Abstract
Background
Acrolein is an extremely electrophilic aldehyde. Increased urinary acrolein adducts have been found in type 2 diabetic patients and people with a smoking habit. The increased blood acrolein was shown in patients who received the cancer drug cyclophosphamide. Both diabetes and smoking are risk factors for skeletal muscle wasting or atrophy. Acrolein has been found to induce myotube atrophy in vitro. The in vitro and in vivo effects and mechanisms of acrolein on myogenesis and the in vivo effect of acrolein on muscle wasting still remain unclear.
Methods
C2C12 myoblasts were used to assess the effects of low‐dose acrolein (0.125–1 μM) on myogenesis in vitro. Mice were exposed daily to acrolein in distilled water by oral administration (2.5 and 5 mg/kg) for 4 weeks with or without glycerol‐induced muscle injury to investigate the effects of acrolein on muscle wasting and regeneration.
Results
Non‐cytotoxic‐concentration acrolein dose dependently inhibited myogenic differentiation in myoblasts (myotube formation inhibition: 0.5 and 1 μM, 66.25% and 46.25% control, respectively, n = 4, P < 0.05). The protein expression for myogenesis‐related signalling molecules (myogenin and phosphorylated Akt: 0.5 and 1 μM, 85.15% and 51.52% control and 62.63% and 56.57% control, respectively, n = 4, P < 0.05) and myosin heavy chain (MHC: 0.5 and 1 μM, 63.64% and 52.53% control, n = 4, P < 0.05) were decreased in acrolein‐treated myoblasts. Over‐expression of the constitutively active form of Akt in myoblasts during differentiation prevented the inhibitory effects of acrolein (1 μM) on myogenesis (MHC and myogenin protein expression: acrolein with or without constitutively active Akt, 64.65% and 105.21% control and 69.14% and 102.02% control, respectively, n = 5, P < 0.05). Oral administration of acrolein for 4 weeks reduced muscle weights (5 mg/kg/day: 65.52% control, n = 6, P < 0.05) and cross‐sectional area of myofibers in soleus muscles (5 mg/kg/day: 79.92% control, n = 6, P < 0.05) with an up‐regulation of atrogin‐1 and a down‐regulation of phosphorylated Akt protein expressions. Acrolein retarded soleus muscle regeneration in a glycerol‐induced muscle regeneration mouse model (5 mg/kg/day: 49.29% control, n = 4, P < 0.05). Acrolein exposure reduced muscle endurance during rotarod fatigue performance in mice with or without glycerol‐induced muscle injury (5 mg/kg/day without glycerol: 30.43% control, n = 4, P < 0.05). Accumulation of acrolein protein adducts could be detected in the soleus muscles of acrolein‐treated mice.
Conclusions
Low‐dose acrolein significantly inhibited myogenic differentiation in vitro, which might be through inhibition of Akt signalling. Acrolein induced muscle wasting and retarded muscle regeneration in mice. These results suggest that acrolein may be a risk factor for myogenesis and disease‐related myopathy.
Keywords: Acrolein, Myoblast, Myogenesis, Akt signalling, Muscle regeneration
Introduction
Acrolein, a highly electrophilic unsaturated aldehyde, is a ubiquitous environmental toxicant, to which humans are exposed though dietary, endogenous, and environmental sources. Acrolein can be formed during the processing of frying food or produced from industry or automobile exhausts.1, 2, 3 Acrolein is classified as a significant priority toxic pollutant of the environment by the US Environmental Protection Agency.4 Endogenous sources of acrolein include lipid peroxidation, degradation of amino acids, and metabolic products of the cancer drug cyclophosphamide.3, 5 Tobacco smoke is also one of the major sources of human acrolein exposure.2 The acrolein levels in cigarette smoke can be up to 90 ppm.6 Alwis et al. have indicated that acrolein is a predominant contributor to cigarette smoke‐induced toxicity.7 Acrolein comprises a reactive carbonyl group that can interact with amino acids, protein, and nucleophilic sites in DNA.2, 6 Previous studies showed that acrolein exposure could be associated with the pathogenesis of diabetes mellitus, cardiovascular disease, and Alzheimer's disease through mechanisms such as the adduction of protein/DNA and induction of mitochondrial/endoplasmic reticulum stress.3, 5
The endogenous sources of acrolein that contribute to various diseases are formed through polyamine degradation and oxidative stress.8 Acrolein is also known to be produced from overheated vegetable matter and fat.8 Chan et al. have demonstrated that cooking can cause significantly higher concentrations of acrolein in grocery stores.9 The tolerable daily intake (TDI) for acrolein has been estimated to be 7.5 μg/kg body weight.1 It has also been estimated that daily dietary consumption of unsaturated aldehydes, such as acrolein, is about 5 mg/kg of food.10 It has been estimated that workers in bars and taverns are exposed to acrolein at the levels of 15 to 1830 μg/day from cigarette smoke.11 The free acrolein in the plasma of uraemic patients with chronic renal failure has been detected to be 1–1.4 μM, which is higher than normal subjects (about 0.5 μM); the levels of acrolein‐protein adducts in the plasma of uraemic patients were 140–170 μM.12 Moreover, the patients who received cancer drug cyclophosphamide (60 mg/kg body weight/day) have been found to have peak blood acrolein levels at 6.2–10.2 μM.11
Skeletal muscle makes up 40–45% of the weight of the adult human body. Skeletal muscle has remarkable plasticity that adapts to physiological and environmental influences by changing its contractile and metabolic properties.13, 14 Responding to injury, skeletal muscle undergoes a regenerative process at the cellular and molecular levels.15, 16 In the initiation of myogenesis, myoblasts change into mononucleated myocytes with an elongated shape and express myogenic regulatory factors (MRFs); at the end of myogenic differentiation, myocytes convert into multinucleated myotubes, which exhibit numerous muscle structure proteins like myosin heavy chain (MHC), creatine kinase, and α‐actin.13, 16
Diabetes is known to impair skeletal muscle function and induces muscle atrophy such that the progression of diabetes not only impacts skeletal muscle growth but also inhibits the restoration of injured skeletal muscle.17, 18 The urinary levels of acrolein adduct were increased in both type 2 diabetes patients and habitual smokers.8 Oral administration of acrolein (1 mg/kg) to mice for 48 days has been shown to induce dilated cardiomyopathy.19 Furthermore, Rom et al. have found that in vitro application of both cigarette smoke in an exposure chamber with 50–600 mmHg negative pressure levels and acrolein at the concentrations of 10–500 μM to C2 skeletal myotubes induces myotube atrophy.20, 21 However, the precise effects of acrolein on skeletal muscle myogenesis and muscle mass remain unclear. In this study, we investigated the influences of acrolein exposure at doses relevant to human exposure on the in vitro and in vivo regulation of muscle regeneration and muscle mass.
Materials and methods
Cell culture
Murine C2C12 skeletal muscle myoblasts were purchased from American Type Culture Collection (Manassas, VA, USA). C2C12 cells were maintained in growth medium [GM; Dulbecco's modified Eagle's medium supplemented with 10% heat‐inactivated foetal bovine serum and antibiotics (penicillin 100 IU/mL, streptomycin 100 μg/mL, and amphotericin B 0.25 μg/mL)] at 37°C and 5% carbon dioxide in a humidified atmosphere.
Myogenic differentiation
Myogenic differentiation was performed as described previously.18 Myoblasts were cultured in differentiation medium [DM; MCDB201 and Ham's F12 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 2% heat‐inactivated horse serum and 1% penicillin/streptomycin/amphotericin B] with or without acrolein (0.125–1 μM; Sigma‐Aldrich, St. Louis, MO, USA) treatment. During differentiation, the medium with or without acrolein was replaced every day for 4 days until the multinucleated myotubes were formed. The morphology of the myotube was observed by haematoxylin and eosin (H&E) staining.
Cell viability
Cells were seeded in 96‐well plates and incubated in GM overnight and then transferred to DM with or without acrolein for 1–4 days. The survival rate of differentiated myoblasts was measured using 3‐(4,5‐dimethyl thiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT; Sigma‐Aldrich) assay that was reduced to purple formazan via mitochondrial dehydrogenase in living cells. The formazan crystals were solubilized by dimethyl sulfoxide, and its absorbance was measured at 570 nm by spectrophotometer.
Western blot analysis
The extraction of cellular protein and western blot analysis were performed as previously described.22 Cell lysates were separated on 8% or 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then electrotransferred onto polyvinylidene difluoride membrane. The membranes were blocked with skim milk (5%) for 1 h and then incubated with the primary antibodies for Akt, phosphorylated Akt (Ser473) (Cell Signaling, Danvers, MA, USA), myogenin, MHC, β‐actin (Santa Cruz, Santa Cruz, CA, USA), and acrolein protein adduct (Novus Biologicals, Littleton, CO, USA) overnight at 4°C. The membranes were incubated with anti‐rabbit or anti‐mouse antibodies conjugated to horseradish peroxidase for 1 h. The blots were visualized with enhanced chemiluminescence reagent (BioRad, Hercules, CA, USA) and exposed to X‐ray film. The densitometric analysis was assessed using ImageJ software.
Measurement of creatine kinase activity
The analysis of creatine kinase activity was performed by using a creatine kinase assay kit (Teco Diagnostics, Anaheim, CA, USA) following the manufacturer's instructions. Creatine kinase activity was calibrated to total protein level determined by a bicinchoninic acid (BCA) protein assay kit.
Transient transfection
Both the control pcDNA3.1 empty vector and the constitutively active form of Akt [myristoylated (myr) Akt], which was a generous gift from M.L. Kuo (National Taiwan University, Taipei, Taiwan),23 were transfected into C2C12 cells using a Lipofectamine 3000 reagent (Invitrogen) according to the manufacturer's instruction. Following transfection of pcDNA3.1 or myr Akt to cells, the GM was substituted for the DM, and C2C12 cells were further incubated for 4 days with or without acrolein. The transfection efficiency (about 40–50%) was confirmed by an equal amount of a plasmid‐encoded green fluorescent protein under the control of the cytomegalovirus promoter.
Animals
Five‐week‐old male ICR mice were obtained from the Experimental Animal Center, College of Medicine, National Taiwan University (Taipei, Taiwan). The laboratory protocol was approved by the ethical review committee of the College of Medicine, National Taiwan University, and was conducted in accordance with the regulations of Taiwan and NIH guidelines on the care and welfare of animals. Mice were treated humanely and with regard for the alleviation of suffering. Mice were separately housed in cages under constant temperature at 22 ± 2°C and 12 h light/dark cycles. The highest no‐observed‐adverse‐effect level (NOAEL) values of acrolein for systemic effects in mice treated with acrolein for 18 months (gavage in water) are 4.5 mg/kg/day.4 Doses of 2.5 and 5 mg/kg were chosen for in vivo study of acrolein. Mice were exposed to acrolein in distilled water by oral gavage daily for 4 weeks. The control mice were daily subjected to distilled water by oral gavage.
Histological and immunohistochemical assessment
The 5 μm soleus muscle sections were assessed by H&E staining. Cross‐sectional areas were calculated using the ImageJ 1.48 software (National Institutes of Health, Bethesda, MD, USA) in five random fields for each section. The immunohistochemistry for atrogin‐1 and phosphorylated Akt was performed in soleus muscles staining with anti‐atrogin‐1 antibody (Abcam, Cambridge, UK) and anti‐phosphorylated Akt antibody (Cell Signaling). For signal visualization, a SuperPicture horseradish peroxidase polymer conjugate kit (Invitrogen) was used.
Muscle fatigue task test
Motor coordination and balance in mice were determined by using a rotarod apparatus (Ugo Basile, Verese, Italy) as previously described.18 Mice were trained for 1 day on the rotarod for acclimation; the training consisted of two trials: the first trial was at a constant speed (13 rpm) for 15 min with a 15 min rest and the second trial was at speeds ramping from 13 to 25 rpm for 15 min. After acclimation training, the muscle fatigue task had mice run at speeds ramping from 13 to 25 rpm and maintained at 25 rpm for 30 min. Mice were replaced on the rotarod if they fell off or stopped running four times within 60 s of each fall. The latency to fall off the rotarod was recorded.
Glycerol‐induced muscle injury and regeneration model
The experiment of glycerol‐induced muscle injury and regeneration was performed as previously described.18, 24 Mice were anaesthetized under ketamine (30 mg/kg) and xylazine (4 mg/kg). Glycerol (100 μL of 50%, v/v) was injected into soleus muscles. The muscle strength was assessed by rotarod performance before and 5 days after glycerol injection. Mice were sacrificed 5 days after glycerol injection, and the soleus muscles were isolated. For histological analysis, the soleus muscles were fixed in 4% paraformaldehyde and embedded in paraffin, and 5 μm tissue slices were stained with H&E. Myofibers with central nuclei were calculated using the ImageJ 1.48 software (National Institutes of Health) in five random fields for each section.
Statistics
All results are given as means ± SEM of at least three independent experiments. The one‐way analysis of variance (ANOVA) followed by post hoc analysis with Bonferroni's test was used to analyse the statistical significance of differences in the means of the experimental groups. In the experiments of transient transfection of (myr) Akt, the two‐way ANOVA followed by post hoc analysis with the Sidak test was used. P < 0.05 was considered to be statistically significant.
Results
Acrolein retards myogenic differentiation in C2C12 myoblasts
Acrolein at concentrations of 0.125–1 μM did not reduce cell viability in C2C12 myoblasts (Figure 1A). For myogenesis, C2C12 myoblasts were incubated with differentiation medium until cell fusion and multinucleated myotube formation. As shown in Figure 1B, acrolein at concentrations of 0.5 and 1 μM significantly inhibited myogenic differentiation and myotube formation from C2C12 myoblasts observed by H&E staining and calculation of nuclei in the myotubes. Myogenin is known to be involved in the initiation of muscle differentiation, and myosin heavy chain (MHC) is a late myogenic differentiation marker.16 We further found that acrolein (0.125–1 μM) suppressed the protein expression of MHC and myogenin in differentiated C2C12 myoblasts (Figure 2A). Acrolein also reduced the creatine kinase activity, a marker of myoblast myogenesis,22 in a dose‐dependent manner (Figure 2B). These results revealed that acrolein was capable of inhibiting myoblast differentiation and myotube formation in cultured C2C12 myoblasts.
Figure 1.
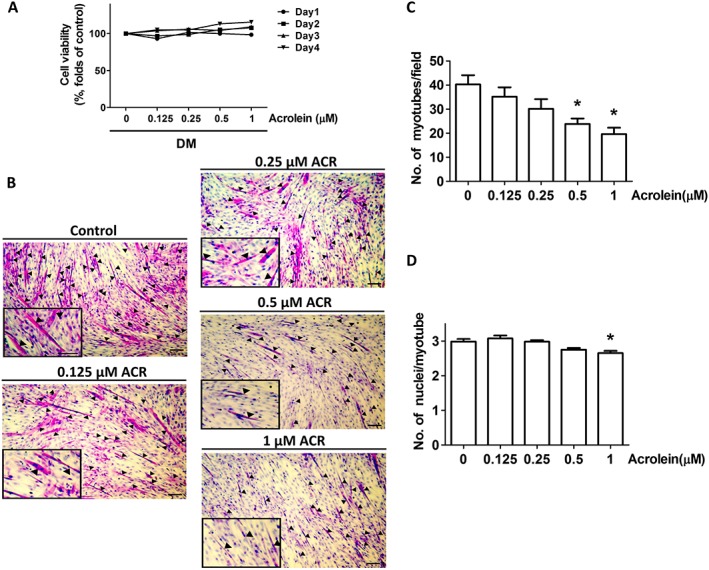
Inhibitory effects of acrolein on myogenic differentiation in C2C12 myoblasts. Cells were incubated in differentiation medium (DM) for 4 days with increasing concentrations of acrolein (ACR; 0.125–1 μM). (A) The effects of acrolein on cell viability determined by 3‐(4,5‐dimethyl thiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay were shown. (B) Cell morphology was inspected by haematoxylin and eosin (H&E) staining. Black arrows indicate the formation of multinucleated myotubes (A). The number of myotubes formed per field (C) and the average of nuclei per myotube (D) are shown. Results are expressed as means ± SEM for three independent experiments. Scale bar = 100 μm. * P < 0.05 compared with control.
Figure 2.
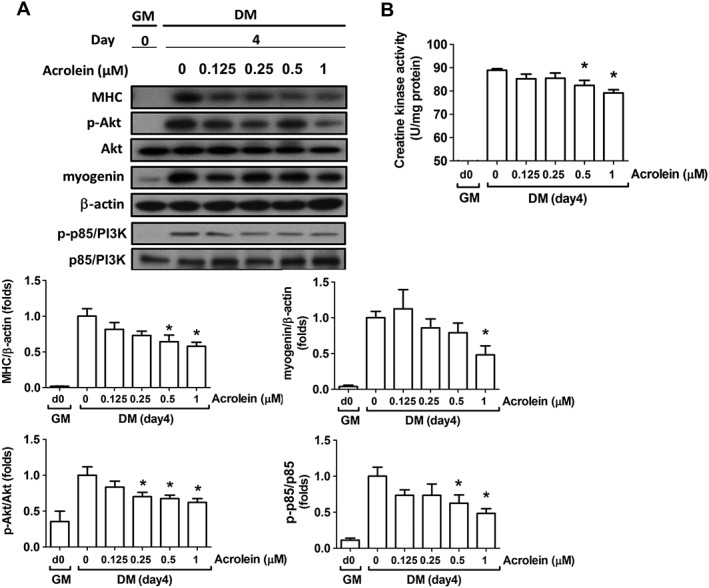
Effects of acrolein on protein expressions of myogenesis markers and creatine kinase activity in C2C12 myoblasts. Cells were incubated in growth medium (GM; Day 0) or differentiation medium (DM) for 4 days with increasing concentrations of acrolein (ACR; 0.125–1 μM). (A) The protein markers for myogenesis are shown. The protein expressions of phosphorylated Akt, Akt, myosin heavy chain (MHC), myogenin, phosphorylated p85/PI3K, and p85/PI3K were determined by western blotting and quantified using densitometric analysis. The β‐actin was regarded as a loading control. (B) The activity of creatine kinase is shown. Results are expressed as means ± SEM for at least three independent experiments. * P < 0.05 compared with control in DM without acrolein treatment.
Acrolein down‐regulated Akt signalling during myogenesis
The phosphatidylinositol 3‐kinase (PI3K)/Akt signal pathway is known to play a pivotal role in myogenesis.25, 26 We next investigated the effect of acrolein on Akt signalling during myogenic differentiation. As shown in Figure 2A, the protein expressions of phosphorylated p85/PI3K and phosphorylated Akt (Ser473) were dose‐dependently decreased by acrolein (0.125–1 μM) treatment in differentiated myoblasts. To verify the role of Akt signalling in acrolein‐induced myogenesis suppression, the constitutively active form of Akt (c.a. Akt) was transiently transfected into C2C12 myoblasts. Transfection with c.a. Akt significantly prevented the inhibition of MHC and myogenin protein expression (Figure 3A) and creatine kinase activity (Figure 3B) by acrolein (1 μM) in differentiated myoblasts. In morphological analysis by H&E staining, the acrolein‐inhibited myotube formation and average nuclei in myotubes were also prevented by c.a. Akt transfection (Figure 4). These results suggest that acrolein that inhibits myogenesis may be through an Akt‐regulated pathway.
Figure 3.
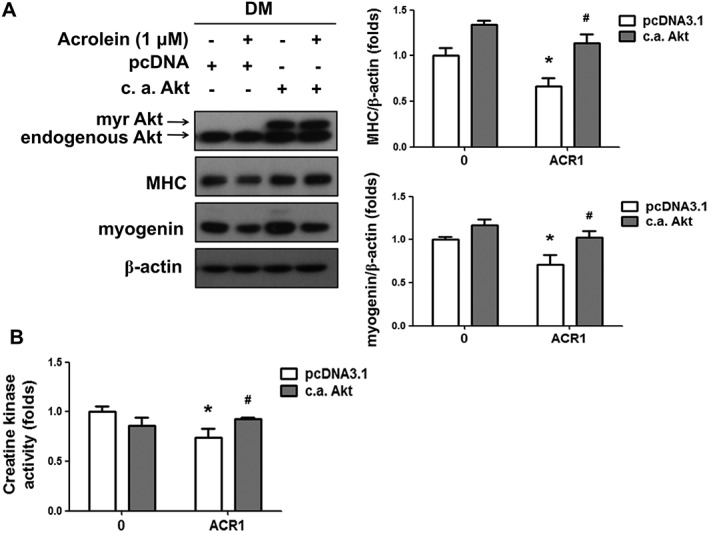
Transfection with a constitutively active form of Akt prevented the acrolein‐inhibited myogenic differentiation. After the transient transfection of a constitutively active form of Akt (c.a. Akt) or control pcDNA3.1 for 24 h, C2C12 myoblasts were consecutively differentiated in differentiation medium (DM) with 1 μM acrolein (ACR) for 4 days. (A) The protein expressions of Akt, myosin heavy chain (MHC), and myogenin were determined by western blotting and quantified using densitometric analysis. The β‐actin was regarded as a loading control. (B) The activity of creatine kinase is shown. Results are expressed as means ± SEM for at least three independent experiments. * P < 0.05 compared with pcDNA control group without acrolein treatment. # P < 0.05 compared with pcDNA control group with acrolein treatment.
Figure 4.
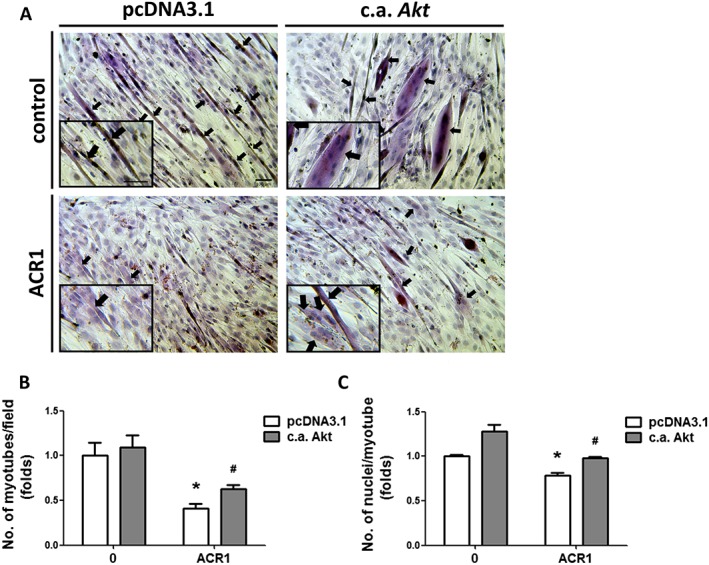
Transfection with a constitutively active form of Akt prevented the acrolein‐inhibited myotube formation. After the transient transfection of a constitutively active form of Akt (c.a. Akt) or control pcDNA3.1 for 24 h, C2C12 myoblasts were consecutively differentiated in differentiation medium (DM) with 1 μM acrolein (ACR) for 4 days. Cell morphology was inspected by haematoxylin and eosin (H&E) staining. Black arrows indicated the formation of multinucleated myotubes (A). The number of myotubes formed per field (B) and the average of nuclei per myotube (C) are shown. Results are expressed as means ± SEM for three independent experiments. Scale bar = 100 μm. * P < 0.05 compared with pcDNA control group without acrolein treatment. # P < 0.05 compared with pcDNA control group with acrolein treatment.
Acrolein decreases muscle weight and cross‐sectional area and retards muscle regeneration in mice
After 2.5 or 5 mg/kg acrolein exposure by oral gavage for 2 and 4 weeks, the body weights of the mice were significantly decreased (Figure 5A). Weight and myofiber cross‐sectional area of soleus muscles in acrolein‐treated mice were also significantly decreased (Figure 5B).
Figure 5.
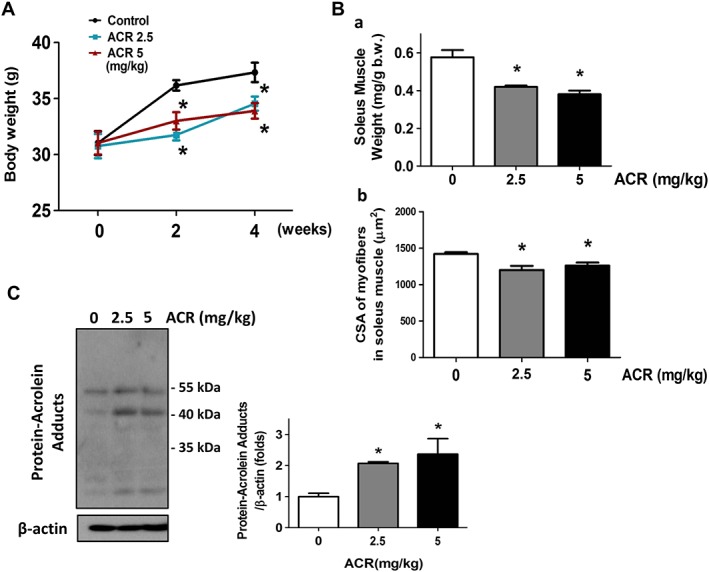
Effects of acrolein on body weight and soleus muscle weight, cross‐sectional area, and acrolein‐protein adduct accumulation in mice. Mice were treated with acrolein (ACR; 2.5 and 5 mg/kg) by daily oral gavage for 4 weeks. The body weights (A) and soleus muscle weights (B‐a) and cross‐sectional areas (CSAs; B‐b) are shown. The acrolein‐protein adducts in soleus muscles (C) were determined by western blotting. Results are expressed as means ± SEM (n = 4–6). * P < 0.05 compared with control group.
Acrolein can interact with protein to form a protein adduct.6 Increased urinary acrolein adduct has been observed in both type 2 diabetic patients and habitual smokers.8 We next detected the acrolein protein adducts in soleus muscles of acrolein‐treated mice. As shown in Figure 5C, the acrolein protein adduct was significantly increased in the soleus muscles of acrolein‐treated mice.
We also investigated whether acrolein exposure affected the muscle atrophy‐related signals in mice. As shown in Figure 6, the soleus muscle isolated from acrolein‐treated mice showed an increased atrogin‐1 and a decreased phosphorylated Akt and a decreased phosphorylated FOXO3A protein expression according to immunohistochemistry staining.
Figure 6.
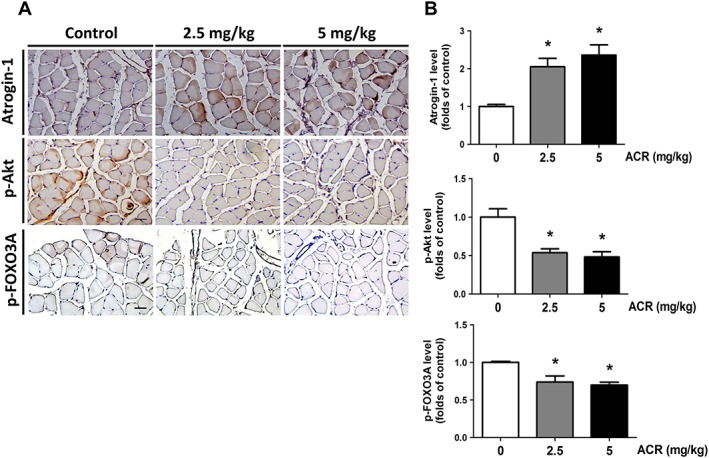
Effects of acrolein on the expressions of atrogin‐1 and phosphorylated Akt and phosphorylated FOXO3A in the soleus muscles of mice. Mice were treated with acrolein (ACR; 2.5 and 5 mg/kg) by daily oral gavage for 4 weeks. The representative immunohistochemical images for atrogin‐1 and phosphorylated Akt and phosphorylated FOXO3A expressions in the soleus muscles isolated from each group are shown (A). Immunohistochemical staining was quantified using densitometric analysis (B). Results are expressed as means ± SEM for at least three independent experiments. * P < 0.05 compared with control group.
To establish the muscle regeneration model, the intramuscular injection of glycerol was performed on soleus muscle, which causes reproducible muscle necrotic injury. Myogenic regeneration is evoked within 2–14 days after glycerol injection.27 The newly formed myofibers with centralized nuclei represent progressive myofiber repair.28 Acrolein significantly inhibited glycerol‐induced soleus muscle regeneration in mice as observed by H&E staining (Figure 7A) and calculation of the percentage of centralized nuclei located in the myofibers (Figure 7B) and the cross‐sectional area (CSA) in the myofibers (Figure 7C). Moreover, the rotarod performance was used to evaluate the motor coordination and the skeletal muscle strength and function.29, 30 As shown in Figure 7D, the latency on the rotarod apparatus during the performance of the muscle fatigue task was shorter in acrolein‐treated mice as compared with control mice. Acrolein exposure also significantly enhanced the glycerol's inhibitory effect on muscle endurance in mice (Figure 7C). We further examined the myogenesis‐related signals in this muscle regeneration model. As shown in Figure 8, the protein expressions of phosphorylated Akt, myogenin, and MHC were significantly increased in myogenic soleus muscles after glycerol‐induced injury. Acrolein treatment significantly reduced myogenesis and protein expression of phosphorylated Akt, myogenin, and MHC in glycerol‐injured soleus muscles. These results indicate that acrolein may retard skeletal muscle regeneration and hamper exercise performance in mice.
Figure 7.
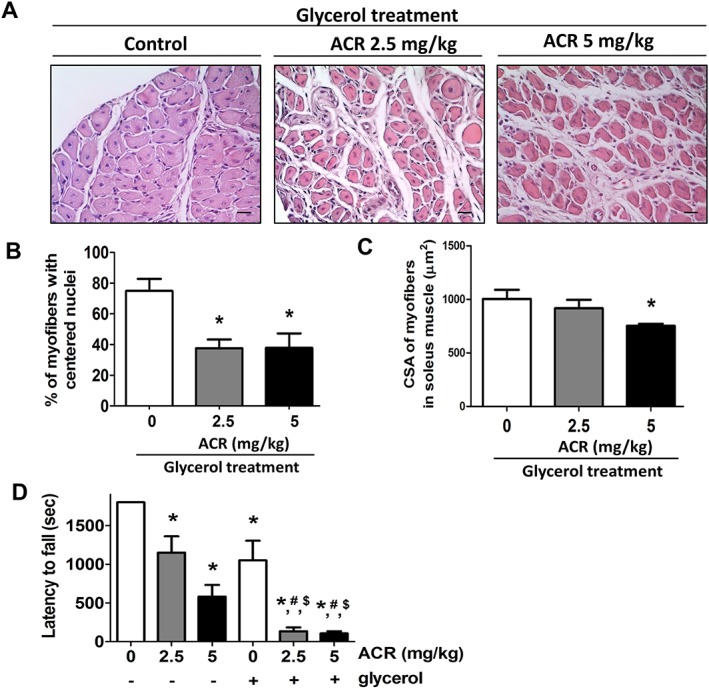
Effects of acrolein on muscle regeneration after glycerol injury in mice. Mice were treated with acrolein (ACR; 2.5 and 5 mg/kg) by daily oral gavage for 4 weeks. The glycerol myopathy model was used to test the effects of acrolein on muscle regeneration. (A) The histology in soleus muscles determined by haematoxylin and eosin (H&E) staining is shown. The muscle regeneration was shown as the percentage of centralized nuclei located in myofibers (B). The cross‐sectional area (CSA) in myofibers was also shown (C). Moreover, the latency on the rotarod apparatus during muscle fatigue task performance in mice with or without acrolein treatment in the presence or absence of glycerol injury is shown (D). Results are expressed as means ± SEM (n = 5). Scale bar = 20 μm. * P < 0.05 compared with control group without both acrolein and glycerol treatment. # P < 0.05 compared with control group with glycerol treatment. $ P < 0.05 compared with acrolein group without glycerol treatment. [Correction added on 19 February 2019 after first online publication: Figure 7C has been corrected in this version]
Figure 8.
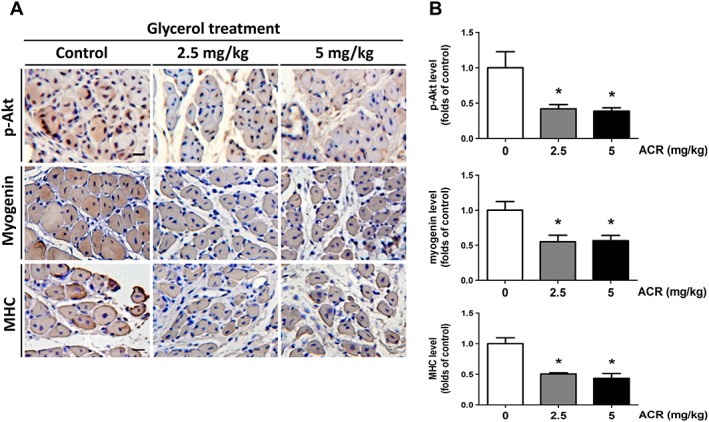
Effects of acrolein on myogenesis‐related signals after glycerol injury in mice. Mice were treated with acrolein (ACR; 2.5 and 5 mg/kg) by daily oral gavage for 4 weeks. The glycerol myopathy model was used to test the effects of acrolein on muscle regeneration. The representative immunohistochemical images for phosphorylated Akt and myogenin and myosin heavy chain (MHC) expressions in the soleus muscles isolated from each group are shown (A). Scale bar = 100 μm. The immunohistochemical staining was quantified using densitometric analysis (B). Results are expressed as means ± SEM for at least three independent experiments. * P < 0.05 compared with control group.
Discussion
In the present study, we investigated the effects of acrolein at doses relevant to human exposure on the in vitro and in vivo regulation of muscle regeneration and muscle mass. Non‐cytotoxic concentrations of acrolein dose dependently inhibited the myogenic differentiation in myoblasts, perhaps through inhibition of Akt signalling. Acrolein induced muscle wasting and retarded muscle regeneration in mice.
Acrolein exposure to mice has been found to induce myocyte hypertrophy and increase apoptosis in the hearts.19 The precise effects of acrolein on skeletal muscle myogenesis and muscle mass in vivo remain unclear. In this study, we found that the low and non‐toxic concentrations of acrolein (0.125–1 μM) suppressed myogenesis from cultured myoblasts by inhibiting morphological myotube formation, protein expressions of MHC and myogenin, and creatine kinase activity in a dose‐dependent manner. We further found that acrolein (2.5 and 5 mg/kg) exposure to mice for 4 weeks caused significant loss of body weight and soleus muscle mass and decreased cross‐sectional area under normal conditions. Increased atrogin‐1 protein expression could also be observed in soleus muscle isolated from acrolein‐treated mice. Moreover, skeletal muscle is known to be capable of regenerating in response to muscle injury in which the inflammation reaction and activation of satellite cells have occurred.13, 15 In the present work, we used an experimental glycerol myopathy mouse model to investigate the effect of acrolein on muscle regeneration. Acrolein also appeared to inhibit muscle regeneration and decreased muscle strength by rotarod trials in mice with glycerol injury. These results indicate that acrolein is capable of hindering myoblast differentiation and muscle regeneration and causing muscle loss. The findings from previous studies and the present work suggest that acrolein may not only enhance catabolism but also inhibit anabolism in skeletal muscle.
In this study, glycerol (100 μL of 50%, v/v) was injected into soleus muscles. In addition to the injured soleus, the other proportions of the calf muscle (gastrocnemius and plantaris) around the soleus might also be injured by infiltrated glycerol in this area, which might cause the observed changes in the fatigue task. Moreover, the soleus muscle has been observed to be rich in myofibers expressing the slow type I myosin heavy chain isoform.31 It has also been observed that adult slow soleus muscle possesses a greater percentage of satellite cells compared with adult fast tibialis anterior or extensor digitorum longus muscles.31 Therefore, in this study, we chose to target the soleus muscle for muscle regeneration observation.
PI3K/Akt has been demonstrated to be important upstream signalling mediators for the process of myogenic differentiation.25, 26 It has also been suggested that Akt can substitute for PI3K in the stimulation of myogenic differentiation.26 Thomas et al. have found that the simulated ischaemia is capable of attenuating PI3K activity, which is associated with the inhibition of Akt phosphorylation in C2C12 myotubes.32 Yen et al. have shown that arsenic exposure effectively retards myogenesis via the inhibition of Akt signalling.24 Down‐regulation of Akt signalling was also demonstrated to be involved in benzo(a)pyrene‐induced myogenic differentiation inhibition.22 In the present study, we found that acrolein dose dependently inhibited Akt phosphorylation during myogenic differentiation. Overexpression of a constitutively active form of Akt in myoblasts effectively prevented the inhibitory effects of acrolein on myogenesis and myotube formation. These findings suggest that the inhibition of an Akt‐dependent signalling pathway may contribute to the acrolein‐retarded myogenesis.
Reactive oxygen species (ROS) are known to be produced in skeletal muscles under physiological and pathological conditions.33 Low‐level ROS could regulate normal cellular signalling, middle‐level ROS is capable of arresting cell growth, and high‐level ROS would induce cell death through either apoptotic or necrotic pathways in skeletal muscles.33, 34 The coordination between ROS and enzymatic antioxidant defence systems plays a role in successful muscle restoration during muscle regeneration after injury or muscular degeneration.33, 35 Acrolein can conjugate with glutathione, which may deplete the cellular concentrations of glutathione, resulting in the increase of ROS.3, 5 Acrolein overload may disrupt the cellular redox balance that alters gene expression or transcription factors, resulting in the induction of oxidative stress and inflammation.6 Acrolein is known as a product of lipid peroxidation. It has been found that acrolein is probably 100 times more reactive than 4‐hydroxynonenal (4‐HNE), which is another α,β‐unsaturated aldehyde produced by lipid peroxidation and can contribute to oxidative stress‐mediated deterioration.36 Kondo et al. also showed that high‐level lipid peroxidation products could induce muscle atrophy.37 The formation of protein‐acrolein adducts is elevated in several pathological lesions.3, 5, 8 Acrolein‐protein adducts have been regarded as a faithful biomarker for estimation of acrolein amount.38 Acrolein exposure to mice has also been shown to increase myocardial oxidative stress.19 Oxidative stress has been demonstrated to be involved in both cigarette smoke and acrolein‐induced myotube atrophy in vitro.20, 21 In the present study, we found that oral acrolein exposure to mice caused an accumulation of acrolein‐protein adducts in soleus muscles. Acrolein may disrupt the redox balance and enhance oxidative stress to inhibit skeletal myogenesis.
Ubiquitin‐proteasome‐dependent proteolysis is known to be an important determinant of muscle wasting/atrophy. The muscle‐specific ubiquitin ligases atrogin‐1/MAFbx and MuRF1 have been demonstrated to contribute to increased muscle protein degradation.39 Akt can phosphorylate the transcription factor Forkhead Box O (FOXO), causing inhibition of the transcription of target genes such as atrogin‐1 and MuRF1.39 It has been shown that in vitro application of both cigarette smoke and acrolein to cultured skeletal myotubes can induce myotube atrophy.20, 21 In the present study, we found that the muscle mass and myofiber cross‐sectional areas of soleus muscles in acrolein‐treated mice were significantly decreased. Soleus muscle isolated from acrolein‐treated mice also showed increased atrogin‐1 and decreased phosphorylated Akt protein expression and decreased phosphorylated FOXO3A protein expression. These results suggest that acrolein exposure may induce muscle atrophy in a mouse model.
This study demonstrated for the first time that low‐dose acrolein significantly inhibited myogenic differentiation in vitro and retarded muscle regeneration and caused muscle loss in vivo. Transfection with a constitutively active form of Akt into myoblasts effectively prevented the acrolein‐induced myogenesis inhibition, indicating that acrolein inhibits myogenesis through an Akt signalling pathway. Taken together, these in vitro and in vivo results suggest that acrolein may be a risk factor for myogenesis and disease‐related myopathy.
Author contributions
All authors approved the final version to be published. S.‐H.L. and R.‐S.Y performed the study conception and design. H.‐J.C. and C.‐C.W performed the acquisition of data. H.‐J.C., C.‐C.W, and C.‐Y.C performed the analysis and interpretation of data. D.‐C.C. and R.‐S.Y. provided reagents and technical support. D.‐C.C., R.‐S.Y., and S.‐H.L wrote the paper.
Conflict of interest
H.‐J.C., C.‐C.W., D.‐C.C., C.‐Y.C., R.‐S.Y., and S.‐H.L. declared that they have no conflict of interest.
Acknowledgements
This study was supported by grants from the Ministry of Science and Technology of Taiwan (NSC105‐2314‐B‐002‐025‐MY3) and the National Taiwan University Hospital (106‐S3442).
The authors certify that they complied with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.40
Chen, H.‐J. , Wang, C.‐C. , Chan, D.‐C. , Chiu, C.‐Y. , Yang, R.‐S. , and Liu, S.‐H. (2019) Adverse effects of acrolein, a ubiquitous environmental toxicant, on muscle regeneration and mass. Journal of Cachexia, Sarcopenia and Muscle, 10: 165–176. 10.1002/jcsm.12362.
Contributor Information
Rong‐Sen Yang, Email: rsyang@ntuh.gov.tw.
Shing‐Hwa Liu, Email: shinghwaliu@ntu.edu.tw.
References
- 1. Abraham K, Andres S, Palavinskas R, Berg K, Appel KE, Lampen A. Toxicology and risk assessment of acrolein in food. Mol Nutr Food Res 2011;55:1277–1290. [DOI] [PubMed] [Google Scholar]
- 2. Bein K, Leikauf GD. Acrolein—a pulmonary hazard. Mol Nutr Food Res 2011;55:1342–1360. [DOI] [PubMed] [Google Scholar]
- 3. Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain C, et al. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci 2015;143:242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agency for Toxic Substances and Disease Registry . Toxicological profile for acrolein. 2007. Accessed at http://www.atsdr.cdc.gov/ToxProfiles/tp124.pdf on Jan 31, 2018.
- 5. Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res 2008;52:7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci 2000;57:6–15. [DOI] [PubMed] [Google Scholar]
- 7. Alwis KU, de Castro BR, Morrow JC, Blount BC. Acrolein exposure in U.S. tobacco smokers and non‐tobacco users: NHANES 2005–2006. Environ Health Perspect 2015;123:1302–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daimon M, Sugiyama K, Kameda W, Saitoh T, Oizumi T, Hirata A, et al. Increased urinary levels of pentosidine, pyrraline and acrolein adduct in type 2 diabetes. Endocr J 2003;50:61–67. [DOI] [PubMed] [Google Scholar]
- 9. Chan WR, Sidheswaran M, Sullivan DP, Cohn S, Fisk WJ. Cooking‐related PM2.5 and acrolein measured in grocery stores and comparison with other retail types. Indoor Air 2015;26:489–500. [DOI] [PubMed] [Google Scholar]
- 10. Wang GW, Guo Y, Vondriska TM, Zhang J, Zhang S, Tsai LL, et al. Acrolein consumption exacerbates myocardial ischemic injury and blocks nitric oxide‐induced PKCε signaling and cardioprotection. J Mol Cell Cardiol 2008;44:1016–1022. [DOI] [PubMed] [Google Scholar]
- 11. Faroon O, Roney N, Taylor J, Ashizawa A, Lumpkin MH, Plewak DJ. Acrolein environmental levels and potential for human exposure. Toxicol Ind Health 2008;24:543–564. [DOI] [PubMed] [Google Scholar]
- 12. Igarashi K, Ueda S, Yoshida K, Kashiwagi K. Polyamines in renal failure. Amino Acids 2006;31:477–483. [DOI] [PubMed] [Google Scholar]
- 13. Zanou N, Gailly P. Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatory factors (MRFs) and insulin‐like growth factors (IGFs) pathways. Cell Mol Life Sci 2013;70:4117–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mounier R, Théret M, Lantier L, Foretz M, Viollet B. Expanding roles for AMPK in skeletal muscle plasticity. Trends Endocrinol Metab 2015;26:275–286. [DOI] [PubMed] [Google Scholar]
- 15. Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 2013;93:23–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jang YN, Baik EJ. JAK‐STAT pathway and myogenic differentiation. JAKSTAT 2013;2:e23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. D'Souza DM, Al‐Sajee D, Hawke TJ. Diabetic myopathy: impact of diabetes mellitus on skeletal muscle progenitor cells. Front Physiol 2013;4:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiu CY, Yang RS, Sheu ML, Chan DC, Yang TH, Tsai KS, et al. Advanced glycation end‐products induce skeletal muscle atrophy and dysfunction in diabetic mice via a RAGE‐mediated, AMPK‐down‐regulated, Akt pathway. J Pathol 2016;238:470–482. [DOI] [PubMed] [Google Scholar]
- 19. Ismahil MA, Hamid T, Haberzettl P, Gu Y, Chandrasekar B, Srivastava S, et al. Chronic oral exposure to the aldehyde pollutant acrolein induces dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 2011;301:H2050–H2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rom O, Kaisari S, Aizenbud D, Reznick AZ. Cigarette smoke and muscle catabolism in C2 myotubes. Mech Ageing Dev 2013a;134:24–34. [DOI] [PubMed] [Google Scholar]
- 21. Rom O, Kaisari S, Aizenbud D, Reznick AZ. The effects of acetaldehyde and acrolein on muscle catabolism in C2 myotubes. Free Radic Biol Med 2013b;65:190–200. [DOI] [PubMed] [Google Scholar]
- 22. Chiu CY, Yen YP, Tsai KS, Yang RS, Liu SH. Low‐dose benzo(a) pyrene and its epoxide metabolite inhibit myogenic differentiation in human skeletal muscle‐derived progenitor cells. Toxicol Sci 2014;138:344–353. [DOI] [PubMed] [Google Scholar]
- 23. Kuo ML, Chuang SE, Lin MT, Yang SY. The involvement of PI3‐K/Akt‐dependent up‐regulation of Mcl‐1 in the prevention of apoptosis of Hep3B cells by interleukin‐6. Oncogene 2001;20:677–685. [DOI] [PubMed] [Google Scholar]
- 24. Yen YP, Tsai KS, Chen YW, Huang CF, Yang RS, Liu SH. Arsenic inhibits myogenic differentiation and muscle regeneration. Environ Health Perspect 2010;118:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang BH, Zheng JZ, Vogt PK. An essential role of phosphatidylinositol 3‐kinase in myogenic differentiation. Proc Natl Acad Sci U S A 1998;95:14179–14183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiang BH, Aoki M, Zheng JZ, Li J, Vogt PK. Myogenic signaling of phosphatidylinositol 3‐kinase requires the serine‐threonine kinase Akt/protein kinase B. Proc Natl Acad Sci U S A 1999;96:2077–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kawai H, Nishino H, Kusaka K, Naruo T, Tamaki Y, Iwasa M. Experimental glycerol myopathy: a histological study. Acta Neuropathol 1990;80:192–197. [DOI] [PubMed] [Google Scholar]
- 28. Folker ES, Baylies MK. Nuclear positioning in muscle development and disease. Front Physiol 2013;4:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shiotsuki H, Yoshimi K, Shimo Y, Funayama M, Takamatsu Y, Ikeda K, et al. A rotarod test for evaluation of motor skill learning. J Neurosci Methods 2010;189:180–185. [DOI] [PubMed] [Google Scholar]
- 30. Burnes LA, Kolker SJ, Danielson JF, Walder RY, Sluka KA. Enhanced muscle fatigue occurs in male but not female ASIC3−/− mice. Am J Physiol Regul Integr Comp Physiol 2008;294:R1347–R1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 2004;84:209–238. [DOI] [PubMed] [Google Scholar]
- 32. Thomas MP, Mills J, Engelbrecht AM. Phosphatidylinositol‐3‐kinase (PI3K) activity decreases in C2C12 myotubes during acute simulated ischemia at a cost to their survival. Life Sci 2012;91:44–53. [DOI] [PubMed] [Google Scholar]
- 33. Kozakowska M, Pietraszek‐Gremplewicz K, Jozkowicz A, Dulak J. The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes. J Muscle Res Cell Motil 2015;36:377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barbieri E, Sestili P. Reactive oxygen species in skeletal muscle signaling. J Signal Transduct 2012;2012:982794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pierce AP, de Waal E, McManus LM, Shireman PK, Chaudhuri AR. Oxidation and structural perturbation of redox‐sensitive enzymes in injured skeletal muscle. Free Radic Biol Med 2007;43:1584–1593. [DOI] [PubMed] [Google Scholar]
- 36. Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer's disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging 2001;22:187–194. [DOI] [PubMed] [Google Scholar]
- 37. Kondo H, Miura M, Itokawa Y. Oxidative stress in skeletal muscle atrophied by immobilization. Acta Physiol Scand 1991;142:527–528. [DOI] [PubMed] [Google Scholar]
- 38. Higashi K, Igarashi K, Toida T. Recent progress in analytical methods for determination of urinary 3‐hydroxypropylmercapturic acid, a major metabolite of acrolein. Biol Pharm Bull 2016;39:915–919. [DOI] [PubMed] [Google Scholar]
- 39. Bilodeau PA, Coyne ES, Wing SS. The ubiquitin proteasome system in atrophying skeletal muscle: roles and regulation. Am J Physiol Cell Physiol 2016;311:C392–C403. [DOI] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


