Abstract
Every year the bites of Anopheles mosquitoes kill hundreds of thousands of people, mostly young African children, by transmitting deadly Plasmodium falciparum malaria parasites. Since the turn of the century, efforts to prevent transmission of these parasites via the mass distribution of insecticide-treated bed nets have been extremely successful, causing an unprecedented reduction in malaria deaths1. However, resistance to insecticides has become widespread in Anopheles populations2–4, threatening a global resurgence of the disease and making the generation of effective new malaria control tools an urgent public health priority. Here, we show that development of P. falciparum can be rapidly and completely blocked when Anopheles gambiae females uptake low concentrations of specific antimalarials from treated surfaces, simulating contact with a bed net. Mosquito exposure to atovaquone prior to or shortly after P. falciparum infection causes full parasite arrest in the female midgut, preventing transmission of infection. Similar transmission-blocking effects are achieved with other cytochrome B inhibitors, demonstrating that parasite mitochondrial function is a good target for parasite killing. Incorporating these effects into a model of malaria transmission dynamics predicts that the inclusion of Plasmodium inhibitors on mosquito nets would significantly mitigate the global health impact of insecticide resistance. This study identifies a powerful new strategy for blocking Plasmodium transmission by Anopheles females, with promising implications for malaria eradication efforts.
Significant strides have been made in malaria control since the introduction of insecticide-based strategies targeting the Anopheles mosquito species that transmit Plasmodium parasites. Long-lasting insecticide treated bed nets (LLINs) alone are predicted to be responsible for 68% of all malaria cases averted since the beginning of the 21st century, and together with indoor residual insecticide spraying (IRS) of house walls represent a cornerstone of malaria control efforts1. The pervasive use of these strategies has, however, caused an alarming spread of resistance to insecticides in all major Anopheles populations in malaria-endemic countries2–5. Containment and management of this issue has been undermined by the lack of approved active ingredients for LLINs, which, until recently6–8, were limited to pyrethroids. Undoubtedly, the rapid decline in insecticide efficacy constitutes a pressing public health emergency threatening to roll back much of the progress made towards eliminating malaria since the introduction of LLINs. Indeed, after a period of steady decline in annual clinical cases, sub-Saharan Africa and other geographies have experienced a plateau or even an increase in malaria incidence9. As many countries move towards a focus on not just malaria control but elimination, it is imperative that more and improved tools to stop parasite transmission by the Anopheles mosquito are generated.
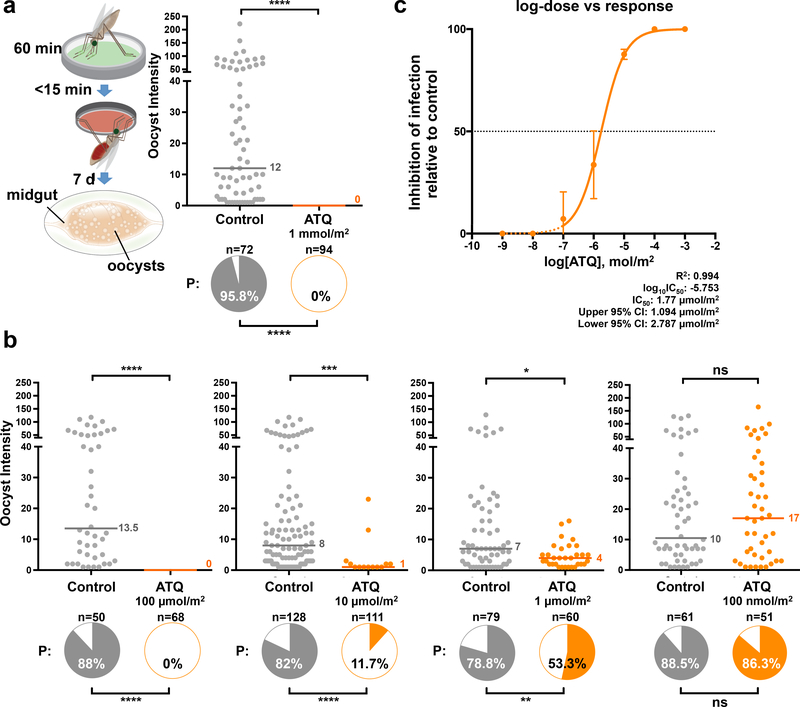
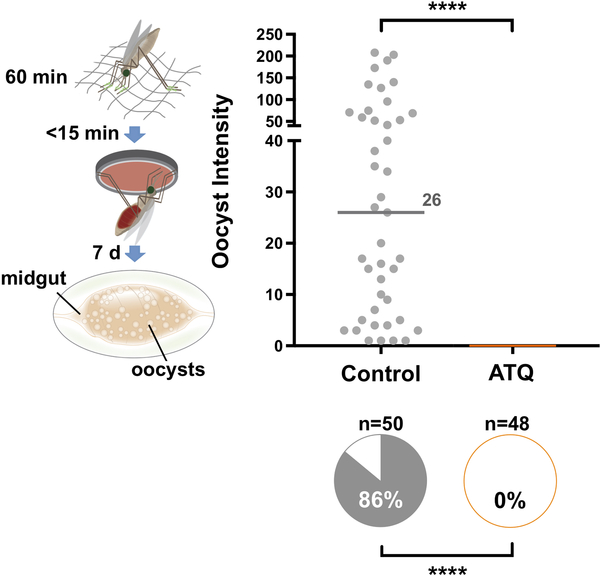
Besides LLINs and IRS, malaria control strategies heavily rely on drugs to cure Plasmodium infections in humans, the current gold standard treatment being the use of artemisinin-based combination therapy (ACT)9. We reasoned that it may be possible to use antimalarial compounds to also clear Plasmodium infections directly in the Anopheles mosquito, employing delivery methods equivalent to mosquito contact with insecticides on a bed net or wall. This rationale exploits the fact that generally fewer than 100 P. falciparum ookinetes successfully cross the midgut epithelium to form oocysts, representing a significant bottleneck to transmission. To test this approach, we coated a glass substrate with the potent parasite cytochrome B inhibitor atovaquone (ATQ) and allowed An. gambiae females to rest on this surface immediately prior to P. falciparum infection. This tarsal exposure (i.e. via the mosquito legs) is based on a modified WHO insecticide assay10 which simulates how mosquitoes uptake insecticides on LLINs and IRS. Due to its highly lipophilic nature, we hypothesized that ATQ would be capable of traversing the insect cuticle, killing the parasite during sporogony. Strikingly, no P. falciparum oocysts were detected in ATQ-treated females (1 mmol/m2 for 60 minutes) at 7 days post an infectious blood meal (pIBM), while control, mock-exposed individuals showed high infection prevalence and intensity (Fig. 1a). To characterize the protective effect of ATQ, we performed a dilution series of exposures, and observed complete blockade of P. falciparum development using a tenfold lower ATQ concentration (100 μmol/m2), while at as low as 10 μmol/m2 we still found significant inhibition of infection prevalence (87.6% inhibition) and intensity (87.5% inhibition) (Fig. 1b). Further ATQ dilutions had a progressively reduced, dose-dependent inhibitory effect (Fig. 1b). By interpolating these data onto a dose-response curve we calculated the IC50 of ATQ exposure as a surface concentration of 1.77 μmol/m2 (Fig. 1c). This is comparable to the LC50 of the potent neurotoxic LLIN insecticide permethrin in susceptible An. gambiae (63 μmol/m2 for a 60-minute tarsal exposure11).
Figure 1: An. gambiae exposure to atovaquone (ATQ) aborts P. falciparum development.
(a) P. falciparum parasites are completely eliminated (0% oocyst intensity, and 0% prevalence of infection, shown in the pie charts) in females exposed to 1 mmol/m2 ATQ for 60 minutes immediately prior to infection (Prevalence: Two-sided Chi2, n = 166, df = 1, χ2 = 155.14, p < 0.0001). The exposure method is shown in the graphic: green represents ATQ coated onto a glass surface. (b) Dose-dependent inhibition (range: 100 μmol/m2 - 100 nmol/m2) of P. falciparum infection by exposure to ATQ. Significant reductions in prevalence and intensity were observed at doses as low as 1 μmol/m2 (Prevalence: Two-sided Chi2. 100 μmol/m2: n = 118, df = 1, χ2 = 95.42, p < 0.0001. 10 μmol/m2: n = 239, df = 1, χ2 = 117.6, p < 0.0001. 1 μmol/m2: n = 139, df = 1, χ2 = 9.85, p = 0.0017. Intensity: Two-sided Mann-Whitney: 10 μmol/m2: n = 239, df = 1, U = 287.5, p = 0.0004. 1 μmol/m2: n = 139, df = 1, U = 686, p = 0.0104). (c) Dose-response curve fit for ATQ exposure (Non-linear regression, n = 13, df = 12, Sum of Squares = 1003, R2 = 0.9441). The IC50 for ATQ pre-infection exposure, calculated by interpolation, is indicated. Mean inhibition relative to control prevalence is indicated. Error bars are 95% CI. Dashed portions of the sigmoidal fit are estimated. In all panels where relevant, statistical significance is indicated as so: ns = not significant, * = p < 0.05, ** = p < 0.01, *** = < 0.001, **** = p < 0.0001. Medians are indicated. For (a) and (b), n indicates the number of biologically independent mosquito samples. For (c), n indicates the relative inhibition observed in ATQ-treated mosquitoes in independent experiments.
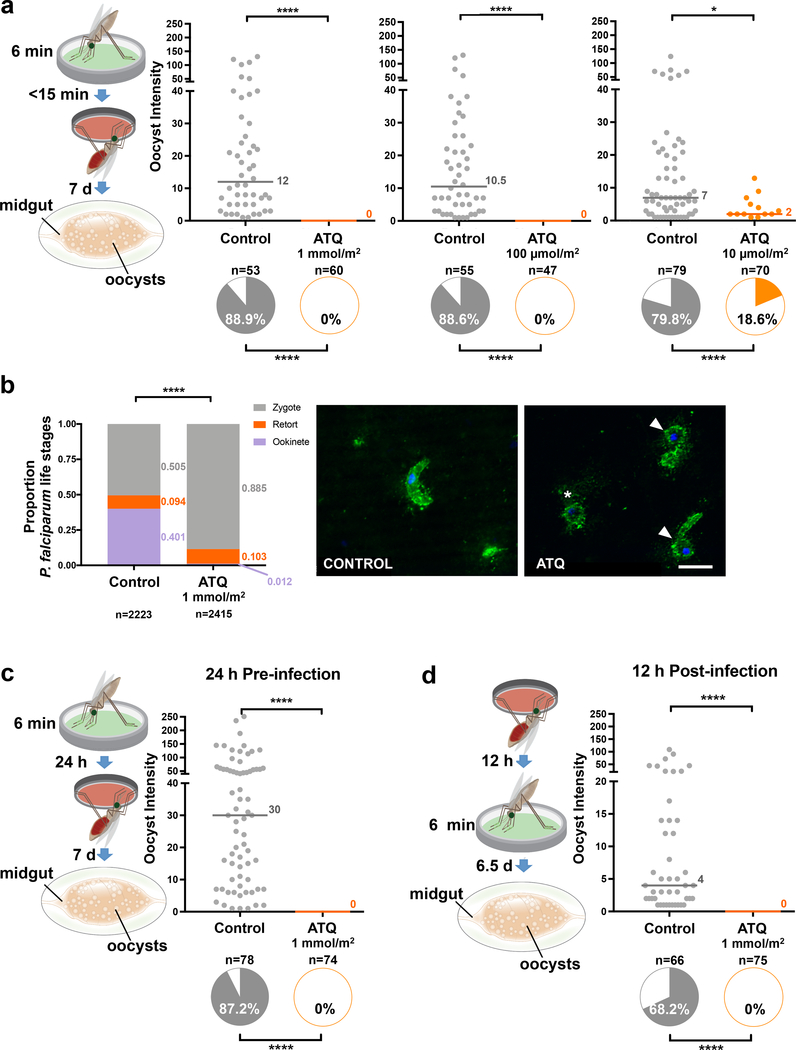
Importantly, killing of P. falciparum parasites was similarly effective when exposure time was reduced to 6 minutes (Fig. 2a), indicating that transmission-blocking doses of ATQ are taken up across the insect cuticle within a short timeframe that is compatible with reported contact times for host-seeking mosquitoes on LLINs12. Parasites were killed at the early zygote-ookinete transition, as determined in immunofluorescent assays (IFAs) of infected midguts (Fig. 2b). These data are consistent with previous studies showing that ookinetes are arrested when mosquitoes feed on P. berghei-infected mice injected with ATQ13 or when parasites are cultured in vitro in the presence of this antimalarial drug14. Parasite development was also completely aborted when mosquitoes were exposed to ATQ either 24 hours prior to or 12 hours after infection (Fig. 2c, d). These findings indicate that ATQ-like antimalarials could be incorporated into other control interventions beside treated nets, including attractive toxic sugar baits where females become exposed to chemicals while sugar feeding15, or IRS where contact occurs while females are resting before or after blood feeding. Notably, ATQ exposure had no fitness costs to the mosquito in terms of survival and reproductive output (Extended Data Fig. 1).
Figure 2: The transmission blocking activity of ATQ is maintained at shorter exposure times and at time points of exposure before and after infection.
(a) P. falciparum parasites are completely eliminated (0% oocyst intensity, and 0% prevalence of infection, shown in the pie charts) in females exposed to either 1 mmol/m2 or 100 μmol/m2 ATQ for 6 min (Prevalence: Two-sided Chi2. 1 mmol/m2: n = 113, df = 1, χ2 = 91.00, p < 0.0001. 100 μmol/m2: n = 102, df = 1, χ2 = 80.59, p < 0.0001). At 10 μmol/m2, prevalence of infection (10 μmol/m2: n = 149, df = 1, χ2 = 55.58, p < 0.0001) and median oocyst intensity (2-sided Mann-Whitney, n = 149, df = 1, U = 258, p = 0.0349) are significantly reduced in the ATQ-treated group. Medians are indicated. (b) IFAs of mosquito midgut lumens 21 h post P. falciparum infection using parasite-specific antibodies (anti-PfS25, green) and DNA (DAPI, blue) staining. Example images from 14 independent mosquito midgut samples (7 control, 7 ATQ-treated); P. falciparum forms are shown. Left panel: mature ookinete in controls. Right panel: zygote (asterisk) and retort forms (white arrows) in ATQ-treated females. ATQ-treated females have few ookinetes (1.2% total parasites) and a large proportion of zygotes (88.5% total parasites), indicating parasite arrest, while controls contain a significantly larger proportion of normal ookinetes (40.1%, Nominal Logistic Regression, n = 5091, df = 14, χ2 = 1620.88, p < 0.0001). Scale bar: 10 μm. (c, d) P. falciparum parasites are completely eliminated also when females are exposed to ATQ (1 mmol/m2, 6 min) either (c) 24 h prior (2-sided Chi2 w/Bonferroni correction, n= 152, df = 1, χ2 = 116.74, p < 0.0001) or (d) 12 h after (2-sided Chi2 w/ Bonferroni correction, n = 141, df = 1, χ2 = 75.11, p < 0.0001) an infectious blood meal. Medians are indicated. Where relevant, statistical significance is indicated as so: * = p < 0.05, ** = p < 0.01, *** = < 0.001, **** = p < 0.0001. For (a), (c) and (d), n indicates the number of biologically independent mosquito samples. For (b), n indicates the number of independent parasite forms.
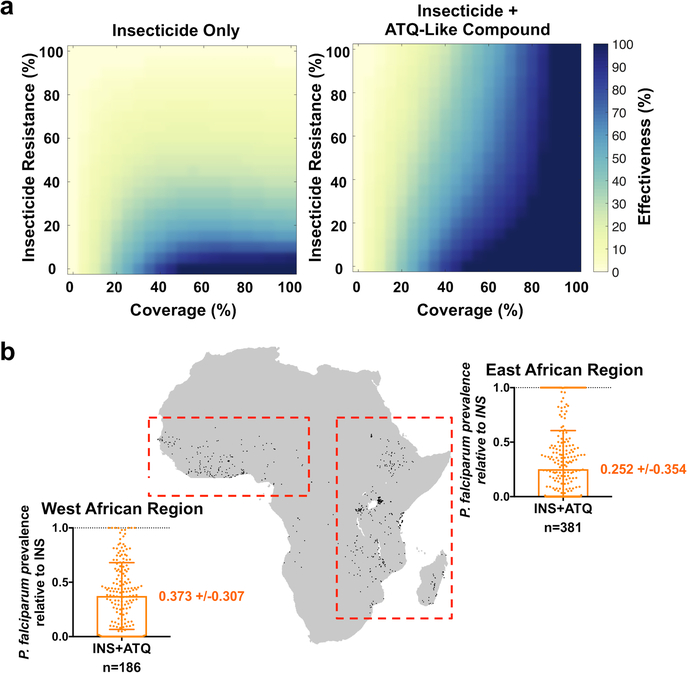
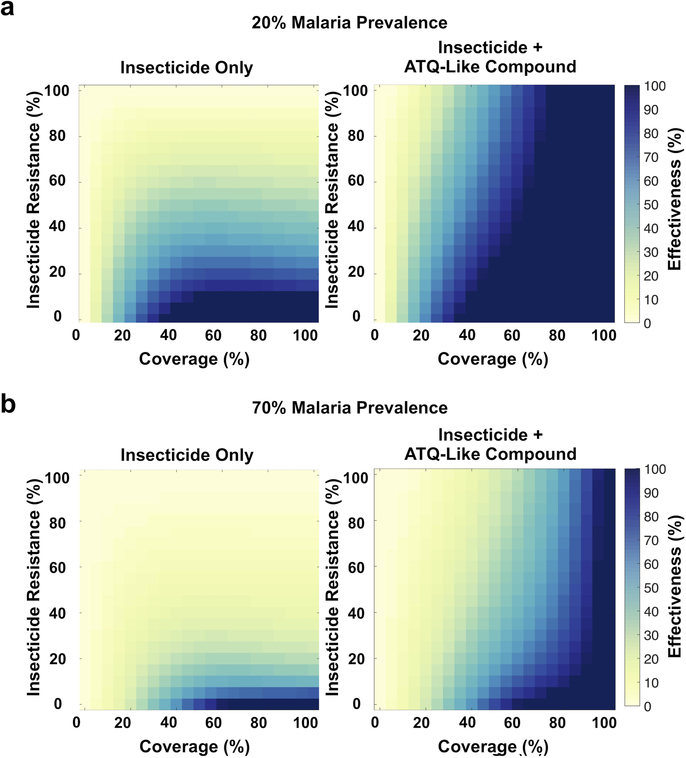
We next incorporated these results into a mathematical model of malaria transmission that includes mosquito population dynamics and human malaria infection16 (Extended Data Fig. 2). The effects of the introduction of either conventional insecticide-treated nets, or nets combining insecticides with a compound that has ATQ-like properties, were modeled in populations with varying malaria transmission intensity (20–80% human infection prevalence), at varying intervention coverage (0–100%) and insecticide resistance levels in the vector population (0–100%). We assumed 100% blocking of new Plasmodium infections on the same day of exposure to the antimalarial compound, and no effects on ongoing infections. In the presence of insecticide resistance, the application of ATQ-like compounds to nets is always predicted to reduce malaria prevalence, with the extent of this reduction being dependent on the level of malaria transmission, the coverage, and the degree of insecticide resistance (Extended Data Fig. 3). ATQ-like compounds significantly increased the effectiveness of the control intervention under a broad range of scenarios, facilitating malaria suppression across transmission settings relevant in Africa and in other malaria-endemic regions (Fig. 3a, Extended Data Fig. 4). Moreover, we modeled 567 specific locations in West and East Africa for which recent (2013–2018) insecticide resistance data is available17, incorporating estimates of P. falciparum prevalence and LLIN coverage from 20151. Consistent with our other model outputs, these data predict that adding an ATQ-like compound to LLINs would appreciably reduce P. falciparum prevalence in these areas (Fig. 3b). Incorporation of ATQ-like compounds would therefore markedly expand the lifespan of insecticide-based strategies, a factor particularly important in transmission hot spots where resistance to pyrethroids is nearly total2.
Figure 3: Malaria transmission model predicts that adding ATQ to insecticide-treated nets would increase bed net effectiveness.
(a) Heat maps of changes in malaria transmission for bed net-like interventions using insecticide alone or insecticide plus an ATQ-like compound, relative to no intervention at varying coverage and varying insecticide resistance levels. The model considers an intermediate 45% prevalence of human infection (effects at lower and higher malaria prevalence are described in Extended Fig. 4). The “effectiveness” of the interventions is defined as (1 - proportion reduction in malaria transmission relative to no intervention) and is represented as colors ranging from yellow (no change in malaria transmission) to dark blue (elimination of malaria transmission) at varying levels of coverage (x-axis) and insecticide resistance (y-axis). Insecticide resistance is the percentage of mosquitoes that are impervious to insecticide; coverage is the probability of a mosquito encountering an intervention during a single feeding episode. (b) Predicted effects of adding ATQ to existing insecticide-treated nets in 567 African locations with available insecticide resistance data (indicated by black dots on the map of Africa). For each location, the model considers the estimated bed net coverage and P. falciparum prevalence in 2–10 year old children reported in 20151, and insecticide resistance levels reported between 2013 and 201817. The graphs show mean malaria prevalence for insecticide/ATQ combination bed nets (INS+ATQ), relative to insecticide only bed nets (dotted line at y = 1), for sampled sites in West and East Africa (red boxes, n = 186 and n = 381, respectively). Error bars represent one standard deviation from the mean prevalence. In both (a) and (b), the model outputs demonstrate that addition of ATQ substantially increases the ability of treated nets to reduce malaria transmission across a broad range of transmission settings.
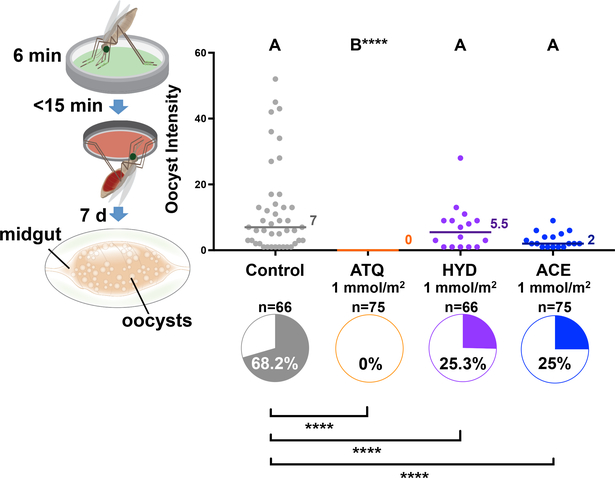
ATQ acts by displacing ubiquinone (CoQ) from the QO site of complex III (Cytochrome bc1) of the mitochondrial electron transport chain (mtETC), disrupting the mitochondrial membrane potential (ΔΨm) and thus inhibiting both mitochondrial ATP production18 and de novo pyrimidine synthesis19. We next tested additional compounds with anti-cytochrome B activity not in clinical use, but with potential to be rapidly adapted for use in mosquito-targeting interventions: the registered insecticides acequinocyl (ACE) and hydramethylnon (HYD), and the veterinary drug decoquinate (DEC). We also included the dihydrofolate reductase inhibitor pyrimethamine (PYR)20, a compound with extremely potent transmission-blocking activity in humans that acts by disrupting parasite DNA replication (Extended Data Table 1). All these compounds have nanomolar activity against P. falciparum asexual stages in vitro20–22, and they lack acute insecticidal activity against An. gambiae, as determined in our experiments (Extended Data Fig. 5a). Remarkably, HYD and ACE showed strong P. falciparum killing activity, reducing oocyst prevalence by 63.9% and 64.3%, respectively, relative to controls (Fig. 4). DEC and PYR on the other hand had no detectable effect on infection (Extended Data Fig. 5b), possibly due to higher polar surface area relative to ATQ, ACE and HYD - and insecticides used in LLINs - which may negatively affect uptake (Extended Data Table 1).
Figure 4: Other cytochrome B inhibitors have P. falciparum transmission-blocking activity.
An. gambiae females exposed to 1 mmol/m2 of the arthropod cytochrome B inhibitors acequinocyl (ACE) and hydramethylnon (HYD), as well as ATQ, for 6 minutes show strongly reduced prevalence (pie charts) of P. falciparum relative to controls (Pairwise, 2-sided Chi2 w/ Bonferroni correction: ATQ: n = 141, df = 1, χ2 = 75.11, p < 0.0001. HYD: n = 132, df = 1, χ2 = 23.85, p < 0.0001. ACE: n = 141, df = 1, χ2 = 26.00, p < 0.0001). HYD and ACE had no impact on the intensity of infection (Wilcoxon with Dunn’s post hoc, n = 282, df = 3, HYD: p = 0.99, ACE: p = 0.19). Letters indicate groups that are statistically different from one another **** = p < 0.0001; n indicates the number of biologically independent mosquito samples.
ATQ is used extensively (in combination with proguanil) for prophylaxis in travelers to malaria-endemic areas, and as a stopgap therapy in the case of treatment failure with ACT and other therapies. A handful of mutations conferring resistance to ATQ in the P. falciparum erythrocytic cycle have been shown to cause parasite arrest early in mosquito infection23, although partial transmissibility has been observed in some ATQ-resistant P. berghei parasites23,24. Similar developmental arrest during mosquito stages is associated with functional knockout of other components of the mtETC - including Type II NADH dehydrogenase25, succinate dehydrogenase (complex II)26 and ATP-synthase (complex V)27 - which are non-essential for asexual growth. These observations demonstrate the critical role for the mtETC during P. falciparum development within the Anopheles female, making mitochondrial function an attractive target for parasite killing. The use of ATQ in mosquito-targeting interventions may, however, not be advisable as mutations conferring resistance to ATQ could nevertheless arise in the event of its widespread incorporation on LLINs, compromising its efficacy as a human therapeutic. The identification of additional effective compounds that can kill mosquito stages of P. falciparum using different modes of action is therefore a priority area of future research. In contrast, mosquito resistance to parasite inhibitors would be unlikely given the lack of observed fitness costs associated with compound exposure in the An. gambiae female, either in terms of fecundity or survival.
Our study demonstrates the vast potential of anti-parasitic compounds for malaria control methods aimed at the Anopheles vector, and greatly expands the library of compounds that can be considered for use on bed nets and other mosquito control interventions such as IRS and attractive toxic sugar baits. It is important to note that there is a significant gap between the proof-of-concept demonstrated here and implementation of a field-ready product. Indeed, while we were able to demonstrate that exposure to ATQ deposited on a net substrate was also able to completely block infection (Extended Data Fig. 6), many additional parameters including compound toxicity, formulation, cost and stability will need to be determined before this strategy can be deployed. Critically, once these hurdles are overcome, the use of Plasmodium inhibitors on LLINs or IRS could be rapidly integrated into the extensive manufacturing and distribution pipelines already in operation in all malaria-endemic regions, thereby providing an effective and safe tool to accelerate the drive toward malaria elimination.
Materials and Methods
Insect lines and rearing
Mosquitoes used for this study were Anopheles gambiae sensu stricto, G3 strain. Adult and larval mosquitoes were maintained in a purpose-built insectary at 27°C ± 2 and 80% RH. Larvae were reared from hatching in 1L ddH2O using an optimized density and feeding regimen. Pupae were collected and placed in cages (Bugdorm™, Megaview Science. Co. Taiwan), and after eclosure adult mosquitoes were provided water and 10% w/v glucose solution ad libitum. For colony maintenance, 5–7 d old adults were provided a blood meal of donated human blood using an artificial membrane feeding system (Hemotek Limited, Great Harwood, UK).
Compound Exposures
Compounds (ATQ, ACE, HYD, PYR, PER, Millipore-Sigma, St. Louis, MO, USA) were dissolved in a suitable, volatile vehicle (Extended Data Table 1) at stock concentrations of 3–10 mg/ml (0.3–1% w/v). Working concentrations of each compound were created through serial dilutions. To generate a compound-coated surface, a volume of working solution containing a known quantity of compound was added to 1 ml excess vehicle and transferred to a 6 cm diameter glass petri dish (0.0283m2). Treated dishes were placed on a lateral shaker and left for 4 h or overnight until evaporation of the volatile vehicle, coating the compound to the glass substrate. Control plates were treated identically using only the vehicle. A translucent plastic cup was placed over the coated surface to contain mosquitoes during exposure. A flap was cut into the base of the cup to allow the introduction of mosquitoes. Plates were used for 1 d and discarded. For exposures, 15–25 mosquitoes were introduced through the cup flap using a mouth aspirator (J. W. Hock & Co., Florida, USA) and incubated on the treated surface for 6–60 min depending on the experimental parameters. Exposure plates were agitated once during exposure to discourage resting on the untreated walls and base of the cup. After exposure, mosquitoes were transferred to a clean 17.5 cm3 cage (Bugdorm™, Megaview Science. Co. Taiwan). For net exposures, 10 × 10 cm squares of 100 denier, polyester netting were dipped in a 0.5 mg/ml solution of ATQ in acetone - or acetone alone - and allowed to air-dry for 10 min. Mosquito exposure was carried out as described above.
Plasmodium falciparum Infection Assays
Infections were carried out using the NF54 P. falciparum cell line provided by Carolina Barillas-Mury at the NIH via a BSL-II MTA from BEI Resources. The original source of this cell line is BEI Resources (MRA-1000). The parasite line was authenticated using a nested PCR protocol that uses primers specific for P. falciparum. Our NF54 cultures were confirmed to be free of any mycoplasma contamination. An. gambiae females (5-d old) were exposed to compounds as described above. Immediately after exposure, females were transferred to a sealed, secure infection glovebox and provided an in vitro culture of P. falciparum (NF54) gametocytes28,29 through a custom made, glass, water-heated membrane feeder. After 60 min, females that failed to engorge fully were vacuum aspirated out of their containers directly into 80% ethanol, and discarded. At 7–9 d pIBM, females that had blood fed were vacuum aspirated into 80% ethanol, incubated for 10 minutes at −20°C, and transferred out of the secure feeding box into PBS on ice. Midguts were dissected out in PBS and stained with 0.2% w/v mercurochrome (in ddH2O) for 17 minutes. After staining, midguts were mounted on glass microscope slides in 0.02% w/v mercurochrome, and oocyst prevalence and intensity were determined by examination at 40x air objective on an inverted compound light microscope (Olympus Corporation, Waltham, MA).
Ookinete Immunofluorescent Staining
21 hr pIBM females (either ATQ- or mock-exposed) were aspirated into PBS at 4°C, beheaded, and transferred to a dissecting microscope. Female midguts including the blood bolus were isolated and transferred to 20 μl PBS on ice. Guts were disrupted by repeated pipetting and the crude isolate homogenized by vortexing briefly (~5 s). 10μl of the homogenate was spotted onto a poly-L-lysine-coated slide and air-dried. Once dry, the tissues were fixed by incubation with 4% paraformaldehyde (PFA) for 15 minutes. Slides were then rinsed with 0.05% w/v BSA in PBS and stained with a mouse antibody raised against the P. falciparum surface protein PfS25 (BEI Resources, Manassas VA, USA). Secondary staining was carried out with a FITC-donkey-anti-mouse antibody (ThermoFisher Scientific, Waltham MA, USA). After staining and rinsing, tissues were mounted in Vectashield™ with DAPI (Vector Laboratories, Burlingame CA, USA) and examined under oil at 63x magnification using a Zeiss Observer.Z1 inverted fluorescent microscope (Carl Zeiss Microscopy GmbH, Jena, Germany).
Survival Assays
To assess acute survival following exposure, 40 females were exposed (as described above) to ATQ, PYR, ACE, HYD, and PER for 1 h at a dosage of 1 mmol/m2 for each compound. Each exposure had an independent negative control of 40 mock exposed females. Immediately after exposure, each treatment group was transferred to a 500 ml paper cup and provided with glucose. At 48 h post exposure the proportion of surviving mosquitoes in each group was determined. Differences in survival between control and compound-exposed mosquitoes were detected using Chi2 analysis. For long-term survival, ~100 ATQ- or control-exposed females were placed in clean 17.5 cm3 cages (Bugdorm™, Megaview Science. Co., Taiwan). Water and a 10% w/v glucose solution were provided ad libitum. Cages were checked daily for mortality, and dead mosquitos were removed and counted. Each experiment continued until all mosquitoes had died. Differences in median time-to-death between treatment groups were analyzed using a Log-Rank Mantell-Cox test.
Egg development assay
Females (5-day old) were exposed to ATQ at 1 mmol/m2 for 60 minutes and provided with an infectious blood meal as described above. Gravid females were collected at 3 d pIBM and the ovaries dissected out in 1x PBS. Developed eggs were liberated from the ovarian tissue by gentle agitation with a fine dissection needle and counted.
Modeling
We built upon a discrete time model of the mosquito life cycle and malaria transmission16. We used a simple model in order to distinguish the qualitative impact on transmission of adding ATQ to bed nets in the presence of insecticide resistance, in comparison with standard approaches. For clarity we use a single, well-mixed mosquito population without spatial structure, waning of efficacy of interventions, seasonality, or outdoor biting behavior. Briefly, mosquitoes progressed through egg, larval, and adult stages, which included four-day gonotrophic cycles (feeding, two days of resting, laying), with a time step of one day (Extended Data Fig. 2a). Malaria transmission was incorporated through a simple Susceptible-Infectious-Susceptible (SIS) framework for human malaria infection. Modifications from the model17 included (i) the possibility for exposure in every feeding compartment, (ii) a revised formulation of age-dependent adult daily mortality, and (iii) updated computations of the mosquito-human transmission risk functions (βM and βH, Extended Data Fig. 2). All simulations were carried out using Matlab 2016a.
During every feeding, mosquitoes could be exposed to insecticide, alone or with ATQ. Insecticide caused the death of a fraction of the feeding population, determined by insecticide resistance (the fraction of mosquitoes that are impervious to insecticide) and coverage level. In the case of no insecticide resistance, all mosquitoes that encountered insecticide along with ATQ were killed by the insecticide. We assumed no lasting effects of insecticide and that mosquito survival after an initial insecticide exposure was not correlated with survival after additional exposures. ATQ induced full refractoriness to Plasmodium falciparum infection, i.e. 100% protection against infection on the same day of exposure.
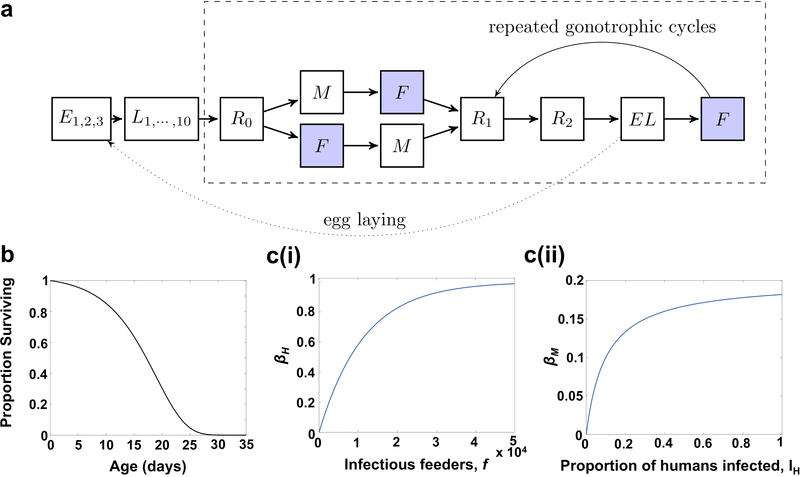
Age-dependent mortality for adult mosquitoes was determined by a Gompertz distribution30 (Extended Data Fig. 1a) with scale parameter b = 0.1868 and shape parameter η = 0.0293 (Extended Data Fig. 2b), such that the survival function, i.e. one minus the cumulative distribution function, was S(x) = exp (η(1 - ebx))
The daily risk of a human becoming infected was computed using βH(t) = 1 - (1 - b)af(t) where b = 0.55 is the probability of infection given a bite from an infectious mosquito31, a is the bites per human per mosquito, and f(t) is the number of infectious feeders on day t (Extended Data Fig. 2c(i)). We fitted the biting rate a to give the desired transmission setting in the absence of intervention assuming a larval carrying capacity of K = 5×105 mosquitoes and a human recovery rate of 25 days. We found a = 1.1×10 −4 in moderate transmission (45% human infection prevalence (HIP)). For other transmission settings, we find a = 4.2×10−5 at 20% HIP, a = 6.4×10−5 at 30% HIP, a = 9.2×10−4 at 40% HIP, a=1.34×10−4 at 50% HIP, a=1.96×10−4 at 60% HIP, a = 3.04×10−4 at 70% HIP, and a = 5.32×10−4 at 80% HIP.
The daily risk of a mosquito becoming infected was computed with βM(t) = k2 (1 - k1 / k2IH(t)+k1). We restricted βM to be between 0 and 0.2 by choosing k1 = 0.02, which controls the initial steepness of the curve, and k2 = 0.2, which restricts the maximum risk to be ~18%32,33 (Extended Data Fig. 2c(ii)).
To calculate a rough estimate of the possible impact of adding antimalarials on insecticide-treated nets in Africa, we examined locations where there was available data on insecticide resistance, malaria prevalence, and bed net coverage. To this end, insecticide resistance measurements in sub-Saharan Africa from 2013 to 2018 from IR Mapper17 were combined with estimates from 2015 for bed net coverage and P. falciparum parasite rate (PfPR) in 2–10 year old children from the Malaria Atlas Project1. The data were aggregated with resampling to a grid square size of 5 km by 5 km at the equator. We considered each grid square to be a location, and only included these grid squares in the analysis, since insecticide resistance is likely to be highly spatially heterogeneous. In total we found 2641 insecticide resistance measurements from 597 locations. For 10 locations there was insufficient data on PfPR and bed net coverage to fit the model. We additionally excluded a further 20 sites outside of our selected geographies for a final total of 567 locations. Insecticide resistance, bed net coverage and PfPR ranged between 0 – 100%, 0 – 100% and 0.13 – 74.4 respectively in these locations. When there were multiple insecticide resistance measurements in a location, we used the average level of insecticide resistance. For simplicity, and because we calculate a relative impact, we assume that PfPR in 2–10 year old children reflects overall prevalence in the population. For each location, given the level of bed net coverage and insecticide resistance, we fit the biting rate to return the reported PfPR value as prevalence for the entire population of that grid square. We compared the PfPR with the prevalence predicted by the model when including an ATQ-like compound (as above, considering 100% blocking of new Plasmodium infections on the same day of exposure to the antimalarial compound, and no effects on ongoing Plasmodium infections) on all insecticide-treated bed nets predicted from the Malaria Atlas Project1. We considered the relative reduction in prevalence in each location and grouped them by West Africa (186 locations) and East Africa (381 locations), to account for broad differences in ecology and epidemiology. This approach is necessarily simplistic, and does not account for the fact that the relationship between current prevalence, bed net use, and insecticide resistance may not be at equilibrium, or that the measurements for the underlying data may not have been taken during the same time period. Further, the estimated impact of our approach may not be generalizable to areas outside of our sample area for which insecticide resistance data is not available. Overall, however, this calculation gives a rough estimate for the relative impact that adding antimalarials may have in the regions for which data exist.
Statistics and Reproducibility
Statistical analyses were carried out using GraphPad Prism v7.0 for MacOSX (GraphPad Software Inc., La Jolla CA, USA) unless otherwise stated. For infections, differences in prevalence were analyzed by Chi2. In experiments where both treatment groups had individuals that produced >0 oocysts, differences in median oocyst burden between groups (intensity of infection) was analyzed using a Mann-Whitney Mean Ranks test. For multiple comparisons (e.g. Fig. 4) differences in prevalence between multiple groups were determined using pair-wise Chi2 corrected for multiple comparisons (Bonferroni). Similarly multiple comparisons of intensity were carried out using Wilcoxon with Dunn’s post hoc. To determine IC50 from dose-response data, the mean relative inhibition (ATQ exposed prevalence/Control prevalence) was calculated for each tested dose and fit with a sigmoidal curve function using non-linear regression. To compare the relative proportions of each parasite form detected in the mosquito midgut at 21 h pIBM we constructed a logistic regression model using JMP Pro 14 (SAS Institute, Cary NC, USA) with “Parasite Form” (ookinete, retort, zygote) as the independent variable and “Treatment” (ATQ/Control) as the dependent variable. We also included the term “Mosquito Sample” (n = 7 per treatment) to account for random between-sample variation. This cofactor was nested within treatment. All infection experiments were replicated a total of three times as independent biological replicates. Survival and fitness experiments were replicated independently twice. All collected data is included in the presented figures.
Data Availability
Raw data for infection experiments are available as a Supplemental Data spreadsheet. All further data is available upon request.
Code Availability
All custom computer code used in this study has been uploaded to GitHub and can be accessed from the following URL: https://github.com/laurenchilds/ATQAnopheles
Extended Data
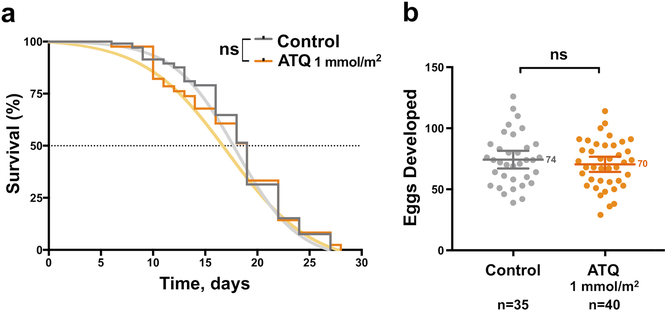
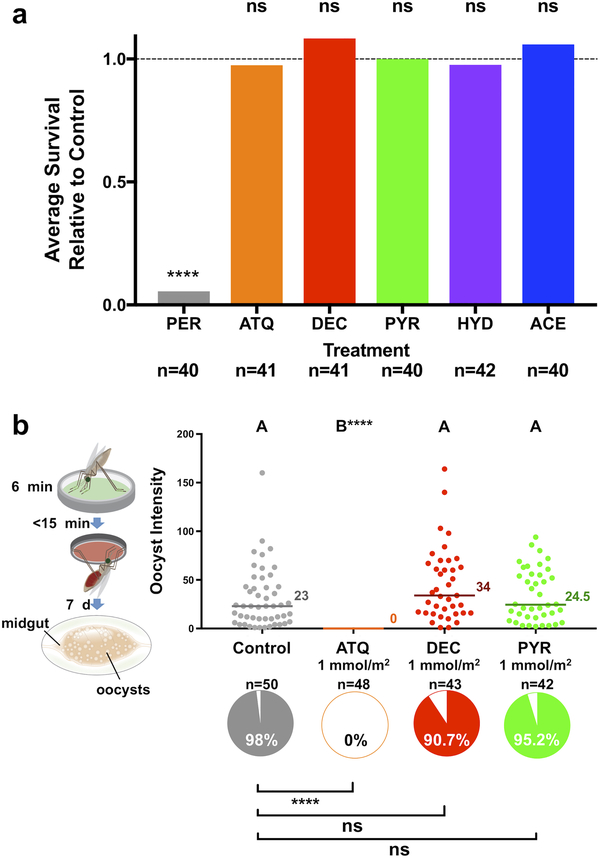
Extended Data Figure 1: Effects of ATQ exposure on survival and post blood-feeding egg production in An. gambiae females.
a) ATQ exposure has no effect on the acute or long-term survival of An. gambiae females (2-sided Log-Rank Mantel-Cox, n = 189, df = 1, χ2 = 0.00, p = 0.9951). The sigmoidal fit used for subsequent modeling is shown. b) The production of eggs after an infections blood meal is unaffected by ATQ exposure (2-sided, unpaired Student’s t, n = 75, df = 1, t = 0.826, p = 0.4115). Means and 95% CI of the mean are indicated. Where relevant, statistical significance is indicated as so: ns = not significant, * = p < 0.05, ** = p < 0.01, *** = < 0.001, **** = p < 0.0001; n indicates the number of biologically independent mosquito samples.
Extended Data Figure 2: Model Structure and Population Parameters.
(a) Schematic representation of the mosquito life cycle model with the time step of one day. Mosquitoes spend three days as eggs (Ei), ten days as larvae (Li), which includes the pupal stage. Adult female mosquito compartments fall within the dashed box and begin with a rest day (R0) followed by mating (M) or feeding (F). After feeding, females undergo two days of rest (Ri) followed by a day for egg laying (EL). Then the cycle repeats. Shaded boxes denote when exposure to insecticide or ATQ could occur. These are the same compartments were mosquitoes can become infected or transmit infections, assuming they have been infected for a period longer than the incubation time. (b) Survival of the mosquito population as a function of age. The curve is a Gompertz distribution with scale parameter b = 0.1868 and shape parameter η = 0.0293. (c) Functions relating human and mosquito infection levels with risk of infection. (i) The risk of a human becoming infected, βH, as a function of the number of infectious feeders, f. (ii) The risk of a mosquito becoming infected, βM, as a function of the fraction of the human population that is infected, IH.
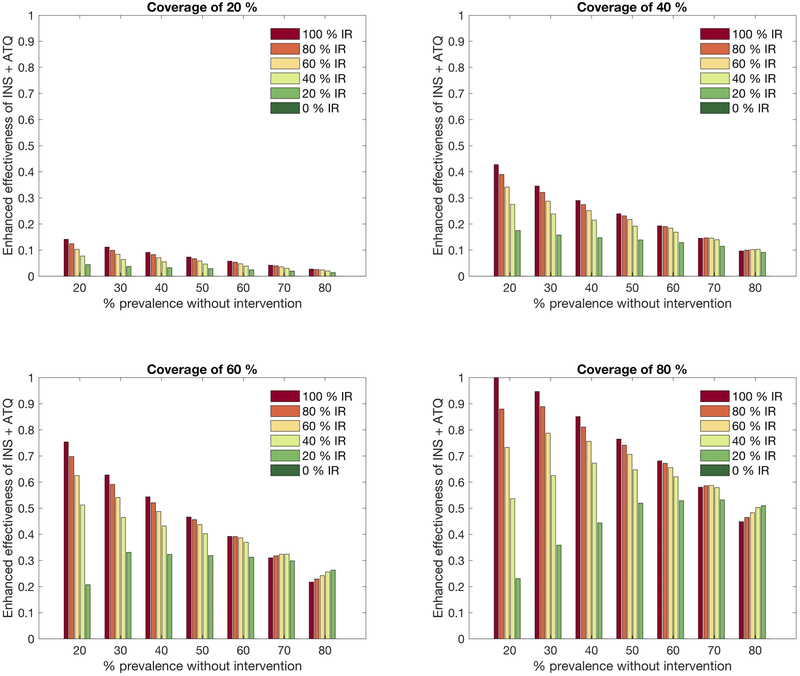
Extended Data Figure 3: Sensitivity of model results to variation in prevalence, coverage and insecticide resistance.
The graphs show the enhanced effectiveness of insecticide combined with ATQ (relative to insecticide alone) in reducing human prevalence under varying levels of coverage (across panels), prevalence (along x-axis), coverage and insecticide resistance (bar color). The enhanced effectiveness of the interventions is defined as (the quantity of human prevalence with only insecticide - human prevalence with insecticide and ATQ over human prevalence with only insecticide) and is represented by positive values when the addition of ATQ is beneficial. Prevalence is quantified after ten years of simulation. The coverage is varied from 20%−80% (upper left panel 20%; upper right panel 20%; lower left panel 60%; and lower right panel 80%). In each panel, the position of the bars determines the malaria prevalence under no intervention, from 20–80%. The bar color represents insecticide resistance levels (dark green 0%; green 20%; light green 40%; yellow 60%; orange 80%; and red 100%). In the complete absence of insecticide resistance all mosquitoes that contact insecticide are killed, and thus, all dark green bars equal zero.
Extended Data Figure 4: Malaria transmission model predicting the effects of adding ATQ to insecticide-treated nets in additional malaria prevalence settings.
The heat maps show changes in malaria transmission for bed net-like interventions using insecticide alone or insecticide plus an ATQ-like compound, relative to no intervention at varying coverage and varying insecticide resistance levels. The model considers both (a) 20% and (b) 70% prevalence of malaria. The effectiveness of the interventions is defined as (1 - proportion reduction in malaria transmission relative to no intervention) and is represented as colors ranging from yellow (no change in malaria transmission) to dark blue (elimination of malaria transmission) at varying levels of coverage (x-axis) and insecticide resistance (y-axis). Insecticide resistance is the percentage of mosquitoes that are impervious to insecticide. Coverage is the probability of a mosquito encountering an intervention during a single feeding episode. The model output demonstrates that addition of ATQ significantly increases the ability of an LLIN-like intervention to reduce and even eliminate malaria transmission.
Extended Data Figure 5: Testing additional compounds for fitness costs and transmission blocking activity through tarsal contact.
(a) Mosquito survival relative to an untreated control after 48 h following exposure to ATQ, DEC, PYR, HYD, ACE and permethrin (PER). The proportion of female An. gambiae surviving exposure to each compound (1 mmol/m2, 60 minutes) relative to the proportion of individuals surviving exposure to an untreated control is shown. PER exposure causes almost complete mortality (proportionate survival relative to controls = 0.055, Pairwise, 2-sided Chi2 w/ Bonferroni correction, n = 80, df = 1, χ2 = 76.10, p < 0.0001), while all other compounds behave comparably to controls. (b) Neither PYR nor DEC (1 mmol/m2, 6 min) are capable of reducing the prevalence P. falciparum through tarsal contact, relative to controls (Pairwise Chi2 w/ Bonferroni correction, DEC: n= 93, df = 1, χ2 = 2.42, p = 0.12. PYR: n = 92, df = 1, χ2 = 0.55, p = 0.46). Similarly, DEC and PYR had no impact on the intensity of infection, compared to a mock-treated control (Wilcoxon with Dunn’s post hoc, n = 183, df = 3, p = 0.31 (DEC) and p = 0.99 (PYR)). Letters indicate groups that are statistically different from one another. Statistical significance is indicated as **** = p < 0.0001. Medians are indicated; n denotes the number of biologically independent mosquito samples.
Extended Data Figure 6: ATQ exposure via a netting substrate completely inhibits P. falciparum development.
An. gambiae females were allowed to rest for 60 min on 100 denier polyester netting that had been treated with either a 0.5 mg/ml (0.05% w/v) solution of ATQ in acetone or acetone alone. Females exposed to ATQ in this way failed to become infected after an infectious P. falciparum blood meal, demonstrating that a netting substrate is also capable of delivering sufficiently high doses of ATQ to inhibit infection (2-sided Chi2, n = 98, df = 1, χ2 = 75.55, p < 0.0001). Medians are indicated; n denotes the number of biologically independent mosquito samples.
Extended Data Table 1: Chemical properties and structures of study compounds and bed-net approved chemicals.
Chemical properties of currently approved insecticides and synergists for bed net use - permethrin, deltamethrin, pyriproxifen, piperonyl butoxide and chlorfenapyr. - and all compounds tested in this study - atovaquone, hydramethylnon, acequinocyl, decoquinate and pyriproxifen.
| Compound | Vehicle | H Donors | H Acceptors | Rotational Bonds | Polar Surface Area (Å2) | Active Tarsally? | Target | Chemical Structure |
|---|---|---|---|---|---|---|---|---|
| Permethrina | Acetone | 0 | 3 | 7 | 35.5 | Yes | Para sodium-gated ion channel (cell membrane) | |
| Deltamethrina | Acetone | 0 | 4 | 7 | 59.3 | Yes | Para sodium-gated ion channel (cell membrane) | |
| Pyriproxifenab | Acetone | 0 | 4 | 7 | 40.6 | Yes | Methoprene Tolerant (nucleus) | 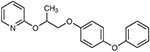 |
| Chlorfenapyrab | Acetone | 0 | 5 | 4 | 38 | Yes | Mitochondrial inter-membrane space |  |
| Piperonyl butoxideab | Acetone | 0 | 5 | 13 | 46.2 | Yes | Cytochrome P450s | 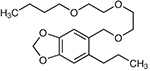 |
| Atovaquone | Acetone | 1 | 3 | 2 | 54.4 | Yesc | Cytochrome B (mitochondrial inner membrane) | 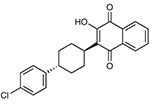 |
| Hydramethylnon | Acetone | 2 | 8 | 6 | 48.8 | Yesc | Cytochrome B (mitochondrial inner membrane) | 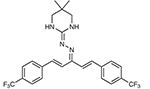 |
| Acequinocyl | Acetone | 0 | 4 | 13 | 60.4 | Yesc | Cytochrome B (mitochondrial inner membrane) |  |
| Decoquinate | Chloroform | 1 | 6 | 15 | 73.89 | Noc | Cytochrome B (mitochondrial inner membrane) | |
| Pyrimethamine | Acetone (sparingly) | 2 | 4 | 2 | 77.8 | Noc | Dihydrofolate Reductase- Thymidylate Synthase (cytosol) |  |
approved for use in long-lasting inseciticide treated nets
only in combination with permethrin/deltamethrin
against Plasmodium falciparum infection
Acknowledgements
The authors would like to thank Naresh Singh, Emily Lund and Kate Thornburg for Plasmodium and Anopheles culture and Manuela Bernardi for help with graphics. Additionally, the authors wish to thank Dyann Wirth, Selina Bopp, Hilary Ranson, and the members of the Catteruccia labs for comments and suggestions on the manuscript. Malaria prevalence and LLIN coverage map data were retrieved from the Malaria Atlas Project (www.map.ox.ac.uk). Insecticide resistance data was retrieved from the IR Mapper database (www.irmapper.com). FC is funded by a Faculty Research Scholar Award by the Howard Hughes Medical Institute (HHMI) and the Bill & Melinda Gates Foundation (BMGF) (Grant ID: OPP1158190), and by the National Institutes of Health (NIH) (R01 AI124165, R01 AI104956). LMC is supported by Simons Foundation Collaboration Grant 524390. COB is supported by NIGMS Maximizing Investigator’s Research Award (MIRA) #R35GM124715–02. The findings and conclusions within this publication are those of the authors and do not necessarily reflect positions or policies of the HHMI, the BMGF, Simons Foundation or the NIH.
References
- 1.Bhatt S et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211, doi: 10.1038/nature15535 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toé KH et al. Increased Pyrethroid Resistance in Malaria Vectors and Decreased Bed Net Effectiveness, Burkina Faso. Emerging Infectious Diseases 20, 1691–1696, doi: 10.3201/eid2010.140619 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Bortel W et al. The insecticide resistance status of malaria vectors in the Mekong region. Malar J 7, 102, doi: 10.1186/1475-2875-7-102 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dykes CL et al. Knockdown resistance (kdr) mutations in Indian Anopheles culicifacies populations. Parasit Vectors 8, 333, doi: 10.1186/s13071-015-0946-7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ondeto BM et al. Current status of insecticide resistance among malaria vectors in Kenya. Parasit Vectors 10, 429, doi: 10.1186/s13071-017-2361-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghavendra K et al. Chlorfenapyr: a new insecticide with novel mode of action can control pyrethroid resistant malaria vectors. Malar J 10, 16, doi: 10.1186/1475-2875-10-16 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.N’Guessan R, Odjo A, Ngufor C, Malone D & Rowland M A Chlorfenapyr Mixture Net Interceptor(R) G2 Shows High Efficacy and Wash Durability against Resistant Mosquitoes in West Africa. PLoS One 11, e0165925, doi: 10.1371/journal.pone.0165925 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngufor C et al. Olyset Duo(R) (a pyriproxyfen and permethrin mixture net): an experimental hut trial against pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus in Southern Benin. PLoS One 9, e93603, doi: 10.1371/journal.pone.0093603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Malaria Report 2018. World Health Organisation (2018).
- 10.Guidelines for testing mosquito adulticides for indoor residual spraying and treatment of mosquito nets. World Health Organisation Pesticide Evaluation Schemes (2006). [Google Scholar]
- 11.Owusu HF, Chitnis N & Muller P Insecticide susceptibility of Anopheles mosquitoes changes in response to variations in the larval environment. Sci Rep 7, 3667, doi: 10.1038/s41598-017-03918-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker JE et al. Infrared video tracking of Anopheles gambiae at insecticide-treated bed nets reveals rapid decisive impact after brief localised net contact. Sci Rep 5, 13392, doi: 10.1038/srep13392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler RE, Billingsley PF, Pudney M & Sinden RE Inhibitory action of the anti-malarial compound atovaquone (566C80) against Plasm8odium berghei ANKA in the mosquito, Anopheles stephensi. Parasitology 108 ( Pt 4), 383–388 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Delves M et al. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med 9, e1001169, doi: 10.1371/journal.pmed.1001169 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiorenzano JM, Koehler PG & Xue RD Attractive Toxic Sugar Bait (ATSB) For Control of Mosquitoes and Its Impact on Non-Target Organisms: A Review. Int J Environ Res Public Health 14, doi: 10.3390/ijerph14040398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Childs LM et al. Disrupting Mosquito Reproduction and Parasite Development for Malaria Control. PLoS Pathog 12, e1006060, doi: 10.1371/journal.ppat.1006060 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knox TB et al. An online tool for mapping insecticide resistance in major Anopheles vectors of human malaria parasites and review of resistance status for the Afrotropical region. Parasit Vectors 7, 76, doi: 10.1186/1756-3305-7-76 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava IK, Rottenberg H & Vaidya AB Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. J Biol Chem 272, 3961–3966 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Painter HJ, Morrisey JM, Mather MW & Vaidya AB Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature 446, 88–91, doi: 10.1038/nature05572 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Richards WH & Maples BK Studies on Plasmodium falciparum in continuous cultivation. I. The effect of chloroquine and pyrimethamine on parasite growth and viability. Ann Trop Med Parasitol 73, 99–108 (1979). [PubMed] [Google Scholar]
- 21.Nam TG et al. A chemical genomic analysis of decoquinate, a Plasmodium falciparum cytochrome b inhibitor. ACS Chem Biol 6, 1214–1222, doi: 10.1021/cb200105d (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witschel M, Rottmann M, Kaiser M & Brun R Agrochemicals against malaria, sleeping sickness, leishmaniasis and Chagas disease. PLoS Negl Trop Dis 6, e1805, doi: 10.1371/journal.pntd.0001805 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodman CD et al. Parasites resistant to the antimalarial atovaquone fail to transmit by mosquitoes. Science 352, 349–353, doi: 10.1126/science.aad9279 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blake LD et al. Menoctone Resistance in Malaria Parasites Is Conferred by M133I Mutations in Cytochrome b That Are Transmissible through Mosquitoes. Antimicrob Agents Chemother 61, doi: 10.1128/AAC.00689-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boysen KE & Matuschewski K Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase. J Biol Chem 286, 32661–32671, doi: 10.1074/jbc.M111.269399 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hino A et al. Critical roles of the mitochondrial complex II in oocyst formation of rodent malaria parasite Plasmodium berghei. J Biochem 152, 259–268, doi: 10.1093/jb/mvs058 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Sturm A, Mollard V, Cozijnsen A, Goodman CD & McFadden GI Mitochondrial ATP synthase is dispensable in blood-stage Plasmodium berghei rodent malaria but essential in the mosquito phase. Proc Natl Acad Sci U S A 112, 10216–10223, doi: 10.1073/pnas.1423959112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Extended Data References
- 28.Trager W, Jensen JB, Human malaria parasites in continuous culture. Science 193, 673–675 (1976). [DOI] [PubMed] [Google Scholar]
- 29.Ifediba T, Vanderberg JP, Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature 294, 364–366 (1981). [DOI] [PubMed] [Google Scholar]
- 30.Christiansen-Jucht C, Erguler K, Shek CY, Basanez MG, Parham PE, Modelling Anopheles gambiae s.s. Population Dynamics with Temperature- and Age-Dependent Survival. Int J Environ Res Public Health 12, 5975–6005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith DL, Drakeley CJ, Chiyaka C, Hay SI, A quantitative analysis of transmission efficiency versus intensity for malaria. Nat Commun 1, 108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boudin C, Olivier M, Molez JF, Chiron JP, Ambroise-Thomas P, High human malarial infectivity to laboratory-bred Anopheles gambiae in a village in Burkina Faso. Am J Trop Med Hyg 48, 700–706 (1993). [DOI] [PubMed] [Google Scholar]
- 33.Killeen GF, Ross A, Smith T, Infectiousness of malaria-endemic human populations to vectors. Am J Trop Med Hyg 75, 38–45 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data for infection experiments are available as a Supplemental Data spreadsheet. All further data is available upon request.