Abstract
Currently, the early preclinical detection of left ventricular dysfunction is difficult because biomarkers are not specific for the cardiomyopathic process. The underlying molecular mechanisms leading to heart failure remain elusive, highlighting the need for identification of cardiac-specific markers. The growth hormone secretagogue receptor (GHSR) and its ligand ghrelin are present in cardiac tissue and are known to contribute to myocardial energetics. Here, we examined tissue ghrelin-GHSR levels as specific markers of cardiac dysfunction in patients who underwent cardiac transplantation. Samples of cardiac tissue were obtained from 10 patients undergoing cardiac transplant at the time of organ harvesting and during serial posttransplant biopsies. Quantitative fluorescence microscopy using a fluorescent ghrelin analog was used to measure levels of GHSR, and immunofluorescence was used to measure levels of ghrelin, B-type natriuretic peptide (BNP), and tissue markers of cardiomyocyte contractility and growth. GHSR and ghrelin expression levels were highly variable in the explanted heart, less in the grafted heart biopsies. GHSR and ghrelin were strongly positively correlated, and both markers were negatively correlated with left ventricular ejection fraction. Ghrelin had stronger positive correlations than BNP with the signaling markers for contractility and growth. These data suggest that GHSR-ghrelin have potential use as an integrated marker of cardiac dysfunction. Interestingly, tissue ghrelin appeared to be a more sensitive indicator than BNP to the biochemical processes that are characteristic of heart failure. This work allows for further use of ghrelin-GHSR to interrogate cardiac-specific biochemical mechanisms in preclinical stages of heart failure (HF).
The peptide hormone ghrelin is well-known as a potent orexigenic hormone. It stimulates food intake by activating hypothalamic neurons that regulate normal feeding behavior [1]. It is the natural ligand of the growth hormone secretagogue (GHSR) receptor 1a, a seven-transmembrane, G protein-coupled receptor, which, in addition to the hypothalamus, is expressed in other brain regions as well as several endocrine organs, such as the anterior pituitary, pancreatic islets, intestine, thyroid, and adipose tissue. In addition, ghrelin and GHSR are both expressed in cardiomyocytes, where they function through an axis that is independent of their role in regulating energy expenditure [2]. Activation of GHSR in cardiomyocytes promotes excitation-contraction coupling by increasing Ca2+ flux through both voltage-dependent Ca2+ channels [3] and the sarcoplasmic reticulum Ca2+-ATPase pump (SERCA2a) [4–6], and promotes cardiomyocyte growth and survival through ERK1/2 [4, 5], and phosphatidylinositol-3-kinase/Akt [5, 7]. We [6], and others [3], have recently shown that levels of GHSR are decreased in rodent models of diabetic cardiomyopathy, indicating that the dynamics of ghrelin and GHSR change even with mild impairments in left ventricular (LV) function.
In contrast, levels of ghrelin and GHSR are dramatically altered throughout the heart in patients with severe HF [8], indicating that myocardial GHSR is altered differently in HF compared with mild cardiomyopathy. The clinical syndrome of HF is most commonly associated with substantial impairment of LV contractility, leading to elevated intracardiac diastolic pressures and extravasation of fluid into the lung parenchyma and other tissues. The early detection and treatment of HF are limited by two issues: a) the specific series of molecular mechanisms leading to impaired contractility remain elusive in patients with idiopathic cardiomyopathies, and b) the responses to guideline-directed medical therapies remain highly variable, such that many patients continue to deteriorate, leading to either the need for cardiac transplantation or ultimately death. Clinically, there is a critical need to prospectively identify groups of patients who will ultimately be at higher risk, particularly in the early stages of LV dysfunction, when the clinical status and ventricular function are not by themselves consistent reliable predictors of disease progression and clinical outcomes. Circulating biomarkers, such as natriuretic peptide type-B (BNP), particularly the N-terminal form, and troponins T and I [9–11], provide some prediction of the progression of HF, by indicating changes within the cardiomyocyte that lead to stress, injury, and apoptosis. However, they are produced whenever heart tissue is damaged by any direct or indirect injury to the myocardium, and there may be discordance between tissue and circulating levels of these biomarkers. There is a need to identify biomarkers that are localized to the myocardium that reflect the cellular and molecular processes within the heart that underlie the progression of HF.
In this study, we evaluated the role of tissue ghrelin-GHSR levels as a specific biomarker of cardiac dysfunction in a cohort of patients who underwent cardiac transplantation. We examined samples from the diseased explanted heart and biopsies from the same individual’s engrafted heart followed through to one-year posttransplantation (PTx). In addition, we aimed to determine the relationship between ghrelin-GHSR and BNP to biochemical signaling molecules in cardiac dysfunction. Given the importance of intracellular Ca2+ homeostasis in atrial and ventricular contractility and the role of phospho-ERK 1/2 in cardiomyocyte growth, we hypothesized that changes in the ghrelin-GHSR axis in myocardial tissue could potentially reflect derangements in cardiomyocyte contractility and initiation of cardiac hypertrophic reprogramming that characterize the progression of HF.
1. Materials and Methods
A. Patient Cohort
Tissue samples were harvested from 10 patients who underwent cardiac transplantation at the London Health Sciences Center between 2011 and 2013. The protocol for sample dissections was approved by Western University’s Health Sciences Research Ethics Board. Samples, roughly 0.5 to 1.5 cm in length, were collected from the right atrium (RA) and left ventricle of the explanted/diseased heart from each cardiac transplant patient. Endomyocardial biopsies, roughly 0.1 to 0.3 cm in length, from the right ventricle of the newly grafted heart were also taken at various time points PTx, generally weekly for the first 4 weeks, monthly for months 2 through 6, and then at 1 year PTx. Patient demographics, cardiac function [LV ejection fraction (LVEF)], and medications pretransplantation and PTx are shown in Tables 2 and 3, respectively. All patient samples and patient data were kept anonymous, and all marker analyses was done before receiving clinical data.
Table 2.
Cardiac Transplant Recipient Patient Demographics
| Recipient Condition | No. of Patients With Condition (n = 10) |
|---|---|
| Male | 6 |
| Mean recipient age, y | 54 |
| Coronary artery disease | 5 |
| Hypertension | 1 |
| High pulmonary artery pressure | 10 |
| Diabetes | 0 |
| Assist device pretransplant | 3 |
| LVEF <30% pretransplant | 10 |
| LVEF >50% 1 mo posttransplant | 8 |
| LVEF >50% ≥ 6 months posttransplant | 10 |
Table 3.
Patient Medications Pre- and Postcardiac Transplant
| Medications | Pretransplant (n = 10) | Posttransplant 1 month (n = 10) | Posttransplant 6 months (n = 10) | Posttransplant 1 year (n = 10) |
|---|---|---|---|---|
| ACE inhibitor | 8 | 2 | 2 | 8 |
| Antiarrhythmic | 5 | 2 | 0 | 0 |
| Angiotensin receptor blocker | 0 | 0 | 0 | 0 |
| Antiplatelet | 5 | 7 | 7 | 8 |
| Coumadin | 7 | 1 | 0 | 0 |
| Beta blocker | 10 | 1 | 0 | 0 |
| Calcium channel blocker | 10 | 2 | 4 | 3 |
| Digoxin | 8 | 0 | 0 | 0 |
| Diuretics | 10 | 7 | 2 | 2 |
| Statins | 5 | 5 | 6 | 6 |
| Nitrates | 0 | 0 | 0 | 0 |
| Nitroglycerin | 0 | 0 | 0 | 0 |
| Antidiabetics | 0 | 0 | 0 | 0 |
| Inotropic support (milrinone/dobutamine) | 1 | 0 | 0 | 0 |
| Tacrolimus | 0 | 9 | 10 | 10 |
| Mycophenolate mofetil | 0 | 9 | 9 | 9 |
| Prednisone | 0 | 7 | 9 | 6 |
Abbreviation: ACE, angiotensin-converting enzyme.
B. Immunofluorescence Microscopy
Samples from both the explanted (diseased) and grafted hearts were frozen and embedded in optimal cutting temperature compound, and subsequently sectioned at 7 μm thickness, as previously described [4, 12]. Immunohistochemistry using primary and fluorophore-conjugated secondary antibodies was conducted as previously described [4, 12]. In brief, tissue sections were incubated with primary polyclonal or monoclonal antibodies [13–22] (Table 1) for 1 hour at room temperature in a humidified chamber. These antibodies were used to identify ghrelin (1:100), BNP (1:1000), pERK1/2 (1:250), SERCA2a (1:300), and collagen I (1:500). Samples were rinsed twice in PBS and incubated for 2 hours at room temperature with secondary antibodies (1:500) (Table 1). To detect GHSR, we used the far-red ghrelin peptide analog, Ghrelin [1-18, Lys18(Cy5)], as we have previously done to quantify GHSR in situ [4]. This analog binds with high specificity to GHSR in mouse cardiac tissue samples [6]. Following incubation with secondary antibodies, this fluorescent peptide analog was added to tissue sections for 30 minutes. Sections were washed with PBS, incubated 8 minutes with 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain (1:1000), and mounted with ProLong Gold antifade (Life Technologies) to prevent the tissues from photobleaching. Images were captured with a Nikon Eclipse TE2000-S fluorescent microscope. Five random fields of view were acquired for each of four tissue sections at ×20 magnification (Nikon NIS Elements v. BR 4.50.00) and used for further image analysis. Higher resolution images were captured using a Nikon A1R confocal microscope at ×60 magnification.
Table 1.
Information on Antibodies Used
| Antigen | Catalog No. | Dilution | Host | Reference No. |
|---|---|---|---|---|
| Ghrelin | sc-10359 | 1:100 | Goat | [13] |
| Serca2A | ab3625 | 1:300 | Rabbit | [14] |
| pERK1/2 | sc-377400 | 1:250 | Mouse | [15] |
| BNP | ab19645 | 1:1000 | Rabbit | [16] |
| Collagen I | ab34710 | 1:500 | Rabbit | [17] |
| DAPI | 62247 | 1:1000 | — | [18] |
| Alexa Fluor 488 | A11055 | 1:500 | Donkey antigoat | [19] |
| Alexa Fluor 594 | A21207 | 1:500 | Donkey antirabbit | [20] |
| Alexa Fluor 594 | A21203 | 1:500 | Donkey antimouse | [21] |
| Alexa Fluor 488 | A21206 | 1:500 | Donkey antirabbit | [22] |
C. Fibrosis Imaging
To assess fibrosis, heart tissue sections were stained with Masson’s trichrome stain by the Core Pathology Laboratory at the London Health Sciences Center. Sections were acquired using bright field microscopy at ×10, ×20, or ×40 magnifications with a Zeiss Axioskop EL-Einsatz microscope and Northern Eclipse software. Fluorescence microscopy was also used to acquire collagen I images as described previously.
D. Data Analysis
Images of GHSR, ghrelin, BNP, SERCA2a, and pERK were analyzed with FIJI v. 1.49v, a distribution of ImageJ software (National Institutes of Health). Fluorescence intensities of each section were quantified using a custom FIJI script that integrates raw density images that represent protein expression levels, as we have previously reported [4, 6, 12, 23, 24]. Briefly, thresholding was conducted for each image to determine the fluorescence intensity (positive pixel count above threshold minus the background). Fibrosis was analyzed using an online script that quantified the percentage of fibrotic tissue in each sample by distinguishing fibrotic tissue from nonfibrotic tissue [25]. Statistical analyses were performed using GraphPad Prism version 7.02 or IBM SPSS statistics 25, as follows: unpaired Student t test, a one-way ANOVA with analysis of variance using Tukey post hoc test to compare differences between diseased hearts and biopsies of the grafted hearts; Pearson correlation and logistic linear regression for correlations between markers; and Spearman bivariate correlation for relationships between LVEF and the following markers: GHSR, ghrelin, BNP, pERK1/2, and SERCA2a, all with significance set at P < 0.05.
2. Results
A. Cardiac Transplant Patient Cohort
Six of the 10 patients who underwent cardiac transplantation were male, with an overall mean age of 54 years (Table 2). Five had substantial coronary artery disease pretransplant, and none had diabetes. All patients had elevated pulmonary artery pressures pretransplant, along with severely reduced LVEF (<30%) by echocardiographic assessment. Serial echocardiographic assessment of LV function following cardiac transplant showed LV recovery to an LVEF >50% in eight patients by 1-month PTx. By 6-month PTx, all patients had normal LV function (Table 2). Although most patients were receiving HF medications (i.e., angiotensin-converting enzyme inhibitors/beta blockers/diuretics) before transplantation, only a small number continued to receive these medications following surgery. Table 3 contains a complete listing of medications pret and PTx.
B. GHSR and Ghrelin Expression in Cardiomyocytes
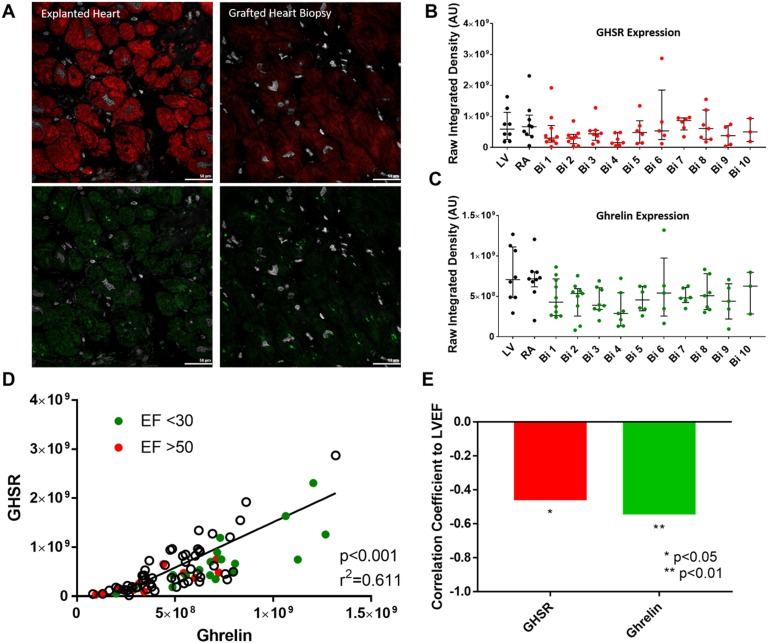
In any given cardiac transplant patient, the expression of GHSR and ghrelin in both LV and RA appeared to be elevated in the explanted heart in comparison with their expression PTx in the grafted heart tissue biopsies over time (Fig. 1A–1C). By logistic regression analysis of all patient specimens, both pretransplantation and PTx, the level of GHSR expression demonstrated a strong and highly significant positive correlation with the level of ghrelin expression (r = 0.7817, P < 0.0001). To examine associations of ghrelin and GHSR with cardiac dysfunction, data were divided into two groups of LVEF <30% (pretransplant) and LVEF > 50% (PTx); there were no midrange values of LVEF. In the pretransplant hearts with LVEF <30%, expression of GHSR and ghrelin clustered toward the higher end of the regression line, whereas in grafted heart biopsies at 1 and 6 months (LVEF >50%), expression of GHSR and ghrelin clustered toward the lower end of the regression line (Fig. 1 D). To more closely examine correlations between ghrelin/GHSR and cardiac dysfunction, we used a Spearman bivariate correlation test to show significant negative correlations between GHSR and LVEF (P = 0.018) and ghrelin and LVEF (P = 0.004) (Fig. 1E).
Figure 1.
Ghrelin and GHSR expression in patients pre- and postcardiac transplant. (A) Representative fluorescent images of GHSR (red), ghrelin (green), and DAPI (white) are shown in explanted heart tissue (left) and grafted heart biopsies (right) taken from the same patient. (B and C) Quantified fluorescence intensity of GHSR (red) and ghrelin (green) from explanted heart tissue (LV and RA, black dots) and grafted heart biopsies (Bi 1-10, colored dots). (D) Positive correlation of GHSR and ghrelin expression is shown in the entire cardiac transplant cohort with EF <30 in green and EF >50 in red, with white dots representing tissue samples without related EF values. Each dot represents one transplant patient sample. (E) Negative correlation between LVEF and GHSR (red) and ghrelin (green). AU, arbitrary unit; Bi, biopsy.
C. Metabolic Markers in Cardiac Tissue
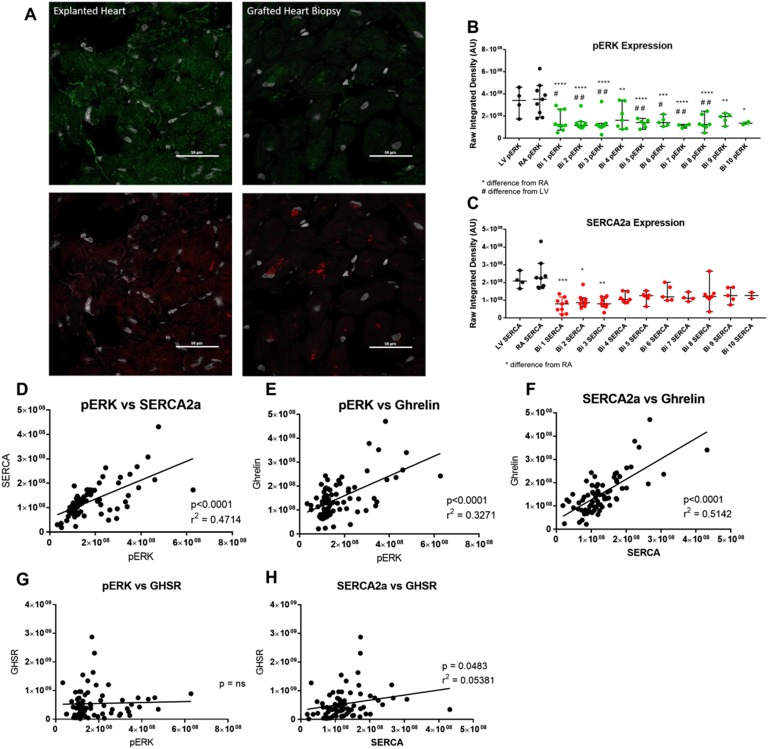
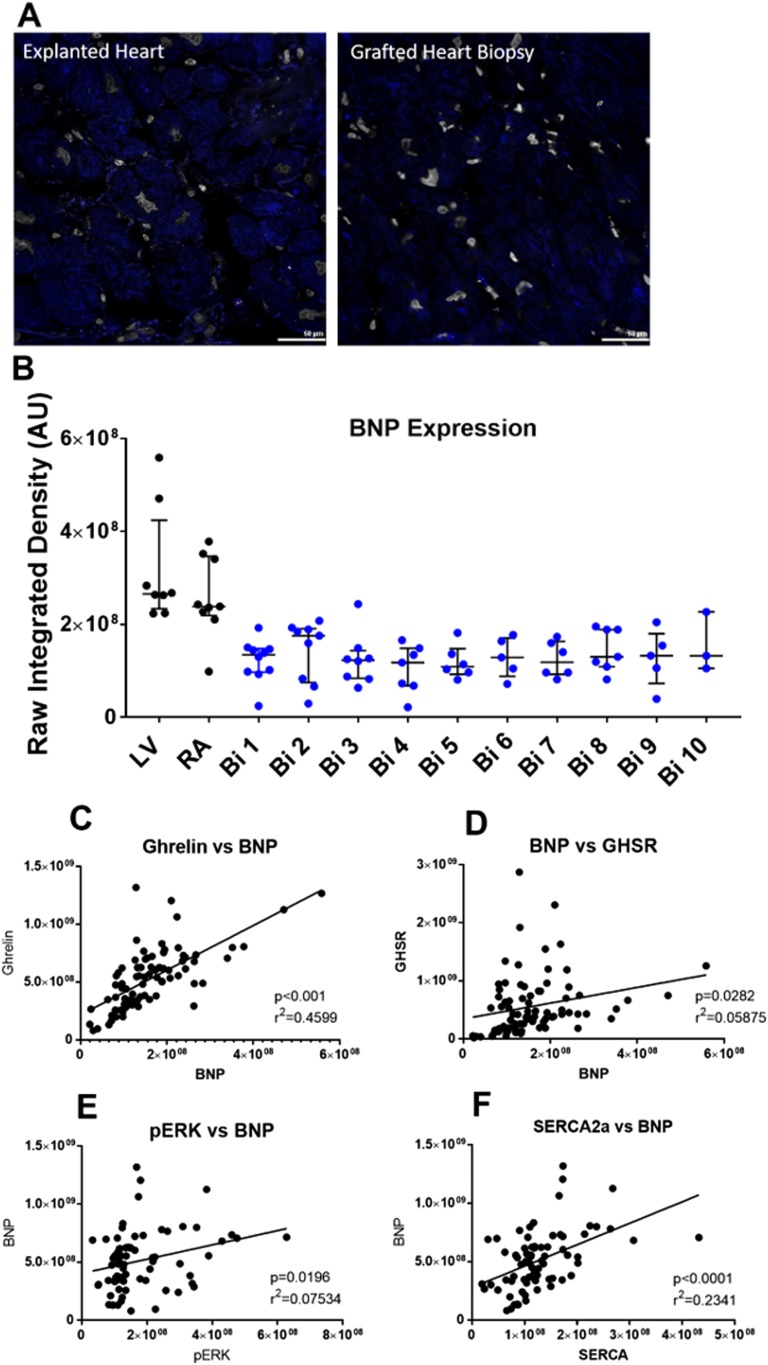
The expression of metabolic markers in cardiac tissue that are associated with cardiac dysfunction were measured by immunofluorescence microscopy. We measured the following markers: (i) SERCA2a, as an index of cardiomyocyte contractility; (ii) pERK1/2, as a marker of cardiomyocyte growth/hypertrophy; and (iii) BNP, as a validated clinical tool used to determine the presence and degree of HF. Representative images of SERCA2a, pERK1/2, and BNP expression are shown in Fig. 2A and Fig. 3A. The expression of SERCA2a was significantly elevated in cardiac tissue from the RA of the diseased heart (Fig. 2B) when compared with its expression in endomyocardial biopsies taken at weeks 1, 2, and 3 PTx by 3.2-, 2.4-, and 2.7-fold, respectively. However, there was no important difference in SERCA2a expression in the RA after the first month PTx. There were also no substantial differences in the expression of SERCA2a in the LV tissue of the diseased heart when compared with the PTx endomyocardial biopsies. pERK1/2 expression was significantly increased in the cardiac tissue of the diseased heart taken from the RA by 1.8- to 3.1-fold and LV by 1.7- to 2.9-fold when compared with the grafted heart tissue biopsies taken at any time point PTx (Fig. 2C). The degree of BNP expression (Fig. 3B) showed similar trends as for GHSR and ghrelin. Logistic regression analyses were performed to determine the association between SERCA2a, pERK1/2, GHSR, and ghrelin levels (Fig. 2D–2H). Highly significant and strong positive correlations were found between pERK1/2 and SERCA2a (r = 0.6867, P < 0.0001), pERK1/2 and ghrelin (r = 0.5719, P < 0.0001), and SERCA2a and ghrelin (r = 0.7171, P < 0.0001). By contrast, there was a much weaker correlation between SERCA2a and GHSR (r = 0.2320, P = 0.0483), and there was no correlation between pERK1/2 with GHSR. Logistic regression analyses were performed between all metabolic markers and BNP to determine any possible relationships (Fig. 3C–3F). There was a positive correlation between ghrelin and BNP (r = 0.6782, P < 0.0001), and SERCA2a and BNP (r = 0.4838, P < 0.0001). However, there were weak correlations between GHSR and BNP (r = 0.2423, P = 0.0282), and pERK1/2 and BNP (r = 0.2745, P = 0.0196). Spearman bivariate correlations (correlation coefficient, CorC) were calculated as described; they indicated highly significant negative associations between LVEF and SERCA2a (P < 0.001, CorC = −0.63), and pERK (P < 0.001, CorC = −0.814), and between LVEF and BNP (P < 0.001, CorC = −0.773).
Figure 2.
Cardiac metabolic markers in patients pre- and postcardiac transplant in entire transplant patient cohort. (A) Representative fluorescent images of pERK1/2 (green) and SERCA2a (red) are shown from explanted heart tissue (left) and grafted heart biopsies (right) samples taken from the same patient. (B and C) Quantified fluorescence intensity of pERK1/2 (green) and SERCA2a (red) are shown for explanted heart tissue (LV and RA, black dots) and grafted heart biopsies (Bi 1-10, colored dots). (D–H) Positive correlation is seen in the entire cardiac transplant cohort for pERK1/2 vs SERCA2a, pERK1/2 vs ghrelin, SERCA2a vs ghrelin, pERK1/2 vs GHSR, and SERCA2a vs GHSR. Each dot represents one transplant patient sample. *P < 0.05 from RA; **P < 0.01 from RA; ***P < 0.001 from RA; ****P < 0.0001 from RA; #P < 0.05 from LV; ##P < 0.01 from LV.
Figure 3.
BNP expression in patients pre- and postcardiac transplant. (A) Representative fluorescent images of BNP are shown from explanted heart tissue (left) and grafted heart biopsies (right) taken from the same patient. (B) Quantified fluorescence intensity of BNP is shown in explanted heart tissue (LV and RA, black dots) and grafted heart biopsies (Bi 1-10, colored dots). (C–F) Positive correlation is shown in the entire cardiac transplant cohort for ghrelin vs BNP, BNP vs GHSR, pERK1/2 vs BNP, and SERCA2a vs BNP. Each dot represents one transplant patient sample.
D. Cardiac Fibrosis
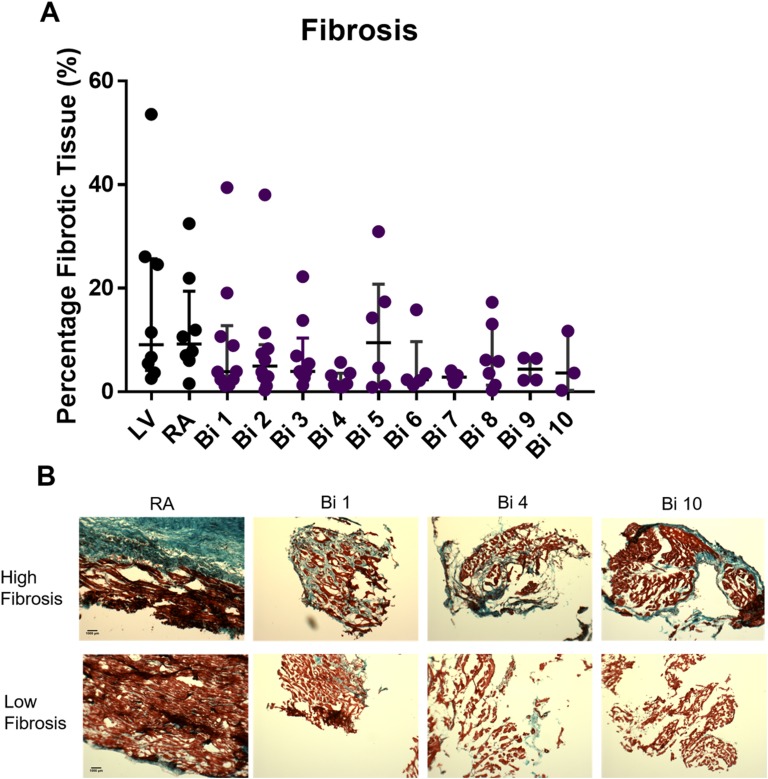
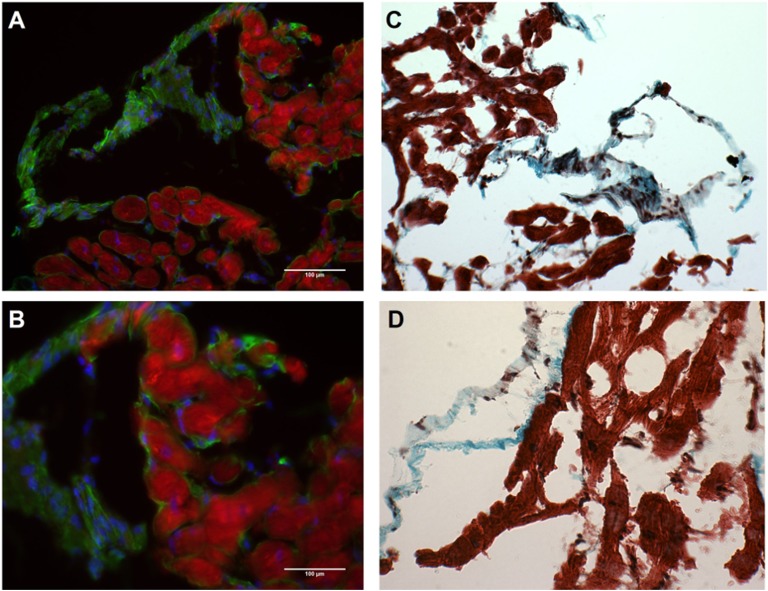
Cardiac fibrosis was determined in all patient samples using Masson’s trichrome stain, which measured the presence of collagen I and III (in blue) and compared that with the nonfibrotic tissue (in red). Quantification of fibrosis is illustrated in Fig. 4A; it revealed a high degree of variability both between and within patients from one time point to another (Table 4). Representative images showing the high degree of variability between patients taken from a single time point are shown in Fig. 4B, in which substantial fibrosis was seen in one patient with large amounts of collagen I and II (blue) and minimal fibrosis was seen in another patient at the same time point. To determine whether GHSR tissue levels were contributed to by fibrosis, cardiac tissue was examined for colocalization between collagen I and GHSR by fluorescence microscopy (Fig. 5A and 5B). These analyses showed no colocalization between collagen I and GHSR in the human tissue samples (Fig. 5C and 5D).
Figure 4.
Cardiac fibrosis in patients pre- and postcardiac transplant. (A) Quantified fibrotic data are shown for explanted heart tissue (LV and RA, black dots) and grafted heart biopsies (Bi 1-10, colored dots). (B) Representative images of the fibrotic variability between patients showing high levels of fibrosis (top) and low levels of fibrosis (bottom) where blue is fibrotic tissue (collagen I and III) and red is healthy cardiac tissue. Images of the RA, Bi 1, Bi 4, and Bi 10 showing different patients at same time point pre- and postcardiac transplant.
Table 4.
Fibrosis Explanted Hearts and Healthy Implanted Heart Biopsies, %
| LV | RA | Bi 1 | Bi 2 | Bi 3 | Bi 4 | Bi 5 | Bi 6 | Bi 7 | Bi 8 | Bi 9 | Bi 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 26.1 | 10.6 | 1.3 | 2.9 | 5.4 | 1.5 | 15.8 | 2.2 | 1.3 | — | — | — |
| Patient 2 | 24.6 | 6.0 | 19.1 | 3.8 | 3.8 | 5.7 | — | — | — | — | — | — |
| Patient 3 | — | 7.0 | 3.9 | 11.4 | 22.2 | 0.9 | — | — | — | 3.6 | 2.2 | — |
| Patient 4 | 2.6 | 32.5 | 1.2 | 0.3 | — | 3.9 | 4.6 | — | — | 13.1 | — | — |
| Patient 5 | 53.6 | — | 2.4 | 7.3 | 3.9 | 1.3 | — | — | — | — | — | — |
| Patient 6 | 3.7 | 7.8 | 2.4 | 38.0 | 1.3 | — | — | — | 4.1 | 5.9 | 2.3 | 11.7 |
| Patient 7 | 5.5 | — | 39.4 | 2.9 | 2.7 | 3.1 | 17.4 | 3.5 | 1.8 | 6.1 | 6.4 | 0.3 |
| Patient 8 | 7.6 | 1.6 | 10.7 | 1.1 | 6.9 | 3.6 | 0.9 | 1.3 | 2.8 | 0.3 | 6.6 | 3.6 |
| Patient 9 | — | 21.9 | 8.9 | 6.1 | 3.5 | — | 1.2 | 2.3 | — | 17.3 | — | — |
| Patient 10 | 11.5 | 11.9 | 3.8 | 8.3 | 13.8 | 0.9 | 30.9 | 2.2 | 3.2 | — | — | — |
Figure 5.
Fibrosis and GHSR in human myocardial tissue. Representative images from grafted heart biopsy 1 showing the same patient sample in all images. (A and B) No colocalization of GHSR (red) and collagen I (green) with DAPI showing nuclei (blue). (C and D) Masson’s trichrome staining of same patient sample, where blue is fibrotic tissue (collagen I and III) and red is healthy tissue. (A and C) ×10 magnification; (B and D) ×20 magnification.
3. Discussion
This study is a comprehensive and longitudinal study that examines tissue GHSR, ghrelin and subsequent downstream biochemical signaling molecules involved in cardiomyocyte contractility and growth, over time, in the same patients with two different hearts. Our findings have demonstrated a relationship between cardiac function, as measured by LVEF, and the expression of both ghrelin and GHSR, suggesting that the cardiac ghrelin-GHSR axis may represent a cardiac-specific biomarker in HF. Our findings further showed an association between some signaling proteins and their relationship to LVEF. Our results demonstrate relationships among GHSR, ghrelin, and biochemical signaling molecules in human cardiac tissues and their relationship to LV function, therefore providing substantial findings related to this myocardial ghrelin-GHSR axis.
Currently, the “gold standard” for cardiac biomarkers of HF are BNP and its N-terminal form, which are markers of myocardial stress. Historically, studies using BNP have focused on characterizing HF with reduced EF as associated with and directly linked to elevated circulating BNP levels [11, 26]. More recent studies have also attempted to understand the mechanisms leading to HF in the setting of preserved EF. To explore this and other etiologies of HF, a variety of biomarkers including hormones (growth hormone) [27, 28], extracellular matrix proteins (matrix metalloproteinase, galectin-3); oxidative stress proteins (8-hydroxy-2,-deoxyguanosine, neopterin); and inflammatory molecules (C-reactive protein, pentraxin 3) [29] have been identified and their association with the presence and degree of HF investigated. Further, circulating biomarkers of HF continue to be discovered through proteomic and gene sequencing of blood serum [30–32]. Many serum biomarkers are being identified [33, 34], and ghrelin-GHSR axis may provide a complementary cardiac tissue-localized indicator of LV function in HF. Certainly, biomarkers that are intimately involved in the different pathological processes in HF would help enhance diagnosis and might have the potential to optimize targeted therapies [29, 35]. We propose that the ghrelin-GHSR axis represents a cardiac-specific indicator of HF.
A prior study has also shown that levels of myocardial GHSR are elevated in chronic HF in humans [8]. In contrast to our findings, in which both GHSR and ghrelin were elevated, that study also reported a decrease in ghrelin expression in chronic HF. The difference in the degree of ghrelin expression between these two studies may be due to the different tissues being compared. Beiras-Fernandez et al. [8] compared tissue obtained from HF patients with those obtained from young adult subjects with no previous history of heart disease. In contrast, our study compared levels of GHSR and ghrelin at end-stage HF with serial biopsies from the engrafted heart under the effects of immunosuppressive therapy taken up to a year after transplant surgery. Furthermore, there were important relationships between LVEF and tissue ghrelin and GHSR levels in two hearts from the same patient. Similar to Beiras-Fernandez et al., our sample size is relatively small cohort of patients with end-stage HF, and may provide challenges in making broad generalizations on the cardiac ghrelin-GHSR system as a biomarker for HF. Now that we have established that higher levels of GHSR and ghrelin associate with end-stage HF with LVEF in the very low range, these results set the stage for a larger study examining changes in cardiac GHSR and ghrelin in patients with a range of LVEF values that reflect the evolution of HF.
Ghrelin is produced in a variety of cardiovascular cell types, including endothelial cells, inflammatory cells, and cardiomyocytes [36]. Ghrelin down-regulates proinflammatory and up-regulates anti-inflammatory signaling pathways in attenuating cardiac hypertrophy [37], postinfarct cardiac remodeling [38], and sepsis [39]. Although we did not study inflammatory signaling pathways, it is possible that the higher levels of ghrelin/GHSR in the diseased heart may indicate up-regulation in response to the inflammatory environment of HF. The posttransplant immunosuppressive regimen may also affect myocardial ghrelin levels and ghrelin-GHSR signaling, particularly through anti-inflammatory pathways.
Circulating ghrelin may also be a potential single biomarker of HF, and from a prognostic perspective, a level of 85 pmol/L or higher was predictive of increased survival [40]. However, circulating ghrelin levels are also elevated in the fasting state and are decreased in both the elderly population and with patients who have a higher body mass index [41], therefore skewing this prognostic cutoff level. We did not obtain blood samples for measurement of circulating ghrelin and therefore could not establish a relationship between serum ghrelin and immunoreactive ghrelin in cardiac tissue. Our data in cardiac tissue samples provide a direct measure of the ghrelin-GHSR axis being elevated in the human myocardium. These data further strengthen the case for the use of GHSR-ghrelin as a cardiac-localized biomarker of HF; however, how tissue levels of ghrelin-GHSR change with age or body mass index in humans is not known.
As mentioned previously, circulating BNP and NT-pro BNP are the currently used clinical biomarkers of HF, because levels rise with decreasing LVEF [42–44]. Our results indicate that levels of BNP in human cardiac tissue trend toward an increase when LVEF is in the very low range and was more strongly correlated with LVEF than was ghrelin, suggesting that tissue levels of BNP could also be a sensitive biomarker for severe HF. Interestingly, in our study, levels of both GHSR and ghrelin were also associated with LVEF, indicating that the ghrelin-GHSR axis may also be a good cardiac biomarker of LV function. As discussed later, ghrelin showed stronger correlations with biochemical signaling molecules, indicating that tissue ghrelin is more closely associated than is BNP with the biochemical mechanisms that underlie the development of HF.
To better understand the potential mechanisms underlying HF, we examined the downstream signaling pathways that link the ghrelin-GHSR axis to cardiomyocyte contractility and growth. The substantial elevation of pERK1/2 levels we observed in end-stage HF is consistent with the reported elevations in pERK1/2 in cardiac tissue in mouse and rat models of HF [45]. Signaling through pERK1/2 is associated with pressure overload-induced myocardial hypertrophy, indicating maladaptive alterations under conditions of persistent myocardial stress. Interestingly, ghrelin and GHSR gene variants are also associated with LV hypertrophy [46, 47]; therefore, our data suggest an upregulated ghrelin-GHSR-pERK1/2 pathway that may mediate HF in humans through myocardial hypertrophy.
The strong positive correlation between ghrelin and SERCA2A is in accordance with the role of ghrelin in the improvement of Ca2+ dynamics in cardiomyocytes isolated from rodents with ischemia-reperfusion myocardial injury [48]. In this study, activation of GHSR by either ghrelin or hexarelin increased SERCA2a expression and activity through increased phosphorylation of its regulatory binding protein, phospholamban, thus replenishing Ca2+ stores in the sarcoplasmic reticulum. However, our results indicating that SERCA2a levels are actually elevated in end-stage HF are in sharp contrast to the literature documenting a decrease in SERCA2a expression and activity in the failing human myocardium [49]. In contrast to the literature, we compared SERCA2a expression between end-stage heart disease and engrafted hearts, and not with healthy controls. The relative decrease in SERCA2a expression in biopsies taken at earlier time points may reflect a subclinical immune response; a recent study has suggested that decreases in tissue SERCA2a correlate with graft rejection [50].
Cardiac fibrosis resulting from deposition of extracellular matrix proteins, including fibroblasts and collagen, occurs in all etiologies of heart disease and HF and is a marker of increased HF severity [51, 52]. Our results indicate increased collagen I and III deposition in the diseased hearts, although there was important variability both between and within patients. The variability could be a consequence of sampling location; a high presence of fibrosis in the apparently healthy implanted hearts likely indicates a considerable degree of geographic heterogeneity within any given patient’s heart. Traditionally, biopsies are acquired from the right ventricle, as was done here, although a recent study found LV biopsies to be not only possible with low risk via radial access, but preferable for determination of heart function through immunohistochemistry and molecular analyses [53]. Because there was such a large degree of variability in the amount of fibrotic tissue in both pretransplant hearts and grafted heart tissue, there was a possibility that GHSR expression originated within the fibrotic tissue, potentially skewing our results. However, we have shown that GHSR was found in nonfibrotic tissue only; therefore, the variability in expression likely lies in HF type and severity, and not in sampling location. Because the diseased heart tends to have larger amounts of collagen deposition, the positive fluorescent signal is only originating from a limited, nonfibrotic component of the whole tissue section. Therefore, the measurement of GHSR we used was affected by the extent of collagen deposition within any given sample, with samples with higher degrees of fibrosis having an artificially depressed measurement of “myocardial” levels.
The current study characterized changes in myocardial GHSR and ghrelin before and after cardiac transplantation. The engrafted hearts were subjected to immunosuppressive therapy, and we did not measure the degree of inflammation or subclinical indicators of rejection in these biopsies. However, that four of these patients were followed up to a year after transplant, with normal EFs, and no dramatic changes in GHSR, ghrelin, SERCA2a, pERK1/2, and fibrosis from the first through to the last biopsy, suggests a normally functioning and stable engrafted heart during this period. Our next step is to characterize the GHSR-ghrelin axis in cardiac tissue obtained from human donors with no history of heart disease.
Overall, we have identified the ghrelin-GHSR axis as a cardiac-localized biomarker of cardiac dysfunction in human HF. This axis was demonstrated to have a higher sensitivity to the downstream signaling molecules linked to cardiomyocyte contractility and hypertrophy when compared with BNP, the gold standard biomarker of HF used clinically. Cardiac fibrosis was highly variable both within and between patients and GHSR was not expressed in fibrotic tissue. We are examining the expression and relationship of GHSR-ghrelin signaling that contribute to defective cardiomyocyte programming in other types of heart disease in humans, as well as in healthy human hearts. Our ongoing work will help to identify the role of myocardial GHSR-ghrelin as a biomarker that may be used to determine the progression of HF at earlier stages.
Acknowledgments
We thank Dr. Peter Pflugfelder, Ms. Anna McDonald, and Ms. Stephanie Fox for assistance in the collection of endomyocardial biopsies, and the Pathology Core Laboratory at the London Health sciences center for their help in fibrosis staining of all tissue samples.
Financial Support: This work was funded by the Canadian Institutes of Health Research and a Natural Sciences and Engineering Research Council grant (to S.D., L.L., and G.W.).
Current Affiliation: Dr. Stoke’s current affiliation is Health Sciences, University of Ottawa, Ottawa, Ontario K1N 6N5, Canada.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CorC
correlation coefficient
- BNP
B-type natriuretic peptide
- DAPI
4′,6-diamidino-2-phenylindole
- EF
ejection fraction
- GHSR
growth hormone secretagogue receptor
- HF
heart failure
- LV
left ventricular
- RA
right atrium
- PTx
posttransplantation
- SERCA2a
sarcoplasmic reticulum Ca2+-ATPase pump
References and Notes
- 1. Yanagi S, Sato T, Kangawa K, Nakazato M. The homeostatic force of ghrelin. Cell Metab. 2018;27(4):786–804. [DOI] [PubMed] [Google Scholar]
- 2. Kishimoto I, Tokudome T, Hosoda H, Miyazato M, Kangawa K. Ghrelin and cardiovascular diseases. J Cardiol. 2012;59(1):8–13. [DOI] [PubMed] [Google Scholar]
- 3.Sun Q, Ma Y, Zhang L, Zhao Y-F, Zang W-J, Chen C. Effects of GH secretagogues on contractility and Ca2+ homeostasis of isolated adult rat ventricular myocytes. Endocrinology 2010;151(9):4446–4454. [DOI] [PubMed]
- 4. Douglas GAF, McGirr R, Charlton CL, Kagan DB, Hoffman LM, Luyt LG, Dhanvantari S. Characterization of a far-red analog of ghrelin for imaging GHS-R in P19-derived cardiomyocytes. Peptides. 2014;54:81–88. [DOI] [PubMed] [Google Scholar]
- 5. Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159(6):1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sullivan R, McGirr R, Hu S, Tan A, Wu D, Charron C, Lalonde T, Arany E, Chakrabarti S, Luyt L, Dhanvantari S. Changes in the cardiac GHSR1a-ghrelin system correlate with myocardial dysfunction in diabetic cardiomyopathy in mice. J Endocr Soc. 2017;2(2):178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuan M-J, Wang T, Kong B, Wang X, Huang C-X, Wang D. GHSR-1a is a novel pro-angiogenic and anti-remodeling target in rats after myocardial infarction. Eur J Pharmacol. 2016;788:218–225. [DOI] [PubMed] [Google Scholar]
- 8. Beiras-Fernandez A, Kreth S, Weis F, Ledderose C, Pöttinger T, Dieguez C, Beiras A, Reichart B. Altered myocardial expression of ghrelin and its receptor (GHSR-1a) in patients with severe heart failure. Peptides. 2010;31(12):2222–2228. [DOI] [PubMed] [Google Scholar]
- 9. Gaggin HK, Januzzi JL Jr. Biomarkers and diagnostics in heart failure. Biochim Biophys Acta. 2013;1832(12):2442–2450. [DOI] [PubMed] [Google Scholar]
- 10. van Kimmenade RRJ, Januzzi JL Jr. Emerging biomarkers in heart failure. Clin Chem. 2012;58(1):127–138. [DOI] [PubMed] [Google Scholar]
- 11. Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358(20):2148–2159. Available at: www.nejm.org.proxy1.lib.uwo.ca/doi/pdf/10.1056/NEJMra0800239. Accessed 11 May 2018. [DOI] [PubMed] [Google Scholar]
- 12. McGirr R, McFarland MS, McTavish J, Luyt LG, Dhanvantari S. Design and characterization of a fluorescent ghrelin analog for imaging the growth hormone secretagogue receptor 1a. Regul Pept. 2011;172(1-3):69–76. [DOI] [PubMed] [Google Scholar]
- 13.RRID. AB_2111733. https://scicrunch.org/resolver/AB_2111733.
- 14.RRID. AB_303961. https://scicrunch.org/resolver/AB_303961.
- 15.RRID. AB_2762850. https://scicrunch.org/resolver/AB_2762850.
- 16.RRID. AB_445037. https://scicrunch.org/resolver/AB_445037.
- 17.RRID. AB_731684. https://scicrunch.org/resolver/AB_731684.
- 18.RRID. AB_2629482. https://scicrunch.org/resolver/AB_2629482.
- 19.RRID. AB_2534102. https://scicrunch.org/resolver/AB_2534102.
- 20.RRID. AB_141637. https://scicrunch.org/resolver/AB_141637.
- 21.RRID. AB_141633. https://scicrunch.org/resolver/AB_141633.
- 22.RRID. AB_141708. https://scicrunch.org/resolver/AB_141708.
- 23. Abbas A, Yu L, Lalonde T, Wu D, Thiessen JD, Luyt LG, Dhanvantari S. Development and characterization of an 18F-labeled ghrelin peptidomimetic for imaging the cardiac growth hormone secretagogue receptor. Mol Imaging. 2018;17:1536012118809587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guizzetti L, McGirr R, Dhanvantari S. Two dipolar α-helices within hormone-encoding regions of proglucagon are sorting signals to the regulated secretory pathway. J Biol Chem. 2014;289(21):14968–14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, Kahaleh MB, Xie Z, Malhotra D, Kolodkin NI, Lakatta EG, Fedorova OV, Bagrov AY, Shapiro JI. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47(3):488–495. [DOI] [PubMed] [Google Scholar]
- 26. Friões F, Lourenço P, Laszczynska O, Almeida PB, Guimarães JT, Januzzi JL, Azevedo A, Bettencourt P. Prognostic value of sST2 added to BNP in acute heart failure with preserved or reduced ejection fraction. Clin Res Cardiol. 2015;104(6):491–499. [DOI] [PubMed] [Google Scholar]
- 27. Arcopinto M, Salzano A, Bossone E, Francesco Ferrara, Emanuele Bobbio, Domenico Sirico, Olga Vriz, Carlo De Vincentiis, Margherita Matarazzo, Lavinia Saldamarco, Francesco Saccà, Raffaele Napoli, Massimo Iacoviello, Vincenzo Triggiani, Andrea M. Isidori, Carlo Vigorito, Jorgen Isgaard, Antonio Cittadiniet al. Multiple hormone deficiencies in chronic heart failure.2015;184(1):421–423. [DOI] [PubMed] [Google Scholar]
- 28.Arcopinto M, Salzano A, Giallauria F, Bossone E, Isgaard J, Marra AM, Bobbio E, Vriz O, Åberg DN, Masarone D, De Paulis A, Saldamarco L, Vigorito C, Formisano P, Niola M, Perticone F, Bonaduce D, Saccà L, Colao A, Cittadini A; T.O.S.CA. (Trattamento Ormonale Scompenso CArdiaco) Investigators. Growth hormone deficiency is associated with worse cardiac function, physical performance, and outcome in chronic heart failure: insights from the T.O.S.CA. GHD Study. PLoS One 2017;12(1):e0170058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeishi Y. Biomarkers in heart failure. Int Heart J 2014;55(6):474–481. Available at: www.jstage.jst.go.jp/article/ihj/55/6/55_14-267/_pdf. Accessed 6 April 2018. [DOI] [PubMed]
- 30. Senthong V, Kirsop JL, Tang WHW. Clinical phenotyping of heart failure with biomarkers: current and future perspectives. Curr Heart Fail Rep. 2017;14(2):106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meder B, Haas J, Sedaghat-Hamedani F, Kayvanpour E, Frese K, Lai A, Nietsch R, Scheiner C, Mester S, Bordalo DM, Amr A, Dietrich C, Pils D, Siede D, Hund H, Bauer A, Holzer DB, Ruhparwar A, Mueller-Hennessen M, Weichenhan D, Plass C, Weis T, Backs J, Wuerstle M, Keller A, Katus HA, Posch AE. Epigenome-wide association study identifies cardiac gene patterning and a novel class of biomarkers for heart failure. Circulation. 2017;136(16):1528–1544. [DOI] [PubMed] [Google Scholar]
- 32. Xuan L, Sun L, Zhang Y, Huang Y, Hou Y, Li Q, Guo Y, Feng B, Cui L, Wang X, Wang Z, Tian Y, Yu B, Wang S, Xu C, Zhang M, Du Z, Lu Y, Yang BF. Circulating long non-coding RNAs NRON and MHRT as novel predictive biomarkers of heart failure. J Cell Mol Med. 2017;21(9):1803–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stenemo M, Nowak C, Byberg L, Sundström J, Giedraitis V, Lind L, Ingelsson E, Fall T, Ärnlöv J. Circulating proteins as predictors of incident heart failure in the elderly. Eur J Heart Fail. 2018;20(1):55–62. [DOI] [PubMed] [Google Scholar]
- 34. van Boven N, Kardys I, van Vark LC, Akkerhuis KM, de Ronde MWJ, Khan MAF, Merkus D, Liu Z, Voors AA, Asselbergs FW, van den Bos EJ, Boersma E, Hillege H, Duncker DJ, Pinto YM, Postmus D. Serially measured circulating microRNAs and adverse clinical outcomes in patients with acute heart failure. Eur J Heart Fail. 2018;20(1):89–96. [DOI] [PubMed] [Google Scholar]
- 35. Bishu K, Redfield MM. Acute heart failure with preserved ejection fraction: unique patient characteristics and targets for therapy. Curr Heart Fail Rep. 2013;10(3):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D’Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Diéguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrère B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschöp MH. Ghrelin. Mol Metab. 2015;4(6):437–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mao Y, Tokudome T, Kishimoto I, Otani K, Nishimura H, Yamaguchi O, Otsu K, Miyazato M, Kangawa K. Endogenous ghrelin attenuates pressure overload-induced cardiac hypertrophy via a cholinergic anti-inflammatory pathway. Hypertension. 2015;65(6):1238–1244. [DOI] [PubMed] [Google Scholar]
- 38. Huang C-X, Yuan M-J, Huang H, Wu G, Liu Y, Yu SB, Li HT, Wang T. Ghrelin inhibits post-infarct myocardial remodeling and improves cardiac function through anti-inflammation effect. Peptides. 2009;30(12):2286–2291. [DOI] [PubMed] [Google Scholar]
- 39. Wu R, Dong W, Cui X, Zhou M, Simms HH, Ravikumar TS, Wang P. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Ann Surg. 2007;245(3):480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen Y, Ji XW, Zhang AY, Lv JC, Zhang JG, Zhao CH. Prognostic value of plasma ghrelin in predicting the outcome of patients with chronic heart failure. Arch Med Res. 2014;45(3):263–269. [DOI] [PubMed] [Google Scholar]
- 41. Rigamonti AE, Pincelli AI, Corrà B, Viarengo R, Bonomo SM, Galimberti D, Scacchi M, Scarpini E, Cavagnini F, Müller EE. Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients. J Endocrinol. 2002;175(1):R1–R5. Available at: http://joe.endocrinology-journals.org/content/175/1/R1.full.pdf. Accessed 14 August 2018. [DOI] [PubMed] [Google Scholar]
- 42. Karakiliç E, Kepez A, Abali G, Coşkun F, Kunt M, Tokgözoğlu L. The relationship between B-type natriuretic peptide levels and echocardiographic parameters in patients with heart failure admitted to the emergency department. Anadolu Kardiyol Derg. 2010;10(2):143–149. [DOI] [PubMed] [Google Scholar]
- 43. Jiang Yanxia MC, Yanxia J, Xingjun C, Xintao T, Lei S. The correlation between left ventricular ejection fraction and peripheral blood MCP-1 NT-pro bnp in patients with acute coronary syndrome. Intern Med. 2014;041–4. [Google Scholar]
- 44. Belagavi AC, Rao M, Pillai AY, Srihari US. Correlation between NT proBNP and left ventricular ejection fraction in elderly patients presenting to emergency department with dyspnoea. Indian Heart J. 2012;64(3):302–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;91(9):776–781. [DOI] [PubMed] [Google Scholar]
- 46. Baessler A, Kwitek AE, Fischer M, Koehler M, Reinhard W, Erdmann J, Riegger G, Doering A, Schunkert H, Hengstenberg C. Association of the ghrelin receptor gene region with left ventricular hypertrophy in the general population: results of the MONICA/KORA Augsburg Echocardiographic Substudy. Hypertension. 2006;47(5):920–927. [DOI] [PubMed] [Google Scholar]
- 47. Ukkola O, Pääkkö T, Kesäniemi YA. Ghrelin and its promoter variant associated with cardiac hypertrophy. J Hum Hypertens. 2012;26(7):452–457. [DOI] [PubMed] [Google Scholar]
- 48.Ma Y, Zhang L, Edwards JN, Launikonis BS, Chen C. Growth hormone secretagogues protect mouse cardiomyocytes from in vitro ischemia/reperfusion injury through regulation of intracellular calcium. PLoS One 2012;7(4):e35265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gorski PA, Ceholski DK, Hajjar RJ. Altered myocardial calcium cycling and energetics in heart failure--a rational approach for disease treatment. Cell Metab. 2015;21(2):183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tarazón E, Ortega A, Gil-Cayuela C, Sánchez-Lacuesta E, Marín P, Lago F, González-Juanatey JR, Martínez-Dolz L, Portolés M, Rivera M, Roselló-Lletí E. SERCA2a: a potential non-invasive biomarker of cardiac allograft rejection. J Heart Lung Transplant. 2017;36(12):1322–1328. [DOI] [PubMed] [Google Scholar]
- 51.Majumder R, Nayak AR, Pandit R. Nonequilibrium arrhythmic states and transitions in a mathematical model for diffuse fibrosis in human cardiac tissue. PLoS One 2012;7(10):e45040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boldt A, Wetzel U, Lauschke J, Weigl J, Gummert J, Hindricks G, Kottkamp H, Dhein S. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90(4):400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frey N, Meder B, Katus HA. Left ventricular biopsy in the diagnosis of myocardial diseases. Circulation. 2018;137(10):993–995. [DOI] [PubMed] [Google Scholar]