Abstract
Background:
Chronic fatigue syndrome is a complex chronic condition with large negative impact on patients’ function and quality of life. Efficacy and cost-effectiveness of cognitive behavioral intervention remain inconclusive.
Objective:
To evaluate the cost-utility of a home-based fatigue self-management (FSM) intervention as compared to usual care among primary care patients with severe chronic fatigue syndrome (CFS).
Methods:
An economic evaluation alongside of a randomized controlled study design was used. Cost and utility data were collected from 137 patients with severe CFS at baseline and 1-year follow-up. The FSM group (n=89) received self-delivered cognitive behavioral self-management intervention and the usual care group (n=48) received regular medical care. Cost was measured by total costs (direct, indirect, and intervention costs) during the follow-up period. Quality-adjusted life years (QALY), as the utility measure, were derived from the Medical Outcomes Survey Short Form-36. A societal perspective was adopted. Bootstrapped incremental cost-utility ratios (ICURs) and net monetary benefit were calculated as measures of cost-effectiveness.
Results:
Baseline individual characteristics were similar between the two groups. The intervention was well-received by the participants with only minimum attrition. At the end of one year post-intervention, FSM dominated usual care in terms of ICUR in both the intention-to-treat analysis and the complete-cases-only analysis. Net monetary benefit analysis showed that FSM has higher probability of achieving positive net monetary across the entire range of possible societal willingness-to-pay for fatigue symptom management.
CONCLUSIONS:
In primary care patients with severe CFS, the low-cost FSM appears to be a cost-effective treatment.
Keywords: cost-utility analysis, cognitive behavioral therapy, self-management, fatigue
INTRODUCTION
Chronic fatigue syndrome (CFS) is a perplexing condition of unknown etiology which has serious impact on patient function, quality of life, and health care costs [1–3]. Patients with CFS have been shown to have more impairment in function than those with hypertension, type 2 diabetes, depression, and even congestive heart failure [4]. Studies have also shown that CFS is associated with significant losses in quality of life [5–7]. The direct and indirect cost of CFS has been estimated at more than $17 billion in the U.S.[8] Treatment options for patients with CFS remain very limited to cognitive behavioral therapy (CBT), exercise therapy, and immune modulator intervention, none of which has shown definitive efficacy for illness reversal across studies [9, 10].
CBT is designed to help patients identify and reduce negative thoughts and to build positive coping skills. While previous research has shown that CBT is effective in reducing symptoms of CFS in primary care [7, 11–20], limited evidence exists regarding the cost-effectiveness of CBT interventions as compared to general counseling, graded exercise, pharmacological therapy, and attention control [7, 21–23].
One recent study found that a CBT group training intervention was cost-effective with a gain of 0.06 QALYs and a €828 reduction in costs over 4 years [24]. In North America, we reported preliminary evidence that a two-session brief CBT-based fatigue self-management intervention is cost-effective as compared to usual care at 12-month post intervention among 73 CFS patients from a primary care practice [25]. However, another study found that a nurse-led self-help treatment for CFS was not cost-effective [26]. Regardless of this mixed evidence for cost-effectiveness, the question remains as to whether the cost of CBT interventions can be further reduced by using a home-based self-management CBT program. If successful, the home-based self-management intervention can be easily made available to a large number of patients due to the low costs and ease of use associated with the home-based self-management.
The purpose of the present study is to compare the cost-utility of a home-based fatigue self-management (FSM) intervention to treatment as usual (TAU) from the societal perspective in the treatment of CFS in primary care. Because the FSM does not involve any patient travel or clinician’s time in delivery of the intervention, we hypothesized that FSM would lead to lower total costs due to a lower intervention cost over the course of the one-year study.
METHODS
Home-Based Fatigue Self-Management Study
The Home-Based Fatigue Self-Management Study is a randomized controlled trial involving 137 primary care patients with CFS conducted in five CFS-specialized group practices in the US (primarily in Utah and North Carolina) between 2012 and 2014. Details of the study have been reported elsewhere [27]. Briefly, the main inclusion criteria for participants were (a) age between 18 and 65 years; (b) at least six months of persistent, unremitting fatigue with secondary symptoms (pain, sleeplessness, exertional malaise, etc.). Exclusion criteria included: (a) Medical: pregnant, fatigue due to identifiable medical conditions (such as autoimmune diseases) or to medications (such as beta blockers); (b) Psychiatric: psychosis or dementia, alcohol or substance abuse, depression with melancholic or psychotic features, and anorexia nervosa or bulimia nervosa. All patients were asked to provide a note from their physicians confirming a diagnosis of CFS. Given that most patients were diagnosed at least one year into the illness [1], these criteria included patients with well-established symptoms.
The main target for the treatment was to reduce the negative influences of: affective distress, absence of pleasant affect and experiences, and maladaptive activity patterns. A behavioral approach targeted to these factors was expected to yield reductions in fatigue symptoms and improvements in functioning. The overarching goal was to help the patient achieve a healthy balance between activity, rest, and leisure [28]. The study protocol was approved by the Stony Brook University Institutional Review Board (IRB).
After written informed consent and baseline assessments were obtained, patients were randomly assigned to one of three groups: FSM with online web diaries and waist-worn actigraphs (n=45), FSM with paper diaries and pedometers (n=44), and usual care (TAU, n=48). The FSM intervention consisted of an evidence-based 56-page self-management booklet with 2 audio CD-ROMs that duplicated the content of the booklet and a relaxation audio CD-ROM. The daily diaries were assigned to monitor fatigue and track compliance with the 12-week intervention. The study began with an initial telephone screening followed by questionnaire assessments mailed to participants’ homes.
Consistent with best practices, health services use data were collected monthly from baseline through the three-month intervention phase and continued through the final follow-up assessment performed at 12-months post-treatment. While patients in the TAU group received no treatment beyond usual medical care, they participated in the study assessments using web diaries and actigraphs. Because participants in the two active treatment groups received essentially the same intervention and modes of data collection (web versus paper diaries) were not essential in determining cost-effectiveness of FSM, the two groups were combined for the purpose of the cost-utility analysis to assess the impact of FSM on patient-reported costs and utility.
Outcome Measures
Quality-adjusted life years (QALYs) were generated from the SF-36 questionnaire (version 2) from baseline and 12 months after randomization. The SF-36 questionnaire measures the multi-dimensional concept of health status related to physical, psychological, and social well-being among the general adult population. Because SF-36 scoring assumes equal importance among the items and it is not based on preferences, we used a published algorithm to convert SF-36 data to utility scores on the scale of 0 to 1 (0 is an anchoring point for death and 1 for perfect health) [29].
Service Use and Costs
Health resource use and costs were identified and valued from the societal perspective for the Reference Case analysis following the “Panel Recommendations” [30]. Health care resource use was measured with a modified version of the Client Service Receipt Inventory (CSRI), a validated health care utilization diary [31], to record health service use as well as employment and informal care status for the 3 month period prior to baseline and during the post-treatment follow-up period. Patients were instructed to record all health services use as they occurred.
To evaluate the economic effects of the prescribed treatments, we identified relevant cost categories of resource use by measuring utilization in each resource category and identifying the unit costs (prices) of the corresponding category. Health care costs and non-health care costs were included in the calculation of total costs for each patient [32]. Health care costs included the costs of health services utilization (hospitalizations, and visits to health care providers such as general practitioner, specialist, physical therapist, alternative medicine providers) and the use of prescription medications. Non-health care costs included out-of-pocket expenses, costs of paid and unpaid help, productivity loss, and cost of delivering the intervention, if any. As part of the modified CSRI, information on the frequency of paid help and the number of illness-related absences from paid or unpaid work was also collected. The intervention costs included costs of the FSM: booklet, CDs, investigator supervision, and the economic consequences of the programs in terms of health services utilization before and after the intervention.
For each category of health care resources, we used standard approaches to estimate costs [33, 34]. Unit costs (prices) for major health care services (e.g. provider office visits) and prescription medications were based on national average of Medicare payment rates, estimated from the 2013 Medical Expenditure Panel Survey (MEPS). Medicare payment rates are widely used as approximate measures of the opportunity costs associated with health services use in economic evaluations. Unit costs of various diagnostic tests were based on 2010 Medicare Physician Fee Schedule Payment Schedule (MPFS) published by the Centers for Medicare and Medicaid Services (CMS).
Costs associated with lost productivity and the use of informal caregivers were also included. Because the traditional human capital approach of valuating productivity loss tends to over-estimate it [35], we valued the productivity loss by using average wages obtained from the U.S. Census Bureau (U.S. Census Bureau, Statistical Abstract of the United States, 2013) instead of actual wages of the participants. We calculated daily wages from annual wages and then estimated the total lost income for each patient as a product of the total number of days missed work and daily wages.
For the cost associated with using informal caregivers, we used the national average hourly wage of home health aides from the 2013 Occupational Employment and Wage Estimates produced by the Bureau of Labor Statistics (BLS). For each patient, total costs were calculated by adding the direct and indirect costs. Unit costs are expressed in US dollars ($) based on 2013 prices. Because the study began in 2012 and ended in 2014, all prices were adjusted by using the medical care component of the Consumer Price Index (CPI) in the US (Appendix 1).
Analysis
Outcomes
Statistical analyses were performed using STATA (Version 12, College Station, TX). We first compared patients’ baseline characteristics in the three groups using appropriate tests of statistical significance (i.e. Chi-square test for binary variables, analysis of variance for continuous variables). For both the quality of life and the cost outcomes, we used multivariate regression models to adjust for the following baseline patient characteristics: age, gender, education, marital status, employment status, number of chronic conditions, and number of symptoms. These variables were selected based on previous research regarding factors potentially associated with patient outcomes [15, 36].
For multivariate analysis, our primary interest was to examine the between-group differences in total health care expenditures among participants in the FSM as compared to those in the control groups. Therefore, we estimated total expenditures using a generalized linear model (GLM) with a gamma distribution for the dependent variable and a log link function to account for the distributional characteristics of costs data. GLM also has the benefit of not requiring retransformation in generating predicted costs after the model is fitted as compared to the two-part model[37].
Cost-Utility Analyses
Incremental cost-utility ratios (ICURs) were conceptualized as the following: ICUR = (ΔC1−ΔC2)/(ΔU1−ΔU2), where ΔC1−ΔC2 is the difference in cost change between two groups (FSM vs TAU) from baseline to 1-year follow-up; and (ΔU1−ΔU2) which is the difference in the average effectiveness between the two groups [38]. To account for a minor unbalance in baseline characteristics between the two groups, multivariate analysis was used with a difference-in-differences approach to estimate an unbiased measure of the treatment effect on both cost and utility measures. We plotted a cost-effectiveness plane (with a cost dimension and a fatigue dimension) to show the incremental change in utility scores and in costs for FSM versus TAU. To account for uncertainty involved in the statistical inference, 3000 ICURs were obtained through bootstrapping of the GLM (for cost) and linear regression model (for utility)[39].
Because negative ICUR may result in ambiguity as to which group dominates, we used the net-benefit approach to evaluate the cost-utility of the treatment group as suggested in the literature [40, 41]. By using a series of hypothetical values for societal willingness-to-pay (λ) for each additional unit of QALY, the net monetary benefit (NMB) the decision-maker is willing to pay per QALY can be calculated as: Net Monetary Benefit = λΔE − ΔC, where ΔE=difference in effectiveness and ΔC=difference in costs[42]. Given each level of willingness-to-pay (often unknown from the societal perspective), a program is deemed cost-effective if Net Monetary Benefit > 0 [33].
In the present study, net benefits were calculated for each patient in the sample using a range of values ($0 to $50000 in $1000 increments) for λ to reflect the uncertainty surrounding the societal willingness-to-pay per QALY. For each value of willingness-to-pay, Net Monetary Benefits were calculated from the 3000 bootstrapped predicted means of costs and effectiveness. The frequency of positive Net Monetary Benefits was then tallied to form the basis for the cost-effectiveness acceptability curves (CEACs). Finally, CEACs were plotted to display the probably that the FSM is cost-effective, given different levels of willingness-to-pay for each QALY gained [43].
Sensitivity Analysis
For the 12 participants who did not complete the study, we used last-value-carried-forward to impute the effectiveness measure. Therefore the intention-to-treat (ITT) analysis provides more conservative estimates (by assuming no change) of the effectiveness measures for the full sample as recommended by a consensus report [44]. To test the robustness of the results, we then conducted sensitivity analyses by repeating the analysis among completers only assuming that the data were missing at random as the result of attrition. Multiple imputation was not conducted given the small amount of missing values (4.4%).
RESULTS
Sample Characteristics
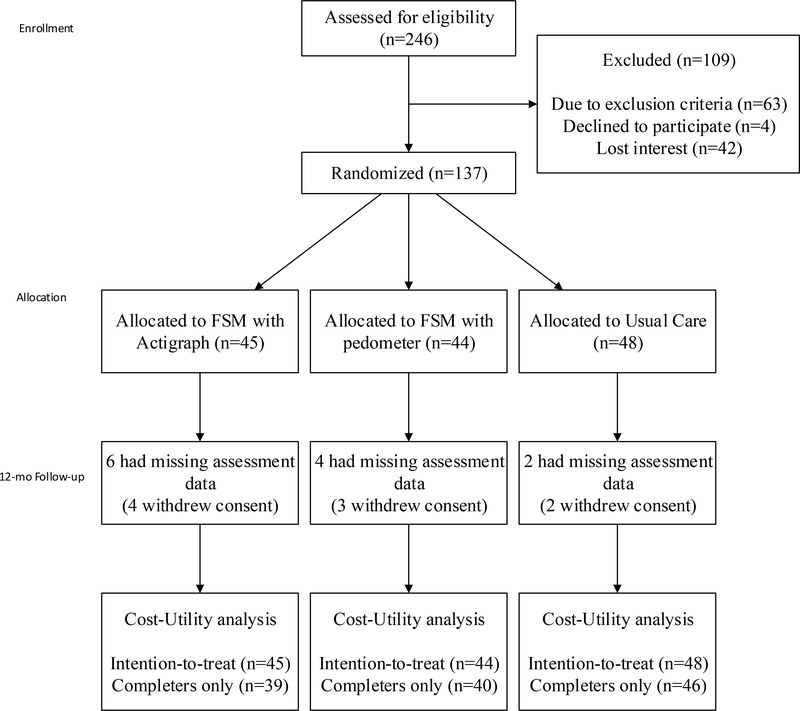
The sample consisted of 137 individuals who were randomized into the FSM (45 with actigraphs combined with 44 with pedometers) and TAU (n=48). A total of 125 (91%) patients completed the 12-month follow-up assessment (Figure 1). The groups are similar in baseline patient characteristics with the exception of length of illness in which the FSM group reported shorter duration (Table 1).
Figure 1.
CONSORT Flow Diagram
Table 1.
Baseline characteristics by intervention group
| Variables | TAU n=48 |
FSM n=89 |
p |
|---|---|---|---|
| Age, mean (SD) | 50.0 (11.3) | 47.5 (11.6) | 0.19 |
| Female, % | 87.5 | 88.8 | 0.79 |
| White, % | 97.9 | 88.8 | 0.10 |
| College or graduate degree, % | 60.4 | 55.1 | 0.59 |
| Married or partnered, % | 52.1 | 57.3 | 0.59 |
| Have Children, % | 47.9 | 62.9 | 0.10 |
| Currently working, % | 81.3 | 86.5 | 0.46 |
| Body Mass Index, mean(SD) | 25.7(6.3) | 25.0(5.8) | 0.55 |
| Length of illness in years, mean (SD) | 17.3(10.6) | 13.1(7.8) | 0.02 |
| Fatigue Severity Scale, mean (SD) | 6.6 (0.4) | 6.5 (0.5) | 0.21 |
| SF-36 Physical Function, mean (SD) | 38.9(22.1) | 37.4(19.8) | 0.70 |
TAU=Treatment as Usual; FSM=Home-Based Fatigue Self-Management; SD=standard deviation; P values are based on analysis for variance for continuous variables and Chi-square test for proportions.
Table 2 presents the average costs by resource use categories pre- and post-treatment for TAU and FSM groups. The total cost of the FSM packet is estimated at $79 per patient (personnel: $27, patient information booklet: $10, CD-ROMs: $15, and overhead: $27). Overall, both groups experienced reduction in total average costs of about 10%, with similar costs of pre- and post- interventions. The FSM group had a 6.5% reduction in costs during the post period ($8712 to $8143) and the TAU group had a 9.7% reduction in costs during the same period ($8928 to $8064).
Table 2.
Costs and changes in costs for TAU and FSM, by category and period
| Pre-Intervention | Post-Intervention | ||||||
|---|---|---|---|---|---|---|---|
| Variables | n (%) users | # of Contacts ± SD† | Cost ($) | n (%) users | # of Contacts ± SD† |
Cost ($) | Changes in Costs ($) |
| Treatment as Usual (TAU, n = 48) | |||||||
| 1. GP visit | 17(35) | 1±.5 | 51 | 39(81) | 0±.6 | 47 | −4 |
| 2. Specialist visit | 13(27) | 2±2.3 | 36 | 33(69) | 1±1.1 | 18 | −18 |
| 3. Other provider visit | 18(38) | 3±1.9 | 65 | 46(96) | 1±2.9 | 86 | 21 |
| 4. Provider (1 + 2 + 3) | 30(63) | 3±2.6 | 152 | 47(98) | 2±3 | 152 | 0 |
| 5. ER visit | 0(0) | − | 0 | 2(4) | 0 | 2 | 2 |
| 6. Hospital admissions | 0(0) | − | 0 | 0(0) | − | 0 | 0 |
| 7. Rx medications | 45(94) | 6±2.9 | 173 | 48(100) | 5±2.7 | 180 | 7 |
| 8. Laboratory test | 21(44) | 3±2.8 | 75 | 47(98) | 1±.6 | 37 | −38 |
| 9. Informal care, hours | 26(54) | 26±32.6 | 159 | 38(79) | 17±24.2 | 157 | −2 |
| 10. Missed work, hours | 38(79) | 10±6.3 | 185 | 48(100) | 6±6.3 | 142 | −43 |
| 11. Total cost (4 + 5 + 6 + 7 + 8 + 9 + 10)* | 744 | 672 | −72 | ||||
| 12. Annualized average cost | 8928 | 8064 | −864 | ||||
| 13. Intervention cost | 0 | 0 | 0 | ||||
| 14. Grand total (12 + 13) | 8928 | 8064 | −864 | ||||
| Home-Based Fatigue Self-Management (FSM, n = 89) | |||||||
| 1. GP visit | 24(27) | 2±1.6 | 62 | 73(82) | 1±3.5 | 79 | 17 |
| 2. Specialist visit | 19(21) | 2±1.4 | 28 | 53(60) | 1±1.2 | 19 | −9 |
| 3. Other provider visit | 20(22) | 2±1.9 | 35 | 65(73) | 1±.7 | 38 | 3 |
| 4. Provider (1 + 2 + 3) | 41(46) | 3±3 | 125 | 82(92) | 2±3.7 | 136 | 11 |
| 5. ER visit | 0(0) | − | 0 | 2(2) | 0±.1 | 3 | 3 |
| 6. Hospital admissions | 0(0) | − | 0 | 1(1) | − | 5 | 5 |
| 7. Rx medications | 79(89) | 5±2.7 | 154 | 81(91) | 5±2.5 | 154 | 0 |
| 8. Laboratory test | 39(44) | 3±2.1 | 71 | 79(89) | 1±.6 | 47 | −24 |
| 9. Informal care, hours | 50(56) | 26±23.7 | 166 | 73(82) | 20±23.6 | 194 | 28 |
| 10. Missed work, hours | 70(79) | 11±10.1 | 210 | 82(92) | 6±5.1 | 134 | −76 |
| 11. Total cost (4 + 5 + 6 + 7 + 8 + 9 + 10)* | 726 | 672 | −94 | ||||
| 12. Annualized average cost | 8712 | 8064 | −648 | ||||
| 13. Intervention cost‡ | 0 | 79 | 79 | ||||
| 14. Grand total (12 + 13) | 8712 | 8143 | −569 | ||||
TAU=Treatment as Usual; FSM=Home-Based Fatigue Self-Management; GP=General Practitioner; ER=Emergency Room; Rx=Prescription;
contacts were calculated among users;
*Total costs during the post- period were standardized to monthly so that the results are comparable to the pre- period.
Intervention costs included: Personnel ($27), Material (booklet, $10 and CD-ROM, $15), and facility and other ($27).
Table 3 summarizes the incremental cost and incremental effectiveness, as well as ICURs for FSM as compared to TAU. At 1-year follow-up, the FSM group had a small positive impact on QALY (0.014, 95% CI: −0.008, 0.036) at lower costs ($64, 95% CI: −208, 77) as compared to TAU, resulting in an ICUR of −4442 (FSM dominated). This means that each additional QALY gained in the FSM were associated with potential saving of $4442 from a societal perspective when compared to TAU. The complete-cases-only analysis showed a lower amount of potential savings at $1455 (95% CI: −18130, 6602), but this is accompanied by a significant improvement in QALY (as indicated by the 95% CI that does not include zero). In both cases, the bootstrapped 95% confidence intervals for these ICURs suggest that there is considerable uncertainty around the point estimates due in part to the modest sample size.
Table 3.
Adjusted incremental cost, effectiveness, and cost-effectiveness ratios
| Intervention | Incremental Cost (95% CI) |
Incremental Utility (95% CI) |
ICUR |
|---|---|---|---|
| ITT analysis, 12 month | |||
| TAU | Reference | Reference | Reference |
| FSM | −$64 (−208, 77) |
0.014 (−0.008, 0.036) |
FSM dominant |
| Complete-case analysis, 12 month | |||
| TAU | Reference | Reference | Reference |
| FSM | −$36 (−190, 97) |
0.025 (0.001, 0.049) |
FSM dominant |
ITT=Intention-to-treat; TAU=Treatment as Usual; FSM=Home-Based Fatigue Self-Management; CI=Confidence Interval.
ICUR = Incremental Cost-Utility Ratio, in 2013 US dollars; ICUR = −4442 for intention-to-treat analysis and −1455 for complete-cases-only analysis. Because the magnitude of negative ICUR do not convey the same information as positive ICUR do, “FSM dominant” is reported to indicate that FSM is more effective at lower costs as compared to TAU.
Effectiveness and costs were obtained from multivariate regression models adjusting for the following baseline characteristics: age, gender, ethnicity, education, marital status, and employment status.
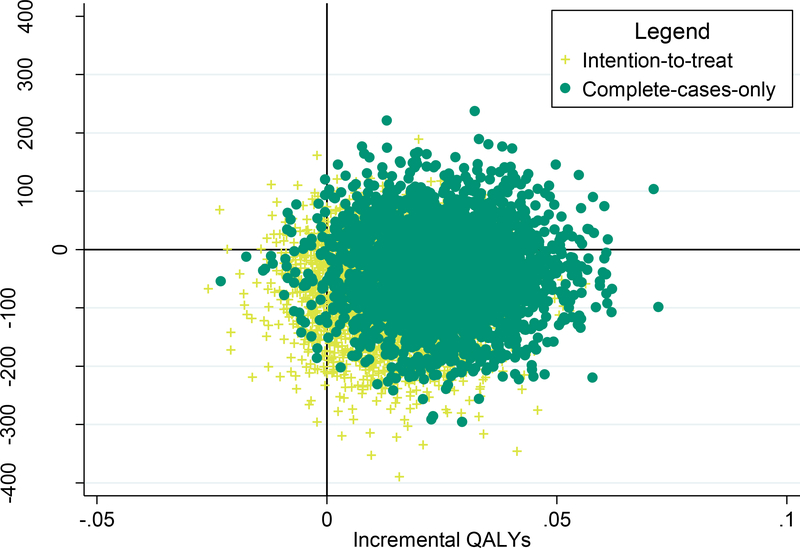
Figure 2 shows the incremental cost-effectiveness for FSM and as compared to TAU in 3000 bootstrapped samples. The majority of the ICUR for FSM vs. TAU fell in the southeast quadrant of the ICUR plane, indicating than FSM is likely to be more effective at lower costs as compared to TAU.
Figure 2.
Scatter plots of incremental cost-utility ratios for FSM versus TAU from bootstrapped samples
Note: FSM = Home-Based Fatigue Self-Management; TAU=Treatment as Usual; QALY=Quality-Adjusted Life Year</p/>Four quadrants: northeast (more effective, more costly), northwest (less effective, more costly), southwest (less effective, less costly), and southeast (more effective, less costly).
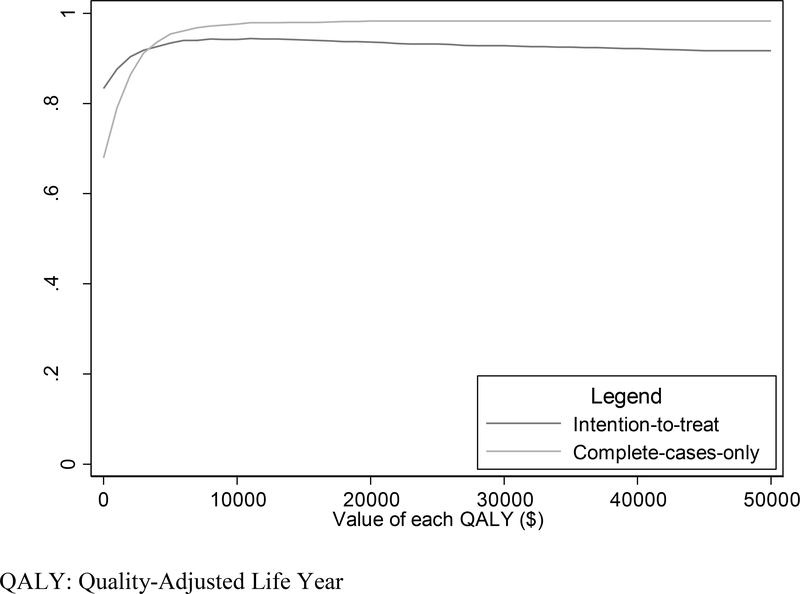
Figure 3 shows the cost-effectiveness acceptability curves (CEACs) as a function of different willingness-to-pay values per QALY. As expected, the probability that net monetary benefit is greater than zero increases as willingness-to-pay per QALY increases. Because complete-case-only analysis yielded slightly higher benefits in terms of QALY, it generated higher net benefit across the majority of the range for willingness-to-pay. Overall, FSM condition has high probability of achieving positive net monetary benefit across the entire range of possible societal willingness-to-pay.
Figure 3.
Cost-Effectiveness Acceptability Curves as a function of different societal willingness-to-pay values per QALY
QALY: Quality-Adjusted Life Year
DISCUSSION
To our knowledge, this study is the first cost-utility analysis conducted alongside a randomized controlled trial for a home-based fatigue self-management intervention among CFS patients. Previous studies have shown that CBT interventions delivered in primary care by trained professionals have moderate effect on fatigue symptoms [22, 23, 45] and may be cost-effective [25]. The present study extends our previous findings by demonstrating for the first time that a low cost home-based fatigue self-management toolkit is well received by patients with small but positive outcomes and is likely to be cost-effective even without contacts with any trained professionals. The value of this intervention may be based on its minimal labor and cost that could serve patients who are less likely to travel to a facility for treatment.
Cognitive behavioral therapy (CBT) has been shown to reduce unexplained fatigue symptoms for people who suffer from chronic fatigue [27]. While CBT has an effect on fatigue symptoms comparable to graded exercise therapy and counseling in primary care, the longer term cost-effectiveness of CBT remains unclear [21–23, 46]. From an economic perspective, the FSM toolkit offers a promising alternative to traditional multi-session CBT (6–16 visits required) delivered by experienced therapists [19], a group-based CBT training [24], or a modified two-session CBT training plus a self-help booklet [45]. Therefore, the evidence of cost-effectiveness in addition to efficacy for this FSM toolkit would offer a new tool for clinicians in the management of chronic fatigue.
The small effect of the FSM on patient utility (QALY) at 1-year post intervention is somewhat expected because unlike previous studies involving trained professionals, the FSM is entirely self-administered by (very low functioning) patients. While future studies should test the efficacy of FSM in more patients from broader geographic areas, the initial evidence of cost-effectiveness, coupled with extremely low cost of the FSM toolkit, suggest that the intervention costs may be offset by cost savings generated from reduced health services utilization during the 1-year follow-up. Therefore, it seems that less labor-intensive modalities of CBT such as the internet-based or brief nurse-led self-management approaches hold promise in lowering intervention costs while still offering some benefits to patients, thus providing greater value for society.
A number of limitations should be considered in interpreting the findings. First, because patients were recruited from several primary care practices, findings may not be generalizable to patients in other geographic locations. A multi-center randomized controlled study may be needed to test whether evidence would support wider use of the FSM toolkit in a broader CFS patient populations. Second, since most patients recruited in the study had severe CFS, the absence of contact with an interventionist may have precluded individual adjustments of the treatment protocol to maximize benefits. In addition, the clinical efficacy of the intervention is relatively small, given that recent research syntheses have indicated mixed results with great heterogeneity with respect to the beneficial effect of CBT[9, 10] Future studies should include larger samples to examine whether modified protocols retaining some elements of face-to-face contact in lieu of the home-based self-management would yield larger effects while at modest costs. Finally, as CBT interventions tend to generate improvement at 3–6 months post intervention, the 1-year time horizon used this analysis may not capture this initial gain and subsequent tapering off.
Despite these limitations, the present study provides important preliminary evidence of cost-effectiveness for a low-cost CBT-based FSM toolkit for home use by CFS patients. It has the strength of incorporating the economic data collection into the clinical outcomes measures by design and as a result, both cost and effectiveness data were collected from the same individuals, representing the good practice for conducting economic evaluation in clinical trials [44]. Nevertheless, additional research is needed to examine whether the toolkit is effective in a larger patient population with less severe symptoms and/or whether some minimal level of interventionist contact is required to optimize the treatment effect demonstrated by earlier interventions delivered in part by trained professionals.
ACKNOWLEDGEMENTS
This study (blinded for review) was supported by NIH grant #R42NR010496 (National Institute of Nursing Research; PI: F. Friedberg). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. All authors declared no conflicts of interest.
Appendix 1.
Unit prices used to value different types of goods/services for the analysis (in 2013 $)
| Service | Unit | Unit Cost ($) |
| Primary care physician | visit | $ 128 |
| Nurse practitioner | visit | $ 96 |
| Specialist | visit | $ 162 |
| Physical/Occupational therapist | visit | $ 96 |
| Social worker | visit | $ 81 |
| Homeopath/Acupuncturist | visit | $ 65 |
| Dentist | visit | $ 162 |
| Emergency room | visit | $ 704 |
| Hospital | visit | $ 2,115 |
| Prescription medication | count | $ 34 |
| MRI | count | $ 443 |
| CT | count | $ 243 |
| Ultrasound | count | $ 55 |
| X-ray | count | $ 84 |
| Blood test | count | $ 38 |
| Child/personal care | hour | $ 11 |
| Hourly wage | hour | $ 23 |
REFERENCE
- 1.IOM, Beyond myalgic encephalomyelitis/chronic fatigue syndrome: Redefining an illness. 2015, Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 2.Jason LA, Taylor RR, Kennedy CL, et al. , Chronic fatigue syndrome: occupation, medical utilization, and subtypes in a community-based sample. J Nerv Ment Dis, 2000. 188(9):568–76. [DOI] [PubMed] [Google Scholar]
- 3.Sabes-Figuera R, McCrone P, Hurley M, et al. , The hidden cost of chronic fatigue to patients and their families. BMC Health Serv Res, 2010. 10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Twisk FN, The status of and future research into Myalgic Encephalomyelitis and Chronic Fatigue Syndrome: the need of accurate diagnosis, objective assessment, and acknowledging biological and clinical subgroups. Front Physiol, 2014. 5:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nijrolder I, van der Horst H, and van der Windt D, Prognosis of fatigue. A systematic review. J Psychosom Res, 2008. 64(4):335–49. [DOI] [PubMed] [Google Scholar]
- 6.Nunez M, Fernandez-Sola J, Nunez E, et al. , Health-related quality of life in patients with chronic fatigue syndrome: group cognitive behavioural therapy and graded exercise versus usual treatment. A randomised controlled trial with 1 year of follow-up. Clin Rheumatol, 2011. 30(3):381–9. [DOI] [PubMed] [Google Scholar]
- 7.McCrone P, Sharpe M, Chalder T, et al. , Adaptive pacing, cognitive behaviour therapy, graded exercise, and specialist medical care for chronic fatigue syndrome: a cost-effectiveness analysis. PLoS One, 2012. 7(8):e40808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collin SM, Crawley E, May MT, et al. , The impact of CFS/ME on employment and productivity in the UK: a cross-sectional study based on the CFS/ME national outcomes database. BMC Health Serv Res, 2011. 11:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith ME, Haney E, McDonagh M, et al. , Treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med, 2015. 162(12):841–50. [DOI] [PubMed] [Google Scholar]
- 10.Larun L, Brurberg KG, Odgaard-Jensen J, et al. , Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev, 2016. 2:Cd003200. [DOI] [PubMed] [Google Scholar]
- 11.Ridsdale L, Darbishire L, and Seed PT, Is graded exercise better than cognitive behaviour therapy for fatigue? A UK randomized trial in primary care. Psychol Med, 2004. 34(1):37–49. [DOI] [PubMed] [Google Scholar]
- 12.Moss-Morris R, Sharon C, Tobin R, et al. , A randomized controlled graded exercise trial for chronic fatigue syndrome: outcomes and mechanisms of change. J Health Psychol, 2005. 10(2):245–59. [DOI] [PubMed] [Google Scholar]
- 13.Twisk FN and Maes M, A review on cognitive behavorial therapy (CBT) and graded exercise therapy (GET) in myalgic encephalomyelitis (ME) / chronic fatigue syndrome (CFS): CBT/GET is not only ineffective and not evidence-based, but also potentially harmful for many patients with ME/CFS. Neuro Endocrinol Lett, 2009. 30(3):284–99. [PubMed] [Google Scholar]
- 14.Wiborg JF, Knoop H, Stulemeijer M, et al. , How does cognitive behaviour therapy reduce fatigue in patients with chronic fatigue syndrome? The role of physical activity. Psychol Med, 2010. 40(8):1281–7. [DOI] [PubMed] [Google Scholar]
- 15.Schreurs KM, Veehof MM, Passade L, et al. , Cognitive behavioural treatment for chronic fatigue syndrome in a rehabilitation setting: effectiveness and predictors of outcome. Behav Res Ther, 2011. 49(12):908–13. [DOI] [PubMed] [Google Scholar]
- 16.White PD, Goldsmith KA, Johnson AL, et al. , Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet, 2011. 377(9768):823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durham RC, Chambers JA, Power KG, et al. , Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland. Health Technol Assess, 2005. 9(42):1–174. [DOI] [PubMed] [Google Scholar]
- 18.O’Dowd H, Gladwell P, Rogers CA, et al. , Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme. Health Technol Assess, 2006. 10(37):iii–iv, ix-x, 1–121. [DOI] [PubMed] [Google Scholar]
- 19.Price JR, Mitchell E, Tidy E, et al. , Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database Syst Rev, 2008(3):CD001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedman E, Andersson E, Lindefors N, et al. , Cost-effectiveness and long-term effectiveness of internet-based cognitive behaviour therapy for severe health anxiety. Psychol Med, 2013. 43(2):363–74. [DOI] [PubMed] [Google Scholar]
- 21.Chisholm D, Godfrey E, Ridsdale L, et al. , Chronic fatigue in general practice: economic evaluation of counselling versus cognitive behaviour therapy. Br J Gen Pract, 2001. 51(462):15–8. [PMC free article] [PubMed] [Google Scholar]
- 22.McCrone P, Ridsdale L, Darbishire L, et al. , Cost-effectiveness of cognitive behavioural therapy, graded exercise and usual care for patients with chronic fatigue in primary care. Psychol Med, 2004. 34(6):991–9. [DOI] [PubMed] [Google Scholar]
- 23.Severens JL, Prins JB, van der Wilt GJ, et al. , Cost-effectiveness of cognitive behaviour therapy for patients with chronic fatigue syndrome. QJM, 2004. 97(3):153–61. [DOI] [PubMed] [Google Scholar]
- 24.Visser MS, Zonneveld LN, Van’t Spijker A, et al. , The Cost-Effectiveness of Cognitive-Behavioral Group Training for Patients with Unexplained Physical Symptoms. Value Health, 2015. 18(5):570–7. [DOI] [PubMed] [Google Scholar]
- 25.Meng H, Friedberg F, and Castora-Binkley M, Cost-effectiveness of chronic fatigue self-management versus usual care: a pilot randomized controlled trial. BMC Fam Pract, 2014. 15:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson G, Epstein D, Chew-Graham C, et al. , Cost-effectiveness of supported self-management for CFS/ME patients in primary care. BMC Fam Pract, 2013. 14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedberg F, Adamowicz J, Caikauskaite I, et al. , Efficacy of two delivery modes of behavioral self-management in severe chronic fatigue syndrome. Fatigue: Biomedicine, Health and Behavior, 2016. 4(3):63–69. [Google Scholar]
- 28.Friedberg F, Chronic fatigue syndrome, fibromyalgia, and related illnesses: a clinical model of assessment and intervention. J Clin Psychol, 2010. 66(6):641–65. [DOI] [PubMed] [Google Scholar]
- 29.Brazier J, Roberts J, and Deverill M, The estimation of a preference-based measure of health from the SF-36. J Health Econ, 2002. 21(2):271–92. [DOI] [PubMed] [Google Scholar]
- 30.Gold M, Siegel J, Russell L, et al. , Cost-effectiveness in health and medicine. 1996, New York, New York: Oxford University Press. [Google Scholar]
- 31.Beecham J and Knapp M, Costing psychiatric interventions, in Measuring mental health needs, Thornicroft G, Editor. 2001. p. 200–224.
- 32.Goossens MEJB, Rutten-Van Mölken MPMH, Kole-Snijders AMJ, et al. , Health economic assessment of behavioural rehabilitation in chronic low back pain: a randomised clinical trial. Health Economics, 1998. 7(1):39–51. [DOI] [PubMed] [Google Scholar]
- 33.Glick HA, Doshi JA, Sonnad SS, et al. , Economic Evaluation in Clinical Trials Handbooks in Health Economic Evaluation Series, ed. Gray A and Briggs A 2007, New York: Oxford University Press. [Google Scholar]
- 34.Oostenbrink JB, Koopmanschap MA, and Rutten FF, Standardisation of costs: the Dutch Manual for Costing in economic evaluations. Pharmacoeconomics, 2002. 20(7):443–54. [DOI] [PubMed] [Google Scholar]
- 35.Krol M and Brouwer W, How to estimate productivity costs in economic evaluations. Pharmacoeconomics, 2014. 32(4):335–44. [DOI] [PubMed] [Google Scholar]
- 36.Burgess M, Andiappan M, and Chalder T, Cognitive behaviour therapy for chronic fatigue syndrome in adults: face to face versus telephone treatment: a randomized controlled trial. Behav Cogn Psychother, 2012. 40(2):175–91. [DOI] [PubMed] [Google Scholar]
- 37.Manning WG and Mullahy J, Estimating log models: to transform or not to transform? Journal of Health Economics, 2001. 20(4):461–494. [DOI] [PubMed] [Google Scholar]
- 38.Drummond M, Sculpher M, Torrance G, et al. , Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. 2005, New York, NY: Oxford University Press. [Google Scholar]
- 39.Mooney CZ, Bootstrap statistical inference: examples and evaluations for political science. American Journal of Political Science, 1996. 40(2):570–602. [Google Scholar]
- 40.Briggs AH, O’Brien BJ, and Blackhouse G, Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health, 2002. 23:377–401. [DOI] [PubMed] [Google Scholar]
- 41.Stinnett AA and Mullahy J, The negative side of cost-effectiveness analysis. Jama, 1997. 277(24):1931–2; author reply 1932–3. [PubMed] [Google Scholar]
- 42.Hoch JS, Rockx MA, and Krahn AD, Using the net benefit regression framework to construct cost-effectiveness acceptability curves: an example using data from a trial of external loop recorders versus Holter monitoring for ambulatory monitoring of “community acquired” syncope. BMC Health Services Research, 2006. 6(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fenwick E, Claxton K, and Sculpher M, Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ, 2001. 10(8):779–87. [DOI] [PubMed] [Google Scholar]
- 44.Ramsey SD, Willke RJ, Glick H, et al. , Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health, 2015. 18(2):161–72. [DOI] [PubMed] [Google Scholar]
- 45.Friedberg F, Napoli A, Coronel J, et al. , Chronic fatigue self management in primary care: A randomized trial. Psychosomatic Medicine, 2013. 75:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabes-Figuera R, McCrone P, Hurley M, et al. , Cost-effectiveness of counselling, graded-exercise and usual care for chronic fatigue: evidence from a randomised trial in primary care. BMC Health Serv Res, 2012. 12:264. [DOI] [PMC free article] [PubMed] [Google Scholar]