Abstract
Objective:
Studies have linked self-reported discrimination to telomere attrition, a biological marker of accelerated cellular aging. However, it is unknown whether intersections between social categories—race, socioeconomic status (SES), sex, and age—influence the association of varying forms of discrimination with telomere length. We examined these associations in a socioeconomically and racially/ethnically diverse urban sample.
Methods:
Cross-sectional data were from 341 middle-aged (30–64 years) African American and White, community participants in the Healthy Aging in Neighborhoods of Diversity across the Life Span Study (HANDLS). Multiple regression models examined up to 3-way interactions between a discrimination measure (i.e., everyday, racial, gender, lifetime burden, and frequency of discrimination across sources) and two social categories.
Results:
After adjusting for depressive symptoms, waist circumference, and lifetime substance use, two themes emerged: 1) among women with higher SES, a) greater lifetime discrimination burden (b = −0.23, p = .011), gender discrimination (b = −0.29, p = .040), and racial discrimination (b = −0.24, p = 0.023) and 2) among younger adults, irrespective of race and sex, greater frequency of discrimination across sources (b = 0.002, p = .008) was associated with shorter telomeres.
Conclusions:
Irrespective of race, women with higher SES and younger adults reporting greater discrimination may be at particular risk for accelerated aging. Telomere attrition promotes and accelerates chronic health conditions for which there are health disparities. Future research explicating intersections among specific discrimination indices and social categories is warranted.
Keywords: Discrimination, Race, Socioeconomic status, Sex, Age, Telomere attrition
1. Introduction
In the United States (U.S.), social stress is a pervasive aspect of daily life for many individuals. Indeed, social stressors are particularly persistent along the lines of marginalized statuses associated with race, age, sex, and socioeconomic status (SES) and are linked to a myriad of aging-related poorer health (e.g., Bosworth, 2018; Cunningham et al., 2017; Meyer, 2003; Schnittker & McLeod, 2005; Williams & Jackson, 2005). Importantly, the cellular mechanisms underlying these linkages remain largely understudied (Epel, 2009). Telomeres represent one such plausible pathway.
Within human somatic cells, telomeres consist of tandem repeats of the TTAGGG DNA sequence as well as specific associated proteins (Chan & Blackburn, 2004). Located at the ends of each chromosome, telomeres confer protection to the underlying genetic material, and thus help safeguard genetic stability within the cell. However, recurring cellular replication, the absence of telomerase activity within human somatic cells, and chronic stress exposure together contribute to a reduction in telomere length (Epel, 2009). Consequently, critically shortened telomeres not only compromise genetic stability within the cell, but also promotes cellular senescence, and ultimately, apoptosis (Calado & Young, 2009; Chan & Blackburn, 2004). Telomere attrition has been prospectively associated with all-cause mortality and morbidity across several disease endpoints, including cancer and cardiovascular disease (Epel et al., 2009; Haycock et al., 2014).
With respect to the contribution of chronic stress exposure to cellular apoptosis, telomeres have been conceptualized as “psychobio-markers,” or biological indices of psychosocial stress (Epel, 2009). A body of work has demonstrated the adverse linkage of psychosocial stress to telomere length across various forms of adversity. Meta-analyses and systematic reviews highlight that stress arising from psychiatric illness, early life adversity, violence exposure, caregiver strain, life events (e.g., divorce or death of a loved one), and poverty contribute to shortened telomeres (Darrow et al., 2016; Mathur et al., 2016; Oliveira et al., 2016; Ridout, Ridout, Price, Sen, & Tyrka, 2016, 2018; Schutte & Malouff, 2016). Importantly, these findings demonstrate that chronic sources of stress may have long-lasting consequences for health as reflected in accelerated biological aging.
Discrimination, is a specific type of chronic stressor reflecting unfair treatment unfolding in interpersonal interactions. Discrimination has been established as a potent and deleterious factor in mental and physical health disparities (Paradies, 2006; Paradies et al., 2015; Pascoe & Smart Richman, 2009). Discriminatory experiences typically vary along the lines of race and ethnicity (hereafter race), age, sex, and SES, paralleling sociohistorical demarcations of social categories in the U.S. (Kessler, Mickelson, & Williams, 1999). Similarly, disparities in health also vary along these established lines, with social categories functioning as robust predictors of disease endpoints (U.S. Department of Health & Human Services, 2014). Recent evidence suggests that telomere attrition is inversely associated with self-reported discrimination and also varies by sociodemographic category.
1.1. Linkages across types of discrimination and telomere length
Two recent reports from the Health and Retirement Study show that different forms of discrimination are linked to telomere length in older (> 50 years) African Americans. First, in analyses exclusive to African Americans, major lifetime discrimination (e.g., not being hired for a job) – but not everyday discrimination (e.g., being treated with less courtesy in day-to-day life) – was inversely linked to telomere length (Lee, Kim, & Neblett, 2017). However, in race-stratified analyses, everyday discrimination – but not major life discrimination – was inversely linked to telomere length in African Americans, but not Whites (Liu & Kawachi, 2017). Some studies of discrimination and telomere length have reported null effects (Geronimus et al., 2015). Others examining specific forms of discrimination, principally racial discrimination, have documented inverse associations with telomere length conditional upon psychological factors, including greater depressive symptoms and perceptions of Anti-Black bias, in middle-aged African American men (Chae et al., 2014, 2016), or as part of a broader stress construct in pregnant Mexican-American women (Ruiz, Trzeciakowski, Moore, Ayers, & Pickler, 2017). Altogether, these findings provide initial evidence that self-reported discrimination may be implicated in the acceleration of telomere attrition.
1.2. Variations in telomere length as a function of social categories
Research has demonstrated sociodemographic variations in telomere length, which do not consistently reflect established variations in U.S. health disparities. For instance, some studies report that African Americans have longer telomeres than Whites from birth into adulthood (e.g., Rewak et al., 2014) but show a greater accelerated decline in older age (Hunt et al., 2008). Yet, there is also evidence that in middle to older age, African Americans have longer telomeres than Whites (Needham et al., 2013). With regard to sex, a meta-analysis demonstrated that men typically have shorter telomeres (Gardner et al., 2014); however, a study in middle-aged to older adults indicated that compared to Whites and men, African American women had the greatest attrition over time (Diez Roux et al., 2009). Similarly, middle-aged African American women were biologically 7.5 years older than White women of the same chronological age assessed by telomere length (Geronimus et al., 2010). While the overall evidence regarding SES and telomere length shows weak or null effects (Robertson et al., 2013), there is evidence that African Americans with higher SES have longer telomeres compared to Whites across all SES levels and African Americans with lower SES (Adler, 2013). For instance, data from the National Health and Nutrition Examination Survey (NHANES) demonstrated that less education was associated with shorter telomeres in African American and Whites, but no associations were observed with income. However, less income has been associated with shorter telomeres in midlife African American men (Schrock et al., 2018). Taken together, these data demonstrate variations in telomere length by social category, some of which are inconsistent with established health disparities. It is unknown whether the associations of these social statuses with telomeres are influenced by social factors such as discrimination.
1.3. Rationale for present study
The present study examines self-reported discrimination and social categories to understand if their interaction yields differential patterning in relation to telomere length. We seek to extend existing research in two ways. First, drawing upon an intersectionality framework, we examine the linkage of discrimination to telomere attrition as conditional upon multiple social categories, specifically, race, SES, age, and age. Health disparities research has begun to use this framework to highlight how interdependent social categories simultaneously converge to inform lived experiences, and in turn, shape health (Williams et al., 2012). Further, the Healthy People 2020 U.S. objectives set forth by the U.S. Department of Health and Human Services (2014), highlight the need for research on social statuses and discrimination to further elucidate health disparities. A prior report on discrimination and telomere length (Lee et al., 2017) observed that neither age nor sex moderated the associations between major life discrimination and telomere length in older African Americans. Perhaps concurrently considering age and sex alongside other social statuses may reveal different effects. Indeed, our group recently published a report using the present study’s sample examining interactive relations between discrimination and sociodemographic variables with telomere length in race-stratified (i.e., within-race) analyses (Pantesco et al., 2018). Findings revealed within-race associations between discrimination and telomere length in African Americans and Whites that varied by age, sex, and/or SES. In light of previous research stressing the importance of examining both within-race and between-race effects in health disparities research (Whitfield, Allaire, Belue, & Edwards, 2008), the present study will expand on our previous work by examining these trends across both African Americans and Whites, including potential moderating effects of race.
Second, we extend the prior research by using a comprehensive examination of discrimination. Prior telomere reports have either focused explicitly on discrimination (e.g., major and/or everyday discrimination; Lee et al., 2017) or attributions for that discrimination (e.g., race, ancestry, or national origin; Liu & Kawachi, 2017 or racial discrimination (Chae et al., 2014, 2016;). Discrimination, however, is a multidimensional construct composed of various forms, experiences, and magnitudes, which in turn, may yield different links with telomere attrition. Health disparities scholars have strongly recommended examining a fuller spectrum of interpersonal discrimination. Although various forms of interpersonal discrimination would be expected to be moderately interrelated, they may also capture unique aspects of the experience of discrimination when concurrently assessed (Krieger, 2014; Lewis, Cogburn, & Williams, 2015). To this end, we assess three categories of interpersonal discrimination; 1) day-to-day, social status non-specific unfair treatment (everyday discrimination) 2) lifetime, social status specific (frequency of discrimination across sources, racial and gender discrimination), and 3) lifetime burden, social status nonspecific (lifetime discrimination burden). Whereas everyday discrimination assesses unfair treatment irrespective of the reason or attribution for the experience, and the lifetime burden measure captures the weight of an individual’s full experience with discrimination, the assessments of social status specific forms of discrimination – e.g., racial and gender discrimination – reflect discrimination rooted in power differentials related to the sociohistorical marginalization of the targeted individual as a function of their low status group membership. To our knowledge, health disparities research has yet to concurrently examine multiple forms of discrimination within the context of social categories. We propose that there are interactive relations of each form of self-reported discrimination with race, SES, age, and sex in relation to telomere attrition. Directional hypotheses were not proposed a priori given the exploratory nature of the research.
2. Methods
2.1. Participants and procedure
Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) is an ongoing longitudinal study of disparities in health and disease attributable to race and SES. Evans et al. (2010) have previously detailed the design of the HANDLS study. Briefly, HANDLS participants are a fixed cohort of urban-dwelling adults, recruited via household screenings from an area probability sample of 13 census segments in the city of Baltimore, Maryland. The census segments were pre-determined for their likelihood of yielding representative samples of individuals who were African American and White, men and women, and with adjusted household incomes above and below 125% of the 2004 U.S. Department of Health and Human Services poverty guidelines. HANDLS participants self-identified as African American or White and were between 30–64 years of age at baseline. The Institutional Review Board at the National Institute of Environmental Health Sciences approved the HANDLS study protocol. After initial selection, potential participants were excluded from HANDLS if they met any of the following criteria at baseline: (1) outside of the age range of 30–64 years, (2) currently pregnant, (3) within six months of active cancer treatment (i.e., chemotherapy, radiation, or biological treatments), (4) diagnosed with AIDS, (5) unable to provide informed consent, (6) unable to provide data for at least five measures, (7) unable to provide valid government-issued identification or were currently without a verifiable address, (8) had uncontrolled high blood pressure (> 160/100).
The first wave of HANDLS occurred between 2004–2009 and consisted of two phases: (1) recruitment, written informed consent, and an interview in participants’ homes, and (2) medical history, physical examination, and other assessments on mobile medical research vehicles parked within participants’ neighborhoods (Evans et al., 2010). Of the 3720 participants selected for the original HANDLS cohort, 2802 completed both phases. A subset of these participants consented to DNA collection, of whom 360 with DNA in the biorepository from Waves 1 and 3 were randomly selected from a cross of race, sex, and baseline age (median-split) for telomere assays. The present study included 341 participants with valid data for relevant variables. Analysis-specific sample sizes varied slightly due to missing data on the different discrimination measures, ranging from 338 to 341 participants. A power analysis using the G*Power software (version 3.1; for more information, see Faul, Erdfelder, Lang, & Buchner, 2007) revealed that all analysis-specific sample sizes were adequately powered (1 – β = 0.80) to detect a small–medium Cohen’s effect size of f 2 = 0.06 for the present analyses, which included a maximum of 12 predictor variables (see Statistical Analyses for a description of the regression models).
2.2. Measures
2.2.1. Discrimination
2.2.1.1. Everyday Discrimination.
The Everyday Discrimination scale (Williams, Yu, Jackson, & Anderson, 1997) is a nine-item measure assessing the frequency of routine experiences of unfair treatment which does not require an explicit attribution (e.g., race) for the experience. Some examples of items are “being treated with less courtesy,” “getting worse service at stores,” or “people acting like you are not smart.” Participants rated the frequency of their experiences on the following scale: (1) “almost every day,” (2) “at least once a week,” (3) “few times a month,” (4) “few times a year,” (5) “less than once per year” and (6) “never”. All responses were reversed scored, such that a score of 6 corresponded to “almost every day.” Possible scores on this measure ranged from 9 to 54, with higher scores indicating greater everyday discrimination. This scale has previously been shown to have good internal consistency (e.g., α = 0.88 in Williams et al., 1997), and internal consistency was similar in our study (α = 0.81).
2.2.1.2. Gender and racial discrimination.
Gender and racial discrimination were assessed with two measures used previously in epidemiologic research (e.g., Krieger, 1990). Gender discrimination was assessed by five items that asked whether participants have ever experienced gender discrimination at school, when getting a job, at work, at home, and when getting medical care. Racial discrimination was measured using a six-item inventory that assessed whether participants have ever experienced racial discrimination at school, when getting a job, at work, when getting housing, when getting medical care, and from police or in courts. For each item in both measures, participants could reply Yes (1) or No (0). Possible scores on the gender and racial discrimination scales ranged from 0 to 5 and 0–6, respectively, with greater summed scores indicating greater levels of discrimination. In our study, internal consistencies for the gender discrimination and racial discrimination scales were α = 0.74 and α = 0.84, respectively.
2.2.1.3. Sources of discrimination.
Sources of discrimination were assessed with a ten-item measure adapted from a previous measure of discrimination in healthcare settings (LaVeist, Rolley, & Diala, 2003). Items asked, “Overall how much have you experienced prejudice or discrimination due to…” gender, race, ethnicity, income, age, religion, physical appearance, sexual orientation, health status, and disability. Participants rated their experiences on a 4-point scale ranging from 1 (not at all) to 4 (a lot). Scores ranged from 10 to 40, with higher scores indicating a higher number of sources of discrimination experienced more frequently. In our study, this scale had good internal consistency (α = 0.83).
2.2.1.4. Lifetime discrimination burden.
Lifetime discrimination burden was assessed with a two-item measure. Specifically, these items asked (1) “Overall, how much has discrimination interfered with you having a full and productive life?” and (2) “Overall, how much harder has your life been because of discrimination?” Participants responded on a 4-point scale ranging from 1 (not at all) to 4 (a lot). Possible scores ranged from 2 to 8, with higher scores indicating greater lifetime discrimination burden. These items have been used previously in epidemiological research (i.e., Jackson Heart Study, Friedman, Williams, Singer, & Ryff, 2009; & Survey of Midlife Development in the United Status, Sims, Wyatt, Gutierrez, Taylor, & Williams, 2009). The two items comprising this scale were strongly correlated in our study, r = .80, p < 0.001.
2.2.2. Sociodemographic information
Sociodemographic information was collected in the household interview component of Phase 1. Participants reported their age, sex, and self-identified race. Participants’ SES was calculated from a composite score that included self-reported annual household income and educational attainment. Participants were classified as higher SES if they reported (1) an annual household income (adjusted for household size) above or equal to 125% of the 2004 U.S. Department of Health and Human Services poverty guidelines, and (2) educational attainment greater than or equal to a high school diploma or GED. Participants were classified as lower SES if they reported (1) an annual household income (adjusted for household size) below 125% of the 2004 Health and Human Services poverty guidelines, or (2) educational attainment less than high school diploma or GED.
2.3. Telomere assay
Telomere length was measured by the quantitative polymerase chain reaction (qPCR)-based method described previously by Cawthon (2002). Briefly, 10 ng of DNA isolated from peripheral blood mononuclear cells (PBMC), was used in each PCR reaction in triplicates for each participant. Both telomere (T) and a single copy gene (36B4) (S) were included in the same 384-well plate using SYBR master mix on an Applied Biosystem 7900 H T system (ThermoFisher). The average cycle threshold (Ct) values of T and S were calculated from the triplicates to generate the average T/S ratio of each sample. To convert T/S ratio into actual telomere length in kilobases (kb), we measured one hundred thirty samples by both qPCR and the Southern method (Lin et al., 2015) and used the resulting conversion equation to calculate telomere length in kb from the T/S ratio.
2.4. Adjustment variables
Depression symptoms, lifetime substance use burden, and waist circumference were selected as adjustment variables based on the inclusion of similar variables in past studies of telomere length (Beach, Lei, Brody, Yu, & Philibert, 2014; Puterman et al., 2016; Wolkowitz et al., 2011). The Center for Epidemiologic Studies Depression (CES-D) scale (Radloff, 1977) was administered to participants during Phase 2. The CES-D is a 20-item inventory used to assess depressive symptoms over the past week. Participants responded to each item on a 4-point scale ranging from 0 (Rarely) to 3 (Mostly). Possible scores ranged from 0 to 60, with higher scores indicating greater depressive symptomatology.
Waist circumference in centimeters (cm) and substance use history were collected during Phase 2. Participants reported their substance use history during the broader medical history assessment. For any specified substance of abuse, participants could reply with one of four response options: Never tried, Tried, never used regularly, Former user (Used > 6 months ago), or Current user (Used in past 6 months). In the present study, responses for cigarette, marijuana, cocaine/crack, and opiate use were collapsed into dichotomous variables that were coded as 0 (Never used; i.e., never tried or tried, never used regularly) and 1 (Ever used; i.e., former or current user). Dichotomous scores for each of the four substances were summed to compute a lifetime substance use burden variable. Scores ranged from 0 to 4, with higher scores indicating greater lifetime substance use burden.
Data imputation was performed for all adjustment variables with < 10% missing within each race, poverty status, and sex subgroup (i.e., CES-D and waist circumference). Multiple linear regression (i.e., using age, sex, race, and poverty status as predictors) was used for imputation for the purpose of replicability.
2.5. Statistical analyses
Statistical analyses were conducted with the Statistical Package for the Social Sciences (SPSS) version 24. Multiple linear regression modeling was used to examine interactive relations of discrimination, SES, and other sociodemographic factors with telomere length. Specifically, we were interested in whether the interaction of discrimination and SES would vary as a function of age, sex, or race to predict telomere length. Therefore, we examined interaction effects up to the three-way interaction level, which included (a) self-reported discrimination scores, (b) SES, and (c) age, sex, or race. All analyses began with fully adjusted models, which contained a three-way interaction effect, two-way interaction effects nested beneath it, as well as all main effects and adjustment variables. If the three-way interaction effect was significant, the fully adjusted model was retained. Conversely, if the three-way interaction effect was nonsignificant, data analysis proceeded through the backward elimination procedure, which guides removal of nonsignificant, higher-level interaction terms from regression analyses (Morrell, Pearson, & Brant, 1997). Consistent with the procedure, the three-way interaction was removed from the regression model if found to be to nonsignificant, and analyses were then rerun. Subsequently, significant two-way interactions were identified and retained in the next step, while nonsignificant two-way interactions were removed from analysis. If no significant three- or two-way interactions were identified in the previous steps, then regression analysis proceeded with only main effects and covariates. Finally, the PROCESS macro for SPSS, Version 2.16 (Hayes, 2013) was used to probe and visualize significant two- and three-way interaction effects.
3. Results
African Americans reported significantly greater gender discrimination, racial discrimination, frequency of discrimination across sources, and lifetime discrimination burden than their White counterparts (all p’s < .001; see Table 1). African Americans also reported greater lifetime substance use burden than Whites (t(339) = −2.13, p < 0.05), whereas Whites had a higher waist circumference than African Americans (t(339) = 4.29, p < 0.001). There were no racial differences in sociodemographic factors (i.e., age, sex, and SES variables), everyday discrimination, or telomere length (all p’s > .05). Unadjusted bivariate correlations between discrimination measures ranged from r = 0.31 to r = 0.70 (all p’s < .01; see Supplementary Table 1 for correlations among all study variables). Overall, telomere length in the present sample ranged from 2.60 to 8.50 kb.
Table 1.
Participant Characteristics in the Overall Sample and Stratified by Race.
| African American (n =176) | White (n =165) | All (n =341) | |
|---|---|---|---|
| % African American | — | — | 51.6% |
| % Women | 48.3% | 52.1% | 50.1% |
| % Lower SES a | 61.4% | 63.6% | 62.5% |
| % < High school diploma or GED | 29.0% | 37.6% | 33.1% |
| % < 125% federal poverty level | 50.0% | 51.5% | 50.7% |
| Age | 47.57 (± 9.40) | 47.89 ( ± 8.41) | 47.72 (± 8.92) |
| Depressive symptoms | 13.52 (± 9.86) | 15.56 ( ± 11.54) | 14.51 (± 10.74) |
| Waist circumference (cm) *** | 95.48 (± 16.97) | 103.71 (± 18.39) | 99.46 (± 18.12) |
| Lifetime substance use burden b * | 1.64 (± 1.24) | 1.36 ( ±1.17) | 1.50 ( ± 1.21) |
| Everyday discrimination | 21.15 (± 7.88) | 19.73 ( ± 7.81) | 20.46 (± 7.86) |
| Gender discrimination *** | 0.96 (± 1.37) | 0.32 ( ±0.74) | 0.65 (1.16) |
| Racial discrimination *** | 1.78 (± 1.96) | 0.34 ( ±0.91) | 1.09 ( ± 1.70) |
| Sources of discrimination c *** | 17.84 (± 6.31) | 14.93 ( ± 4.60) | 16.43 (± 5.73) |
| Lifetime discrimination burden *** | 3.94 (± 1.90) | 2.93 ( ±1.54) | 3.45 ( ± 1.80) |
| Telomere length (kb) | 5.62 (± 0.75) | 5.69 ( ±0.69) | 5.66 ( ± 0.72) |
Note. Racial differences in all study variables were examined with independent samples t-tests and chi-square tests of independence.
p < .05
p < 0.01
p < .001.
Participants were considered to have lower SES if they reported having an educational attainment less than a high school diploma or GED and/or adjusted household incomes below 125% of the 2004 federal poverty level.
Number of substances (cigarettes, marijuana, cocaine/crack, or opiates) participants ever used.
Refers to frequency of discrimination across sources.
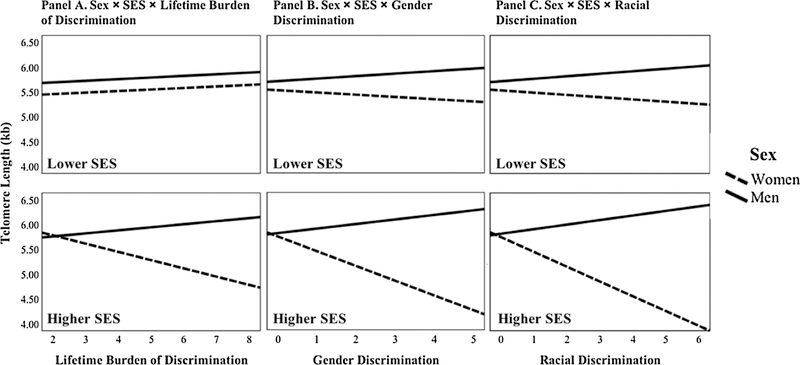
Findings revealed four significant three-way interactions: (a) Sex × SES × Lifetime Discrimination Burden, b = −0.23, p = .011; (b) Sex × SES × Gender Discrimination, b = −0.29, p = .040; (c) Sex × SES × Racial Discrimination, b = −0.24, p = .023 (see Supplementary Table 2 for full regression model results). As shown in Fig. 1, among women with higher SES, shorter telomeres were associated with greater (a) lifetime discrimination burden, b = −0.17, p = .003 (b) gender discrimination, b = −0.30, p = .001; and (c) racial discrimination, b = −0.30, p < .001. Across these three measures, every 1-point increase in discrimination was associated with a 0.17–0.30 kb decrease in telomere length among women with higher SES. Discrimination was not associated with telomere length among women with lower SES, or men of either SES group (all p’s > .05). Other models with three-way interaction terms were nonsignificant, thus, three-way interactions were eliminated from all subsequent models.
Fig. 1.
Significant moderating effects of sex and SES on the association between telomere length and (A) lifetime discrimination burden, (B) gender discrimination, and (C) racial discrimination.
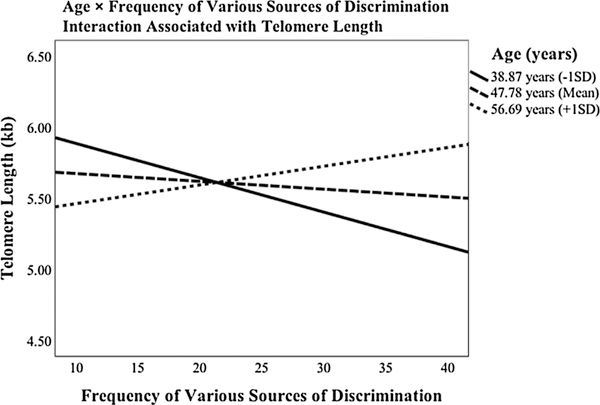
Next, findings revealed a significant two-way interaction of Age × Frequency of Discrimination across Sources with telomere length, b = .002, p = 0.008 (see Supplementary Table 3 for full regression results). As depicted in Fig. 2, among younger participants (38.87 years), greater frequency of discrimination across sources was related to shorter telomeres, b = −0.02, p = 0.020. Frequency of discrimination across sources was not associated with telomere length among middle-aged (47.78 years), b = −0.01, p = .458, or older participants (56.69 years), b = 0.01, p = .173.
Fig. 2.
Significant moderating effect of age on the association between frequency of discrimination across various sources and telomere length.
Everyday discrimination was not associated with telomere length, neither as a main effect nor within interactions. However, backward elimination of nonsignificant interaction terms in the everyday discrimination models revealed a significant two-way interaction of Sex × Race with telomere length, b = 0.35, p = .025 (Supplementary Table 4). As depicted in Supplementary Fig. 1, African American women had shorter telomeres than African American men, b = 0.45, p < .001. In contrast, there were no significant differences in telomere length between White women and White men, b = .10, p = 0.353. In addition, there was a significant main effect of age in this model (as well as all other models), such that greater age was related to shorter telomeres, b = −0.01, p = 0.025. Notably, findings revealed no racial differences in telomere length, neither as a main effect nor within interactions (all p’s > .05).
4. Discussion
In a sample of middle-aged African American and White adults, women with higher SES and younger adults (38.87 years old) reporting greater exposure to discrimination had shorter telomeres. Specifically, greater lifetime discrimination burden and gender and racial dis-crimination were each associated with shorter telomere length among women with higher SES. Among younger participants, greater frequency of discrimination across sources was associated with shorter telomeres. Associations were independent of race, as well as depressive symptoms, waist circumference, and substance use. These findings highlight the importance of considering the interwoven nature of historically demarcated social categories with the social experience of discrimination, as well as how these linkages may bear upon health.
The current findings extend the applications of extant stress theories (Clark, Anderson, Clark, & Williams, 1999; Paradies, 2006) and complement previous work showing inverse links between discrimination and specifically, racial discrimination and telomere length (Chae et al., 2014, 2016; Lee et al., 2017; Liu & Kawachi, 2017). They also expand upon prior reports in several ways. Most studies of discrimination and telomere length do not consider additional contextual factors, such as race, SES, sex, and age, especially in combination with each other. In the current study, discrimination was associated with shorter telomeres in both African American and White women with higher SES. Elevated health risk among those falling at the intersection of a high status (e.g., high SES) and low status social category (e.g., female sex) may represent what Bowleg (2012) refers to as an intersectionality paradox. These data reflect a pattern similar to the work showing diminishing returns for African Americans ascending the ranks of SES who experience poorer, not better, health (Diez-Roux, Nieto, Tyroler, Crum, & Szklo, 1995; Farmer & Ferraro, 2005; Waldstein et al., 2016). Diminishing returns is posited to be influenced by the price of economic progress for a minority group. Specifically, achieving greater SES typically situates African Americans in predominately White settings, which may lead to heightened exposure to chronic, interpersonal discrimination and, in turn, compromised health. A similar pattern may be at play in women with higher SES. Greater access by way of higher SES may increase the likelihood that these women are met with discriminatory interactions steeped in traditional expectations of gender roles. This dynamic may pertain to both African American and White women due to the shared challenges arising from male privilege. For instance, as women continue to make gains in male-dominated settings (Pew Research Center, 2013), men increasingly see themselves as disadvantaged and see women as becoming more advantaged at their expense (Kehn & Ruthig, 2013). Such an orientation may be particularly aversive for women with higher SES. It is also plausible that some women are mistreating other women (Reuben, Sapienza, & Zingales, 2014), which could be a consequence of competition for resources in a patriarchal society.
Reports of racial discrimination were associated with shorter telomeres among women with higher SES, irrespective of race. The evidence that African American women with higher SES reported higher levels of racial discrimination and had shorter telomeres is not surprising. Indeed, it is consistent with the literature showing the physical costs of racism among African Americans overall (e.g., Paradies, 2006), and particularly those with more socioeconomic resources (Farmer & Ferraro, 2005).
This association was also observed in White women with higher SES, which was an unexpected finding. Several explanations may exist for this finding (e.g., Apfelbaum, Norton, & Sommers, 2012; Craig & Richeson, 2017; DiAngelo, 2011; Norton & Sommers, 2011; Wilkins & Kaiser, 2013). Of late, a growing number of Whites in the U.S. have been reported as perceiving an increase in Anti-White bias and racial discrimination toward their group (see report byNNational Public Radio, Robert Wood Johnson Foundation, & Harvard T. H. Chan School of Public Health, 2017; Norton & Sommers, 2011). In addition, these perceptions have been linked with poor health outcomes in Whites (e.g., Peterson, Matthews, Derby, Bromberger, & Thurston, 2016. Thus, researchers have sought to elucidate the underlying psychological mechanisms for these attributions to racial discrimination among Whites. Individual construal’s of explanations for mistreatment in interpersonal interactions are in part shaped by social and cultural ideologies and the broader societal milieu. In this regard, an emerging body of work highlights how the current and impending demographic shift in the U.S. – wherein by 2050 Whites will be the “majority-minority” – are contributing to concerns of a fundamental change in American culture (Craig & Richeson, 2017). Thus, perceptions of progress among racial minorities have been found to stoke concerns of destabilization of the traditional social hierarchy among non-racial minorities (Wilkins, Hirsch, Kaiser, & Inkles, 2016). Nevertheless, altogether, these reports and the emerging linkages to health outcomes are unfolding in a context in which racial minorities have long fared poorly across multiple domains (e.g., health, education, criminal justice, and wealth; Alexander, 2010; Hoffman, Trawalter, Axt, & Oliver, 2016; Pager & Shepherd, 2008; Washington, 2006), indicative of embedded multilevel racial discrimination, which are largely not observed in Whites.
The second novel finding was that greater frequency of discrimination across sources was associated with shorter telomere length among younger participants. One prior study (Lee et al., 2017) reported that age did not modify the linkage of major life discrimination to telomeres, but the sample consisted of older African Americans. While telomere length declines with age (Epel, 2009), the current findings suggest that social factors may be associated with telomere length earlier in the life course and could possibly point to a cascading stress-health effect emerging in young adulthood. Considering the shared meaning of youth across race could shed light on these findings. Urban enclaves around the U.S. report an increase in racial, political, economic, and cultural frustration (Dobuzinskis, 2015). Younger Americans of different races may be acutely aware of these tensions. This may raise vigilance for bias, whether the bias is actual or not (Sewell, Horsford, Coleman, & Watkins, 2016), possibly explaining why these associations emerged irrespective of race. Given data showing that telomere shortening may contribute to accelerated aging and that racial minorities experience an earlier onset of poorer health, as well as emerging research highlighting health disparities in middle-aged Whites (Case & Deaton, 2015), examination of the relationship between discrimination and health earlier in the life course is an important next step.
4.1. Limitations, strengths, and future directions
The study has some limitations. First, the data were cross-sectional, and conclusions regarding causation, as well as the temporal links among the factors, are not possible. Future work should examine discrimination and telomere length within the context of social categories across the life course to establish temporal patterns. Such data may also shed light on epidemiological inconsistencies, such as African Americans having longer telomeres than Whites throughout the lifespan. Second, this work focused explicitly on individual-level discrimination. While the measures employed share a moderate amount of variance because they represent dimensions of the same underlying latent construct, they also have substantial unique variance. Assessing other forms of discrimination, especially at the structural-level, may lead to a better understanding of health disparity trajectories. Third, the study sample size was small (n = 341), which raises potential power-related concerns. Given that underpowered studies can produce biased findings (Crutzen & Peters, 2017; Simonsohn, 2015), ensuring that studies are powered adequately for the analyses being conducted is an important consideration. In the present study, we ran a power analysis (see Methods) that revealed our sample was powered to detect a small–medium f 2 effect size, suggesting that our sample, although small, was acceptable for drawing conclusions from the present analyses.
However, given the sample size, we opted to not test beyond 3-way interaction models. Future studies with larger samples should examine interactions that are more complex and investigate additional social categories, such as sexual orientation, in more depth. Fourth, there is no established analytical strategy for examining intersectionality. While interaction modeling allows some insight into how social categories may influence each other, it may not fully capture subtle nuances in these linkages (Cole, 2009). Fifth, findings may not be generalizable to African Americans and Whites living in non-urban settings. Different communities facilitate different types of social interactions and, in turn, may yield different linkages between discrimination and health. Finally, exploring the factors underlying perceptions of unfair treatment in different social groups will be important in future work.
Our study has several strengths. Participants were recruited from an area probability sample representing an economically diverse group of working aged African American and white adults. Participants included in our analyses were sampled randomly from the parent study. In addition, we assessed several specific forms of discrimination. In line with a conceptualization of discrimination as a multidimensional construct, we examined various forms of discrimination such as lifetime burden and gender. Our analyses used an intersectional approach to examine complex interactions among race, SES, sex, and age.
Our findings from midlife adults in an urban setting suggest a need for more research on the potential effects of discrimination and social statuses on telomere length. We investigated various forms of discrimination and showed that less commonly studied types matter. Our results also point to the value of considering an intersectional approach when examining discrimination and health endpoints which influence the perceptions and management of unfair treatment. Speculatively, the difference in telomere length across women with higher SES and younger adults, suggests a physiological age deterioration for these individuals when reporting greater exposure to particular types of discrimination. Thus, these subgroups may assume health trajectories that are paradoxical to what is expected as a function of social statuses they occupy (Rehkopf et al., 2016). In the absence of telomerase activity that would allow estimation of metric of years lost physiologically, the current findings are underscored by prior studies demonstrating that the difference in telomere length intimates impending health outcomes (Cherkas et al., 2006; Geronimus et al., 2010).
5. Conclusions
The current findings show that various forms of interpersonal discrimination are associated with accelerated biological aging, as indexed by telomere length, among African American and White adults in the U.S. In concurrently demonstrating the relevance of multiple forms of interpersonal discrimination this work may promote the conceptualization of discrimination as a multidimensional construct, which has unique effects in groups falling at the intersections of multiple statuses. Here, such an approach uncovered a subgroup (specifically, African American and White women with higher SES) with the strongest evidence of biological aging in relation to discrimination, which may have relevance for understanding future patterns of health risk as women continue to ascend the SES ladder. The observation that race may not always contribute to differential associations between discrimination and health endpoints demonstrates a need to more comprehensively assess ideological values that underlie expectations of treatment, opportunity, and fairness. In sum, if telomeres function as “psychobiomarkers,” reflecting exposure to discrimination-related stress at the cellular level, the current findings hold promise in revealing linkages to later life health disparities in understudied subgroups.
Supplementary Material
Acknowledgements
We wish to thank the HANDLS participants for their continued commitment to the study, as well the HANDLS research team. The National Institute on Aging Intramural Research Program of the National Institutes of Health (NIH) directed this research. We would like to acknowledge our funding sources: K01AG043581 (Beatty Moody), R01AG034161 (Waldstein), the National Institute on Aging’s Intramural Research ProgramZIAG000513 (Evans), and the Claude D. Pepper Older Americans Independence CenterP30AG028747 (Katzel).
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.biopsycho.2018.12.004.
References
- Adler NE (2013). Health disparities: Taking on the challenge. Perspectives on Psychological Science, 8(6), 679–681. [DOI] [PubMed] [Google Scholar]
- Alexander M (2010). The New jim crow: Mass incarceration in the age of colorblindness. New York: New Press. [Google Scholar]
- Apfelbaum EP, Norton MI, & Sommers SR (2012). Racial colorblindness: Emergence, practice, and implications. Current Directions in Psychological Science, 21, 205–209. 10.1177/0963721411434980. [DOI] [Google Scholar]
- Beach SR, Lei MK, Brody GH, Yu T, & Philibert RA (2014). Non-supportive parenting affects telomere length in young adulthood among African Americans: Mediation through substance use. Journal of Family Psychology, 28, 967–972. 10.1037/fam0000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosworth B (2018). Increasing disparities in mortality by socioeconomic status. Annual review of public health, 39, 237–251. [DOI] [PubMed] [Google Scholar]
- Bowleg L (2012). The problem with the phrase women and minorities: Intersectionality—an important theoretical framework for public health. American Journal of Public Health, 102, 1267–1273. 10.2105/AJPH.2012.300750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado RT, & Young NS (2009). Telomere diseases. New England Journal of Medicine, 361, 2353–2365. 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A, & Deaton A (2015). Rising morbidity and mortality in midlife among White non-Hispanic Americans in the 21st century. Proceedings of The National Academy of Sciences of The United States of America, 112, 15078–15083. 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM (2002). Telomere measurement by quantitative PCR. Nucleic Acids Research, 30, e47 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Epel ES, Nuru-Jeter AM, Lincoln KD, Taylor RJ, Lin J, … Thomas SB (2016). Discrimination, mental health, and leukocyte telomere length among African American men. Psychoneuroendocrinology, 63, 10–16. 10.1016/j.psyneuen.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Nuru-Jeter AM, Adler NE, Brody GH, Lin J, Blackburn EH, … Epel ES (2014). Discrimination, racial bias, and telomere length in African-American men. American Journal of Preventive Medicine, 46, 103–111. 10.1016/j.amepre.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SR, & Blackburn EH (2004). Telomeres and telomerase. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 359, 109–122. 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, … Spector TD (2006). The effects of social status on biological aging as measured by white‐blood‐cell telomere length. Aging Cell, 5, 361–365. [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, & Williams DR (1999). Racism as a stressor for African Americans: A biopsychosocial model. American Psychologist, 54, 805–816. 10.1037/0003-066X.54.10.805. [DOI] [PubMed] [Google Scholar]
- Cole ER (2009). Intersectionality and research in psychology. American Psychologist, 64, 170–180. 10.1037/a0014564. [DOI] [PubMed] [Google Scholar]
- Craig MA, & Richeson JA (2017). Information about the U.S. racial demographic shift triggers concerns about anti-White discrimination among the prospective White “minority”. PloS One, 12, 1–20. 10.1371/journal.pone.0185389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutzen R, & Peters GJY (2017). Scale quality: Alpha is an inadequate estimate and factor- analytic evidence is needed first of all. Health Psychology Review, 11(3), 242–247. [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Croft JB, Liu Y, Lu H, Eke PI, & Giles WH (2017). Vital signs: Racial disparities in age-specific mortality among blacks or African Americans—United States, 1999–2015. MMWR. Morbidity and mortality weekly report, 66(17), 444–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow SM, Verhoeven JE, Révész D, Lindqvist D, Penninx BWJH, Delucchi KL, … Mathews CA (2016). The association between psychiatric disorders and telomere length: A meta-analysis involving 14,827 persons. Psychosomatic Medicine, 78, 776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAngelo R (2011). White fragility. The International Journal of Critical Pedagogy, 3, 54–70. Retrieved from http://libjournal.uncg.edu/ijcp/article/view/249. [Google Scholar]
- Diez Roux AV, Ranjit N, Jenny NS, Shea S, Cushman M, Fitzpatrick A, & Seeman T (2009). Race/ethnicity and telomere length in the multi‐ethnic study of Atherosclerosis. Aging Cell, 8, 251–257. 10.1111/j.1474-9726.2009.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Roux AV, Nieto FJ, Tyroler HA, Crum LD, & Szklo M (1995). Social inequalities and atherosclerosis: The atherosclerosis risk in communities study. American Journal of Epidemiology, 141, 960–972. 10.1093/oxfordjournals.aje.a117363. [DOI] [PubMed] [Google Scholar]
- Dobuzinskis A (2015). Nearly half of young Americans lack confidence in justice system: Survey. Reuters. Retrieved from http://www.reuters.com/article/us-usa-justice-study/nearly-half-of-young-americans-lack-confidence-in-justice-system-survey-idUSKBN0NK2BS20150429.
- Epel ES (2009). Telomeres in a life-span perspective: A new “psychobiomarker”? Current Directions in Psychological Science, 18, 6–10. 10.1111/j.14678721.2009.01596.x. [DOI] [Google Scholar]
- Epel ES, Adler NE, Merkin SS, Seeman TE, Cawthon R, Blackburn EH, … Pletcher MJ (2009). The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging-Us, 1, 81–88. 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, & Zonderman AB (2010). Healthy aging in neighborhoods of diversity across the life span (HANDLS): Overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethnicity & Disease, 20, 267–275. [PMC free article] [PubMed] [Google Scholar]
- Farmer MM, & Ferraro KF (2005). Are racial disparities in health conditional on socioeconomic status? Social Science & Medicine, 60, 191–204. 10.1016/j.socscimed.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Williams DR, Singer BH, & Ryff CD (2009). Chronic discrimination predicts higher circulating levels of E-selectin in a national sample: The MIDUS study. Brain, Behavior, and Immunity, 23, 684–692. 10.1016/j.bbi.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, … Bekaert S (2014). Gender and telomere length: Systematic review and meta-analysis. Experimental Gerontology, 51, 15–27. 10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, & Cruz TD (2010). Do US black women experience stress-related accelerated biological aging? Human Nature, 21, 19–38. 10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Pearson JA, Linnenbringer E, Schulz AJ, Reyes AG, Epel ES, … Blackburn EH (2015). Race-ethnicity, poverty, urban stressors, and telomere length in a Detroit community-based sample. Journal of Health and Social Behavior, 56, 199–224. 10.1177/0022146515582100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, & Willeit P (2014). Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ, 349, 1–11. 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press. [Google Scholar]
- Hoffman KM, Trawalter S, Axt JR, & Oliver MN (2016). Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between Blacks and Whites. Proceedings of The National Academy of Sciences of The United States of America, 113, 4296–4301. 10.1073/pnas.1516047113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, … Aviv A (2008). Leukocyte telomeres are longer in African Americans than in Whites: The National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell, 7, 451–458. 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehn A, & Ruthig JC (2013). Perceptions of gender discrimination across six decades: The moderating roles of gender and age. Sex Roles, 69, 289–296. 10.1007/s11199-013-0303-2. [DOI] [Google Scholar]
- Kessler RC, Mickelson KD, & Williams DR (1999). The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. Journal of Health and Social Behavior, 40, 208–230. 10.2307/2676349. [DOI] [PubMed] [Google Scholar]
- Krieger N (2014). Discrimination and health inequities. International Journal of Health Services, 44, 643–710. 10.2190/HS.44.4.b. [DOI] [PubMed] [Google Scholar]
- Krieger N (1990). Racial and gender discrimination: Risk factors for high blood pressure? Social Science & Medicine, 30, 1273–1281. 10.1016/02779536(90)90307-E. [DOI] [PubMed] [Google Scholar]
- LaVeist TA, Rolley NC, & Diala C (2003). Prevalence and patterns of discrimination among U.S. health care consumers. International Journal of Health Services, 33, 331–344. 10.2190/TCAC-P90F-ATM5-B5U0. [DOI] [PubMed] [Google Scholar]
- Lee DB, Kim ES, & Neblett EW Jr (2017). The link between discrimination and telomere length in African American adults. Health Psychology, 36, 458–467 doi:/hea0000450. [DOI] [PubMed] [Google Scholar]
- Lewis TT, Cogburn CD, & Williams DR (2015). Self-reported experiences of discrimination and health: Scientific advances, ongoing controversies, and emerging issues. Annual Review of Clinical Psychology, 11, 407–440. 10.1146/annurev-clinpsy-032814-112728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Damjanovic A, Metter EJ, Nguyen H, Truong T, Najarro K, … Hodes RJ (2015). Age-associated telomere attrition of lymphocytes in vivo is co-ordinated with changes in telomerase activity, composition of lymphocyte subsets and health conditions. Clinical Science, 128, 367–377. 10.1042/CS20140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SY, & Kawachi I (2017). Discrimination and telomere length among older adults in the United States: Does the association vary by race and type of discrimination? Public Health Reports, 132, 220–230. 10.1177/0033354916689613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur MB, Epel E, Kind S, Desai M, Parks CG, Sandler DP, … Khazeni N (2016). Perceived stress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain, behavior, and immunity, 54, 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer IH (2003). Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: Conceptual issues and research evidence. Psychological bulletin, 129(5), 674–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell CH, Pearson JD, & Brant LJ (1997). Linear transformations of linear mixed- effects models. The American Statistician, 51, 338–343 doi:/00031305.1997.10474409. [Google Scholar]
- National Public Radio, Robert Wood Johnson Foundation, & Harvard T. H. Chan School of Public Health (2017). Discrimination in America: Experiences and views of African Americans. Retrieved from http://www.npr.org/assets/img/2017/10/23/discriminationpoll-african-americans.pdf.
- Needham BL, Adler N, Gregorich S, Rehkopf D, Lin J, Blackburn EH, & Epel ES (2013). Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Social Science & Medicine, 85, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton MI, & Sommers SR (2011). Whites see racism as a zero-sum game that they are now losing. Perspectives on Psychological Science, 6, 215–218. 10.1177/1745691611406922. [DOI] [PubMed] [Google Scholar]
- Oliveira BS, Zunzunegui MV, Quinlan J, Fahmi H, Tu MT, & Guerra RO (2016). Systematic review of the association between chronic social stress and telomere length: A life course perspective. Ageing research reviews, 26, 37–52. [DOI] [PubMed] [Google Scholar]
- Pager D, & Shepherd H (2008). The sociology of discrimination: Racial discrimination in employment, housing, credit, and consumer markets. Annual Review of Sociology, 34, 181–209. 10.1146/annurev.soc.33.040406.131740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantesco EJ, Leibel DK, Ashe JJ, Waldstein SR, Katzel LI, Liu HB, … Beatty Moody DLB (2018). Multiple forms of discrimination, social status, and telomere length: Interactions within race. Psychoneuroendocrinology, 98, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies Y (2006). A systematic review of empirical research on self-reported racism and health. International Journal of Epidemiology, 35, 888–901. 10.1093/ije/dyl056. [DOI] [PubMed] [Google Scholar]
- Paradies Y, Ben J, Denson N, Elias A, Priest N, Pieterse A, … Gee G (2015). Racism as a determinant of health: A systematic review and meta-analysis. PLoS ONE, 10, e0138511 10.1371/journal.pone.0138511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe EA, & Smart Richman L (2009). Perceived discrimination and health: A meta-analytic review. Psychological Bulletin, 135, 531–554. 10.1037/a0016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LM, Matthews KA, Derby CA, Bromberger JT, & Thurston RC (2016). The relationship between cumulative unfair treatment and intima media thickness and adventitial diameter: The moderating role of race in the study of women’s health across the nation. Health Psychology, 35, 313–321. 10.1037/hea0000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Research Center (2013). Women make significant gains in the workplace and educational attainment, but lag in pay. Retrieved from http://www.pewresearch.org/fact-tank/2013/03/08/women-make-significant-gains-in-the-workplace-and-educationalattainment-but-lag-in-pay/.
- Puterman E, Gemmill A, Karasek D, Weir D, Adler NE, Prather AA, … Epel ES (2016). Lifespan adversity and later adulthood telomere length in the nationally representative U.S. Health and retirement Study. Proceedings of The National Academy of Sciences of The Unites States of America, 113 10.1073/pnas.1525602113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale a self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. 10.1177/014662167700100306. [DOI] [Google Scholar]
- Rehkopf DH, Needham BL, Lin J, Blackburn EH, Zota AR, Wojcicki JM, … Epel ES (2016). Leukocyte telomere length in relation to 17 biomarkers of cardiovascular disease risk: A cross-sectional study of US adults. PLoS Medicine, 13, e1002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben E, Sapienza P, & Zingales L (2014). How stereotypes impair women’s careers in science. Proceedings of The National Academy of Sciences of The United States of America, 111, 4403–4408. 10.1073/pnas.1314788111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewak M, Buka S, Prescott J, De Vivo I, Loucks EB, Kawachi I, … Kubzansky LD (2014). Race-related health disparities and biological aging: Does rate of telomere shortening differ across Blacks and Whites? Biological Psychology, 99, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, Price LH, & Tyrka AR (2018). Early life adversity and telomere length: A meta-analysis. Molecular psychiatry, 23, 858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout KK, Ridout SJ, Price LH, Sen S, & Tyrka AR (2016). Depression and telomere length: A meta-analysis. Journal of affective disorders, 191, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson T, Batty GD, Der G, Fenton C, Shiels PG, & Benzeval M (2013). Is socioeconomic status associated with biological aging as measured by telomere length? Epidemiologic Reviews, 35, 98–111. 10.1093/epirev/mxs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz RJ, Trzeciakowski J, Moore T, Ayers KS, & Pickler RH (2017). Acculturation predicts negative affect and shortened telomere length. Biological Research for Nursing, 19, 28–35. 10.1177/1099800416672005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittker J, & McLeod JD (2005). The social psychology of health disparities. Annual Review of Sociology, 31, 75–103. [Google Scholar]
- Schrock JM, Adler NE, Epel ES, Nuru-Jeter AM, Lin J, Blackburn EH, … Chae DH (2018). Socioeconomic status, financial strain, and leukocyte telomere length in a sample of African American midlife men. Journal of racial and ethnic health disparities, 5(3), 459–467. [DOI] [PubMed] [Google Scholar]
- Schutte NS, & Malouff JM (2016). The relationship between perceived stress and telomere length: A meta‐analysis. Stress and health, 32, 313–319. [DOI] [PubMed] [Google Scholar]
- Sewell W, Horsford CE, Coleman K, & Watkins CS (2016). Vile vigilance: An integrated theoretical framework for understanding the state of Black surveillance. Journal of Human Behavior in the Social Environment, 26, 287–302. 10.1080/10911359.2015.1127735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims M, Wyatt SB, Gutierrez ML, Taylor HA, & Williams DR (2009). Development and psychometric testing of a multidimensional instrument of perceived discrimination among African Americans in the Jackson Heart Study. Ethnicity & Disease, 19(1), 56. [PMC free article] [PubMed] [Google Scholar]
- Simonsohn U (2015). Small telescopes: Detectability and the evaluation of replication results. Psychological science, 26(5), 559–569. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2014). Leading health indicators. Healthy People. Retrieved from https://www.healthypeople.gov/2020/Leading-Health-Indicators.
- Waldstein SR, Beatty Moody DL, McNeely JM, Allen AJ, Sprung MR, Shah MT, … Zonderman AB (2016). Cross-sectional relations of race and poverty status to cardiovascular risk factors in the healthy aging in neighborhoods of diversity across the lifespan (HANDLS) study. BMC Public Health, 16 10.1186/s12889-016-2945-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington HA (2006). Medical apartheid: The dark history of medical experimentation on Black Americans from colonial times to the present. New York: Doubleday. [Google Scholar]
- Whitfield KE, Allaire JC, Belue R, & Edwards CL (2008). Are comparisons the answer to understanding behavioral aspects of aging in racial and ethnic groups? The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 63(5), P301–P308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins CL, & Kaiser CR (2014). Racial progress as threat to the status hierarchy: Implications for perceptions of anti-White bias. Psychological Science, 25(2), 439–446. [DOI] [PubMed] [Google Scholar]
- Wilkins CL, Hirsch AA, Kaiser CR, & Inkles MP (2016). The threat of racial progress and the self-protective nature of perceiving anti-White bias. Group Processes & Intergroup Relations, 20, 801–812. 10.1177//1368430216631030. [DOI] [Google Scholar]
- Williams DR, & Jackson PB (2005). Social sources of racial disparities in health. Health Affairs, 24(2), 325–334. [DOI] [PubMed] [Google Scholar]
- Williams David R., Yu Yan, Jackson James S., & Anderson Norman B. (1997). Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of health psychology, 2(3), 335–351. [DOI] [PubMed] [Google Scholar]
- Williams DR, Kontos EZ, Viswanath K, Haas JS, Lathan CS, MacConaill LE, … Ayanian JZ (2012). Integrating multiple social statuses in health disparities research: the case of lung cancer. Health Services Research, 47, 1255–1277. 10.1111/j.1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, & Blackburn EH (2011). Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress: Preliminary findings. PloS One, 6, 1–8. 10.1371/journal.pone.001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.