Abstract
Rectal cancer is prone to local recurrence and systemic metastasis. However, owing to improvements in TNM staging and treatment, including a more widespread use of rectal MRI and increased radiologist awareness of the key rectal cancer TNM staging features, the mortality rate of rectal cancer has been declining over the past few decades in adults over 50 years of age. Currently, rectal MRI plays a key role in the pre- and posttreatment evaluation of rectal cancer, assisting the multidisciplinary team in tailoring the most appropriate treatment option. The benefits achieved with rectal MRI are strictly dependent on obtaining good-quality images, which is important for the characterization of the main anatomic structures and their relationship with the tumor. In primary staging, rectal MRI helps the radiologist (a) describe the tumor location and morphology, (b) provide its T and N categories, (c) detect the presence of extramural vascular invasion, and (d) identify its relationship with surrounding structures, including the sphincter complex and involvement of the mesorectal fascia. These features help diagnose locally advanced rectal tumors (categories T3c-d, T4, N1, and N2), for which neoadjuvant chemoradiotherapy (CRT) is indicated. In restaging after neoadjuvant CRT, in addition to reassessing the features noted during primary staging, rectal MRI can help in the assessment of treatment response, especially with the emergence of nonsurgical approaches such as “watch and wait.”
©RSNA, 2019
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Identify the anatomic landmarks relevant for local staging of rectal cancer at MRI.
■ Recognize the optimal rectal MRI protocol indicated for primary tumor staging and restaging.
■ List the key points to include in the radiologic report for primary staging, restaging after neoadjuvant CRT, and local recurrence.
Introduction
Colorectal cancer is the third most common cancer in men and the second most common in women (1). In the United States, it represents the third leading cause of new cancer cases and cancer-related deaths in both men and women. For 2018, it was projected that there would be 97–220 new cases of colorectal cancer and 44% would have occurred in the rectum (2). The prevalence is considerably higher in more developed countries than in less developed countries. However, the mortality rate in more developed countries is lower, reflecting increased screening and improvements in rectal cancer staging and treatment (2). On the other hand, the prevalence has increased among patients younger than 50 years (3), and specifically in this group, the death rate has increased by 1% per year (2).
The prognosis of rectal cancer is directly related to tumor infiltration into the mesorectum and the ability to surgically achieve negative circumferential resection margins (CRMs) (4). The use of total mesorectal excision (TME) as the standard treatment of rectal cancer and the adoption of neoadjuvant chemoradiotherapy (CRT) for patients with locally advanced rectal cancers (LARCs), diagnosed on the basis of MRI features, has led to substantial improvements in local disease control (5–9). Currently, rectal MRI is the preferred imaging modality for local staging of rectal cancer.
The standard treatment in patients with MRI-staged LARC is neoadjuvant CRT followed by TME (10,11). In approximately half of patients, the disease is downstaged after CRT, and almost one-third of patients demonstrate complete pathologic response after undergoing TME (12–15). Habr-Gama et al (13) and other authors (16–18) have shown that select patients with clinical complete response to CRT can be safely followed up in a nonsurgical approach.
Rectal MRI may add value in patient care in various scenarios. In primary staging (preoperative setting), MRI can assist in (a) selecting patients with LARC who are suitable for treatment with neoadjuvant CRT; (b) guiding surgeons in surgical planning; and (c) identifying poor prognostic factors, including extramural vascular invasion (EMVI), mucin content, and involvement of the mesorectal fascia (MRF) (19,20). In the restaging setting (after treatment with neoadjuvant CRT), rectal MRI can help in (a) evaluating tumor regression; (b) tailoring surgical planning; (c) detecting a complete clinical response, along with a review of the results of digital rectal examinations and endoscopic procedures; and (d) monitoring patients undergoing the nonsurgical treatment approach. Finally, after local treatment, performing rectal MRI is relevant during follow-up for the early diagnosis of local recurrence. MRI in the recurrence setting may contribute to management by outlining disease extension within the pelvis and providing a road map to determine the resectability of lesions and the best surgical approach (21).
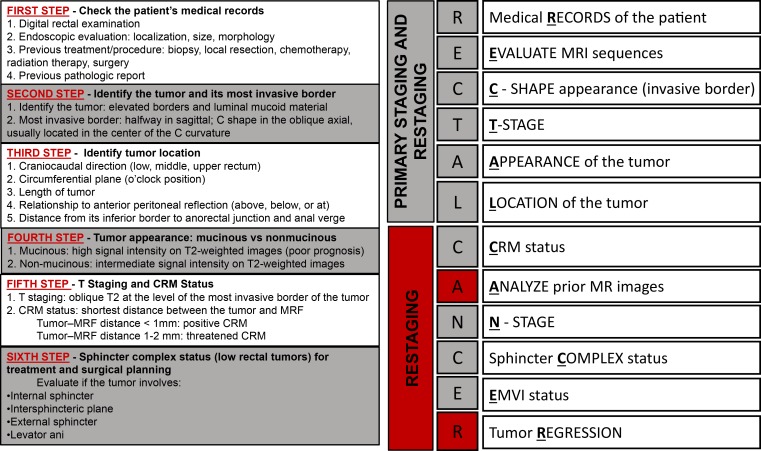
In this article, we review rectal MRI techniques, relevant anatomic landmarks with correlating MRI findings, and current concepts in the management of patients with rectal cancer, including the most common surgical techniques. Furthermore, we discuss the role of MRI in the assessment of local staging, restaging, and recurrence and the future directions of imaging research in rectal cancer. We also propose a schematic step-by-step approach to facilitate comprehension of the key features to address in a rectal MRI radiologic report, represented by the mnemonic RECTAL CANCER (for medical records, evaluate MRI sequences, C-shape appearance, T category, appearance and location of the tumor, CRM status, analyze prior MR images, N category, sphincter complex status, EMVI status, and tumor regression).
Managing Rectal Cancer and Current Concepts
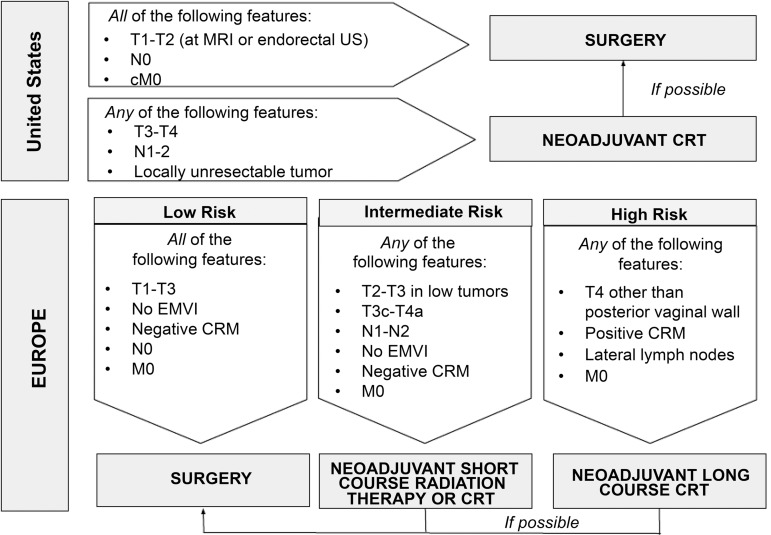
Table 1 demonstrates TNM staging of rectal cancer, where T represents the tumor, N represents the lymph nodes near the tumor, and M represents whether the tumor has metastasized (11,12). The prefixes c, p, and y represent clinical, pathologic, and postneoadjuvant therapy, respectively. The management concepts of rectal cancer in the United States and Europe are summarized in Figure 1. Surgical resection is still considered the curative treatment of rectal cancer. The surgical techniques may vary depending on the location and extent of the disease.
Table 1:
TNM Classification of Rectal Cancer
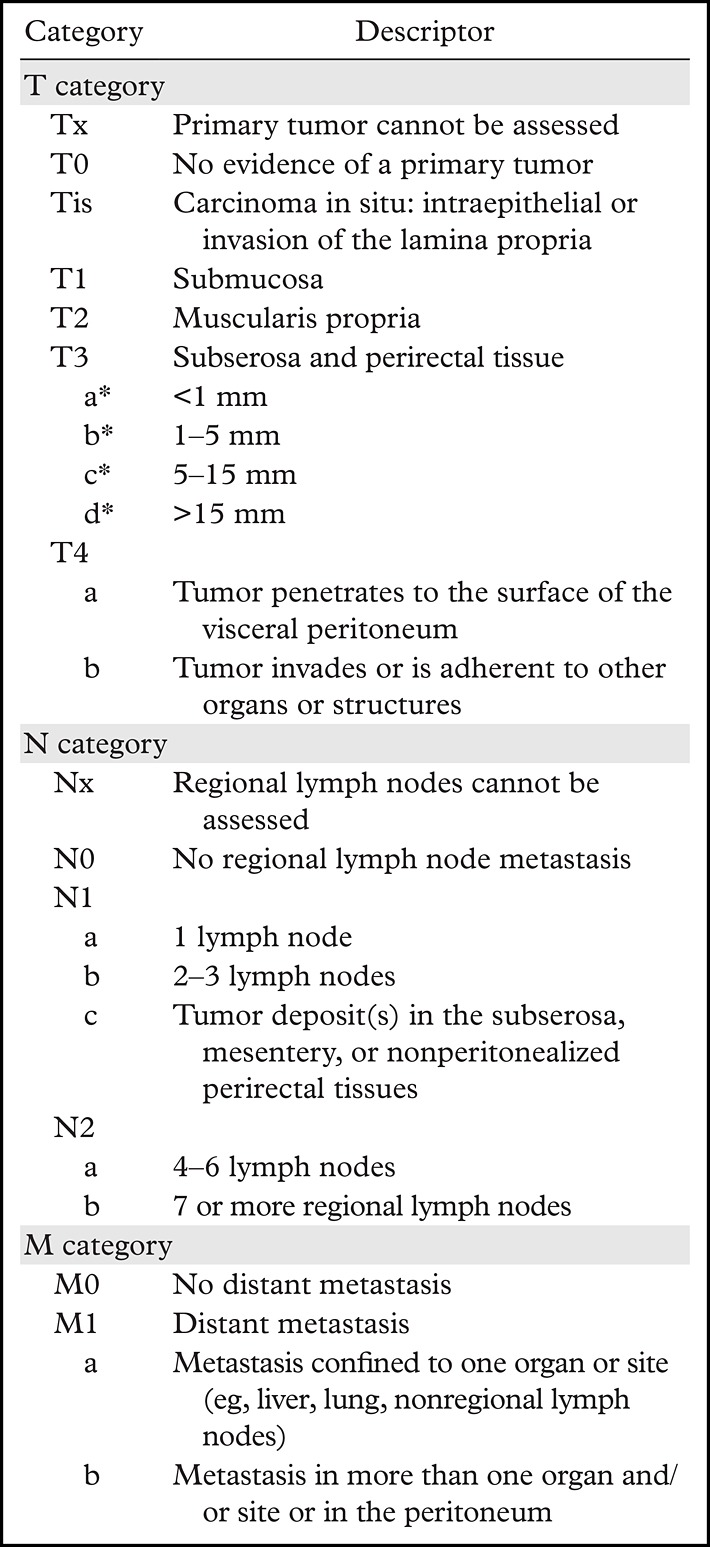
*The subclassification of the T3 category is determined on the basis of an MRI evaluation and is used in the European guidelines for treatment recommendations (11).
Figure 1.
Schematic flowchart summarizes the current management concepts of rectal cancer in the United States and Europe.
Transanal endoscopic microsurgery (Fig 2a) is characterized by a focal endoscopic resection of the tumor and can be indicated for select patients with early rectal cancer. The selection criteria for transanal endoscopic microsurgery include well- or moderately differentiated rectal cancers and tumors that are categorized as cT1 or cN0, are less than 3 cm, are within 8 cm of the anal verge, or involve less than 30% of the wall circumference (12,23).
Figure 2a.
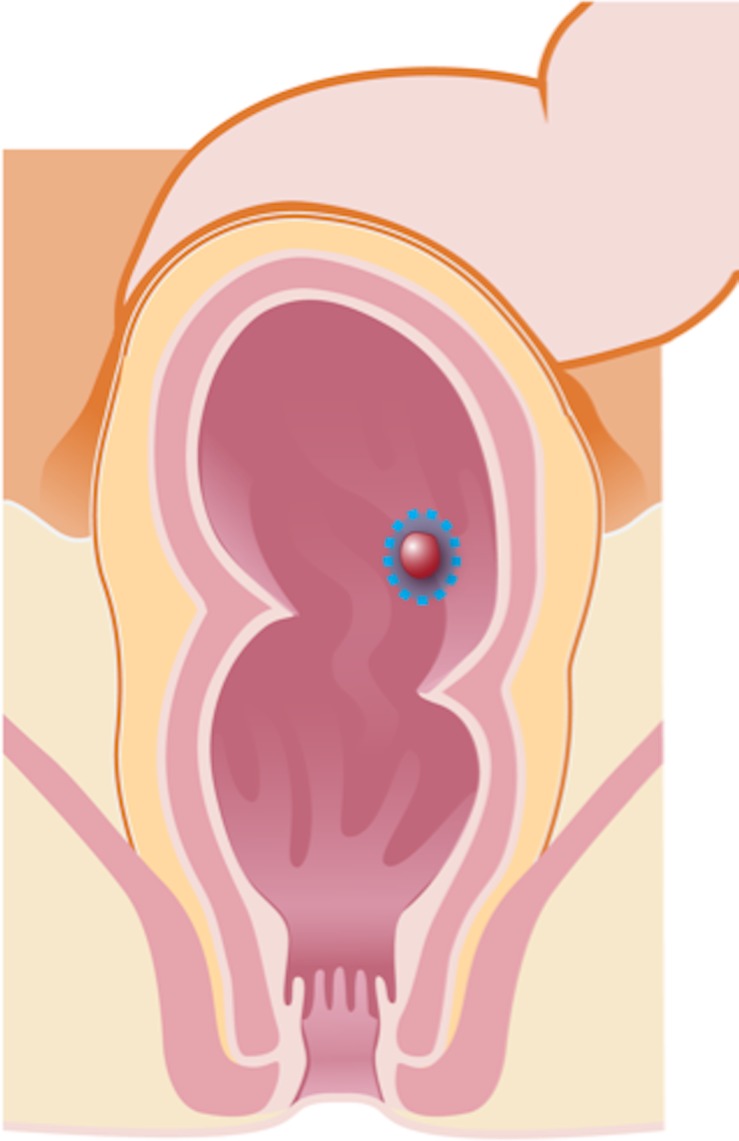
Illustrations of the anatomy of the rectum depict various surgical techniques used to treat rectal cancer. Dotted blue lines = anatomic structures removed during the procedure. Red area = rectal tumor. (a) Illustration shows a transanal endoscopic microsurgery with focal endoscopic resection of a tumor. (b) Illustration depicts a low anterior resection and TME and resection of the whole sigmoid or part of it, which preserves the sphincter complex. (c) Illustration depicts an abdominoperineal resection and TME, with resection of the sphincter complex. (d) Illustration depicts an intersphincteric abdominoperineal resection and TME, with dissection within the intersphincteric plane and a portion of the internal sphincter. The entire external sphincter is preserved. (e) Illustration depicts an extralevator abdominoperineal resection and TME, with a broader dissection of the sphincter complex. (Reprinted, under a CC BY-ND 4.0 license, from Memorial Sloan Kettering Cancer Center.)
TME is the standard transabdominal surgery indicated for the curative treatment of rectal cancer (10). After its implementation, patient outcome and quality of life improved considerably. TME involves a complete resection of the mesorectum along the MRF plane.
Low anterior resection (Fig 2b) is the most common transabdominal resection indicated for tumors located in the middle or upper rectum. This technique is characterized by TME and the resection of the whole sigmoid or part of it.
Figure 2b.
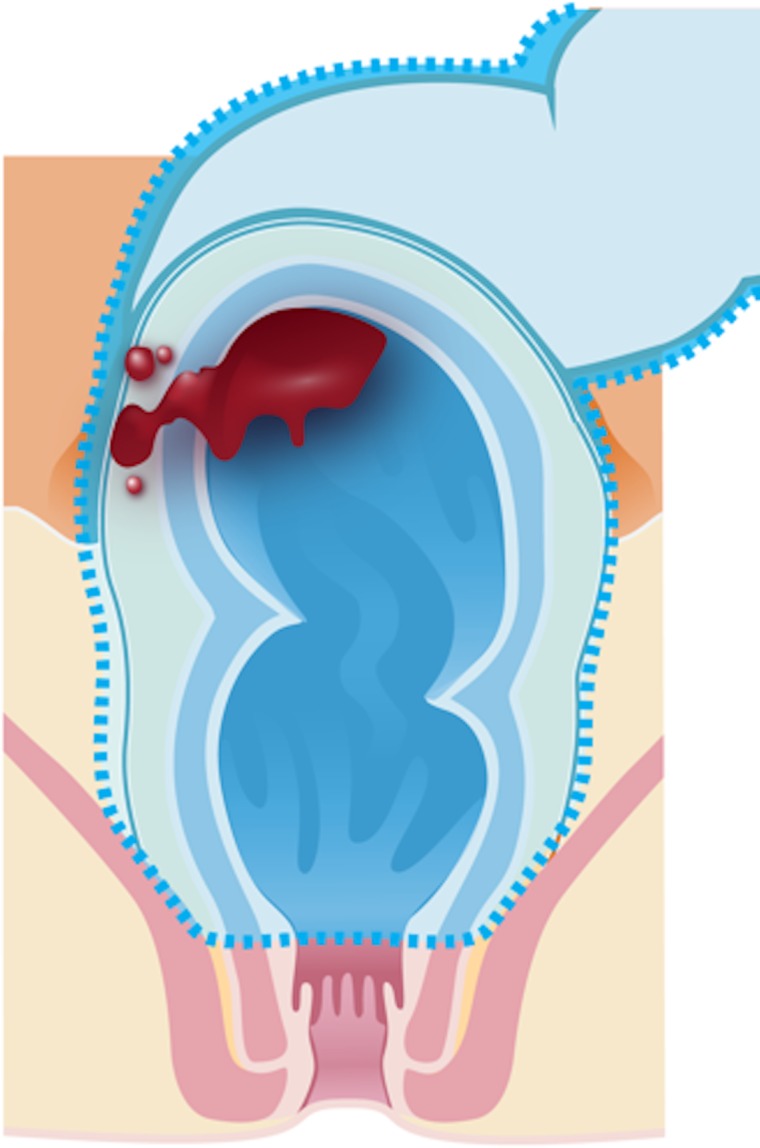
Illustrations of the anatomy of the rectum depict various surgical techniques used to treat rectal cancer. Dotted blue lines = anatomic structures removed during the procedure. Red area = rectal tumor. (a) Illustration shows a transanal endoscopic microsurgery with focal endoscopic resection of a tumor. (b) Illustration depicts a low anterior resection and TME and resection of the whole sigmoid or part of it, which preserves the sphincter complex. (c) Illustration depicts an abdominoperineal resection and TME, with resection of the sphincter complex. (d) Illustration depicts an intersphincteric abdominoperineal resection and TME, with dissection within the intersphincteric plane and a portion of the internal sphincter. The entire external sphincter is preserved. (e) Illustration depicts an extralevator abdominoperineal resection and TME, with a broader dissection of the sphincter complex. (Reprinted, under a CC BY-ND 4.0 license, from Memorial Sloan Kettering Cancer Center.)
Ultra-low anterior resection is a sphincter-sparing surgery that can be performed in patients with low rectal cancer above the anorectal junction. The coloanal anastomosis is created 1 cm distal to the lower edge of the tumor in this procedure.
Standard abdominoperineal resection (Fig 2c) with TME is indicated for tumors that infiltrate the anal canal or the levator ani and/or external sphincter, located less than 1 cm from the anal verge or in cases where the resection will result in incontinence. It is characterized by the resection of the sphincter complex, resulting in a permanent colostomy.
Figure 2c.
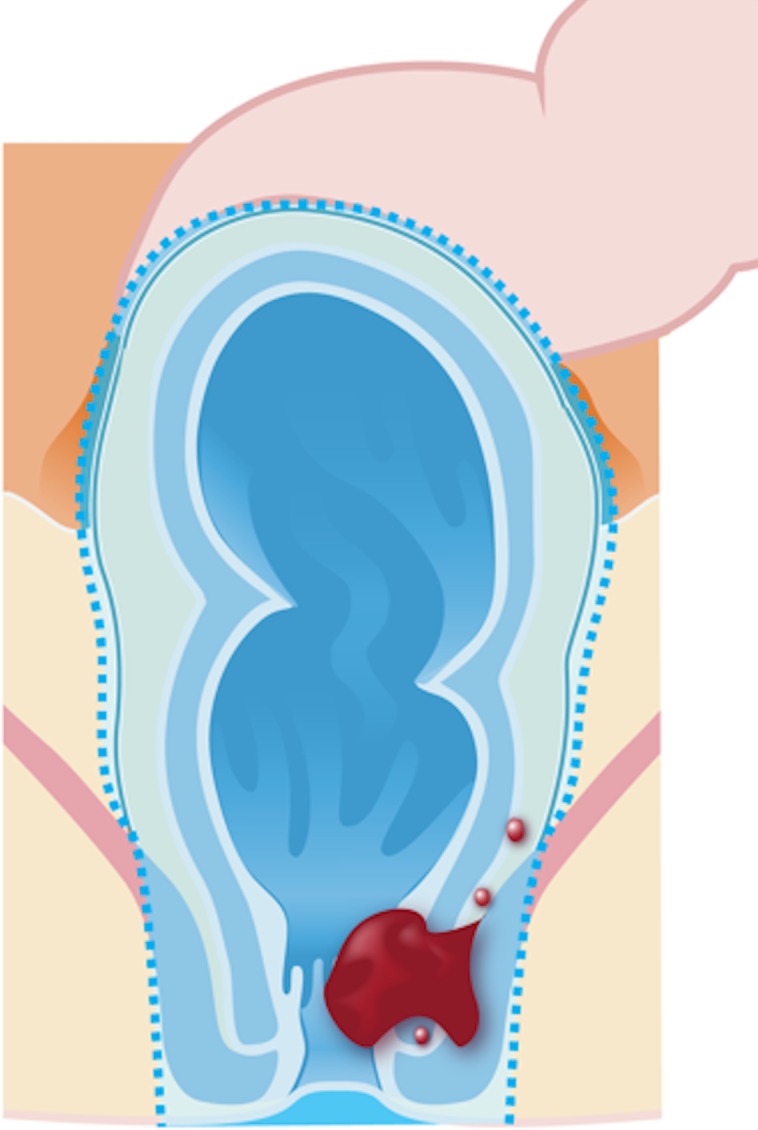
Illustrations of the anatomy of the rectum depict various surgical techniques used to treat rectal cancer. Dotted blue lines = anatomic structures removed during the procedure. Red area = rectal tumor. (a) Illustration shows a transanal endoscopic microsurgery with focal endoscopic resection of a tumor. (b) Illustration depicts a low anterior resection and TME and resection of the whole sigmoid or part of it, which preserves the sphincter complex. (c) Illustration depicts an abdominoperineal resection and TME, with resection of the sphincter complex. (d) Illustration depicts an intersphincteric abdominoperineal resection and TME, with dissection within the intersphincteric plane and a portion of the internal sphincter. The entire external sphincter is preserved. (e) Illustration depicts an extralevator abdominoperineal resection and TME, with a broader dissection of the sphincter complex. (Reprinted, under a CC BY-ND 4.0 license, from Memorial Sloan Kettering Cancer Center.)
Intersphincteric abdominoperineal resection (Fig 2d) is a sphincter-sparing surgery that can be considered in cases where the intersphincteric plane is not infiltrated by the tumor. Therefore, the dissection is performed within the intersphincteric plane and the external sphincter is preserved.
Figure 2d.
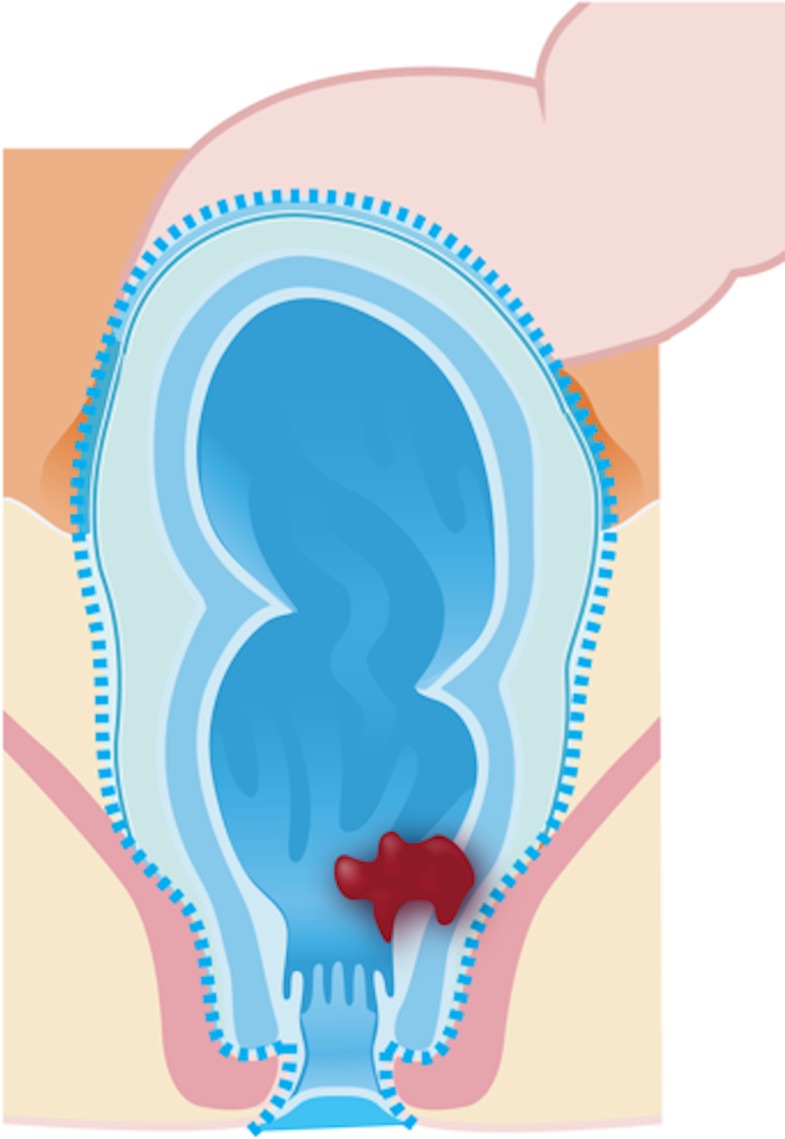
Illustrations of the anatomy of the rectum depict various surgical techniques used to treat rectal cancer. Dotted blue lines = anatomic structures removed during the procedure. Red area = rectal tumor. (a) Illustration shows a transanal endoscopic microsurgery with focal endoscopic resection of a tumor. (b) Illustration depicts a low anterior resection and TME and resection of the whole sigmoid or part of it, which preserves the sphincter complex. (c) Illustration depicts an abdominoperineal resection and TME, with resection of the sphincter complex. (d) Illustration depicts an intersphincteric abdominoperineal resection and TME, with dissection within the intersphincteric plane and a portion of the internal sphincter. The entire external sphincter is preserved. (e) Illustration depicts an extralevator abdominoperineal resection and TME, with a broader dissection of the sphincter complex. (Reprinted, under a CC BY-ND 4.0 license, from Memorial Sloan Kettering Cancer Center.)
Extralevator abdominoperineal resection (Fig 2e) is indicated for tumors that infiltrate the intersphincteric plane and external sphincter and/or levator ani. The technique consists of a broader dissection of the sphincter complex and consequently avoids the “waist” effect that is created in a standard abdominoperineal resection, thus creating a cylindrical specimen. This surgery aims to reduce bowel and tumor perforation during the surgery and to avoid positive CRM.
Figure 2e.
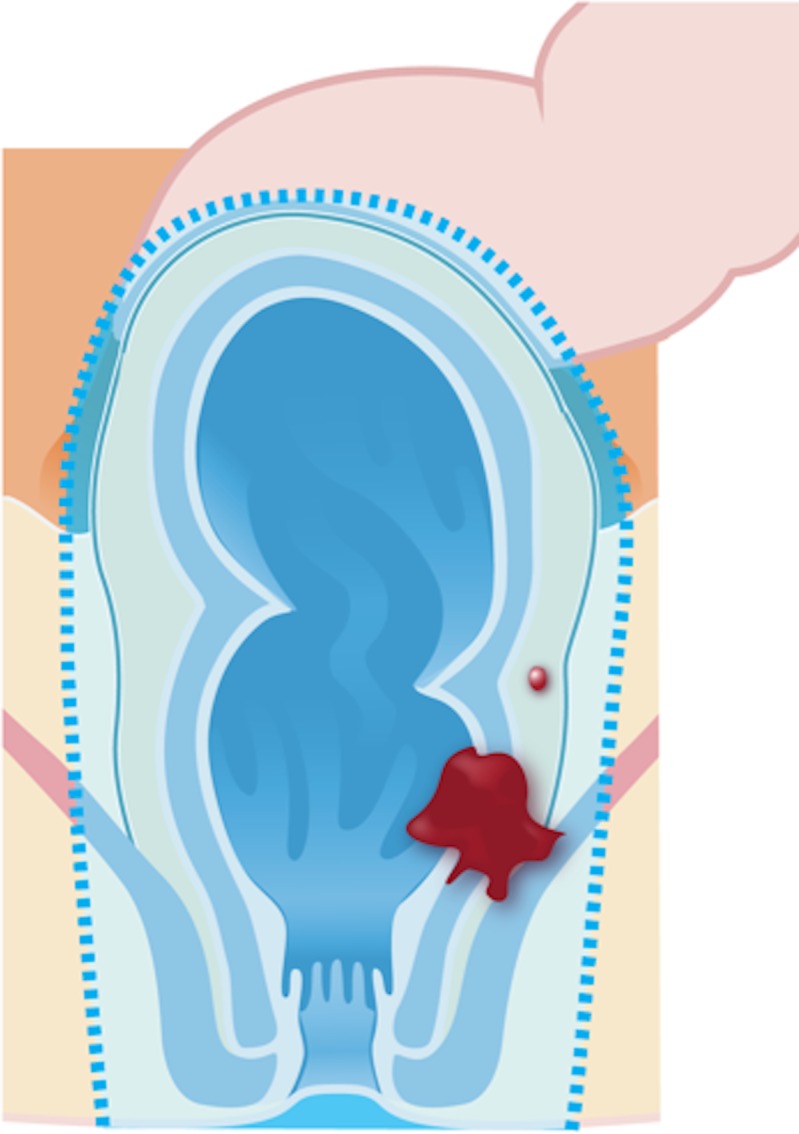
Illustrations of the anatomy of the rectum depict various surgical techniques used to treat rectal cancer. Dotted blue lines = anatomic structures removed during the procedure. Red area = rectal tumor. (a) Illustration shows a transanal endoscopic microsurgery with focal endoscopic resection of a tumor. (b) Illustration depicts a low anterior resection and TME and resection of the whole sigmoid or part of it, which preserves the sphincter complex. (c) Illustration depicts an abdominoperineal resection and TME, with resection of the sphincter complex. (d) Illustration depicts an intersphincteric abdominoperineal resection and TME, with dissection within the intersphincteric plane and a portion of the internal sphincter. The entire external sphincter is preserved. (e) Illustration depicts an extralevator abdominoperineal resection and TME, with a broader dissection of the sphincter complex. (Reprinted, under a CC BY-ND 4.0 license, from Memorial Sloan Kettering Cancer Center.)
Rectal MRI Protocol
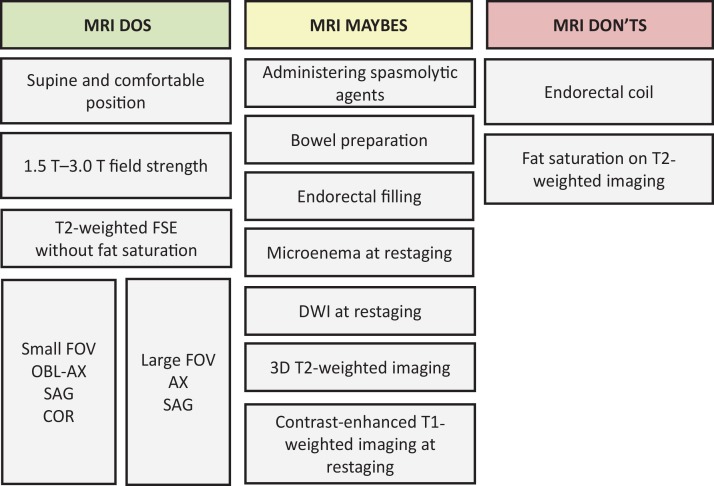
The potential benefits achieved with rectal MRI are strictly dependent on obtaining good-quality images to allow for characterization of the main anatomic structures and their relation to the tumor. High-spatial-resolution T2-weighted imaging is the most important MRI sequence in the evaluation of rectal cancer and anatomic structures. Standardized imaging protocols also allow for more accurate and reproducible interpretations, which facilitate the widespread use of this technique (24). Figure 3 summarizes the MRI techniques frequently recommended (“Dos”), those that are not recommended (“Don’ts”), and some that are controversial practices (“Maybes”) that may be performed in select cases.
Figure 3.
Chart categorizes rectal MRI protocol according to techniques that are frequently recommended (“Dos”), those that are not recommended (“Don’ts”), and some that are controversial practices (“Maybes.”) T2-weighted imaging with fat saturation is rarely necessary but may be useful for imaging mucinous tumors. AX = axial, COR = coronal, DWI = diffusion-weighted imaging, FOV = field of view, FSE = fast spin-echo, OBL-AX = oblique axial, SAG = sagittal, 3D = three dimensional.
Dos
Patients must be informed about the time required for imaging, and they must be positioned comfortably in the supine position in the MR imager (20). High-field-strength MRI provides fast image acquisition, high spatial resolution, and high signal-to-noise ratio, improving the visibility of the rectal wall (25). Ideally, higher field strengths (eg, 1.5 T or 3.0 T) are preferred, with some studies demonstrating similar accuracies for staging for both (26–28). While 1.5 T is the most widely available and used, 3.0 T may improve spatial resolution, with an increase of the signal-to-noise ratio, and may be preferable to 1.5 T. However, some experts cite greater magnetic susceptibility artifact at 3.0 T, which may occur during DWI, as a potential disadvantage (29,30). Pelvic phased-array surface coils are recommended and must cover from the aortic bifurcation to the anal verge.
The standard rectal MRI protocol in the evaluation of rectal cancer includes performing two-dimensional (2D) FSE T2-weighted sequences without fat suppression, using a small field of view and a section thickness less than 3 mm (high-resolution protocol) (26). Images in this sequence should be obtained in the (a) oblique axial plane (perpendicular to the tumor), as incorrect plane obliquity leads to blurring of the muscularis propria, which can cause incorrect T staging (31); (b) sagittal plane, which is determined by the longitudinal tumor axis; and (c) oblique coronal plane (parallel to the anal canal), which is important to depict low rectal tumors and to better evaluate their relationship with the anal sphincter. These sequences have a proven high diagnostic accuracy, between 90% and 100%, for the evaluation of tumor invasion into the MRF and adjacent organs and are recommended by the Magnetic Resonance Imaging and Rectal Cancer European Equivalence (MERCURY) group (19).
FSE T2-weighted MRI with a large field of view without fat suppression obtained in the axial plane of the entire pelvis, from the aortic bifurcation to the sphincter, allows for evaluation of distant lymph node chains (eg, inferior mesenteric, lateral, and inguinal). In the sagittal plane, from one side of the pelvic wall to the other, FSE T2-weighted MRI allows for localization of the primary tumor, enabling the measurement of its height and its relationship to the midline structures, such as the anal verge (25,28).
Don’ts
It is not recommended to use a routine bowel preparation such as air insufflation to distend the rectum with any contrast material or to use intravenous contrast material (20,25,26,31–34). The use of an endorectal coil it is also not endorsed owing to patient comfort and cost (20,28). In regard to MRI sequences, T2-weighted imaging with fat suppression is not routinely recommended (28,33).
Maybes
For certain MRI techniques in imaging rectal cancer, there is no consensus. Administering spasmolytic agents such as glucagon (1 mg administered intravenously, intramuscularly, or subcutaneously) or hyoscine butylbromide (20 mg administered intravenously) is not mandatory but may reduce artifacts caused by peristalsis when administered immediately before the examination or just before the most motion-sensitive sequences (eg, DWI or dynamic contrast material–enhanced [DCE] sequences) are obtained (26).
Endorectal filling is not routinely used because, although it may facilitate detection of small tumors with rectal distention, it may alter staging owing to compression of the mesorectal fat. This can change the distance of the tumor to the MRF, possibly leading to nonvisualization of the mesorectal nodes (35). However, there are some studies that favor endorectal filling that can be found in the literature (28,36).
Another optional MRI sequence includes DWI with a high b value (≥800 sec/mm2), which may improve the diagnostic performance of MRI for tumor restaging after CRT. For primary staging, it may improve tumor and lymph node detection, although it is not officially recommended (20,25,26,31,37). Using a microenema 15 minutes before performing DWI may help remove rectal air and reduce artifacts, which can be particularly helpful for assessing residual tumor at restaging MRI (38).
Three-dimensional T2-weighted MRI is not routinely recommended but may be useful for evaluating response to neoadjuvant therapy. However, 2D imaging still seems preferable (39). T1-weighted imaging with a wider field of view may help in assessment of the common iliac and lower para-aortic nodes or incidental findings in the pelvis (with the same principle of FSE T2-weighted MRI with a large field of view) and especially the bones. It may also be useful in cases of mucinous neoplasm when T2 signal intensities can be identical to those of fat.
It is known that contrast-enhanced T1-weighted imaging does not improve the diagnostic accuracy of local staging of rectal cancer (20). Studies found no difference for distinction between T1-T2 and borderline T3 tumors for the evaluation of tumor extension into the MRF (40). However, particularly at restaging, the use of intravenous contrast material may help identify local recurrence, depicted on images as heterogeneous enhancement (41).
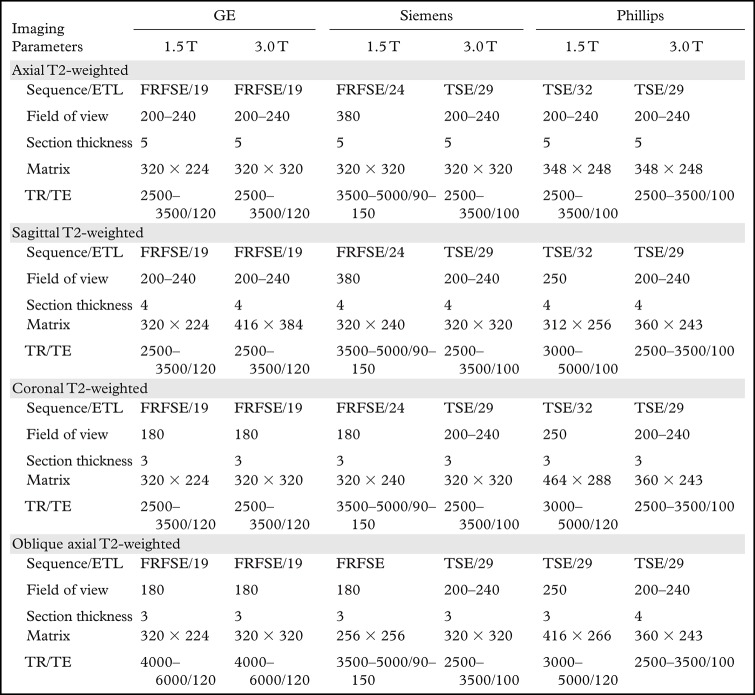
The main MRI parameters of the most important rectal MRI sequences among common MRI equipment vendors are summarized in Table 2.
Table 2:
Rectal MRI Parameters among Common Vendors
Note.—Field of view and section thickness are measured in millimeters. TR/TE is measured in milliseconds. ETL = echo train length, FRFSE = fast relaxation FSE, TE = echo time, TR = repetition time, TSE = turbo spin-echo.
Anatomic MRI Features
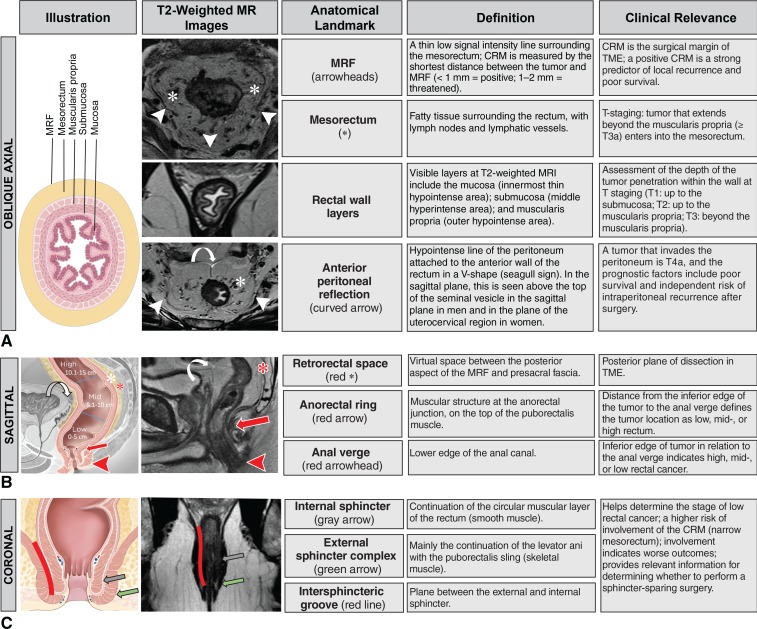
It is imperative for radiologists to be familiar with key anatomic landmarks of the rectum to provide an accurate local staging of rectal cancer. High-resolution T2-weighted MRI is the main sequence performed for the evaluation of relevant structures. Figure 4 summarizes the main anatomic landmarks seen at rectal MRI, provides details regarding which imaging plane is best suited for the identification of each landmark, and describes their clinical relevance.
Figure 4.
Chart shows the anatomic landmarks of the rectum, describes their clinical relevance, and summarizes their imaging appearance. A, Illustration and MR images in the oblique axial view best depict the MRF, mesorectum, rectal wall layers, and anterior peritoneal reflection. B, Illustration and MR image in the sagittal view best depict the retrorectal space, anorectal ring, and anal verge. Curved arrows = anterior peritoneal reflection, white * = mesorectum. C, Illustration and MR image in the coronal view best depict the internal sphincter, external sphincter complex, and intersphincteric space. (Illustrations adapted and reprinted, under a CC BY-ND 4.0 license, from Memorial Sloan Kettering Cancer Center.)
Staging Rectal Cancer with MRI
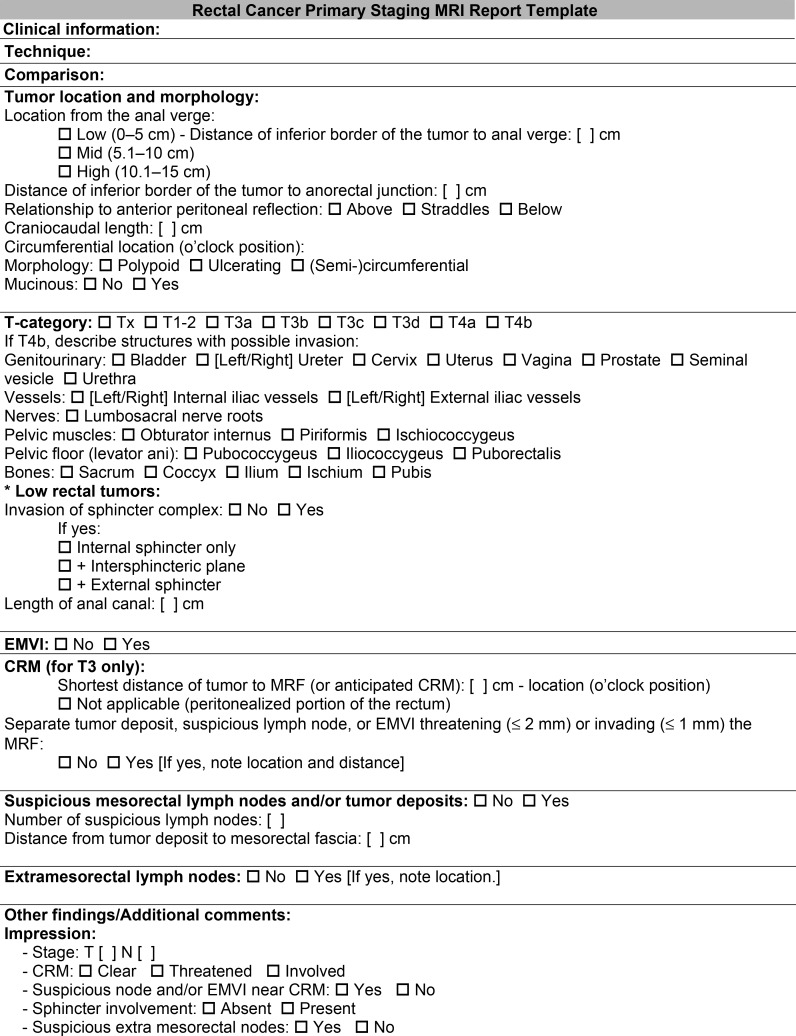
In the context of primary tumor staging, performing rectal MRI is important for the evaluation of tumor location and morphology, T category, anal sphincter complex involvement, CRM status, involvement of the pelvic sidewall, EMVI, and N category. These features should be included in the rectal MRI report. A suggested radiologic report template for primary staging can be found in Figure 5.
Figure 5.
Radiologic report template lists the key imaging findings and features that should be evaluated at primary staging and included in the rectal MRI report.
Location and Morphology
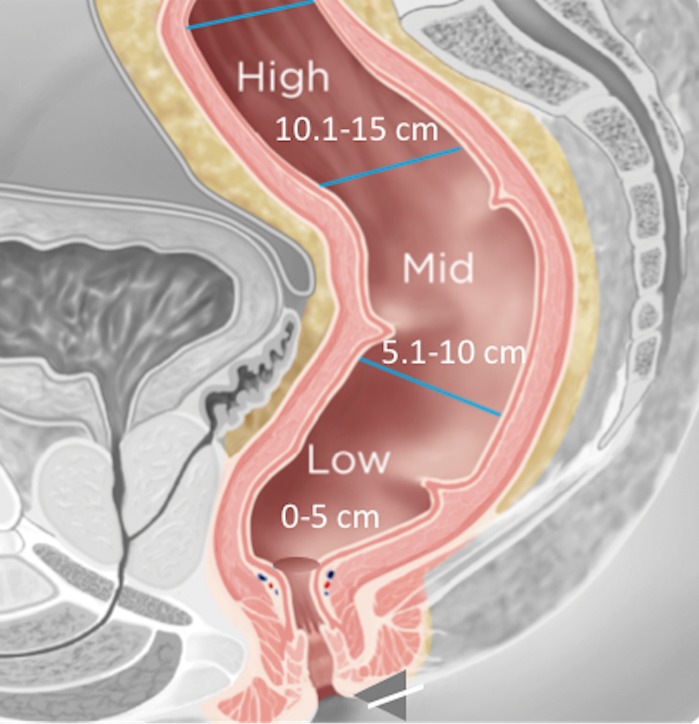
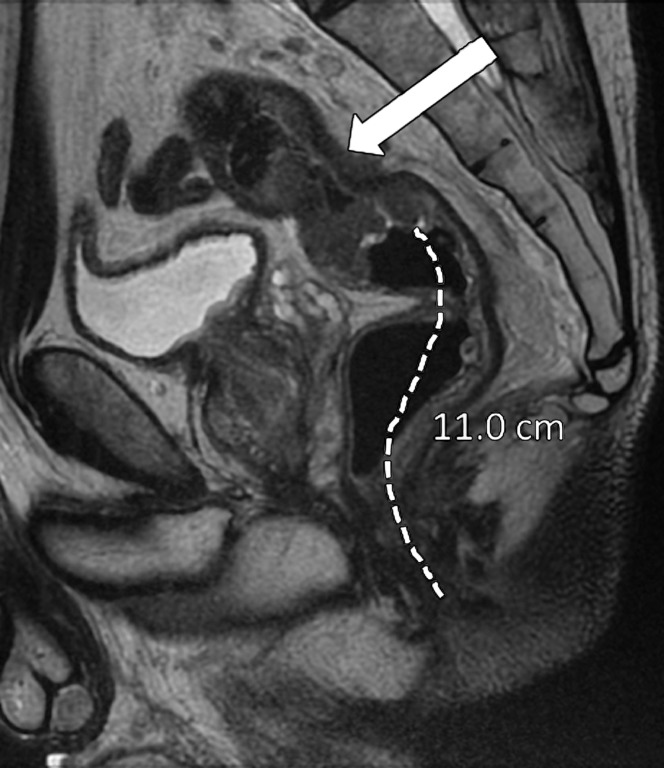
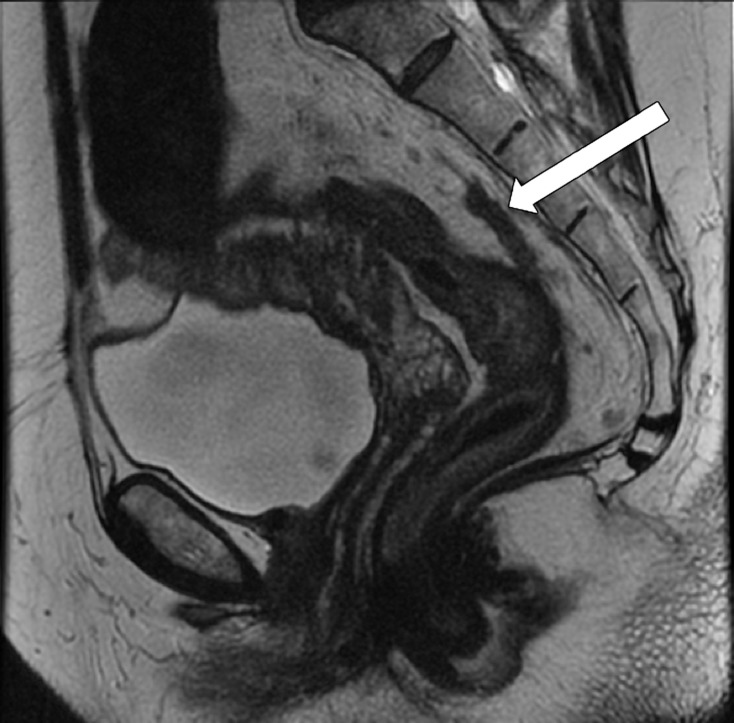
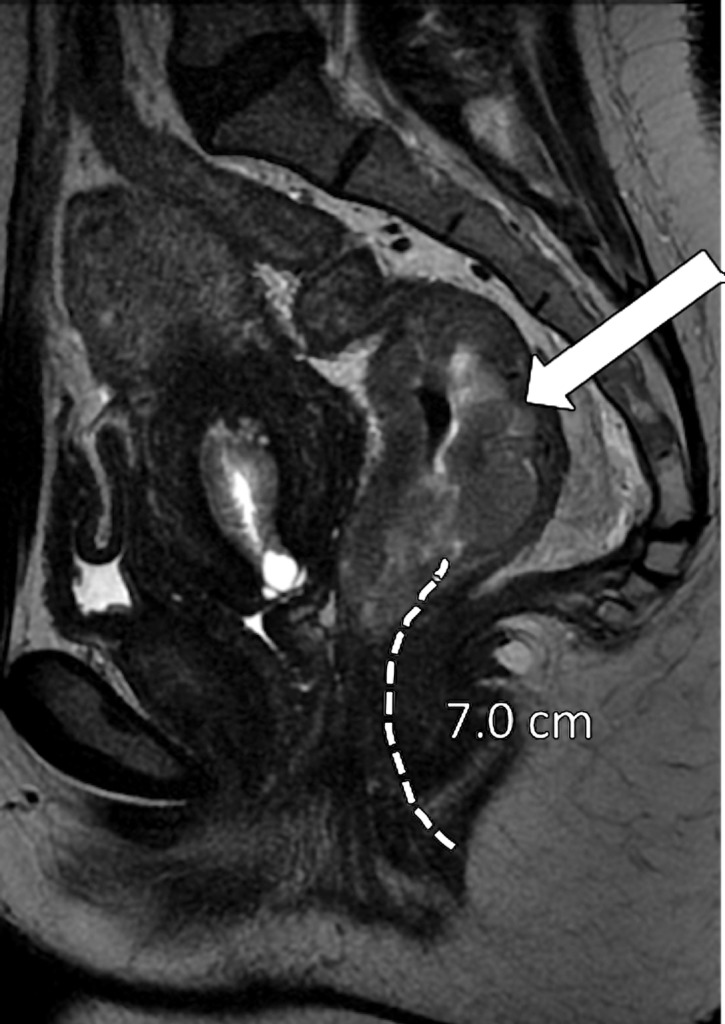
It is crucial to describe the tumor location in the craniocaudal direction (lower, middle, or upper rectum) and in the circumferential plane (clock-face position), as well as its length, relationship to the anterior peritoneal reflection, and distance from the inferior border of the tumor to the anal verge and the anorectal junction. This information helps determine the best surgical approach. The location of the tumor is categorized as low (0–5 cm from the anal verge), middle (5.1–10 cm from the anal verge), and high (10.1–15 cm from the anal verge) (Fig 6). Tumors located above 15 cm from the anal verge are treated as colon cancer and, consequently, their staging and treatment differ from those of rectal cancer.
Figure 6a.
Tumor location in the craniocaudal direction. (a) Illustration depicts the sagittal view of the rectum and provides the measurements of the tumor from the anal verge, which help categorize tumor location. Blue lines separate the low, mid-, and high rectum. (Figure 6a reprinted, under a CC BY-ND 4.0 license, from Memorial Sloan Kettering Cancer Center.) (b–d) Sagittal T2-weighted MR images show tumors (arrow) in the high (b), mid- (c), and low (d) rectum. Dotted line = measurement from the rectum entrance to the tumor location.
Figure 6b.
Tumor location in the craniocaudal direction. (a) Illustration depicts the sagittal view of the rectum and provides the measurements of the tumor from the anal verge, which help categorize tumor location. Blue lines separate the low, mid-, and high rectum. (Figure 6a reprinted, under a CC BY-ND 4.0 license, from Memorial Sloan Kettering Cancer Center.) (b–d) Sagittal T2-weighted MR images show tumors (arrow) in the high (b), mid- (c), and low (d) rectum. Dotted line = measurement from the rectum entrance to the tumor location.
Figure 6c.
Tumor location in the craniocaudal direction. (a) Illustration depicts the sagittal view of the rectum and provides the measurements of the tumor from the anal verge, which help categorize tumor location. Blue lines separate the low, mid-, and high rectum. (Figure 6a reprinted, under a CC BY-ND 4.0 license, from Memorial Sloan Kettering Cancer Center.) (b–d) Sagittal T2-weighted MR images show tumors (arrow) in the high (b), mid- (c), and low (d) rectum. Dotted line = measurement from the rectum entrance to the tumor location.
Figure 6d.
Tumor location in the craniocaudal direction. (a) Illustration depicts the sagittal view of the rectum and provides the measurements of the tumor from the anal verge, which help categorize tumor location. Blue lines separate the low, mid-, and high rectum. (Figure 6a reprinted, under a CC BY-ND 4.0 license, from Memorial Sloan Kettering Cancer Center.) (b–d) Sagittal T2-weighted MR images show tumors (arrow) in the high (b), mid- (c), and low (d) rectum. Dotted line = measurement from the rectum entrance to the tumor location.
The tumor’s morphologic pattern (polypoid, ulcerating, circumferential, or semicircumferential) and especially its appearance (nonmucinous or mucinous) should also be described (Fig 7). Mucinous tumors show high signal intensity at T2-weighted MRI and have a worse prognosis than that of nonmucinous tumors, with a higher metastatic propensity and often a higher stage at the time of diagnosis (42).
Figure 7a.
Mucinous and nonmucinous tumors. Axial oblique T2-weighted MR images in two different patients show a mucinous tumor (arrow in a) and a nonmucinous tumor (arrow in b). Mucinous tumors typically show high signal intensity, and nonmucinous tumors show intermediate signal intensity.
Figure 7b.
Mucinous and nonmucinous tumors. Axial oblique T2-weighted MR images in two different patients show a mucinous tumor (arrow in a) and a nonmucinous tumor (arrow in b). Mucinous tumors typically show high signal intensity, and nonmucinous tumors show intermediate signal intensity.
Sometimes identifying the tumor may be challenging for radiologists who are not experienced in reading rectal MR images in daily practice. Two imaging characteristics can be helpful in tumor identification: rectal cancer usually appears with elevated borders and can accumulate mucoid material in the rectal lumen of the tumoral region, which can also be visualized in nonmucinous lesions (Fig 8).
Figure 8a.
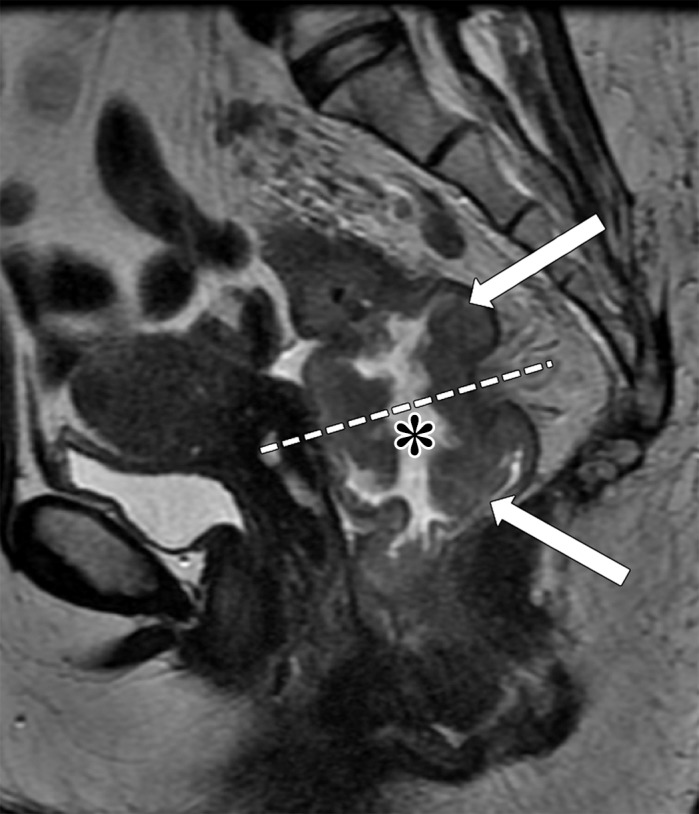
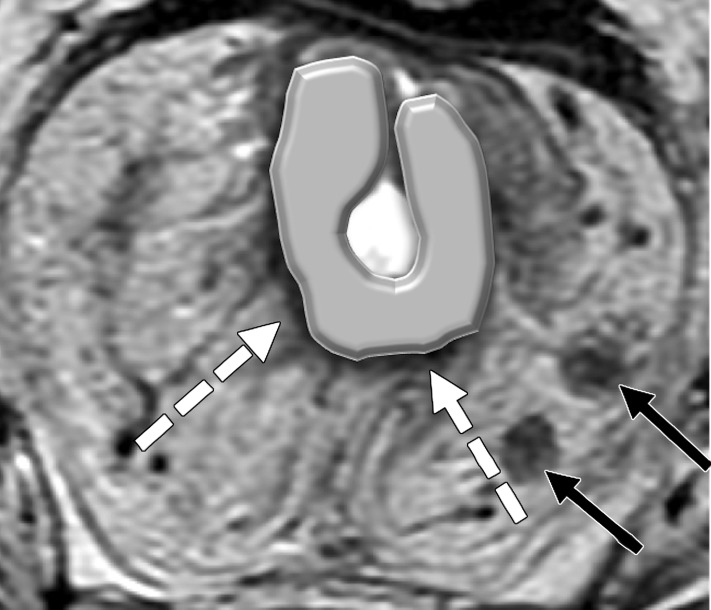
Tumor within the middle rectum clinically staged as T3b, with positive mesorectal lymph nodes, accumulation of mucoid material within the rectal lumen (* in a and b), and elevated borders in the superior and inferior edge of the tumor. (a) Sagittal T2-weighted MR image shows a tumor with elevated borders (arrows) in the superior and inferior edge of the tumor. Dashed line = plane of the axial oblique MR image shown in b. (b, c) Axial oblique T2-weighted MR image (b) and magnified area of interest (square outline in b) (c) obtained perpendicular to the tumor, halfway in the craniocaudal direction, show a C-shape tumor (dashed arrows). An added gray overlay in c depicts the shape of the tumor. The most invasive portion of the tumor is frequently located around the center of the C shape. The tumor infiltrates beyond the muscularis propria, 2 mm into the mesorectum (T3b) (arrowhead in b), and two 7-mm round heterogeneous mesorectal lymph nodes (solid arrows in c) are depicted, making this tumor positive for lymph node involvement.
Mid- and High Rectal Cancer Tumor Staging
Figure 9 demonstrates the location of rectal cancer and its corresponding T category. Diagnostic accuracy, sensitivity, and specificity of high-resolution rectal MRI in assessing T category are 85%, 87%, and 75%, respectively (43). T category is characterized by the depth of tumor penetration into the rectal wall and extramural spread into the mesorectum and adjacent structures. It is important to identify the most invasive portion of the tumor, corresponding to the area of deepest infiltration, which is usually located halfway in the craniocaudal direction and at the center of the C shape depicted on oblique axial images (Fig 8). The T category is better applied to mid- and high rectal cancers and differs from that of low rectal cancer, especially owing to the narrowing of the mesorectum, which is a barrier to circumferential tumor spread (25,44), with a resultant higher risk of involvement of the MRF (45).
Figure 9.
Illustration depicts the anatomy of the rectum and the possible locations of rectal cancer, along with corresponding T categories and potential tumor sizes for each location.
Figure 8b.
Tumor within the middle rectum clinically staged as T3b, with positive mesorectal lymph nodes, accumulation of mucoid material within the rectal lumen (* in a and b), and elevated borders in the superior and inferior edge of the tumor. (a) Sagittal T2-weighted MR image shows a tumor with elevated borders (arrows) in the superior and inferior edge of the tumor. Dashed line = plane of the axial oblique MR image shown in b. (b, c) Axial oblique T2-weighted MR image (b) and magnified area of interest (square outline in b) (c) obtained perpendicular to the tumor, halfway in the craniocaudal direction, show a C-shape tumor (dashed arrows). An added gray overlay in c depicts the shape of the tumor. The most invasive portion of the tumor is frequently located around the center of the C shape. The tumor infiltrates beyond the muscularis propria, 2 mm into the mesorectum (T3b) (arrowhead in b), and two 7-mm round heterogeneous mesorectal lymph nodes (solid arrows in c) are depicted, making this tumor positive for lymph node involvement.
T1 tumors infiltrate the submucosa, and T2 tumors extend into the muscularis propria. Rectal MRI does not provide a reliable distinction between these two categories, except in some patients with T1 tumors when it is possible to identify a preserved submucosal layer (hyperintense signal) beneath the lesion (46,47). Therefore, patients should undergo endorectal US owing to its superior diagnostic performance in these cases (26).
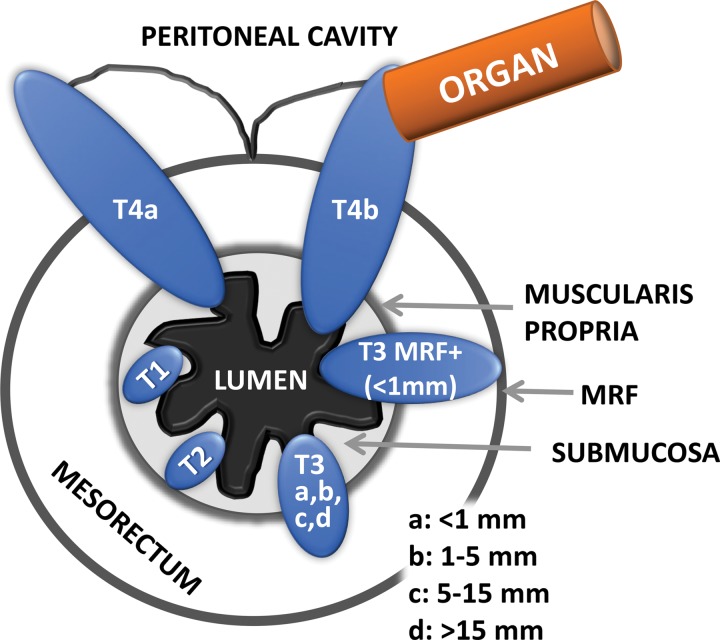
T3 tumors are characterized by a discontinuity of the muscularis propria, with extension of the tumor into the mesorectum without infiltration of the MRF or adjacent organs (20) (Fig 10c). They are classified into four categories dependent on the distance between the outermost edge of the muscularis propria and the maximum extramural spread of the tumor (T3a, <1 mm; T3b, 1–5 mm; T3c, 5–15 mm; and T4d, >15 mm).
Figure 10c.
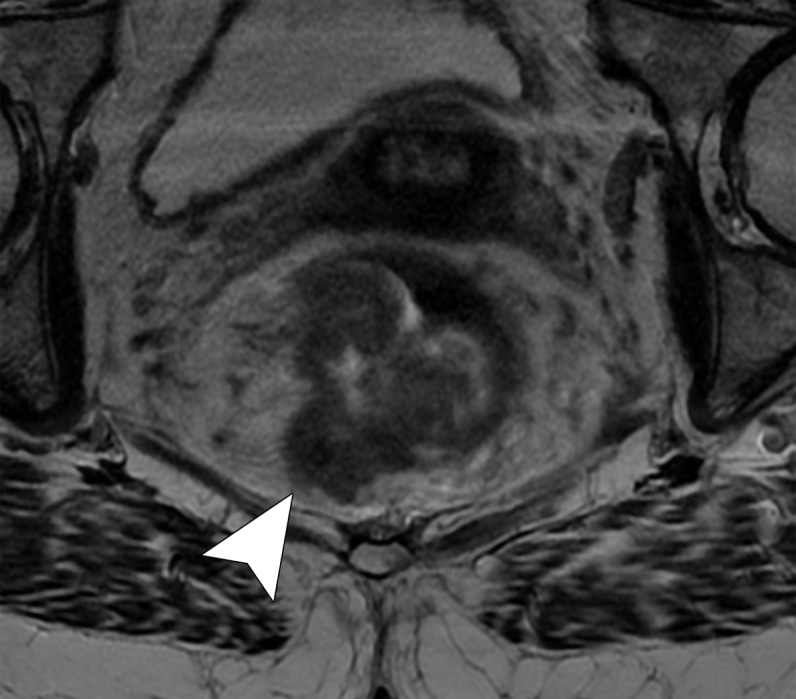
Rectal MR images that show distinct tumor stages obtained from three different patients. (a, b) Sagittal (a) and axial (b) T2-weighted MR images show a polypoid lesion (solid arrow) surrounded by mucoid material, with a thin stalk attached to the rectal wall and the intact muscularis propria (dashed arrow), findings characteristic of a T1 or T2 tumor. (c) Oblique axial T2-weighted MR image in another patient shows a tumor infiltrating 7 mm beyond the muscularis propria (T3c), with positive MRF infiltration (arrowhead). (d) Oblique axial T2-weighted MR image in a third patient shows a tumor invading the anterior peritoneal reflection (arrowhead), a characteristic finding of a T4a grade tumor.
Figure 10a.
Rectal MR images that show distinct tumor stages obtained from three different patients. (a, b) Sagittal (a) and axial (b) T2-weighted MR images show a polypoid lesion (solid arrow) surrounded by mucoid material, with a thin stalk attached to the rectal wall and the intact muscularis propria (dashed arrow), findings characteristic of a T1 or T2 tumor. (c) Oblique axial T2-weighted MR image in another patient shows a tumor infiltrating 7 mm beyond the muscularis propria (T3c), with positive MRF infiltration (arrowhead). (d) Oblique axial T2-weighted MR image in a third patient shows a tumor invading the anterior peritoneal reflection (arrowhead), a characteristic finding of a T4a grade tumor.
Figure 10b.
Rectal MR images that show distinct tumor stages obtained from three different patients. (a, b) Sagittal (a) and axial (b) T2-weighted MR images show a polypoid lesion (solid arrow) surrounded by mucoid material, with a thin stalk attached to the rectal wall and the intact muscularis propria (dashed arrow), findings characteristic of a T1 or T2 tumor. (c) Oblique axial T2-weighted MR image in another patient shows a tumor infiltrating 7 mm beyond the muscularis propria (T3c), with positive MRF infiltration (arrowhead). (d) Oblique axial T2-weighted MR image in a third patient shows a tumor invading the anterior peritoneal reflection (arrowhead), a characteristic finding of a T4a grade tumor.
Figure 10d.
Rectal MR images that show distinct tumor stages obtained from three different patients. (a, b) Sagittal (a) and axial (b) T2-weighted MR images show a polypoid lesion (solid arrow) surrounded by mucoid material, with a thin stalk attached to the rectal wall and the intact muscularis propria (dashed arrow), findings characteristic of a T1 or T2 tumor. (c) Oblique axial T2-weighted MR image in another patient shows a tumor infiltrating 7 mm beyond the muscularis propria (T3c), with positive MRF infiltration (arrowhead). (d) Oblique axial T2-weighted MR image in a third patient shows a tumor invading the anterior peritoneal reflection (arrowhead), a characteristic finding of a T4a grade tumor.
Differentiating T2 tumors from early T3 tumors can be difficult (48). Penetration into the muscular layers by small vessels and desmoplastic reaction are common pitfalls that can lead to overstaging a T2 tumor as a T3 tumor (20). Desmoplastic reaction is depicted as spicules with low signal intensity at T2-weighted imaging, while T3 tumors have a broad-based or nodular appearance with intermediate signal intensity at T2-weighted imaging (20). Lastly, T4 tumors are those that infiltrate the peritoneal reflection (T4a) or other pelvic organs and structures (T4b).
Low Rectal Cancer and Anal Sphincter Complex Status
In patients with low rectal cancer, radiologists play a pivotal role in preoperative evaluation. Accurate staging is required to determine the need for neoadjuvant CRT or more extensive surgery and to provide the surgeon with a guide for planes of excision (45,49). Conventional staging is insufficient because tumors in the lower rectum are in close proximity to the anal sphincter complex and are more likely to invade the MRF and adjacent organs, with positive surgical margins in about 30% of cases owing to the narrowing of the mesorectum in this location (49).
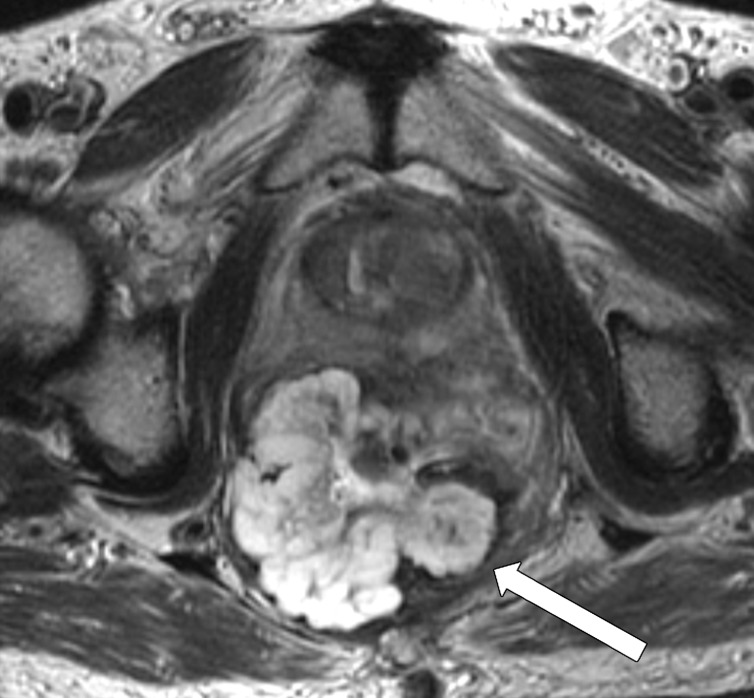
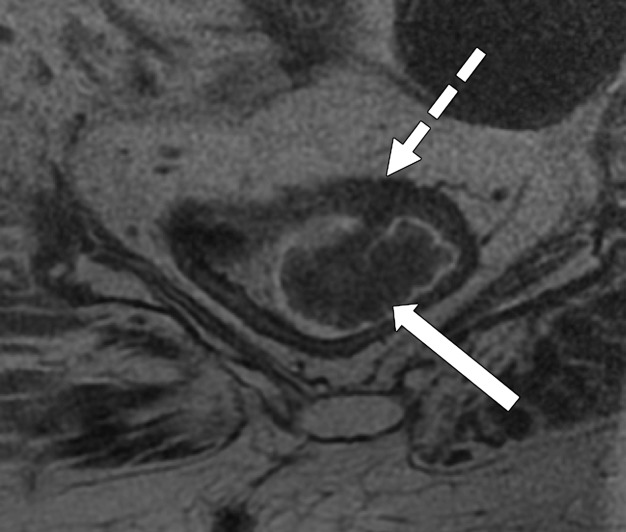
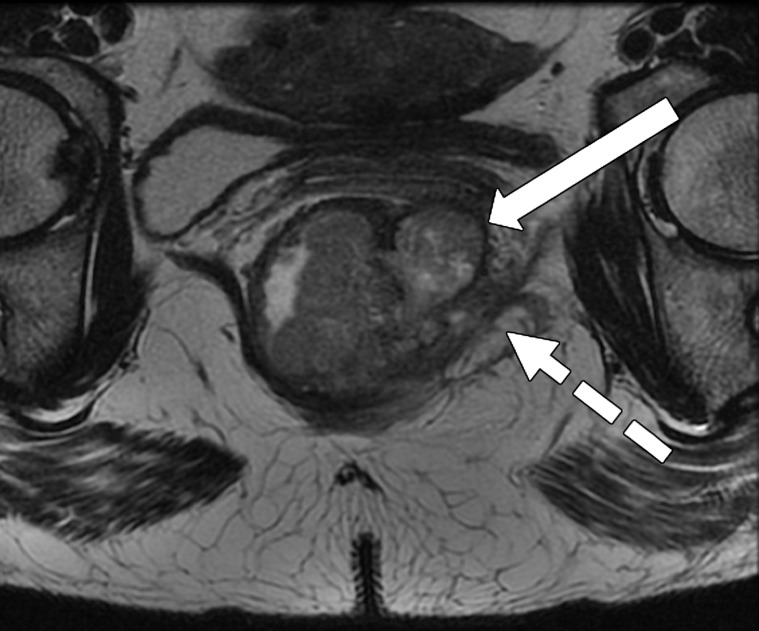
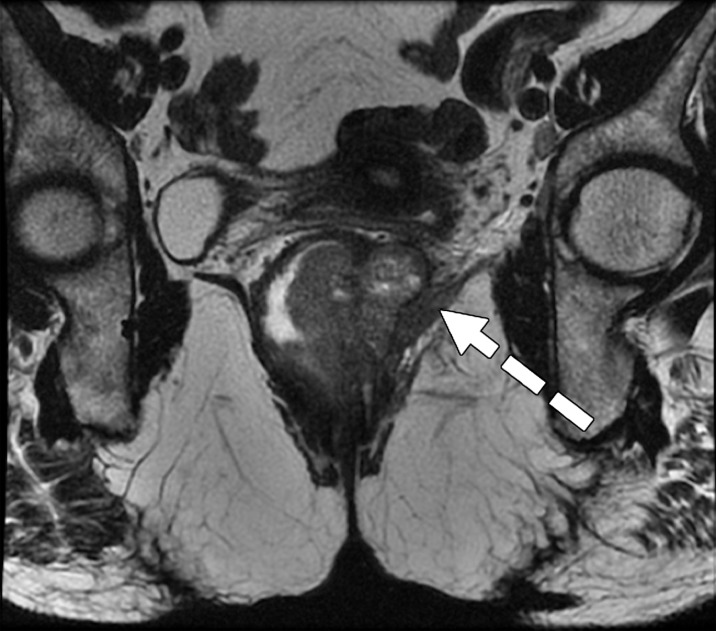
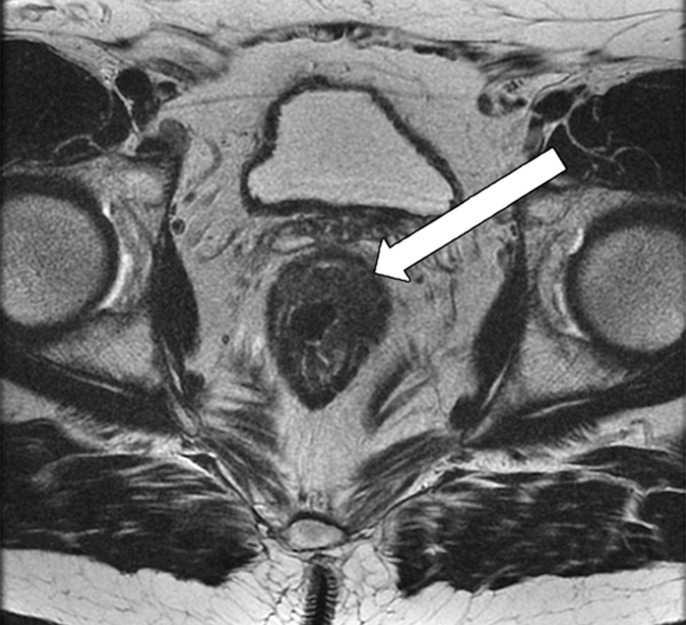
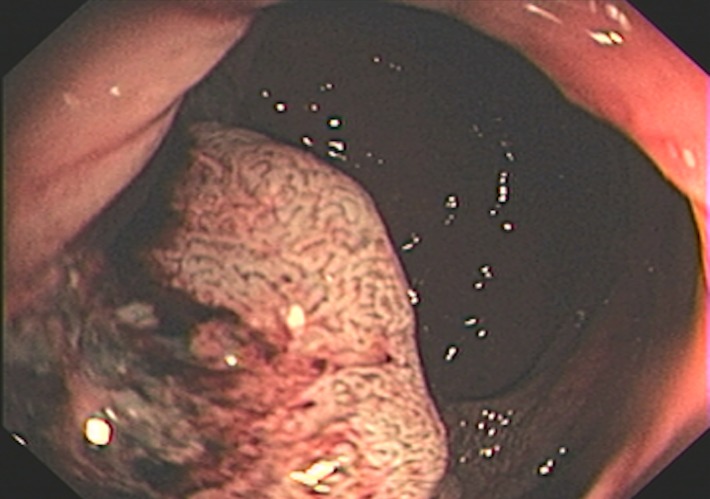
Taylor et al (50) revised a specific staging system on the basis of invasion of the anal sphincter complex owing to the extension through the muscular layer for surgical planning and the risk of traditional abdominoperineal resection. The report should describe if the tumor invades the internal sphincter, intersphincteric plane, and external sphincter and/or levator ani (Fig 11). The coronal oblique plane is the best plane for this evaluation at T2-weighted MRI.
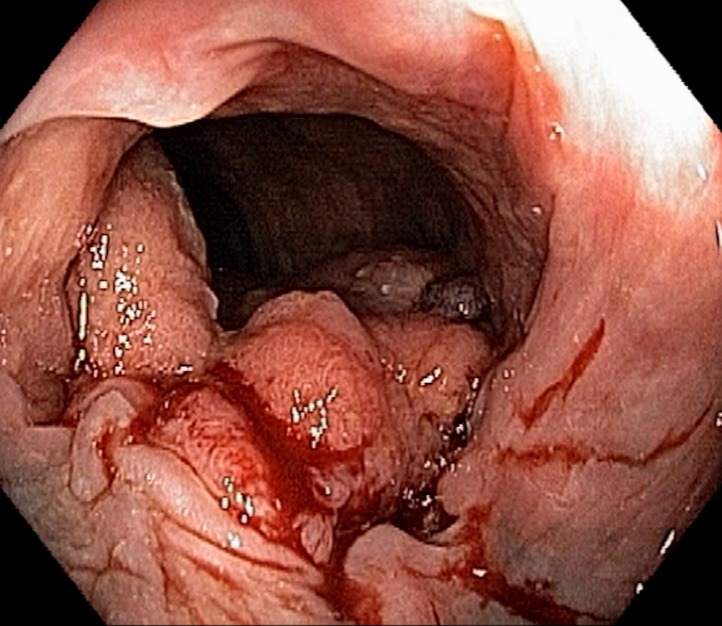
Figure 11a.
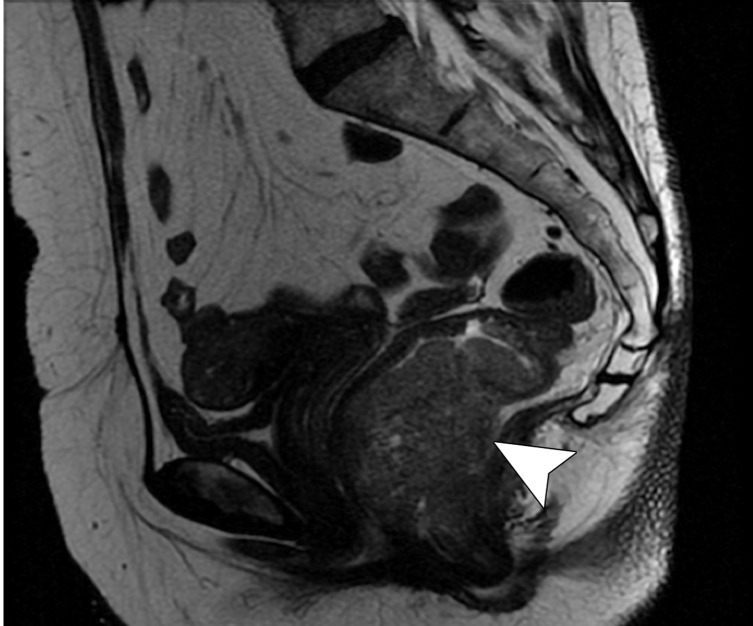
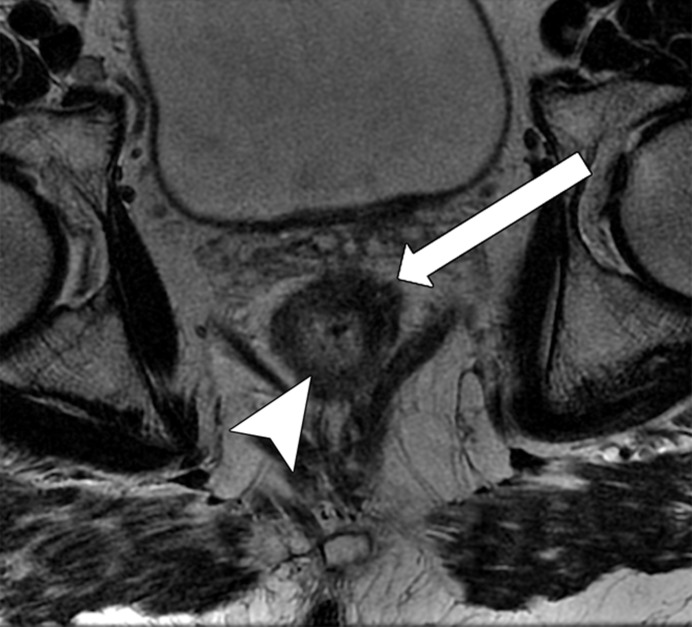
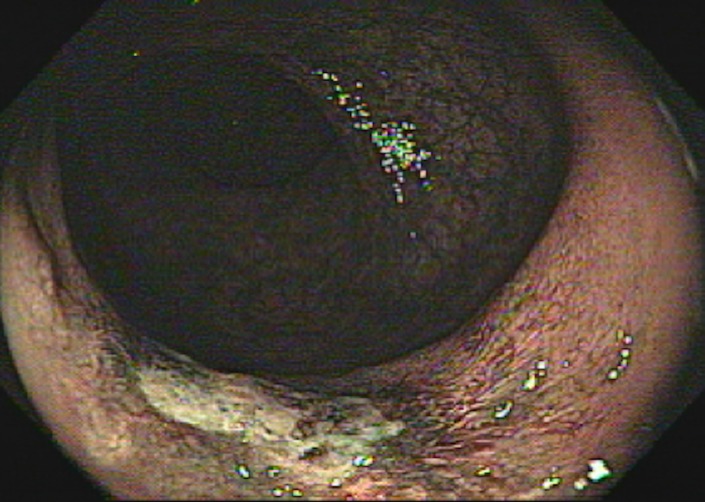
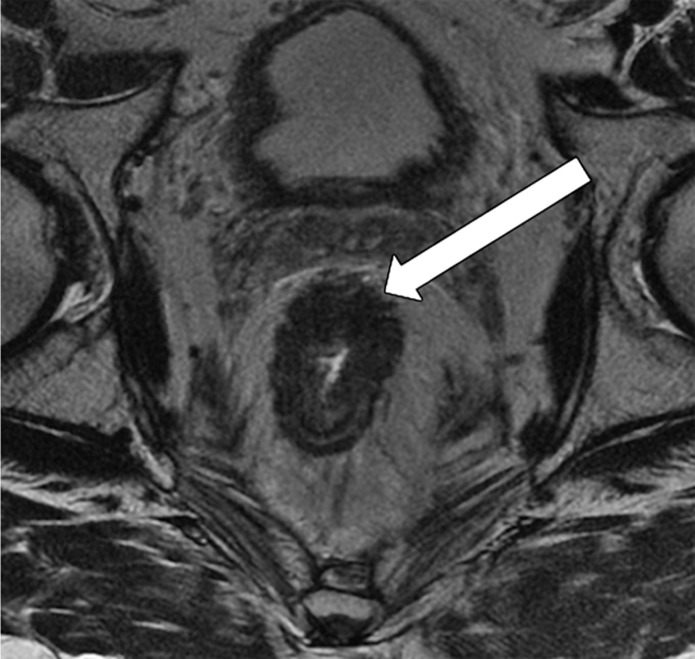
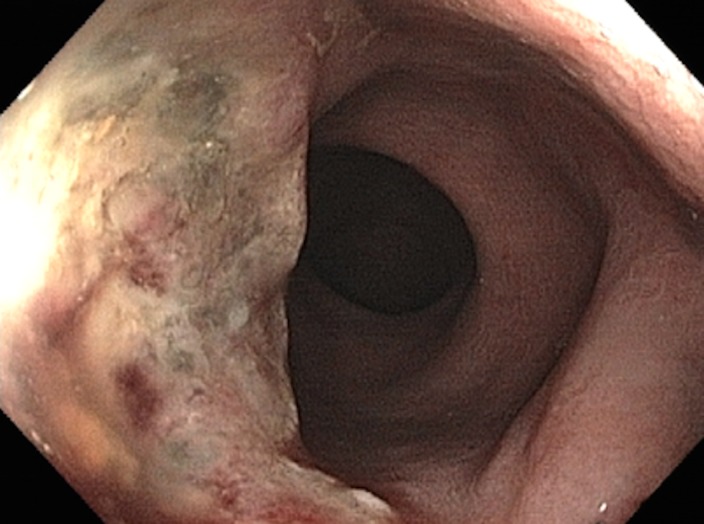
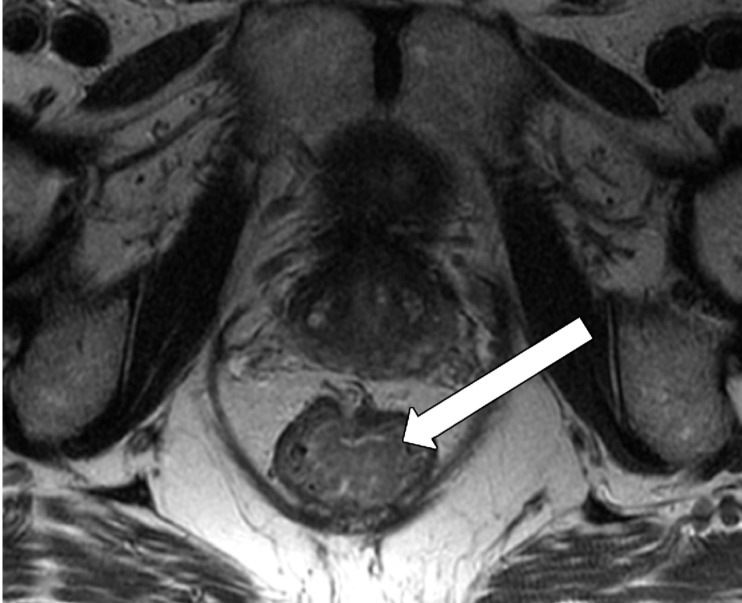
Tumor in the lower rectum. (a) Colonoscopic image shows a semicircumferential ulcerated tumor. Rectal MRI was performed for local staging. (b) Sagittal T2-weighted MR image shows a semicircumferential tumor (arrowhead) in the lower rectum. (c, d) Axial oblique (c) and coronal (d) T2-weighted MR images show the tumor (solid arrow in c) infiltrating beyond the muscularis propria and invading the left external sphincter and levator ani muscle (dashed arrow), which are thickened and have intermediate signal intensity.
Figure 11b.
Tumor in the lower rectum. (a) Colonoscopic image shows a semicircumferential ulcerated tumor. Rectal MRI was performed for local staging. (b) Sagittal T2-weighted MR image shows a semicircumferential tumor (arrowhead) in the lower rectum. (c, d) Axial oblique (c) and coronal (d) T2-weighted MR images show the tumor (solid arrow in c) infiltrating beyond the muscularis propria and invading the left external sphincter and levator ani muscle (dashed arrow), which are thickened and have intermediate signal intensity.
Figure 11c.
Tumor in the lower rectum. (a) Colonoscopic image shows a semicircumferential ulcerated tumor. Rectal MRI was performed for local staging. (b) Sagittal T2-weighted MR image shows a semicircumferential tumor (arrowhead) in the lower rectum. (c, d) Axial oblique (c) and coronal (d) T2-weighted MR images show the tumor (solid arrow in c) infiltrating beyond the muscularis propria and invading the left external sphincter and levator ani muscle (dashed arrow), which are thickened and have intermediate signal intensity.
Figure 11d.
Tumor in the lower rectum. (a) Colonoscopic image shows a semicircumferential ulcerated tumor. Rectal MRI was performed for local staging. (b) Sagittal T2-weighted MR image shows a semicircumferential tumor (arrowhead) in the lower rectum. (c, d) Axial oblique (c) and coronal (d) T2-weighted MR images show the tumor (solid arrow in c) infiltrating beyond the muscularis propria and invading the left external sphincter and levator ani muscle (dashed arrow), which are thickened and have intermediate signal intensity.
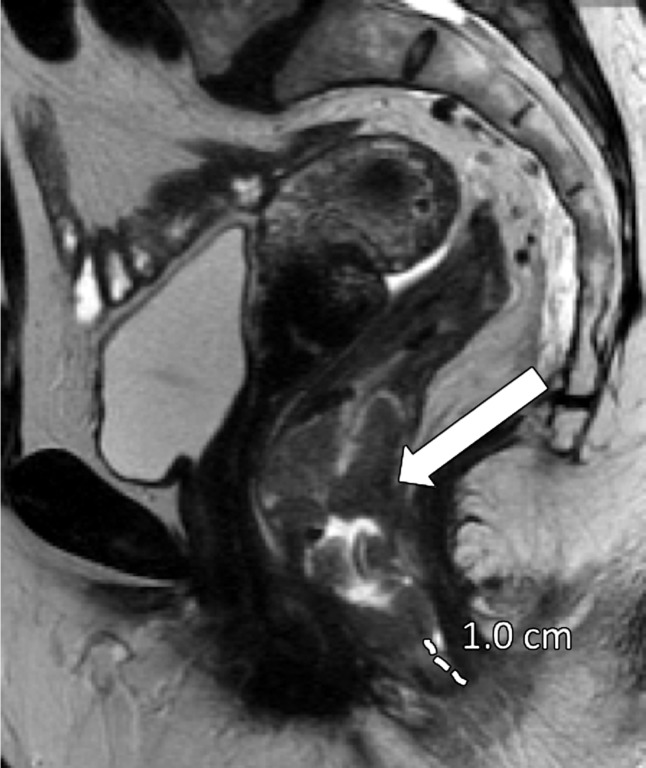
CRM Status
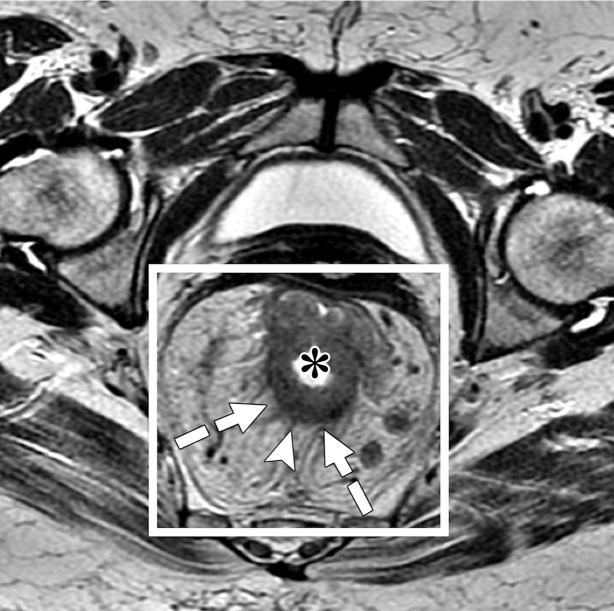
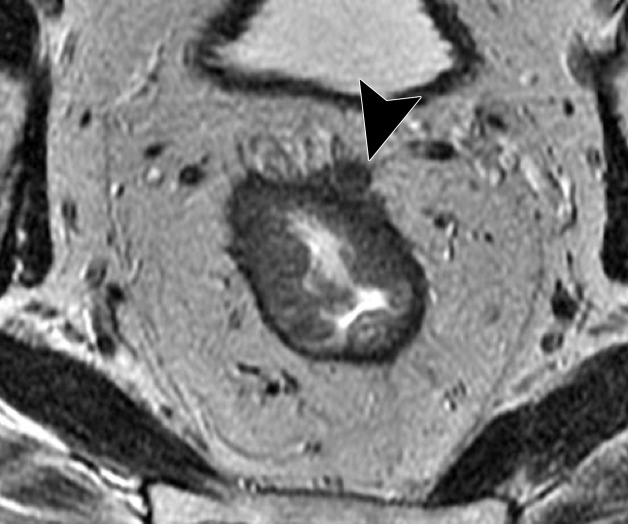
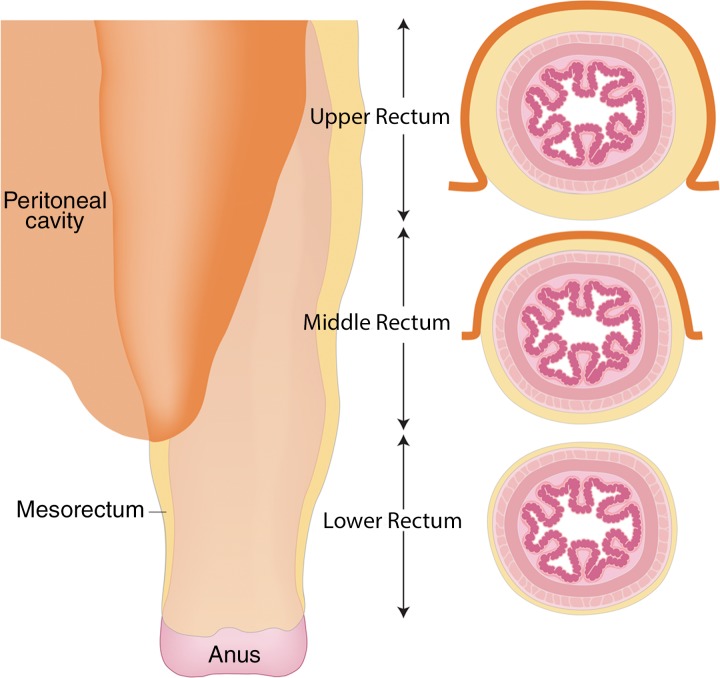
CRM is the surface of the nonperitonealized part of the rectum that is resected during surgery. MRI is the most reliable imaging modality to determine potential CRM involvement (43,51). At MRI, CRM status can be obtained by measuring the shortest distance between the outermost part of the rectal tumor and the MRF (52). The CRM status is potentially positive if this measurement is less than 1 mm, and threatened if it is between 1 and 2 mm (33). It is important to highlight that the rectum is not entirely surrounded by MRF (Fig 12), and thus CRM status is not applicable if the tumor is situated in a peritonealized aspect of the rectal wall.
Figure 12.
Illustration depicts sagittal and axial views of the peritoneal and MRF coverage of the rectum. Note that the potential CRM described in the radiologic report corresponds to the distance between the tumor and the MRF and does not include the portions of the rectum surrounded by peritoneum. (Reprinted, under a CC BY-ND 4.0 license, from Memorial Sloan Kettering Cancer Center.)
A tumor–MRF distance of more than 1 mm is a reliable predictor for negative margins after TME (50). On the other hand, a positive CRM is the most important predictor of local recurrence and poor survival (53). Therefore, every report should include the CRM status and the location of potential involvement (clock-face method).
Pelvic Organs and Sidewall Involvement
In T4b tumors, it is important to describe if adjacent structures are involved, including the uterus, vagina, prostate gland, seminal vesicles, ureters, presacral fascia, sacral nerve roots, sacrum, iliac vessels, and pelvic muscles.
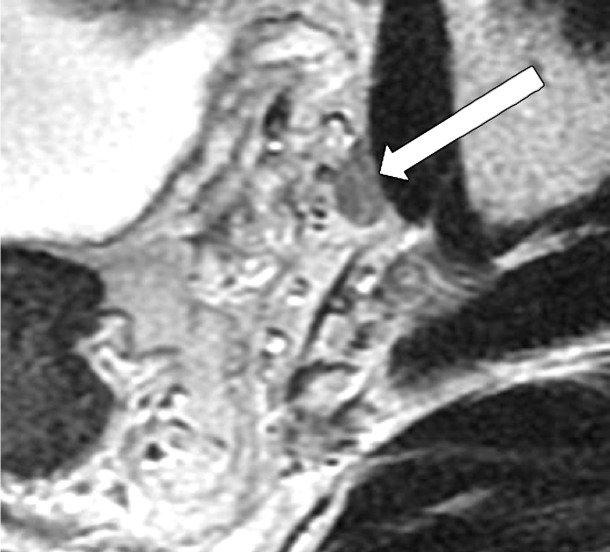
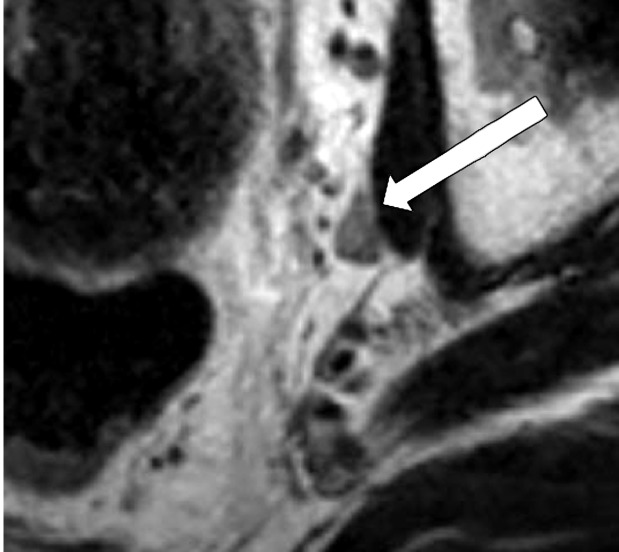
Extramural Vascular Invasion
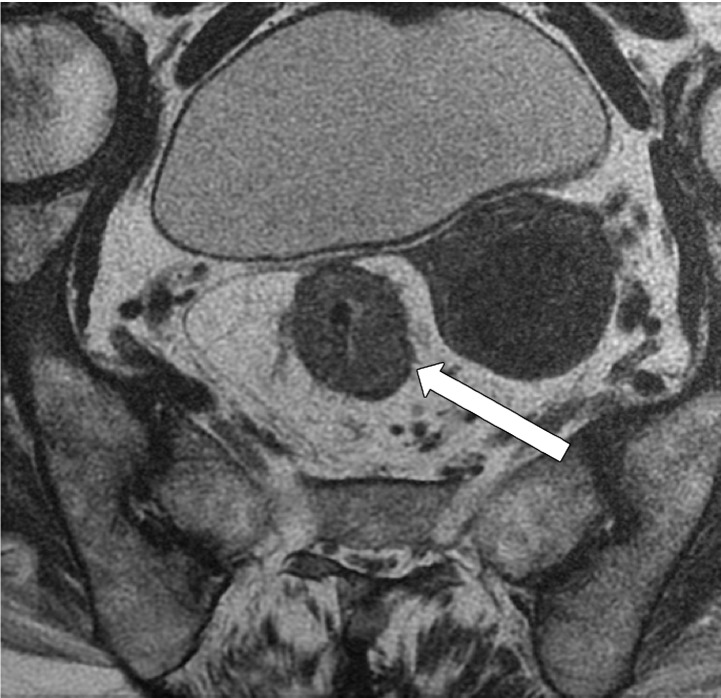
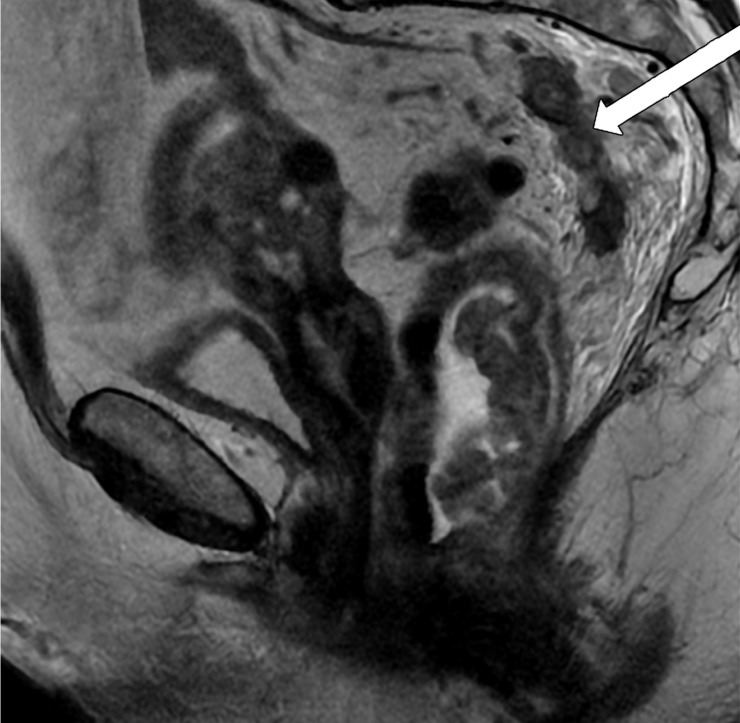
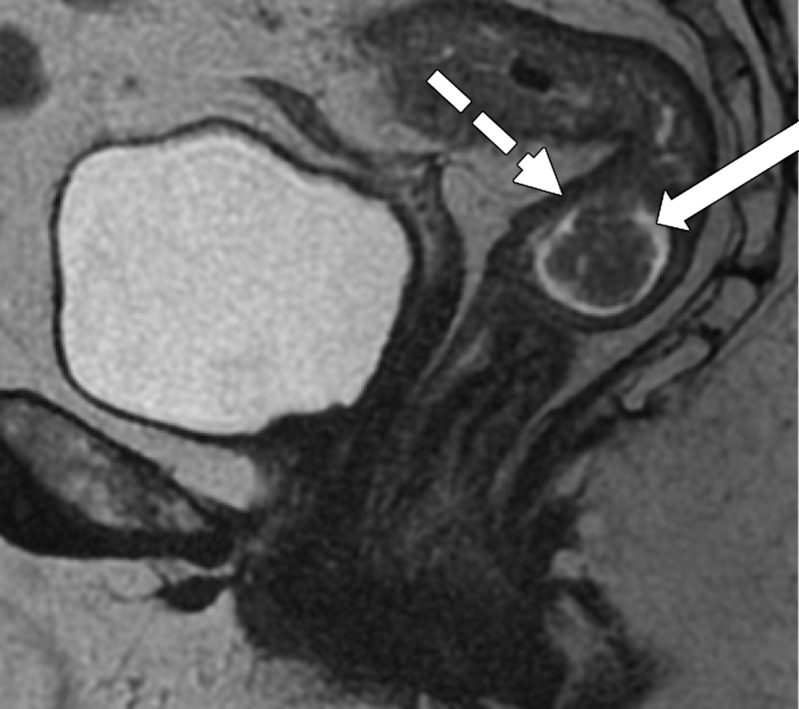
MRI can depict EMVI with moderate sensitivity and high specificity, which is an important prognostic factor and predictor of metastatic disease (54–56). EMVI is an extension of the tumor to the vessels in the mesorectum, resulting in wall irregularity, focal enlargement, and/or signal intensity of the tumor (intermediate at T2-weighted imaging) within the vessel (56) (Fig 13).
Figure 13a.
EMVI. Sagittal T2-weighted MR images in two different patients show signs of EMVI, characterized by focal enlargement of the vessel, signal intensity of the tumor replacing the flow void, and wall irregularity (arrow).
Figure 13b.
EMVI. Sagittal T2-weighted MR images in two different patients show signs of EMVI, characterized by focal enlargement of the vessel, signal intensity of the tumor replacing the flow void, and wall irregularity (arrow).
Lymph Node Involvement
Compared with the accuracy of MRI in tumor staging, the accuracy of MRI in assessing the involvement of metastatic lymph nodes in rectal cancer is less accurate, which is an important prognostic factor and indicator for the use of neoadjuvant CRT (43). The presence, number, and precise location of suspicious lymph nodes should be reported.
The proximity between the suspicious lymph nodes and the MRF is also important to report for surgical planning, although it has been shown that it does not confer poor prognosis in the same manner as that of the primary tumor (12).
As a large proportion of metastatic lymph nodes in rectal cancer measure less than 5 mm, size is not a reliable criterion (57,58). However, some studies have demonstrated that lymph nodes measuring greater than 8 mm in the short axis are highly specific for metastatic involvement (26,59,60). Therefore, it has been proposed for nodal assessment to include size and morphologic characteristics of malignancy, including the presence of irregular borders, heterogeneous signal intensity, and round shape (Table 3) (Fig 8) (33,57).
Table 3:
Malignant Morphologic Criteria and Lymph Node Size
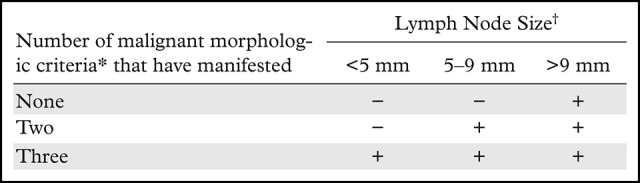
*Irregular borders, heterogeneous signal intensity, and round shape.
†Measured in the largest short axis. − indicates not suspicious for malignancy, + indicates suspicious for malignancy.
Figure 8c.
Tumor within the middle rectum clinically staged as T3b, with positive mesorectal lymph nodes, accumulation of mucoid material within the rectal lumen (* in a and b), and elevated borders in the superior and inferior edge of the tumor. (a) Sagittal T2-weighted MR image shows a tumor with elevated borders (arrows) in the superior and inferior edge of the tumor. Dashed line = plane of the axial oblique MR image shown in b. (b, c) Axial oblique T2-weighted MR image (b) and magnified area of interest (square outline in b) (c) obtained perpendicular to the tumor, halfway in the craniocaudal direction, show a C-shape tumor (dashed arrows). An added gray overlay in c depicts the shape of the tumor. The most invasive portion of the tumor is frequently located around the center of the C shape. The tumor infiltrates beyond the muscularis propria, 2 mm into the mesorectum (T3b) (arrowhead in b), and two 7-mm round heterogeneous mesorectal lymph nodes (solid arrows in c) are depicted, making this tumor positive for lymph node involvement.
Regional lymph nodes involved in rectal cancer include the mesorectal, superior rectal, middle rectal, inferior rectal, sigmoid mesenteric, inferior mesenteric, lateral sacral, presacral, sacral promontory, or internal iliac (61). Lymph nodes out of these chains are considered distant metastases (M1). Extramesorectal nodes are important to describe, including those along the pelvic sidewall, as they are a negative prognostic predictor and are not routinely resected (62). Lesions that infiltrate the presacral space can manifest with retroperitoneal lymph nodes; therefore, those chains are also important to evaluate. A group of tumor cells not associated with lymphoid or vascular tissues, defined as tumor deposits, are characterized as N1c.
Restaging Rectal Cancer with MRI
For patients with LARC, neoadjuvant CRT is considered the standard treatment. It has been shown to improve local control, inducing tumor downstaging in approximately 50% of patients, and results in pathologic complete response in 15%–38% of cases (13–15,63,64). This can allow for a sphincter-preserving surgery to be performed or may even offer a nonsurgical treatment approach in some patients.
Digital rectal examination and endoscopy have been used to evaluate pathologic complete response, but these assessments are limited to the luminal view, leaving residual tumors in other layers of the bowel wall undetected (65). In this context, MRI has an important role in the assessment of tumor response after neoadjuvant CRT.
Before the restaging of rectal cancer after rectal MRI is performed, it is important to verify the neoadjuvant treatment the patient underwent and to evaluate the results of previous examinations (digital rectal examination, endoscopy, and pretreatment MRI) to understand the primary tumor’s location and morphology. The normal rectal wall adjacent to the tumor can manifest with post-CRT changes such as submucosal edema (thickened and intermediate to high signal intensity on T2-weighted images) that can lead to a common pitfall usually misinterpreted as residual tumor (Fig 14c). After treatment, the tumor may be similar in appearance to that of the pretreatment tumor or may appear atrophic and fibrotic, with low signal intensity on T2-weighted images, which is dependent on the type of response (66).
Figure 14a.
Low rectal cancer in a 56-year-old man who underwent neoadjuvant CRT and had clinical complete response, with tumor regrowth 9 months later. (a, b) Oblique axial T2-weighted MR image (a) obtained during primary staging shows an infiltrative tumor (arrow) with the most invasive border between the 1-o’clock and 3-o’clock positions, infiltrating 1 mm beyond the muscularis propria (T3a), which corresponds to a polypoid lesion seen on the colonoscopic image (b). (c, d) Oblique axial T2-weighted MR image (c) obtained after neoadjuvant CRT shows an area with low signal intensity (arrow), and colonoscopic image (d) shows a scar in the tumor bed, without residual tumor. Note the wall thickening and mucosal edema within the normal rectal wall, which were caused by CRT (arrowhead in c). The patient was selected for a watch-and-wait protocol. (e) Oblique axial T2-weighted MR image obtained 9 months later shows thickening in the tumor bed, with areas of intermediate signal intensity (arrow), a finding suspicious for tumor regrowth. (f) Colonoscopic image shows tumor regrowth.
Figure 14b.
Low rectal cancer in a 56-year-old man who underwent neoadjuvant CRT and had clinical complete response, with tumor regrowth 9 months later. (a, b) Oblique axial T2-weighted MR image (a) obtained during primary staging shows an infiltrative tumor (arrow) with the most invasive border between the 1-o’clock and 3-o’clock positions, infiltrating 1 mm beyond the muscularis propria (T3a), which corresponds to a polypoid lesion seen on the colonoscopic image (b). (c, d) Oblique axial T2-weighted MR image (c) obtained after neoadjuvant CRT shows an area with low signal intensity (arrow), and colonoscopic image (d) shows a scar in the tumor bed, without residual tumor. Note the wall thickening and mucosal edema within the normal rectal wall, which were caused by CRT (arrowhead in c). The patient was selected for a watch-and-wait protocol. (e) Oblique axial T2-weighted MR image obtained 9 months later shows thickening in the tumor bed, with areas of intermediate signal intensity (arrow), a finding suspicious for tumor regrowth. (f) Colonoscopic image shows tumor regrowth.
Figure 14c.
Low rectal cancer in a 56-year-old man who underwent neoadjuvant CRT and had clinical complete response, with tumor regrowth 9 months later. (a, b) Oblique axial T2-weighted MR image (a) obtained during primary staging shows an infiltrative tumor (arrow) with the most invasive border between the 1-o’clock and 3-o’clock positions, infiltrating 1 mm beyond the muscularis propria (T3a), which corresponds to a polypoid lesion seen on the colonoscopic image (b). (c, d) Oblique axial T2-weighted MR image (c) obtained after neoadjuvant CRT shows an area with low signal intensity (arrow), and colonoscopic image (d) shows a scar in the tumor bed, without residual tumor. Note the wall thickening and mucosal edema within the normal rectal wall, which were caused by CRT (arrowhead in c). The patient was selected for a watch-and-wait protocol. (e) Oblique axial T2-weighted MR image obtained 9 months later shows thickening in the tumor bed, with areas of intermediate signal intensity (arrow), a finding suspicious for tumor regrowth. (f) Colonoscopic image shows tumor regrowth.
Figure 14d.
Low rectal cancer in a 56-year-old man who underwent neoadjuvant CRT and had clinical complete response, with tumor regrowth 9 months later. (a, b) Oblique axial T2-weighted MR image (a) obtained during primary staging shows an infiltrative tumor (arrow) with the most invasive border between the 1-o’clock and 3-o’clock positions, infiltrating 1 mm beyond the muscularis propria (T3a), which corresponds to a polypoid lesion seen on the colonoscopic image (b). (c, d) Oblique axial T2-weighted MR image (c) obtained after neoadjuvant CRT shows an area with low signal intensity (arrow), and colonoscopic image (d) shows a scar in the tumor bed, without residual tumor. Note the wall thickening and mucosal edema within the normal rectal wall, which were caused by CRT (arrowhead in c). The patient was selected for a watch-and-wait protocol. (e) Oblique axial T2-weighted MR image obtained 9 months later shows thickening in the tumor bed, with areas of intermediate signal intensity (arrow), a finding suspicious for tumor regrowth. (f) Colonoscopic image shows tumor regrowth.
Figure 14e.
Low rectal cancer in a 56-year-old man who underwent neoadjuvant CRT and had clinical complete response, with tumor regrowth 9 months later. (a, b) Oblique axial T2-weighted MR image (a) obtained during primary staging shows an infiltrative tumor (arrow) with the most invasive border between the 1-o’clock and 3-o’clock positions, infiltrating 1 mm beyond the muscularis propria (T3a), which corresponds to a polypoid lesion seen on the colonoscopic image (b). (c, d) Oblique axial T2-weighted MR image (c) obtained after neoadjuvant CRT shows an area with low signal intensity (arrow), and colonoscopic image (d) shows a scar in the tumor bed, without residual tumor. Note the wall thickening and mucosal edema within the normal rectal wall, which were caused by CRT (arrowhead in c). The patient was selected for a watch-and-wait protocol. (e) Oblique axial T2-weighted MR image obtained 9 months later shows thickening in the tumor bed, with areas of intermediate signal intensity (arrow), a finding suspicious for tumor regrowth. (f) Colonoscopic image shows tumor regrowth.
Figure 14f.
Low rectal cancer in a 56-year-old man who underwent neoadjuvant CRT and had clinical complete response, with tumor regrowth 9 months later. (a, b) Oblique axial T2-weighted MR image (a) obtained during primary staging shows an infiltrative tumor (arrow) with the most invasive border between the 1-o’clock and 3-o’clock positions, infiltrating 1 mm beyond the muscularis propria (T3a), which corresponds to a polypoid lesion seen on the colonoscopic image (b). (c, d) Oblique axial T2-weighted MR image (c) obtained after neoadjuvant CRT shows an area with low signal intensity (arrow), and colonoscopic image (d) shows a scar in the tumor bed, without residual tumor. Note the wall thickening and mucosal edema within the normal rectal wall, which were caused by CRT (arrowhead in c). The patient was selected for a watch-and-wait protocol. (e) Oblique axial T2-weighted MR image obtained 9 months later shows thickening in the tumor bed, with areas of intermediate signal intensity (arrow), a finding suspicious for tumor regrowth. (f) Colonoscopic image shows tumor regrowth.
Mucin Response
As described previously, mucin within the tumor appears as an area of high signal intensity on T2-weighted images. Regarding this characteristic, tumors can manifest with one of three different mucin responses after CRT:
Mucin (or colloid degeneration) response can occur in nonmucinous tumors that become mucinous after CRT (66). It indicates a response to treatment and better prognosis (4).
Acellular mucin response represents a pathologic response of a mucinous tumor with no impact on recurrence-free survival (67). Until recently, there has been no reliable imaging method to differentiate cellular from acellular mucin.
Mucinous tumor without response is characterized as a mucinous tumor at the primary staging that did not respond to CRT. It is related to an increased risk of local recurrence and poor outcome (4,66).
Comparing pretreatment MR images with posttreatment MR images is important to differentiate colloid degeneration in a nonmucinous tumor from a genuinely mucinous tumor.
Residual Tumor and Fibrosis
A residual tumor has intermediate signal intensity on T2-weighted images whereas fibrosis and/or scaring has low signal intensity. However, differentiation is still challenging as residual tumor may occur within a scar. Additional functional MRI sequences, including DWI, have demonstrated promising results in tumor restaging at MRI. Fibrosis demonstrates low signal intensity on high b-value diffusion-weighted images, while residual tumor shows high signal intensity (Fig 15). In the tumor restaging setting, performing a microenema is suggested before performing rectal MRI to reduce the amount of gas within the rectum to decrease artifacts at DWI (68,69).
Figure 15a.
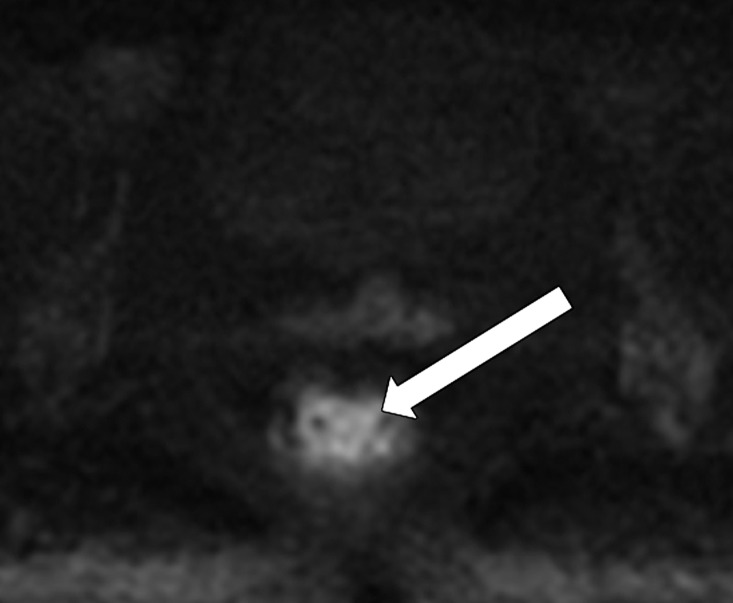
Partial response after neoadjuvant CRT. (a, b) Oblique axial T2-weighted MR image (a) and diffusion-weighted image (b) obtained during primary staging shows a tumor (arrow) in the lower rectum, with intermediate signal intensity in a and restricted diffusion in b. (c) Oblique axial T2-weighted MR image obtained after neoadjuvant CRT at restaging shows areas of low signal intensity (black arrow) in the tumor bed and residual tumor (white arrows) with intermediate signal intensity. (d, e) Axial diffusion-weighted image (d) shows restricted diffusion within the areas of the residual tumor (arrowhead), which was confirmed on the corresponding ADC map (e).
Figure 15b.
Partial response after neoadjuvant CRT. (a, b) Oblique axial T2-weighted MR image (a) and diffusion-weighted image (b) obtained during primary staging shows a tumor (arrow) in the lower rectum, with intermediate signal intensity in a and restricted diffusion in b. (c) Oblique axial T2-weighted MR image obtained after neoadjuvant CRT at restaging shows areas of low signal intensity (black arrow) in the tumor bed and residual tumor (white arrows) with intermediate signal intensity. (d, e) Axial diffusion-weighted image (d) shows restricted diffusion within the areas of the residual tumor (arrowhead), which was confirmed on the corresponding ADC map (e).
Figure 15c.
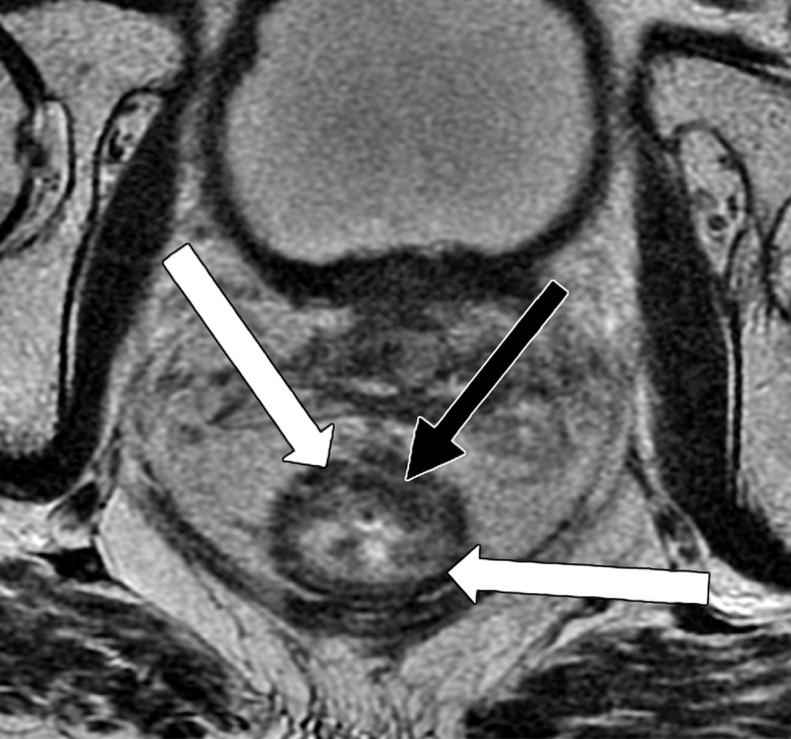
Partial response after neoadjuvant CRT. (a, b) Oblique axial T2-weighted MR image (a) and diffusion-weighted image (b) obtained during primary staging shows a tumor (arrow) in the lower rectum, with intermediate signal intensity in a and restricted diffusion in b. (c) Oblique axial T2-weighted MR image obtained after neoadjuvant CRT at restaging shows areas of low signal intensity (black arrow) in the tumor bed and residual tumor (white arrows) with intermediate signal intensity. (d, e) Axial diffusion-weighted image (d) shows restricted diffusion within the areas of the residual tumor (arrowhead), which was confirmed on the corresponding ADC map (e).
Figure 15d.
Partial response after neoadjuvant CRT. (a, b) Oblique axial T2-weighted MR image (a) and diffusion-weighted image (b) obtained during primary staging shows a tumor (arrow) in the lower rectum, with intermediate signal intensity in a and restricted diffusion in b. (c) Oblique axial T2-weighted MR image obtained after neoadjuvant CRT at restaging shows areas of low signal intensity (black arrow) in the tumor bed and residual tumor (white arrows) with intermediate signal intensity. (d, e) Axial diffusion-weighted image (d) shows restricted diffusion within the areas of the residual tumor (arrowhead), which was confirmed on the corresponding ADC map (e).
Figure 15e.
Partial response after neoadjuvant CRT. (a, b) Oblique axial T2-weighted MR image (a) and diffusion-weighted image (b) obtained during primary staging shows a tumor (arrow) in the lower rectum, with intermediate signal intensity in a and restricted diffusion in b. (c) Oblique axial T2-weighted MR image obtained after neoadjuvant CRT at restaging shows areas of low signal intensity (black arrow) in the tumor bed and residual tumor (white arrows) with intermediate signal intensity. (d, e) Axial diffusion-weighted image (d) shows restricted diffusion within the areas of the residual tumor (arrowhead), which was confirmed on the corresponding ADC map (e).
Assessing Other Posttreatment Features
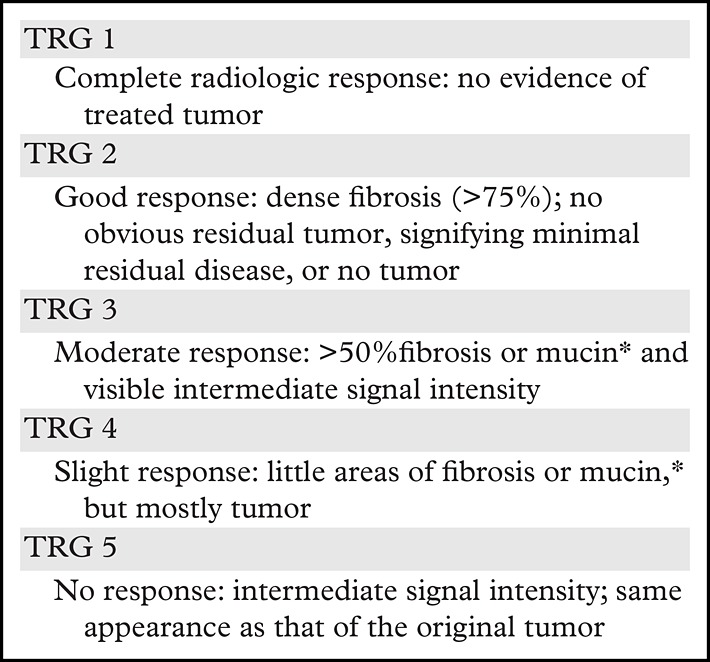
A tumor regression grading system for rectal cancer has been proposed (Table 4) and demonstrates a correlation with survival outcomes (19). Regression grades range from 1 to 5, with grade 1 indicating complete radiologic response to treatment and grade 5 indicating no response.
Table 4:
Tumor Regression Grade Features at MRI
The accuracy of rectal cancer staging at MRI in post-CRT tumors is lower than that of primary staging at MRI (70). Therefore, it is important to use a multidisciplinary approach and a combination of modalities to assess response to treatment, including MRI, clinical assessment, and endoscopy. During tumor restaging, it is also important to evaluate the sphincter complex and pelvic side wall status in a similar fashion to that which is performed at primary staging.
The CRM status and the smallest distance between the remaining tumor and the MRF must also be described in the radiologic report, although this evaluation is less accurate than that of the pretreatment assessment (51). The importance of reporting EMVI at rectal cancer restaging, which can disappear after treatment and be replaced by fibrotic tissue, is still unclear (66).
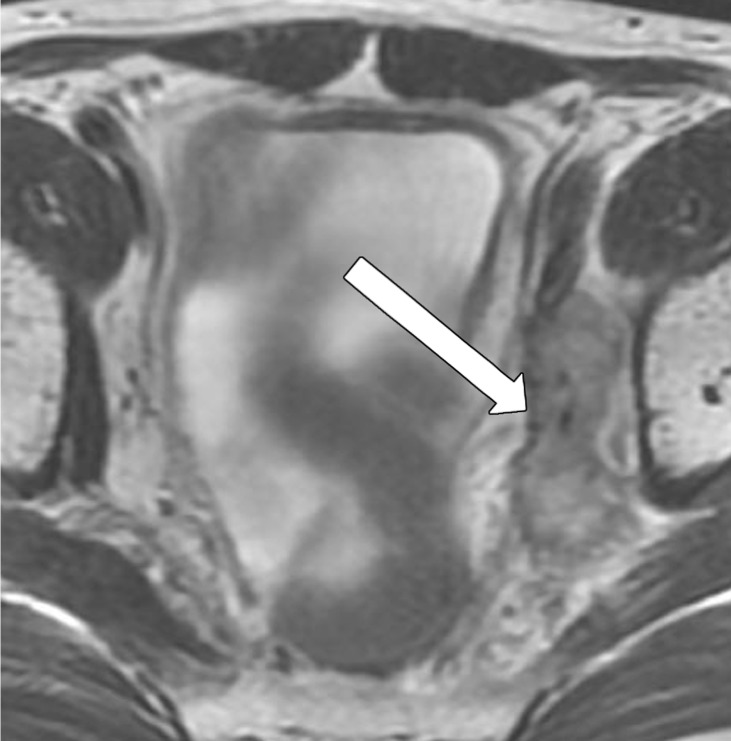
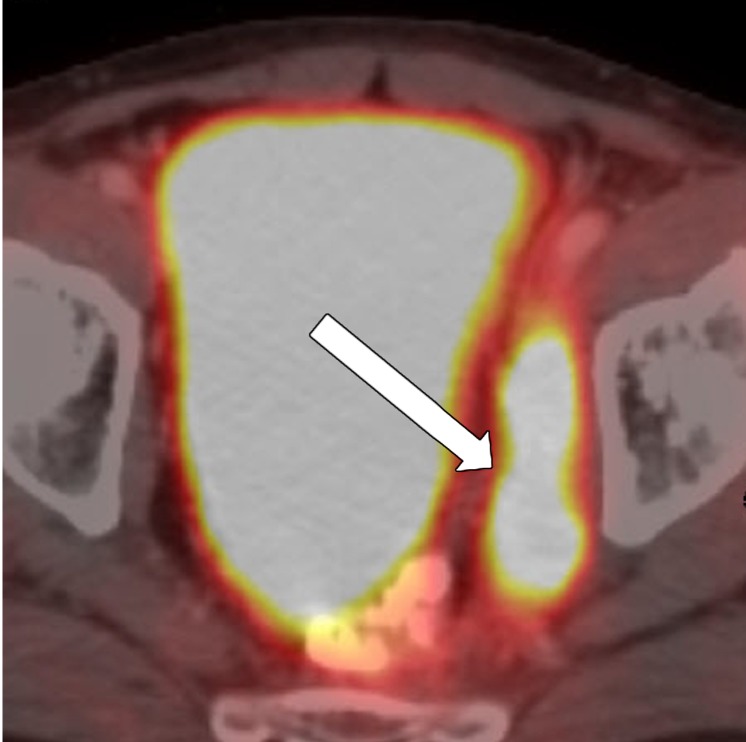
The number of remaining suspicious nodes must be reported at restaging. Many irradiated lymph nodes disappear, and the majority of the remaining nodes are sterilized. After CRT, evaluating nodal size in the short axis is more reliable than evaluating borders and shape to assess for residual malignancy (33). The absence of lymph nodes at DWI, the decrease in size in at least 70% of lymph nodes, and a nodal size less than 2.5 mm in the short axis have been shown to be reliable predictors of negative node status after surgery (Fig 16) (71).
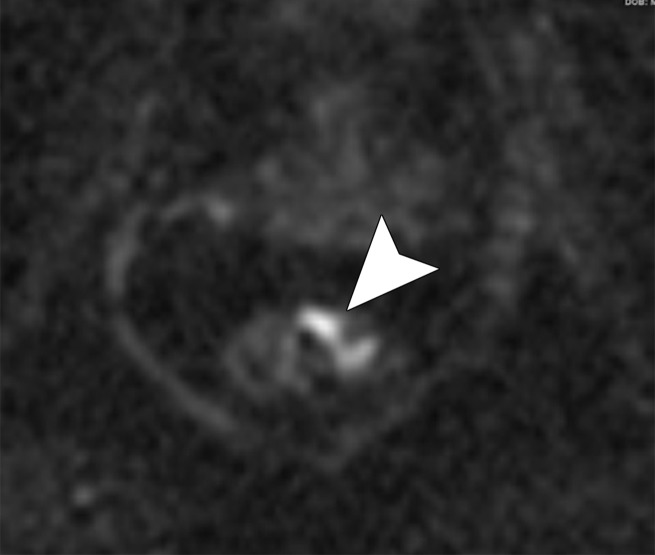
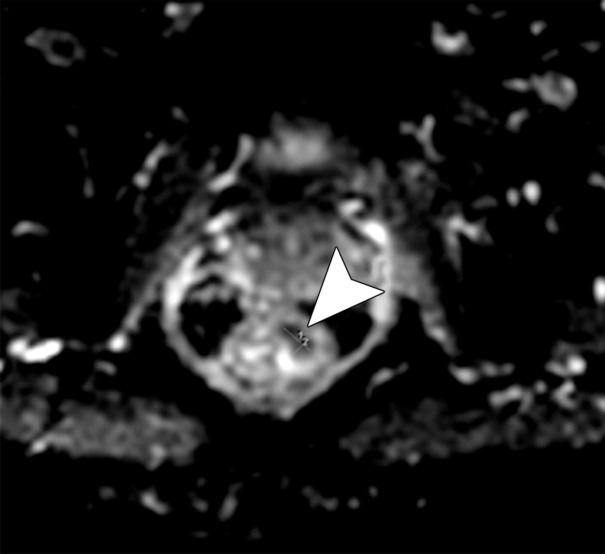
Figure 16a.
No significant change in lymph node size after CRT. (a) Axial T2-weighted MR image obtained during primary staging shows a suspicious left lateral node (arrow). (b) Axial T2-weighted MR image obtained after CRT shows the lymph node (arrow), which did not reduce in size. (c, d) Axial T2-weighted MR image (c) and axial PET/CT image (d) obtained 10 months after TME without lateral pelvic lymph node dissection shows the metastatic node with marked enlargement (arrow in c) and FDG uptake (arrow in d).
Figure 16b.
No significant change in lymph node size after CRT. (a) Axial T2-weighted MR image obtained during primary staging shows a suspicious left lateral node (arrow). (b) Axial T2-weighted MR image obtained after CRT shows the lymph node (arrow), which did not reduce in size. (c, d) Axial T2-weighted MR image (c) and axial PET/CT image (d) obtained 10 months after TME without lateral pelvic lymph node dissection shows the metastatic node with marked enlargement (arrow in c) and FDG uptake (arrow in d).
Figure 16c.
No significant change in lymph node size after CRT. (a) Axial T2-weighted MR image obtained during primary staging shows a suspicious left lateral node (arrow). (b) Axial T2-weighted MR image obtained after CRT shows the lymph node (arrow), which did not reduce in size. (c, d) Axial T2-weighted MR image (c) and axial PET/CT image (d) obtained 10 months after TME without lateral pelvic lymph node dissection shows the metastatic node with marked enlargement (arrow in c) and FDG uptake (arrow in d).
Figure 16d.
No significant change in lymph node size after CRT. (a) Axial T2-weighted MR image obtained during primary staging shows a suspicious left lateral node (arrow). (b) Axial T2-weighted MR image obtained after CRT shows the lymph node (arrow), which did not reduce in size. (c, d) Axial T2-weighted MR image (c) and axial PET/CT image (d) obtained 10 months after TME without lateral pelvic lymph node dissection shows the metastatic node with marked enlargement (arrow in c) and FDG uptake (arrow in d).
Local Recurrence at MRI
Due to the advent of neoadjuvant CRT and improvements in rectal surgery, the prevalence of recurrent rectal cancer began to decline in the past decade, occurring in approximately 4%–8% of patients who underwent surgery performed with a curative intent, especially in the first 3 years after treatment (72). Early diagnosis is fundamental to avoid progression and enable surgical resection (21).
The main risk factors for local recurrence are lack of preoperative radiation therapy, CRM positivity, EMVI, close proximity of the tumor to the anal verge, perforation of the tumor at surgery, anastomotic leak, higher pathologic TNM stage, and lower tumor differentiation (73). Although 30% of patients can be asymptomatic, the majority of patients with local recurrence manifest with symptoms and an increased carcinoembryonic antigen level.
Local recurrence can occur in four locations: (a) axial, with recurrence in the anastomotic, residual mesorectum, or perirectal soft tissue in the center of the pelvis or perineum, including the pelvic floor; (b) anterior, with recurrence in the bladder, vagina, uterus, seminal vesicles, or prostate; (c) posterior, with recurrence in the presacral fascia, sacrum, coccyx, or sacral root sheaths; and (d) lateral, with recurrence in the pelvic ureters, iliac vessels, lateral lymph nodes, pelvic nerves, sidewall muscles, or lateral pelvic bones.
Most local recurrences are anastomotic and therefore are easily identified at clinical evaluation and/or endoscopy. However, other recurrence sites can be difficult to diagnose by these methods. In this context, imaging is an important tool in early diagnosis, especially in asymptomatic patients. The main role of imaging is to verify the extent and precise localization of the disease, as well as the presence of metastases.
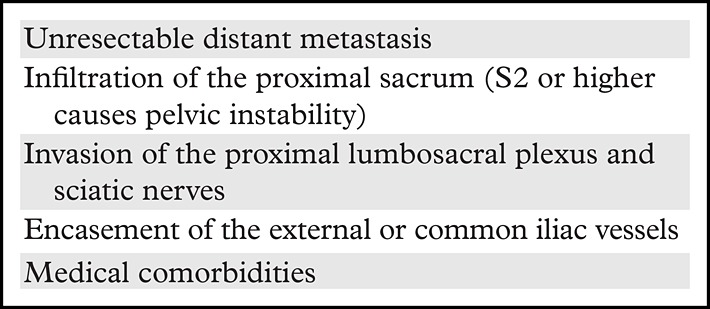
MRI is the most accurate imaging modality for local staging, while CT and PET/CT are more useful in detecting distant recurrence. However, posttreatment (surgery or CRT) changes may be difficult to differentiate from local recurrence, as they can share similar imaging features and may also appear fluorodeoxyglucose avid at PET. An increase in size and early, heterogeneous, marked contrast enhancement, invasive behavior, and asymmetric appearance are suspicious for local recurrence (21,47,74). The contraindications for pelvic exenteration are shown in Table 5 (75,76) and should be described in the radiologic report.
Table 5:
Contraindications for Pelvic Exenteration and Features to Report in Patients with Local Recurrence
Schematic Approach to Reporting Rectal Cancer at MRI
Figure 17 summarizes a schematic step-by-step approach to facilitate comprehension of the key features that should be addressed and described in a rectal cancer MRI report. The key features are also represented by the mnemonic RECTAL CANCER.
Figure 17.
Chart shows a step-by-step approach to imaging and staging rectal cancer. The mnemonic RECTAL CANCER facilitates the comprehension of the key features that should be addressed in a rectal MRI report. Red areas = features to identify and steps to complete at restaging.
Future Directions and Research
Some novel MRI techniques, which are not currently used in routine clinical practice, have been studied to overcome some limitations of MRI in the evaluation of rectal cancer during primary staging and restaging. The main novel techniques that have been studied in the evaluation of response after CRT are DCE MRI, magnetization transfer ratio (MTR), and textural analysis (eg, radiomics). In regard to lymph node assessment, novel contrast material and PET/MRI have been described as possible means of improving the evaluation of nodal status. Furthermore, some of these techniques have been assessed as imaging biomarkers to predict clinical outcomes.
DCE MRI assesses tumor vascularization, which can help in tumor identification and may correlate with the degree of angiogenesis and tumor aggressiveness and may predict response to neoadjuvant CRT. Volume transfer content (Ktrans) is the volume transfer coefficient that reflects vascular permeability of gadolinium from blood into the extravascular extracellular space. Dijkhoff et al (77) published a systematic review revealing that high Ktrans values at primary staging and a decrease in Ktrans values after CRT are significant predictors of response (77).
MTR is a technique that evaluates differences in magnetization interaction between the protons bound to macromolecules and those in free water, which corresponds to the efficiency of this exchange. Tissues that are rich in macromolecules (such as those in collagen that occur in fibrosis) will express a high MTR. Studies have demonstrated the potential of MTR in the assessment of tumor response after CRT (78,79).
Radiomics consists of computer-aided extraction of many quantitative features from large imaging datasets (“big data”) derived from CT, MRI, and PET, with the potential to correlate these image phenotypes that are otherwise not detectable by conventional radiologic human interpretation with disease outcomes (80). In rectal cancer, radiomics using MRI has demonstrated promising pilot results in the prediction of complete response after CRT (81–83) and using PET/CT as a predictor of survival (84).
The use of lymph node MRI contrast material, such as ultrasmall superparamagnetic iron oxide (USPIO) and gadofosveset, is promising for differentiating benign from metastatic lymph nodes, but availability and safety concerns have mostly prevented their use (85,86). USPIO is an iron-based nanoparticle that is taken up by normal cells, decreasing their signal intensity at T2-weighted imaging. Malignant nodes do not take up USPIO particles and have a higher signal intensity relative to that of benign nodes and are enhanced relative to that of normal tissue. Gadofosveset is an albumin-bound gadolinium chelate that is taken up by normal and reactive lymph nodes, which enhance like vessels. Thus, malignant nodes show less enhancement.
PET/MRI has demonstrated higher accuracy in T staging and at least comparable accuracy in N and M staging compared with those of PET/CT owing to the high soft-tissue contrast enhancement depicted at MRI (87). This strength may optimize local and distant staging and potentially improve the diagnostic performance of MRI by adding the functional capabilities of PET. However, studies with larger sample sizes are required to evaluate the importance of this modality in rectal cancer evaluation.
FDG PET/CT and PET/MRI may also be helpful for restaging (67). In a meta-analysis with a total of 538 patients with locally advanced rectal cancer, Rymer et al (88) found that post-CRT PET/CT showed a statistically significant reduction in the maximum standardized uptake value in histopathologic responders and higher response index values. The sensitivities of PET/CT in the prediction of pathologic complete response and identification of distant metastases were 75% and 97%, respectively (89).
Although these techniques are promising and may add value in patient care, further research is still required to provide consistent results and standardized technical parameters before PET/MRI can be implemented into clinical practice for patients with rectal cancer.
Conclusion
Currently, rectal MRI plays a key role in management of patients with rectal cancer in local staging, identifying risk factors for local and distant recurrence to help tailor treatment, and improving patient outcome. A systematic analysis allows for a uniform and reproducible interpretation.
Acknowledgments
Acknowledgments
The authors would like to express their deepest gratitude to Joanne Chin, MFA, for her editorial support and to MSK Design and Creative Services.
N.H. supported by the National Institutes of Health Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Presented as an education exhibit at the 2017 RSNA Annual Meeting.
For this journal-based SA-CME activity, the authors, editor, and reviewers have disclosed no relevant relationships.
Abbreviations:
- CRM
- circumferential resection margin
- CRT
- chemoradiotherapy
- DCE
- dynamic contrast material–enhanced
- DWI
- diffusion-weighted imaging
- EMVI
- extramural vascular invasion
- FSE
- fast spin-echo
- LARC
- locally advanced rectal cancer
- MRF
- mesorectal fascia
- TME
- total mesorectal excision
- 2D
- two-dimensional
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):e359–e386. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society . Cancer facts and figures: 2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf. Accessed May 1, 2018.
- 3.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 2015;150 (1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagtegaal I, Gaspar C, Marijnen C, Van De Velde C, Fodde R, Van Krieken H. Morphological changes in tumour type after radiotherapy are accompanied by changes in gene expression profile but not in clinical behaviour. J Pathol 2004;204(2):183–192. [DOI] [PubMed] [Google Scholar]
- 5.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1(8496): 1479–1482. [DOI] [PubMed] [Google Scholar]
- 6.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991;324(11):709–715. [DOI] [PubMed] [Google Scholar]
- 7.Gastrointestinal Tumor Study Group . Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med 1985;312(23):1465–1472. [DOI] [PubMed] [Google Scholar]
- 8.National Institutes of Health . Adjuvant therapy for patients with colon and rectum cancer. NIH Consensus Statement 1990;8(4):1–25. [PubMed] [Google Scholar]
- 9.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351(17):1731–1740. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network . Clinical practice guidelines in oncology (NCCN Guidelines): colon cancer—version 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Published 2018. Accessed January 30, 2018.
- 11.Glimelius B, Tiret E, Cervantes A, Arnold D; ESMO Guidelines Working Group . Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24(suppl 6):vi81–vi88. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network . NCCN guidelines: version 2.2017—rectal cancer. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Published September 10, 2017. Accessed May 1, 2018.
- 13.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004;240(4):711–717; discussion 717–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11(9):835–844. [DOI] [PubMed] [Google Scholar]
- 15.Påhlman L, Bohe M, Cedermark B, et al. The Swedish rectal cancer registry. Br J Surg 2007;94(10):1285–1292. [DOI] [PubMed] [Google Scholar]
- 16.Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011;29(35):4633–4640. [DOI] [PubMed] [Google Scholar]
- 17.Renehan AG, Malcomson L, Emsley R, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol 2016;17(2):174–183. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Liu H, Yin J, et al. Wait-and-see or radical surgery for rectal cancer patients with a clinical complete response after neoadjuvant chemoradiotherapy: a cohort study. Oncotarget 2015;6(39):42354–42361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011;29(28):3753–3760. [DOI] [PubMed] [Google Scholar]
- 20.Taylor FG, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol 2008;191(6):1827–1835. [DOI] [PubMed] [Google Scholar]
- 21.Sinaei M, Swallow C, Milot L, Moghaddam PA, Smith A, Atri M. Patterns and signal intensity characteristics of pelvic recurrence of rectal cancer at MRI. RadioGraphics 2013;33(5):e171–e187. [DOI] [PubMed] [Google Scholar]
- 22.Glimelius B, Oliveira J; ESMO Guidelines Working Group . Rectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2008;19(suppl 2):ii31–ii32. [DOI] [PubMed] [Google Scholar]
- 23.Willett CG, Compton CC, Shellito PC, Efird JT. Selection factors for local excision or abdominoperineal resection of early stage rectal cancer. Cancer 1994;73(11):2716–2720. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki C, Torkzad MR, Tanaka S, et al. The importance of rectal cancer MRI protocols on interpretation accuracy. World J Surg Oncol 2008;6(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jhaveri KS, Hosseini-Nik H. MRI of rectal cancer: an overview and update on recent advances. AJR Am J Roentgenol 2015;205(1):W42–W55. [DOI] [PubMed] [Google Scholar]
- 26.Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2018;28(4):1465–1475. [Published correction appears in Eur Radiol 2018;28(6):2711.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delli Pizzi A, Basilico R, Cianci R, et al. Rectal cancer MRI: protocols, signs and future perspectives radiologists should consider in everyday clinical practice. Insights Imaging 2018;9(4):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gollub MJ, Arya S, Beets-Tan RG, et al. Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017. Abdom Radiol (NY) 2018 May 21 [Epub ahead of print]. [DOI] [PubMed]
- 29.Kim H, Lim JS, Choi JY, et al. Rectal cancer: comparison of accuracy of local-regional staging with two- and three-dimensional preoperative 3T MRI. Radiology 2010;254(2):485–492. [DOI] [PubMed] [Google Scholar]
- 30.Gowdra Halappa V, Corona Villalobos CP, Bonekamp S, et al. Rectal imaging: part 1—high-resolution MRI of carcinoma of the rectum at 3 T. AJR Am J Roentgenol 2012;199(1):W35–W42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoeffel C, Mulé S, Laurent V, Bouché O, Volet J, Soyer P. Primary rectal cancer local staging. Diagn Interv Imaging 2014;95(5):485–494. [DOI] [PubMed] [Google Scholar]
- 32.Beets-Tan RG, Lambregts DM, Maas M, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2013;23(9):2522–2531. [DOI] [PubMed] [Google Scholar]
- 33.Brown G, Daniels IR, Richardson C, Revell P, Peppercorn D, Bourne M. Techniques and trouble-shooting in high spatial resolution thin slice MRI for rectal cancer. Br J Radiol 2005;78(927):245–251. [DOI] [PubMed] [Google Scholar]
- 34.Okizuka H, Sugimura K, Yoshizako T, Kaji Y, Wada A. Rectal carcinoma: prospective comparison of conventional and gadopentetate dimeglumine enhanced fat-suppressed MRI. J Magn Reson Imaging 1996;6(3):465–471. [DOI] [PubMed] [Google Scholar]
- 35.Slater A, Halligan S, Taylor SA, Marshall M. Distance between the rectal wall and mesorectal fascia measured by MRI: effect of rectal distension and implications for preoperative prediction of a tumour-free circumferential resection margin. Clin Radiol 2006;61(1):65–70. [DOI] [PubMed] [Google Scholar]
- 36.Ye F, Zhang H, Liang X, Ouyang H, Zhao X, Zhou C. Journal club: preoperative MRI evaluation of primary rectal cancer: intrasubject comparison with and without rectal distention. AJR Am J Roentgenol 2016;207(1):32–39. [DOI] [PubMed] [Google Scholar]
- 37.Soyer P, Lagadec M, Sirol M, et al. Free-breathing diffusion-weighted single-shot echo-planar MRI using parallel imaging (GRAPPA 2) and high b value for the detection of primary rectal adenocarcinoma. Cancer Imaging 2010;10(1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Griethuysen JJM, Bus EM, Hauptmann M, et al. Gas-induced susceptibility artefacts on diffusion-weighted MRI of the rectum at 1.5 T: effect of applying a micro-enema to improve image quality. Eur J Radiol 2018;99:131–137. [DOI] [PubMed] [Google Scholar]
- 39.Hausmann D, Liu J, Budjan J, et al. Image quality assessment of 2D versus 3D T2WI and evaluation of ultra-high b-value (b=2,000 mm/s2) DWI for response assessment in rectal cancer. Anticancer Res 2018;38(2):969–978. [DOI] [PubMed] [Google Scholar]
- 40.Maas M, Lambregts DM, Lahaye MJ, et al. T-staging of rectal cancer: accuracy of 3.0 Tesla MRI compared with 1.5 Tesla. Abdom Imaging 2012;37(3):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vliegen RF, Beets GL, von Meyenfeldt MF, et al. Rectal cancer: MRI in local staging—is gadolinium-based contrast material helpful? Radiology 2005;234(1):179–188. [DOI] [PubMed] [Google Scholar]
- 42.Consorti F, Lorenzotti A, Midiri G, Di Paola M. Prognostic significance of mucinous carcinoma of colon and rectum: a prospective case-control study. J Surg Oncol 2000;73(2):70–74. [DOI] [PubMed] [Google Scholar]
- 43.Al-Sukhni E, Milot L, Fruitman M, et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol 2012;19(7):2212–2223. [DOI] [PubMed] [Google Scholar]
- 44.Salerno G, Daniels I, Heald RJ, Brown G, Moran BJ. Management and imaging of low rectal carcinoma. Surg Oncol 2004;13(2-3):55–61. [DOI] [PubMed] [Google Scholar]
- 45.Shihab OC, Moran BJ, Heald RJ, Quirke P, Brown G. MRI staging of low rectal cancer. Eur Radiol 2009;19(3):643–650. [DOI] [PubMed] [Google Scholar]
- 46.Costa-Silva L, Brown G. Magnetic resonance imaging of rectal cancer. Magn Reson Imaging Clin N Am 2013;21(2):385–408. [DOI] [PubMed] [Google Scholar]
- 47.Furey E, Jhaveri KS. Magnetic resonance imaging in rectal cancer. Magn Reson Imaging Clin N Am 2014;22(2):165–190, v–vi. [DOI] [PubMed] [Google Scholar]
- 48.Beets-Tan RG, Beets GL, Vliegen RF, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet 2001;357(9255):497–504. [DOI] [PubMed] [Google Scholar]
- 49.Shihab OC, Brown G, Daniels IR, Heald RJ, Quirke P, Moran BJ. Patients with low rectal cancer treated by abdominoperineal excision have worse tumors and higher involved margin rates compared with patients treated by anterior resection. Dis Colon Rectum 2010;53(1):53–56. [DOI] [PubMed] [Google Scholar]
- 50.Taylor FG, Quirke P, Heald RJ, et al. One millimetre is the safe cut-off for magnetic resonance imaging prediction of surgical margin status in rectal cancer. Br J Surg 2011;98(6):872–879. [DOI] [PubMed] [Google Scholar]
- 51.Oberholzer K, Junginger T, Heintz A, et al. Rectal cancer: MRI of the mesorectal fascia and effect of chemoradiation on assessment of tumor involvement. J Magn Reson Imaging 2012;36(3):658–663. [DOI] [PubMed] [Google Scholar]
- 52.Hermanek P, Junginger T. The circumferential resection margin in rectal carcinoma surgery. Tech Coloproctol 2005;9(3):193–199; discussion 199–200. [DOI] [PubMed] [Google Scholar]
- 53.Taylor FG, Quirke P, Heald RJ, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg 2011;253(4):711–719. [DOI] [PubMed] [Google Scholar]
- 54.Horn A, Dahl O, Morild I. Venous and neural invasion as predictors of recurrence in rectal adenocarcinoma. Dis Colon Rectum 1991;34(9):798–804. [DOI] [PubMed] [Google Scholar]
- 55.Bugg WG, Andreou AK, Biswas D, Toms AP, Williams SM. The prognostic significance of MRI-detected extramural venous invasion in rectal carcinoma. Clin Radiol 2014;69(6):619–623. [DOI] [PubMed] [Google Scholar]
- 56.Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg 2008;95(2):229–236. [DOI] [PubMed] [Google Scholar]
- 57.Brown G, Richards CJ, Bourne MW, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MRI with histopathologic comparison. Radiology 2003;227(2):371–377. [DOI] [PubMed] [Google Scholar]
- 58.Kotanagi H, Fukuoka T, Shibata Y, et al. The size of regional lymph nodes does not correlate with the presence or absence of metastasis in lymph nodes in rectal cancer. J Surg Oncol 1993;54(4):252–254. [DOI] [PubMed] [Google Scholar]
- 59.Kim JH, Beets GL, Kim MJ, Kessels AGH, Beets-Tan RGH. High-resolution MRI for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol 2004;52(1):78–83. [DOI] [PubMed] [Google Scholar]
- 60.Beets-Tan RG. Pretreatment MRI of lymph nodes in rectal cancer: an opinion-based review. Colorectal Dis 2013;15(7):781–784. [DOI] [PubMed] [Google Scholar]
- 61.Kaur H, Choi H, You YN, et al. MRI for preoperative evaluation of primary rectal cancer: practical considerations. RadioGraphics 2012;32(2):389–409. [DOI] [PubMed] [Google Scholar]
- 62.Sugihara K, Kobayashi H, Kato T, et al. Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum 2006;49(11):1663–1672. [DOI] [PubMed] [Google Scholar]
- 63.Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol 2008;15(10):2661–2667. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Aguilar J, Marcet J, Coutsoftides T, et al. Impact of neoadjuvant chemotherapy following chemoradiation on tumor response, adverse events, and surgical complications in patients with advanced rectal cancer treated with TME. J Clin Oncol 2011;29(15 suppl):3514. [Google Scholar]
- 65.Duldulao MP, Lee W, Streja L, et al. Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer. Dis Colon Rectum 2013;56(2):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel UB, Blomqvist LK, Taylor F, et al. MRI after treatment of locally advanced rectal cancer: how to report tumor response—the MERCURY experience. AJR Am J Roentgenol 2012;199(4):W486–W495. [DOI] [PubMed] [Google Scholar]
- 67.Shia J, McManus M, Guillem JG, et al. Significance of acellular mucin pools in rectal carcinoma after neoadjuvant chemoradiotherapy. Am J Surg Pathol 2011;35(1):127–134. [DOI] [PubMed] [Google Scholar]
- 68.van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MRI: a systematic review and meta-analysis. Radiology 2013;269(1):101–112. [DOI] [PubMed] [Google Scholar]
- 69.Maas M, Lambregts DM, Nelemans PJ, et al. Assessment of clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for organ-saving treatment. Ann Surg Oncol 2015;22(12):3873–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen CC, Lee RC, Lin JK, Wang LW, Yang SH. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum 2005;48(4):722–728. [DOI] [PubMed] [Google Scholar]
- 71.van Heeswijk MM, Lambregts DMJ, Palm WM, et al. DWI for assessment of rectal cancer nodes after chemoradiotherapy: is the absence of nodes at DWI proof of a negative nodal status? AJR Am J Roentgenol 2017;208(3):W79–W84. [DOI] [PubMed] [Google Scholar]
- 72.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345(9):638–646. [DOI] [PubMed] [Google Scholar]
- 73.Jörgren F, Johansson R, Damber L, Lindmark G. Risk factors of rectal cancer local recurrence: population-based survey and validation of the Swedish rectal cancer registry. Colorectal Dis 2010;12(10):977–986. [DOI] [PubMed] [Google Scholar]
- 74.Torkzad MR, Kamel I, Halappa VG, Beets-Tan RG. Magnetic resonance imaging of rectal and anal cancer. Magn Reson Imaging Clin N Am 2014;22(1):85–112. [DOI] [PubMed] [Google Scholar]
- 75.Moore HG, Shoup M, Riedel E, et al. Colorectal cancer pelvic recurrences: determinants of resectability. Dis Colon Rectum 2004;47(10):1599–1606. [DOI] [PubMed] [Google Scholar]
- 76.Sagebiel TL, Viswanathan C, Patnana M, Devine CE, Frumovitz M, Bhosale PR. Overview of the role of imaging in pelvic exenteration. RadioGraphics 2015;35(4):1286–1294. [DOI] [PubMed] [Google Scholar]
- 77.Dijkhoff RAP, Beets-Tan RGH, Lambregts DMJ, Beets GL, Maas M. Value of DCE-MRI for staging and response evaluation in rectal cancer: a systematic review. Eur J Radiol 2017;95:155–168. [DOI] [PubMed] [Google Scholar]
- 78.Martens MH, Lambregts DM, Papanikolaou N, et al. Magnetization transfer imaging to assess tumour response after chemoradiotherapy in rectal cancer. Eur Radiol 2016;26(2):390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martens MH, Lambregts DM, Papanikolaou N, et al. Magnetization transfer ratio: a potential biomarker for the assessment of postradiation fibrosis in patients with rectal cancer. Invest Radiol 2014;49(1):29–34. [DOI] [PubMed] [Google Scholar]
- 80.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016;278(2):563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horvat N, Veeraraghavan H, Khan M, et al. MRI of rectal cancer: radiomics analysis to assess treatment response after neoadjuvant therapy. Radiology 2018;287(3):833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cusumano D, Dinapoli N, Boldrini L, et al. Fractal-based radiomic approach to predict complete pathological response after chemo-radiotherapy in rectal cancer. Radiol Med (Torino) 2018;123(4):286–295. [DOI] [PubMed] [Google Scholar]
- 83.Liu Z, Zhang XY, Shi YJ, et al. Radiomics analysis for evaluation of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Clin Cancer Res 2017;23(23):7253–7262. [DOI] [PubMed] [Google Scholar]
- 84.Lovinfosse P, Polus M, Van Daele D, et al. FDG PET/CT radiomics for predicting the outcome of locally advanced rectal cancer. Eur J Nucl Med Mol Imaging 2018;45(3):365–375. [DOI] [PubMed] [Google Scholar]
- 85.Choi SH, Moon WK. Contrast-enhanced MRI of lymph nodes in cancer patients. Korean J Radiol 2010;11(4):383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lambregts DM, Heijnen LA, Maas M, et al. Gadofosveset-enhanced MRI for the assessment of rectal cancer lymph nodes: predictive criteria. Abdom Imaging 2013;38(4):720–727. [DOI] [PubMed] [Google Scholar]
- 87.Paspulati RM, Partovi S, Herrmann KA, Krishnamurthi S, Delaney CP, Nguyen NC. Comparison of hybrid FDG PET/MRI compared with PET/CT in colorectal cancer staging and restaging: a pilot study. Abdom Imaging 2015;40(6):1415–1425. [DOI] [PubMed] [Google Scholar]
- 88.Rymer B, Curtis NJ, Siddiqui MR, Chand M. FDG PET/CT can assess the response of locally advanced rectal cancer to neoadjuvant chemoradiotherapy: evidence from meta-analysis and systematic review. Clin Nucl Med 2016;41(5):371–375. [DOI] [PubMed] [Google Scholar]
- 89.Cho YB, Chun HK, Kim MJ, et al. Accuracy of MRI and 18F-FDG PET/CT for restaging after preoperative concurrent chemoradiotherapy for rectal cancer. World J Surg 2009;33(12):2688–2694. [DOI] [PubMed] [Google Scholar]