Abstract
Healthcare-associated transmission of monkeypox has been observed on multiple occasions in areas where the disease is endemic. Data collected by the US Centers for Disease Control and Prevention (CDC) from an ongoing CDC-supported program of enhanced surveillance in the Tshuapa Province of the Democratic Republic of the Congo, where the annual incidence of human monkeypox is estimated to be 3.5–5/10,000, suggests that there is approximately one healthcare worker infection for every 100 confirmed monkeypox cases. Herein, we describe a study that commenced in February 2017, the intent of which is to evaluate the effectiveness, immunogenicity, and safety of a third-generation smallpox vaccine, IMVAMUNE®, in healthcare personnel at risk of monkeypox virus (MPXV) infection. We describe procedures for documenting exposures to monkeypox virus infection in study participants, and outline lessons learned that may be of relevance for studies of other investigational medical countermeasures in hard to reach, under-resourced populations.
Keywords: Monkeypox, IMVAMUNE, Smallpox vaccine, Clinical trial, Democratic Republic of the Congo
1. Introduction
It is estimated that more than 1000 cases of human monkeypox (MPX) occur in the Democratic Republic of the Congo (DRC) each year, leading to frequent exposures of healthcare workers (HCWs) to the disease. In this paper, we describe a collaboration among the U.S. Centers for Disease Control and Prevention (CDC), the DRC Ministry of Health (MOH), and the Kinshasa School of Public Health (KSPH), to perform a vaccine study in adult HCWs in Tshuapa, DRC to evaluate the effectiveness of the attenuated smallpox vaccine, IMVAMUNE®, to prevent infection with monkeypox virus (MPXV).
2. Human monkeypox
Human monkeypox was discovered in 1970, when regional elimination of smallpox revealed the occurrence of sporadic cases of a similar disease in rural areas of DRC (Ladnyj et al., 1972). Since that time, the epidemiology and clinical features of the disease have been extensively characterized but no specific medical countermeasures have been introduced. Prevention efforts have been hampered by the fact that MPX is a zoonosis for which a definitive animal reservoir host has not been identified. In addition to the DRC, cases have recently been reported from multiple countries including Nigeria, the Republic of the Congo (ROC), Sierra Leone, Liberia, Cameroon, and the Central African Republic (CAR)(reviewed in (Durski et al., 2018)). In many of these countries, human MPX had not been reported for decades. It has been hypothesized that the increased incidence of MPX may be due to the cessation of routine smallpox vaccination leading to waning orthopoxvirus immunity (Rimoin et al., 2010). Routine smallpox vaccination was discontinued in DRC after smallpox eradication around 1982 (McCollum and Damon, 2014).
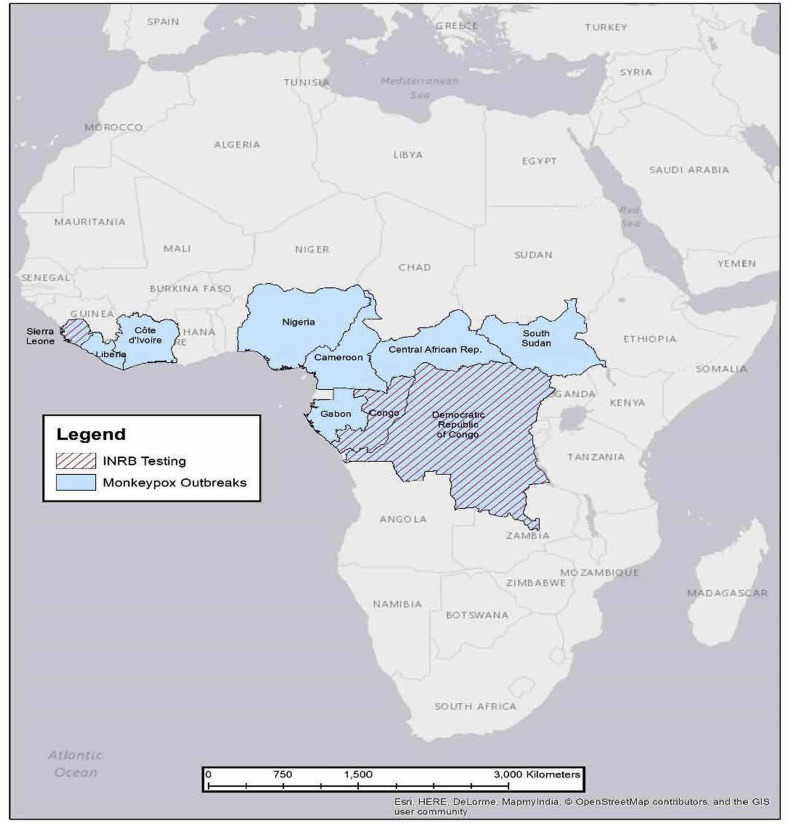
CDC's Poxvirus and Rabies Branch has worked with the DRC MOH and KSPH to strengthen MPX surveillance in Tshuapa Province to further our understanding of the disease. This enhanced surveillance program for human MPX enables routine detection of MPX cases throughout the province. Suspected cases meeting a standardized case definition are investigated by trained personnel using a standardized case report form. Lesion specimens (swabs and/or crusts) are collected from those with active rash illness. Specimens are transported to Kinshasa where laboratory diagnostic testing for orthopoxviruses is performed by the Institut National de Biorecherche (INRB). Laboratory confirmation has provided additional information on the extent of disease in the country (Fig. 1 ), resulting in increased vigilance and more rapid response to situations of public health concern. The INRB has also provided assistance to other African countries, including the ROC and Sierra Leone, for the diagnosis of MPX in suspected cases, and serves as a regional reference facility (Hoff et al., 2017) (Fig. 2 ).
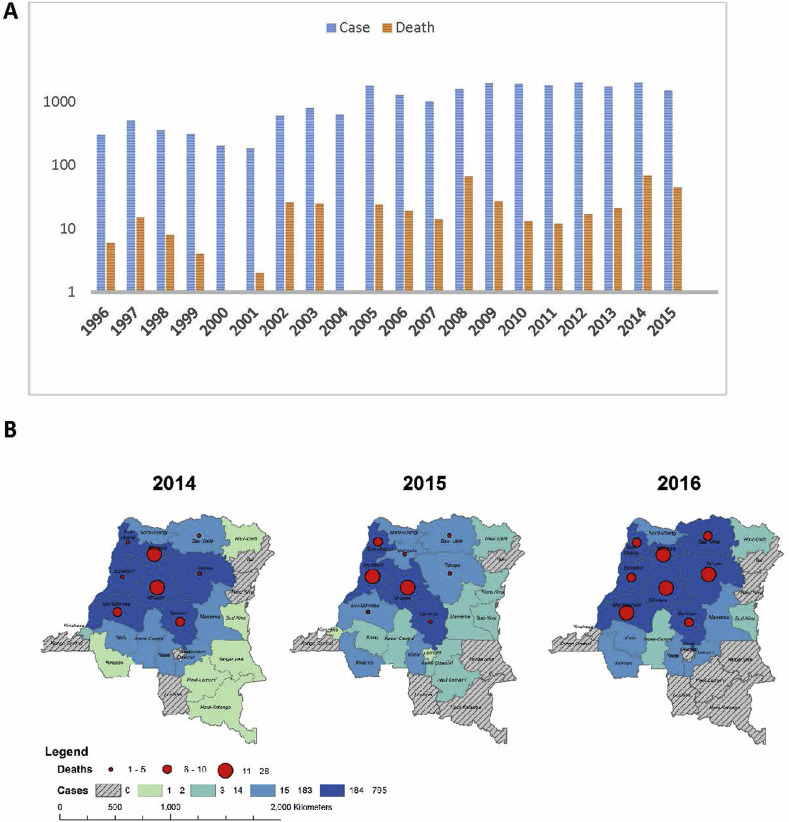
Fig. 1.
(A) Suspect monkeypox case notifications and deaths in the DRC from 1996 to 2016. Cases are often not followed to outcome; thus, this does not represent a fatality rate. (B) Distribution of monkeypox cases and deaths in the DRC from 1996 to 2015 (Nakazawa et al., 2013).
Fig. 2.
Countries that have reported cases of human MPX, including those that have sent samples to the INRB Laboratory in Kinshasa for diagnostic testing from 2007 to 2017.
The establishment of this robust surveillance platform has made it possible to undertake a number of research studies that have contributed to our understanding of the epidemiology of MPX in the region (Shiferaw et al., 2017), and has allowed for passive monitoring of risk groups. This led to the recognition of healthcare workers (HCWs) as a group at enhanced risk for infection (Bass et al., 2013). The platform of surveillance for MPX in Tshuapa also provides a foundation for evaluating whether medical countermeasures developed for smallpox can also be successfully used for MPX.
2.1. Monkeypox in healthcare workers
In many parts of Africa, frontline HCWs are at risk of contracting (and propagating) serious communicable infections such as monkeypox, Ebola, or Marburg. Strengthening infection prevention and control (IPC) in vulnerable clinical setting is a key objective of pandemic preparedness. Healthcare workers risk infection with MPXV when they provide consultation and care for patients with MPX. Risks are heightened if workers are unable to apply adequate infection control measures (i.e., a combination of standard, contact and droplet precaution), due to lack of personal protective equipment or sanitary supplies.
Between 2010 and 2014, 1266 suspect MPX cases were investigated in Tshuapa, wherein eleven instances the case identified themselves as a ‘healthcare worker’ (Table 1 ). Considering all suspect cases between January 2010 and August 2014, the overall proportion of HCW cases was 0.9% (range of 0.6–1.8% by year). Among the 699 confirmed cases during this time period, six confirmed cases of MPX among HCWs represented a proportion of 0.9% (range of 0.3–3.1% by year). On average, 1.5 HCWs were infected per year during the observation period, yielding an estimated annual HCW incidence rate of 17.4/10,000. The elevated incidence of MPX disease among HCWs relative to the general population in Tshuapa District is concerning.
Table 1.
Summary of characteristics of the suspect monkeypox cases among healthcare workers in Tshuapa, DRC, 2010–2014.
| Total (n = 14) | Confirmed MPX Casea (n = 7) | Confirmed VZV Casea (n = 3) | |
|---|---|---|---|
| Male | 13 | 7 | 3 |
| Female | 1 | 0 | 0 |
| Age (n = 12) | |||
| 30-39 | 6 | 3 | 2 |
| 40-49 | 2 | 1 | 1 |
| 50+ | 4 | 3 | 0 |
| Vaccination scar present (n = 12) | 7 | 5 | 1 |
| Exposure to a suspect caseb | |||
| Contact at work | 6 | 3 | 1 |
| Contact with family or friend | 9 | 4 | 2 |
| Lived with the patient | 6 | 4 | 0 |
| Shared a bed with the patient | 2 | 1 | 0 |
| Animal exposureb | |||
| Bushmeat contact | 6 | 3 | 0 |
| Rodents in the home | 3 | 1 | 0 |
Case 4 was confirmed with both MPXV and VZV infections; thus, Case 4 contributed to the counts noted for both columns.
Potential human and zoonotic exposures (not mutually exclusive) in the three weeks prior to symptom onset.
To understand the risk environment for healthcare workers in Tshuapa, we retrospectively investigated 14 instances of suspect HCW infections that occurred between 2009 and 2014. Diagnostic specimens had been collected and tested for 12 of the 14 ill HCWs, 7 were confirmed to have MPX, 3 had chickenpox (varicella-zoster virus, VZV); one of these individuals was found to be co-infected with both. Cases of MPXV and VZV coinfection have been reported in other regions of DRC (Hoff et al., 2017). The presence of a smallpox vaccination scar was examined for 12 cases; 7 cases (58.3%) had a vaccination scar present (Table 1). Five of the 7 confirmed MPX cases (71.4%) had a vaccination scar and one of the 3 confirmed VZV cases (33.3%) had a vaccination scar.
Each HCW reported exposure to a person who had a similar illness; one HCW reported exposure to five suspect cases. Six HCWs (42.9%) reported exposure during the course of their official duties as a HCW, including three of the MPX confirmed cases (42.8%). However, providing healthcare in the home to family members was also a prominent source for potential exposure: nine suspect cases (64.3%) reported potential exposure while caring for an ill family member or friend.
3. Vaccination of healthcare workers
Preventing the occurrence of MPX in HCWs presents a number of challenges. Provision of personal protective equipment appropriate for MPX and training in their use are essential measures for primary prevention of MPX in HCWs. Secondary prevention through the use of smallpox vaccine is another strategy. Studies performed during and in the immediate aftermath of smallpox eradication demonstrated that smallpox vaccination could also confer protection against infection with MPXV (Jezek et al., 1988). However, smallpox vaccines have not been used primarily due to concerns about adverse events (Rimoin and Graham, 2011). For example, the uncertain prevalence of human immunodeficiency virus (HIV) infection or other forms of immunosuppression in monkeypox-endemic areas presents a risk of serious vaccine complications including eczema vaccinatum (Reed et al., 2012) and progressive vaccinia, the latter an adverse event in which uncontrolled vaccinia virus replication commonly results in death (Bray and Wright, 2003). Contact transmission of vaccine virus, fetal vaccinia, and the existence of dermatological risk factors are also major concerns.
Significant advances have been made in vaccine technology since the eradication of smallpox (Table 2 ). First-generation smallpox vaccines used during the eradication were propagated in calf skin and purified from calf lymph. In contrast, second-generation vaccines are propagated in tissue cell culture and produced using modern good manufacturing practices. As such, they have less risk of contamination with adventitious agents. However, both first- and second-generation vaccines contain replication-competent vaccinia virus, which poses a risk of adverse events. Third-generation vaccines are also propagated in tissue culture and produced using modern good manufacturing practices but use attenuated vaccinia viruses with favorable safety profiles.
Table 2.
Characteristics of first-, second- and third-generation smallpox vaccines.
| Vaccine generation | Characteristics of vaccine | Potential Adverse Events |
|---|---|---|
| First (e.g., Dryvax, Aventis Pasteur Smallpox Vaccine, Lister, Tiantan/Temple of Heaven, and EM63 among others) | Vaccines used during the eradication campaign included several different strains of vaccinia virus. Nearly all were propagated in calf lymph. All first-generation vaccines used live, replication-competent virus. A successful vaccination produced a lesion at the site of administration that generated infectious virus. |
|
| Second (e.g., ACAM2000, Lister vaccine produced in primary rabbit kidney cells (RIVM), Elstree-BN, VV Lister/CEP, and CJ-50300) | Second-generation vaccines are propagated in tissue cell culture and produced under good manufacturing practices. As such, they have less risk of contamination with adventitious agents. However, second generation vaccines still contain live, replication-competent vaccinia virus and as such are assumed to present the same risk of adverse events as first-generation vaccines. | Same as above |
| Third (e.g., IMVAMUNE and LC16m8) | IMVAMUNE is derived from Modified Vaccinia Ankara (MVA), a vaccinia virus that has lost the ability to replicate in mammalian cells. Consequently, it does not produce a lesion at the site of vaccination and no longer presents a risk of autoinoculation, inadvertent transmission, or systemic spread. IMVAMUNE was developed for use in persons with increased risk factors for adverse events. |
|
IMVAMUNE is a third-generation smallpox vaccine that has been tested in persons infected with HIV and in persons who have atopic dermatitis (Petersen et al., 2015a). Six published clinical trials have demonstrated the safety and immunogenicity of IMVAMUNE in these populations (Frey et al., 2007; Frey et al., 2013; Greenberg et al., 2013; Vollmar et al., 2006; von et al., 2010; Walsh et al., 2013). Furthermore, protection against monkeypox has also been demonstrated in several animal model studies (Earl et al., 2008; Keckler et al., 2011; Stittelaar et al., 2005). In the United States, the second-generation smallpox vaccine ACAM2000 has been licensed by the Food and Drug Administration (FDA) and purchased for the Strategic National Stockpile (SNS) to be used during an emergency involving smallpox. Vaccination with ACAM2000 is also recommended for select laboratory and healthcare personnel (Petersen et al., 2015b).
Third-generation vaccines are still under development and remain unlicensed in the United States. Nonetheless, IMVAMUNE has completed multiple preclinical and clinical studies and has been purchased for the U.S. SNS to be used under an Emergency Use Authorization (EUA) in the event of a smallpox emergency (Petersen et al., 2015a). In addition, IMVAMUNE (under the trade name IMVANEX) has been granted marketing authorization in the European Union under exceptional circumstances for immunization against smallpox. The development of third-generation vaccines with improved safety profiles shifts the risk/benefit ratio significantly such that they are now feasible for the prevention of MPX. However, because they were developed after the eradication of smallpox, their ability to prevent natural human orthopoxvirus infections has never been demonstrated.
3.1. IMVAMUNE vaccine study
To determine whether the concept of vaccinating HCWs to prevent MPX is feasible and acceptable, the authors of this paper approached two key groups of stakeholders in the DRC to gauge their opinions. First, we approached HCWs in Tshuapa, a sample of whom were systematically surveyed to assess their perceptions of personal risk for MPX and willingness to be vaccinated. The second group queried was a broad group of stakeholders, including representatives of the Congolese Ministry of Health, WHO, Kinshasa School of Public Health, National Institute for Biomedical Research, the Directorate of Pharmacy and Medicines, and the Directorate of Disease Control Immunization Program. For the stakeholder group, we convened a two-day workshop with presentations addressing monkeypox epidemiology in DRC, risks to health workers, vaccine considerations, etc. The workshop concluded with an open discussion about the risk and potential benefits of vaccination as a means to prevent occupationally-acquired MPX infections. Based on feedback received, it was decided to study the ability of vaccination with IMVAMUNE to prevent MPX in DRC HCWs. The study commenced February 2017, and is currently ongoing while study participants undergo immunologic monitoring and follow-up for exposure to MPXV.
The study is a prospective cohort of HCWs, including laboratory workers, aged 18 years and older. Participants receive two doses of IMVAMUNE on days 0 and 28 (Fig. 3 ). Target enrollment for the study is 1000 persons, representing ∼80% of registered HCWs combined in the Boende, Wema, Bokungu and Mondombe health zones within Tshuapa. Study participation is open to male and non-pregnant female HCWs over the age of 18 (some additional exclusion criteria are noted). The primary objectives are to determine the number of suspected and confirmed cases of MPX and the number of MPXV exposures among vaccinated HCWS over a period of observation of two years. The development of MPX disease among participants will be monitored using existing surveillance infrastructure plus health interview and serologic monitoring during follow up visits. Exposures to MPXV will be solicited during follow-up visits and via exposure diaries.
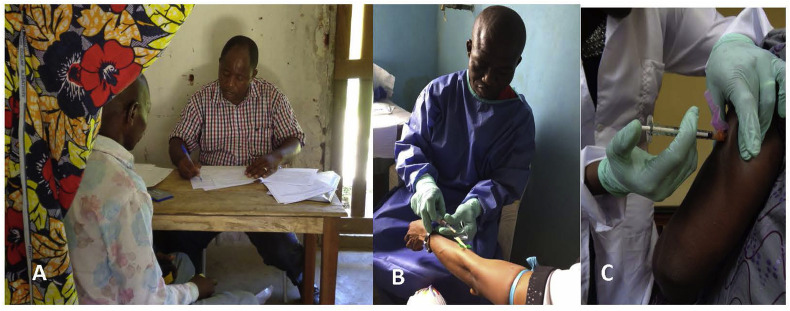
Fig. 3.
IMVAMUNE vaccine study. (A) Prospective participant reviewing study consent documents with local study coordinator. (B) Serum collection prior to vaccination. (C) Vaccination of the first participant in the study.
This data and comparisons to retrospective surveillance data from the same locales will provide the basis for evaluating the effectiveness of the vaccine to prevent MPX. The study is powered to detect a statistically significant reduction in HCW cases based on the historical incidence and case ascertainment rates from the enhanced surveillance data. In addition, serum samples for immunogenicity evaluations will be collected prior to each vaccination (days 0 and 28) and on days 14, 42, 180, 365, 545, and 730 after the receipt of the first dose of vaccine. Antibody titers may serve as a surrogate for effectiveness and provide additional confidence in the vaccine. The time points distant from vaccination may also provide some evidence for the duration of immunity.
The safety of the vaccine is also monitored. Participants maintain an adverse event diary to record systemic and local adverse events (AEs) for 7 days after each immunization. Monitoring safety in this context is important, given that IMVAMUNE has never been studied in Africa and the population in Tshuapa differs in many respects from the population that have received IMVAMUNE in clinical trials to date. All previous clinical trials took place in Europe or the United States and involved populations that typically have different background health concerns than those in Africa where MPX occurs (i.e., populations in the United States and Western Europe generally experience a lower burden of infectious and chronic conditions) (Norheim et al., 2015).
3.2. Ethical considerations
There are many species of animal, in addition to the theoretical reservoir host, that are susceptible to MPXV infection and are competent to transmit the virus to humans. This effectively prevents consideration of disease eradication, meaning there will always be a need for interventions against this disease. IMVAMUNE may prove to be an important armament for protection of high-risk groups, such as HCWs, hunters or other persons who may be frequently exposed to MPXV.
HCWs were selected to be the participants in this vaccine study for several reasons. Surveillance data have shown that HCWs in Tshuapa are at increased risk of MPX infection. Thus, from a technical perspective, focusing on HCWs allows the best chance to demonstrate the effectiveness of vaccine to prevent disease. Infection with MPXV remains a relatively rare event, even among HCWs in this endemic region. A properly powered case-control study would require such large numbers of participants as to make it unfeasible. On the other hand, a prospective cohort study has the potential to achieve statistical significance.
From an ethical standpoint, the selection of HCWs upholds the principles of beneficence and justice. Beneficence requires an investigator to maximize benefits for the individual participant and/or society, while minimizing risk of harm to the individual. Participants in the study are expected to acquire protection against MPX infection based on current immunogenicity and efficacy data in preclinical and clinical studies. As such, protection from MPX would benefit the individual participant as well as society by preventing forward transmission to others and providing greater safety to HCWs who care for MPX patients. Furthermore, the risks of harm from IMVAMUNE are lower compared to traditional smallpox vaccines and likely outweighed by the potential benefits. Justice demands the equitable selection of participants such that those who undertake the burdens of research must be likely to benefit from the research. HCWs in this study are likely to directly benefit from this research if the vaccine proves to be effective given the increased risk of MPXV infection among this population. This study is registered in ClinicalTrials.gov under identifier NCT02977715.
3.3. Experience to date and lessons learned
Performing clinical studies in rural, resource-limited settings such as Tshuapa presents numerous challenges. Finding practical solutions will become increasingly important as new, investigational medical countermeasures are developed for diseases such as MPX, Ebola virus disease and cholera. The IMVAMUNE vaccine study has encountered difficulties in augmenting the existing cold chain to accommodate both distribution of vaccine as well as temporary storage of blood samples after collection. These were overcome by the use of solar-powered refrigerators in conjunction with cold boxes such as the Credo cube for vaccine transport. The transition of IMVAMUNE from a liquid frozen to a lyophilized formulation has the potential to improve the cold chain parameters and simplify usage in the field (Frey et al., 2015). Other challenges included those that were foreseen, such as how to avoid of exhaustion of supply inventories, and others we did not anticipate, such as staff fatigue which mounted over the 16 weeks required for initial enrollment of participants.
Another difficulty encountered early on was the amount of time required to explain to each prospective participant the risks and benefits of participating in the study, answer questions, and complete both the informed consent and medical screening forms. These steps are vitally important to ensure that each prospective enrollee is thoroughly informed about the study and about his/her rights as a research subject. Recognizing this as an imperative, we attempted to streamline the process, while maintaining its rigor, by partitioning the informed consent and enrollment process into three defined steps. In the first step, prospective enrollees were gathered successively into groups of about 25 persons for demonstration sessions, during which a short video about the study was shown. These sessions were led by a trained health educator. Next, each large group was broken into 3–5 smaller groups, each of which met with a study coordinator who explained the specific elements of informed consent to the prospective participants.
Lastly, each person met individually with a senior Kinshasa-based member of the study team for a question and answer session, at the end of which the prospective enrollee either provided consent or declined to participate in the study. Having the step performed by Kinshasa-based personnel offered additional reassurance of confidentiality for participants who might have felt uncomfortable discussing sensitive topics (e.g., HIV infection status) with locally-based study staff. Afterward, prospective participants were medically screened in private. They were encouraged to ask questions at each step throughout the process, both in the large group and in the private sessions. By applying this streamlined process, were able to routinely enroll >100 participants per day.
4. Next steps
Serologic monitoring of study participants will continue at roughly 6-month intervals until the two-year timepoint is reached. Until that time, the study team will continue to collect information pertaining to participants' occupational exposures to MPX cases—presumptive and confirmed—, MPX infection status, and possible vaccine adverse events. Finally, as a surrogate measure for immunization efficacy, we will measure participants' serum orthopoxvirus antibody titers and will assess whether specific participant characteristics (e.g., age, sex, prior smallpox immunization) are associated with more enduring antibody levels.
Despite advances in medical countermeasures for smallpox, including new vaccines and antivirals, the potential for the use of these products against other orthopoxviruses (e.g., bovine vaccinia, buffalopox) has not been fully explored, nor has there been extensive consideration of their use in a post-exposure setting (e.g., for ring vaccination). While extensive preclinical and clinical studies are required for licensure of these products, the “animal rule” established by the FDA allows certain products to be licensed without directly demonstrating efficacy in humans (Burns, 2012). The frequent occurrence of human MPX infection in the DRC provides an opportunity to fill this gap. Understanding the performance parameters of IMVAMUNE under conditions of natural orthopoxvirus transmission will build confidence in its use as a preventive measure for many populations potentially at risk, including HCWs, and, in the future, perhaps hunters or other groups with elevated risk.
In addition to gathering information on effectiveness and safety, administering the vaccine in the field provides further logistical experience that can be applied to preparedness planning for smallpox as well as MPX. It is worth noting that since the eradication of smallpox, MPX virus is the only orthopoxvirus to cause an outbreak in the United States (Reed et al., 2004). It is prudent to be prepared for outbreaks of both smallpox and MPX.
The MPX surveillance program and IMVAMUNE vaccine study in Tshuapa highlight the unique opportunity to put smallpox medical countermeasures to good use in populations who need them, while at the same time gathering valuable information to ensure their appropriate and efficient use. Lessons learned pertaining to maintenance of a cold chain, the need to mitigate staff fatigue and ways to streamline and strengthen the informed consent process may be of value to others embarking upon investigational vaccine trials in rural DRC, and in other regions affected by MPX.
Funding
Funding for this project comes from the US Centers for Disease Control and Prevention.
Acknowledgements
The authors of this paper would like to thank colleagues from the US Centers for Disease Control and Prevention, the Kinshasa School of Public Health, the Congolese Ministry of Health and the University of Kinshasa for their contributions to this study.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Bass J., Tack D.M., McCollum A.M., Kabamba J., Pakuta E., Malekani J., Nguete B., Monroe B.P., Doty J.B., Karhemere S., Damon I.K., Balilo M., Okitolonda E., Shongo R.L., Reynolds M.G. Enhancing healthcare worker ability to detect and care for patients with monkeypox in the Democratic Republic of the Congo. Int. Health. 2013;5:237–243. doi: 10.1093/inthealth/iht029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M., Wright M.E. Progressive vaccinia. Clin. Infect. Dis. 2003;36:766–774. doi: 10.1086/374244. [DOI] [PubMed] [Google Scholar]
- Burns D.L. Licensure of vaccines using the animal rule. Curr. Opin. Virol. 2012;2:353–356. doi: 10.1016/j.coviro.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Durski K.N., McCollum A.M., Nakazawa Y., Petersen B.W., Reynolds M.G., Briand S., Djingarey M.H., Olson V., Damon I.K., Khalakdina A. Emergence of monkeypox - West and Central Africa, 1970-2017. MMWR Morb. Mortal. Wkly. Rep. 2018;67:306–310. doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl J.L., Americo P.L., Wyatt L.S., et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10889–10894. doi: 10.1073/pnas.0804985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S.E., Newman F.K., Kennedy J.S., Sobek V., Ennis F.A., Hill H., Yan L.K., Chaplin P., Vollmar J., Chaitman B.R., Belshe R.B. Clinical and immunologic responses to multiple doses of IMVAMUNE (Modified Vaccinia Ankara) followed by Dryvax challenge. Vaccine. 2007;25:8562–8573. doi: 10.1016/j.vaccine.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S.E., Winokur P.L., Salata R.A., El-Kamary S.S., Turley C.B., Walter E.B., Jr., Hay C.M., Newman F.K., Hill H.R., Zhang Y., Chaplin P., Tary-Lehmann M., Belshe R.B. Safety and immunogenicity of IMVAMUNE(R) smallpox vaccine using different strategies for a post event scenario. Vaccine. 2013;31:3025–3033. doi: 10.1016/j.vaccine.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S.E., Wald A., Edupuganti S., Jackson L.A., Stapleton J.T., El S.H., El-Kamary S.S., Edwards K., Keyserling H., Winokur P., Keitel W., Hill H., Goll J.B., Anderson E.L., Graham I.L., Johnston C., Mulligan M., Rouphael N., Atmar R., Patel S., Chen W., Kotloff K., Creech C.B., Chaplin P., Belshe R.B. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naive subjects. Vaccine. 2015;33:5225–5234. doi: 10.1016/j.vaccine.2015.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R.N., Overton E.T., Haas D.W., Frank I., Goldman M., von K.A., Virgin G., Badeker N., Vollmar J., Chaplin P. Safety, immunogenicity, and surrogate markers of clinical efficacy for modified vaccinia Ankara as a smallpox vaccine in HIV-infected subjects. J. Infect. Dis. 2013;207:749–758. doi: 10.1093/infdis/jis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff N.A., Morier D.S., Kisalu N.K., Johnston S.C., Doshi R.H., Hensley L.E., Okitolonda-Wemakoy E., Muyembe-Tamfum J.J., Lloyd-Smith J.O., Rimoin A.W. Varicella coinfection in patients with active monkeypox in the democratic Republic of the Congo. EcoHealth. 2017 Sep;14(3):564–574. doi: 10.1007/s10393-017-1266-5. [DOI] [PubMed] [Google Scholar]
- Jezek Z., Grab B., Szczeniowski M.V., Paluku K.M., Mutombo M. Human monkeypox: secondary attack rates. Bull. World Health Organ. 1988;66:465–470. [PMC free article] [PubMed] [Google Scholar]
- Keckler M.S., Carroll D.S., Gallardo-Romero N.F., et al. Establishment of the black-tailed prairie dog (Cynomys ludovicianus) as a novel animal model for comparing smallpox vaccines administered preexposure in both high- and low-dose monkeypox virus challenges. J. Virol. 2011;85:7683–7698. doi: 10.1128/JVI.02174-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladnyj I.D., Ziegler P., Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972;46:593–597. [PMC free article] [PubMed] [Google Scholar]
- Lane J.M., et al. Complications of smallpox vaccination, 1968: results of ten statewide surveys. J. Infect. Dis. 1970;122(4):303–309. doi: 10.1093/infdis/122.4.303. [DOI] [PubMed] [Google Scholar]
- Lane J.M., et al. Complications of smallpox vaccination, 1968. N. Engl. J. Med. 1969;281(22):1201–1208. doi: 10.1056/NEJM196911272812201. [DOI] [PubMed] [Google Scholar]
- McCollum A.M., Damon I.K. Human monkeypox. Clin. Infect. Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora L.F., Khan A.H., Sperling L.S. Cardiac complications after smallpox vaccination. South Med. J. 2009;102(6):615–619. doi: 10.1097/SMJ.0b013e31819fe55b. [DOI] [PubMed] [Google Scholar]
- Morgan J., et al. Myocarditis, pericarditis, and dilated cardiomyopathy after smallpox vaccination among civilians in the United States, January-October 2003. Clin. Infect. Dis. 2008;46(Suppl 3):S242–S250. doi: 10.1086/524747. [DOI] [PubMed] [Google Scholar]
- Nakazawa Y., Lash R.R., Carroll D.S., Damon I.K., Karem K.L., Reynolds M.G., Osorio J.E., Rocke T.E., Malekani J.M., Muyembe J.J., Formenty P., Peterson A.T. Mapping monkeypox transmission risk through time and space in the Congo Basin. PloS One. 2013;8 doi: 10.1371/journal.pone.0074816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norheim O.F., Jha P., Admasu K., Godal T., Hum R.J., Kruk M.E., Gomez-Dantes O., Mathers C.D., Pan H., Sepulveda J., Suraweera W., Verguet S., Woldemariam A.T., Yamey G., Jamison D.T., Peto R. Avoiding 40% of the premature deaths in each country, 2010-30: review of national mortality trends to help quantify the UN sustainable development goal for health. Lancet. 2015;385:239–252. doi: 10.1016/S0140-6736(14)61591-9. [DOI] [PubMed] [Google Scholar]
- Petersen B.W., Damon I.K., Pertowski C.A., Meaney-Delman D., Guarnizo J.T., Beigi R.H., Edwards K.M., Fisher M.C., Frey S.E., Lynfield R., Willoughby R.E. Clinical guidance for smallpox vaccine use in a postevent vaccination program. MMWR Recomm. Rep. 2015;64:1–26. [PubMed] [Google Scholar]
- Petersen B.W., Harms T.J., Reynolds M.G., Harrison L.H. Use of vaccinia virus smallpox vaccine in laboratory and healthcare personnel at risk for occupational exposure to orthopoxviruses - recommendations of the advisory committee on immunization practices (ACIP) MMWR Morb. Mortal. Wkly. Rep. 2016. 2015;65:257–262. doi: 10.15585/mmwr.mm6510a2. [DOI] [PubMed] [Google Scholar]
- Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., Kazmierczak J.J., Stratman E.J., Li Y., Fairley J.A., Swain G.R., Olson V.A., Sargent E.K., Kehl S.C., Frace M.A., Kline R., Foldy S.L., Davis J.P., Damon I.K. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- Reed J.L., Scott D.E., Bray M. Eczema vaccinatum. Clin. Infect. Dis. 2012;54:832–840. doi: 10.1093/cid/cir952. [DOI] [PubMed] [Google Scholar]
- Rimoin A.W., Graham B.S. Whither monkeypox vaccination. Vaccine. 2011;29(Suppl. 4):D60–D64. doi: 10.1016/j.vaccine.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoin A.W., Mulembakani P.M., Johnston S.C., Lloyd Smith J.O., Kisalu N.K., Kinkela T.L., Blumberg S., Thomassen H.A., Pike B.L., Fair J.N., Wolfe N.D., Shongo R.L., Graham B.S., Formenty P., Okitolonda E., Hensley L.E., Meyer H., Wright L.L., Muyembe J.J. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M.A., Seward J.F., T. Smallpox Vaccine in Pregnancy Registry, Pregnancy, birth, and infant health outcomes from the National Smallpox Vaccine in Pregnancy Registry, 2003-2006. Clin. Infect. Dis. 2008;46(Suppl 3):S221–S226. doi: 10.1086/524744. [DOI] [PubMed] [Google Scholar]
- Shiferaw M.L., Doty J.B., Maghlakelidze G., Morgan J., Khmaladze E., Parkadze O., Donduashvili M., Wemakoy E.O., Muyembe J.J., Mulumba L., Malekani J., Kabamba J., Kanter T., Boulanger L.L., Haile A., Bekele A., Bekele M., Tafese K., McCollum A.A., Reynolds M.G. Frameworks for preventing, detecting, and Controlling zoonotic diseases. Emerg. Infect. Dis. 2017;23 doi: 10.3201/eid2313.170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stittelaar K.J., van Amerongen G., Kondova I., Kuiken T., van Lavieren R.F., Pistoor F.H., Niesters H.G., van Doornum G., van der Zeijst B.A., Mateo L., Chaplin P.J., Osterhaus A.D. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J. Virol. 2005 Jun;79(12):7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar J., Arndtz N., Eckl K.M., Thomsen T., Petzold B., Mateo L., Schlereth B., Handley A., King L., Hulsemann V., Tzatzaris M., Merkl K., Wulff N., Chaplin P. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine. 2006;24:2065–2070. doi: 10.1016/j.vaccine.2005.11.022. [DOI] [PubMed] [Google Scholar]
- von K.A., Vollmar J., Pokorny R., Rapp P., Wulff N., Petzold B., Handley A., Mateo L., Siersbol H., Kollaritsch H., Chaplin P. A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE. Vaccine. 2010;28:1209–1216. doi: 10.1016/j.vaccine.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S.R., Wilck M.B., Dominguez D.J., Zablowsky E., Bajimaya S., Gagne L.S., Verrill K.A., Kleinjan J.A., Patel A., Zhang Y., Hill H., Acharyya A., Fisher D.C., Antin J.H., Seaman M.S., Dolin R., Baden L.R. Safety and immunogenicity of modified vaccinia Ankara in hematopoietic stem cell transplant recipients: a randomized, controlled trial. J. Infect. Dis. 2013;207:1888–1897. doi: 10.1093/infdis/jit105. [DOI] [PMC free article] [PubMed] [Google Scholar]