Abstract
Hibernoma is a benign adipocytic tumor with predilection for subcutaneous tissue of the thigh, upper trunk, and neck of middle-aged adults. 11q13 rearrangement resulting in MEN1/AIP codeletion is characteristic. Hibernomas are composed, in varying proportions, of brown fat cells, mature adipocytes, and microvacuolated lipoblast-like cells. Examples containing predominantly multivacuolated lipoblast-like cells are uncommon and distinction from atypical lipomatous tumor (ALT) is important for clinical management. We herein present the clinicopathologic features of 64 hibernomas histologically mimicking ALT. MDM2 and CDK4 immunohistochemistry as well as MDM2 fluorescence in-situ hybridization (FISH) were performed in a subset of cases. Clinical and follow-up information were obtained from referring pathologists. Thirty-four patients were male and 30 female, with a median age of 43 years (range, 24–78 years). The tumors were well- circumscribed and mostly deeply located (53/64 cases, 83%) with a median tumor size of 12.9 cm (range, 3.5–23 cm) and predilection for the thigh (42/64 cases, 66%). Histologically, large cells with prominent lipoblast-like cytoplasmic fatty vacuoles and small central nuclei were present to a prominent degree in all cases, along with mature univacuolated adipocytes and smaller numbers of large, finely vacuolated cells with eosinophilic granular cytoplasm. Nuclear atypia and mitoses were absent. None of the 39 cases tested showed CDK4 and MDM2 overexpression or MDM2 amplification. Follow-up, available for 16/64 cases (median, 47 months; range, 1–165 months), revealed no recurrences or metastases. Hibernoma mimicking ALT shows predilection for deep soft tissue, especially in the thigh. These tumors behave in a benign fashion and MDM2/CDK4 negativity may be useful in excluding ALT.
Keywords: Hibernoma, brown fat, atypical lipomatous tumor, liposarcoma
Introduction
Hibernoma, a benign brown fat tumor, was first described as “pseudolipoma” by Merkel in 19061 and was termed “hibernoma” by Gery in 1914 because of its morphologic resemblance to the brown fat observed in hibernating animals.2 Hibernomas are uncommon and account for <2% of benign lipomatous tumors and 1% of all adipocytic tumors.3 They mostly occur in middle-aged adults with equal gender distribution, tend to be large with an average tumor size of 9.3 cm (range, 1–24 cm), and show a predilection for subcutaneous tissue of the thigh, upper trunk, and neck.4 Deep seated hibernomas account for <15% of cases.4 Hibernomas are benign tumors that do not recur after complete resection and no metastases have been reported.4 11q13 rearrangement resulting in MEN1 and AIP co-deletion is a characteristic finding.5, 6 Only a single case of hibernoma with (9;11)(q34;q13) translocation has been reported.7 Hibernomas are composed, in varying proportions, of three cell types which include large, finely vacuolated cells with eosinophilic granular cytoplasm (brown fat cells), cells with larger fatty vacuoles resembling lipoblasts, and mature adipocytes. In addition, rare myxoid, lipoma-like, and spindle cell variants of hibernoma have been described.4 Immunohistochemically, most hibernomas are positive for S- 100 protein and usually negative for CD34.4
We herein present the clinicopathologic features of 64 cases of a distinct morphologic subset of hibernomas containing predominantly multivacuolated lipoblast-like cells, which may therefore be confused histologically with atypical lipomatous tumor (ALT).
Materials and Methods
Sixty-four cases diagnosed as hibernoma mimicking ALT between 2000 and 2017 were retrieved from the consultation file of one of the authors (C. D. M. F.). Hematoxylin and eosin stained sections were reviewed when available, and pathologic features were re-evaluated by at least two of the authors. Immunohistochemical staining was performed on 4-μm-thick formalin-fixed paraffin-embedded tissue sections, using monoclonal antibodies directed against MDM2 (EMD Millipore, Billerica, MA, clone 1F2, 1:15, citrate buffer pressure cooker) and CDK4 (Cell Signaling, Danvers, MA, clone D9G3E, 1:400, citrate buffer pressure cooker). Appropriate controls were used throughout. MDM2 fluorescence in-situ hybridization (FISH) was performed in a subset of cases at the time of diagnosis. Clinicopathologic and follow-up data, when available, were kindly provided by the referring pathologists and clinicians (see Acknowledgements).
This study was performed with approval of the Institutional Review Board at Brigham and Women’s Hospital.
Results
Clinicopathologic Findings
Table 1 summarizes the clinicopathologic data of 64 cases of hibernoma mimicking ALT. Thirty-four patients were male and 30 female (M:F=1.1:1), with a median age at diagnosis of 43 years (range, 24–78 years). Grossly, the hibernomas were well-circumscribed and mostly subfascial (i.e., deeply located) (53/64 cases, 83%) or superficial (11/64 cases, 17%). The majority of tumors occurred in the thigh (42/64 cases, 66%), followed by buttock (8/64 cases), inguinal region/groin (6/64 cases), trunk (3/64 cases), and less commonly axilla, foot, popliteal fossa, leg (not specified), and stomach (one case each). Most patients presented clinically with a painless palpable mass. The median tumor size was rather large, with a median largest dimension of 12.9 cm (range, 3.5–23 cm).
Table 1.
Clinicopathologic findings in 64 cases of hibernoma mimicking atypical lipomatous tumor.
| Case no. | Age | Sex | Tumor site (side) | Tumor size (cm) | Margin status |
MDM2 IHC |
MDM2 FISH |
CDK4 IHC |
Follow up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | F | Inguinal region (R) | 6 | - | Negative | - | Negative | - |
| 2 | 53 | M | Thigh | 10 | - | Negative | - | Negative | - |
| 3 | 37 | M | Thigh (L) | 18 | - | - | - | - | - |
| 4 | 37 | M | Thigh (L) | 18 | Negative | - | - | - | - |
| 5 | 74 | M | Thigh (L) | 23 | - | - | - | - | - |
| 6 | 49 | M | Thigh (R) | 14 | - | - | - | - | - |
| 7 | 43 | F | Thigh (L) | 15 | Positive | - | - | - | ANED (84) |
| 8 | 56 | M | Thigh (L) | 19 | - | - | - | - | - |
| 9 | 50 | M | Inguinal region (L) | 3.5 | Negative | Negative | - | Negative | ANED (165) |
| 10 | 57 | F | Thigh (L) | 12.5 | - | - | - | - | ANED (135) |
| 11 | 42 | M | Thigh (R) | 10 | Negative | Negative | - | Negative | ANED (76) |
| 12 | 39 | M | Thigh (L) | 8.7 | Negative | - | - | - | - |
| 13 | 25 | F | Axilla (L) | 11 | Negative | - | - | - | ANED (150) |
| 14 | 39 | M | Thigh (R) | 20.5 | Positive | - | - | - | ANED (118) |
| 15 | 40 | F | Thigh (R) | 17 | - | - | - | - | - |
| 16 | 61 | F | Thigh (L) | 15 | - | Negative | - | Negative | - |
| 17 | 42 | F | Thigh (L) | 20 | - | - | - | - | - |
| 18 | 49 | M | Thigh (L) | 13 | Negative | Negative | - | Negative | - |
| 19 | 33 | F | Thigh (R) | 11 | Negative | Negative | - | Negative | - |
| 20 | 40 | M | Thigh (R) | 14.7 | - | - | - | - | - |
| 21 | 38 | M | Popliteal fossa (L) | 6 | - | - | - | - | - |
| 22 | 39 | F | Thigh (R) | 14 | Positive | Negative | - | Negative | - |
| 23 | 44 | F | Lower back | 4 | - | Negative | - | Negative | - |
| 24 | 28 | M | Thigh (L) | 14.2 | Negative | - | - | - | - |
| 25 | 42 | M | Thigh (L) | 18.5 | - | - | - | - | - |
| 26 | 24 | M | Thigh (R) | 8 | - | - | - | - | ANED (48) |
| 27 | 65 | M | Foot (R) | 7 | - | Negative | - | Negative | - |
| 28 | 28 | F | Groin (R) | 11 | Negative | - | - | - | - |
| 29 | 59 | M | Thigh (R) | 6.2 | - | Negative | - | Negative | - |
| 30 | 66 | F | Thigh (L) | 6 | Positive | Negative | - | Negative | - |
| 31 | 50 | F | Thigh (R) | 18 | Positive | Negative | - | Negative | - |
| 32 | 56 | F | Thigh (L) | 12.7 | Negative | - | - | - | - |
| 33 | 39 | F | Thigh (L) | 14 | Positive | Negative | - | Negative | - |
| 34 | 43 | F | Thigh (L) | 8.1 | Positive | Negative | - | Negative | - |
| 35 | 52 | M | Thigh (R) | 10 | Negative | Negative | - | Negative | - |
| 36 | 78 | F | Inguinal region (L) | 8 | Negative | - | - | - | ANED (40) |
| 37 | 39 | M | Buttock (R) | 17.3 | Negative | Negative | - | Negative | - |
| 38 | 58 | F | Thigh (L) | 13.5 | - | Negative | - | Negative | - |
| 39 | 50 | F | Buttock (R) | 15.5 | - | Negative | - | Negative | ANED (1) |
| 40 | 40 | M | Thigh (L) | 15 | Negative | Negative | - | Negative | ANED (45) |
| 41 | 38 | M | Thigh (L) | 11 | - | Negative | - | Negative | - |
| 42 | 61 | M | Abdominal wall (R) | 14 | - | - | - | - | - |
| 43 | 43 | F | Leg (L) | 7 | - | - | - | - | - |
| 44 | 46 | F | Thigh (L) | 7.7 | - | - | - | - | - |
| 45 | 46 | F | Thigh (R) | 20 | - | Negative | - | Negative | ANED (9) |
| 46 | 26 | M | Buttock (R) | 14 | Positive | - | Negative | - | - |
| 47 | 47 | M | Buttock/lower back | 15 | - | Negative | - | Negative | - |
| 48 | 31 | F | Thigh (L) | 14 | Positive | Negative | - | Negative | - |
| 49 | 68 | F | Thigh (L) | 10 | - | Negative | - | Negative | - |
| 50 | 31 | M | Thigh (R) | 14 | - | Negative | - | Negative | - |
| 51 | 44 | M | Thigh (L) | 7.3 | Positive | - | Negative | - | ANED (23) |
| 52 | 43 | F | Thigh (R) | 10 | - | Negative | - | Negative | - |
| 53 | 34 | F | Thigh (L) | 9.5 | - | Negative | - | Negative | - |
| 54 | 47 | M | Thigh (L) | 12 | Negative | Negative | - | Negative | - |
| 55 | 45 | F | Groin (L) | 11 | Positive | Negative | - | Negative | - |
| 56 | 51 | M | Buttock (R) | 14 | - | Negative | - | Negative | - |
| 57 | 48 | M | Buttock (R) | 15.8 | - | - | - | - | ANED (7) |
| 58 | 30 | F | Buttock (R) | 14.5 | Negative | - | - | - | ANED (2.5) |
| 59 | 45 | M | Thigh (L) | 3.8 | - | Negative | - | Negative | - |
| 60 | 28 | M | Thigh (L) | 6.7 | - | Negative | - | Negative | - |
| 61 | 36 | F | Inguinal region | 23 | - | - | Negative | - | - |
| 62 | 69 | M | Buttock (L) | 23 | - | - | Negative | Negative | - |
| 63 | 41 | M | Abdominal wall (L) | 8.4 | Positive | - | Negative | - | ANED (1) |
| 64 | 38 | F | Stomach | 10 | Negative | - | Negative | - | ANED (5) |
ANED: alive, no evidence of disease; IHC: immunohistochemistry; L: left; R: right
Grossly, the tumors were lobulated, well circumscribed, and surrounded partially or completely by a thin capsule in a subset of cases (21/64 cases), whereas the remaining cases were circumscribed but not clearly encapsulated. The cut surface varied from yellow to brown, and only 5/64 cases showed focal “necrosis”. Microscopically, the tumor cells were arranged in lobules separated by thin fibrous septa and were composed of three cell populations, to varying degrees (Fig. 1): finely microvacuolated cells with clear cytoplasm and central or less frequently eccentrically located small vesicular nuclei with single central nucleolus (lipoblast-like cells) without scalloping of the nuclei (Fig. 2), mature adipocytes, and brown fat cells with granular eosinophilic cytoplasm. The relative proportions of lipoblast-like cells per case ranged from 10% to 80%, but in areas of all cases these cells were strikingly predominant. No cytologic atypia, mitoses, or areas of necrosis were observed histologically in any of the cases. The hibernomas contained variable numbers of thin-walled, blood vessels of varying size and 7/64 cases contained occasional thick-walled vessels. Resection margins were positive in 12/64 cases (19%). None of the tumors tested expressed MDM2 (0/33) or CDK4 (0/34) by immunohistochemistry (Fig. 3) and FISH, performed in six cases, revealed no MDM2 amplification.
Figure 1.
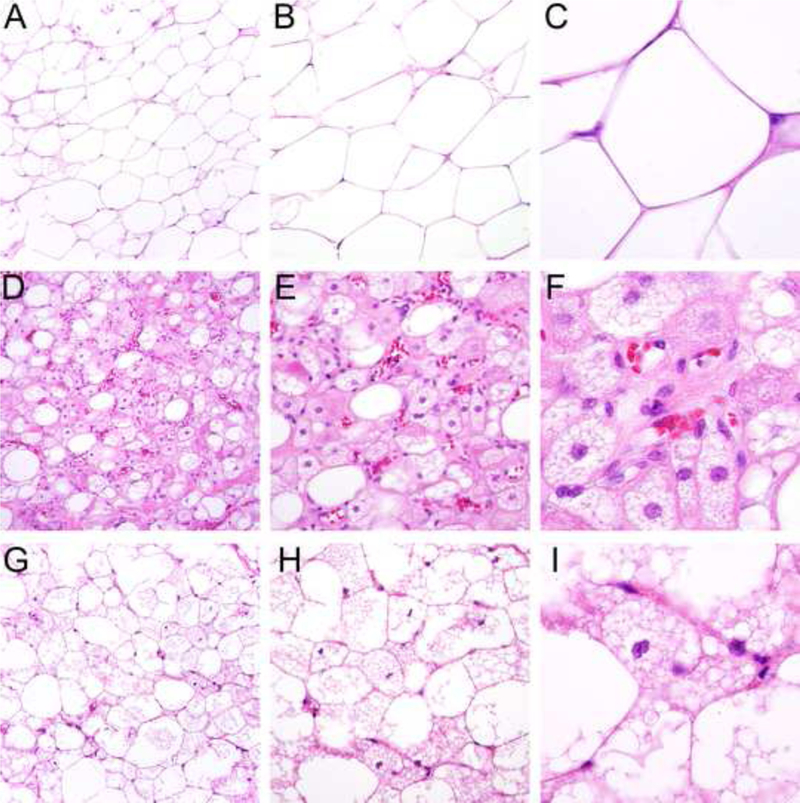
Morphologic spectrum of hibernoma: tumors consist in variable proportions of mature adipocytes containing a single cytoplasmic vacuole (A-C), large, finely vacuolated cells with eosinophilic granular cytoplasm (hibernoma cells) resembling brown fat (D-F) and multivacuolated lipoblastlike cells with small central nuclei (G-I), which were especially prominent in this case series (see Figure 2).
Figure 2.
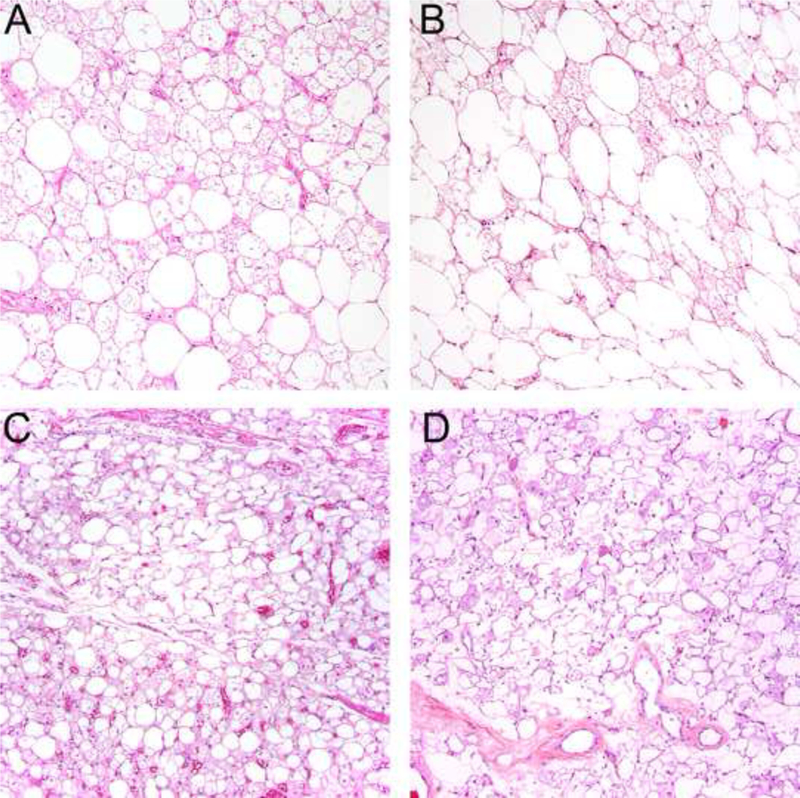
Areas of each tumor contained numerous multivacuolated lipoblast-like cells, often in sheets (A,B), but in others areas admixed brown fat cells and mature adipocytes were admixed (C-D).
Figure 3.
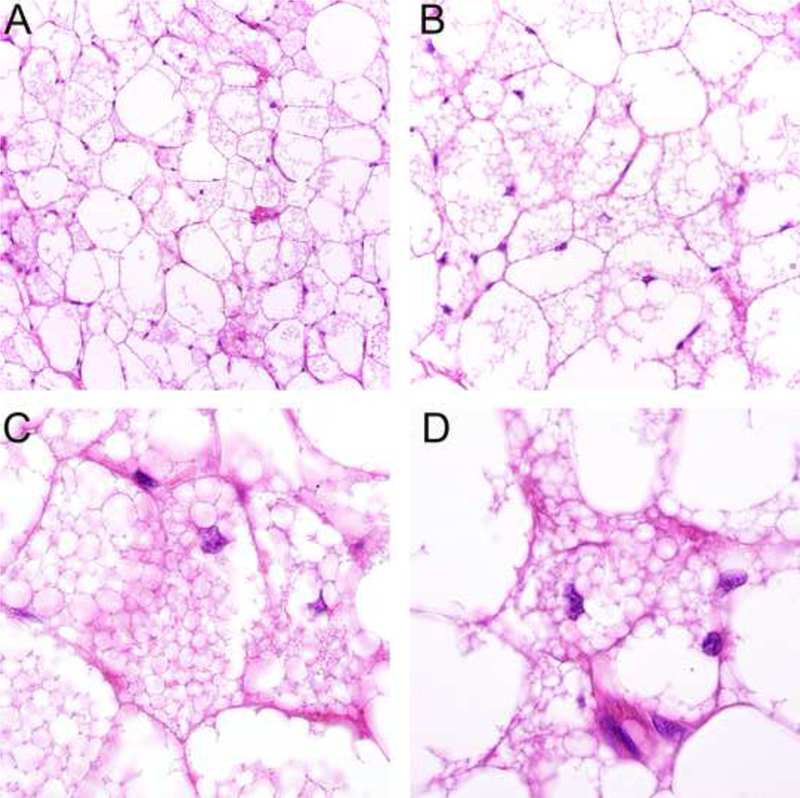
Prominent multivacuolated lipoblast-like cells in hibernoma mimicking atypical lipomatous tumor exhibit small nuclei without hyperchromasia or atypia and contain multiple small fatty vacuoles with minimal scalloping of the nuclei (A-D).
Follow-up
Following a benign diagnosis, follow-up information was available for only 16/64 (25%) cases with a median follow-up time of 47 months (range, 1–165 months) and revealed that none of the patients developed local recurrence or metastasis, emphasizing the benign behavior of hibernoma. At the time of follow-up, all patients were alive without evidence of disease. Most patients had not been seen again after initial treatment.
Four cases were considered recurrent by the referring institution, two of which had known positive margins in the initial resection, suggesting incomplete resection rather than true recurrence.
Discussion
Hibernoma is an uncommon benign tumor composed of brown fat and most frequently arises in the thigh, upper trunk, and neck. Rare cases occurring in the axilla, groin, supraclavicular area, buttock, scalp, abdominal wall, breast, flank, pleura, adrenal, spine, larynx, bone, and spermatic cord have been described.4, 8–22
We present herein the clinicopathologic findings in 64 cases of hibernoma with predominant microvacuolated lipoblast-like cells, referred in consultation with a diagnostic concern for ALT in 42/64 (66%) of cases, leading to preoperative radiation therapy in one case (#52). In addition to the classical variant of hibernoma, accounting for ~80% of cases, myxoid (9%), lipoma-like (7%), and spindle cell (2%), variants have been described in the literature4, but tumors with such striking ALT-like features have not been emphasized previously. Because of the biased nature of consultation material, among which “classical” hibernomas are rare, it is not possible to determine or estimate the proportion of hibernomas which mimic ALT. It is also impossible for us to reliably assess the extent of lipoblast-like cells in these 64 cases, given the variability in sampling and number of slides submitted in the setting of consultation cases. It is abundantly clear, however, that most of the referring pathologists were concerned about excluding ALT, suggesting that, in the slides they saw, lipoblast-like cells were a major and striking component (which was often mentioned in the referral letters).
The median patient age of 43 years and a slight male predominance observed in our study are in concordance with the findings in a large series of 170 hibernomas described by Furlong et al.4 In their study, the authors find that only 30% of all hibernomas occurred in the thigh and 11% were deep-seated.4 In our series, hibernoma mimicking ALT shows a clear predilection for the thigh (66%) and deep/intramuscular location (83%). Of note, in two smaller case series of hibernoma, the thigh was reported as the most common location (in 21% and 76% of cases, respectively).18, 19
All cases in our series were diagnosed based on morphologic features and additional immunohistochemical workup is generally not needed to establish the diagnosis of hibernoma. None of the cases tested (in total 39) showed MDM2/CDK4 overexpression or MDM2 amplification which is useful to rule out ALT as the main histologic differential diagnosis in this hibernoma subtype.
ALT frequently occurs in the lower extremities and is a locally aggressive tumor that comprises adipocytic (or lipoma-like), sclerosing, inflammatory, and spindle cell histologic subtypes.3 The designation ALT is used for lesions that are considered amenable to surgical resection, whereas well-differentiated liposarcoma is used to designate lesions at non-extremity sites, e. g. retroperitoneum, which require more radical surgery in order to achieve clear margins. ALT does not have the potential to metastasize and in the extremities, rarely undergoes dedifferentiation, but local recurrence is observed in 20–30% of cases.
Histologically, ALT is composed of a mature adipocytic component to varying degrees, showing significant variation in cell size and at least focal nuclear atypia and hyperchromasia, usually in both adipocytes and stromal cells. The presence of scattered hyperchromatic, often multinucleate, stromal cells or multivacuolated lipoblasts (defined by the presence of single or multiple sharply marginated cytoplasmic vacuoles scalloping an enlarged hyperchromatic nucleus) contributes to the morphologic diagnosis.3 In contrast, the multivacuolated lipoblast-like cells observed in hibernoma exhibit small nuclei which are neither hyperchromatic nor atypical, the vacuoles tend to be smaller than those observed in true lipoblasts and the scalloping of the nuclei is minimal. Furthermore, in ALT, true multivacuolated lipoblasts are usually not as numerous as the lipoblast-like cells in hibernoma mimicking ALT. An additional morphologic feature favoring hibernoma is the presence of scattered cells with more granular eosinophilic cytoplasm (brown fat cells). It is worth noting that exceedingly rare examples of well-differentiated liposarcoma (with MDM2 gene amplification) exhibiting hibernoma-like areas have been reported. 23
MDM2 and CDK4 overexpression resulting from ring or giant marker chromosomes and high level 12q13–15 amplification (including the MDM2, CDK4, and HMGA2 loci) help distinguish ALT from hibernoma, which instead shows 11q13 rearrangements involving AIP and MEN1. Recently published data have shown that loss of AIP is likely responsible for the brown fat phenotype.24
Distinction of hibernoma from ALT is clinically important as ALT requires complete resection and harbors a risk of local recurrence in ~30% of cases, whereas none of the hibernomas mimicking ALT in our series recurred locally. However, two thirds of cases were initially diagnosed as ALT (or had a differential diagnosis that included ALT) and the fact that one patient received preoperative radiation highlights the importance of establishing the correct diagnosis to prevent unnecessary and potentially harmful treatment.
In summary, hibernoma consisting predominantly of multivacuolated lipoblast-like cells, shows predilection for the thigh and other deep locations, and may be confused histologically with ALT. Negativity for MDM2 and CDK4 by immunohistochemistry and lack of MDM2 amplification are useful in the distinction of these lesions.
Figure 4.
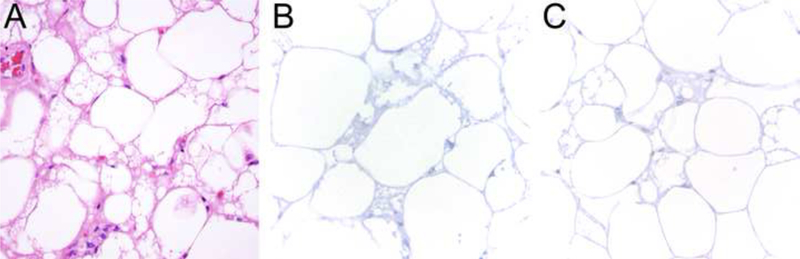
The lipoblast-like cells in hibernoma mimicking atypical lipomatous tumor (A) are negative for MDM2 (B) and CDK4 (C) by immunohistochemistry.
Acknowledgements
The authors thank the following pathologists and clinicians who kindly provided case material and clinical follow-up when available: Dr. Neil A. Abrahams, Hollywood, FL; Dr. Manuel Alvarez, Santiago, Chile; Dr. Robin Andree, Greensboro, NC; Dr. Rebecca R. Balaj, Dayton, OH; Dr. N.K. de Boer, Groningen, The Netherlands; Dr. T. David Bourne, Louisville, KY, Prof. Adriaan P. de Bruine, Venlo, The Netherlands; Dr. Norberto Cartagena, Jr., Miami, FL; Dr. Julio C. Cespedes, Jackson, MS; Dr. Jey-Hsin Chen, Seattle, WA; Dr. George H. Clarke, Carey, NC; Dr. Vincenzo Ciocca, Wynnewood, PA; Prof. Charles Eugene Connolly, Galway, Ireland; Dr. John V. Cooney, Quincy, MA; Dr. Ediz F. Cosar, Worcester, MA; Dr. Haytham H. Dimashkieh, Greenville, SC; Dr. Alain Dumas, Shelton, CT; Dr. Chad Ellermeier, Seattle, WA; Dr. Gary Ellwein, Idaho Falls, ID; Dr. Paul R. Ferbend, Providence, RI; Dr. Ashley D. Gable, Tulsa, OK; Dr. David L. Gardner, Monterey, CA; Dr. J.M.H.H. van Gorp, Utrecht, The Netherlands; Dr. Michael P. Greeff, Lethbridge, Canada; Dr. A.D. Groote, Groningen, The Netherlands; Dr. Meri C. Guerry, Greenville, SC; Dr. Anthony Guichard, Burlingame, CA; Dr. Steven D. Gurley, Scottsdale, AZ; Dr. Min Han, Hackensack, NJ; Dr. Sami Harawi, Hackensack, NJ; Dr. Jihad Hayek, Boston, MA; Dr. Susan Homan, Albany, NY; Dr. Heng Hong, Greenville, NC; Dr. Nancy J. Hunter, Morristown, NJ; Dr. Mary N. Kilo, Portland, OR; Dr. Robin Kirby, Pittsfield, MA; Dr. Eric Lang, Columus, OH; Dr. John Sang Jin Lee, San Diego, CA; Dr. Jeffrey F. Lipton, Brooklyn, NY; Dr. Ciaran Mannion, Hackensack, NJ; Dr. Holly L. McDaniel, Bangor, ME; Dr. Grace D. Moore, Louisville, KY; Dr. Harry F. Moussouris, Flushing, NY; Dr. Sophie Otto, Adelaide, Australia; Dr. Ronald W. Oxenhandler, Chattanooga, TN; Dr. Amy Rapkiewicz, New York, NY; Dr. Douglas Reale, Miami, FL; Dr. Lorinda A. Soma, Sacramento, CA; Dr. Kunchang Song, Hackensack, NJ; Rabbi Sorotzkin, Jerusalem, Israel; Dr. Janet Stallman, Newport Beach, CA; Dr. Yanyu (Helen) Sun, Brooklyn, NY; Dr. Cheryl A. Szpak, Raleigh, NC; Dr. Sigurd J. Torgerson, Colorado Springs, CO; Dr. Tien Anh N. Tran, Orlando, FL; Dr. Jonathan L. Vitsky, Chesterfield, MO; Dr. J.E. van der Wal, Groningen, The Netherlands; Dr. A.E. Wassenaar, Amsterdam, The Netherlands; Dr. Jon D. Wilson, Royal Oak, MI.
Footnotes
Financial disclosure: The authors have no competing interests to disclose
Conflict of interest
The authors have no conflict of interest to disclose.
References
- 1.Merkel H On a pseudolipoma of the breast. Beitr Pathol Anat. 1906;39:152–157. [Google Scholar]
- 2.Discussions Gery L.. Bull Mem Soc Anat Paris. 1914;89:111–112. [Google Scholar]
- 3.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (Eds.). WHO Classification of Tumours of Soft Tissue and Bone. 4th edition, IARC, Lyon; 2013. [Google Scholar]
- 4.Furlong MA, Fanburg-Smith JC, Miettinen M. The morphologic spectrum of hibernoma: a clinicopathologic study of 170 cases. Am J Surg Pathol. 2001;25:809–814. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher CD, Akerman M, Dal Cin P, et al. Correlation between clinicopathological features and karyotype in lipomatous tumors. A report of 178 cases from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. Am J Pathol. 1996;148:623–630. [PMC free article] [PubMed] [Google Scholar]
- 6.Nord KH, Magnusson L, Isaksson M, et al. Concomitant deletions of tumor suppressor genes MEN1 and AIP are essential for the pathogenesis of the brown fat tumor hibernoma. Proc Natl Acad Sci U S A. 2010;107:21122–21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turaga KK, Silva-Lopez E, Sanger WG, et al. A (9;11)(q34;q13) translocation in a hibernoma. Cancer Genet Cytogenet. 2006;170:163–166. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher CD, Cole RS, Gower RL, et al. Hibernoma of the spermatic cord: the first reported case. Br J Urol. 1986;58:99–100. [DOI] [PubMed] [Google Scholar]
- 9.Naik R, Panda KM, Kushwaha AK, et al. Intramuscular Hibernoma: A Rare Tumour in Buttock. J Clin Diagn Res. 2015;9:ED01–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shackelford RE, Al Shaarani M, Ansari J, et al. A Twenty-Four-Year-Old Woman with Left Flank Lipoma-Like Hibernoma. Case Rep Oncol. 2017;10:438–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HS, Lee SG, Son S, et al. Hibernoma in the thoracic back muscle accompanied by neurilemmoma. Korean JSpine. 2012;9:362–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantisani V, Mortele KJ, Glickman JN, et al. Large retroperitoneal hibernoma in an adult male: CT imaging findings with pathologic correlation. Abdom Imaging. 2003;28:721–724. [DOI] [PubMed] [Google Scholar]
- 13.Jaroszewski DE, Petris GD. Giant hibernoma of the thoracic pleura and chest wall. World J Clin Cases. 2013;1:143–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ugalde PA, Guilbault F, Vaillancourt R, et al. Subpleural hibernoma. Ann Thorac Surg. 2007;84:1376–1378. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz BF, Wasson L. Hibernoma arising from the adrenal gland. Urology. 2003;61:1035. [DOI] [PubMed] [Google Scholar]
- 16.Chitoku S, Kawai S, Watabe Y, et al. Intradural spinal hibernoma: case report. Surg Neurol. 1998;49:509–512; discussion 512–503. [DOI] [PubMed] [Google Scholar]
- 17.Minni A, Barbaro M, Vitolo D, et al. Hibernoma of the para-glottic space: an unusual tumour of the larynx. Acta Otorhinolaryngol Ital. 2008;28:141–143. [PMC free article] [PubMed] [Google Scholar]
- 18.Beals C, Rogers A, Wakely P, et al. Hibernomas: a single-institution experience and review of literature. Med Oncol. 2014;31:769. [DOI] [PubMed] [Google Scholar]
- 19.Mavrogenis AF, Coll-Mesa L, Drago G, et al. Hibernomas: clinicopathological features, diagnosis, and treatment of 17 cases. Orthopedics. 2011;34:e755–759. [DOI] [PubMed] [Google Scholar]
- 20.Song B, Ryu HJ, Lee C, et al. Intraosseous Hibernoma: A Rare and Unique Intraosseous Lesion. J Pathol Transl Med. 2017;51:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westacott L, Collins A, Dickenson I. Intraosseous Hibernoma in the Sacrum of an Adult. Int J Surg Pathol. 2016;24:749–752. [DOI] [PubMed] [Google Scholar]
- 22.Neufeld M, Medlicott S, Nickerson D. Case of hibernoma in the right supraclavicular fossa. Can JPlast Surg. 2005;13:33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallin M, Schneider N, Thway K. Well-differentiated liposarcoma with hibernoma-like morphology. Int J Surg Pathol 2016; 24: 620–622. [DOI] [PubMed] [Google Scholar]
- 24.Magnusson L, Hansen N, Saba KH et al. Loss of the tumor suppressor gene AIP mediates the browning of human brown fat tumors. J Pathol 2017; 243: 160–164. [DOI] [PubMed] [Google Scholar]


