Abstract
Background
Many liver transplantation programs require documented alcohol sobriety prior to United Organ Network Sharing (UNOS) listing. This pilot study examined the feasibility of the first mobile, alcohol relapse-prevention intervention for liver transplant patients with alcoholic liver disease (ALD).
Method
Randomized 8-week pilot feasibility trial of a text message-based alcohol intervention. In-treatment assessment was conducted at 4-weeks (4W) and immediate post-treatment assessment was conducted at 8-weeks (8W). Participants were liver transplant candidates (N = 15) diagnosed with ALD who reported at least one drinking episode in the past year. Primary feasibility outcomes were percent of messages responded to and post-treatment intervention satisfaction ratings. Preliminary clinical efficacy outcomes were any biologically confirmed alcohol consumption, stress, abstinence self-efficacy, and alcohol craving.
Results
On feasibility outcomes, participants responded to 81% of messages received and reported high rates of intervention satisfaction, looked forward to receiving the messages, and found it easy to complete the intervention. On preliminary efficacy outcomes, zero participants in the text message (TM) had positive urine alcohol tests at 8W. 2 of the 6 participants in standard care (SC) tested positive at 8W. No effects were seen on craving. For stress, a condition × time interaction emerged. TM participants had less stress at 4W and 8W compared to SC at baseline. They maintained their stress level during the intervention. For self-efficacy, a trend for condition effect emerged. TM participants had higher self-efficacy than SC participants.
Conclusion
Participants reported high satisfaction with the intervention, looked forward to the messages, and found it easy to complete. Participants who received the intervention had better treatment outcomes than those who received standard care. They maintained higher levels of self-efficacy and lower stress. Mobile alcohol interventions may hold significant promise to help ALD liver transplant patients maintain sobriety.
Keywords: liver transplant, mobile intervention, alcohol, alcoholic liver disease
In the United States, chronic liver disease leads to over 2 million outpatient physician visits and over 750,000 hospitalizations per year (Kim, 2002; Larson et al., 2006). Each year, over 40,000 patients progress to end-stage liver disease (ESLD), liver failure and death. Liver transplantation is the only definitive treatment for the complications of cirrhosis and liver failure (Alquahtani & Larson, 2011). However, approximately 5-10% of patients listed by the United Network of Organ Sharing will die without receiving an organ (Alquahtani & Larson, 2011).
Alcohol-related liver disease (ALD) alone accounts for nearly 30% of all liver transplants (Belle et al., 1997; Organ Procurement and Transplantation Network, 2010). Most commonly, ALD develops from 10-20 years of heavy, sustained drinking, yet the level is variable and not all meet criteria for alcohol dependence (DiMartini et al., 2001).
Up to 50% of liver transplantation patients with alcoholism return to drinking within 5 years (DiMartini et al., 2006). Survival rates of patients who resume excessive drinking post-transplant are significantly lower than those of abstinent patients or patients who have a minor lapse post-transplant (Pfitzmann et al., 2007). Abstinence duration of less than 6-months pre-transplant best predicts post-transplant alcohol outcomes (see DiMartini et al., 2010 & Miguet et al., 2004). Consequently, most transplant centers require a minimum of 6 months of alcohol abstinence prior to putting candidates on the active UNOS waiting list. Exceptions to this rule can be made, however, highlighting the urgent need to develop interventions that can reduce relapse to alcohol in liver transplant candidates.
Because of their hepatotoxicity and concerns about prescribing an additional medication for patients already burdended with polypharmacy, pharmacotherapies for alcohol dependence are very rarely utilized in this population. Thus, the development of novel behavioral interventions is particularly important. Prior small-scale studies have shown that psychosocial behavioral interventions can be integrated into liver clinics, are acceptable to patients (Georgiou et al., 2003), and can reduce drinking (Weinrieb et al., 2011). Yet, liver transplant patients face particular and unique challenges that make traditional substance use treatment difficult and necessitate consideration of novel treatment modalities.
Harnessing Mobile Technology for Behavioral Alcohol Interventions
Mobile technologies are ubiquitous and utilization of short-message service (SMS: e.g., text message) is exceptionally high (Center, 2014; CTIA, 2014). SMS consists of near instant delivery of short messages (160 character maximum). Because mobile phone usage is particularly high in socioeconomically disadvantaged populations (Faulkner & Culwin, 2005), SMS is an optimal and promising intervention strategy for patients in poorer health and socioeconomically disadvantaged, including liver transplantation patients.
Review studies have found support for the use of SMS-based interventions for disease prevention (e.g., weight loss, smoking cessation, exercise) and disease management (e.g., diabetes and asthma management) (Cole-Lewis & Kershaw, 2010; Fjeldsoe, Marshall, & Miller, 2009). These SMS interventions have been effectively implemented without any additional treatment support (Weitzel et al., 2007; Whiitaker et al., 2008). Compared to the development of mobile-health (mHealth) interventions for these health conditions, the development of SMS-based interventions for alcohol use is relatively nascent. One large-scale study (n=765) assessed the effectiveness of an SMS intervention for hazardous drinking young adults who presented to the emergency department (Suffoletto et al., 2014). Results indicated that young adults that received the stand-alone SMS intervention decreased binge drinking days and drinks per drinking day from baseline to 3-months post-intervention. The authors concluded that the utilization of this treatment modality could allow for better dissemination of an alcohol intervention in a particularly busy treatment setting. Another small-scale trial of a stand-alone SMS intervention examined treatment outcomes in patients with dual diagnoses of major depression and alcohol dependence (Agyapong, Ahern, McLoughlin, & Farren, 2012). Results indicated that patients who received the SMS intervention, compared to receipt of a fornightly thank you SMS message, had lower depression scores and a trend for greater cumulative alcohol abstinence days at the end of the 3-month intervention. Yet another stand-alone SMS intervention for alcohol use in young adults found that participants in the intervention condition drank fewer drinks per drinking day (Weitzel, Bernhardt, Mays, & Glanz, 2007). Thus, stand-alone SMS interventions have been found to be efficacious for reducing alcohol consumption in young adults, and promising results have been found in dual-diagnosis patients with alcohol dependence. No study has examined the use of an SMS-based alcohol intervention for liver transplantation patients.
SMS-based interventions also have promise to overcome some of the significant and particular burdens faced by liver transplantation patients. Because SMS messages can reach patients beyond a fixed clinic location, they have shown particular promise for chronically ill patients (Wei et al., 2011). Given that ALD patients have a high medical treatment burden, mobile technology may be able to provide additional treatment for patients with limited ability to attend in-person appointments. The role of being a transplant patient is often accompanied by significant stress and frequently requires adjustment to major losses across life domains (e.g., physical, social, etc.) (Olbrisch, Benedict, Ashe, & Levenson, 2002). It can pose sudden and unexpected challenges (e.g., financial), and patients can face years of medical testing, procedures, and evaluation while waiting for a organ to be available (Olbrisch et al., 2002). As a result of these significant stressors, it has been found that 41% of all transplant recipients have poor psychosocial outcomes, characterized by increased psychological stress, anxiety and depression, and that these outcomes can last for up to two years post-transplant (Goetzmann et al., 2008). Evidence suggests that patients with stronger coping skills to deal with this stress have better short and long-term adjustment (Tix & Frazier, 1998). Because of the high level of stress and anxiety in organ transplant populations, the literature consistently emphasizes the importance of the development of skills to build self-control and solve the problems posed by the transplantation process, which can reduce the overall stress experienced by a transplantation patient (Levenson & Olbrisch, 2000; Reid, 1990). Because SMS-interventions occur in a patient’s natural environment, they can provide real-time support in the real world and at specific moments (Heron & Smyth, 2010) and could provide a source of stress reduction. For a population with high medical treatment burden, mobile technology may be able to provide additional treatment for patients with limited ability to attend in-person appointments.
Given the urgent need to reduce alcohol use in liver transplantation candidates and the simultaneous need to develop interventions that do not add to treatment burden, the goal of this pilot study was to develop a mobile, SMS-based intervention to reduce the incidence of alcohol relapse and decrease stress in pre-liver transplant ALD patients trying to achieve the mandatory 6-months of abstinence for Transplantation Center listing. As no other study to-date has developed a mobile intervention for this population, we also sought to evaluate the feasibility, acceptability, and perceived helpfulness of an SMS-based intervention for ALD patients. To assess feasibility and acceptability, we sought to evaluate patterns of usage (e.g., percent of messages responded) and end of treatment satisfaction. To assess the effect of the intervention, we sought to compare differences in biologically confirmed, objective alcohol consumption (e.g., ethyl glucuronide (EtG)) and subjective characteristics of alcohol consumption (e.g., drinking self reports, craving, and stress) between patients that received the text message intervention and those that received standard care in the liver transplantation clinic.
Method
Study Design
This pilot study compared the feasibility and initial efficacy of an 8-week text message, alcohol relapse prevention intervention with standard care in pre-liver transplantation candidates with ALD in evaluation for addition to the transplant wait list. We assessed patient satisfaction with the text message intervention and preliminary impact of the intervention on drinking behavior, craving, and stress during treatment.
A convenience sample of participants was recruited during their initial visit with the liver transplantation team (LTT) at the Yale-New Haven Hospital Transplantation Center in New Haven, CT. Each week, physicians and social workers of the LTT met to review new cases being referred to the transplantation clinic and identified those patients who had consumed alcohol in the past year. Those patients were identified as being likely eligible and were referred to our study Research Assistant. At the patient’s next scheduled medical appointment, our study research assistant would meet with him/her to discuss participation in the trial and obtain participant consent. The study statistician created a randomization list to assign participants to the 8-week Text Message (TM) condition or the Standard Care (SC) condition. Condition assignments were placed in individual envelopes that were only opened by the study research assistant upon participant enrollment. The study PI and all other personnel were blinded to condition. All participants met with LTT psychologists or psychology fellows for individual psychotherapy. All participants completed self-report assessments and provided urine EtG prior to intervention at baseline (BL), 4 weeks (4W; mid-treatment), and 8 weeks (8W; immediate post-treatment). TM participants completed treatment satisfaction ratings at 8W. Enrollment in the trial occurred between March 2013 and December 2013. Follow-up assessments were completed by the end of March 2014. This trial was funded by pilot funds from the Department of Psychological Medicine at Yale-New Haven Hospital. Pilot funds for the trial were available for 1 year. Written informed consent was obtained from all participants. Participants were all informed that their volunatary participation in the trial would have no impact on their UNOS listing eligibility (see additional details under description of EtG methodology). The study protocol was approved by the Human Investigation Committee of Yale Medical School.
Participants
Treatment participants were 15 liver transplantation candidates. Participants were eligible for the pilot trial if they: (1) reported ≥ 1 drinking episode in the previous calendar year; (2) were diagnosed with ALD; (3) were in the evaluation phase for liver transplantation listing. Participants were excluded if they had unstable psychiatric conditions, including active psychosis and suicidal ideation.
Of the 15 patients who were randomized to treatment, 8 were assigned to the TM condition and 7 were assigned to the SC condition. One SC patient died shortly after baseline, leaving 6 participants eligible to complete assessments at 4W and 8W. Of those who initiated treatment, 11 (73%; 6 TM patients and 5 SC patients) patients completed all assessments at 4W, and 14 (93%; 8 TM patients and 6 SC patients) patients completed assessments at 8W.
Interventions
Standard Care
All aspects of care received by SC participants were also provided to the TM condition participants. Medical care was managed by medical specialty providers. Patients in the Yale Liver Transplantation Clinic who are identified by the LTT as needing assistance to achieve the Center-required minimum of 6 months of sobriety pre-listing receive psychological addiction counseling by behavioral health clinicians (licensed clinical psychologists/psychiatrists and doctoral-level clinical psychology interns) within the liver transplantation clinic. While patients are permitted to elect to participate in addictions treatment outside the transplantation center, patients who need weekly therapy or have significant medical comorbidities that make participation in structured external treatment difficult elect to participate in treatment within the transplantation center. The level of treatment (i.e., number of weekly/biweekly sessions, content of sessions) is determined on the individual patient level by his/her provider. Patients who are identified as needing a higher level of care (e.g., inpatient detoxification or intensive outpatient (IOP) treatment) complete this treatment prior to beginning outpatient therapy in the LTT. No outside addiction counseling (e.g., participation in Alcoholic’s Anonymous) is required by the clinic.
Text Messaging
In addition to the SC interventions, TM condition participants received SMS messages. As no trials of SMS messaging exist with this population, the intervention message schedule was based on a large-scale, randomized controlled trial of an SMS intervention for smoking cessation (Free et al, 2001) and a smaller study on a SMS intervention for reducing drinking in college students (Weitzel et al., 2007). Participants received 3 text messages per day for the first four weeks of the study and 3 messages per week for the last four weeks.
The content of the intervention was developed prior to the commencement of the trial. Key domains of message topics were chosen based on the content of evidence-based, relapse prevention treatment, particularly the Marlatt and Gordon relapse prevention model (1985). Messages included motivational content and tailored behavior-change content. Four key domains were addressed in the messages: (1) identification of cravings; (2) mood; (3) identification of high-risk situations; (4) coping strategies, including drink refusal skills. A fifth domain, termed the “general” domain, included messages on exercise, diet, and overall health. The lead author drafted an initial text message bank of 180 messages. Written feedback was elicited from the entire LTT, including surgeons, hepatologists, psychologists, psychiatrists, social workers, and research assistants. Critically, consensus from experts indicated concerns that most liver transplant patients do not understand the concept of alcohol craving, do not understand how to refuse drinks, and have a difficult time understanding that the association of abstinence and health is not immediate. As a result of this feedback, updates to the message bank were completed. An additional domain was added to include messages on trigger identification (e.g., “Triggers are anything that was associated with drinking. People you drank with, places you drank, etc. Write down what used to be associated with drinking”). Messages were added that included specific drink refusal statements. We also updated the schedule of message delivery. For the first two weeks of the intervention, participants would receive messages only from the trigger identification and the general domains. For the second two weeks, they would also receive messages from the other domains. Messages in the second month of the study would continue to be from the general domain. The final message bank included 219 messages.
For the four weeks, the first message of the day was an assessment message that assessed participants’ status that morning (e.g., “Do you think it will be hard to remain sober? Reply with HARD, SO (i.e., so-so), EASY” or “When you are craving alcohol, wait 15 minutes. Cravings always pass. How bad are your cravings today? Reply: HI, MED, or LOW”). Participants texted back their response and would receive a tailored intervention message response. For example, if a participant responded to the question above with “HARD,” he/she would receive the following response, “We know it’s hard. It’s a decision you will not regret. Keep getting the support you need. Remind yourself of your sober reasons.” The third message of the day was a content message (i.e., not an assessment message) from one of the content domains. During the second 4 weeks of the trial, all messages were content messages. For all 8 weeks, participants were asked to respond to text messages with a “1” to indicate that the message was read. Example text messages are presented in Table 1.
Table 1.
Examples of Text Messages from the Text Message Alcohol Intervention
| Message Category and Participant Responses | Example Messages |
|---|---|
| FIRST STATUS | Do you think it will be hard to remain sober? Reply with: HARD, SO, EASY |
| HARD | We know it’s hard. It’s a decision you will not regret. Keep getting the support you need. Remind yourself of your sober reasons. |
| SO | Do one day at a time and practice avoiding your triggers. You can do it! |
| EASY | You are on the right track. Quitting drinking is hard but stay confident. You can do this! |
| CRAVING IDENTIFICATION | Triggers are anything that was associated with drinking. People you drank with, places you drank in, etc. Write down what used to be associated with drinking. |
| Triggers are individualized. Is there a particular drink you always had? Emotions you felt when/before you drank? Think about your drinking situations. | |
| By identifying triggers, you can better avoid them. Triggers can be anything that was associated with drinking – places, people, emotions, things. | |
| When you drank, you connected drinking with people, places, and things. These can become triggers for cravings. What are your people, places and things? | |
| MOOD 1 | Feeling down/angry? Negative emotions can trigger cravings. Do a pleasant activity to increase mood. Reply with your mood: GOOD, OK, BAD |
| GOOD | Glad you are feeling good! Keep up your positive attitude and staying strong. |
| OK | Hang in there! It isnt easy to stay sober but it is worth it. |
| BAD | We know staying sober is hard but stay strong! We all have bad days. You will get through this. Do something to boost your mood – just don’t drink. |
Note. Bolded/italicized text represents message category and are not seen by participants. Responses (e.g., HARD, OK, BAD) are responses to the first prompt of the category. Messages in the same row as the responses are messages sent in reply to participants’ answers.
All participants received a mobile phone to use while they were enrolled in the study. Specifically, they received the LG enV2, which is designed with large external keys and a QWERTY keyboard. Participants also received a handout with pictures of the phone screen and written instructions on how to read and send text messages. The study research assistant reviewed this and practiced at baseline to ensure understanding.
All messages were sent by the study research assistant using Google Voice Techology. All messages were sent by the study research assistant using Google Voice technology. Participants were informed that they were receiving messages from and sending messages to an automated system. Voice and data were disabled on the study phones to minimize use of the phones for purposes unrelated to the study.
Several measures were taken to specifically protect participants’ privacy during their participation in this technology-based intervention. The phones purchased for the study were selected in cooperation with Yale’s Informational Technology Services (ITS) department and met Yale University standards for technological security. Text messages contained no personal information, and all phones were set-up with participant numbers rather than names. This ensured that no personal health transmission (PHI or ePHI) was transmitted, stored, or received via text message.
Measures
Alcohol Use History
At baseline, alcohol use histories were obtained via self-report items on age at first drink, average drinks per drinking day in the past 12 months, lifetime maximum drinks consumed, and lifetime alcohol-related hospital admissions.
Urine Ethyl Glucuronide (EtG)
Voluntary urine samples for measurement of EtG, a minor metabolite of ethanol that can be used to document recent drinking (Jatlow et al., 2014), were obtained at each appointment. As required by the Human Investigation Committee, participants were assured that EtG test results and all study assessments would not be included in their medical chart and would not be communicated to members of the transplant team, thus ensuring that study participation did not impact placement on the active wait list for liver transplant. Urine specimens were stored at −70C until measurement of EtG by High Performance Liquid Chromatography coupled with Mass Spectrometery with deuterated internal standards, as adapted from published procedures (Weinmann et al., 2004). Values that exceeded 500 μg/l were considered positive for recent alcohol consumption. EtG provides a biologically confirmed methodology for the assessment of recent alcohol consumption, compared to self-reported alcohol use which have been shown to be inaccurate in liver transplantation samples (Erim et al., 2007). EtG analysis has been shown to detect significantly more instances of drinking in this population and therefore is used as the primary outcome for this pilot study.
Breathalyzer
Prior to all appointments (e.g., BL, 4W, 8W), all participants were screened using an alcohol breathalyzer. Positive values were coded as “1.” Negative readings were coded as “0.”
Timeline Follow-Back (TLFB)
The Timeline Follow-Back Interview (TLFB; Sobell & Sobell, 2000) assessed self-reported daily alcohol consumption. It was completed at baseline for the preceding 30 days. At each subsequent visit (4W, 8W), the TLFB assessed drinking since the prior appointment. Due to the low frequency of drinking, TLFB data were dichotomized into: (1) any drinking at baseline and (2) any drinking during treatment.
Alcohol Use Self-Efficacy
Alcohol use self-efficacy was assessed with a single, self-report item. Participants rated on a scale of 1 (not at all confident) to 10 (extremely confident) how confident they were that they would remain abstinent from alcohol for the next 30 days. Single item measures are predictive of relapse up to 6 months post-treatment discharge (Hoeppner et al., 2011).
Obsessive Compulsive Drinking Scale (OCDS)
The Obsessive Compulsive Drinking Scale (OCDS: Anton et al., 1995) is a 12-item self-report scale designed to assess obsessionality and compulsivity related to craving and drinking behavior. It has is sensitive to, and specific of, the obsessive and compulsive characteristics of urges to drink and the ability to resist those urges. The OCDS is scored by summing all items; total range is 0-48. Mean total score in a sample of newly admitted patients with alcohol dependence was 20.6 (SD = 7.3; Anton et al., 1995). Cronbach’s alpha in the current sample was 0.96.
Perceived Stress Scale (PSS)
The Perceived Stress Scale (PSS; Cohen et al., 1983) is a 4-item self-report scale that assesses thoughts and feelings during the previous 30 days. The scale is scored on a 0 (never) to 4 (very often) Likert scale. Summary scores are obtained by reverse-scoring the positively stated items and then creating a sum score. Summary scores range from 0-16, with higher scores indicating higher degrees of stress. The measure does not have clinical cut-off scores.
Intervention Satisfaction
An investigator-generated form assessed overall satisfaction with the text message intervention. Participants were asked to rate how much they enjoyed getting the messages, the degree to which they found the messages helpful for staying abstinent and for coping with urges. Participants also rated the frequency of the messages (1 = 3 texts per week was too many, 2 = 3 texts per week was appropriate, 3 = 3 texts per week was too few) and how satisfied (1 = not at all, 5 = extremely) they were with the intervention.
Statistical Analyses
Feasibility
Outcome variables for feasibility were established a priori. Feasibility was assessed by (1) examining the number of text messages to which participants responded and (2) assessing mean ratings on intervention satisfaction (e.g., ratings of helpfulness, ease of use, and satisfaction) as assessed on the end-of-treatment satisfaction survey.
Preliminary Intervention Efficacy
All outcome variables were specified a priori. The primary intervention alcohol efficacy variable, established a priori, was biologically confirmed rates of abstinence from alcohol (e.g., EtG). Simple count assessments were used to compare the two conditions on rates of positive EtG results. Secondary alcohol efficacy variables were: (1) self-reports of any drinking days, alcohol craving, and alcohol self-efficacy. Finally, the third specific aim of the study was to assess initial efficacy of the intervention on stress reduction. The primary intervention stress outcome variable was self-reported perceived stress.
Outcome analyses fit generalized estimating equations (GEE) for each variable. Models used all available data on subjects and parameter estimates were obtained using maximum likelihood estimation. All models included main effects of Time and Treatment and a Treatment × Time interaction term. GEE models are less sensitive to covariance structure than generalized linear mixed models and provide consistent parameter estimates even when covariance structure is misspecified. Given the small sample size and preliminary nature of the study, GEE models were utilized. All statistical analyses were conducted in SPSS, version 21 (IBM Corporation).
Results
Participant Characteristics
Table 2 presents participant demographics. Participants reported a mean age of 50.80 years (SD=7.86). The majority were male (n=11, 73%), Caucasian (n=14, 93%), and married (n=9, 60%). Most participants reported a highest education level of high school or less (n=10; 67%) and were unemployed or disabled (n=10; 67%). There were no significant demographic differences between the conditions.
Table 2.
Demographic Information and Baseline Alcohol Use Data for Whole Sample (N = 15) and by Text Message Condition
| Whole Sample (N = 15) |
TM Condition (n = 8) |
SC Condition (n = 7) |
|
|---|---|---|---|
| Demographics | |||
| Age (M, SD) | 50.80 (7.86) | 49.88 (6.71) | 51.86 (9.44) |
| Gender (n, % male) | 11 (73%) | 6 (75%) | 5 (72%) |
| Race (n, % Caucasian) | 14 (93%) | 8 (100%) | 6 (86%) |
| Marital Status | |||
| Single | 2 (13%) | 0 (0%) | 2 (29%) |
| Married | 9 (60%) | 5 (63%) | 4 (57%) |
| Sep/Divorced | 4 (27%) | 3 (37%) | 1 (14%) |
| Highest Education | |||
| HS or Less | 10 (67%) | 6 (73%) | 4 (57%) |
| Some College | 5 (33%) | 2 (25%) | 3 (43%) |
| Unemployed/Disabled | 10 (67%) | 6 (57%) | 4 (57%) |
| Current Smoking Status (n, % yes) | 6 (40%) | 3 (38%) | 3 (43%) |
|
Alcohol Use | |||
| Age of First Drink (M, SD) | 16.27 (3.01) | 15.38 (2.93) | 17.29 (3.20) |
| Past Year Frequency | |||
| Daily | 10 (67%) | 5 (71%) | 5 (71%) |
| 5-6 times/week | 1 (7%) | 0 (0%) | 1 (14%) |
| 3-4 times/week | 1 (7%) | 1 (14%) | 0 (0%) |
| Twice/week | 1 (7%) | 0 (0%) | 1 (14%) |
| 3-11 times/year | 1 (7%) | 1 (14%) | 0 (0%) |
| Drinks per Drinking Day (Past Year) | 1 (7%) | 1 (14%) | 0 (0%) |
| 25 or more | 2 (13%) | 2 (29%) | 0 (0%) |
| 19-24 drinks | 2 (13%) | 0 (0%) | 2 (29%) |
| 9-15 drinks | 3 (20%) | 2 (29%) | 1 (14%) |
| 5-8 drinks | 6 (40%) | 2 (29%) | 4 (57%) |
| 1-4 drinks | |||
| Binge Frequency (Past Year) | |||
| Daily | 4 (27%) | 3 (43%) | 1 (14%) |
| 2-4x/week | 2 (14%) | 0 (0%) | 2 (29%) |
| 1 day/week | 1 (7%) | 1 (14%) | 0 (0%) |
| 0 days | 7 (47%) | 3 (43%) | 4 (57%) |
| Abstinence Self-Efficacy | 9.67 (0.72) | 10.00 (0.00) | 9.29 (0.36) |
| Alcohol Craving (OCDS) | 5.90 (10.02) | 8.62 (13.13) | 3.57 (6.60) |
| Perceived Stress Scale (PSS) | 5.85 (3.11) | 4.86 (3.39) | 6.86 (2.67) |
Participants reported a mean age of first drink of 16.27 years (SD=3.01) and began to drink regularly at 21.60 years (SD=6.72). Participants reported a lifetime average of 3.20 (SD=4.14) alcohol-related inpatient admissions and 2.73 (SD = 4.42) outpatient or emergency room visits for alcohol use. The two conditions were not significantly different (all ps > 0.05) with respect to outpatient treatment (SC = 2.29 (4.54); TM = 3.13 (4.58)) or inpatient admissions (SC = 3.29 (4.31); TM = 3.13 (4.29)).
Per eligibility requirements, all participants reported alcohol use in the past year. On drinks per drinking day in the past 12 months (rated on a categorical Likert scale: 0 = did not drink; 5 = 9-11 drinks; 11 = 25 or more drinks), 33% reported drinking 1 or 2 drinks, 20% reported between 3-6 drinks, 21% reported between 7-15 drinks, and 20% reported at least 19 drinks on a typical drinking day. TLFB assessed drinking in the 30 days before intake. Participants reported an average of 2.47 (SD = 7.83, Median = 0) drinking days in the past month, an average of 0.20 (SD = 0.56, Median = 0) drinks on heaviest day in the past month, and an average of 92.04% (SD = 25.25, Median = 100) days abstinent in the past month. Over one-third of the sample consumed 24 or more drinks (n=5, 36%) on their lifetime day of maximum alcohol consumption. There were no significant differences between the two conditions on any alcohol variables at baseline (all ps>0.05).
Intervention Satisfaction and Dosage
As noted, 6 of the 8 participants in the TM condition completed intervention satisfaction ratings. Participants were very satisfied with the intervention (M=4.17, SD=1.17) and looked forward to receiving the messages (M=3.33, SD=1.03). Participants found it easy to respond to (M=4.33, SD=0.52) and read the messages (M=3.67, SD=1.03). On a Likert scale of 1 (too many) to 3 (too few), 88% (n = 7) reported that the frequency of the daily texts was appropriate, and 1 participant said that there were too few daily texts. On a Likert scale of 1 (too many texts) to 3 (too few texts), participants found 3 texts per week appropriate (M=2.00, SD=0.52). They found the messages helpful for abstinence (M=3.67, SD=1.51), coping with cravings (M=3.67, SD=1.51), and stress (M=3.67, SD=1.37).
Intervention feasibility and dose was assessed in two ways. First, participants rated on a 0 (0%) to 6 (100%) scale the percentage of messages they read. One participant reported reading 80-90% of messages; the other 5 reported reading 100% of messages [5/6 (83%)]. Intervention dose was also assessed via the percent of messages to which they actually responded. It was possible to receive a total of 96 messages. The overall, actual rate of response was 78%, without adjustment. During the study, two participants were hospitalized (1 participant for 6 days and 1 for 4 days); both responded to fewer messages during hospitalization. Removing their hospitalization days yields an adjusted rate of response of 81%. Thus, there was was a high rate of congruency between participants’ reported rate of response and objective data on response rates.
Preliminary Efficacy Findings
Alcohol Use
Both conditions had participants with positive EtG results at BL. A total of 2 of the 8 TM participants had positive results; 3 of the 7 participants in the SC condition tested positive. At 8W, 0 of the TM participants had positive EtG results, whereas 2 of the 6 participants in the SC condition tested positive.
Alcohol consumption was detected more often by EtG than by breathalyzer or TLFB. Across all time-points, only 1 participant recorded a positive breathalyzer BAC. Less alcohol use was also reported via TLFB than by EtG. At BL, one participant in each condition reported any alcohol consumption in the prior 30 days (χ2(1)=0.01, p=0.92). During treatment, zero SC participants and two TM participants reported drinking. There was no difference between the conditions during treatment ((χ2(1)=1.75, p=0.19).
Stress
GEE models indicated a significant interaction of condition and time on stress (Wald χ2 (2)=6.01; p<0.05). At BL, the two conditions reported equivalent levels of stress (p=0.118). Compared to SC baseline, the TM condition had a trend of less stress at 4W (p=0.076) and at 8W (p=0.098). The conditions were equivalent at 4W and 8W. Examining main effects, in the SC condition, there was a decrease in stress from BL to 4W (p<0.05) and a non-significant increase from 4W to 8W (p=0.364). In the TM condition, there was no significant change from BL to 4W (p=0.93) and no change from 4W to 8W (p=0.99).
Abstinence Self-Efficacy
There was a trend for a condition effect (Wald χ2 (1)=3.00; p=0.08). The BL level of the TM condition was higher than the SC condition at all time-points (all ps<0.05). There was no difference between the conditions at 4W (p=0.209) or 8W (p=0.174). Examining main effects, in the SC condition, there was no difference between BL and 4W (p=0.16); there was a trend for a significant increase from 4W to 8W (p=0.088). In the TM condition, there was no significant change from BL to 4W (p=0.303) or from 4W to 8W (p=0.587).
Craving
GEE models were run as negative binomial models with a log link function because OCDS scores were zero-inflated. There were no significant effects of condition (Wald χ2 (1)=0.951; p=0.33), time (Wald χ2 (2)=0.209; p=0.90), or time × condition (Wald χ2 (2)=0.76; p=0.68). Examination of means indicates that the TM condition reported higher levels of craving at all time points, but there were no differences between the conditions.
Discussion
This study sought to examine the preliminary feasibility of a SMS-based alcohol relapse prevention intervention for pre-listing liver transplantation patients. To our knowledge, this is the first study of a mobile alcohol intervention for liver transplantation patients. The TM condition reported high levels of intervention satisfaction, completed the intervention, and found the dose adequate. TM participants had fewer positive urine EtG results at Week 8 than SC participants. TM participants maintained a consistent level of stress during the intervention, suggesting again that this intervention did not add a stress burden. Overall, these results provide early evidence that mobile interventions could be a beneficial augment to standard treatment for this population.
Few mobile alcohol interventions have been developed (Cohn et al., 2011); none target liver transplant patients. A review of alcohol applications found that most focus on tracking drinks and few utilized empirically-based components of alcohol treatment (Cohn et al., 2011). Most focus on changing drinking behavior in young adults (e.g., Carra et al., 2016). Other vulnerable populations could benefit from a mobile intervention that can provide adjunctive care outside of a usual clinic. In our study, patients had numerous health complications and medical appointments. Despite this high medical burden, Our patients reported very high levels of satisfaction with the intervention and found it easy to respond and complete the intervention. Consistent with this, our patients reported high levels of participation in the intervention, which was coorborated by our analytics data on their response rates. They found the rate of messages received to be appropriate and only one participant rated that they would have changed the frequency of the messages. Overall, the SMS intervention was a feasible and positive intervention for our patients to complete. These high intervention satisfaction ratings could have been because the intervention did not require additional medical appointments and provided real-time behavioral strategies.
Our results provide preliminary evidence that the SMS-based intervention was helpful for liver transplant patients for abstaining from alcohol and maintaining stress level. While our patients did not reduce their stress level, they also did not experience a significant increase in stress. This is consistent with the previous study that tested an alcohol intervention for this population (Weinrieb et al., 2011). It provides further evidence that incorporating relapse-prevention interventions into a transplantation clinic could provide significant benefit for ALD patients. Our results also provide the first preliminary evidence that an SMS-intervention can be helpful for stress in this population. Our results showed that TM participants did not reduce stress, but their stress levels remained consistently lower than the SC condition. The SC condition showed significantly variable stress levels. Given that ALD pre-listing patients have numerous health, social, and environmental stressors, maintaining a consistently lower stress level could be important for health outcomes.
A noteworthy finding is the discrepancy between self-reported alcohol use and biological data. Detection rates were much lower with self-reported drinking and breathalyzer testing. This is consistent with findings on the increased sensitivity of EtG testing results in liver transplantation patients (Erim et al., 2007) and high-risk populations (Wurst et al., 2003). In our study, participants were reassured that any self-disclosure of drinking would not be impact their UNOS listing status. Despite this reassurance, only two participants reported any instance of alcohol use during treatment. It is noteworthy that the two people that reported alcohol use were in the TM condition, whereas our biological data indicated that the SC condition had higher rates of recent drinking. Therefore, it could be that participation in the intervention helped participants to be more honest about their drinking through increased understanding of their drinking patterns and triggers; it could also be that participation in the intervention provided evidence that their data was truly protected from the medical team. Given that most transplantation centers have site-specific requirements of six months of sobriety prior to UNOS listing, this highlights the importance of including sensitive, biological testing at clinic visits to detect recent alcohol use and/or to reinforce abstinence.
There was no effect on craving despite tailoring the initial intervention phase to provide information about cravings. All participants reported low levels at BL, and scores remained consistently low during treatment. This is consistent with other studies examining craving in ALD transplantation patients (e.g., Weinreib et al., 2001). Comparing craving between ALD transplantation patients and patients in alcohol treatment receiving naltrexone therapy, transplantation patients denied alcohol craving (Weinreib et al., 2001). Transplant patients may be highly reluctant to report alcohol craving for fear of its impact on UNOS listing. Patients may not want to disclose that they are struggling to maintain sobriety. Yet, alcohol craving is a significant predictor of relapse (Sinha et al., 2011). Much more work needs to be done to address how to understand alcohol craving in this population.
Study limitations should be noted. The sample size was very small; effect size estimates from small pilot studies should be interpreted with caution (Kraemer et al., 2006). The sample was also a convenience sample, generated from potential patients that were identified as likely to be eligible by the LTT. A full trial that was able to screen all patients in a liver clinic would provide more complete data on feasibility. Time spent on intervention content could not be assessed and could only be measured via participants’ response to SMS messages. Participants could have responded that they read the messages without reading them. The study duration was very short. Given that the listing requirement is six months of sobriety, more work needs to be done to build an intervention for that entire time. Additionally, given data suggesting that treatment post-transplant can be as important as pre-transplant (Lisson et al., 2005), work needs to be done to build an intervention tailored for post-transplant.
Maintenance of sobriety for ALD liver transplant patients is a site-specific requirement for UNOS listing at many transplantation centers in the United States. A mobile, SMS-based intervention for alcohol relapse prevention shows promise for reducing alcohol consumption and maintaining lower stress levels. Further development of behavioral alcohol interventions for this vulnerable population remains an urgent clinical need; mobile interventions may provide a beneficial care adjunct.
Figure 1.
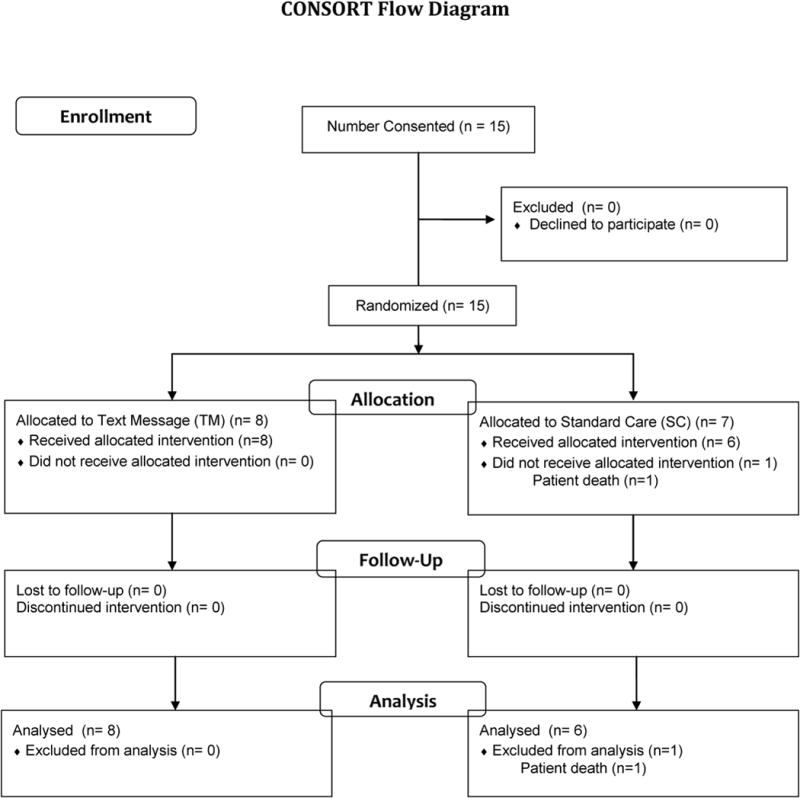
Consolidated Standards of Reporting Trials diagram
Acknowledgments
Grant and Financial Support
The project described was supported by T32 AA015496 and P50 AA012870 (KSD) and by an internal grant from the Psychological Medicine Service of Yale-New Haven Hospital (BAT).
Footnotes
DR. KELLY S. DEMARTINI (Orcid ID : 0000-0003-0943-2507)
Conflicts of Interest/Disclosures
Stephanie O’Malley, Ph.D. is a member of the American Society of Clinical Psychopharmacology’s (ASCP’s) Alcohol Clinical Trials Initiative, supported by Alkermes, Amygdala, Arbor Pharma, Ethypharm, Indivior, Lundbeck, Otsuka; Consultant/advisory board member, Alkermes, Amygdala, Opiant; Mitsubishi Tanabe; Medication supplies, Astra Zeneca, Pfizer; DSMB member, Indiana University, Emmes Corporation.
Michael Schilsky, M.D. declares no conflicts. He is a speaker for Gilead and has grant support from Wilson therapeutics.
Benjamin Toll, Ph.D. received an investigator initiated grant from Pfizer for medicine only, and he has testified as an expert witness in litigation filed against the tobacco companies.
All other authors report no conflicts.
Contributor Information
Kelly S. DeMartini, Yale School of Medicine, Dept. of Psychiatry & Smilow Cancer Hospital at Yale-New Haven.
Michael L. Schilsky, Yale School of Medicine, Depts of Medicine and Surgery, Divisions of Digestive Diseases and Transplantation and Immunology, Yale-New Transplantation Center
Amanda Palmer, Moffitt Cancer Center, University of South Florida
Dwain C Fehon, Yale-New Haven Hospital, Liver Transplantation Center
Paula Zimbrean, Yale-New Haven Hospital, Liver Transplantation Center
Stephanie S. O’Malley, Yale School of Medicine, Dept. of Psychiatry
Hochang B. Lee, Yale-New Haven Hospital, Dept of Psychological Medicine
Benjamin A. Toll, Hollings Cancer Center, Medical University of South Carolina
References
- Alqahtani SA, Larson AM. Adult liver transplantation in the USA. Current opinion in gastroenterology. 2011;27(3):240–247. doi: 10.1097/MOG.0b013e3283457d5d. [DOI] [PubMed] [Google Scholar]
- Belle SH, Beringer KC, Detre KM. Liver transplantation for alcoholic liver disease in the United States 1988-1995. Liver Transplant Surg. 1997;3:212–209. doi: 10.1002/lt.500030304. [DOI] [PubMed] [Google Scholar]
- Bravata DM, Olkin I, Barnato AE, Keeffe EB, Owens DK. Employment and alcohol use after liver transplantation for alcoholic and nonalcoholic liver disease: a systematic review. Liver Transplantation. 2001;7(3):191–203. doi: 10.1053/jlts.2001.22326. [DOI] [PubMed] [Google Scholar]
- Carrà G, Crocamo C, Bartoli F, Carretta D, Schivalocchi A, Bebbington PE, Clerici M. Impact of a Mobile E-Health Intervention on Binge Drinking in Young People: The Digital–Alcohol Risk Alertness Notifying Network for Adolescents and Young Adults Project. Journal of Adolescent Health. 2016;58(5):520–526. doi: 10.1016/j.jadohealth.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Center P. R. Mobile Technology Fact Sheet. 2014 [cited 2015 May 15]; Available from: http://www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/
- Cohn AM, Hunter-Reel D, Hagman BT, Mitchell J. Promoting behavior change from alcohol use through mobile technology: the future of ecological momentary assessment. Alcoholism: Clinical and Experimental Research. 2011;35(12):2209–2215. doi: 10.1111/j.1530-0277.2011.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BH, Pirsch JD, Becker YT, Hanaway MJ, Van der Werf WJ, D’Alessandro AM, Armbrust M. Long-term results of liver transplantation in patients over 60 years of age and older. Transplantation. 2000;70(5):780–783. doi: 10.1097/00007890-200009150-00012. [DOI] [PubMed] [Google Scholar]
- CTIA. Annual Wireless Industry Survey. 2014 [cited 2015 May 9]; Available from: http://www.ctia.org/your-wireless-life/how-wireless-works/annual-wireless-industry-survey.
- DiMartini A, Dew MA, Day N, Fitzgerald MG, Jones BL, DeVera ME, Fontes P. Trajectories of alcohol consumption following liver transplantation. American Journal of Transplantation. 2010;10(10):2305–2312. doi: 10.1111/j.1600-6143.2010.03232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMartini A, Day N, Dew MA, Javed L, Fitzgerald MG, Jain A, Fung JJ, Fontes P. Alcohol consumption patterns and predictors of use following liver transplantation for alcoholic liver disease. Liver Transpl. 2006;12:813–820. doi: 10.1002/lt.20688. [DOI] [PubMed] [Google Scholar]
- DiMartini A, Day N, Dew MA, Lane T, Fitzgerald MG, Magill J, Jain A. Alcohol use following liver transplantation: a comparison of follow-up methods. Psychosomatics. 2001;42(1):55–62. doi: 10.1176/appi.psy.42.1.55. [DOI] [PubMed] [Google Scholar]
- Erim Y, Böttcher M, Dahmen U, Beck O, Broelsch CE, Helander A. Urinary ethyl glucuronide testing detects alcohol consumption in alcoholic liver disease patients awaiting liver transplantation. Liver transplantation. 2007;13(5):757–761. doi: 10.1002/lt.21163. [DOI] [PubMed] [Google Scholar]
- Faulkner X, Culwin F. When fingers do the talking: a study of text messaging. Interacting with Computers. 2005;17(2):167–185. [Google Scholar]
- Free C, Knight, Robertson S, Whittaker R, Edwards P, Zhou W, Rodgers A, Cairns J, Kenward MG, Roberts I. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomized trial. Lancet. 2011;378:49–55. doi: 10.1016/S0140-6736(11)60701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou G, Webb K, Griggs K, Copello A, Neuberger J, Day E. First report of a psychosocial intervention for patients with alcohol-related liver disease undergoing liver transplantation. Liver Transpl. 2003;9:772–775. doi: 10.1053/jlts.2003.50152. [DOI] [PubMed] [Google Scholar]
- Heron KE, Smyth JM. Ecological momentary interventions: Incorporating mobile technology into psychosocial and health behaviour treatments. British Journal of Health Psychology. 2010;15:1–39. doi: 10.1348/135910709X466063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. The burden of hepatitis C in the United States. Hepatology. 2002;36(S1) doi: 10.1053/jhep.2002.36791. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Archives of general psychiatry. 2006;63(5):484–489. doi: 10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- Larson AM, Curtis JR. Integrating palliative care for liver transplant candidates:“too well for transplant, too sick for life”. JAMA. 2006;295(18):2168–2176. doi: 10.1001/jama.295.18.2168. [DOI] [PubMed] [Google Scholar]
- Mackie J, Groves K, Hoyle A, Garcia C, Garcia R, Gunson B, Neuberger J. Orthotopic liver transplantation for alcoholic liver disease: a retrospective analysis of survival, recidivism, and risk factors predisposing to recidivism. Liver Transpl. 2001;7:418–427. doi: 10.1053/jlts.2001.23789. [DOI] [PubMed] [Google Scholar]
- Miguet M, Monnet E, Vanlemmens C, Gache P, Messner M, Hruskovksy S, et al. Predictive factors of alcohol relapse after orthotopic liver transplantation for alcoholic liver disease. Gastroenterol Clin Bio. 2004;28:845–851. doi: 10.1016/s0399-8320(04)95146-9. [DOI] [PubMed] [Google Scholar]
- Pfitzmann R, Schwenzer J, Rayes N, Seehofer D, Neuhaus R, Nussler NC. Long-term survival and predictors of relapse after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl. 2007;13:197–205. doi: 10.1002/lt.20934. [DOI] [PubMed] [Google Scholar]
- Roisen R, Kerr WC, Filmore KM. Cirrhosis mortality and per capita consumption of distilled spirits, United States 1949-94 trend analysis. BMJ. 1999;319:666–70. doi: 10.1136/bmj.319.7211.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KIA, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress-and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Archives of general psychiatry. 2011;68(9):942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back - a Technique for Assessing Self-Reported Alcohol-Consumption. Measuring Alcohol Consumption. 1992:41–72. [Google Scholar]
- Wagner CC, Haller DL, Olbrisch ME. Relapse prevention treatment for liver transplant patients. Journal of Clinical Psychology in Medical Settings. 1996;3(4):387–398. doi: 10.1007/BF01994021. [DOI] [PubMed] [Google Scholar]
- Wei J, Hollin I, Kachnowski S. A review of the use of mobile phone text messaging in clinical and healthy behaviour interventions. J Telemed Telecare. 2011;17(1):41–8. doi: 10.1258/jtt.2010.100322. [DOI] [PubMed] [Google Scholar]
- Weinrieb RM, Van Horn DHA, Lynch KG, Lucey MR. A randomized controlled study of treatment for alcohol dependence in patients awaiting liver transplantation. LiverTranspl. 2011;17:539–547. doi: 10.1002/lt.22259. [DOI] [PubMed] [Google Scholar]
- Weitzel J, Bernhardt J, Usdan S, Mays D, Glanz K. Using wireless handheld computers and tailored text messaging to reduce negative consequences of drinking alcohol. J Stud Alc Drug. 2007;68:534–537. doi: 10.15288/jsad.2007.68.534. [DOI] [PubMed] [Google Scholar]
- Whittaker R, Maddison R, McRobbie H, Bullen C, Denny S, Dorey E, et al. A multimedia mobile phone-based youth smoking cessation intervention: Findings from content development and piloting studies. J Med Internet Research. 2008;10:e49. doi: 10.2196/jmir.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst FM, Skipper GE, Weinmann W. Ethyl glucuronide—the direct ethanol metabolite on the threshold from science to routine use. Addiction. 2003;98(s2):51–61. doi: 10.1046/j.1359-6357.2003.00588.x. [DOI] [PubMed] [Google Scholar]


