Abstract
Manganese (Mn) is essential for several species and daily requirements are commonly met by an adequate diet. Mn overload may cause motor and psychiatric disturbances and may arise from an impaired or not fully developed excretion system, transporter malfunction and/or exposure to excessive levels of Mn. Therefore, deciphering processes regulating neuronal Mn homeostasis is essential to understand the mechanisms of Mn neurotoxicity. In the present study we selected two small molecules (with opposing effects on Mn transport) from a previous high throughput screen of 40,167 to test their effects on Mn toxicity parameters in vivo using Caenorhabditis elegans. We pre-exposed worms to VU0063088 and VU0026921 for 30 min followed by co-exposure for 1 h with Mn and evaluated Mn accumulation, dopaminergic (DAergic) degeneration and worm survival. Control worms were exposed to vehicle (DMSO) and saline only. In pdat-1::GFP worms, with GFP labeled DAergic neurons, we observed a decrease of Mn-induced DAergic degeneration in the presence of both small molecules. This effect was also observed in an smf-2 knockout strain. SMF-2 is a regulator of Mn transport in the worms and this strain accumulates higher Mn levels. We did not observe improved survival in the presence of small molecules. Our results suggest that both VU0063088 and VU0026921 may modulate Mn levels in the worms through a mechanism that does not require SMF-2 and induce protection against Mn neurotoxicity.
Introduction
Manganese (Mn) is an essential metal that acts as a cofactor to several enzymes. When in excess Mn accumulates in basal ganglia structures and may cause motor and psychiatric disturbances both in human and mammalian experimental models. Mn overload may arise from an impaired or not fully developed excretion system, transporter malfunction and/or exposure to excessive levels of Mn by air, water, food, ephedrone abuse or total parenteral nutrition (TPN) (Aschner et al. 2015; Roth 2006; Stepens et al. 2008). Therefore Mn transport possesses intricate regulation.
To date, several Mn importers have been identified. Among them, the divalent metal transporter 1 (DMT1), transferrin receptor (TfR), Zn transporters-ZIP8 and ZIP14, dopamine transporter (DAT), Ca channels, choline transporter and citrate transporter. Ferroportin and solute carrier family 30, member 10 (SLC30A10) are cell membrane transporters that facilitate Mn export (Horning et al. 2015). These proteins also transport other divalent cations such as iron (Fe) or zinc (Zn); as of this moment SLC30A10 is the only known Mn specific transporter. However, SLC39A14 was recently demonstrated to be crucial for efficient Mn uptake by the liver and pancreas, and its deficiency results in impaired Mn excretion and accumulation of the metal in blood, brain and bone (Aydemir et al. 2017; Jenkitkasemwong et al. 2018; Liu et al. 2017; Xin et al. 2017).
Mn accumulation in the nervous system leads to the development of a progressive parkinsonian syndrome, termed manganism, which is analogous to idiopathic Parkinson’s disease (PD). Notably, the brain regions where Mn accumulates differ in manganism and PD. T1-weighted magnetic resonance imaging (MRI) of patients shows excessive levels of Mn accumulation preferentially in the basal ganglia, especially in the globus pallidus. In PD Mn accumulates is substantia nigra (Lucchini et al. 2000). Manganism is irresponsive to levodopa treatment and there are limited options to treat this condition (Koller et al. 2004).
A high throughput screen of 40,167 small molecules was performed to identify modifiers of cellular Mn content in a mouse striatal neuron cell line. The screen identified 41 small molecule modifiers of neuronal Mn transport, both small molecules that increase and decrease net Mn accumulation were identified (Kumar et al. 2014). These data argue that intracellular Mn levels are actively controlled by the cell and not exclusively due to system level homeostasis mechanisms such as at the blood-brain barrier (BBB) or blood-cerebrospinal fluid barrier. Furthermore, mechanisms regulating Mn content might be developmentally regulated in DAergic neurons reflecting the changing physiological demand for Mn (Kumar et al. 2014). According to the initial in vitro screen, at both toxicological and physiological Mn exposures, VU0063088 is a Mn-level decreasing molecule and VU0026921 is a Mn-level increasing molecule (Kumar et al. 2014). We selected those two molecules for our in vivo investigation.
C. elegans is an important tool for in vivo studies, due to its genetic amenability and homology with mammals. In C. elegans, excess Mn induces oxidative stress and leads to DAergic degeneration (Benedetto et al. 2010). The roles of different Mn transporters have been tested in C. elegans. SMF-2 is a divalent metal transporter-1 (DMT-1) orthologue involved in metal content regulation. This transporter is expressed in worm pharynx and senses Mn levels in the media, controlling pharyngeal pumping to avoid excessive Mn intake (Au et al. 2009; Chen et al. 2015; Leyva-Illades et al. 2014).
VU0026921 was shown to increase Mn accumulation by 2 fold in vitro (at both physiological and toxicological Mn exposures). VU0063088 decreased Mn accumulation by 1.2 fold at physiological Mn levels and by 0.5 fold at toxicological Mn levels (Kumar et al. 2014). Using C. elegans as a model organism, the main goal of this study was to test VU0063088 and VU0026921 effects on Mn toxicity parameters in vivo and to determine whether SMF-2 could play a part in these Mn content modifiers mechanism of action.
Methodology
Chemicals
Manganese chloride (MnCl2), cholesterol and bovine serum albumin (BSA) were purchased from Sigma (St. Louis, MO, USA). Agar, peptone and agarose were purchased from BD (Franklin Lakes, NJ, USA). VU0026921 was synthetized at Vanderbilt University, Nashville, TN, USA. VU0063088 was purchased from Vitas-M Laboratory (Champaign, IL, USA). Small molecules stock solutions were prepared in DMSO at 10 mM and were kept at −20°C. All other reagents were of analytical grade.
C. elegans culture, handling of the worms and exposure to small molecules and/or Mn
BY200 [pdat-1::gfp (vtIs1)] was kindly provided by the Blakely laboratory, Vanderbilt University Medical Center, and was used as the wild-type (WT) strain for this work. BY200 worms were crossed with VC171 [smf-2(gk133) X] worms to create MAB300 [smf-2(gk133) X, dat-1::GFP(vtIs1) V] strain (Leyva-Illades et al. 2014). VC171 was from the Caenorhabditis Genetics Center (CGC - University of Minnesota, Twin Cities, MN, USA).
Worms were maintained in standard culture conditions at 20°C (Brenner 1974) in plastic petri dishes containing agar 8P seeded with NA22 Escherichia coli (as food). During experiments worms were maintained on plates containing nematode growth medium (NGM) and E. coli OP50–1 streptomycin resistant strain was used as food. Synchronized populations at the L1 larval stage were obtained by isolating eggs from gravid hermaphrodites using 1% NaOCl, 0.25M NaOH. Eggs were isolated from cell debris by a 30% sucrose gradient (Sulston and Hodgkin 1988).
Small molecules (1, 5, 10 and 50 μM) effects were first tested in the DAergic neurodegeneration assay (data not shown). The lowest concentration that decreased DAergic neurodegeneration was chosen for further experiments. Small molecules at 100 μM working solutions were prepared in 85 mM NaCl. Final small molecule concentration was 5 μM and DMSO concentration was 0.05%. Care was taken to avoid exposure to concentrations above 0.5% DMSO, which has been shown to influence C. elegans lifespan (Wang et al. 2010). Worms were pre-exposed for 30 min to VU0063088 or VU0026921 followed by 1 h co-exposure with 25 or 50 mM MnCl2 prepared in 85 mM NaCl. Vehicle was 0.05% DMSO in NaCl. Acute exposure to MnCl2 was conducted in populations of 2500 worms at the L1 larval stage. All exposures were performed in siliconized tubes with constant rotation at 20°C. The worms were then pelleted by centrifugation and washed 3 times with 85 mM NaCl.
Mn content in worms
Mn levels were determined by inductively coupled plasma-mass spectrometry (ICP-MS) using 8800 ICP-QQQ (Agilent, Santa Clara, CA) with the instrumental parameters and ashing protocol previously described (Bornhorst et al. 2014). Briefly, after treatments, 40,000 L1 worms per condition were washed five times in 85 mM NaCl to remove excess Mn from the medium. Worms were re-suspended in 1 mL 85 mM NaCl supplemented with 1% protease inhibitor and sonicated. An aliquot was taken for protein quantification with bicinchoninic acid (BCA) assay. The remaining samples were dried and ashed before the diluted ash (with 2% HNO3 including 10 μg/L Rh) were subjected to the ICP-MS analysis.
Fluorescence microscopy for DAergic degeneration assay
Two hours after treatments, worms were anesthetized with 30 μM levamisole hydrochloride and mounted on slides containing 2% agarose pads. Worms were observed using a BX41 fluorescence microscope (Olympus, PA, USA). Sixty to ninety worms per treatment group were analyzed. Discontinuous GFP marking in the BY200 strain exposed to Mn is indicative of DAergic neurodegeneration as previously described (Benedetto et al. 2010). Each worm was scored for the absence (considered normal), or presence of any of the following morphological changes: puncta formation along dendritic processes, shrunk and/or lost soma, loss of dendrites (considered degenerated). Results were expressed as percentage of degenerated worms by treatment group as previously described (Bornhorst et al. 2014). Representative images were obtained with a PerkinElmer spinning disk confocal, 60 × objective (PerkinElmer, Waltham, MA, USA).
Basal slowing response (BSR)
To evaluate worms’ DAergic system functionality we employed BSR test 48 h after treatments. In this behavioral assay, worms reduce their mobility upon reaching their food source (E. coli). This basal slowing depends on DAergic signaling and the three classes of DAergic neurons (CEP, ADE and PDE) participate in this response. (Sawin et al. 2000). BSR assay was conducted as previously described (Peres et al. 2018). Briefly, five worms were placed on the center of a 60 mm NGM plate containing bacteria spread in a ring shape. Another five worms were placed on the center of a NGM plate without bacteria. The number body bends was counted over a period of 20 s. Results were expressed as the difference (Δ) between the numbers of body bends in the plate with bacteria and without bacteria. A low Δ value means greater mobility on food, indicating deficits in DAergic function. The number of body bends off-food was used as a measure of locomotion. The cat-2 mutant strain was used as a positive control in this test. This strain is deficient in tyrosine hydroxylase (CAT-2) homologue and has reduced levels of dopamine.
Survival assay
Immediately after exposure, 40 to 60 worms were placed on 60 mm plates containing NGM and seeded with OP50–1 E. coli. Each condition was performed in triplicate. The surviving worms were scored 48 h after treatment. Results were expressed as percent live worms relative to day 0. Experiments were repeated independently at least three times. Mn concentration was chosen based on previous works by our group (Benedetto et al. 2010).
Statistical analysis
Results were expressed as mean ± S.E.M. All graphs and statistical analysis were prepared with GraphPad Prism 7.0 (GraphPad Software Inc., La Jolla, CA, USA). Data for each of the small molecules compared to vehicle control treatment were analyzed separately by two-way analysis of variance (ANOVA) followed by Dunnet’s post hoc test. Results were considered significant when p <0.05.
Results
No difference in total body Mn accumulation was observed in worms pre-exposed to VU0063088 or VU0026921
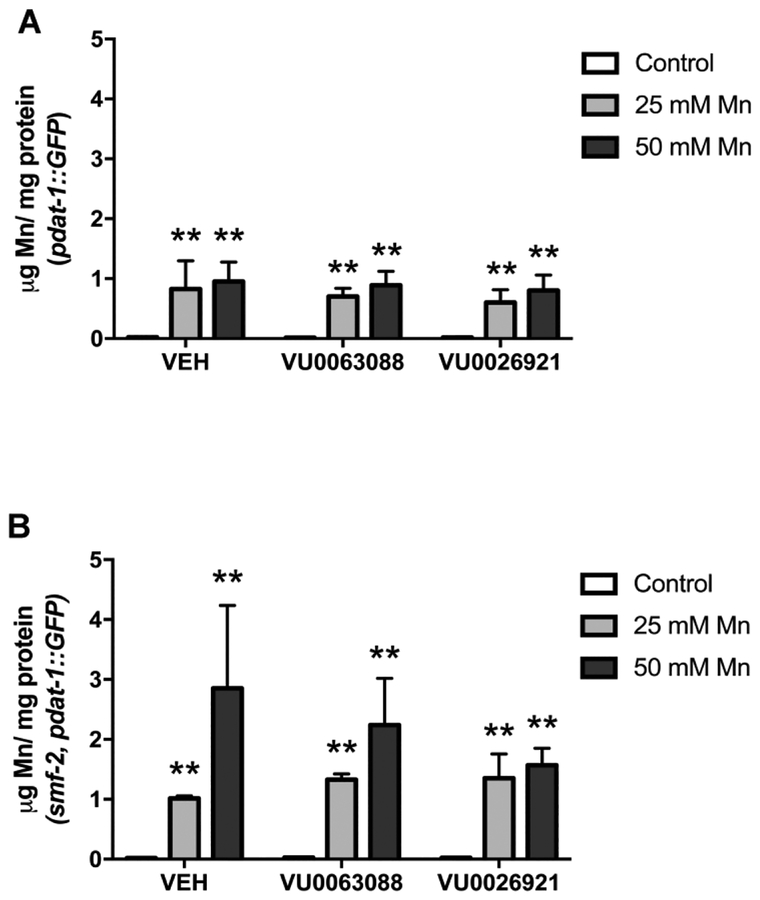
Mn accumulation significantly increased immediately after exposure to 25 or 50 mM Mn in pdat-1::GFP worms with a significant effect of Mn treatment [F (2, 12) = 6.93; p <0.01] for VU0063088 and [F (2, 12) = 5.63; p <0.01] for VU0026921 (Fig. 1A). In smf-2, pdat-1::GFP worms this increase in Mn levels was more pronounced when worms were pre-exposed to vehicle compared to worms pre-exposed to VU0026921 [F (2, 11) = 5.64; p <0.001], however it was not statistically significant, with a significant effect of Mn treatment (Fig. 1B). No significant interaction between Mn treatment and small molecule was observed.
Fig. 1:
Intraworm manganese content after 30 min pre-exposure with 5 μM VU0063088 or VU0026921 1 h co-exposure with 25 or 50 mM MnCl2 was quantified by ICP-MS immediately after treatment. (A) Pdat-1::GFP worms, (B) smf-2, Pdat-1::GFP worms. Metal content is expressed as μg Mn/mg protein. Data are expressed as mean ± S.E.M. from three independent experiments. Statistical analysis by two-way ANOVA. **p < 0.01, compared to no Mn controls.
Mn-induced DAergic degeneration was attenuated in worms pre-exposed to VU0063088 or VU0026921
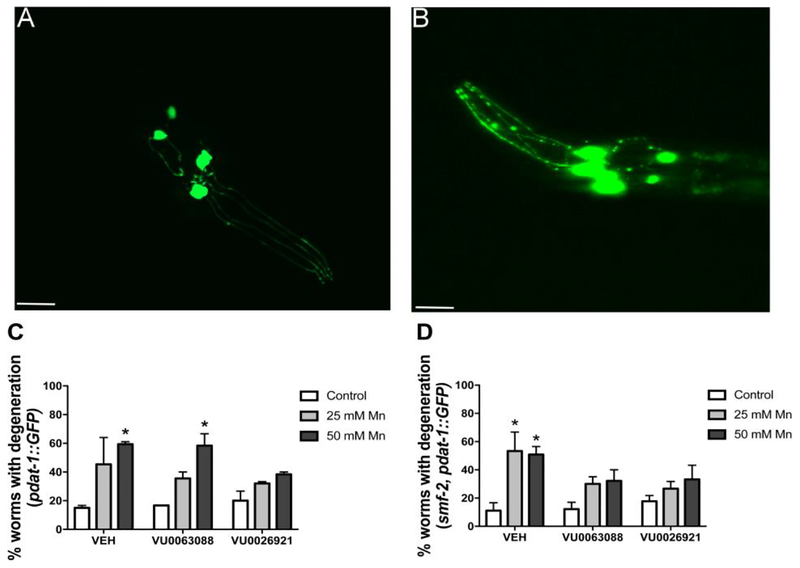
Worms were observed under fluorescent microscope and scored for DAergic neurodegeneration (puncta, shrunken soma, loss of soma or dendrites). Figure 2A represents worms with healthy neurons (scored as normal). Figure 2B represents worms with puncta (discontinuous marking of the dendrite). The latter were scored as degenerated. An increase in the percentage of WT worms with DAergic neurodegeneration was observed, with a significant effect of Mn treatment [F (2, 6) = 12.61; p <0.01] for VU0063088 and [F (2, 6) = 7.62; p <0.05] for VU0026921 (Fig. 2 C). Post hoc test revealed significant Mn-induced (50 mM) DAergic degeneration in vehicle (59.4 ± 2.4 %; p <0.05) and VU0063088 (58.3 ± 8.3 %; p <0.05) pre-exposed. In contrast, the percentage of degenerated WT worms pre-treated with VU0026921 did not significantly increase (Fig. 2C), however no significant interaction between Mn treatment and small molecule was observed.
Fig. 2:
L1 worms were pre-treated for 30 min with 5 μM VU0063088 or VU0026921 and then exposed to 25 or 50 mM MnCl2 for 1 h. At least 60 worms per condition were observed under fluorescent microscope and scored for DAergic degeneration. (A) Depicts the head of a L1 worm with healthy neurons, scored as normal. (B) Neurons with puncta (discontinuous marking in the dendrite), loss or cell body shrinkage or loss of dendrites in their dopaminergic neurons were quantified as containing degeneration. Results are expressed as mean ± S.E.M. of 2 to 3 experiments with (C) Pdat-1::GFP worms or (D) smf-2, Pdat-1::GFP worms. * P <0.05 compared to vehicle (VEH) control group. Twoway ANOVA followed by post hoc Dunnet’s test. Representative images were obtained with a PerkinElmer spinning disk confocal, 60× objective. Scale bar represents 13 μm.
smf-2, pdat-1::GFP worms displayed increase in neurodegeneration, with a significant effect of Mn treatment [F (2, 12) = 10.11; p <0.001] for VU0063088 and [F (2, 12) = 7.35; p <0.01] for VU0026921. Post hoc test indicated vehicle pre-exposed group displayed 53.3 ± 9.5 % (p <0.05) and 50.8 ± 4.0 % (p <0.05) degenerated worms when exposed to 25 or 50 mM Mn, respectively. Both VU0063088 and VU0026921 small molecules partially prevented this effect (Fig. 2D). No significant interaction between Mn treatment and small molecule was observed.
BSR was impaired in worms exposed to Mn but not in worms pre-exposed to small molecules
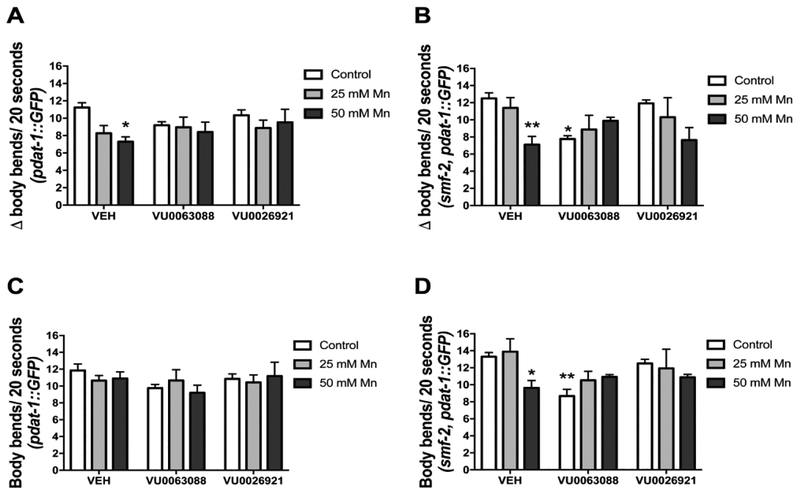
BSR was evaluated in WT or smf-2, pdat-1::GFP worms by counting the number of body bends off-food and on-food. The difference (Δ) between the two conditions expressed BSR behavior. BSR impairment was observed in WT worms, with a significant effect of Mn treatment [F (2, 18) = 4.07; p <0.05] for VU0063088 and [F (2, 18) = 4.32; p <0.05] for VU0026921 (Fig. 3A and 3B). Post hoc analysis revealed significant differences in vehicle pre-exposed, 50 mM Mn exposed worms (Δ = 7.2 ± 0.4; p <0.05; Fig 3A). This impairment was not observed in worms pre-exposed to VU0063088 or VU0026921 (p >0.05)
Fig. 3:
Basal slowing response (BSR) was evaluated in pdat-1::GFP (A) or smf-2, pdat-1::GFP worms (B) pre-treated for 30 min with 5 μM VU0063088 or VU0026921 and then exposed to 25 or 50 mM MnCl2 for 1 h. Controls were incubated with 85 mM NaCl. The test was performed 48 h after treatments. Worms were washed off plates and 5 worms were pipetted in test plates with or without OP-50 E. coli (food). After 5 min acclimation period, body bends were counted in 20 s intervals for each worm. BSR was expressed as number (average for all worms) of body bends off-food minus the number of body bends on food (Δ). Locomotion was expressed as the number of body bends/20 s on NGM plates without food (C) pdat-1::GFP, (D) smf-2, pdat-1::GFP. Data are expressed as mean ± S.E.M. of 3 to 4 experiments. * p <0.05, ** p <0.01 compared to the vehicle (VEH) control group. Two-way ANOVA followed by post hoc Dunnet’s test.
In smf-2 worms pre-exposed to VU0063088 we observed BSR impairment with a significant interaction [F (2, 11) = 8.27; p <0.01]. Post hoc analysis revealed significant differences between 50 mM Mn vehicle exposed group (Δ = 7.1 ± 0.7, p <0.05) and control. VU0063088 control group was also significantly impaired (Δ = 7.7 ± 0.27, p <0.05) compared to vehicle control. In smf-2 worms pre-exposed to VU0026921 there were no statistically significant BSR impairment (Fig 3B). Cat-2 mutants were used as positive control and displayed a low Δ as expected (Δ = 4.75 ± 0.37, p <0.001, data not shown).
Locomotion was assessed by the number of body bends off-food. Locomotion was not impaired by Mn exposure in the conditions tested herein (Fig 3C and 3D; p >0.05). In smf-2 worms exposed to VU0063088, controls displayed decreased locomotion (Fig. 3D), with a significant effect of small molecule treatment [F (1, 11) = 10.75; p <0.01], and significant interaction [F (2, 11) = 7.49; p <0.01].
Mn-induced lethality was not attenuated in worms pre-exposed to small molecules
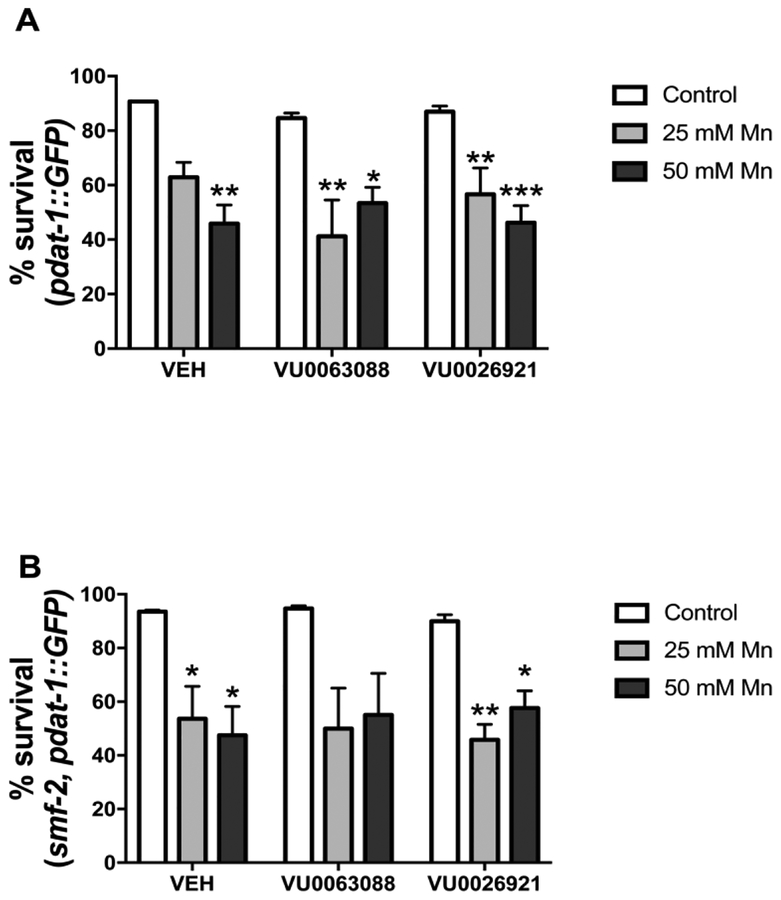
Survival was evaluated in L1 worms pre-exposed to VU0063088 or VU0026921 and exposed to 25 or 50 mM Mn for 1 h. A significant effect of Mn treatment was observed on WT worms survival [VU0063088 F (2, 12) = 18.81; p <0.001] and [VU0026921 F (2, 12) = 26.97; p <0.001]. Post hoc analysis revealed 45.87 ± 6.79 % worms pre-exposed to vehicle and exposed to 50 mM Mn survived, versus 90.74 ± 0.22 % worms in the vehicle control group (p <0.01). Similar decrease in survival was observed in worms exposed to small molecules and 25 or 50 mM Mn (Fig. 4A)
Fig. 4:
Synchronized L1 worms were pre-treated for 30 min with 5 μM VU0063088 or VU0026921 and then exposed to 25 or 50 mM MnCl2 for 1 h, followed by plating 30–60 worms in NGM and scoring lethality. Results are expressed as the percentage of animals alive 48 h post-treatment relative to day 0 and are expressed as mean ± S.E.M. of 3 experiments. * p <0.05, ** p <0.01, *** p <0.001 compared to vehicle (VEH) control group. Two-way ANOVA followed by post hoc Dunnet’s test.
In smf-2 worms, decreased survival was observed, with a significant effect of Mn treatment [VU0063088 F (2, 11) = 11.13; p <0.01] and [VU0026921 F (2, 12) = 19.48; p <0.001]. Post hoc revealed significant differences between worms pre-exposed to vehicle and exposed to 25 mM Mn (53.65 ± 12.01 % survival) or 50 mM Mn (47.45 ± 10.76 % survival), p <0.05. When worms were pre-exposed to VU0026921 a decrease in survival was observed with 25 mM Mn and 50 mM Mn in comparison with VEH control group (Fig. 4B).
Discussion
This study provides evidence to support a potential protective role for VU0063088 and VU0026921 against Mn-induced DAergic neurodegeneration in worms. We observed a partial recovery of DAergic neurons morphology supported by evidence of BSR. This effect does not require SMF-2, a homologue of DMT-1, since worms lacking this transporter displayed similar results to WT. The role of other Mn transporters in small molecule exposed worms remains to be determined. However, small molecules can be important tools to determine Mn-regulating mechanisms in vivo.
As described before by Au et al., 2009, SMF-2 deficient worms accumulate higher Mn levels than pdat-1::GFP worms. Our ICPMS data supports this effect. This higher Mn accumulation was not accompanied by increased DAergic degeneration, suggesting Mn effects are not entirely dependent on total body Mn levels. Further, in this model, DAergic degeneration and survival upon Mn exposure are not always correlated. We observed that small molecule pre-treatment partially rescued Mn-induced neurodegeneration, but had no effect on worm survival. Notably, this effect was apparent even with little influence on Mn levels in worms. Therefore, treatments that rescue DAergic neurons may not always be sufficient for survival.
Despite the protective effect on DAergic neurodegeneration, counter to our expectations, VU0063088 significantly impaired smf-2 worms locomotion compared to VEH exposed worms, in the absence of Mn treatment. In worms lacking this transporter there could be imbalance on other ions that we did not address in this work. The presence of VU0063088 may have enhanced this characteristic. The neuronal circuit that controls locomotion in worms is small (75 motor neurons), but complex. Cholinergic and GABAergic neurons distributed along the ventral nerve cord act on muscle cells to induce forward or backward movements (Zhen and Samuel 2015). The precise mechanism of VU0063088 on smf-2 worms needs further investigation.
Mutations in human subjects with deregulated Mn homeostasis have elucidated the critical role of metal transporters. Tuschl et al. (2008) reported a new form of familial parkinsonism in a 12-year old girl with hypermanganesaemia, liver cirrhosis, an extrapyramidal motor disorder and polycythaemia. Mn concentration in her blood was 10 times higher than normal (>3000 nmol/L), although she had never been exposed to high Mn levels. Whole-genome mapping identified SLC30A10 as the affected gene (Tuschl et al. 2012). SLC30A10 was later described to function primarily as an exporter at the cell membrane to transport cytoplasmic Mn ions across the membrane to the extracellular space (Leyva-Illades et al. 2014).
We did not observe alterations in Mn accumulation patterns in the conditions tested herein, but with other small molecules, or higher concentrations of these molecules, effects could be apparent. Manipulation of Mn levels with small molecules coupled with genetic manipulation of the biological targets of these molecules may uncover transport mechanisms and reveal therapeutic targets against heavy metal toxicity. Further testing with mutants for other Mn transporters (such as SMF-1/3, SLC30A10, among others), or even different signaling proteins (MAPKs, AKT), could elucidate small molecules targets for modifying intracellular Mn concentrations.
Conclusion
We have identified two small molecules that suppress Mn-induced DAergic degeneration, although these molecules did not impact Mn levels at the level of the whole worm. It is possible VU0063088 and VU0026921 alter Mn compartmentalization or even bind Mn. Also, higher concentrations of these molecules, and/or different exposure protocols could show alterations in Mn accumulation. The mechanisms of action are currently being explored.
Highlights.
This study demonstrates a potential protective role against DAergic neurodegeneration for VU0063088 and VU0026921 in Mn exposed worms.
This effect is not dependent on SMF-2, a Mn transporter.
Small molecules can be important tools to determine Mn-regulating mechanisms in vivo.
Acknowledgments
MA and ABB were supported by National Institute of Health (NIH) R01 ES10563 and R01 ES07331. MA was also supported by R01 ES020852. We thank the “Deutsche Forschungsgemeinschaft” (DFG) further for the financial support of Schw 903/9–1 and BO 4103/2–1. Images were obtained at the Analytical Imaging Facility of the Albert Einstein College of Medicine [NCI cancer center support grant (P30CA013330)]. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). Funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. In addition, we thank the members of the Vanderbilt Chemical Synthesis Core.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Bibliography
- Aschner JL et al. (2015) Neuroimaging identifies increased manganese deposition in infants receiving parenteral nutrition Am J Clin Nutr 102:1482–1489 doi: 10.3945/ajcn.115.116285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au C, Benedetto A, Anderson J, Labrousse A, Erikson K, Ewbank JJ, Aschner M (2009) SMF-1, SMF-2 and SMF-3 DMT1 Orthologues Regulate and Are Regulated Differentially by Manganese Levels in C. elegans PLoS One 4:e7792 doi: 10.1371/journal.pone.0007792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydemir TB et al. (2017) Metal Transporter Zip14 (Slc39a14) Deletion in Mice Increases Manganese Deposition and Produces Neurotoxic Signatures and Diminished Motor Activity J Neurosci 37:5996–6006 doi: 10.1523/JNEUROSCI.0285-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetto A, Au C, Avila DS, Milatovic D, Aschner M (2010) Extracellular dopamine potentiates Mn-induced oxidative stress, lifespan reduction, and dopaminergic neurodegeneration in a BLI-3-dependent manner in Caenorhabditis elegans. PLoS Genet 6 (8) doi: 10.1371/journal.pgen.1001084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornhorst J et al. (2014) The effects of pdr1, djr1.1 and pink1 loss in manganese-induced toxicity and the role of [small alpha]-synuclein in C. elegans Metallomics 6:476–490 doi: 10.1039/C3MT00325F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans Genetics 77:71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Bowman AB, Mukhopadhyay S, Aschner M (2015) SLC30A10: A novel manganese transporter Worm 4:e1042648 doi: 10.1080/21624054.2015.1042648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M (2015) Manganese Is Essential for Neuronal Health Annu Rev Nutr 35:71–108 doi:doi: 10.1146/annurev-nutr-071714-034419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkitkasemwong S et al. (2018) SLC39A14 deficiency alters manganese homeostasis and excretion resulting in brain manganese accumulation and motor deficits in mice Proc Natl Acad Sci U S A 115:E1769–E1778 doi: 10.1073/pnas.1720739115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller WC, Lyons KE, Truly W (2004) Effect of levodopa treatment for parkinsonism in welders: A double-blind study Neurology 62:730–733 [DOI] [PubMed] [Google Scholar]
- Kumar KK et al. (2014) Cellular manganese content is developmentally regulated in human dopaminergic neurons Scientific Reports 4:6801 doi:10.1038/srep06801 10.1038/srep06801http://www.nature.com/articles/srep06801 - supplementary-informationhttp://www.nature.com/articles/srep06801 - supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-Illades D et al. (2014) SLC30A10 Is a Cell Surface-Localized Manganese Efflux Transporter, and Parkinsonism-Causing Mutations Block Its Intracellular Trafficking and Efflux Activity The Journal of Neuroscience 34:14079–14095 doi: 10.1523/JNEUROSCI.2329-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C et al. (2017) Hypothyroidism induced by loss of the manganese efflux transporter SLC30A10 may be explained by reduced thyroxine production J Biol Chem doi: 10.1074/jbc.M117.804989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini R, Albini E, Placidi D, Gasparotti R, Pigozzi MG, Montani G, Alessio L (2000) Brain magnetic resonance imaging and manganese exposure Neurotoxicology 21:769–775 [PubMed] [Google Scholar]
- Peres TV et al. (2018) Role of Caenorhabditis elegans AKT-1/2 and SGK-1 in Manganese Toxicity Neurotox Res doi: 10.1007/s12640-018-9915-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JA (2006) Homeostatic and toxic mechanisms regulating manganese uptake, retention, and elimination Biol Res 39:45–57 [DOI] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR (2000) C. elegans Locomotory Rate Is Modulated by the Environment through a Dopaminergic Pathway and by Experience through a Serotonergic Pathway Neuron 26:619–631 doi: 10.1016/S0896-6273(00)81199-X [DOI] [PubMed] [Google Scholar]
- Stepens A et al. (2008) A Parkinsonian Syndrome in Methcathinone Users and the Role of Manganese N Engl J Med 358:1009–1017 doi:doi: 10.1056/NEJMoa072488 [DOI] [PubMed] [Google Scholar]
- Sulston J, Hodgkin J (1988) Methods. 1988.
- Tuschl K et al. (2012) Syndrome of Hepatic Cirrhosis, Dystonia, Polycythemia, and Hypermanganesemia Caused by Mutations in SLC30A10, a Manganese Transporter in Man The American Journal of Human Genetics 90:457–466 doi: 10.1016/j.ajhg.2012.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuschl K, Mills PB, Parsons H, Malone M, Fowler D, Bitner-Glindzicz M, Clayton PT (2008) Hepatic cirrhosis, dystonia, polycythaemia and hypermanganesaemia--a new metabolic disorder J Inherit Metab Dis 31:151–163 doi: 10.1007/s10545-008-0813-1 [DOI] [PubMed] [Google Scholar]
- Wang X, Wang X, Li L, Wang D (2010) Lifespan extension in Caenorhabditis elegans by DMSO is dependent on sir-2.1 and daf-16 Biochem Biophys Res Commun 400:613–618 doi: 10.1016/j.bbrc.2010.08.113 [DOI] [PubMed] [Google Scholar]
- Xin Y et al. (2017) Manganese transporter Slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice Cell Discov 3:17025 doi: 10.1038/celldisc.2017.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen M, Samuel ADT (2015) C. elegans locomotion: small circuits, complex functions Curr Opin Neurobiol 33:117–126 doi: 10.1016/j.conb.2015.03.009 [DOI] [PubMed] [Google Scholar]