4 toxicities were neutropenia (14.1%), diarrhea (17.6%), asthenia (9.4%), vomiting (5.6%), and hypertension (16.5%).
Conclusion
This update shows that intensive regimens such as FIrB/FOx are also feasible options for first-line treatment of mCRC patients in the “real-life” setting.
Keywords: metastatic colorectal cancer, intensive chemotherapy regimen, bevacizumab, clinical practice, 5-fluorouracil infusion
Introduction
In recent years, several regimens of cytotoxic agents combined with biological compounds have been investigated, and the regimens have become a common routine in the treatment of patients with unresectable or metastatic colorectal cancer (mCRC). The anti-angiogenic monoclonal antibody bevacizumab was the first targeted agent successfully used in schedules containing a fluoropyrimidine (5-fluorouracil [5-FU] or capecitabine) in combination with either irinotecan1–3 or oxaliplatin,4–6 reaching objective response rates (ORR) even higher than 50%, median progression free survivals (PFS) between 8.3 and 11.1 months, and median overall survivals (OS) between 20.3 and 22.2 months.
Several clinical trials investigated intensive regimens, initially made of triplet chemotherapy,7–9 and then combined with targeted agents. Overall, all the Phase I and Phase II trials that investigated the safety and activity of triplet chemotherapy regimens in association with EGFR inhibitors (cetuximab and panitumumab) in first-line setting showed poor feasibility, with frequent dose reductions due to adverse events.10–15
However, regarding combination regimens with bevacizumab, Phase II studies demonstrated the effectiveness of combining FOLFOXIRI plus bevacizumab, with acceptable safety profiles.16–18 The first study was developed by the GONO group (Gruppo Oncologico Nord Ovest);16 with a 10-month PFS rate of 74%, G3/G4 neutropenia of 49%, and G3/G4 diarrhea of 14%, it had laid the foundations for the development of similar regimens. In the OLIVIA trial, FOLFOXIRI plus bevacizumab showed higher response rate, higher resection rate, and longer PFS vs mFOLFOX-6 plus bevacizumab in patients with unresectable liver metastases, with manageable toxicities in the experimental arm.17 In the single-arm OPAL trial, FOLFOXIRI plus bevacizumab achieved similar efficacy results, but with a better toxicity profile.18
The GONO group subsequently developed the TRIBE study, a randomized Phase III trial which compared, in K/NRAS and BRAF unselected patients, the first-line treatment with FOLFOXIRI plus bevacizumab with FOLFIRI plus bevacizumab, both followed by a maintenance therapy with bevacizumab and 5-FU.19 The study met its primary end point, with a statistically significant benefit in PFS (12.1 months vs 9.7 months, P=0.006); ORR and median OS were 65% and 31.0 months, respectively, in the experimental arm.
When it became clear that KRAS exon 2 mutant patients (subsequently K/NRAS and BRAF mutant patients) did not benefit from EGFR inhibitors combined with chemotherapy, things have changed.20–23 The genotype assessment became mandatory for the proper selection of first-line treatments, and wild-type patients were treated more and more with doublet regimens plus EGFR inhibitors.
In the OS update, with molecular subgroup analyses of the TRIBE study, the experimental arm reached median PFS and median OS of 12.3 and 29.8 months, respectively. Median OS, median PFS, and ORR were 41.7 months, 13.7 months, and 65% in K/NRAS and BRAF wild-type patients, respectively, while 27.3 months, 12.0 months, and 66% in K/NRAS mutant patients, respectively. In BRAF-mutant patients, median OS, median PFS, and ORR were 19.0 months, 7.5 months, and 56%, respectively.24
In order to increase the tolerability of 5-FU in combination with irinotecan in mCRC patients, we previously developed an alternative way of administrating 5-FU, called timed-flat infusion (TFI), which is a 12-hour nocturnal flat infusion (from 10:00 PM to 10:00 AM), without 5-FU bolus and folinic acid.25 Indeed, no experimental evidence have supported the fact that folinic acid administration enhances the antitumoral activity of infusional 5-FU at its maximum tolerated dose.26–28 TFI/5-FU exploits the increased activity of dihydropyrimidine dehydrogenase, the enzyme involved in 5-FU intracellular catabolism in mononuclear cells, as well as the reduced proliferation of the healthy tissues most damaged by 5-FU (the bone marrow and oral/rectal mucosa) during the night hours.29–32 We then developed a triplet schedule, called FIrB/FOx, containing irinotecan and oxaliplatin administered every other week, in association with TFI/5-FU two nights a week,33 and the FIrB/FOx schedule, by adding bevacizumab to this intensive regimen.34 In the Phase II study of FIrB/FOx (K/NRAS and BRAF unselected patients), ORR was 82%, median PFS 12 months, and median OS 28 months. The toxicity profile was favorable, except for G3/G4 diarrhea (28%). The received dose intensities (rDIs) were higher than 80% of the planned dose for each drug.
Here we report a clinical update of patients previously enrolled in a Phase II study, and of those subsequently treated in clinical practice with FIrB/FOx regimen, to confirm the activity, safety, and feasibility of this intensive regimen in the “real-life” setting.
Materials and methods
Patient eligibility
The present retrospective analysis evaluated mCRC patients who had been treated with first-line FIrB/FOx regimen, at Medical Oncology department of St Salvatore Hospital of L’Aquila, from February 2006 to 2018. Patients were eligible if they had histologically confirmed diagnosis of CRC, clinically measurable disease, age 18–75 years, Eastern Cooperative Oncology Group Performance Status (ECOG-PS) ≤1; adequate hematological, renal, and hepatic functions, and life expectancy longer than 3 months. Treatment schedules were tailored to be in keeping with patients’ fitness, which was defined according to age, ECOG-PS, and comorbidities. Comorbidities were evaluated by Cumulative Index Rating Scale (CIRS).35
Schedule
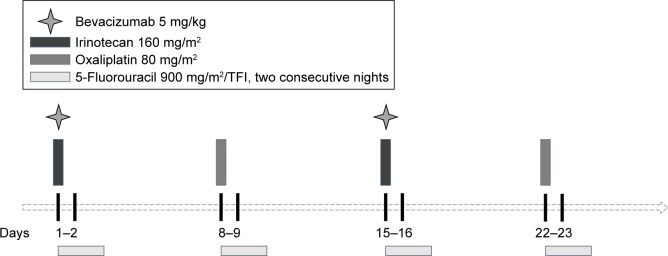
Standard FIrB/FOx regimen is a schedule of weekly TFI/5-FU (12 hours for two consecutive nights: from 10:00 PM to 10:00 AM) at 900 mg/m2/night, combined with weekly alternating irinotecan 160 mg/m2 plus bevacizumab (Avastin®; Roche, Welwyn Garden City, UK) 5 mg/kg on days 1 and 15 and oxaliplatin 80 mg/m2 on days 8 and 22. Cycles were repeated every 28 days (4 weeks; Figure 1). 5-FU was administered by a portable pump (CADD Plus, SEVIT) using a central venous access device (port-a-cath or peripherally inserted central catheter). Modified FIrB/FOx was defined by any projected dose reduction compared to the standard one based on age, PS, and/or comorbidities. The doses level reductions were 800 mg/m2/night and 750 mg/m2/night for 5-FU, 140 mg/m2 and 120 mg/m2 for irinotecan, and 70 mg/m2 for oxaliplatin (seven patients took part at the dose-finding step for oxaliplatin in the Phase II study that started from 60 mg/m2). No dose adjustments for bevacizumab were allowed (bevacizumab doses were temporarily discontinued in case of G3 hypertension, wound complications, ≥G2 thromboembolic events, and anyhow at least 8 weeks from a scheduled surgery).
Figure 1.
Graphic representation of the FIrB/FOx schedule.
Abbreviation: TFI, timed-flat infusion.
Mutational assessment
KRAS (exons 2–4), NRAS (exons 2–4), and BRAF (exon 15) analyses were performed on paraffin embedded tissue blocks obtained from the primary tumor and/or metastatic site, using direct sequencing, pyrosequencing, and real-time polymerases chain reaction techniques (SNaPshot® multiplex assay, Cobas® Z480 analyzer) in clinical practice. The molecular analysis was retrospectively performed in patients treated before 2014. In eight patients, genotype was not assessed due to difficulties in obtaining tumor tissues.
Study design and statistical analysis
In this “single-institution”, retrospective study, clinical outcomes (ORR, disease control rate [DCR], PFS, and OS) were assessed in “real life” conditions; thus, activity and efficacy were influenced not only by FIrB/FOx but also by other factors, such as metastasectomies, locoregional treatments, and subsequent treatments. With this in mind, we considered more appropriate to define clinical outcomes collection as “effectiveness analysis”. Subgroup analyses were performed among patients treated with standard/modified regimens, among K/NRAS wild-type/mutant patients, and among patients with left-side (descending and sigmoid colon, rectum) and right-side (caecum, ascending and transverse colon) primary tumors. Clinical evaluation of response was made by computed tomography scan every 3 months; positron emission tomography was added based on investigators’ choice. ORR was defined as the portion of patients that experienced an objective response (complete response or partial response) as best response; DCR was defined as the portion of patients that experienced an objective response or stable disease as best response. Responses to treatment were evaluated according to RECIST criteria (version 1.0 before 2010 and version 1.1 subsequently).36,37 To properly assess responses, we planned two evaluations of ORR and DCR: at 3 and 6 months. The second evaluation was performed just in patients who experienced at least a stable diesease, according to RECIST criteria, at the 3 month evaluation, and in patients who underwent at least six consecutive cycles of therapy, without discontinuations for any cause (even planned surgeries). PFS was defined as the length of time between treatment commencement and disease progression or death (resulting from any cause) or to the last contact; OS was defined as the length of time between treatment commencement and death or to last contact. Toxicity was reported according to National Cancer Institute Common Toxicity Criteria (version 3.0 before 2011 and version 4.0 subsequently). Median rDI was computed “per cycle” as mg/m2/week. Data cut-off period was April 2018. Median PFS and median OS were evaluated using the Kaplan–Meier method.38 Median period of follow-up was calculated according to the reverse Kaplan–Meier method.39 In the subgroup analysis, Fisher’s exact test40 was used to compare ORR and log-rank test41 to compare PFS and OS among subgroups. All statistical analyses were performed using MedCalc Statistical Software version 18.2.1 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2018).
Ethical statement
Being a retrospective update of data previously collected in the Phase II study, together with those deriving from the subsequent clinical practice, this collection was not considered a clinical trial. Therefore, approval by institutional review boards was not required, although a notification was sent (normative ref Gazzetta Ufficiale della Repubblica Italiana n. 76 of 31-3-2008). All patients provided written, informed consent to the proposed treatment option. The procedures followed were in accordance with the precepts of Good Clinical Practice and the ethical standards of the local responsible committee on human experimentation (Comitato Etico per le province di L’Aquila e Teramo).
Results
Patients’ features
From February 2006 to February 2018, 85 consecutive mCRC patients were treated with first-line FIrB/FOx regimen (50 previously enrolled in the Phase II study and 35 subsequently treated in clinical practice): 58 with standard regimen and 27 with modified ones. Male/female ratio was 47/38 and median age was 62 years; 58 (68.2%) patients had ECOG-PS 0 and 27 (31.8%) had ECOG-PS ≥1. Among the 77 (90.6%) K/NRAS and BRAF evaluable patients, 21 (24.7%) were K/NRAS and BRAF wild type, 53 (62.4%) were K/NRAS mutant, and 3 (3.5%) were BRAF mutant. Clinical features of the overall population are summarized in Table 1.
Table 1.
Patients’ features
| Overall patients, n (%) | Standard FIrB/FOx, n (%) | Modified FIrB/FOx, n (%) | |
|---|---|---|---|
|
| |||
| Patients (n) | 85 | 58 | 27 |
| Age, years | |||
| Range | 40–73 | 40–73 | 44–73 |
| Median | 62 | 61 | 63 |
| Elderly (≥65 years) | 26 (30.6) | 16 (27.6) | 10 (37) |
| Sex | |||
| Male | 47 (55.3) | 31 (53.4) | 16 (59.2) |
| Female | 38 (44.7) | 27 (46.6) | 11 (40.8) |
| ECOG-PS | |||
| 0 | 58 (68.2) | 44 (75.9) | 14 (51.9) |
| 1 | 27 (31.8) | 14 (24.1) | 13 (48.1) |
| CIRS (comorbidity) | |||
| Primary | 30 (35.2) | 26 (44.8) | 4 (14.8) |
| Intermediate | 49 (57.7) | 29 (50.0) | 20 (74.1) |
| Secondary | 6 (7.1) | 3 (5.2) | 3 (11.1) |
| Metastatic disease | |||
| Metachronous | 15 (17.6) | 13 (22.4) | 2 (7.4) |
| Synchronous | 70 (82.4) | 45 (77.6) | 25 (92.6) |
| Primary tumor | |||
| Left | 60 (70.6) | 39 (67.2) | 21 (77.8) |
| Right | 25 (29.4) | 19 (32.8) | 6 (22.2) |
| Sites of metastasis | |||
| Liver | 55 (64.8) | 35 (60.3) | 20 (74) |
| Lung | 24 (28.2) | 16 (27.6) | 8 (29.6) |
| Peritoneum, Ascites | 17 (20.0) | 12 (20.7) | 5 (18.5) |
| Lymph nodes | 29 (34.1) | 21 (36.2) | 8 (29.6) |
| Other | 21 (24.7) | 20 (34.5) | 1 (3.7) |
| Liver-limited | 25 (29.4) | 14 (24.1) | 11 (40.7) |
| Primary rectal tumor | 13 (15.3) | 5 (8.6) | 8 (29.6) |
| un-resected | |||
| K/NRAS mutational status | |||
| Wild type | 21 (24.7) | 17 (29.3) | 4 (14.8) |
| Mutant | 53 (62.4) | 34 (58.6) | 19 (70.4) |
| Not available | 8 (9.4) | 7 (12.1) | 1 (3.7) |
| BRAF mutant patients | 3 (3.5) | – | 3 (11.1) |
Effectiveness analysis
Response to FIrB/FOx was not evaluable in two patients among 85: one patient had not yet evaluated the disease at the data cut-off and the other died during the second cycle (intestinal perforation in patient with peritoneal carcinomatosis). Activity analysis is summarized in Table 2. In the overall population, 3-month ORR was 75.9% (63/83) and 3-month DCR was 93.9% (78/83), while among the 56 evaluable patients, 6-month ORR was 55.3% (31/56) and 6-month DCR was 87.5% (49/56). As shown in Table 2, 3-month ORR/DCR and 6-month ORR/DCR were similar among patients treated with standard and modified regimens, while there was a tendency of a greater activity in K/NRAS wild-type patients compared to K/NRAS mutant ones, even if without statistically significant differences.
Table 2.
Three-month and 6-month activities based on standard/modified FIrB/FOx regimens and based on K/NRAS mutational status (binomial confidence interval)
| Overall | Standard FIrB/FOx | Modified FIrB/FOx | K/NRAS, BRAF wild type | K/NRAS mutant | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Enrolled patients | 85 | 100 | 58 | 100 | 27 | 100 | 21 | 100 | 53 | 100 |
| Evaluable (3 months) | 83 | 97.6 | 58 | 100 | 25 | 92.6 | 21 | 100 | 51 | 96.2 |
| Partial response Complete response |
55 8 |
38 6 |
17 2 |
19 1 |
31 7 |
|||||
| 3-month ORR | 75.9 (95% CI: 65.2–84.6) |
75.8 (95% CI: 62.8–86.1) |
76.0 (95% CI: 54.8–90.6) |
95.2 (95% CI: 76.1–99.8) |
74.5 (95% CI: 60.3–85.6) |
|||||
| P-value | – | P=1.0000 | P=0.0526 | |||||||
| Stable disease Progression disease |
15 5 |
10 4 |
5 1 |
1 – |
10 3 |
|||||
| 3-month DCR | 93.9 (95% CI: 86.5–98.0) | 93.1 (95% CI: 83.2–98.1) | 96 (95% CI: 79.6–99.9) | 100 (95% CI: nd) | 94.1 (95% CI: 83.7–98.7) | |||||
| P-value | – | P=1.0000 | P=0.5510 | |||||||
| Evaluable (6 months) | 56 | 65.8 | 35 | 60.3 | 21 | 84 | 14 | 66.6 | 36 | 70.6 |
| Partial response Complete response |
23 8 |
16 6 |
7 2 |
10 1 |
12 6 |
|||||
| 6-month ORR | 55.3 (95% CI: 41.4–68.6) | 62.8 (95% CI: 44.9–78.5) | 42.8 (95% CI: 21.8–65.9) | 78.5 (95% CI: 49.2–95.3) | 50 (95% CI: 32.9–67.1) | |||||
| P-value | – | P=0.1733 | P=0.0657 | |||||||
| Stable disease Progression disease |
18 7 |
8 5 |
10 2 |
3 – |
14 4 |
|||||
| 6-month DCR | 87.5 (95% CI: 75.9–94.8) | 85.7 (95% CI: 69.7–95.2) | 90.4 (95% CI: 69.6–98.8) | 100 (95% CI: nd) | 88.8 (95% CI: 73.9–96.8) | |||||
| P-value | – | P=0.6997 | P=0.5647 | |||||||
Abbreviations: DCR, disease control rate; nd, not defined; ORR, objective response rate.
Among the 24 evaluable patients with right-side primary tumor, 3-month ORR/DCR were 79.7% (95% CI: 67.1–89.0) and 93.2% (95% CI: 83.5–98.1), respectively, while among the 59 evaluable patients with left-side primary tumor, 3-month ORR/DCR were 66.7% (95% CI: 44.7–84.4) and 95.8% (95% CI: 78.8–99.8), respectively, without significant differences (data not shown). Median PFS of patients with right-side and left-side primary tumors were 12.8 months (95% CI: 8.8–19.3; 21 events) and 15.3 months (95% CI: 13.8–17.8; 51 events), respectively, without statistically significant difference (P=0.7044). Median OS of patients with right-side and left-side primary tumors were 20.1 months (95% CI: 13.9–55.4; 10 censored patients) and 37.8 months (95% CI: 26.7–47.7; 23 censored patients), respectively, without statistically significant difference (P=0.6943).
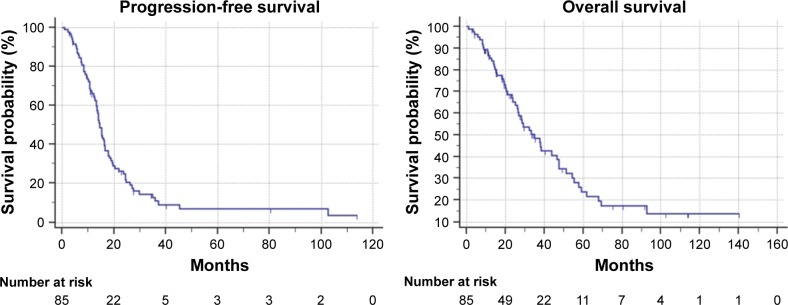
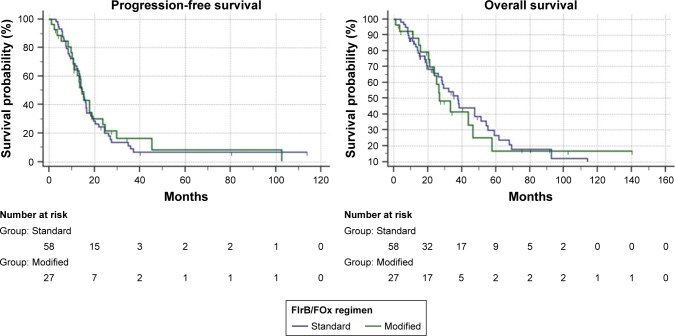
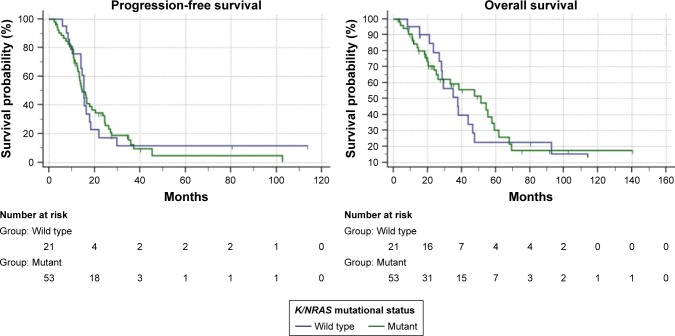
Table 3 summarizes survival analysis; in the overall population, after a median follow-up of 75.4 months (1–140), median PFS was 14.4 months (95% CI: 13.2–16.5) and median OS was 34.9 months (95% CI: 26.3–47.6) (Figure 2). Among the 58 patients who underwent first-line standard FIrB/Fox, median PFS was 14.4 months and median OS was 37.8 months. Among the 27 patients who underwent modified FIrB/Fox, median PFS was 14.4 months and median OS 26.6 months (Figure 3). Among the 21 K/NRAS wild-type patients, median PFS was 15.3 months and median OS was 37.8 months. Among the 53 K/NRAS mutant patients, median PFS was 14.4 months and median OS 51.4 months (Figure 4). As shown in Table 3, there were no statistically significant differences among subgroups.
Table 3.
Efficacy data of the overall population based on standard/modified FIrB/FOx regimens K/NRAS mutational status
| Overall | Standard FIrB/FOx | Modified FIrB/FOx | K/NRAS, BRAF wild type | K/NRAS mutant | |
|---|---|---|---|---|---|
|
| |||||
| Enrolled patients | 85 | 58 | 27 | 21 | 53 |
| Median PFS (months) | 14.4 | 14.4 | 14.4 | 15.3 | 14.4 |
| 95% CI | 13.2–16.5 | 13.2–16.5 | 10.9–18.7 | 13.9–17.7 | 12.8–20.2 |
| Progression events | 72 | 50 | 22 | 17 | 45 |
| P-value | – | P=0.8589 | P=0.8753 | ||
| Median OS (months) | 34.9 | 37.8 | 26.6 | 37.8 | 51.4 |
| 95% CI | 26.3–47.6 | 28.2–54.1 | 23.8–46.6 | 28.2–46.6 | 26.1–59.0 |
| Deaths | 52 | 37 | 15 | 15 | 28 |
| P-value | – | P=0.7746 | P=0.8527 | ||
Abbreviations: PFS, progression-free survival; OS, overall survival.
Figure 2.
Kaplan–Meier survival estimate of the overall treated patients: progression-free survival and overall survival.
Figure 3.
Kaplan–Meier (log-rank test) survival estimate: standard vs modified FIrB/FOx regimens.
Notes: Progression-free survival: 14.4 months vs 14.4 months; P=0.8589. Overall survival: 37.8 months vs 26.6 months; P=0.7746.
Figure 4.
Kaplan–Meier (log-rank test) survival estimate: K/NRAS wild-type vs K/NRAS mutant patients.
Notes: Progression-free survival: 15.3 months vs 14.4 months; P=0.8753. Overall survival: 37.8 months vs 51.4 months; P=0.8527.
Toxicity
All the patients were evaluable, but among the 438 administered cycles, only 426 were evaluable for toxicity. In the overall population, the most relevant treatment-related grade 3 adverse events were neutropenia (11.8%), diarrhea (17.6%), asthenia (9.4%), vomiting (5.6%), and hypertension (16.5%). No febrile neutropenia was reported. Grade 4 adverse events were leukopenia (1.2%), neutropenia (2.3%), and increased transaminases (1.2%). Granulocyte-colony stimulating factor (G-CSF) were used in case of grade 4 neutropenia. One death was suspected to be related to adverse event (bowel perforation in a patient with peritoneal carcinomatosis). Proteinuria was not mentioned because it was not reported in our records. All toxicity data are summarized in Table 4.
Table 4.
Cumulative toxicity of standard and modified FIrB/FOx regimens
| Overall patients | Standard FIrB/FOx | Modified FIrB/FOx | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Number | 85 | 58 | 27 | |||||||||
|
| ||||||||||||
| NCI-CTC Grade | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Nausea (%) | 39 (45.8) | 23 (27.1) | 2 (2.3) | – | 30 (51.7) | 16 (27.6) | 2 (3.4) | – | 9 (33.3) | 7 (25.9) | – | – |
| Vomiting (%) | 18 (21.1) | 11 (12.9) | 5 (5.6) | – | 10 (17.2) | 8 (13.8) | 4 (6.9) | – | 8 (29.6) | 3 (11.1) | 1 (3.7) | – |
| Diarrhea (%) | 33 (29.4) | 21 (24.7) | 15 (17.6) | – | 25 (43.1) | 13 (22.4) | 10 (17.2) | – | 8 (29.6) | 8 (29.6) | 5 (18.5) | – |
| Anorexia (%) | 11 (12.9) | 7 (8.2) | 2 (2.3) | – | 6 (10.3) | 3 (5.2) | 2 (3.4) | – | 5 (18.5) | 4 (14.8) | – | – |
| Mucositis (%) | 21 (24.7) | 5 (5.9) | 1 (1.2) | – | 17 (29.3) | 4 (6.9) | – | – | 4 (14.8) | 1 (3.7) | 1 (3.7) | – |
| Asthenia (%) | 26 (30.6) | 18 (21.2) | 8 (9.4) | – | 18 (31) | 10 (17.2) | 8 (13.8) | – | 8 (29.6) | 8 (29.6) | – | – |
| Increased | 9 (10.6) | 6 (7.1) | 2 (2.3) | 1 (1.2) | 8 (13.8) | 5 (8.6) | 2 (3.4) | 1 (1.7) | 1 (3.7) | 1 (3.7) | – | – |
| transaminases (%) | ||||||||||||
| Peripheral | 43 (50.6) | 18 (21.2) | – | – | 27 (46.5) | 10 (17.2) | – | – | 16 (59.2) | 8 (29.6) | – | – |
| neuropathy (%) | ||||||||||||
| Leukopenia (%) | 24 (28.2) | 24 (28.2) | 3 (3.5) | 1 (1.2) | 16 (27.6) | 18 (31) | 1 (1.7) | – | 8 (29.6) | 6 (22.2) | 2 (7.4) | 1 (3.7) |
| Neutropenia (%) | 18 (21.2) | 11 (12.9) | 10 (11.8) | 2 (2.3) | 15 (25.9) | 9 (15.5) | 8 (13.8) | 1 (1.7) | 3 (11.1) | 2 (7.4) | 2 (7.4) | 1 (3.7) |
| Febrile neutropenia (%) | – | – | – | – | – | – | – | – | – | – | – | – |
| Anemia (%) | 14 (16.5) | 6 (7.1) | 2 (2.3) | – | 14 (24.1) | 3 (5.2) | – | – | – | 3 (11.1) | 2 (7.4) | – |
| Thrombocytopenia (%) | 11 (12.9) | 3 (3.5) | – | – | 7 (12.1) | 2 (3.4) | – | – | 4 (14.8) | 1 (3.7) | – | – |
| Hypertension (%) | 23 (27.1) | 8 (9.4) | 14 (16.5) | – | 17 (29.3) | 5 (8.6) | 13 (22.4) | – | 6 (22.2) | 3 (11.1) | 1 (3.7) | – |
| Thromboembolic | 1 (1.2) | 1 (1.2) | 1 (1.2) | – | 1 (1.7) | 1 (1.7) | – | – | – | – | 1 (3.7) | – |
| events (%) | ||||||||||||
| Cardiac arrhythmia (%) | 1 (1.2) | 1 (1.2) | – | – | 1 (1.7) | 1 (1.7) | – | – | – | – | – | – |
| Wound | – | 2 (2.3) | – | – | – | 1 (1.7) | – | – | – | 1 (3.7) | – | – |
| complication (%) | ||||||||||||
| Epistaxis (%) | 16 (18.2) | 2 (2.3) | – | – | 14 (24.1) | 2 (3.4) | – | – | 2 (7.4) | – | – | – |
Abbreviation: NCI-CTC, National Cancer Institute Common Terminology Criteria.
Dose intensity
Among the overall population, the median number of administered cycles was six (range 1–14), as well as in standard (range 1–9) and modified (range 1–14) FIrB/FOx subgroups; among the 438 administered cycles, 426 were evaluable for dose intensities. Median rDIs per cycle in the overall population were as follows: irinotecan 64.8 (22.4–80) mg/m2/w (81% of standard DI), oxaliplatin 34.2 (11.4–40) mg/m2/w (85% of standard DI), 5-FU 1,465 (600–1,800) mg/m2/w (81% of standard DI), and bevacizumab 2.2 (0.2–2.5) mg/kg/w (88% of standard DI). Table 5 summarizes the rDIs according to standard and modified regimens.
Table 5.
Received dose intensities
| Overall FIrB/FOx | Standard FIrB/FOx | Modified FIrB/FOx | ||||
|---|---|---|---|---|---|---|
|
Received dose intensity/cycle – mg/m2(or kg)/week | ||||||
| Median (range) | % of standard full dose | Median (range) | % of standard full dose | Median (range) | % of standard full dose | |
|
| ||||||
| Bevacizumab | 2.2 (0.2–2.5) | 88 | 2.1 (0.2–2.5) | 84 | 2.2 (1–2.5) | 88 |
| Irinotecan | 64.8 (22.4–80) | 81 | 64.9 (22.4–80) | 81 | 64.4 (36–80) | 80 |
| Oxaliplatin | 34.2 (11.4–40) | 85 | 33.1 (11.4–40) | 83 | 34.4 (20–40) | 86 |
| 5-fluorouracil | 1,465 (600–1,800) | 81 | 1,476 (600–1,800) | 82 | 1,445 (600–1,800) | 80 |
Subsequent treatments
At the moment of data cut-off analysis, the induction phase with FIrB/FOx was ongoing in three patients. After first-line FIrB/FOx, 24 (28.2%) patients underwent ablative locoregional treatments or surgeries. Overall, 14 (16.5%) patients underwent hepatic surgery (alone or in combination), nine (10.6%) primary tumor or locoregional recurrence resections, two (2.3%) lung metastasectomies, three (3.5%) rectal primary tumors radiation therapies followed by surgical resection, one (1.2%) radiation therapy to ret-roperitoneal lymph nodes, and three (3.5%) synchronous surgery of liver metastases and primary tumor. Overall, just eight (9.4%) patients maintained their therapy with 5-FU (or capecitabine) plus bevacizumab after FIrB/FOx induction. Among the 72 patients who progressed after first–line therapy, 51 (70.8%) underwent a second-line chemotherapy: 14 (27.4%) rechallenged with FIrB/FOx, 13 (25.5%) EGFR inhibitor-based regimen, 9 (17.6%) other bevacizumab-based regimens, and 6 (11.8%) aflibercept-based regimens (Table 6). Twenty-seven patients underwent a third-line chemotherapy and 13 patients a fourth-line therapy.
Table 6.
Subsequent systemic treatments after first-line FIrB/FOx
| Overall (85 patients) | |
|---|---|
|
| |
| Maintenance therapy (5-fluorouracil/ | 8 (9.4) |
| capecitabine–bevacizumab), n (%) | |
| Progression events, n | 72 (84.7) |
| Second-line chemotherapy, n (%) | 51 (70.8) |
| FIrB/FOx rechallenge | 14 (27.4) |
| AntiEGFR-based regimens | 13 (25.5) |
| Other bevacizumab-based regimens | 9 (17.6) |
| Aflibercept-based regimens | 6 (11.8) |
| Third-line systemic therapy, n (%) | 27 (37.5) |
| Fourth-line systemic therapy, n (%) | 13 (18.1) |
Discussion
The major problem of combination regimens is the designing of the proper schedule, which should ensure the balance between dose intensity, efficacy, and tolerability of each drug. Few institutions have begun to use intensive regimens made of triplet chemotherapy plus bevacizumab in their clinical practice; the significant increase in efficacy is obtained at the cost of a greater toxicity and this has probably led clinicians to perceive a poor reproducibility of these regimens in the “real-life” setting.
In our case series, 55 patients (64.7%) had one or more significant comorbidities and 26 patients (30.6%) were elderly (from 65 to 74 years old). We achieved comparable results between patients treated with standard and those treated with modified FIrB/FOx (due to age, PS, and/or comorbidities), thanks to the awareness that intensive regimens require patients to be carefully monitored during their treatment. As shown in Table 1, among patients treated with modified regimens, 48.1% had ECOG-PS 1, whereas among the patients treated with standard FIrB/FOx, 24.1% had ECOG-PS 1. Similarly, 85.2% of patients treated with modified FIrB/FOx had an intermediate or secondary CIRS stage, while it was 55.2% among patients who were treated with the standard regimen.
This update comes from our clinical practice, and as a reflection that not every mCRC patient can be treated with a four-drug regimen, patients had been enrolled over a period of 12 years. To better explain the slowdown of the accrual after the Phase II study, we have to consider that the advent of EGFR inhibitors has changed the game. K/NRAS wild-type patients were treated more and more with EGFR inhibitor–based regimens, even more considering that a clinical trial of FIrB/FOx regimen combined with cetuximab (EudraCT 2009-016793-32) has been ongoing since 2009 at our institution.
Table 7 summarizes the most relevant Phase II and Phase III trials of triplet chemotherapy regimens, alone and in combination with either EGFR inhibitors or bevacizumab, together with the Phase II study of FIrB/FOx and this update. Even though is it not proper to compare results of different studies, and considering all the limitations of our experience (retrospective/observational nature, sample size, single institution, selection bias, absence of radiological updated revision of responses), we beg to make some speculations. Global incidence of G3/G4 neutropenia with FIrB/FOx is by far the lowest, as well as the incidence of G3/G4 diarrhea, even more considering that no G4 diarrhea was observed. FIrB/FOx showed a good safety profile, even looking at G1/G2 ones, without significant differences between patients treated with standard and modified regimens. According to the good safety profile, rDIs were ≥80% of standard doses for each drug in overall population and in both standard and modified FIrB/FOx subgroups, confirming that dose adjustments allow an effective chemotherapy administration, even in more frail patients, who are frequent in the “real-life” setting.
Table 7.
Comparison of clinical trials of triplet chemotherapies plus bevacizumab
| Trial (ref) | Type of study | Regimen | Study population | Primary end point | ORR (%) | mPFS (months) | mOS (months) | Neutropenia (%) G3/G4* (exp arm) | Diarrhea (%) G3/G4* (exp arm) | Enrolled patients |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Souglakos et al7 | Randomized, Phase III, prospective | FOLFOXIRI vs FOLFIRI | mCRC, genotype unselected | OS | 43 | 8.4 | 21.5 | 35 | 27.7 | 137: exp arm; 146: control arm |
| Falcone et al8 | Randomized, Phase III, | FOLFOXIRI vs | mCRC, genotype unselected | Response rate | 66 | 9.8 | 22.6 | 50 | 20 | 122: exp arm; |
| prospective | FOLFIRI | 122: control arm | ||||||||
| Ychou et al9 | Single-arm, Phase II, prospective | FOLFIRINOX | Liver-limited, genotype unselected | R0 resection rate | 70.6 | 13.9 | 36 | 64.7 | 29.4 | 34 |
| Fornaro et al12 | Sinlge-arm, Phase II, prospective | mFOLFOXIRI- Panitumumab | mCRC, K/NRAS, and BRAF wild type | ORR | 89 | 11.3 | NR | 48.6 | 35.1 | 37 |
| Saridaki et al13 | Single-arm, Phase II, prospective | mFOLFOXIRI- Cetuximab | mCRC, KRAS wild type | ORR | 70 | 10.2 | 30.3 | 23.3 | 53 | 30 |
| Assenat et al14 | Single-arm, Phase II, prospective | FOLFIRINOX- Cetuximab | mCRC, genotype unselected | CR rate | 80.9 | 9.5 | 24.7 | 38 | 52 | 42 |
| MACBETH15 | Randomized, Phase II, prospective | mFOLFOXIRI- Cetuzimab (induction) | mCRC, K/NRAS, and BRAF wild type | 10 mPFR | 76 | 11.2 | NA | 33 | 19 | 116 |
| OLIVIA17 | Randomized, Phase II, prospective | mFOLFOX-6-Bev vs FOLFOXIRI-Bev | Liver-limited, genotype unselected | Resection rate | 82 | 18.6 | NR | 50 | 30 | 41: exp arm; |
| 39: control arm | ||||||||||
| OPAL18 | Single-arm, Phase II, prospective | FOLFOXIRI-Bev | mCRC, genotype | PFS | 64 | 11.1 | 32.2 | 26 | 11 | 90 |
| unselected | ||||||||||
| TRIBE19,24 | Randomized, Phase III, prospective | FOLFOXIRI-Bev vs | mCRC, genotype | PFS | 65 | 12.3 | 29.8 | 50 | 18.8 | 252: exp arm; |
| FOLFIRI-Bev | unselected | 256: control arm | ||||||||
| Cheng et al46 | Retrospective, two arm | FOLFOXIRI-Bev vs XELOXIRI-Bev | mCRC, genotype unselected | – | 71 | 13.5 | 31.3 | 46.4 | 20.3 | 69: exp arm; 69: control arm |
| Poker34 | Single-arm, Phase II, prospective | Standard FIrB/FOx | mCRC, genotype unselected | ORR | 82 | 12.0 | 28.0 | 10 | 28 (G3) | 50 |
| FIrB/FOx | Retrospective, | Standard and | mCRC, genotype | – | 75.9 | 14.4 | 34.9 | 14.1 | 17.6 (G3) | 85 |
| UPDATE | single arm | modulated FIrB/FOx | unselected | |||||||
Note:
Grading of adverse events according to the National Cancer Institute Common Terminology Criteria.
Abbreviations: Bev, bevacizumab; exp, experimental; ORR, objective response rate; NA, not available; NR, not reached; PFS, progression-free survival; mCRC, metastatic colorectal cancer; mPFS, mean progression-free survival; mOS, mean overall survival.
We can say that bevacizumab is the preferred biological combination partner for intensive chemotherapy because of the absence of cumulative toxicities, even if it adds distinctive ones. In clinical practice, with “less selected patients”, we had to pay particular attention to these class-specific toxicities. An early G3 pulmonary embolism, two G2 fistulas, and 14 G3 hypertension differed from what was reported in the Phase II study of FIrB/FOx [34]. These occurrences, together with the 23 scheduled surgeries, could explain the 2.2 mg/kg/w (range: 0.2–2.5) bevacizumab rDI.
In our experience, just eight patients maintained their therapy with 5-FU (or capecitabine) plus bevacizumab after the induction with FIrB/FOx, and the median number of administered cycles was six in both subgroups. Longest treatment duration was 14 months among patients treated with modified regimens and 9 months among those treated with standard ones. With the “right patient”, standard FIrB/FOx is a well-tolerated treatment, which could be administered for a long period.
In the study population, the predictive and prognostic roles of K/NRAS mutations and of primary tumor location42 were not confirmed, given the absence of statistically significant differences in ORR, PFS, and OS among subgroups. However, we must recognize that the sample size could have affected these results.
Our analysis showed that FIrB/FOx regimen is a valid option for a multimodal approach to mCRC patients. The 3-month ORR of 75.9% shows that FIrB/FOx regimen is suitable in the conversion setting. Indeed, 24 patients overall (28.2%) underwent ablative locoregional treatments or surgeries after first-line FIrB/FOx. The 6-month ORR of 55.3% suggests that it leads to long-lasting responses even in “not radicalizable” patients. Thanks to this differentiated analyses, we could roughly estimate how much chemotherapy and other subsequent locoregional treatments count in the “effectiveness” (PFS in clinical practice is influenced not only by chemotherapy but also by surgeries and locoregional treatments).
One of the most frequent criticisms of intensive regimens is that if four drugs are used in the first-line setting, patients will suffer from a lack of therapeutic options at the moment of a second-line chemotherapy. On the other hand, among 72 patients who progressed after first-line FIrB/FOx, 51 patients (70.8%) underwent a second-line chemotherapy, 27 patients a third-line chemotherapy, and 13 patients a fourth-line chemotherapy. Another criticism of FIrB/FOx regimen is the weekly administration. Although it represents a greater stress for both patients and their families, and a greater workload for outpatient clinics, it has allowed us to carefully monitor treatment and adverse events. To prove that knowing how to improve therapeutic strategies in this setting is still a subject of interest; several studies are ongoing on the topic. The CHARTA study is a randomized, Phase II trial that compares FOLFOXIRI plus bevacizumab with FOLFOX plus bevacizumab as first-line treatment. The primary end point is 9-month median PFS and, interestingly, health-related quality of life is among the secondary end points which may add information regarding patients-reported outcomes of such intensive regimens.43 In the PERIMAX study, patients previously untreated for liver metastatic disease were randomized to resection followed by postoperative FOLFOX or perioperative (pre- and post-resection) FOLFOXIRI plus bevacizumab for 3 months. However, the study was withdrawn due to insufficient recruitment.43 The STEAM trial is a Phase II study, which investigates the concurrent or sequential FOLFOXIRI (alternating treatment every two cycles of FOLFOX and FOLFIRI) plus bevacizumab with FOLFOX plus bevacizumab in patients with previously untreated mCRC.44 The Phase III trial TRIBE-2 compares two different therapeutic strategies as first-line treatment for mCRC patients: FOLFOXIRI plus bevacizumab, followed by the reintroduction of FOLFOXIRI plus bevacizumab at progression of disease, and FOLFOX plus bevacizumab, followed by FOLFIRI plus bevacizumab at progression of disease.45
In order to confirm the better manageability of TFI/5-FU and reduce the workload that a weekly regimen requires, we planned a single-arm, multicenter, Phase II prospective study of the so-called TFI/FOXIRI-bevacizumab regimen, as first-line treatment of K/NRAS and BRAF unselected mCRC patients. The schedule consists of TFI/5-FU at dose of 800 mg/m2/night (12-hour infusion for four consecutive nights, 3200 mg/m2, days 1–4), combined with infusion of irinotecan (dose finding: 120–140–160 mg/m2), oxaliplatin (80 mg/m2), and bevacizumab (5 mg/kg) every 2 weeks, without folinic acid and 5-FU bolus. Prophylactic administration of G-CSF was also planned (approval number: 2025 of 16/11/2017, “Direzione generale ASL1 Abruzzo, Comitato Etico per le province di L’Aquila e Teramo” – NCT identifier not yet assigned – EudraCT number 2017-004789-91).
Conclusion
This update confirms that FIrB/FOx regimen is a feasible option for first-line treatment of mCRC patients also in the “real-life” setting. Dose modulation allowed to reach comparable clinical outcomes in frail patients, regardless of the K/NRAS genotype. Thanks to the TFI/5-FU and the weekly alternating rate, we achieved similar results to those of clinical trials, with a good safety profile. Not every mCRC patient can be treated with a four-drug regimen and not every cancer care center has the clinical skills to manage these treatments, but the more you handle such complex regimens, the better your everyday practice becomes. Other studies are still needed in this setting: the Phase II study of TFI/FOXIRI-bevacizumab will try to answer some of the open questions on the topic.
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: The addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23(16):3706–3712. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 3.Sobrero A, Ackland S, Clarke S, et al. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology. 2009;77(2):113–119. doi: 10.1159/000229787. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 5.Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE study. J Clin Oncol. 2008;26(21):3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 6.van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20(11):1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 7.Souglakos J, Androulakis N, Syrigos K, et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG) Br J Cancer. 2006;94(6):798–805. doi: 10.1038/sj.bjc.6603011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25(13):1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 9.Ychou M, Viret F, Kramar A, et al. Tritherapy with fluorouracil/leucovorin, irinotecan and oxaliplatin (FOLFIRINOX): a phase II study in colorectal cancer patients with non-resectable liver metastases. Cancer Chemother Pharmacol. 2008;62(2):195–201. doi: 10.1007/s00280-007-0588-3. [DOI] [PubMed] [Google Scholar]
- 10.Folprecht G, Hamann S, Schütte K, et al. Dose escalating study of cetuximab and 5-FU/folinic acid (FA)/oxaliplatin/irinotecan (FOLF-OXIRI) in first line therapy of patients with metastatic colorectal cancer. BMC Cancer. 2014;14:521. doi: 10.1186/1471-2407-14-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garufi C, Torsello A, Tumolo S, et al. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer. 2010;103(10):1542–1547. doi: 10.1038/sj.bjc.6605940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fornaro L, Lonardi S, Masi G, et al. FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO) Ann Oncol. 2013;24(8):2062–2067. doi: 10.1093/annonc/mdt165. [DOI] [PubMed] [Google Scholar]
- 13.Saridaki Z, Androulakis N, Vardakis N, et al. A triplet combination with irinotecan (CPT-11), oxaliplatin (LOHP), continuous infusion 5-fluorouracil and leucovorin (FOLFOXIRI) plus cetuximab as first-line treatment in KRAS WT, metastatic colorectal cancer: a pilot Phase II trial. Br J Cancer. 2012;107(12):1932–1937. doi: 10.1038/bjc.2012.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assenat E, Desseigne F, Thezenas S, et al. Cetuximab plus FOLFIRINOX (ERBIRINOX) as first-line treatment for unresectable metastatic colorectal cancer: a phase II trial. Oncologist. 2011;16(11):1557–1564. doi: 10.1634/theoncologist.2011-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antoniotti C, Cremolini C, Loupakis F, et al. Modified FOLFOXIRI (mFOLFOXIRI) plus cetuximab (cet), followed by cet or bevacizumab (bev) maintenance, in RAS/BRAF wild-type (wt) metastatic colorectal cancer (mCRC): Results of the phase II randomized MACBETH trial by GONO. J Clin Oncol. 2016;34(15 Suppl):3543. [Google Scholar]
- 16.Masi G, Loupakis F, Salvatore L, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 2010;11(9):845–852. doi: 10.1016/S1470-2045(10)70175-3. [DOI] [PubMed] [Google Scholar]
- 17.Gruenberger T, Bridgewater J, Chau I, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26(4):702–708. doi: 10.1093/annonc/mdu580. [DOI] [PubMed] [Google Scholar]
- 18.Stein A, Atanackovic D, Hildebrandt B, et al. Upfront FOLFOXIRI + bevacizumab followed by fluoropyrimidin and bevacizumab maintenance in patients with molecularly unselected metastatic colorectal cancer. Br J Cancer. 2015;113(6):872–877. doi: 10.1038/bjc.2015.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371(17):1609–1618. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 20.Richman SD, Seymour MT, Chambers P, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27(35):5931–5937. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 21.Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 22.van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692–700. doi: 10.1200/JCO.2014.59.4812. [DOI] [PubMed] [Google Scholar]
- 23.Tie J, Gibbs P, Lipton L, et al. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF (V600E) mutation. Int J Cancer. 2011;128(9):2075–2084. doi: 10.1002/ijc.25555. [DOI] [PubMed] [Google Scholar]
- 24.Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 tribe study. Lancet Oncol. 2015;16(13):1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 25.Ficorella C, Ricevuto E, Morelli MF, et al. Increased tolerability of bimonthly 12-hour timed flat infusion 5-fluorouracil/irinotecan regimen in advanced colorectal cancer: a dose-finding study. Oncol Rep. 2006;15(5):1345–1350. doi: 10.3892/or.15.5.1345. [DOI] [PubMed] [Google Scholar]
- 26.Ficorella C, Bruera G, Cannita K, et al. Triplet chemotherapy in patients with metastatic colorectal cancer: toward the best way to safely administer a highly active regimen in clinical practice. Clin Colorectal Cancer. 2012;11(4):229–237. doi: 10.1016/j.clcc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Leichman CG, Fleming TR, Muggia FM, et al. Phase II study of fluorouracil and its modulation in advanced colorectal cancer: a Southwest Oncology Group study. J Clin Oncol. 1995;13(6):1303–1311. doi: 10.1200/JCO.1995.13.6.1303. [DOI] [PubMed] [Google Scholar]
- 28.Schmoll H, Köhne C, Lorenz M, et al. Weekly 24-h infusion of high-dose 5-fluorouracil with or without folinic acid vs bolus 5-FU/FA (NCCTG/Mayo) in advanced colorectal cancer: a randomized phase III study of the EORTC GITCCG and the AIO. Proc Am Soc Clin Oncol 10. 2000;287:a935. [Google Scholar]
- 29.O’Dwyer PJ, Manola J, Valone FH, et al. Fluorouracil modulation in colorectal cancer: lack of improvement with N-phosphonoacetyl-l-aspartic acid or oral leucovorin or interferon, but enhanced therapeutic index with weekly 24-hour infusion schedule-an Eastern Cooperative Oncology Group/Cancer and Leukemia Group B Study. J Clin Oncol. 2001;19(9):2413–2421. doi: 10.1200/JCO.2001.19.9.2413. [DOI] [PubMed] [Google Scholar]
- 30.Lévi F. Chronopharmacology of anticancer agents. In: Redfern PH, Lemmer B, editors. Handbook of Exeperimental Pharmacology: Physiology and Pharmacology of Biological Rhythms – Cancer Chemotherapy. Berlin: Springer-Verlag; 1997. pp. 299–301. [Google Scholar]
- 31.Harris BE, Song R, Soong SJ, Diasio RB. Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion. Cancer Res. 1990;50(1):197–201. [PubMed] [Google Scholar]
- 32.Smaaland R, Abrahamsen JF, Svardal AM, Lote K, Ueland PM. DNA cell cycle distribution and glutathione (GSH) content according to circadian stage in bone marrow of cancer patients. Br J Cancer. 1992;66(1):39–45. doi: 10.1038/bjc.1992.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morelli MF, Santomaggio A, Ricevuto E, et al. Triplet schedule of weekly 5-fluorouracil and alternating irinotecan or oxaliplatin in advanced colorectal cancer: a dose-finding and phase II study. Oncol Rep. 2010;23(6):1635–1640. doi: 10.3892/or_00000805. [DOI] [PubMed] [Google Scholar]
- 34.Bruera G, Santomaggio A, Cannita K, et al. “Poker” association of weekly alternating 5-fluorouracil, irinotecan, bevacizumab and oxaliplatin (FIr-B/FOx) in first line treatment of metastatic colorectal cancer: a phase II study. BMC Cancer. 2010;10(1):567. doi: 10.1186/1471-2407-10-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16(4):1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 36.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for research and treatment of cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 37.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 39.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 40.Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J R Stat Soc. 1922;85(1):87–94. [Google Scholar]
- 41.Peto R, Peto J. Asymptotically efficient RANK invariant test procedures. J R Stat Soc Ser A. 1972;135(2):185–206. [Google Scholar]
- 42.Venook AP. Right-sided vs left-sided colorectal cancer. Clin Adv Hematol Oncol. 2017;15(1):22–24. [PubMed] [Google Scholar]
- 43.Stein A, Glockzin G, Wienke A, et al. Treatment with bevacizumab and FOLFOXIRI in patients with advanced colorectal cancer: presentation of two novel trials (CHARTA and PERIMAX) and review of the literature. BMC Cancer. 2012;12:356. doi: 10.1186/1471-2407-12-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.STEAM A study of sequential and concurrent FOLFOXIRI/Avastin (bevacizumab) regimens versus FOLFOX/Avastin in first-line in patients with metastatic colorectal cancer. [Accessed January 4, 2019]. Available from: https://clinicaltrials.gov/ct2/show/NCT01765582.
- 45.Cremolini C, Marmorino F, Loupakis F, et al. TRIBE-2: a phase III, randomized, open-label, strategy trial in unresectable metastatic colorectal cancer patients by the GONO group. BMC Cancer. 2017;17(1):408. doi: 10.1186/s12885-017-3360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng Y, Song W. Efficacy of FOLFOXIRI versus XELOXIRI plus bevacizumab in the treatment of metastatic colorectal cancer. Int J Clin Exp Med. 2015;8(10):18713–18720. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.