Abstract
Background:
Accurate diagnosis and risk stratification of traumatic brain injury (TBI) at time of presentation remains a clinical challenge. The Head Injury Serum Markers for Assessing Response to Trauma study (HeadSMART) aims to examine blood-based biomarkers for diagnosing and determining prognosis in TBI.
Methods:
HeadSMART is a 6-month prospective cohort study comparing emergency department patients evaluated for TBI (exposure group) to (1) emergency department patients evaluated for traumatic injury without head trauma and (2) healthy persons. Study methods and characteristics of the first 300 exposure participants are discussed.
Results:
Of the first 300 participants in the exposure arm, 70% met the American Congress of Rehabilitation Medicine criteria for TBI, with the majority (80.1%) classified as mild TBI. The majority of subjects in the exposure arm had Glasgow Coma Scale scores of 13–15 (98.0%), normal head computed tomography (81.3%) and no prior history of concussion (71.7%).
Conclusion:
With systematic phenotyping, HeadSMART will facilitate diagnosis and risk-stratification of the heterogeneous group of individuals currently diagnosed with TBI.
Keywords: Traumatic brain injury, TBI, blunt head trauma, biomarkers
Introduction
Each year in the US, incidence of traumatic brain injury (TBI) among civilians is estimated to be 2.5 million [1], with an estimated prevalence of 5.3 million Americans living with a TBI-related disability, costing the nation over $60 billion in annual direct and indirect medical costs [2]. Cognitive, emotional, behavioural, neurologic and physical impairments are common sequelae of TBI, with studies showing ~ 80% of individuals with TBI experiencing at least one neuropsychiatric symptom (NPS) [3–5]. The most common point of evaluation for individuals with TBI is the emergency department (ED), with a recent study from the National Hospital Ambulatory Medical Care Survey (NHAMCS) reporting TBI evaluation during 4.8 million ED visits per year [6].
Frequently forgotten is the difference between the diagnoses of blunt head trauma and TBI, the former denoting the occurrence of a blow to the head and the latter that this blow has resulted in injury to the brain. Diagnosis of blunt head trauma is relatively straightforward, whereas accurate diagnosis of TBI at time of presentation remains a clinical challenge, especially in cases of mild TBI (mTBI). Clinicians must often make a diagnosis of TBI based on imprecise clinical symptoms/ physical exam findings and/or neuroradiographic evidence. Head computed tomography (CT) is the initial diagnostic test of choice; however, sub-optimal tissue characterization prevents accurate capturing of some TBI injuries such as white matter shearing and associated axonal injury. Behavioural presentations after TBI are heterogeneous and complex, requiring a level of observation and multidimensional assessment not routinely available in acute care settings [7]. There is an unmet clinical need for accurate, accessible biomarkers that can reliably detect TBI and predict TBI-associated outcomes.
Blood-based biomarker measurement is a relatively non-invasive and inexpensive method for diagnosing numerous clinical conditions. The identification of blood-based biomarkers for central nervous system (CNS) disease is more challenging due to the presence of the blood–brain barrier. Efforts to characterize TBI by examining one blood-based biomarker at a time have yielded conflicting results and, as TBI is a heterogeneous disorder affecting different cellular components of the brain, multi-biomarker panels may increase the sensitivity and specificity for detecting TBI and predicting outcomes [8]. To date, there is no US Food and Drug Administration-approved blood-based biomarker for use in patients with suspected TBI.
The Head Injury Serum Markers for Assessing Response to Trauma study (HeadSMART) seeks to examine the utility of blood-based biomarkers in diagnosis of TBI, while also collecting data on cognitive and other NPS longitudinally to analyse the prognostic utility of these biomarkers. Insights from this work will inform on the sub-classification of the heterogeneous group of individuals currently diagnosed with TBI. Studying the biochemical profile of recovery from TBI may also help elucidate mechanisms underlying TBI symptomatology and may assist in clinical decision-making.
It is hypothesized that candidate blood-based biomarkers will be differentially expressed post-TBI and that their levels will be associated with TBI-related outcomes (e.g. functional impairment, cognitive impairment, NPS) and associated features (e.g. intracranial haemorrhage on head CT). This study describes the design and methods of HeadSMART and discusses the characteristics of the first 300 participants enrolled in the exposure arm.
Methods
HeadSMART is an ongoing, 6-month prospective cohort study sponsored by ImmunArray with one exposure group (ED patients evaluated for TBI) and two comparison groups: (1) emergency department patients evaluated for traumatic injury without head trauma and (2) healthy persons. Two sites, Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center, are participating in enrollment. The use of two different sites, in demographically distinct parts of Baltimore, MD, promotes representation from diverse ethnic minority groups. A Johns Hopkins University School of Medicine Institutional Review Board (IRB) gave approval for the study.
Recruitment, eligibility and consent
Trained research staff review the electronic medical record of ED patients with trauma-related complaints to determine eligibility and consult with the treating clinicians to obtain permission to approach eligible patients. Prospective participants are assessed for entry criteria, as outlined in Table I, and their ability to provide written informed consent. In the setting of acute TBI, capacity to give written informed consent may be compromised. If Glasgow Coma Scale (GCS) score is < 15 or the participant is unable to consent for another reason, a legally authorized representative (LAR) provides written informed consent. In cases where consent is obtained from a LAR, the consent procedure is repeated if the participant recovers the ability to consent.
Table 1.
Inclusion and exclusion criteria
| Inclusion Criteria for suspected TBI cases |
| • Adults between the ages of 18 and 90 |
| • Blunt traumatic head injury presenting to the ED within 24 hours of injury |
| • Meet the ACEP criteria* for receiving a head CT scan in the setting of suspected TBI, namely: |
| ○ Loss of consciousness (LOC) or post-traumatic amnesia (PTA) and one or more of the following: focal neurologic deficit, headache, vomiting, age >60 years, drug or alcohol intoxication, deficits in short-term memory, physical evidence of trauma above the clavicle, posttraumatic seizure, Glasgow Coma Score (GCS) <15, or coagulopathy |
| ○ No LOC or PTA, but one of the following: focal neurologic deficit, severe headache, vomiting, age >65 years, physical signs of a basilar skull fracture, GCS < 15, coagulopathy, dangerous mechanism of injury (ejection from a motor vehicle, a pedestrian struck, or a fall from a height >3 feet or 5 stairs) |
| • Receive a routine head CT scan in the ED |
| Exclusion Criteria for suspected TBI cases |
| • Initial blood sample not obtained within 24 hours of injury |
| • Received blood transfusion before initial blood draw |
| • Head injury is seizure-induced |
| • Exclusion criteria common to all groups (see below) |
| Inclusion Criteria for traumatic injury without evidence of head trauma |
| • Adults between the ages of 18 and 90 |
| • Traumatic injury including fracture, sprain, ligamentous injury, laceration |
| • No direct injury to head or significant accelerating/decelerating forces associated with injury mechanism |
| Exclusion Criteria for traumatic injury without evidence of head trauma |
| • Unable to recall whether head was injured |
| • Altered mental status or unreliable historian |
| • Initial blood sample not obtained within 24 hours of injury |
| • Received blood transfusion before initial blood draw |
| • Traumatic injury is seizure-induced |
| • Past medical history of stroke with neurological deficits, demyelinating disease, neurodegenerative disease |
| • Exclusion criteria common to all groups (see below) |
| Inclusion Criteria for healthy persons |
| • Adults between the ages of 18 and 90 |
| Exclusion Criteria for healthy persons |
| • Any active illness |
| • Recent blood transfusion |
| • Past medical history of stroke with neurological deficits, demyelinating disease, neurodegenerative disease, or renal failure |
| • Blood pressure currently over 140/80 |
| • Recreational drug use within last 2 weeks (alcohol acceptable as long as not clinically intoxicated) |
| • Exclusion criteria common to all groups (see below) |
| Exclusion Criteria common to all groups |
| • Cannot communicate in English |
| • No working telephone number |
| • Currently pregnant |
| • Past medical history of intracranial surgery, intracranial hemorrhage (traumatic or non-traumatic), brain tumor, dementia (mild and moderate dementia allowed in TBI group) |
ACEP Criteria = American College of Emergency Physicians criteria for a head CT in the setting of suspected TBI [13].
Study data collection procedures
Collection of demographic and clinical information occurs in accordance with the National Institute of Neurological Disorders and Stroke (NINDS) common data elements for TBI (CDE v.2) [9]. The Galveston Orientation and Amnesia Test (GOAT) [10] and Rivermead Post Concussion Symptoms Questionnaire (RPQ) [11] are also completed at the baseline visit. To minimize data entry errors, study data are entered directly at bedside with an iPad. Study data are collected and managed using the Research Electronic Data Capture (REDCap) tool hosted by The Johns Hopkins University Bloomberg School of Public Health [12]. REDCap is a secure, web-based application designed to support data capture for research studies.
Head CT imaging
All participants in the exposure arm meet American College of Emergency Physician (ACEP) criteria (see Table I) for receiving a head CT scan in the setting of suspected TBI [13]. Head CT scans are read by a board-certified neuroradiologist using definitions from the NINDS CDE for radiologic imaging of TBI [14]. Traumatic abnormalities extracted from the non-contrast head CT include, but are not limited to: skull fracture, penetrating injury, haemorrhage, midline shift, ventricle compression, effacement, oedema/brain swelling, diffuse axonal injury, hypoxic-ischaemic injury, cervicomedullary junction or brain-stem injury and brain atrophy or encephalomalacia.
Blood sample collection
The optimal time point(s) for biomarker measurements in TBI remain(s) unclear. As such, samples are obtained acutely (0, 4 and 24 hours), sub-acutely (72 hours, 1 week and 1 month) and medium-term (3 and 6 months) after injury. The initial (0 hour) blood draw refers to samples obtained shortly after consent. Subsequent blood draws are timed from the initial blood draw. Blood is drawn at 0 and 4 hours on all participants in all study groups. Blood draws at 24 hours, 72 hours and 1 week are obtained only from those participants in the suspected TBI group and traumatic injury without head trauma group who remain hospitalized for those periods. Later blood draws are collected for those in the suspected TBI group only. Steps are taken to avoid haemolysis (e.g. using small syringes, drawing blood slowly from existing IVs) and samples are processed, aliquoted and stored at −80°C within 2 hours of blood draw.
TBI diagnostic criteria and severity criteria
TBI is diagnosed at the baseline visit according to the definition proposed by the Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health, also referred to as the American Congress of Rehabilitation Medicine (ACRM) criteria [15]. In those meeting ACRM criteria, TBI severity is classified as mild, moderate or severe based on Veterans Health Administration and Department of Defense (VA/DoD) criteria [16]. There is a group of individuals in the exposure arm meeting ACEP criteria for receiving a head CT, but not meeting ACRM criteria for a diagnosis of TBI. We have coined the term ‘HIBRID’ (Head Injury, but BRain Injury Debatable) to refer to this group.
Overview of follow-up outcome assessments
In-person outcome assessments occur at 1, 3 and 6 months post-injury (Table II) by trained research staff, under the supervision of a board-certified neuropsychologist. One of three board-certified neuropsychiatrists performs a neuropsychiatric assessment. Participants who are unable to attend in-person follow-up assessments receive follow-up telephone calls to complete the Glasgow Outcome Scale Extended (GOSE), RPQ and Patient Health Questionnaire 9 (PHQ-9).
Table 2.
HeadSMART visit schedule.
| Visit | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| Time | Baseline | 1 month | 3 months | 6 months | |
| Procedures | |||||
| Consent | T, I, H | – | – | – | |
| Demographics | T, I, H | – | – | – | |
| Common data elements | T, I, H | – | – | – | |
| Blood draw* | T, I, H | T | T | T | |
| Routine clinical brain CT scan | T | – | – | – | |
| Complete psychiatric assessment | – | T | T | T | |
| Participant Assessments | |||||
| Galveston Orientation and Amnesia Test | T | – | – | – | |
| Rivermead Post Concussion Symptoms Questionnaire | T, I, H | T, I, H | T, I, H | T, I, H | |
| Glasgow Outcome Scale Extended | – | T, I, H | T, I, H | T, I, H | |
| Global Medical Health Rating | – | T | T | T | |
| Neurobehavioral Rating Scale - Revised | – | T | T | T | |
| Montreal Cognitive Assessment | – | T | T | T | |
| Wechsler Test of Adult Reading | – | T | T | T | |
| Brief Test of Attention | – | T | T | T | |
| Trail Making Test, Part A & B | – | T | T | T | |
| Stroop Test | – | T | T | T | |
| Controlled Oral Word Association Test | – | T | T | T | |
| Wisconsin Card Sorting Test | – | T | T | T | |
| Hopkins Verbal Learning Test | – | T | T | T | |
| Brief Visuospatial Memory Test | – | T | T | T | |
| Patient Health Questionnaire 9 | – | T, I, H | T, I, H | T, I, H | |
| Generalized Anxiety Disorder 7 | – | T | T | T | |
| Davidson Trauma Scale | – | T | T | T | |
| Pittsburgh Sleep Quality Index | – | T | T | T | |
| Satisfaction with Life Scale | – | T | T | T | |
| Social Ties Checklist | – | T | T | T | |
| McGill Pain Questionnaire | – | T | T | T |
T = suspected TBI; I = traumatic injury without evidence of head trauma; H = healthy
In all groups, blood draws occur at 0 and 4 hours; in suspected TBI group, if hospitalized, blood draws will also occur at 24 and 72 hours, and 1 week
Cognitive assessment
The tests in the neurocognitive battery are meant to: maximize the comparison potential of these study findings to other TBI studies, capture a breadth of cognitive functions that can be affected by TBI and minimize the participant time burden. The GOAT [10] is administered at the baseline visit. The following neurocognitive tests are completed at 1, 3 and 6 months: Brief Test of Attention [17], Brief Visuospatial Memory Test [18], Controlled Oral Word Association Test [19], Hopkins Verbal Learning Test [20], Stroop Test [21], Trail Making Test [22], Wechsler Test of Adult Reading [23] and Wisconsin Card Sorting Test [24]. The Montreal Cognitive Assessment (MOCA) [25] is also completed.
Symptom and functional outcome questionnaires
The RPQ [11] is used to determine the presence and severity of post-concussion syndrome, a set of somatic, cognitive and emotional symptoms following TBI. The GOSE [26] allows standardized descriptions of the objective degree of recovery after brain injury by using a global scale for functional outcome. The Neurobehavioural Rating Scale–Revised [27] assesses multiple types of neuropsychiatric symptomatology. Standardized symptom self-report questionnaires are completed as follows: PHQ-9 [28] for depression, Generalized Anxiety Disorder 7 (GAD-7) [29] for anxiety, Davidson Trauma Scale (DTS) [30] for post-traumatic stress disorder (PTSD) symptoms, McGill Pain Questionnaire [31] for pain and Pittsburgh Sleep Quality Index [32] for sleep quality. To assess the context in which the TBI is being experienced, the Satisfaction with Life Scale (SWLS) [33], which rates perceived satisfaction with life, and Social Ties Checklist (STC) [34], which assesses the number of established social connections an individual has, are completed.
Neuropsychiatric assessment
This assessment includes family history of psychiatric illness, behavioural problems in childhood, legal history, substance use history, personal past psychiatric history, history of present illness and complete mental state examination. The Global Medical Health Rating [35], a rapid global rating scale for medical comorbidity originally validated in patients with dementia, is also completed.
Data monitoring and quality control
The accuracy of data collection is ensured by: (1) using a standardized electronic data collection tool, (2) entering data directly at the participant’s bedside, (3) automating the validation of data fields to check for clerical errors and (4) using fixed options where possible instead of free-text fields. Data inconsistencies are resolved by a review of the electronic medical record and notes written by trained research assistants to make a final determination.
Safety monitoring
Study personnel have frequent contact with participants, either in-person or by phone. If at any time during the study a participant expresses suicidal or homicidal ideation, or is felt to be a threat to him/herself for any other reason, appropriate referrals to ensure safety are made. One of the study’s neuropsychiatrists is on-call to assist as needed. In addition, since complete neuropsychiatric assessments are performed during the study, participants are referred for care as needed.
Candidate biomarkers
A number of review articles detail what is currently known about the utility of serum biomarkers in TBI [36,37]. The initial candidate biomarkers being measured in HeadSMART are described below. Biomarkers will be measured by ImmunArray using ELISA. De novo discovery of novel TBI biomarkers are ongoing and utilize cutting-edge proteomic techniques. The investigators included measures of primary (e.g. glial, neuronal, endothelial, vascular damage) and secondary (e.g. inflammatory, haemorrhagic, oedematous changes) brain injury to fully characterize the extent of TBI and predict outcomes.
Glial Fibrillary Acidic Protein (GFAP): A monomeric intermediate filament that constitutes the cytoskeleton of astrocytes and helps to maintain the integrity of the blood–brain barrier. Increased levels of GFAP have been related to outcome in patients with severe TBI [38].
S100 calcium binding protein B (S100B): A glial specific calcium-binding protein expressed primarily in astrocytes. Elevations of S100B have been associated with unfavourable neurological outcomes post-TBI [39].
Neurogranin (NRGN): A small neuronal protein that plays an important role in synaptic signalling by regulating cal-modulin availability. In a recently published manuscript, this group found NRGN levels to be increased after TBI [40].
Brain-derived neurotrophic factor (BDNF): Supports the survival of existing neurons and encourages the growth and differentiation of new neurons and synapses. Low serum BDNF levels on the day of injury are prognostic of poor recovery from TBI at 6 months after injury [41]. Failla et al. [42] found a reduction in serum BDNF levels post-TBI and a concomitant elevation in cerebrospinal fluid (CSF) BDNF levels, with the elevated CSF BDNF correlated with time until death.
Metallothionein 3 (MT3): Located in the Golgi apparatus and binds to heavy metals. This isoform is particularly enriched in the CNS [43]. Levels of other isoforms of MT in the blood (MT1 & MT2) have been found to decrease days 1–3 post-TBI and increase days 4–8 post-TBI [44] and, as of this writing, other groups have not yet studied the more CNS-specific form of MT.
Neuron specific enolase (NSE): One of the three enolase isoenzymes found in mammals. Present in mature neurons and cells of neuronal origin. Although utility in TBI remains unclear, elevated levels of NSE in the CSF have been correlated with poor outcome in patients with post-cardiac arrest hypoxic-ischaemic encephalopathic injuries [45].
Beta synuclein (SNCB): A post-synaptic protein of unclear function that is highly expressed in the cerebral cortex neutrophil. It is known that alpha- and gamma-synuclein levels change after brain injury in mouse models [46]. However, SNCB has not been well-studied in TBI.
Intracellular adhesion molecule 5 (ICAM-5): Expressed on the surface of telencephalic neurons and assists with haemophilic binding between neurons and heterophilic binding between neurons and leukocytes. Although utility in TBI is unknown, blood-based ICAM-5 levels could be a measure of disruption of the intercellular adhesion apparatus or a measure of the neuroinflammatory consequences of TBI [47].
Outcome measures
Outcome domains assessed include:
Global functional outcome: As measured by the GOSE and meant to capture the overall impact of TBI.
Post-concussive symptoms: As measured by the RPQ and NBRS and include somatic, cognitive and emotional symptoms resulting from TBI.
Cognitive functioning: Cognitive ability will be divided into measures of global cognitive functioning (e.g. MOCA), attention (e.g. Brief Test of Attention, Trail Making Test Part A), learning and memory (e.g. Hopkins Verbal Learning Test, Brief Visuospatial Memory Test) and executive function (e.g. Controlled Oral Word Association Test, Wisconsin Card Sorting Test, Stroop Test, Trail Making Test Part B).
Psychiatric symptoms: As measured by the PHQ-9, GAD-7, DTS and the neuropsychiatrist-completed assessment.
Perceived health-related quality-of-life: As measured by the SWLS and STC.
Analytic plan
Clinical and demographic characteristics of the ACRM-defined TBI group will be compared to the traumatic injury without head trauma and healthy groups using descriptive statistics. Continuous variables will be summarized using means with standard deviations (normally distributed data) or medians with inter-quartile ranges (non-normally distributed data). Differences between groups will be assessed with t-tests (normally distributed data) or Kruskal-Wallis test (non-normally distributed data). Categorical variables will be summarized using proportions and differences between study groups will be assessed using a χ2 test.
Our primary aims are to determine the ability of candidate biomarkers to diagnose TBI as defined by ACRM criteria, identify suspected TBI cases without intracranial abnormalities on head CT (patients in whom head CT can be safely avoided), and predict long-term cognitive and other neuropsychiatric consequences of TBI. We will first compare the distribution of biomarker levels in those with ACRM-defined TBI to age and gender matched participants from both comparison groups (traumatic injury without head trauma and healthy) and determine the discriminative ability of the biomarkers using the c-statistic. To evaluate the prognostic value of candidate biomarkers, we will fit logistic regressions for each candidate biomarker with the outcome as the dependent variable. We will then build multivariate models that include any of the biomarkers that were associated with the outcome in the univariate models and utilize ensemble methods for combining multiple models. From each model, we will generate sensitivity, specificity, positive and negative predictive values, and area under receiver operator characteristic (ROC) curves. The accuracy of the logistic regression-based model will be compared to that of a model derived using supervised machine learning methods. Specifically, we will use the WEKA package [48] to implement the j48/c4.5 classifier [49]. This tree-based classifier creates nodes that maximize information gain.
A secondary aim is to examine whether biomarker profile and outcomes differ between the ACRM-defined TBI group and the group of patients we are calling “HIBRID” that meet the ACEP criteria for head CT, but not ACRM definition of TBI. Another secondary outcome is to study the biochemical profile of recovery from TBI. We will fit longitudinal random effects models to characterize trajectories of each biomarker acutely, sub-acutely, and medium-term with models adjusted for age, sex, socioeconomic status, and prior substance abuse. We will test whether acute phase trajectory predicts long-term cognitive and other neuropsychiatric outcome by regressing outcomes on fitted random slopes and determine whether longitudinal changes in biomarkers differs between TBI participants with good recovery and those with poor recovery.
Although there is no gold standard for the diagnosis of TBI, we will be using the ACRM criteria given its common use in clinical practice. As we do not feel this is ultimately a sufficient way to diagnosis TBI, another secondary aim is to search for a “LEAD” (Longitudinal, Expert, All Data) standard. The concept of a LEAD standard was originally proposed by Spitzer [50] and advocates for making a diagnosis after multiple examinations (longitudinal), utilizing consensus agreement from expert clinicians (expert), and utilizing any available data in the diagnosis (all data). This will be discussed in detail in a future manuscript.
Power estimation
In calculating a sample size for this study, we elected to focus on precisely measuring the negative predictive value (NPV) of candidate biomarkers for ruling out intracranial abnormalities on head CT in patients evaluated for TBI. The goal is to identify biomarkers that can be safely used to triage the need for a head CT scan without missing a significant number of patients with intracranial abnormalities. Assuming the prevalence of head CTs with intracranial abnormalities is 10%, a targeted NPV of 99%, and an anticipated specificity of 85%, by enrolling 493 participants (i.e. 381 participants who are biomarker negative) we will be able to precisely estimate the NPV of candidate biomarkers with a 95% confidence interval within 1% of the true NPV. We will therefore enroll at least 500 participants with suspected TBI.
Preliminary results
Comparison of consented to non-consented participants with suspected TBI
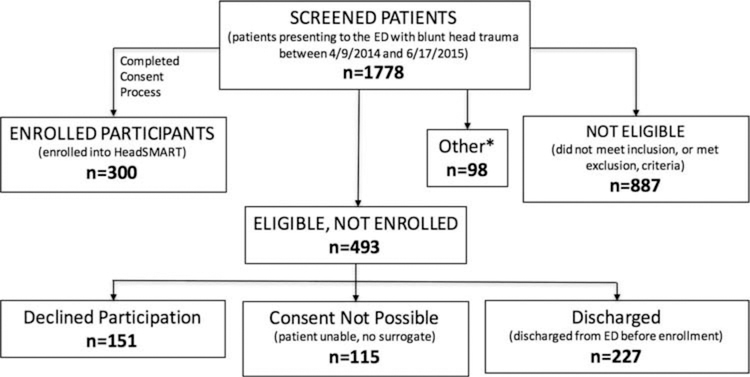
Here we discuss the first 300 participants with suspected TBI (recruited between 4/9/2014 and 6/17/2015). During that time period, 1778 patients presenting to the ED for TBI evaluation were screened with Figure 1 detailing reasons for those not enrolled. Thus far, 84 traumatic injury without head trauma and 51 healthy participants have been enrolled. These comparisons groups are not discussed in detail here.
Figure 1.
This figure is a flowchart that summarizes how 300 subjects were enrolled after screening 1,778 potential subjects.
We compared (Table 3) consenting participants with suspected TBI who were eligible and completed enrollment (n=300) to those who were eligible, but not enrolled (n=493). Enrolled participants were similar to eligible participants not enrolled in age, gender, race, and injury mechanism. Compared to non-enrolled participants, those enrolled were more likely to be married (30.7% versus 21.1%, p=<0.01) and less likely to be intoxicated with drugs or alcohol at time of injury (18% vs. 25.9%, p=<0.01).
Table 3.
Baseline descriptors of enrolled and non-enrolled individuals with suspected TBI.
| Characteristic* | Suspected TBI Consented (n = 300) | Suspected TBI Eligible, Not Consented (n = 493) | P Value |
|---|---|---|---|
| Enrollment Location | 0.872 | ||
| Johns Hopkins Hospital | 177 (59.0) | 288 (58.4) | |
| Johns Hopkins Bayview | 123 (41.0) | 205 (41.6) | |
| Median Age in Years (IQR) | 48 (28–64) | 45 (30–60) | 0.606 |
| Sex | 0.631 | ||
| Male | 175 (58.3) | 279 (56.6) | |
| Female | 125 (41.7) | 214 (43.3) | |
| Race | 0.297 | ||
| Non-Hispanic, white | 166 (55.3) | 225 (45.6) | |
| Hispanic, white | 1 (0.3) | 5 (1.0) | |
| Non-Hispanic, black | 118 (39.3) | 219 (44.4) | |
| Hispanic, black | 1 (0.3) | 1 (0.2) | |
| Asian | 3 (1.0) | 6 (1.2) | |
| Other | 11 (3.8) | 16 (3.2) | |
| Marital Status | 0.006 | ||
| Never Married | 139 (46.3) | 268 (54.4) | |
| Married /Domestic Partnership | 92 (30.7) | 104 (21.1) | |
| Other1 | 69 (23.0) | 72 (14.6) | |
| Mode of Arrival to Emergency Department | 0.052 | ||
| Self Transport | 47 (15.7) | 106 (21.5) | |
| Ambulance | 245 (81.7) | 382 (77.5) | |
| Transfer from Other Facility | 8 (2.6) | 4 (0.8) | |
| Mode of Injury | 0.217 | ||
| Pedestrian Struck | 29 (9.7) | 21 (4.3) | |
| MVC2 | 86 (28.7) | 135 (27.6) | |
| Fall | 123 (41.0) | 214 (43.8) | |
| Assault | 45 (15.0) | 91 (18.6) | |
| Struck By /Against | 12 (4.0) | 13 (2.7) | |
| Pedal Bike | 5 (1.6) | 6 (1.2) | |
| Exercise /Sport /Work-related | 0 | 2 (0.4) | |
| Other | 0 | 7 (1.4) | |
| Intoxicated on Drugs /Alcohol | 0.002 | ||
| Yes | 54 (18.0) | 125 (25.9) | |
| No | 244 (81.3) | 342 (71.0) | |
| Unsure | 2 (0.7) | 15 (3.1) |
Other includes divorced, separated, or widowed status
MVC = motor vehicle collision, including motorcycle
characteristics are number (%) unless listed otherwise
Injury and symptom characteristics of consented participants with suspected TBI
Of the first 300 participants with suspected TBI 100% (300/300) met ACEP criteria for receiving a head CT scan in the ED [13] and 70% (210/300) met ACRM criteria [15] for TBI (Table 4). Of those meeting ACRM criteria, the majority, 80.9% (170/ 210), were classified as mTBI according to VA/DoD criteria [16]. Among all participants with suspected TBI, 98% (294/300) had GCS scores of 13–15, 81.3% (244/300) had normal head CTs, and 71.7% (215/300) had no prior history of concussion.
Table 4.
Injury characteristics and further description of enrolled participants with suspected TBI (n = 300).
| Characteristic | No. (%) |
|---|---|
| Met ACRM1 Criteria | 210 (70.0) |
| Met VA/DoD2 Criteria (if met ACRM criteria for TBI) | |
| Mild | 170 (80.9) |
| Moderate | 38 (18.1) |
| Severe | 2 (1.0) |
| Glasgow Coma Scale Score | |
| 13–15 | 294 (98.0) |
| 9–12 | 3 (1.0) |
| 3–8 | 3 (1.0) |
| CT Findings | |
| Normal | 244 (81.3) |
| Skull Fracture Only | 7 (2.3) |
| Traumatic Brain Abnormality | 37 (12.3) |
| Both* | 12 (4.0) |
| Loss of Consciousness | 153 (51.0) |
| Post-Traumatic Amnesia | 147 (49.0) |
| Altered Mental Status after Injury | 159 (53.0) |
| Deficits in Short-term Memory | 39 (13.0) |
| Headache | 239 (79.7) |
| Vomiting since Injury | 29 (9.7) |
| Seizure since Injury | 4 (1.3) |
| Focal Neurological Deficit | 25 (8.3) |
| Education | |
| Did Not Complete High School | 61 (20.3) |
| Completed High School or Equivalent, No College | 132 (44.0) |
| Some College | 43 (14.3) |
| Obtained College Degree or Higher | 63 (21.0) |
| Unknown | 1 (0.4) |
| Employment Status | |
| Full-time (≥35hr/wk) | 116 (38.7) |
| Part-time (20–34hr/wk) | 25 (8.3) |
| Unemployed | 47 (15.7) |
| Not in Paid Workforce | 108 (36.0) |
| Other | 4 (1.3) |
| Prior Concussion History | |
| None | 215 (71.7) |
| One Prior | 74 (24.7) |
| More Than One Prior | 11 (3.6) |
| Endorsed Pre-Injury Psychiatric History | |
| Depression Diagnosis | 88 (29.3) |
| Depression Medication | 60 (20.0) |
| Prior Suicide Attempts | 16 (5.3) |
| Prior Homicide Attempts | 1 (0.3) |
| Other Psychiatric Diagnosis | 54 (18.0) |
Discussion
HeadSMART is a six-month prospective cohort study that aims to examine the utility of blood-based biomarkers in TBI diagnosis, while also collecting longitudinal data on cognitive and other NPS to analyze the prognostic utility of these blood-based biomarkers. Our first 300 enrolled participants with suspected TBI were discussed.
HeadSMART is unique in its depth of collaboration between emergency medicine, neuropsychiatry, neuropsychology, neurology, and radiology. The complete neuropsychiatric evaluation of all participants by a TBI specialist allows for examination of the mental state of these participants at the highest level of detail and nuance. Inclusion of all individuals meeting ACEP criteria for a head CT scan in the setting of head injury, not just individuals meeting ACRM criteria for TBI, allows us to comment on the population currently receiving head CT scans in the ED. In future manuscripts, we will refer to this group meeting ACEP criteria for head CT, but not ACRM criteria for TBI as “HIBRID.” The serial blood-based biomarker measurements allow us to look at longitudinal variation in biomarker levels and to comment on how these variations relate to outcome (e.g., transient, permanent, or fluctuating symptoms and sequelae).
HeadSMART adds to the literature on TBI using the NINDS CDE v.2 for TBI originally validated in the TRACK-TBI study [51]. The HeadSMART participants are similar to the population-based estimates of individuals evaluated in EDs nationally for TBI (our target population); differences likely reflect our urban recruitment setting. Some differences from the population described in NHAMCS [6] are that HeadSMART is not enrolling individuals <18, has a higher proportion of black participants (68% vs. 39%) and lower proportion of Hispanic participants (0.6% vs. 16%), a higher proportion of individuals arriving by ambulance (82% vs. 42%, although this was the most common mode of arrival in NHAMCS), and a higher proportion of individuals intoxicated on drugs /alcohol (18% vs. 6%). Mechanism of injury was similar, with falls being most common followed by motor vehicle collisions. In both studies, mTBI is by far the most common severity level. These key similarities and differences between HeadSMART and national estimates of the population patients evaluated for TBI in the ED have important implications for the generalizability of study findings.
In conclusion, HeadSMART aims to follow patients longitudinally from a “real world” point of evaluation for TBI, namely the ED. The investigators hope that collection of data at multiple time points will have diagnostic and prognostic value. The in-depth collection of cognitive and neuropsychiatric data allow for extensive assessment of biomarker prognostic utility.
Acknowledgments
Declaration of interest statement
Frederick Korley, M.D., Ph.D. was supported by the Harold Amos Medical Faculty Development Award from the Robert Wood Johnson Foundation. HeadSMART was funded by ImmunArray. ImmunArray has licensed patents to biomarkers described in this article. Under a licensing agreement between ImmunArray and the Johns Hopkins University, Drs. Everett, Van Eyk and Korley are entitled to royalties on biomarkers described in this article. Dr. Batty is an officer, shareholder, and employee of ImmunArray.
References
- 1.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics. 2010. \,; 2010 National Hospital Ambulatory Medical Care Survey (NHAMCS), 2010.
- 2.Centers for Disease Control and Prevention(CDC) NCfIPaC. 2003. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- 3.Kim E, Lauterbach EC, Reeve A, Arciniegas DB, Coburn KL, Mendez MF, Rummans TA, Coffey EC, Research ACo. 2007. Neuropsychiatric complications of traumatic brain injury: a critical review of the literature (a report by the ANPA Committee on Research). J Neuropsychiatry Clin Neurosci 19(2):106–27. [DOI] [PubMed] [Google Scholar]
- 4.Rao V, Koliatsos V, Ahmed F, Lyketsos C, Kortte K. 2015. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol 35(1):64–82. [DOI] [PubMed] [Google Scholar]
- 5.McMahon P, Hricik A, Yue JK, Puccio AM, Inoue T, Lingsma HF, Beers SR, Gordon WA, Valadka AB, Manley GT et al. 2014. Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. J Neurotrauma 31(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korley FK, Kelen GD, Jones CM, Diaz-Arrastia R. 2015. Emergency Department Evaluation of Traumatic Brain Injury in the United States, 2009–2010. J Head Trauma Rehabil. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnould A, Dromer E, Rochat L, Van der Linden M, Azouvi P. 2015. Neurobehavioral and self-awareness changes after traumatic brain injury: Towards new multidimensional approaches. Ann Phys Rehabil Med. [DOI] [PubMed] [Google Scholar]
- 8.Zetterberg H, Smith DH, Blennow K. 2013. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol 9(4):201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hicks R, Giacino J, Harrison-Felix C, Manley G, Valadka A, Wilde EA. 2013. Progress in developing common data elements for traumatic brain injury research: version two–the end of the beginning. J Neurotrauma 30(22):1852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin HS, O’Donnell VM, Grossman RG. 1979. The Galveston Orientation and Amnesia Test. A practical scale to assess cognition after head injury. J Nerv Ment Dis 167(11):675–84. [DOI] [PubMed] [Google Scholar]
- 11.King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. 1995. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 242(9):587–92. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagoda AS, Bazarian JJ, Bruns JJ Jr., Cantrill SV, Gean AD,Howard PK, Ghajar J, Riggio S, Wright DW, Wears RL et al. 2009. Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. J Emerg Nurs 35 (2):e5–40. [DOI] [PubMed] [Google Scholar]
- 14.Duhaime AC, Gean AD, Haacke EM, Hicks R, Wintermark M, Mukherjee P, Brody D, Latour L, Riedy G, Common Data Elements Neuroimaging Working Group Members PWGM. 2010. Common data elements in radiologic imaging of traumatic brain injury. Arch Phys Med Rehabil 91(11):1661–6. [DOI] [PubMed] [Google Scholar]
- 15.Menon DK, Schwab K, Wright DW, Maas AI, Demographics, Clinical Assessment Working Group of the I, Interagency Initiative toward Common Data Elements for Research on Traumatic Brain I, Psychological H. 2010. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil 91 (11):1637–40.21044706 [Google Scholar]
- 16.O’Neil ME, Carlson K, Storzbach D, Brenner L, Freeman M, Quinones A, Motu’apuaka M, Ensley M, Kansagara D. 2013. Complications of Mild Traumatic Brain Injury in Veterans and Military Personnel: A Systematic Review. Washington (DC). [PubMed] [Google Scholar]
- 17.Schretlen D, Bobholz JH, Brandt J. 1996. Development and psychometric properties of the Brief Test of Attention. Clinical Neuropsychologist 10(1):80–89. [Google Scholar]
- 18.Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B. 1996. Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability, and validity. Psychological Assessment 8(2):145–153. [Google Scholar]
- 19.Ruff RM, Light RH, Parker SB, Levin HS. 1996. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol 11(4):329–38. [PubMed] [Google Scholar]
- 20.Brandt J 1991. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. Clinical Neuropsychologist 5(2):125–142. [Google Scholar]
- 21.Golden CJ. 1978. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago,IL: Skoelting; p. 1–32. [Google Scholar]
- 22.Arbuthnott K, Frank J. 2000. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol 22(4):518–28. [DOI] [PubMed] [Google Scholar]
- 23.Holdnack HA. 2001. Wechsler Test of Adult Reading: WTAR. San Antonio: The Psychological Corporation. [Google Scholar]
- 24.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. 1981. Wisconsin Card Sorting Test Manual. Torrance, CA: WPS. [Google Scholar]
- 25.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. 2005. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–9. [DOI] [PubMed] [Google Scholar]
- 26.Jennett B, Bond M. 1975. Assessment of outcome after severe brain damage. Lancet 1(7905):480–4. [DOI] [PubMed] [Google Scholar]
- 27.McCauley SR, Levin HS, Vanier M, Mazaux JM, Boake C, Goldfader PR, Rockers D, Butters M, Kareken DA, Lambert J et al. 2001. The neurobehavioural rating scale-revised: sensitivity and validity in closed head injury assessment. J Neurol Neurosurg Psychiatry 71(5):643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JB. 2001. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spitzer RL, Kroenke K, Williams JB, Lowe B. 2006. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 166(10):1092–7. [DOI] [PubMed] [Google Scholar]
- 30.Davidson JR, Book SW, Colket JT, Tupler LA, Roth S, David D, Hertzberg M, Mellman T, Beckham JC, Smith RD et al. 1997. Assessment of a new self-rating scale for post-traumatic stress disorder. Psychol Med 27(1):153–60. [DOI] [PubMed] [Google Scholar]
- 31.Melzack R 1975. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1(3):277–99. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. 1989. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 33.Diener E, Emmons RA, Larsen RJ, Griffin S. 1985. The Satisfaction With Life Scale. J Pers Assess 49(1):71–5. [DOI] [PubMed] [Google Scholar]
- 34.Starr LB, Robinson RG, Price TR. 1983. Reliability, validity, and clinical utility of the social functioning exam in the assessment of stroke patients. Exp Aging Res 9(2):101–6. [DOI] [PubMed] [Google Scholar]
- 35.Lyketsos CG, Galik E, Steele C, Steinberg M, Rosenblatt A, Warren A, Sheppard JM, Baker A, Brandt J. 1999. The General Medical Health Rating: a bedside global rating of medical comorbidity in patients with dementia. J Am Geriatr Soc 47(4):487–91. [DOI] [PubMed] [Google Scholar]
- 36.Dash PK, Zhao J, Hergenroeder G, Moore AN. 2010. Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurotherapeutics 7(1):100–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hergenroeder GW, Redell JB, Moore AN, Dash PK. 2008. Biomarkers in the clinical diagnosis and management of traumatic brain injury. Mol Diagn Ther 12(6):345–58. [DOI] [PubMed] [Google Scholar]
- 38.Lumpkins KM, Bochicchio GV, Keledjian K, Simard JM, McCunn M, Scalea T. 2008. Glial fibrillary acidic protein is highly correlated with brain injury. J Trauma 65 (4):778–82; discussion 782–4. [DOI] [PubMed] [Google Scholar]
- 39.Di Battista AP, Buonora JE, Rhind SG, Hutchison MG, Baker AJ, Rizoli SB, Diaz-Arrastia R, Mueller GP. 2015. Blood Biomarkers in Moderate-To-Severe Traumatic Brain Injury: Potential Utility of a Multi-Marker Approach in Characterizing Outcome. Front Neurol 6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J, Korley FK, Dai M, Everett AD. 2015. Serum neurogranin measurement as a biomarker of acute traumatic brain injury. Clin Biochem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korley FK, Diaz-Arrastia R, Wu AH, Yue JK, Manley GTMDPD, Sair HI, Van Eyk J, Everett AD, Okonkwo DO, Valadka A et al. 2015. Circulating Brain Derived Neurotrophic Factor (BDNF) Has Diagnostic and Prognostic Value in Traumatic Brain Injury. J Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Failla MD, Conley YP, Wagner AK. 2015. Brain-Derived Neurotrophic Factor (BDNF) in Traumatic Brain Injury-Related Mortality: Interrelationships Between Genetics and Acute Systemic and Central Nervous System BDNF Profiles. Neurorehabil Neural Repair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmiter RD, Findley SD, Whitmore TE, Durnam DM. 1992. MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci U S A 89(14):6333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kukacka J, Vajtr D, Huska D, Prusa R, Houstava L, Samal F, Diopan V, Kotaska K, Kizek R. 2006. Blood metallothionein, neuron specific enolase, and protein S100B in patients with traumatic brain injury. Neuro Endocrinol Lett 27 Suppl 2:116–20. [PubMed] [Google Scholar]
- 45.Chou SH, Robertson CS, Participants in the International Multi-disciplinary Consensus Conference on the Multimodality M. 2014. Monitoring biomarkers of cellular injury and death in acute brain injury. Neurocrit Care 21 Suppl 2:S187–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Surgucheva I, He S, Rich MC, Sharma R, Ninkina NN, Stahel PF, Surguchov A. 2014. Role of synucleins in traumatic brain injury an experimental in vitro and in vivo study in mice. Mol Cell Neurosci 63:114–23. [DOI] [PubMed] [Google Scholar]
- 47.Buonora JE, Yarnell AM, Lazarus RC, Mousseau M, Latour LL, Rizoli SB, Baker AJ, Rhind SG, Diaz-Arrastia R, Mueller GP. 2015. Multivariate analysis of traumatic brain injury: development of an assessment score. Front Neurol 6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hornik K, Buchta C, Hothorn T, Karatzoglou A, Meyer D, Zeileis A. Package ‘RWeka’ [Internet] Available from: https://cran.r-project.org/web/packages/RWeka/RWeka.pdf
- 49.Quinlan JR. 1993. J. R. C4.5. Programs for Machine Learning. Morgan Kaufmann Publishers. [Google Scholar]
- 50.Spitzer RL. 1983. Psychiatric diagnosis: are clinicians still necessary? Compr Psychiatry 24(5):399–411. [DOI] [PubMed] [Google Scholar]
- 51.Yue JK, Vassar MJ, Lingsma HF, Cooper SR, Okonkwo DO, Valadka AB, Gordon WA, Maas AI, Mukherjee P, Yuh EL et al. 2013. Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J Neurotrauma 30 (22):1831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]