Abstract
Background
Measures of skeletal muscle function decline at a faster rate with ageing than do indices of skeletal muscle mass. These observations have been attributed to age‐related changes in muscle quality, another functional determinant separate from skeletal muscle mass. This study tested the hypothesis that improved predictions of skeletal muscle strength can be accomplished by combining clinically available measures of skeletal muscle mass and quality.
Methods
The participants included 146 healthy adult (age ≥ 18 years, range 18–77 years; X ± SD 47 ± 17 years and body mass index 16.5–51.8 kg/m2; 27.7 ± 6.2 kg/m2) men (n = 60) and women (n = 86) in whom skeletal muscle mass was estimated as appendicular lean soft tissue (LST) measured by dual‐energy X‐ray absorptiometry and skeletal muscle quality as bioimpedance analysis‐derived phase angle and B‐mode‐evaluated echogenicity of mid‐thigh skeletal muscle. Strength of the right leg and both arms was quantified as knee isokinetic extension and handgrip strength using dynamometers. The statistical significance of adding phase angle or echogenicity to strength prediction multiple regression models that included extremity‐specific LST and other covariates (e.g. age and sex) was evaluated to test the study hypothesis.
Results
Right leg LST mass alone was significantly (P < 0.0001) correlated with isokinetic right leg strength (R 2 = 0.57). The addition of segmental phase angle measured in the right leg at 50 kHz increased the R 2 of this model to 0.66 (P < 0.0001); other phase angle frequencies (5 and 250 kHz) did not contribute significantly to these models. Results were similar for both right and left arm handgrip strength prediction models. Adding age and sex as model covariates increased the R 2 values of these models further (e.g. right leg strength model R 2 increased to 0.71), but phase angle continued to remain a significant (all P < 0.01) predictor of extremity strength. Similarly, when predicting isokinetic right leg strength, mid‐thigh skeletal muscle echogenicity added significantly (P < 0.0001) to right leg LST, increasing R 2 from 0.57 to 0.64; age was a significant (P < 0.0001) covariate in this model, increasing R 2 further to 0.68.
Conclusions
The hypothesis of the current study was confirmed, strongly supporting and extending earlier reports by quantifying the combined independent effects of skeletal muscle mass and quality on lower‐body and upper‐body measures of strength. These observations provide a clinically available method for future research aimed at optimizing sarcopenia and frailty risk prediction models.
Keywords: Phase angle, Bioimpedance analysis, Echogenicity, Strength, Sarcopenia, Muscle quality
Introduction
Loss of skeletal muscle mass and strength with ageing is the hallmarks of increasingly recognized sarcopenia1 and dynapenia.2 These structural and functional senescence‐related changes in skeletal muscle are accompanied by an increased risk of morbidity3 and mortality.4
At present, identifying people with sarcopenia is accomplished in the clinical setting using anatomic and functional measurements alone or in combination.5, 6, 7 Debate still centres on the optimal clinical measures for identifying patients who have age‐related skeletal muscle changes that place them at risk for developing adverse health outcomes such as falls, skeletal fractures, or impaired activities of daily living.
One concern with diagnostic markers is the cost and practicality involved in measuring skeletal muscle mass in the clinical setting. Bioimpedance analysis (BIA), dual‐energy X‐ray absorptiometry (DXA), computed tomography, D3‐creatine dilution, and magnetic resonance imaging (MRI) are among the available approaches for quantifying skeletal muscle mass.2, 8 Of these methods, DXA estimates of appendicular lean soft tissue (LST) mass are most often used in clinical research protocols as a practical surrogate measure of total body and regional skeletal muscle mass.8, 9, 10 Appendicular LST mass as measured by DXA is highly correlated with total body skeletal muscle mass as quantified by MRI.11, 12, 13, 14 Additionally, DXA‐measured LST is associated with functional limitations and adverse health outcomes.15, 16
An increasingly recognized problem with anatomic skeletal muscle mass measurements is the potential dissociation between mass and function. Substantial decreases in skeletal muscle function with ageing can occur with only minimal loss of skeletal muscle mass.17, 18 This loss of function is often attributed to changes in muscle ‘quality’ independent of ‘mass’.19 Muscle quality is affected by changes in skeletal muscle composition with ageing including alterations in muscle fibre type, expansion of the extracellular space with relative reductions in myofiber diameter, and loss of mitochondrial mass and function.20, 21
Several methods of estimating skeletal muscle composition are available for use in the clinical setting. Computed tomography, MRI, and magnetic resonance spectroscopy can be used to derive multiple measures of skeletal muscle quality such as adipose tissue and lipid infiltration, loss in tissue elasticity, and relative fluid expansion.8 However, these valuable research approaches are not usually practical to apply in the clinical setting. Another measure that relates to ageing and skeletal muscle, phase angle, can be quantified with BIA.22, 23 Changes in phase angle in otherwise healthy and physically active adults appear to reflect subtle metabolic and structural features of muscle fibres with low phase angle values characteristic of older age.22 Similarly, B‐mode ultrasound can be used to quantify ‘echogenicity’, a measure thought to represent adipose and connective tissue infiltration into the skeletal muscle compartment.24 As with BIA systems, ultrasound devices are often available and practical to apply in clinical settings.
A reasonable hypothesis emerging from these earlier observations is that combining measures of skeletal muscle ‘mass’ and ‘quality’ will significantly improve prediction of skeletal muscle function. We tested this hypothesis in the current study by examining the associations between DXA‐measured extremity‐specific LST alone and in combination with phase angle or echogenicity in relation to leg and handgrip strength measured with leg and hand dynamometers, respectively.
Materials and methods
Participants
The data for this study were collected as part of the ongoing National Institutes of Health Shape Up! Adults research programme (R01DK109008). The study was approved by the Pennington Biomedical Research Center Institutional Review Board, and all subjects signed an informed consent prior to participation. Adult participants at or over the age of 18 years were recruited on the centre's website and excluded if they had any body composition abnormalities, had medical implants, had joint replacements, had underlying chronic diseases, had weight over the DXA system limit of 200 kg, or were pregnant. The study was registered at ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT03637855).
All participant measurements were made by trained and certified technicians, including DXA, BIA, and ultrasonography. Once enrolled, participants were asked to fast overnight and to report to the laboratory early on the evaluation day. A screening medical evaluation ensured they were in good health, following which they were asked to evacuate their bladder prior to body composition measurements and skeletal muscle function testing. The testing protocol lasted between 3 and 4 h during which the participants were allowed to eat a light snack.
The key variables to test the study hypothesis included DXA‐measured extremity‐specific LST, BIA‐measured phase angle, B‐mode ultrasound‐measured mid‐thigh skeletal muscle echogenicity, and dynamometer‐measured handgrip and leg strength.
Skeletal muscle mass and quality
Dual‐energy X‐ray absorptiometry
Each participant completed two whole‐body DXA (Hologic Discovery/A, Hologic Inc., Marlborough, MA, USA) scans, which were analysed with Hologic Apex version 5.5 software according to the manufacturer's operation manual for LST mass, fat mass, and bone mineral content of each anatomic segment (head, left arm, right arm, left leg, right leg, and trunk). The National Health and Nutrition Survey, Body Composition Analysis (NHANES BCA) option was enabled, which moves 5.4% lean mass to fat mass.25 The coefficients of variation (CVs) for LST were 1.5%, 2.1%, and 2.5% for the right leg, left arm, and right arm, respectively. Scans with artefacts and motion were excluded from the statistical analysis. The first of the two scans was used for the analysis unless the first scan was excluded because of an artefact then the second scan was used.
Bioimpedance analysis
Phase angles corresponding to DXA extremity measurements were quantified in recumbent subjects with a segmental multifrequency BIA system (S10, InBody Co., Ltd, Seoul, South Korea). Touch‐type electrodes were attached between the heel and the ankle bone of the participants' right and left feet and on their right and left middle finger and thumb. Participants were asked to extend their extremities so as not to touch each other or the torso; they then rested quietly in the supine position for 7 min prior to the BIA measurements. Resistance and reactance of the right arm, left arm, right leg, left leg, and trunk were measured at frequencies of 5, 50, and 250 kHz. Model development was initiated at the most commonly evaluated frequency, 50 kHz. Phase angles of each body segment at each frequency were calculated by the BIA system software as the arctangent of reactance divided by resistance and then transformed from radian grade.
Ultrasound
Thigh skeletal muscle characteristics were quantified with a B‐mode ultrasound system (Aplio 80, Toshiba, Otawara, Japan). Echogenicity, also known as echo intensity, was measured using images taken from the B‐mode ultrasound system. A 7.5 MHz transducer was placed in the transverse position at the midpoint of the right thigh while the participant was lying in the supine position. The midpoint of the thigh was measured as the midpoint between the inguinal crease and the proximal border of the patella. The transducer was gently placed at this point so as to not compress the thigh. Transducer depth was increased until the femur was visible on the system's monitor. The femur was not visible at any depth in some participants who had large thighs, so the depth was set at 10 cm. Images were exported as DICOM files and opened in ImageJ26 for echogenicity measurements. Once opened in ImageJ, a 10 × 10 mm2 was created just below but not including the fascia that separates the subcutaneous adipose tissue from the muscle. The histogram analysis function was used to calculate mean intensity within the created square.
Skeletal muscle strength
Leg
Isokinetic right leg strength measurements were performed with the Biodex (Biodex System 4, Biodex Medical Systems Inc., Shirley, NY, USA) resistance set at 60° per second. Participants walked on a treadmill for 5 min to warm up prior to the leg strength measurements. They were then fastened into the Biodex system with a seatbelt for measurement of right leg strength through extension and flexion. The participants then practiced with one set of three repetitions at an endurance of 50% of maximal effort. For the actual measurements, the participants performed three sets of five repetitions at their maximal effort. Peak torque through extension was measured in Newton‐metres and taken as the maximum torque achieved during the three sets. We refer to this measurement as leg strength, although it has also been termed knee extension strength.21, 24, 27 The CV for leg strength measurements was 6.1%. Participants with a history of right knee arthritis or an operation on the right knee were excluded from analysis.
Handgrip
Grip strength for the right and left arms was measured with a handgrip dynamometer (JAMAR 5030J1, Sammons Preston Rolyan, Nottinghamshire, UK). The participants were asked to position their elbow at a 90° angle and then asked to squeeze the dynamometer as hard as they could and then encouraged to squeeze even harder. The strength of each hand was measured in kilograms, and the average of three measurements was taken. The CVs for grip strength of the right and left hands were 6.1% and 6.7%, respectively. Participants with a history of hand problems (i.e. arthritis and pain) were excluded from data analysis.
Statistical methods
Demographic, DXA, BIA, and ultrasound results are presented as the group means and standard deviations stratified by sex. All statistical analyses were performed with SAS software (version 9.4, SAS Institute Inc., Cary, NC, USA). All key measurements with the exception of participant age were normally distributed after excluding two outliers whose data are not included in this report. The outlier data were excluded as no physiological or technical explanation could be found for these aberrant data points and analyses described below showed no measurable influence when these two subjects were removed from the dataset. To further examine the effects of the non‐normally distributed age variable on study outcomes, we stratified participants into three age groups: 18–39, 40–59, and 60–85 years. We then tested regression coefficients across the three age groups, and there were no significant between‐group differences present. Age was therefore included as a continuous variable in the subsequently developed regression models.
The initial descriptive analyses examined associations between LST, strength, phase angle, echogenicity, and age. We next examined the associations between extremity‐specific LST and corresponding anatomic site strength. Strength prediction models for the right leg and both arms were first developed with LST alone as a predictor variable. The significance of adding segmental phase angle or thigh muscle echogenicity to these models was evaluated next with testing of the additional potential covariates age and sex. Height and weight were also explored as other potential strength prediction covariates but were not included in the final models as they did not add measurably to the study conclusions.
These steps were implemented in the form of six stepwise multiple linear regression analysis model groups. Model 1 included extremity‐specific strength (right leg and arm and left arm) as dependent variable and the corresponding extremity LST as independent variable. Phase angle and echogenicity were then added as covariates in Models 2 and 5, respectively. Model 3 included age and sex as predictor variables in Model 1. Phase angle and echogenicity were then added to Model 3 as covariates in Models 4 and 6, respectively. Variables with P < 0.1 were selected to enter the models and P < 0.05 to be included in the final models.
All models that included phase angle only show values in the regression model tables generated at 50 kHz as neither 5 nor 250 kHz results proved to be significant predictors of strength. No evidence of interactions was found between the variables in the final models. Model β‐weights and standard errors are presented in the tables.
A consideration for use of concepts developed in the current study is development of clinically applicable strength prediction formulas. We therefore separately tested the predictive power of the strength models by completing a five‐fold cross‐validation analysis for Models 1–6.28 A five‐fold cross‐validation randomly divides the subjects into five groups where four are training and one is validation through five iterations where each group is the validation group for one of the iterations. The resulting coefficients and R 2 values of the prediction models from the five iterations were averaged to create the final cross‐validation model. This approach assesses how the prediction models will perform in an independent dataset.
Results
Participant characteristics
There were a total of 148 subjects enrolled in the study, and 146 subjects were included in analyses after exclusion of the two participants with outlying data. The evaluated sample included 60 men and 86 women ranging in age from 18 to 77 years (47 ± 17 years) and body mass index from 16.5 to 51.8 kg/m2 (27.7 ± 6.2 kg/m2) (Table 1).
Table 1.
Participant characteristics
| Men (n = 60) | Women (n = 86) | |
|---|---|---|
| Age [years]a | 45 (18) | 49 (16) |
| Height [cm] | 176.9 (6.9) | 162.8 (6.8) |
| Weight [kg] | 87.3 (17.0) | 72.9 (17.6) |
| BMI [kg/m2] | 27.9 (5.2) | 27.6 (6.9) |
| Right leg | ||
| Leg strength [Nm] | 189 (55) | 104 (28) |
| LST [kg] | 9.8 (1.6) | 6.7 (1.3) |
| 5 kHz phase angle [°] | 3.3 (0.7) | 3.0 (0.8) |
| 50 kHz phase angle [°] | 7.0 (1.4) | 6.4 (1.1) |
| 250 kHz phase angle [°] | 4.5 (1.1) | 4.6 (1.4) |
| Echogenicity | 62 (23) | 110 (26) |
| Right arm | ||
| Grip strength [kg] | 41 (11) | 23 (6) |
| LST [kg] | 4.0 (0.8) | 2.2 (0.5) |
| 5 kHz phase angle [°] | 3.5 (0.7) | 2.7 (0.5) |
| 50 kHz phase angle [°] | 6.7 (0.9) | 5.5 (0.6) |
| 250 kHz phase angle [°] | 5.9 (0.9) | 5.3 (0.9) |
| Left arm | ||
| Grip strength [kg] | 41 (11) | 22 (6) |
| LST [kg] | 3.7 (0.8) | 2.0 (0.5) |
| 5 kHz phase angle [°] | 3.4 (0.7) | 2.7 (0.5) |
| 50 kHz phase angle [°] | 6.6 (0.9) | 5.4 (0.6) |
| 250 kHz phase angle [°] | 5.8 (1.0) | 5.1 (1.0) |
BMI, body mass index; LST, lean soft tissue.
Results are in mean (standard deviation).
As age was not normally distributed, we also give here the median age of 45 years for men and 53 years for women with interquartile ranges of 27–61 and 34–64 years, respectively.
As an example of skeletal muscle mass (i.e. appendicular LST), quality, and strength associations with age, results for the right leg are shown in Figure 1. Right leg LST was not significantly correlated with participant age (R 2 = 0.02, P = 0.13). In contrast, right leg strength and phase angle were both inversely correlated with participant age (R 2 = 0.15 and 0.20, respectively, both P < 0.0001) while echogenicity was positively correlated with age (R 2 = 0.07, P < 0.01).
Figure 1.
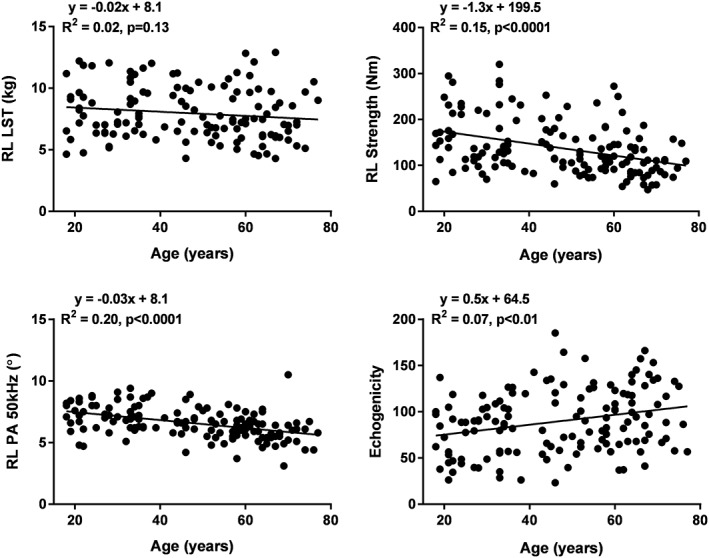
Right leg LST (top left), right leg strength (top right), right leg phase angle at 50 kHz (bottom left), and mid‐thigh echogenicity (bottom right) vs. age. The data are fit with linear regression models with results shown in each panel. LST, lean soft tissue; PA, phase angle; RL, right leg.
Strength models
Phase angle
Leg strength
Right leg LST was significantly associated with right leg strength (Model 1, Table 2: R 2 = 0.57, P < 0.0001; Figure 2). When phase angle at 50 kHz was added as a covariate in Model 2, R 2 increased from 0.57 to 0.66. When covariates for age and sex were added (Models 3 and 4), the effect of phase angle on predicted strength was less pronounced but still significant (P < 0.0001), R 2 increasing from 0.67 to 0.71, respectively.
Table 2.
Strength prediction models including phase angle
|
Right leg Strength [Nm] |
Right arm Grip strength [kg] |
Left arm Grip strength [kg] |
||||
|---|---|---|---|---|---|---|
| β | SE | β | SE | β | SE | |
| Model 1 | ||||||
| Intercept | −25.21 | 13.16 | 4.56** | 1.88 | 7.49** | 1.87 |
| LST [kg] | 20.45*** | 1.60 | 8.52*** | 0.60 | 8.29*** | 0.65 |
| R 2 | 0.57 | 0.60 | 0.55 | |||
| Model 2 | ||||||
| Intercept | −102.56*** | 17.95 | −9.01** | 4.33 | −3.64 | 4.39 |
| LST [kg] | 17.69*** | 1.50 | 6.67*** | 0.79 | 6.39*** | 0.93 |
| PA 50 kHz [°] | 14.62*** | 2.57 | 3.16** | 0.92 | 2.77** | 0.99 |
| R 2 | 0.66 | 0.64 | 0.58 | |||
| Model 3 | ||||||
| Intercept | 86.35** | 20.69 | 20.90*** | 4.33 | 22.45*** | 4.29 |
| Age [years] | −0.87*** | 0.17 | −0.09** | 0.04 | −0.08** | 0.04 |
| Sex [female = 1] | −32.52** | 8.20 | −6.99** | 2.10 | −6.75** | 2.08 |
| LST [kg] | 13.96*** | 1.74 | 5.81*** | 0.95 | 5.63*** | 0.99 |
| R 2 | 0.67 | 0.65 | 0.60 | |||
| Model 4 | ||||||
| Intercept | 6.07 | 28.24 | 2.77 | 5.62 | 7.25 | 5.49 |
| Age [years] | −0.57** | 0.18 | — | — | — | — |
| Sex [female = 1] | −30.36** | 7.94 | −6.52** | 2.07 | −6.44** | 2.05 |
| LST [kg] | 12.82*** | 1.90 | 4.46*** | 1.04 | 4.13*** | 1.15 |
| PA 50 kHz [°] | 11.12*** | 2.58 | 2.93** | 0.89 | 2.62** | 0.96 |
| R 2 | 0.71 | 0.66 | 0.61 | |||
LST, lean soft tissue; PA, phase angle at 50 kHz; SE, standard error.
All phase angles were measured in respective extremity described in the model.
P < 0.05.
P < 0.01.
P < 0.0001.
Figure 2.
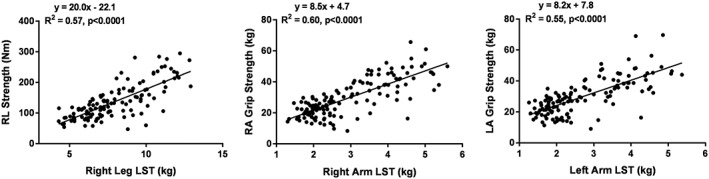
Correlations between right leg strength and right leg LST (left), right arm strength and right arm LST (middle), and left arm strength and left arm LST (right). The data are fit with linear regression models with results shown in each panel. LA, left arm; LST, lean soft tissue; RA, right arm; RL, right leg.
Handgrip strength
Grip strength for both the right and left hands (Model 1) was significantly associated with right and left arm LST (R 2 0.60 and 0.55, respectively, both P < 0.0001; Figure 2). Similar to leg strength, right and left arm phase angle measured at 50 kHz added as a significant covariate in Model 2 and increased R 2 to 0.64 and 0.58, respectively (Table 2). Adding age and sex without phase angle to the regression analysis in Model 3 increased the R 2 values (0.65 and 0.60 for right and left hands, respectively). When both age and phase angle were included in the model (Model 4), age was no longer a significant covariate but sex further increased the R 2 to 0.66 and 0.61 for the right and left arms, respectively.
Echogenicity
Echogenicity measured on the right mid‐thigh was moderately correlated with phase angle of the right leg (R 2 = 0.17, P < 0.0001) and right leg strength (R 2 = 0.37, P < 0.0001; Table 3 and Figure 3). Similar to phase angle, thigh muscle echogenicity added significantly to right leg LST to increase the R 2 from 0.57 to 0.64 (Model 5, Table 3). Age added to the model as a covariate increased R 2 to 0.68 in Model 6, although sex was not a significant predictor variable. The comparable right leg strength model for phase angle (Model 4, Table 2) had a higher R 2 (0.71) and included both age and sex as significant covariates. Both echogenicity and phase angle added significantly to Model 6, although the model R 2 was not measurably increased.
Table 3.
Strength prediction models including thigh echogenicity
|
Right leg Strength (Nm) |
||
|---|---|---|
| β | SE | |
| Model 5 | ||
| Intercept | 51.82* | 20.37 |
| LST (kg) | 16.41*** | 1.72 |
| Echogenicity | −0.51*** | 0.11 |
| R 2 | 0.64 | |
| Model 6 | ||
| Intercept | 79.39** | 20.62 |
| Age (years) | −0.69*** | 0.18 |
| Sex (female = 1) | — | — |
| LST (kg) | 16.15*** | 1.63 |
| Echogenicity | −0.44** | 0.10 |
| R 2 | 0.68 | |
LST, lean soft tissue of the leg; SE, standard error.
P < 0.05.
P < 0.01.
P < 0.0001.
Figure 3.
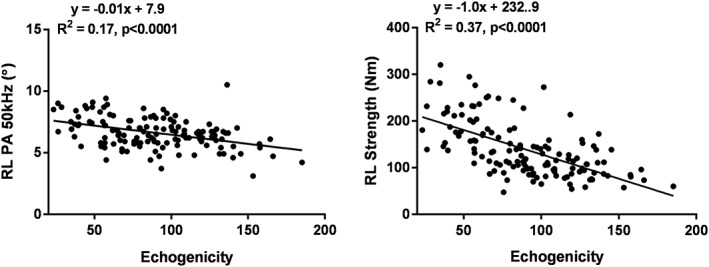
Correlation between right leg phase angle at 50 kHz and echogenicity of the mid‐right leg (left) and right leg strength and echogenicity (right). The data are fit with linear regression models with results shown in each panel. PA, phase angle; RL, right leg.
Cross‐validation
Results from the five‐fold cross‐validation prediction models are displayed for right leg strength and right and left arm strength in Supporting Information, Tables S1 and S2. The same models presented in Table 2 are included in the cross‐validation analysis in Supporting Information, Table S1, and the models presented in Table 3 are included in the cross‐validation analysis in Supporting Information, Table S2. Results for the cross‐validation analysis were similar to the stepwise regression analysis with phase angle and echogenicity still improving prediction equations for strength; however, some R 2 values were slightly lower in the cross‐validation analysis. For example, R 2 values for echogenicity Models 5 and 6 were 0.63 and 0.67 for the cross‐validation compared with the stepwise regression analysis values of 0.64 and 0.68, respectively.
Discussion
Strength prediction models
This study tested the hypothesis that, after controlling for skeletal muscle mass, clinically available measures of muscle quality add significantly to the prediction of strength. Our results strongly support this hypothesis: leg and handgrip strength were significantly associated with BIA‐measured extremity phase angle and ultrasound‐measured thigh muscle echogenicity in regression models after controlling for LST and other covariates including age and sex.
Many cross‐sectional and longitudinal studies report age‐related reductions in muscle mass,14, 29, 30, 31 changes in muscle quality,18, 32 and a wide range of related functional losses.17, 33 Older age groups appear to experience functional changes, including reductions in strength and endurance, at rates that exceed those of decrements in muscle mass.17, 33 These differing rates of muscle senescence have been attributed to changes in tissue quality, a broadly applied term that includes loss of myofibers,20 changes in the proportion of Type I and II fibres,18 alterations in mitochondrial function,34, 35, 36 changes in neural innervation,37 inter‐muscular adipose and connective tissue infiltration,21, 24, 32, 38 and relative increases in extracellular fluid.21 Sarcopenia is thus a multidimensional condition that includes loss of muscle mass and changes in muscle quality that lead to functional deficits and ultimately to morbidity and mortality.15, 16 The two measures evaluated in the current study, phase angle and echogenicity, fit directly into this increasingly recognized paradigm as measures of skeletal muscle quality.
Our interest in phase angle began with the observation that this easily acquired BIA measurement becomes lower with age, even after controlling for multiple determining variables, across large healthy adult groups differing in demographic characteristics.22 The specific mechanisms leading to this age‐related effect are largely unknown but likely reflect some of the wide‐ranging aforementioned changes in muscle composition and quality accompanying ageing.39 These quality effects may influence phase angle through their actions on cell membrane integrity,40 the relationship between cell mass and total muscle mass,41 and multiple other not yet fully characterized mechanisms. Our finding of phase angle measured at 50 kHz as a significant strength model covariate is consistent with that frequency's proximity to the characteristic or critical frequency. The critical frequency is the frequency at which the largest reactance and cell membrane capacitance is observed and the one closest linked to function and outcomes.42, 43
In support of phase angle as a surrogate measure of muscle quality and consistent with the overall results of the current study, Tomeleri et al.44 recently reported significant associations between phase angle, muscle quality, and functional capacity in a cohort of elderly women. The authors defined muscle ‘quality’ as total strength per unit muscle mass, specifically as 1‐repetition max tests (chest press, knee extension, and preacher curl exercises)/LST.
Phase angle is notably reactive in disease states; particularly when acute illness is present, low values are accompanied by poor clinical outcomes.45, 46 Phase angle ideally should therefore be used as a muscle quality measure only in people who are medically stable.
Lacking a definitive anatomic or metabolic mechanism, at present, we can only endorse phase angle as a general measure of skeletal muscle quality.23 Nevertheless, phase angle is easily measured in the clinical setting with relatively inexpensive equipment and significantly adds to LST as a predictor of handgrip and leg strength.
Echogenicity of skeletal muscle is another commonly evaluated measure of muscle quality that can be acquired in the clinical setting, although ultrasound equipment tends to be costly and requires more technical training than for measuring phase angle. However, many medical facilities now have advanced ultrasound units that are operated by highly trained and certified personnel. As with phase angle, there is not a specific anatomic feature of skeletal muscle that can be attributed to a high level of echogenicity, although suggested mechanisms include adipose and fibrous tissue infiltration of the muscle compartment.24 We detected a significant correlation between thigh muscle echogenicity and leg phase angle, although the R 2 was relatively low (0.17), likely reflecting a combination of shared mechanisms and measurement error. We also observed entry of both echogenicity and phase angle into strength prediction models, suggesting some independence between the two measures of muscle quality.
Concordance with earlier studies
The current observations confirm and extend studies by Yamada et al.21, 27 and Taniguchi et al.24 that included men and women with mean group ages in the seventh or eighth decades. Skeletal muscle mass or size was estimated in these studies using ultrasound21, 24 or BIA prediction formulas.21, 27 Phase angle was not specifically measured in these studies, rather quality indices calculated at different frequencies21 or predicted values for extracellular and intracellular water were derived from bioimpedance spectroscopy measurements.21, 24 When controlling for the various estimates of muscle mass, BIA or ultrasound measures of poor skeletal muscle quality were associated with lower levels of physical performance.21, 24 Our findings confirm and extend these observations in both the lower and upper extremities in adults ranging in age from 18 to 77 years with a commonly used sarcopenia diagnostic tool, DXA,5, 10 to measure appendicular LST as the reference for muscle mass and both BIA‐phase angle and ultrasound echogenicity as two estimates of muscle quality. Our study and those of Yamada et al.21, 27 and Taniguchi et al.24 clearly show that combinations of skeletal muscle mass and quality that are practical to acquire in the clinical setting provide more information related to subject strength than either mass or quality estimates alone.
An important implication of our findings and those of Yamada et al.,21, 27 Taniguchi et al.,24 and Tomerli et al.44 is that estimates of skeletal muscle mass can be augmented with BIA measurements when evaluating patients for the presence of sarcopenia and related functional limitations. We anticipate that measures of muscle mass and quality could eventually be combined to create a score that would predict the risk of developing sarcopenia or related disorders rather than be used to simply predict muscle function. The kinds of pathways involved are shown in Figure 4 relating the different mass and quality measures to functional estimates and clinical outcomes.
Figure 4.
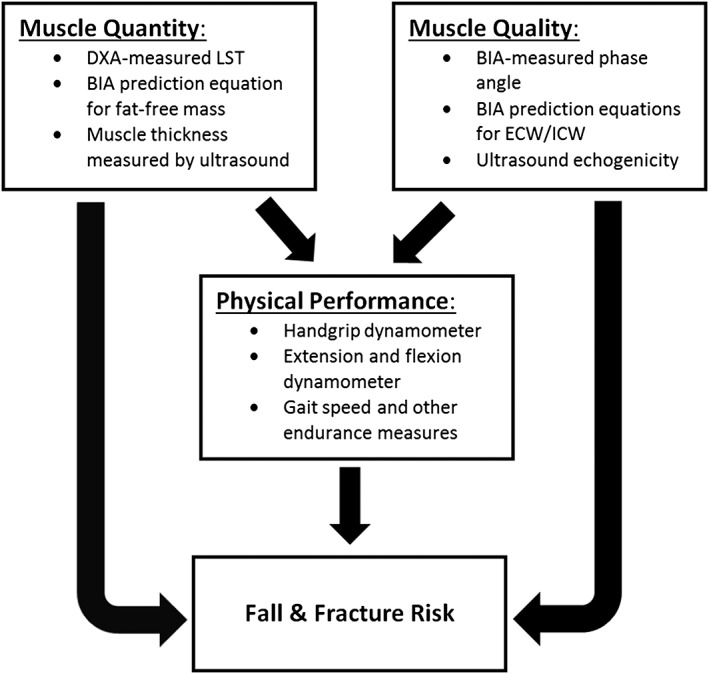
Pathways through which measures of muscle mass/quantity and quality relate to physical performance and ultimately to clinical outcomes such as falls and fractures. Selected muscle measures are shown that were evaluated in the current study and by Yamada et al.21, 27 and Taniguchi et al.24 BIA, bioimpedance analysis; DXA, dual‐energy X‐ray absorptiometry; ECW, extracellular water; ICW, intracellular water; LST, extremity lean soft tissue.
Future studies are needed to sort out the optimal BIA measurements (e.g. impedance ratios and phase angle) and the extent to which BIA and echogenicity measurements are additive in strength prediction models. Our cross‐validation analysis revealed similar R 2 to our stepwise regression analysis, indicating that the covariates in the current study have potential for predicting extremity strength. Our exploratory analysis where measures of body size (e.g. height and weight) were added as covariates revealed that even stronger prediction models may be possible; however, results were inconsistent and showed that these measures added to arm strength predictions but not leg strength. More information on these topics would be useful in moving towards longitudinal studies relating combination mass–quality formulas with clinical outcomes, including mortality. Studies such as these have the potential to establish if combinations of muscle mass and quality measurements could potentially replace or add to functional estimates that capture a subject's strength and endurance. One advantage of phase angle and echogenicity estimates over those provided by strength and most functional measurements is that both are acquired without relying on participant performance.
Study limitations
There are several limitations of the present study. Echogenicity was measured in a small section of the thigh rectus femoris muscle instead of using the entire quadriceps femoris muscle because the ultrasound images on a number of participants did not include the femur. This ultrasound biopsy captures only a small volume of the quadriceps muscle and leaves muscles of the upper extremity unmeasured.
Our finding that muscle strength prediction can be improved by combining DXA‐measured appendicular LST with phase angle or echogenicity, two measures of tissue quality, reveals important often‐unappreciated features of DXA body composition estimates. The appendicular LST compartment as quantified by DXA includes not only the functional myofibers but also other components such as connective tissue that are known to increase in relative amount with age.20, 47 Methods that quantify muscle cell mass, such as 24 h urinary creatine excretion48, 49 or D3‐creatine dilution,50 might improve strength predictions without addition of other ‘quality’ measures. In support of this suggestion, Yamada et al.47 recently showed that an impedance spectroscopy approach predicted ~85% of the age‐related decrease in extremity skeletal muscle power compared with ~49% for available DXA measures.
Additionally, while we were able to show that adding a measure of muscle quality to LST mass improved predictions of strength, the current study was based on a cross‐sectional sample that did not allow us to confirm age‐related loss in measures of muscle quality (e.g. phase angle and echogenicity) in a longitudinal analysis. Lastly, we did not explore clinical outcomes, and we were unable to conclude whether phase angle measurements improve outcome predictions such as falls or fractures.
Conclusions
In sum, the current study strongly supports the position that clinically available measures of skeletal muscle mass and quality can be combined to predict functional strength across the adult age span. These observations provide a rationale for future studies aimed at determining which of these measures alone or in combination maximally predict physical performance and clinical outcomes.
Author Contributions
B. B., B. F., N. J., M. C. G., B. K. N., M. J. S., J. A. S., and S. B. H. conceived and designed the experiments; B. B. and S. H. performed the experiments; B. B., B. F., N. J., M. C. G., B. K. N., M. J. S., J. A. S., and S. B. H. analysed and interpreted the data; S. B. H. and J. A. S. provided necessary logistical support; B. B., B. F., N. J., M. C. G., B. K. N., M. J. S., J. A. S., and S. B. H. edited the manuscript for intellectual content and provided critical comments on the manuscript.
Conflict of interest
Dr Heymsfield reports his membership on the Tanita Corporation Medical Advisory Board. The other authors have no conflict of interest.
Supporting information
Table S1. Strength prediction models including phase angle (with 5‐fold cross validation).
Table S2. Strength prediction models including echogenicity (with 5‐fold cross validation).
Acknowledgements
The authors acknowledge Melanie Peterson for her assistance in preparing this manuscript. This work was partially supported by two National Institutes of Health NORC Center grants P30DK072476, Pennington/Louisiana, and P30DK040561, Harvard; and NIDDK R01DK109008, Shape Up! Adults. The authors of this manuscript certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia, and Muscle: update 2017.51
Bourgeois, B. , Fan, B. , Johannsen, N. , Gonzalez, M. C. , Ng, B. K. , Sommer, M. J. , Shepherd, J. A. , and Heymsfield, S. B. (2019) Improved strength prediction combining clinically available measures of skeletal muscle mass and quality. Journal of Cachexia, Sarcopenia and Muscle, 10: 84–94. 10.1002/jcsm.12353.
References
- 1. Cruz‐Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clark RV, Walker AC, O'Connor‐Semmes RL, Leonard MS, Miller RR, Stimpson SA, et al. Total body skeletal muscle mass: estimation by creatine (methyl‐d3) dilution in humans. J Appl Physiol (1985) 2014;116:1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fuggle N, Shaw S, Dennison E, Cooper C. Sarcopenia. Best Pract Res Clin Rheumatol 2017;31:218–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Springer J, Springer JI, Anker SD. Muscle wasting and sarcopenia in heart failure and beyond: update 2017. ESC Heart Fail 2017;4:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the Foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci 2014;69:576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heymsfield SB, Gonzalez MC, Lu J, Jia G, Zheng J. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc 2015;74:355–366. [DOI] [PubMed] [Google Scholar]
- 9. Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 2018;9:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res 2016;28:1047–1060. [DOI] [PubMed] [Google Scholar]
- 11. Bosy‐Westphal A, Jensen B, Braun W, Pourhassan M, Gallagher D, Muller MJ. Quantification of whole‐body and segmental skeletal muscle mass using phase‐sensitive 8‐electrode medical bioelectrical impedance devices. Eur J Clin Nutr 2017;71:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim J, Heshka S, Gallagher D, Kotler DP, Mayer L, Albu J, et al. Intermuscular adipose tissue‐free skeletal muscle mass: estimation by dual‐energy X‐ray absorptiometry in adults. J Appl Physiol (1985) 2004;97:655–660. [DOI] [PubMed] [Google Scholar]
- 13. Kim J, Shen W, Gallagher D, Jones A Jr, Wang Z, Wang J, et al. Total‐body skeletal muscle mass: estimation by dual‐energy X‐ray absorptiometry in children and adolescents. Am J Clin Nutr 2006;84:1014–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total‐body skeletal muscle mass: estimation by a new dual‐energy X‐ray absorptiometry method. Am J Clin Nutr 2002;76:378–383. [DOI] [PubMed] [Google Scholar]
- 15. Cederholm T, Cruz‐Jentoft AJ, Maggi S. Sarcopenia and fragility fractures. Eur J Phys Rehabil Med 2013;49:111–117. [PubMed] [Google Scholar]
- 16. Landi F, Cruz‐Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing 2013;42:203–209. [DOI] [PubMed] [Google Scholar]
- 17. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 18. McGregor RA, Cameron‐Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan 2014;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Correa‐de‐Araujo R, Harris‐Love MO, Miljkovic I, Fragala MS, Anthony BW, Manini TM. The need for standardized assessment of muscle quality in skeletal muscle function deficit and other aging‐related muscle dysfunctions: a symposium report. Front Physiol 2017;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15‐ to 83‐year‐old men. J Neurol Sci 1988;84:275–294. [DOI] [PubMed] [Google Scholar]
- 21. Yamada Y, Yoshida T, Yokoyama K, Watanabe Y, Miyake M, Yamagata E, et al. The extracellular to intracellular water ratio in upper legs is negatively associated with skeletal muscle strength and gait speed in older people. J Gerontol A Biol Sci Med Sci 2017;72:293–298. [DOI] [PubMed] [Google Scholar]
- 22. Gonzalez MC, Barbosa‐Silva TG, Bielemann RM, Gallagher D, Heymsfield SB. Phase angle and its determinants in healthy subjects: influence of body composition. Am J Clin Nutr 2016;103:712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tomeleri CM, Cavaglieri CR, de Souza MF, Cavalcante EF, Antunes M, Nabbuco HCG, et al. Phase angle is related with inflammatory and oxidative stress biomarkers in older women. Exp Gerontol 2018;102:12–18. [DOI] [PubMed] [Google Scholar]
- 24. Taniguchi M, Yamada Y, Fukumoto Y, Sawano S, Minami S, Ikezoe T, et al. Increase in echo intensity and extracellular‐to‐intracellular water ratio is independently associated with muscle weakness in elderly women. Eur J Appl Physiol 2017;117:2001–2007. [DOI] [PubMed] [Google Scholar]
- 25. Schoeller DA, Tylavsky FA, Baer DJ, Chumlea WC, Earthman CP, Fuerst T, et al. QDR 4500A dual‐energy X‐ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr 2005;81:1018–1025. [DOI] [PubMed] [Google Scholar]
- 26. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamada M, Kimura Y, Ishiyama D, Nishio N, Abe Y, Kakehi T, et al. Differential characteristics of skeletal muscle in community‐dwelling older adults. J Am Med Dir Assoc 2017;18():807:e9–e16. [DOI] [PubMed] [Google Scholar]
- 28. Geisser S. Predictive inference: an introduction In Monographs on Statistics and Applied Probability, vol 55. New York: Chapman & Hall; 1993. [Google Scholar]
- 29. Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol (1985) 2003;95:1717–1727. [DOI] [PubMed] [Google Scholar]
- 30. Landi F, Calvani R, Tosato M, Martone AM, Fusco D, Sisto A, et al. Age‐related variations of muscle mass, strength, and physical performance in community‐dwellers: results from the Milan EXPO survey. J Am Med Dir Assoc 2017;18:88.e17–88.e24. [DOI] [PubMed] [Google Scholar]
- 31. Marzetti E, Hwang AC, Tosato M, Peng LN, Calvani R, Picca A, et al. Age‐related changes of skeletal muscle mass and strength among Italian and Taiwanese older people: results from the Milan EXPO 2015 survey and the I‐Lan Longitudinal Aging Study. Exp Gerontol 2018;102:76–80. [DOI] [PubMed] [Google Scholar]
- 32. Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez‐Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 2009;90:1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keller K, Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. MLTJ 2013;3:346–350. [PMC free article] [PubMed] [Google Scholar]
- 34. Conley KE, Jubrias SA, Cress ME, Esselman P. Exercise efficiency is reduced by mitochondrial uncoupling in the elderly. Exp Physiol 2013;98:768–777. [DOI] [PubMed] [Google Scholar]
- 35. Gonzalez‐Freire M, Scalzo P, D'Agostino J, Moore ZA, Diaz‐Ruiz A, Fabbri E, et al. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: the Baltimore Longitudinal Study of Aging. Aging Cell 2018;17:e12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johannsen DL, Conley KE, Bajpeyi S, Punyanitya M, Gallagher D, Zhang Z, et al. Ectopic lipid accumulation and reduced glucose tolerance in elderly adults are accompanied by altered skeletal muscle mitochondrial activity. J Clin Endocrinol Metab 2012;97:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arnold WD, Taylor RS, Li J, Nagy JA, Sanchez B, Rutkove SB. Electrical impedance myography detects age‐related muscle change in mice. PLoS One 2017;12:e0185614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kent‐Braun JA, Ng AV, Young K. Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol (1985) 2000;88:662–668. [DOI] [PubMed] [Google Scholar]
- 39. Aaron R, Esper GJ, Shiffman CA, Bradonjic K, Lee KS, Rutkove SB. Effects of age on muscle as measured by electrical impedance myography. Physiol Meas 2006;27:953–959. [DOI] [PubMed] [Google Scholar]
- 40. Foster KR, Lukaski HC. Whole‐body impedance—what does it measure? Am J Clin Nutr 1996;64:388S–396S. [DOI] [PubMed] [Google Scholar]
- 41. Shizgal H, inventor Twyman, D., assignee. Method for measuring total body cell mass and total extracellular mass by bioelectrical resistance and reactance. 1987. United States patent US Patent Number 4911175.
- 42. De Lorenzo A, Andreoli A, Matthie J, Withers P. Predicting body cell mass with bioimpedance by using theoretical methods: a technological review. J Appl Physiol (1985) 1997;82:1542–1558. [DOI] [PubMed] [Google Scholar]
- 43. Lukaski HC. Evolution of bioimpedance: a circuitous journey from estimation of physiological function to assessment of body composition and a return to clinical research. Eur J Clin Nutr 2013;67:S2–S9. [DOI] [PubMed] [Google Scholar]
- 44. Tomeleri CM, Cavalcante EF, Antunes M, Nabuco HCG, de Souza MF, Teixeira DC, et al. Phase angle is moderately associated with muscle quality and functional capacity, independent of age and body composition in older women. J Geriatr Phys Ther 2017;1. [DOI] [PubMed] [Google Scholar]
- 45. Norman K, Wirth R, Neubauer M, Eckardt R, Stobaus N. The bioimpedance phase angle predicts low muscle strength, impaired quality of life, and increased mortality in old patients with cancer. J Am Med Dir Assoc 2015;16(2):173 e17–22, 173.e17, 173.e22. [DOI] [PubMed] [Google Scholar]
- 46. Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, et al. Electrical impedance myography correlates with standard measures of ALS severity. Muscle Nerve 2014;49:441–443. [DOI] [PubMed] [Google Scholar]
- 47. Yamada Y, Buehring B, Krueger D, Anderson RM, Schoeller DA, Binkley N. Electrical properties assessed by bioelectrical impedance spectroscopy as biomarkers of age‐related loss of skeletal muscle quantity and quality. J Gerontol A Biol Sci Med Sci 2017;72:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S. Measurement of muscle mass in humans: validity of the 24‐hour urinary creatinine method. Am J Clin Nutr 1983;37:478–494. [DOI] [PubMed] [Google Scholar]
- 49. Proctor DN, O'Brien PC, Atkinson EJ, Nair KS. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am J Physiol 1999;277:E489–E495. [DOI] [PubMed] [Google Scholar]
- 50. Cawthon PM, Orwoll ES, Peters KE, Ensrud KE, Cauley JA, Kado DM, et al. Strong relation between muscle mass determined by D3‐creatine dilution, physical performance and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Strength prediction models including phase angle (with 5‐fold cross validation).
Table S2. Strength prediction models including echogenicity (with 5‐fold cross validation).


