Abstract
Gastric cancer (GC) is one of the most prevalent and malignant types of cancer worldwide. In China, it is the second most common type of cancer and the malignancy with the highest incidence and mortality rate. Chemotherapy for GC is not always effective due to the development of drug resistance. Drug resistance, which is frequently observed in GC, undermines the success rate of chemotherapy and the survival of patients with GC. The dysregulation of non-coding RNAs (ncRNAs), primarily microRNAs (miRNAs or miRs) and long non-coding RNAs (lncRNAs), is involved in the development of GC drug resistance via numerous mechanisms. These mechanisms contribute to the involvement of a large and complex network of ncRNAs in drug resistance. In this review, we focus on and summarize the latest research on the specific mechanisms of action of miRNAs and lncRNAs that modulate drug resistance in GC. In addition, we discuss future prospects and clinical applications of ncRNAs as potential targeted therapies against the chemoresistance of GC.
Keywords: non-coding RNA, microRNA, long non-coding RNA, drug resistance, gastric cancer
1. Introduction
Gastric cancer (GC) is one of the most common malignant types of cancer and the third leading cause of cancer-related death worldwide in the last two decades (1). In many Asian countries, particularly in China, GC is the second most common type of cancer and the leading malignancy as regards the incidence and mortality rate (2). Although advances in surgical techniques have improved the prognosis of patients with early-stage GC, the majority of patients are diagnosed at the advanced stages of the disease and thus have to undergo chemotherapy and radiotherapy (3). However, the 5-year-survival rate has not improved significantly due to the development of chemoresistance to anti-cancer drugs (4). Drug resistance, which is frequently observed in GC, threatens the success rate of chemotherapy and the survival of patients with GC. The underlying mechanisms of drug resistance remain to be fully understood (5-7). One well-known mechanism is the modulation of the epithelial-mesenchymal transition (EMT) process which converts epithelial cells to mesenchymal cells. Studies have confirmed that EMT can induce drug resistance by decelerating the proliferation rate of cancer cells, upregulating the expression of ATP binding cassette (ABC) transporters which mediate drug efflux, and increasing the expression of anti-apoptotic proteins (8-10). It has also been demonstrated that EMT can reconstruct the tumor microenvironment, thereby inducing resistance to immunotherapies (11). Another well-known mechanism related to drug resistance is cancer stem cells (CSCs). CSCs are a cell population with high tumorigenic potential in tumors, and these cells modulate cancer initiation, development and metastasis (12-14). Extensive studies have indicated that CSCs induce drug resistance by expelling intracellular therapeutic drugs to the extracellular space through transport proteins (15-17). A high aldehyde dehydrogenase (ALDH) activity is another element related to drug resistance in CSCs (18,19). Beyond EMT and CSCs, a number of studies have indicated a close association between non-coding RNAs (ncRNAs) and drug resistance in GC, a possibility that has received substantial research attention.
ncRNAs are a type of RNA that does not encode proteins, and they are associated with cell development, metastasis, invasion, proliferation and apoptosis (20). ncRNAs are thought to be involved in drug resistance in human GC (21). Among ncRNAs, microRNAs (miRNAs or miRs) and long non-coding RNAs (lncRNAs), two main families of ncRNAs, play a role in cancer drug resistance (22). miRNAs are approximately 19-25 nucleotides in length and can bind to the 3′-untranslated region (3′-UTR) of target genes to regulate their expression (23). Studies have analyzed the association between miRNAs and drug resistance in GC (21,24,25). lncRNAs are >200 nucleotides in length and are always transcribed by RNA polymerase II, and they lack opening reading frames (ORFs) (26,27). lncRNAs exert their effects by interacting with DNA, RNA and protein (28). Several studies have investigated the function of lncRNAs in drug resistance in GC (21,28,29). In this review, we summarize the mechanisms underlying the role of miRNAs and lncRNAs in regulating drug resistance in GC.
2. Multi-drug resistance and single drug resistance
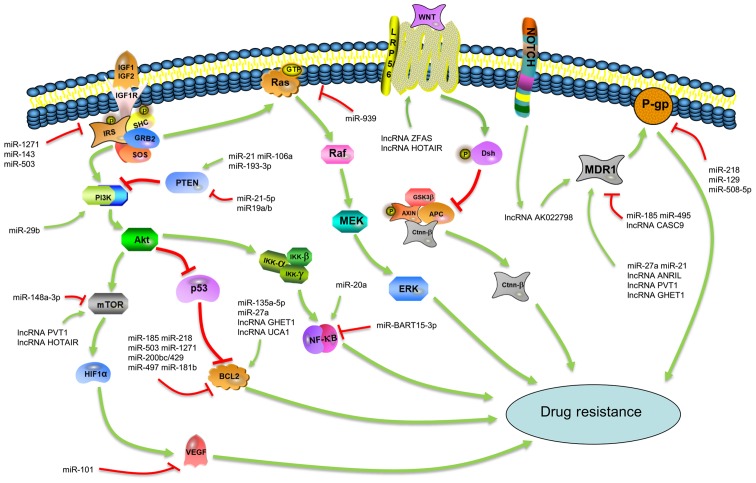
Over the past decades, several chemotherapeutic regimens based on different anti-cancer drugs have improved the survival of a multitude of patients GC. However, chemotherapy in patients with GC often fails due to the development of multi-drug resistance (MDR) and single drug resistance (30-32). Both MDR and single drug resistance in GC are regulated by complex mechanisms, including some classic approaches (Fig. 1). One mechanism of MDR is the modulation of apoptosis and autophagy in cancer cells induced by anti-cancer drugs (25). For example, miR-218 has been shown to inhibit drug-induced apoptosis by increasing the expression of Bcl-2-associated X protein (Bax) and decreasing the expression of B-cell lymphoma-2 (Bcl-2) (33). miR-23b-3p targets autophagy-related gene 12 (ATG12) and high mobility group box 2 (HMGB2) to prevent autophagy and increase the sensitivity of GC cells to 5-fluorouracil (5-FU), vincristine (VCR) and cisplatin (DDP/CDDP) (34). Another important mechanism leading to MDR is the induction of drug efflux in cancer cells by increasing specific energy-dependent transporters, such as P-glycoprotein 1 (P-gp; also known as ABCB1) (25). For instance, miR-106a expedites the efflux of adriamycin (ADR/ADM) to promote ADR resistance in GC by targeting runt-related transcription factor 3 (RUNX3) directly (35). Other mechanisms that may lead to drug resistance have been reported, including DNA damage repair, the mutation of drug targets and the modification of stem cells among others (36-43).
Figure 1.
Classic mechanisms involved in drug resistance in gastric cancer. miRNAs and lncRNAs modulate drug resistance in gastric cancer through classic pathways, such as the PTEN/PI3K/Akt, IGF1R/IRS1, MDR1/P-gp, Wnt/β-catenin, MAPK/ERK, NF-κB and Bcl-2/Bax pathways, which contribute to a complex regulatory network. The arrows represent activation effect and the T symbols indicate inhibition effect. miRNAs, microRNAs; lncRNAs, long non-coding RNAs; IGF1, insulin-like growth factor 1; IGF2, insulin-like growth factor 2; IGF1R, insulin-like growth factor 1 receptor; SHC, SH2 containing protein; GRB2, growth factor receptor-bound protein 2; SOS, son of sevenless; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; mTOR, mammalian target of rapamycin; HIF1α, hypoxia-inducible factor 1α; Iκκ, IκB kinase; Dsh, dishevelled; GSK3β, glycogen synthase kinase 3β; AXIN, axis inhibitor; APC, adenomatous polyposis coli; ctnn-β, β-catenin; P-gp, P-glycoprotein 1.
In addition to these ncRNAs that lead to MDR, there are other ncRNAs that are related to the chemosensitivity of single anti-cancer drugs in GC, including DDP, 5-FU, and VCR. Although some of these ncRNAs play roles in more than one type of drug resistance, the identification of ncRNAs related to single drug resistance is an area worth exploring to promote the development of more effective chemotherapeutic drugs for the treatment of GC.
3. Non-coding RNAs related to multi-drug resistance in gastric cancer
Dysregulated miRNAs
In recent years, several studies have focused on the association between miRNAs and MDR in GC. A number of miRNAs are involved in drug sensitivity in GC via common mechanisms such as apoptosis, the mammalian target of rapamycin (mTOR) pathway and the EMT pathway. miR-185 has been shown to be downregulated in GC and to be related to the resistance to multiple drugs such as ADR, 5-FU, oxaliplatin (L-OHP/OXA), doxorubicin (DOX) and CDDP through the regulation of zinc finger protein 139 (ZNF139) and apoptosis repressor (44,45). miR-218 targets smoothened (SMO) directly to reverse the drug resistance of GC cells to ADM, L-OHP and 5-FU (33). It has been demonstrated that miR-218 is upregulated in patients with GC undergoing cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC), and it targets mTOR to promote the chemosensitivity of GC cells to CDDP in vitro (46). Zeng et al demonstrated that miR-145 targets the 3′-UTR of CD44 directly to promote chemosensitivity to 5-FU and DDP in GC (47). miR-101 enhances the sensitivity to DDP or VCR by regulating Annexin A2 (ANXA2) and vascular endothelial growth factor (VEGF)-C negatively to inhibit viability and promote apoptosis of GC cells (48,49). miR-495 targets the multi-drug resistance protein 1 (MDR1) gene to enhance the sensitivity of GC cells to paclitaxel (PTX) and DOX (50). By targeting enhancer of zeste homolog 2 (EZH2) directly, miR-126 increases the sensitivity of GC cells to VCR and ADR (51). Shang et al demonstrated that miR-508-5p targets ABCB1 and Zinc ribbon domain-containing 1 (ZNRD1) directly to regulate the MDR of GC cells (52). In addition, the upregulation of miR-27b has been shown to promote miR-508-5p expression via cyclin G1 (CCNG1) and p53 in GC cells, enhancing the sensitivity to chemotherapeutic agents, such as ADR, VCR, 5-FU and CDDP (53). Other miRNAs, including miR-30a (30,54,55), miR-107 (56), miR-BART20-5p (57), miR-23b-3p (34) and miR-129-5p (58) have been shown to promote chemotherapeutic sensitivity in GC, and these miRNAs and the brief mechanisms are presented in Table I.
Table I.
miRNAs which reverse multi-drug resistance in gastric cancer.
| MicroRNA | Dysregulation | Effect on drug resistance | Corresponding drugs | Pathway/target | Refs. |
|---|---|---|---|---|---|
| miR-185 | ↓ | Reversing | ADR, 5-FU, OXA; DOX, DDP | MDR1/P-gp, MRP, Bcl-2; ARC/RUNX3 | (44,45) |
| miR-218 | ↓ | Reversing | ADR, OXA, 5-FU | SMO/P-gp/Bax/Bcl2 | (33,46) |
| miR-145 | ↓ | Reversing | 5-FU, DDP | CD44 | (47) |
| miR-101 | ↓ | Reversing | DDP, VCR | ANXA2; VEGF-C | (48,49) |
| miR-495 | ↓ | Reversing | PTX, DOX | MDR1/ABCB1 | (50) |
| miR-126 | ↓ | Reversing | ADR, VCR | EZH2 | (51) |
| miR-508-5p | ↓ | Reversing | / | ABCB1/ZNRD1 | (52) |
| miR-27b | ↓ | Reversing | ADR, VCR, 5-FU, DDP | CCNG1/P53/miR-508-5p | (53) |
| miR-30a | ↓ | Reversing | DDP, 5-FU | EMT | (30,54,55) |
| miR-107 | ↓ | Reversing | OXA, PTX, ADR, 5-FU | Lin28 | (56) |
| miR-BART20-5p | ↓ | Reversing | 5-FU, docetaxel | BAD | (57) |
| miR-23b-3p | ↓ | Reversing | 5-FU, VCR, DDP | ATG12/HMGB2/autophagy | (34) |
| miR-129-5p | ↓ | Reversing | VCR, ADR | ABCB1/ABCC5/ABCG1 | (58) |
ADR, adriamycin; 5-FU, 5-fluorouracil; OXA, oxaliplatin; DOX, doxorubicin; DDP, cisplatin; VCR, vincristine; PTX, paclitaxel; MDR1, multi- drug resistance protein 1; P-gp/ABCB1, P-glycoprotein 1; MRP, multi-drug resistance-associated protein; Bcl-2, B-cell lymphoma-2; ARC, apoptosis repressor with caspase recruitment domain; RUNX3, runt-related transcription factor 3; SMO, smoothened; Bax, Bcl-2-associated X protein; ANXA2, Annexin A2; VEGF-C, vascular endothelial growth factor C; EZH2, Enhancer of Zeste Homolog 2; ZNRD1, Zinc ribbon domain-containing 1; EMT, epithelial-mesenchymal transition; BAD, Bcl-2-associated death promoter; ATG12, autophagy-related gene 12; HMGB2, high mobility group box 2;ABCC5, multi-drug resistance-associated protein 5; ABCG1, ATP-binding cassette sub-family G member 1; /, not mentioned; ↓, downregulation.
Numerous miRNAs have opposite functions in MDR through classical signaling pathways, such as the phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB/Akt) and nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) pathways (59,60). miR-20a is an important upregulated miRNA in GC (59-62). Zhou et al reported that miR-20a not only decreased the sensitivity of GC cells to VCR, ADR, 5-FU and CDDP by directly targeting leucine-rich repeats and immunoglobulin-like domains 1 (LRIG1), but also affected the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and PI3K/Akt signaling pathways, which are mediated by epidermal growth factor receptor (EGFR) to regulate GC cell MDR (59). Other studies have indicated that miR-20a inhibits the expression of cylindromatosis (CYLD) and NFKBIB (also known as IκBβ), activating the NF-κB pathway and two downstream targets, livin and survivin, to promote GC chemoresistance to DDP (60,61). Another study indicated that miR-20a enhanced the resistance of GC cells to docetaxel (DOC) (62). miR-363 has been reported to target F-box and WD repeat domain-containing 7 (FBW7) directly to reduce the sensitivity of GC cells to the DOC + DDP + 5-FU (DCF) regimen (63). miR-106a promotes ADR and DDP resistance in GC cells by expediting the efflux of ADR, targeting RUNX3 to inhibit apoptosis induced by ADR, and modulating phosphatase and tensin homologue (PTEN) and its downstream PI3K/Akt signaling pathway (35,64). miR-20b, miR-27a and miR-181a have been shown to enhance the resistance of GC cells to epirubicin/oxaliplatin/capecitabine (EOX) by targeting hypoxia inducible factor-1 (HIF1A/HIF-1α), MDR1 and homeodomain-interacting protein kinase-2 (HIPK2) (65). miR-27a, which acts as a biomarker to predict 5-FU-based chemotherapy responses in GC, induces resistance to ADR in GC cells via P-gp, cyclin D1 and p21, and it is inhibited by upstream HIF-1α to suppress MDR1/P-gp, lipoprotein receptor-related protein (LRP) and Bcl-2 to reduce L-OHP resistance (66-68). miR-223 negatively regulates the expression of F-box and WD repeat domain-containing 7 (FBXW7) to influence cell cycle progression in DDP-resistant cells, thereby modulating DDP resistance, and its overexpression in HER2-positive GC cells inhibits FBXW7 expression and enhances the resistance to trastuzumab (TZ) by preventing TZ-induced apoptosis (69,70). miR-21 enhances the resistance to several drugs, such as DDP, PTX and TZ by targeting PTEN, the PI3K/Akt signaling pathway and P-gp (71-73). miRNAs involved in MDR in GC are summarized in Table II.
Table II.
miRNAs inducing multi-drug resistance in gastric cancer.
| MicroRNA | Dysregulation | Effect on drug resistance | Corresponding drugs | Pathway/target | Refs. |
|---|---|---|---|---|---|
| miR-20a | ↑ | Inducing | VCR, ADR, 5-FU, DDP; docetaxel | PI3K/Akt, MAPK/ERK; CYLD/NFKBIB (IκBβ)/ NF-κB/livin/survivin | (59-62) |
| miR-363 | ↑ | Inducing | DCF (docetaxel+ DDP+5-FU) | FBXW7 | (63) |
| miR-106a | ↑ | Inducing | DDP, ADR | RUNX3 | (35,64) |
| miR-20b/27a/181a | / | Inducing | EOX | HIF1A/MDR1/HIPK2/hypoxia | (65) |
| miR-27a | ↑ | Inducing | VCR, PTX | P-gp, cyclin D1, p21; HIF-1α/MDR1/P-gp/LRP/Bcl-2 | (66-68) |
| miR-223 | ↑ | Inducing | DDP, TZ | FBXW7/apoptosis | (69,70) |
| miR-21 | ↑ | Inducing | DDP, PTX, TZ | PTEN, PI3K/Akt pathway; P-gp | (71-73) |
VCR, vincristine; ADR, Adriamycin; 5-FU, 5-fluorouracil; DDP, cisplatin; OXA, oxaliplatin; EOX, epirubicin + OXA + capecitabine; PTX, paclitaxel; TZ, trastuzumab; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; CYLD, cylindromatosis; NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; FBXW7, F-box and WD repeat domain-containing 7; RUNX3, runt-related transcription factor 3; HIF1A/HIF-1α, hypoxia inducible factor-1; MDR1, multi-drug resistance protein 1; HIPK2, homeodomain-interacting protein kinase-2; LRP, lipoprotein receptor-related protein; PTEN, phosphatase and tensin homologue; /, not mentioned; ↑, upregulation.
The role of dysregulated miRNAs in MDR in GC cells is mediated by numerous regulatory mechanisms, including the ABCB1/MDR1/P-gp pathway, apoptosis induced by Bcl-2 family proteins, cell autophagy and the EMT process. By regulating these dysregulated miRNAs, the drug resistance of GC to chemotherapy may be reversed, and this may provide some novel ideas for the treatment of GC.
Dysregulated long non-coding RNAs
Over the past decades, studies have indicated that lncRNAs play significant roles in the biological behaviors of GC cells, such as carcinogenesis, development, proliferation, invasion and metastasis, as well as chemosensitivity and drug resistance (21,74). To provide new directions for the research of MDR in GC cells, in this review, we focus on the complex molecular mechanisms underlying anti-cancer drug resistance modulated by various lncRNAs (Table III).
Table III.
lncRNAs involved in multi-drug resistance in gastric cancer.
| lncRNA | Expression in GC | Effect on drug resistance | Drugs | Mechanism | Refs. |
|---|---|---|---|---|---|
| CASC9 | ↑ | Reversing | PTX; ADR | Targeting MDR1 | (75) |
| MRUL | ↑ | Reversing | ADR; VCR | Targeting ABCB1 | (76) |
| ZFAS | ↑ | Inducing | DDP; PTX | Modulating Wnt/β-catenin pathway | (77) |
| MALAT1 | ↑ | Inducing | VCR, DDP | Targeting miR-23b-3p and ATG12 | (78) |
| UCA1 | ↑ | Inducing | ADR, DDP, 5-FU | Targeting miR-27b/Bcl-2/ caspase-3 and | (79,80) |
| cleaved PARP/Bcl2/apoptosis pathway | |||||
| ANRIL | ↑ | Inducing | DDP; 5-FU | Targeting MDR1 and MRP1 | (81) |
PTX, paclitaxel; ADR, adriamycin; VCR, vincristine; DDP, cisplatin; 5-FU, 5-fluorouracil; MDR1, multi-drug resistance protein 1; ABCB1, P-glycoprotein 1; ATG12, autophagy-related gene 12; Bcl-2, B-cell lymphoma-2; PARP, poly ADP-ribose polymerase; MRP1, multi-drug resistance-associated protein 1; ↑, upregulation.
Shang et al reported that lncRNA CASC9 downregulated the expression of MDR1 to reverse the MDR of GC cells to PTX and ADR (75). Wang et al reported that lncRNA MRUL increased the expression of ABCB1 to enhance the sensitivity of GC cells to ADR and VCR and suppress the MDR phenotype (76). Xu et al demonstrated that lncRNA ZFAS, whose expression was upregulated in GC cells, enhanced DDP and PTX resistance in SGC7901 cells by modulating the Wnt/β-catenin signaling pathway (77). lncRNA MALAT1 competes with miR-23b-3p to promote the expression of ATG12 and enhance the resistance to VCR and DDP (78). The overexpression of lncRNA UCA1, which negatively correlates with miR-27b expression, enhances drug resistance to ADR, DDP and 5-FU by inhibiting miR-27b and Bcl-2 and inducing cleaved caspase-3 (79). Another study demonstrated that the downregulation of lncRNA UCA1 promoted the sensitivity of SGC7901/ADR cells to ADR by inducing cleaved Poly ADP-ribose polymerase (PARP) and inhibiting Bcl-2 (80). lncRNA ANRIL is upregulated both in BGC823/DDP and BGC823/5-FU cells, and the silencing of lncRNA ANRIL reverses MDR in GC cells by targeting MDR1 and multi-drug resistance-associated protein 1 (MRP1) (81).
In summary, lncRNAs modulate the MDR of GC cells through various mechanisms, such as the Wnt/β-catenin signaling pathway, the MDR1/MRP1 pathway, Bcl-2/caspase-3 induced apoptosis and by acting as competitive endogenous RNA (ceRNA). Notably, these reports suggest that the dysregulation of all lncRNAs involved in MDR in GC consists of upregulation, and no downregulated lncRNAs leading to MDR in GC cells have been identified to date, at least to the best of our knowledge.
4. Non-coding RNAs related to single drug resistance in gastric cancer
Cisplatin
DDP/CDDP is an anti-cancer agent that can destroy the target DNA of tumor cells (82). It is one of the most widely used first-line anti-cancer agents in clinical therapy, and CDDP resistance, which often occurs in GC, can result in therapeutic failure (83). Chemoresistance has attracted the attention of scientists, and many complex molecular mechanisms of DDP resistance in GC cells have been elucidated to date.
miRNAs can increase the chemosensitivity of GC cells to anti-cancer drugs. For example, the overexpression of miR-125b has been shown to enhance the chemosensitivity to DDP in GC by targeting human epidermal growth factor receptor-2 (HER2) (84). Wang and Ji found that miR-17-5p targeted p21 to modulate apoptosis caused by DDP in GC, and the downregulation of miR-17-5p enhanced the sensitivity of GC cells to DDP (85). miR-22 upregulates the expression of the glycolytic enzyme enolase1 (ENO1) by targeting its 3′-UTR to attenuate the DDP resistance of GC cells (86). miR-129 decreases the expression of P-gp to abate the resistance of GC cells to CDDP (87). The overexpression of miR-148a-3p reduces the resistance of GC cells to CDDP by inducing mitochondrial fission and downregulating A-kinase anchoring protein 1 (AKAP1), preventing cyto-protective autophagy by decreasing Ras-related protein Rab-12 (RAB12) expression and mTOR1 activation (88). AKAP1 inhibits dynamin-related protein 1 (DRP1) dephosphorylation, which is mediated by p53, to enhance CDDP resistance (88). miR-320a targets a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) directly to abate the sensitivity of GC cells to CDDP (89). The overexpression of miR-181a prevents the autophagy of GC cells and enhances the sensitivity of GC cells to CDDP by targeting autophagy related 5 (ATG5) (90). Other miRNAs that enhance the chemosensitivity of DDP in GC include miR-203 (91), miR-149 (92), miR-143 (93), miR-26a (94), miR-1271 (95) and miR-503 (96). These miRNAs and a brief description of their mechanisms are presented in Table IV.
Table IV.
ncRNAs related to cisplatin resistance in gastric cancer.
| ncRNA | Dysregulation | Effect on drug resistance | Pathway/target | Refs. |
|---|---|---|---|---|
| miR-125b | ↓ | Reversing | HER2 | (84) |
| miR-17-5p | ↓ | Reversing | p21 | (85) |
| miR-22 | ↓ | Reversing | ENO1 | (86) |
| miR-129 | ↓ | Reversing | P-gp | (87) |
| miR-148a-3p | ↓ | Reversing | Mitochondrial fission/AKAP1/RAB12/mTOR | (88) |
| miR-320a | ↓ | Reversing | ADAM10 | (89) |
| miR-181a | ↓ | Reversing | Autophagy/ATG5 | (90) |
| miR-203 | ↓ | Reversing | Beiberine/bcl-w | (91) |
| miR-149 | ↓ | Reversing | FoxM1 | (92) |
| miR-143 | ↓ | Reversing | IGF1R/Bcl2 | (93) |
| miR-26a | ↓ | Reversing | NRAS/E2F2 | (94) |
| miR-1271 | ↓ | Reversing | IGF1R/IRS1/mTOR/Bcl-2 | (95) |
| miR-503 | ↓ | Reversing | IGF1R/Bcl-2 | (96) |
| miR-25 | ↑ | Inducing | FOXO3a | (97) |
| miR-214 | ↑ | Inducing | EXO-anti-214 | (98) |
| miR-99a | ↑ | Inducing | CAPNS1/calpain1/calpain2/caspase-3/PARP1 | (83) |
| miR-491 | ↑ | Inducing | CAPNS1/calpain1/calpain2/caspase-3/PARP2 | (83) |
| miR-493 | ↑ | Inducing | DKK1 | (99) |
| miR-421 | ↑ | Inducing | E-cadherin/caspase-3 | (100) |
| miR-29b | ↑ | Inducing | PI3K/Akt pathway | (101) |
| miR-141 | ↑ | Inducing | KEAP1 | (102) |
| miR-132 | ↓ | Inducing | SIRT1/CREB/ABCG2 | (103) |
| miR-524-5p | ↓ | Inducing | SOX9 | (104) |
| miR-34a | ↓ | Inducing | MET | (105) |
| miR-200c | ↓ | Inducing | ZEB2/RhoE; E-cadherin | (106-108) |
| lncRNA HOTAIR | ↑ | Inducing | miR-34a/PI3K/Akt and Wnt/β-catenin pathway; miR-126/VEGFA/PIK3R2 and PI3K/Akt/MRP1 pathway | (109,110) |
| lncRNA PVT1 | ↑ | Inducing | mTOR/HIF-1α/P-glycoprotein/MRP1 and MDR1/MRP/mTOR/HIF-1α pathway | (111,112) |
| lncRNA GHET1 | ↑ | Inducing | Bax/Bcl2/MDR1/MRP1 | (113) |
| lncRNA BCAR4 | ↑ | Inducing | Wnt signaling pathway | (114) |
| lncRNA AK022798 | ↑ | Inducing | Notch1/MRP1/P-gp/caspase-3/caspase-8 | (115) |
| lncRNA CASC2 | ↓ | Reversing | miR-19a | (116) |
HER2, human epidermal growth factor receptor-2; ENO1, enolase1; P-gp, P-glycoprotein 1; AKAP1, a-kinase anchoring protein 1; RAB12, Ras-related protein Rab-12; mTOR, mammalian target of rapamycin; ADAM10, a disintegrin and metalloproteinase domain-containing protein 10; ATG5, autophagy related 5; FoxM1, forkhead box protein M1; IGF1R, insulin-like growth factor 1 receptor; Bcl-2, B-cell lymphoma-2; IRS1, insulin receptor substrate 1; FOXO3a, forkhead box O3a; CAPNS1, calpain small subunit 1; PARP, Poly ADP-ribose polymerase; DKK1, Dickkopf-1; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; KEAP1, Kelch-like ECH-associated protein 1; SIRT1, sirtuin 1; CREB, cAMP response element-binding protein; ABCG2, ATP-binding cassette super-family G member 2; ZEB2, zinc finger E-box binding homeobox 2; VEGFA, vascular endothelial growth factor A; PIK3R2, phosphatidylinositol 3-kinase regulatory subunit beta; MRP1, multidrug resistance-associated protein 1; MDR1, multidrug resistance protein 1; HIF-1α, hypoxia inducible factor-1; Bax, Bcl-2-associated X protein; Notch 1, Notch homolog 1; ↑, upregulation; ↓, downregulation.
Certain miR NAs exert opposite effects on chemosensitivity in DDP-resistant GC cells. miR-25 has been shown to decrease the sensitivity of GC cells to cisplatin by targeting forkhead box O3a (FOXO3a) directly (97). The chemoresistance of GC to DDP induced by miR-214 is reversed by exosomes, which act as nanoparticles (98). A low expression of miR-99a and miR-491 enhances the sensitivity of GC cells to cisplatin by targeting calpain small subunit 1 (CAPNS1)/calpain1/calpain2/caspase-3/PARP1 signaling pathway directly (83). miR-493 prevents Dickkopf-1 (DKK1) expression to enhance the CDDP resistance of GC cells (99). The expression level of miR-421 in GC cells is upregulated by HIF-1α and it promotes DDP resistance through E-cadherin and caspase-3 (100). miR-29b promotes cisplatin resistance in GC cells through the PI3K/Akt signaling pathway (101). miR-141 overexpression inhibits the expression of Kelch-like ECH-associated protein 1 (KEAP1) and promotes the cisplatin resistance of SGC7901/DDP cells (102). Other miRNAs, such as miR-132 (103), miR-524-5p (104), miR-34a (105) and miR-200c (106-108) also promote resistance to DDP in GC. These miRNAs related to DDP resistance are summarized in Table IV.
A number of lncRNAs also modulate the chemosensitivity of GC cells to DDP. lncRNA HOTAIR inhibits the expression of miR-34a to promote DDP resistance in GC through the Wnt/β-catenin and PI3K/Akt signaling pathways (109). Another study indicated that lncRNA HOTAIR functions as a ceRNA of miR-126 by inhibiting its expression and increasing vascular endothelial growth factor A (VEGFA) and phosphatidylinositol 3-kinase regulatory subunit beta (PIK3R2) expression, thereby activating the PI3K/Akt/MRP1 signaling pathway (110). lncRNA PVT1 may promote resistance to DDP by increasing mTOR/HIF-1α/P-gp/MRP1 expression or increasing MDR1/MRP/mTOR/HIF-1α expression (111,112). lncRNA GHET1 enhances the resistance of GC cells to DDP by inhibiting Bax and increasing Bcl-2, MDR1 and MRP1 expression (113). lncRNA BCAR4 enhances resistance to DDP by upregulating certain unknown biomarkers through the Wnt signaling pathway (114). lncRNA AK022798 promotes the resistance of SGC7901/DDP and BGC823/DDP cells to DDP by upregulating MRP1 and P-gp and downregulating caspase-3 and caspase-8 (115). lncRNA CASC2 decreases the resistance of GC to DDP by acting as a sponge for miR-19a (116). These lncRNAs related to DDP resistance in GC are summarized in Table IV.
Apart from DDP, other anti-cancer drugs are used in the treatment of GC in clinical therapy, including 5-FU, ADR, VCR, OXA, DOX, PTX and molecularly-targeted drugs. These anti-cancer drugs also play significant roles in the treatment of GC. However, similar to DDP-resistance, chemoresistance to these drugs frequently occurs in the clinical treatment of GC and can lead to therapeutic failure. Improving our understanding of the mechanisms leading to chemoresistance and reversing the process is therefore critical. Below we discuss the associations between dysregulated ncRNAs and the single anti-cancer drugs mentioned above in GC.
5-Fluorouracil
The overexpression of miR-204 has been shown to enhance sensitivity to 5-FU in GC by inhibiting the EMT signaling pathway, and this effect is mediated by transforming growth factor-β (TGF-β) and by targeting TGF-β receptor 2 (TGF-βR2) directly (117). miR-939 inhibits the Raf/mitogen-activated protein kinase kinase (MAP2K/MEK/MAPKK)/ERK signaling pathway to target solute carrier family 34 member 2 (SLC34A2), thereby decreasing the resistance of GC cells to 5-FU (118). The overexpression of miR-31 weakens resistance to 5-FU by decreasing the expression of RhoA in GC (119). miR-BART15-3p increases the chemosensitivity to 5-FU by targeting the tax1-binding protein 1 (TAX1BP1) gene and modulating NF-κB activity in GC cells (120). The overexpression of miR-197 reverses resistance to 5-FU by targeting the MAPK1 gene (121).
However, miR-193-3p, which is upregulated in GC cells, enhances the 5-FU resistance of GC cells by targeting the PTEN gene directly (122). Few lncRNAs related to 5-FU resistance in GC cells have been identified in recent years. For example, lncRNA LEIGC has been shown to decrease the resistance of GC cells to 5-FU by modulating the EMT pathway (123).
All these ncRNAs and brief descriptions of their mechanisms of action as regards 5-FU resistance are presented in Table V.
Table V.
ncRNAs related to 5-fluorouracil resistance in gastric cancer.
| ncRNA | Dysregulation | Effect on drug resistance | Pathway/target | Refs. |
|---|---|---|---|---|
| miR-204 | ↓ | Reversing | EMT pathway/TGF-β/TGF-βR2 | (117) |
| miR-939 | ↓ | Reversing | SLC34A2 and Raf/mitogen-activated protein kinase kinase/ERK pathway | (118) |
| miR-31 | ↓ | Reversing | RhoA | (119) |
| miR-BART15-3p | ↓ | Reversing | TAX1BP1/NF-κB | (120) |
| miR-197 | ↓ | Reversing | MAPK1 | (121) |
| miR-193-3p | ↑ | Inducing | PTEN | (122) |
| lncRNA LEIGC | ↓ | Reversing | EMT pathway | (123) |
EMT, epithelial-mesenchymal transition; TGF-β, transforming growth factor beta; TGF-βR2, TGF beta receptor 2; SLC34A2, solute carrier family 34 member 2; ERK, extracellular signal-regulated kinase; TAX1BP1, tax1-binding protein 1; NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; MAPK1, mitogen-activated protein kinase; PTEN, phosphatase and tensin homologue; ↑, upregulation; ↓, downregulation.
Adriamycin/doxorubicin
The overexpression of miR-103/107 enhances the sensitivity of SGC7901/ADR cells to DOX by inhibiting P-gp function via the modulation of caveolin-1 (CAV-1) (124). miR-520h is upregulated by DOX in GC cells and inhibits histone deacetylase 1 (HDAC1) to reverse DOX resistance in GC cells (125).
In GC cells and patient tissue samples, the knockdown of miR-215p has been shown to increase PTEN and the tissue inhibitor of matrix metalloproteinases 3 (TIMP3) expression to weaken the drug resistance of GC to DOX (126). miR-140 is downregulated in GC cells and downregulates the expression of SOX4 and resistance-associated factors ATP-binding cassette sub-family C member 1 (ABCC1) and ATP-binding cassette super-family G member 2 (ABCG2) to promote the resistance of GC cells to DOX (127). miR-135a-5p reduces the sensitivity of BGC-823 cells to apoptosis to enhance ADR resistance by targeting activator protein (AP)-2α and Bcl-2 (128). miR-19a/b induces ADR resistance by targeting PTEN directly (129). It also upregulates mdr1 and P-gp to promote the efflux of ADR, and regulates Bax and Bcl-2 to inhibit ADR-induced apoptosis (129).
lncRNA D63785 acts as a ceRNA of miR-422a, and prevents the suppression of myocyte enhancer factor 2D (MEF2D), which is dependent on miR-422a, inducing chemoresistance in GC (130). DOX promotes the apoptosis of GC cells, and the knockdown of miR-422a increases the sensitivity of GC cells to this process to reverse drug resistance (130). The expression level of lncRNA NEAT1 in SGC7901/ADR cells is upregulated, and it enhances ADR resistance by prevent apoptosis induced by ADR, although the potential target gene of NEAT1 remains unknown (131).
Vincristine
miR-647 is downregulated in GC, and the overexpression of miR-647 suppresses ankyrin-B (ANK2) activation and downregulates the expression of focal adhesion kinase (FAK), matrix metalloproteinase (MMP)-2 and MMP12 to reverse VCR resistance in GC (132). miR-1284 upregulates the expression of MYC and inhibits the expression of JUN and MMP12 by targeting eukaryotic initiation factor-4A (eIF4A1) to reduce the VCR resistance of GC cells (133). The ectopic expression of miR-497 has been shown to inhibit Bcl-2 to enhance the sensitivity of SGC7901/VCR cells to apoptosis induced by VCR (134). The overexpression of miR-181b inhibits Bcl-2 expression via the 3′-UTR to decrease the resistance of SGC7901/VCR cells to VCR (135). The overexpression of miR-15b and miR-16 has been shown to prevent Bcl-2 expression to modulate VCR-induced apoptosis and decrease the resistance of SGC7901/VCR cells to VCR (136).
Zhu et al demonstrated that the miR-200bc/429 cluster, which is downregulated in MDR SGC7901/VCR cells, increased Bcl-2 and X-linked inhibitor of apoptosis protein (XIAP) expression to decrease the sensitivity of MDR SGC7901/VCR cells to apoptosis induced by VCR (137).
Oxaliplatin
The expression level of miR-135a in GC cells is upregulated and it inhibits the expression of E2F transcription factor 1 (E2F1) and the Sp1/Death-associated protein kinase 2 (DAPK2) signaling pathway to promote the resistance of GC cells to OXA (138).
lncRNA BLACTA1 is upregulated in GC cells and tissues exhibiting resistance to OXA (139). Furthermore, BLACTA1 targets miR-361 directly to induce the expression of the ABCB1 protein and promote the OXA resistance of GC (139).
Taxanes
Taxanes include classical drugs, such as PTX and DOC, as well as some novel ones, including cabazitaxel and abraxane (ABX) (140). PTX and DOC, known as classical potent cytotoxic drugs, have been used in the treatment of a number of types of cancer, including GC. Wu et al demonstrated that miR-34c-5p was downregulated in PTX-resistant GC cells and tissues (141). The overexpression of miR-34c-5p decreased microtubule-associated protein tau (MAPT) expression and enhanced the sensitivity of GC cells to PTX (141). Tian et al reported that the overexpression of miR-361-5p modulated the expression of FOXM1 by targeting the PI3K/Akt/mTOR pathway, thereby preventing chemoresistance to DOC induced by autophagy in GC (142). Cabazitaxel, a second-generation taxane that stabilizes microtubule, exhibits a broad spectrum anti-cancer activity (143). ABX, a new commercial albumin that binds paclitaxel nanoparticle, can be used in cytopharmaceuticals that induce tumor suppression in ovarian, breast, lung and pancreatic cancer, among others (144,145). However, to date, there have been no reports of cabazitaxel resistance or ABX resistance in GC, at least to the best of our knowledge. The association between ncRNAs and resistance to taxanes in GC is summarized in Table VI.
Table VI.
ncRNAs involved in taxane resistance in gastric cancer.
| Drug | NcRNA | Dysregulation | Effect on drug resistance | Target/pathway | Refs. |
|---|---|---|---|---|---|
| Paclitaxel | miR-34c-5p | ↓ | Reversing | MAPT | (141) |
| Docetaxel | miR-361-5p | ↓ | Reversing | Autophagy/PI3K/Akt/mTOR | (142) |
| Cabazitaxel | / | / | / | / | / |
| Abraxane | / | / | / | / | / |
MAPT, microtubule-associated protein tau; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; mTOR, mammalian target of rapamycin; /, not mentioned; ↑, upregulation; ↓, downregulation.
Molecular targeted drugs
As one of the most rapidly developing fields, molecular targeted drugs play an important role in the treatment of GC. TZ and ramucirumab, which are first-line and second-line therapies for GC, and other specific targeted drugs, such as fibroblast growth factor receptor 2 (FGFR2) inhibitors and bromodomain and extra terminal (BET) inhibitors, are widely used in the clinical treatment of GC (146). Resistance to molecular targeted drugs has been reported previously. Venturutti et al reported that ectopically expressed miR-16 in TZ- and lapatinib-resistant GC cells targets cyclin J (CCNJ) and far upstream element-binding protein 1 (FUBP1) to reverse TZ and lapatinib resistance in ErbB-2-positive GC cells (147).
Another newly developing area, immune checkpoint molecules, has begun to attract increasing attention. Inhibiting the immune response of patients to cancer-specific antigens is a major mechanism of cancer development (148). The dysregulation of immune checkpoint molecules, including cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed cell death protein-1 (PD-1) and its ligand PD-L1, can activate or suppress T lymphocytes, resulting in immune tolerance and cellular immune escape (149,150). Therefore, immune checkpoint therapy, which is based on immune checkpoint inhibitors, can regulate immune response in tumor microenvironment and kill tumor cells indirectly (151). Several immune checkpoint inhibitors, including pembrolizumab, nivolumab, ipilimumab, atezolizumab, avelumab and durvalumab, which target CTLA-4, PD-1 and PD-L1, have been used in the clinical treatment of cancers such as GC, metastatic melanoma, non-small-cell lung cancer (NSCLC), head and neck squamous cell cancer, urothelial carcinoma and Hodgkin's lymphoma (152). Pembrolizumab was the first and only immune checkpoint inhibitor to be approved by the US Food and Drug Administration (FDA) as a third-line treatment for locally advanced and metastatic GC (153). However, to the best of our knowledge, there are no reports on pembrolizumab resistance in GC to date.
Immune checkpoint molecules and miRNAs are not separate. miRNAs have been reported to regulate the expression of immune checkpoint molecules to target its related pathways (154). For example, miR-138 has been shown to inhibit the expression of PD-1 and CTLA-4 on the surface of both regulatory T cells, thus promoting anti-cancer immunity in glioma cell lines (155). A low expression of miR-197, which correlates with a high PD-L1 expression, promotes chemoresistance in NSCLC (156). Beyond miRNAs, some lncRNAs can also modulate immune checkpoint function (157,158). However, the mechanisms how miRNAs and lncRNAs regulate the expression of immune checkpoint molecules in tumor microenvironment remain unclear.
In summary, the molecular mechanisms underlying the involvement of ncRNAs in single drug resistance in GC are varied and specific. However, certain common pathways and target genes involved in these mechanisms have been identified. A number of miRNAs can target the PTEN/PI3K/Akt, insulin-like growth factor 1 receptor (IGF1R)/insulin receptor substrate 1 (IRS1), MDR1/P-gp, Wnt/β-catenin and mTOR pathways to modulate the chemoresistance of GC cells. Some of these ncRNAs can alter the sensitivity of GC cells to apoptosis induced by anti-cancer drugs via the Bcl-2/Bax/caspase family. Targeting E-cadherin to modulate EMT in GC cells is another frequently used approach. However, the majority of lncRNAs involved in single drug resistance are upregulated in GC cells. They modulate single drug resistance through several mechanisms, including the modulation of the PI3K/Akt pathway, the Wnt/β-catenin pathway, the mTOR pathway and Bcl-2/Bax-induced apoptosis, and by acting as ceRNAs. The results described may provide novel potential biomarkers for the diagnosis and treatment of GC. These mechanisms contribute to the involvement of the large and complex network of ncRNAs in drug resistance.
5. Conclusions and future directions
Both miRNAs and lncRNAs play significant roles in modulating cancer chemosensitivity to regulate drug resistance in GC and form a complex regulating network (Fig. 2). The underlying molecular mechanisms include drug efflux, apoptosis dysfunction, DNA damage-induced repair, drug target modulation and proliferation triggering, among others (22). These miRNAs, lncRNAs, critical genes and proteins may be useful to predict the chemosensitivity of GC cells and to establish therapeutic strategies for different patients with GC.
Figure 2.
ncRNAs related to drug resistance in gastric cancer. Many ncRNAs are related to drug resistance in gastric cancer by modulating the sensitivity of gastric cancer cells to drugs, and the molecular mechanisms contribute to a complex regulatory network. Drug resistance related to other miRNAs (oxaliplatin resistance, paclitaxel resistance, trastuzumab resistance, lapatinib resistance, epirubicin resistance, oxaliplatin resistance, capecitabine resistance), drug resistance related to lncRNAs (cisplatin resistance, adriamycin resistance, oxaliplatin resistance, 5-fluorouracil resistance, paclitaxel resistance, vincristine resistance). The arrows represent an inducing effect and the T symbols indicate a reversing effect. ncRNAs, non-coding RNAs; ADR/DOX, adriamycin/doxorubicin; 5-FU, 5-fluorouracil; MDR, multi-drug resistance; VCR, vincristine; DDP, cisplatin.
Changes in ncRNA expression have been confirmed to contribute to the drug resistance of GC. Probably the selective regulation of ncRNA expression can partly enhance the sensitivity of GC cell lines to chemotherapy. At present, miRNA mimics and antagonists, which can imitate and silence the activity of specific miRNA, have been demonstrated to restore or antagonize miRNA functions (159,160). Drugs based on these molecules have not been applied widely in clinical trials, apart from Miravirsen, the first miRNA-targeted drug for the treatment of hepatitis C virus (HCV) (161). Miravirsen can inhibit miR-122 to reduce the HCV RNA level by comprising a locked-nucleic acids (LNA) against miR-122 and now this drug has been examined in a phase II clinical trial (162). As previously described, lncRNAs can act as ceRNAs of some specific miRNAs to modulate their expression. This procession may provide a novel direction that modified or artificial lncRNAs can be designed and transmitted into cell lines to regulate expression of targeted miRNAs, although there is no report about drugs based on this mechanism to date, at least to the best of our knowledge. Another promising clinical application of ncRNAs is acting as potential biomarkers for diagnosis and chemosensitivity in GC. When circulating in the blood as free RNAs, miRNAs are quite stable and they can be easily detected in patients' biofluids (163). Some miRNAs, including miR-223, miR-16 and miR-100, have an increased expression in serum samples of patients with GC, which is associated with clinical characteristics, such as TNM stages (21,164). Certain other miRNAs, such as miR-363, miR-519e and miR-520d have been reported to exhibit differential expression patterns in clinical samples derived from patients who can or cannot respond to chemotherapy (25,165). A successful prediction of treatment response can be made, which would help design an effective and individual therapy planning rather than invalid chemotherapy, which has the risk of potential severe side-effects.
Although ncRNAs provide a novel direction for research on drug resistance in GC, they have not been extensively applied in clinical settings, such as in the diagnosis and treatment of GC. Chemical modification and delivery of ncRNA regulators into tumors are two well-known limitations to the clinical application of ncRNAs (24,166). However, we believe that additional limitations exist. The safety of ncRNAs is an important problem that must be understood. RNA interference, another technique involving RNA, has been found to be toxic in preclinical mouse models (167,168). Whether these specific markers could maintain their function continually during clinical application, in another word, the unexpected mutations, as well as polymorphisms of ncRNAs, may be another problem worth considering. Even slight mutation at some sites may alter the expression of miRNAs and lncRNAs, and influence their macro function. Real-time PCR and next-generation sequencing remain the most effective technologies for the accurate quantitative analysis of ncRNAs (22). Both technologies require precise instruments and skilled technical personnel and therefore are difficult and costly. If these limitations are overcome in the future, we believe that ncRNAs may be a novel field which can be used to reverse drug resistance and improve the prognosis of patients with GC.
In recent years, the ceRNA hypothesis has attracted increasing attention, although many of the functions of ceRNAs in tumors remain unknown, including their involvement in the drug resistance of cancer cells. Studies have indicated that lncRNAs can act as ceRNAs of miRNAs, and compete with the corresponding miRNAs for binding to targets to regulate drug resistance (78,110,130). In addition, studies on the association between exosomes and drug resistance in cancer cells have been published recently. Exosomes play a role in drug resistance through different mechanisms, such as excreting anti-cancer drugs from tumor cells, interacting with oncogenic targets after competing with antibody-based anti-cancer drugs to reduce the curative effect, and contributing to transmit chemoresistance from drug-resistant cells to sensitive ones (169-172). For instance, Wang et al pointed out that drug resistance in GC was modulated by exosomes loaded with miRNA, mRNA and other ncRNAs via fusing with GC cells and modulating biological behaviors such as viability, migration, and apoptosis (98). In renal cell carcinoma cells, lncARSR transmitted by exosomes acts as a ceRNA to enhance sunitinib resistance by binding to miR34/miR-449 competitively (173). Another member of the ncRNA family, circular RNAs (circRNAs), has become the subject of research worldwide. circRNAs are related to carcinogenesis in breast cancer, bladder cancer, GC and colorectal cancer. circRNAs function by sponging miRNAs and regulating gene transcription and encoding proteins (174). For example, circPVT1 increases the expression of ABCB1 to enhance DOX and DDP resistance in osteosarcoma cells (175). Although drug resistance induced by circRNAs in GC has not been reported to date, at least to the best of our knowledge, it is reasonable to believe that drug resistance related to circRNAs and GC will be identified in the future.
These results provide new research areas and may help the design of potential therapeutic strategies with which to reverse drug resistance in different patients in the future. The mechanisms of drug resistance are complex, and more extensive and comprehensive studies are required in order to elucidate the mechanisms of anti-cancer drug resistance in GC in the future.
Acknowledgments
Not applicable.
Funding
This study was supported by the grant of Jilin Provincial Health and Family Planning Commission Training Plan of Young and Technological Core Project (2016Q023), Jilin Provincial Science and Technology Development Planning Project (20180101169JC), Jilin Provincial Health and Family Planning Commission Project (2016C051-2).
Availability of data and materials
Not applicable.
Authors' contributions
All authors (CC, XT, YL, JZ and JL) were involved in the conception and design of the study, and have taken the responsibility for publishing this review article. CC searched literature, wrote the manuscript, and designed the pictures; XT and YL were involved in designing the tables; CC, JZ and JL were involved in the revision of the manuscript. JZ was responsible for technical or material support. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. MAGIC Trial Participants: Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Marin JJ, Al-Abdulla R, Lozano E, Briz O, Bujanda L, Banales JM, Macias RI. Mechanisms of resistance to chemotherapy in gastric cancer. Anticancer Agents Med Chem. 2016;16:318–334. doi: 10.2174/1871520615666150803125121. [DOI] [PubMed] [Google Scholar]
- 5.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrido M, Fonseca PJ, Vieitez JM, Frunza M, Lacave AJ. Challenges in first line chemotherapy and targeted therapy in advanced gastric cancer. Expert Rev Anticancer Ther. 2014;14:887–900. doi: 10.1586/14737140.2014.915194. [DOI] [PubMed] [Google Scholar]
- 7.Zheng T, Wang J, Chen X, Liu L. Role of microRNA in anticancer drug resistance. Int J Cancer. 2010;126:2–10. doi: 10.1002/ijc.24782. [DOI] [PubMed] [Google Scholar]
- 8.Dembinski JL, Krauss S. Characterization and functional analysis of a slow cycling stem cell-like subpopulation in pancreas adenocarcinoma. Clin Exp Metastasis. 2009;26:611–623. doi: 10.1007/s10585-009-9260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxena M, Stephens MA, Pathak H, Rangarajan A. Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2011;2:e179. doi: 10.1038/cddis.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vega S, Morales AV, Ocaña OH, Valdés F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terry S, Savagner P, Ortiz-Cuaran S, Mahjoubi L, Saintigny P, Thiery JP, Chouaib S. New insights into the role of EMT in tumor immune escape. Mol Oncol. 2017;11:824–846. doi: 10.1002/1878-0261.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toledo-Guzmán ME, Bigoni-Ordóñez GD, Ibáñez Hernández M, Ortiz-Sánchez E. Cancer stem cell impact on clinical oncology. World J Stem Cells. 2018;10:183–195. doi: 10.4252/wjsc.v10.i12.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catalano V, Di Franco S, Iovino F, Dieli F, Stassi G, Todaro M. CD133 as a target for colon cancer. Expert Opin Ther Targets. 2012;16:259–267. doi: 10.1517/14728222.2012.667404. [DOI] [PubMed] [Google Scholar]
- 16.Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2:3. doi: 10.1186/2001-1326-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eyre R, Harvey I, Stemke-Hale K, Lennard TW, Tyson-Capper A, Meeson AP. Reversing paclitaxel resistance in ovarian cancer cells via inhibition of the ABCB1 expressing side population. Tumour Biol. 2014;35:9879–9892. doi: 10.1007/s13277-014-2277-2. [DOI] [PubMed] [Google Scholar]
- 18.Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, Tamaki Y, Noguchi S. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15:4234–4241. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J. Cancer stem cells and chemoresistance: The smartest survives the raid. Pharmacol Ther. 2016;160:145–158. doi: 10.1016/j.pharmthera.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahu A, Singhal U, Chinnaiyan AM. Long noncoding RNAs in cancer: From function to translation. Trends Cancer. 2015;1:93–109. doi: 10.1016/j.trecan.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Q, Zhang RW, Sui PC, He HT, Ding L. Dysregulation of non-coding RNAs in gastric cancer. World J Gastroenterol. 2015;21:10956–10981. doi: 10.3748/wjg.v21.i39.10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayers D, Vandesompele J. Influence of microRNAs and long non-coding RNAs in cancer chemoresistance. Genes (Basel) 2017;8:8. doi: 10.3390/genes8030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. doi: 10.1007/978-94-007-5590-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riquelme I, Letelier P, Riffo-Campos AL, Brebi P, Roa JC. Emerging role of miRNAs in the drug resistance of gastric cancer. Int J Mol Sci. 2016;17:424. doi: 10.3390/ijms17030424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matuszcak C, Haier J, Hummel R, Lindner K. MicroRNAs: Promising chemoresistance biomarkers in gastric cancer with diagnostic and therapeutic potential. World J Gastroenterol. 2014;20:13658–13666. doi: 10.3748/wjg.v20.i38.13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geisler S, Coller J. RNA in unexpected places: Long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14:699–712. doi: 10.1038/nrm3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delás MJ, Hannon GJ. lncRNAs in development and disease: From functions to mechanisms. Open Biol. 2017;7:7. doi: 10.1098/rsob.170121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heery R, Finn SP, Cuffe S, Gray SG. Long non-coding RNAs: Key regulators of epithelial-mesenchymal transition, tumour drug resistance and cancer stem cells. Cancers (Basel) 2017;9:9. doi: 10.3390/cancers9040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan L, Xu B, Yuan P, Zhou J, Qin P, Han L, Chen G, Wang Z, Run Z, Zhao P, et al. Tumor-infiltrating CD4+ T cells in patients with gastric cancer. Cancer Cell Int. 2017;17:114. doi: 10.1186/s12935-017-0489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang LL, Zhang XH, Zhang X, Chu JK. MiR-30a increases cisplatin sensitivity of gastric cancer cells through suppressing epithelial-to-mesenchymal transition (EMT) Eur Rev Med Pharmacol Sci. 2016;20:1733–1739. [PubMed] [Google Scholar]
- 31.Archie V, Kauh J, Jones DV, Jr, Cruz V, Karpeh MS, Jr, Thomas CR., Jr Gastric cancer: Standards for the 21st century. Crit Rev Oncol Hematol. 2006;57:123–131. doi: 10.1016/j.critrevonc.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 33.Zhang XL, Shi HJ, Wang JP, Tang HS, Cui SZ. MiR-218 inhibits multidrug resistance (MDR) of gastric cancer cells by targeting Hedgehog/smoothened. Int J Clin Exp Pathol. 2015;8:6397–6406. [PMC free article] [PubMed] [Google Scholar]
- 34.An Y, Zhang Z, Shang Y, Jiang X, Dong J, Yu P, Nie Y, Zhao Q. miR-23b-3p regulates the chemoresistance of gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis. 2015;6:e1766. doi: 10.1038/cddis.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Lu Q, Cai X. MicroRNA-106a induces multidrug resistance in gastric cancer by targeting RUNX3. FEBS Lett. 2013;587:3069–3075. doi: 10.1016/j.febslet.2013.06.058. [DOI] [PubMed] [Google Scholar]
- 36.Kondo T, Wakayama T, Naiki T, Matsumoto K, Sugimoto K. Recruitment of Mec1 and Ddc1 checkpoint proteins to double-strand breaks through distinct mechanisms. Science. 2001;294:867–870. doi: 10.1126/science.1063827. [DOI] [PubMed] [Google Scholar]
- 37.Özeş AR, Miller DF, Özeş ON, Fang F, Liu Y, Matei D, Huang T, Nephew KP. NF-κB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene. 2016;35:5350–5361. doi: 10.1038/onc.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin CT, Lyu YL, Xiao H, Lin WH, Whang-Peng J. Suppression of gene amplification and chromosomal DNA integration by the DNA mismatch repair system. Nucleic Acids Res. 2001;29:3304–3310. doi: 10.1093/nar/29.16.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferguson DO, Alt FW. DNA double strand break repair and chromosomal translocation: Lessons from animal models. Oncogene. 2001;20:5572–5579. doi: 10.1038/sj.onc.1204767. [DOI] [PubMed] [Google Scholar]
- 40.Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clin Ther. 2016;38:1551–1566. doi: 10.1016/j.clinthera.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 41.Schlösser HA, Drebber U, Kloth M, Thelen M, Rothschild SI, Haase S, Garcia-Marquez M, Wennhold K, Berlth F, Urbanski A, et al. Immune checkpoints programmed death 1 ligand 1 and cytotoxic T lymphocyte associated molecule 4 in gastric adenocarcinoma. OncoImmunology. 2015;5:e1100789. doi: 10.1080/2162402X.2015.1100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jabbour E, Kantarjian H, Cortes J. Use of second- and third-generation tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia: An evolving treatment paradigm. Clin Lymphoma Myeloma Leuk. 2015;15:323–334. doi: 10.1016/j.clml.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szakács G, Jakab K, Antal F, Sarkadi B. Diagnostics of multidrug resistance in cancer. Pathol Oncol Res. 1998;4:251–257. doi: 10.1007/BF02905214. [DOI] [PubMed] [Google Scholar]
- 44.Tan B, Li Y, Zhao Q, Fan L, Wang D. ZNF139 increases multidrug resistance in gastric cancer cells by inhibiting miR-185. Biosci Rep. 2018;38:38. doi: 10.1042/BSR20181023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q, Wang JX, He YQ, Feng C, Zhang XJ, Sheng JQ, Li PF. MicroRNA-185 regulates chemotherapeutic sensitivity in gastric cancer by targeting apoptosis repressor with caspase recruitment domain. Cell Death Dis. 2014;5:e1197. doi: 10.1038/cddis.2014.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang XL, Shi HJ, Wang JP, Tang HS, Wu YB, Fang ZY, Cui SZ, Wang LT. MicroRNA-218 is upregulated in gastric cancer after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy and increases chemosensitivity to cisplatin. World J Gastroenterol. 2014;20:11347–11355. doi: 10.3748/wjg.v20.i32.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng JF, Ma XQ, Wang LP, Wang W. MicroRNA-145 exerts tumor-suppressive and chemo-resistance lowering effects by targeting CD44 in gastric cancer. World J Gastroenterol. 2017;23:2337–2345. doi: 10.3748/wjg.v23.i13.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li G, Yang F, Gu S, Li Z, Xue M. MicroRNA-101 induces apoptosis in cisplatin-resistant gastric cancer cells by targeting VEGF-C. Mol Med Rep. 2016;13:572–578. doi: 10.3892/mmr.2015.4560. [DOI] [PubMed] [Google Scholar]
- 49.Bao J, Xu Y, Wang Q, Zhang J, Li Z, Li D, Li J. miR-101 alleviates chemoresistance of gastric cancer cells by targeting ANXA2. Biomed Pharmacother. 2017;92:1030–1037. doi: 10.1016/j.biopha.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Zou Z, Zou R, Zong D, Shi Y, Chen J, Huang J, Zhu J, Chen L, Bao X, Liu Y, et al. miR-495 sensitizes MDR cancer cells to the combination of doxorubicin and taxol by inhibiting MDR1 expression. J Cell Mol Med. 2017;21:1929–1943. doi: 10.1111/jcmm.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang P, Li Z, Liu H, Zhou D, Fu A, Zhang E. MicroRNA-126 increases chemosensitivity in drug-resistant gastric cancer cells by targeting EZH2. Biochem Biophys Res Commun. 2016;479:91–96. doi: 10.1016/j.bbrc.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 52.Shang Y, Zhang Z, Liu Z, Feng B, Ren G, Li K, Zhou L, Sun Y, Li M, Zhou J, et al. miR-508-5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Oncogene. 2014;33:3267–3276. doi: 10.1038/onc.2013.297. [DOI] [PubMed] [Google Scholar]
- 53.Shang Y, Feng B, Zhou L, Ren G, Zhang Z, Fan X, Sun Y, Luo G, Liang J, Wu K, et al. The miR27b-CCNG1-P53-miR-508-5p axis regulates multidrug resistance of gastric cancer. Oncotarget. 2016;7:538–549. doi: 10.18632/oncotarget.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Du X, Liu B, Luan X, Cui Q, Li L. miR-30 decreases multidrug resistance in human gastric cancer cells by modulating cell autophagy. Exp Ther Med. 2018;15:599–605. doi: 10.3892/etm.2017.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li C, Zou J, Zheng G, Chu J. miR-30a decreases multidrug resistance (MDR) of gastric cancer cells. Med Sci Monit. 2016;22:4509–4515. doi: 10.12659/MSM.898415. [DOI] [PubMed] [Google Scholar]
- 56.Teng R, Hu Y, Zhou J, Seifer B, Chen Y, Shen J, Wang L. Overexpression of Lin28 decreases the chemosensitivity of gastric cancer cells to oxaliplatin, paclitaxel, doxorubicin, and fluorouracil in part vi a microRNA-107. PLoS One. 2015;10:e0143716. doi: 10.1371/journal.pone.0143716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim H, Choi H, Lee SK. Epstein-Barr virus miR-BART20-5p regulates cell proliferation and apoptosis by targeting BAD. Cancer Lett. 2015;356:733–742. doi: 10.1016/j.canlet.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 58.Wu Q, Yang Z, Xia L, Nie Y, Wu K, Shi Y, Fan D. Methylation of miR-129-5p-CpG island modulates multi-drug resistance in gastric cancer by targeting ABC transporters. Oncotarget. 2014;5:11552–11563. doi: 10.18632/oncotarget.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou L, Li X, Zhou F, Jin Z, Chen D, Wang P, Zhang S, Zhuge Y, Shang Y, Zou X. Downregulation of leucine-rich repeats and immunoglobulin-like domains 1 by microRNA-20a modulates gastric cancer multidrug resistance. Cancer Sci. 2018;109:1044–1054. doi: 10.1111/cas.13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du Y, Zhu M, Zhou X, Huang Z, Zhu J, Xu J, Cheng G, Shu Y, Liu P, Zhu W, et al. miR-20a enhances cisplatin resistance of human gastric cancer cell line by targeting NFKBIB. Tumour Biol. 2016;37:1261–1269. doi: 10.1007/s13277-015-3921-1. [DOI] [PubMed] [Google Scholar]
- 61.Zhu M, Zhou X, Du Y, Huang Z, Zhu J, Xu J, Cheng G, Shu Y, Liu P, Zhu W, et al. miR-20a induces cisplatin resistance of a human gastric cancer cell line via targeting CYLD. Mol Med Rep. 2016;14:1742–1750. doi: 10.3892/mmr.2016.5413. [DOI] [PubMed] [Google Scholar]
- 62.Li X, Zhang Z, Yu M, Li L, Du G, Xiao W, Yang H. Involvement of miR-20a in promoting gastric cancer progression by targeting early growth response 2 (EGR2) Int J Mol Sci. 2013;14:16226–16239. doi: 10.3390/ijms140816226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang PF, Sheng LL, Wang G, Tian M, Zhu LY, Zhang R, Zhang J, Zhu JS. miR-363 promotes proliferation and chemo-resistance of human gastric cancer via targeting of FBW7 ubiquitin ligase expression. Oncotarget. 2016;7:35284–35292. doi: 10.18632/oncotarget.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fang Y, Shen H, Li H, Cao Y, Qin R, Long L, Zhu X, Xie C, Xu W. miR-106a confers cisplatin resistance by regulating PTEN/Akt pathway in gastric cancer cells. Acta Biochim Biophys Sin (Shanghai) 2013;45:963–972. doi: 10.1093/abbs/gmt106. [DOI] [PubMed] [Google Scholar]
- 65.Danza K, Silvestris N, Simone G, Signorile M, Saragoni L, Brunetti O, Monti M, Mazzotta A, De Summa S, Mangia A, et al. Role of miR-27a, miR-181a and miR-20b in gastric cancer hypoxia-induced chemoresistance. Cancer Biol Ther. 2016;17:400–406. doi: 10.1080/15384047.2016.1139244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Q, Li Y, Tan BB, Fan LQ, Yang PG, Tian Y. HIF-1α induces multidrug resistance in gastric cancer cells by inducing miR-27a. PLoS One. 2015;10:e0132746. doi: 10.1371/journal.pone.0132746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao X, Yang L, Hu J. Down-regulation of miR-27a might inhibit proliferation and drug resistance of gastric cancer cells. J Exp Clin Cancer Res. 2011;30:55. doi: 10.1186/1756-9966-30-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang D, Wang H, Liu R, Li H, Ge S, Bai M, Deng T, Yao G, Ba Y. miRNA27a is a biomarker for predicting chemosensitivity and prognosis in metastatic or recurrent gastric cancer. J Cell Biochem. 2014;115:549–556. doi: 10.1002/jcb.24689. [DOI] [PubMed] [Google Scholar]
- 69.Zhou X, Jin W, Jia H, Yan J, Zhang G. MiR-223 promotes the cisplatin resistance of human gastric cancer cells via regulating cell cycle by targeting FBXW7. J Exp Clin Cancer Res. 2015;34:28. doi: 10.1186/s13046-015-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eto K, Iwatsuki M, Watanabe M, Ishimoto T, Ida S, Imamura Y, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, et al. The sensitivity of gastric cancer to trastuzumab is regulated by the miR-223/FBXW7 pathway. Int J Cancer. 2015;136:1537–1545. doi: 10.1002/ijc.29168. [DOI] [PubMed] [Google Scholar]
- 71.Yang SM, Huang C, Li XF, Yu MZ, He Y, Li J. miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology. 2013;306:162–168. doi: 10.1016/j.tox.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 72.Jin B, Liu Y, Wang H. Antagonism of miRNA-21 sensitizes human gastric cancer cells to paclitaxel. Cell Biochem Biophys. 2015;72:275–282. doi: 10.1007/s12013-014-0450-2. [DOI] [PubMed] [Google Scholar]
- 73.Eto K, Iwatsuki M, Watanabe M, Ida S, Ishimoto T, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, Yoshida N, et al. The microRNA-21/PTEN pathway regulates the sensitivity of HER2-positive gastric cancer cells to trastuzumab. Ann Surg Oncol. 2014;21:343–350. doi: 10.1245/s10434-013-3325-7. [DOI] [PubMed] [Google Scholar]
- 74.Chen QN, Wei CC, Wang ZX, Sun M. Long non-coding RNAs in anti-cancer drug resistance. Oncotarget. 2017;8:1925–1936. doi: 10.18632/oncotarget.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shang C, Sun L, Zhang J, Zhao B, Chen X, Xu H, Huang B. Silence of cancer susceptibility candidate 9 inhibits gastric cancer and reverses chemoresistance. Oncotarget. 2017;8:15393–15398. doi: 10.18632/oncotarget.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Zhang D, Wu K, Zhao Q, Nie Y, Fan D. Long noncoding RNA MRUL promotes ABCB1 expression in multidrug-resistant gastric cancer cell sublines. Mol Cell Biol. 2014;34:3182–3193. doi: 10.1128/MCB.01580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu W, He L, Li Y, Tan Y, Zhang F, Xu H. Silencing of lncRNA ZFAS1 inhibits malignancies by blocking Wnt/β-catenin signaling in gastric cancer cells. Biosci Biotechnol Biochem. 2018;82:456–465. doi: 10.1080/09168451.2018.1431518. [DOI] [PubMed] [Google Scholar]
- 78.YiRen H, YingCong Y, Sunwu Y, Keqin L, Xiaochun T, Senrui C, Ende C, XiZhou L, Yanfan C. Long noncoding RNA MALAT1 regulates autophagy associated chemoresistance via miR-23b-3p sequestration in gastric cancer. Mol Cancer. 2017;16:174. doi: 10.1186/s12943-017-0743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang Q, Chen X, Zhi X. Long Non-Coding RNA (LncRNA) Urothelial Carcinoma Associated 1 (UCA1) Increases Multi-Drug Resistance of Gastric Cancer via Downregulating miR-27b. Med Sci Monit. 2016;22:3506–3513. doi: 10.12659/MSM.900688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shang C, Guo Y, Zhang J, Huang B. Silence of long noncoding RNA UCA1 inhibits malignant proliferation and chemotherapy resistance to adriamycin in gastric cancer. Cancer Chemother Pharmacol. 2016;77:1061–1067. doi: 10.1007/s00280-016-3029-3. [DOI] [PubMed] [Google Scholar]
- 81.Lan WG, Xu DH, Xu C, Ding CL, Ning FL, Zhou YL, Ma LB, Liu CM, Han X. Silencing of long non-coding RNA ANRIL inhibits the development of multidrug resistance in gastric cancer cells. Oncol Rep. 2016;36:263–270. doi: 10.3892/or.2016.4771. [DOI] [PubMed] [Google Scholar]
- 82.Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3:1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Y, Xu W, Ni P, Li A, Zhou J, Xu S. MiR-99a and MiR-491 regulate cisplatin resistance in human gastric cancer cells by targeting CAPNS1. Int J Biol Sci. 2016;12:1437–1447. doi: 10.7150/ijbs.16529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang X, Yao J, Guo K, Huang H, Huai S, Ye R, Niu B, Ji T, Han W, Li J. The functional mechanism of miR-125b in gastric cancer and its effect on the chemosensitivity of cisplatin. Oncotarget. 2017;9:2105–2119. doi: 10.18632/oncotarget.23249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Z, Ji F. Downregulation of microRNA-17-5p inhibits drug resistance of gastric cancer cells partially through targeting p21. Oncol Lett. 2018;15:4585–4591. doi: 10.3892/ol.2018.7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qian X, Xu W, Xu J, Shi Q, Li J, Weng Y, Jiang Z, Feng L, Wang X, Zhou J, et al. Enolase 1 stimulates glycolysis to promote chemore-sistance in gastric cancer. Oncotarget. 2017;8:47691–47708. doi: 10.18632/oncotarget.17868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu C, Shan Z, Li C, Yang L. MiR-129 regulates cisplatin-resistance in human gastric cancer cells by targeting P-gp. Biomed Pharmacother. 2017;86:450–456. doi: 10.1016/j.biopha.2016.11.139. [DOI] [PubMed] [Google Scholar]
- 88.Li B, Wang W, Li Z, Chen Z, Zhi X, Xu J, Li Q, Wang L, Huang X, Wang L, et al. MicroRNA-148a-3p enhances cisplatin cytotoxicity in gastric cancer through mitochondrial fission induction and cyto-protective autophagy suppression. Cancer Lett. 2017;410:212–227. doi: 10.1016/j.canlet.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ge X, Cui H, Zhou Y, Yin D, Feng Y, Xin Q, Xu X, Liu W, Liu S, Zhang Q. miR-320a modulates cell growth and chemosensitivity via regulating ADAM10 in gastric cancer. Mol Med Rep. 2017;16:9664–9670. doi: 10.3892/mmr.2017.7819. [DOI] [PubMed] [Google Scholar]
- 90.Zhao J, Nie Y, Wang H, Lin Y. MiR-181a suppresses autophagy and sensitizes gastric cancer cells to cisplatin. Gene. 2016;576:828–833. doi: 10.1016/j.gene.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 91.You HY, Xie XM, Zhang WJ, Zhu HL, Jiang FZ. Berberine modulates cisplatin sensitivity of human gastric cancer cells by upregulation of miR-203. In Vitro Cell Dev Biol Anim. 2016;52:857–863. doi: 10.1007/s11626-016-0044-y. [DOI] [PubMed] [Google Scholar]
- 92.Li X, Liang J, Liu YX, Wang Y, Yang XH, Luan BH, Zhang GL, Du J, Wu XH. miR-149 reverses cisplatin resistance of gastric cancer SGC7901/DDP cells by targeting FoxM1. Pharmazie. 2016;71:640–643. doi: 10.1691/ph.2016.6696. [DOI] [PubMed] [Google Scholar]
- 93.Zhuang M, Shi Q, Zhang X, Ding Y, Shan L, Shan X, Qian J, Zhou X, Huang Z, Zhu W, et al. Involvement of miR-143 in cisplatin resistance of gastric cancer cells via targeting IGF1R and BCL2. Tumour Biol. 2015;36:2737–2745. doi: 10.1007/s13277-014-2898-5. [DOI] [PubMed] [Google Scholar]
- 94.Wen L, Cheng F, Zhou Y, Yin C. MiR-26a enhances the sensitivity of gastric cancer cells to cisplatin by targeting NRAS and E2F2. Saudi J Gastroenterol. 2015;21:313–319. doi: 10.4103/1319-3767.166206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang M, Shan X, Zhou X, Qiu T, Zhu W, Ding Y, Shu Y, Liu P. miR-1271 regulates cisplatin resistance of human gastric cancer cell lines by targeting IGF1R, IRS1, mTOR, and BCL2. Anticancer. Agents Med Chem. 2014;14:884–891. doi: 10.2174/1871520614666140528161318. [DOI] [PubMed] [Google Scholar]
- 96.Wang T, Ge G, Ding Y, Zhou X, Huang Z, Zhu W, Shu Y, Liu P. MiR-503 regulates cisplatin resistance of human gastric cancer cell lines by targeting IGF1R and BCL2. Chin Med J (Engl) 2014;127:2357–2362. [PubMed] [Google Scholar]
- 97.He J, Qi H, Chen F, Cao C. MicroRNA-25 contributes to cisplatin resistance in gastric cancer cells by inhibiting forkhead box O3a. Oncol Lett. 2017;14:6097–6102. doi: 10.3892/ol.2017.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang X, Zhang H, Bai M, Ning T, Ge S, Deng T, Liu R, Zhang L, Ying G, Ba Y. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol Ther. 2018;26:774–783. doi: 10.1016/j.ymthe.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jia X, Li N, Peng C, Deng Y, Wang J, Deng M, Lu M, Yin J, Zheng G, Liu H, et al. miR-493 mediated DKK1 down-regulation confers proliferation, invasion and chemo-resistance in gastric cancer cells. Oncotarget. 2016;7:7044–7054. doi: 10.18632/oncotarget.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ge X, Liu X, Lin F, Li P, Liu K, Geng R, Dai C, Lin Y, Tang W, Wu Z, et al. MicroRNA-421 regulated by HIF-1α promotes metastasis, inhibits apoptosis, and induces cisplatin resistance by targeting E-cadherin and caspase-3 in gastric cancer. Oncotarget. 2016;7:24466–24482. doi: 10.18632/oncotarget.8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen DD, Feng LC, Ye R, He YQ, Wang YD. miR-29b reduces cisplatin resistance of gastric cancer cell by targeting PI3K/Akt pathway. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2015;37:514–519. doi: 10.3881/j.issn.1000-503X.2015.05.005. In Chinese. [DOI] [PubMed] [Google Scholar]
- 102.Zhou X, Su J, Zhu L, Zhang G. Helicobacter pylori modulates cisplatin sensitivity in gastric cancer by down-regulating miR-141 expression. Helicobacter. 2014;19:174–181. doi: 10.1111/hel.12120. [DOI] [PubMed] [Google Scholar]
- 103.Zhang L, Guo X, Zhang D, Fan Y, Qin L, Dong S, Zhang L. Upregulated miR-132 in Lgr5+ gastric cancer stem cell-like cells contributes to cisplatin-resistance via SIRT1/CREB/ABCG2 signaling pathway. Mol Carcinog. 2017;56:2022–2034. doi: 10.1002/mc.22656. [DOI] [PubMed] [Google Scholar]
- 104.Wang J, Xue X, Hong H, Qin M, Zhou J, Sun Q, Liang H, Gao L. Upregulation of microRNA-524-5p enhances the cisplatin sensitivity of gastric cancer cells by modulating proliferation and metastasis via targeting SOX9. Oncotarget. 2017;8:574–582. doi: 10.18632/oncotarget.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Z, Kong Y, Yang W, Ma F, Zhang Y, Ji S, Ma EM, Liu H, Chen Y, Hua Y. Upregulation of microRNA-34a enhances the DDP sensitivity of gastric cancer cells by modulating proliferation and apoptosis via targeting MET. Oncol Rep. 2016;36:2391–2397. doi: 10.3892/or.2016.5016. [DOI] [PubMed] [Google Scholar]
- 106.Jiang T, Dong P, Li L, Ma X, Xu P, Zhu H, Wang Y, Yang B, Liu K, Liu J, et al. MicroRNA-200c regulates cisplatin resistance by targeting ZEB2 in human gastric cancer cells. Oncol Rep. 2017;38:151–158. doi: 10.3892/or.2017.5659. [DOI] [PubMed] [Google Scholar]
- 107.Chang L, Guo F, Wang Y, Lv Y, Huo B, Wang L, Liu W. MicroRNA-200c regulates the sensitivity of chemotherapy of gastric cancer SGC7901/DDP cells by directly targeting RhoE. Pathol Oncol Res. 2014;20:93–98. doi: 10.1007/s12253-013-9664-7. [DOI] [PubMed] [Google Scholar]
- 108.Chen Y, Zuo J, Liu Y, Gao H, Liu W. Inhibitory effects of miRNA-200c on chemotherapy-resistance and cell proliferation of gastric cancer SGC7901/DDP cells. Chin J Cancer. 2010;29:1006–1011. doi: 10.5732/cjc.010.10236. [DOI] [PubMed] [Google Scholar]
- 109.Cheng C, Qin Y, Zhi Q, Wang J, Qin C. Knockdown of long non-coding RNA HOTAIR inhibits cisplatin resistance of gastric cancer cells through inhibiting the PI3K/Akt and Wnt/β-catenin signaling pathways by up-regulating miR-34a. Int J Biol Macromol. 2018;107:2620–2629. doi: 10.1016/j.ijbiomac.2017.10.154. [DOI] [PubMed] [Google Scholar]
- 110.Yan J, Dang Y, Liu S, Zhang Y, Zhang G. LncRNA HOTAIR promotes cisplatin resistance in gastric cancer by targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumour Biol. 2016;37:16345–16355. doi: 10.1007/s13277-016-5448-5. [DOI] [PubMed] [Google Scholar]
- 111.Zhou DD, Liu XF, Lu CW, Pant OP, Liu XD. Long non-coding RNA PVT1: Emerging biomarker in digestive system cancer. Cell Prolif. 2017;50:50. doi: 10.1111/cpr.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang XW, Bu P, Liu L, Zhang XZ, Li J. Overexpression of long non-coding RNA PVT1 in gastric cancer cells promotes the development of multidrug resistance. Biochem Biophys Res Commun. 2015;462:227–232. doi: 10.1016/j.bbrc.2015.04.121. [DOI] [PubMed] [Google Scholar]
- 113.Zhang X, Bo P, Liu L, Zhang X, Li J. Overexpression of long non-coding RNA GHET1 promotes the development of multidrug resistance in gastric cancer cells. Biomed Pharmacother. 2017;92:580–585. doi: 10.1016/j.biopha.2017.04.111. [DOI] [PubMed] [Google Scholar]
- 114.Wang L, Chunyan Q, Zhou Y, He Q, Ma Y, Ga Y, Wang X. BCAR4 increase cisplatin resistance and predicted poor survival in gastric cancer patients. Eur Rev Med Pharmacol Sci. 2017;21:4064–4070. [PubMed] [Google Scholar]
- 115.Hang Q, Sun R, Jiang C, Li Y. Notch 1 promotes cisplatin-resistant gastric cancer formation by upregulating lncRNA AK022798 expression. Anticancer Drugs. 2015;26:632–640. doi: 10.1097/CAD.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 116.Li Y, Lv S, Ning H, Li K, Zhou X, Xv H, Wen H. Down-regulation of CASC2 contributes to cisplatin resistance in gastric cancer by sponging miR-19a. Biomed Pharmacother. 2018;108:1775–1782. doi: 10.1016/j.biopha.2018.09.181. [DOI] [PubMed] [Google Scholar]
- 117.Li LQ, Pan D, Chen Q, Zhang SW, Xie DY, Zheng XL, Chen H. Sensitization of gastric cancer cells to 5-FU by microRNA-204 through targeting the TGFBR2-mediated epithelial to mesenchymal transition. Cell Physiol Biochem. 2018;47:1533–1545. doi: 10.1159/000490871. [DOI] [PubMed] [Google Scholar]
- 118.Zhang JX, Xu Y, Gao Y, Chen C, Zheng ZS, Yun M, Weng HW, Xie D, Ye S. Decreased expression of miR-939 contributes to chemoresistance and metastasis of gastric cancer via dysregulation of SLC34A2 and Raf/MEK/ERK pathway. Mol Cancer. 2017;16:18. doi: 10.1186/s12943-017-0586-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119.Korourian A, Roudi R, Shariftabrizi A, Madjd Z. MicroRNA-31 inhibits RhoA-mediated tumor invasion and chemotherapy resistance in MKN-45 gastric adenocarcinoma cells. Exp Biol Med (Maywood) 2017;242:1842–1847. doi: 10.1177/1535370217728460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choi H, Lee SK. TAX1BP1 downregulation by EBV-miR-BART15-3p enhances chemosensitivity of gastric cancer cells to 5-FU. Arch Virol. 2017;162:369–377. doi: 10.1007/s00705-016-3109-z. [DOI] [PubMed] [Google Scholar]
- 121.Xiong HL, Zhou SW, Sun AH, He Y, Li J, Yuan X. MicroRNA 197 reverses the drug resistance of fluorouracil induced SGC7901 cells by targeting mitogen activated protein kinase 1. Mol Med Rep. 2015;12:5019–5025. doi: 10.3892/mmr.2015.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jian B, Li Z, Xiao D, He G, Bai L, Yang Q. Downregulation of microRNA-193-3p inhibits tumor proliferation migration and chemoresistance in human gastric cancer by regulating PTEN gene. Tumour Biol. 2016;37:8941–8949. doi: 10.1007/s13277-015-4727-x. [DOI] [PubMed] [Google Scholar]
- 123.Han Y, Ye J, Wu D, Wu P, Chen Z, Chen J, Gao S, Huang J. LEIGC long non-coding RNA acts as a tumor suppressor in gastric carcinoma by inhibiting the epithelial-to-mesenchymal transition. BMC Cancer. 2014;14:932. doi: 10.1186/1471-2407-14-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y, Qu X, Li C, Fan Y, Che X, Wang X, Cai Y, Hu X, Liu Y. miR-103/107 modulates multidrug resistance in human gastric carcinoma by downregulating Cav-1. Tumour Biol. 2015;36:2277–2285. doi: 10.1007/s13277-014-2835-7. [DOI] [PubMed] [Google Scholar]
- 125.Shen Q, Yao Q, Sun J, Feng L, Lu H, Ma Y, Liu L, Wang F, Li J, Yue Y, et al. Downregulation of histone deacetylase 1 by microRNA-520h contributes to the chemotherapeutic effect of doxorubicin. FEBS Lett. 2014;588:184–191. doi: 10.1016/j.febslet.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 126.Chen J, Zhou C, Li J, Xiang X, Zhang L, Deng J, Xiong J. miR-21-5p confers doxorubicin resistance in gastric cancer cells by targeting PTEN and TIMP3. Int J Mol Med. 2018;41:1855–1866. doi: 10.3892/ijmm.2018.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zou J, Xu Y. MicroRNA-140 inhibits cell proliferation in gastric cancer cell line HGC-27 by suppressing SOX4. Med Sci Monit. 2016;22:2243–2252. doi: 10.12659/MSM.896633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pan Y, Ren F, Zhang W, Liu G, Yang D, Hu J, Feng K, Feng Y. Regulation of BGC-823 cell sensitivity to adriamycin via miRNA-135a-5p. Oncol Rep. 2014;32:2549–2556. doi: 10.3892/or.2014.3546. [DOI] [PubMed] [Google Scholar]
- 129.Wang F, Li T, Zhang B, Li H, Wu Q, Yang L, Nie Y, Wu K, Shi Y, Fan D. MicroRNA-19a/b regulates multidrug resistance in human gastric cancer cells by targeting PTEN. Biochem Biophys Res Commun. 2013;434:688–694. doi: 10.1016/j.bbrc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 130.Zhou Z, Lin Z, He Y, Pang X, Wang Y, Ponnusamy M, Ao X, Shan P, Tariq MA, Li P, et al. The long noncoding RNA D63785 regulates chemotherapy sensitivity in human gastric cancer by targeting miR-422a. Mol Ther Nucleic Acids. 2018;12:405–419. doi: 10.1016/j.omtn.2018.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang J, Zhao B, Chen X, Wang Z, Xu H, Huang B. Silence of long noncoding RNA NEAT1 inhibits malignant biological behaviors and chemotherapy resistance in gastric cancer. Pathol Oncol Res. 2018;24:109–113. doi: 10.1007/s12253-017-0233-3. [DOI] [PubMed] [Google Scholar]
- 132.Cao W, Wei W, Zhan Z, Xie D, Xie Y, Xiao Q. Regulation of drug resistance and metastasis of gastric cancer cells via the microRNA647-ANK2 axis. Int J Mol Med. 2018;41:1958–1966. doi: 10.3892/ijmm.2018.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cao W, Wei W, Zhan Z, Xie Y, Xiao Q. MiR-1284 modulates multidrug resistance of gastric cancer cells by targeting EIF4A1. Oncol Rep. 2016;35:2583–2591. doi: 10.3892/or.2016.4643. [DOI] [PubMed] [Google Scholar]
- 134.Zhu W, Zhu D, Lu S, Wang T, Wang J, Jiang B, Shu Y, Liu P. miR-497 modulates multidrug resistance of human cancer cell lines by targeting BCL2. Med Oncol. 2012;29:384–391. doi: 10.1007/s12032-010-9797-4. [DOI] [PubMed] [Google Scholar]
- 135.Zhu W, Shan X, Wang T, Shu Y, Liu P. miR-181b modulates multidrug resistance by targeting BCL2 in human cancer cell lines. Int J Cancer. 2010;127:2520–2529. doi: 10.1002/ijc.25260. [DOI] [PubMed] [Google Scholar]
- 136.Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372–379. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 137.Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J, Jiang B, Shu Y, Liu P. miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemother Pharmacol. 2012;69:723–731. doi: 10.1007/s00280-011-1752-3. [DOI] [PubMed] [Google Scholar]
- 138.Yan LH, Chen ZN, Li-Li, Chen J, Wei WE, Mo XW, Qin YZ, Lin Y, Chen JS. miR-135a promotes gastric cancer progression and resistance to oxaliplatin. Oncotarget. 2016;7:70699–70714. doi: 10.18632/oncotarget.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu X, Zheng Y, Han B, Dong X. Long noncoding RNA BLACAT1 modulates ABCB1 to promote oxaliplatin resistance of gastric cancer via sponging miR-361. Biomed Pharmacother. 2018;99:832–838. doi: 10.1016/j.biopha.2018.01.130. [DOI] [PubMed] [Google Scholar]
- 140.Yared JA, Tkaczuk KH. Update on taxane development: New analogs and new formulations. Drug Des Devel Ther. 2012;6:371–384. doi: 10.2147/DDDT.S28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu H, Huang M, Lu M, Zhu W, Shu Y, Cao P, Liu P. Regulation of microtubule-associated protein tau (MAPT) by miR-34c-5p determines the chemosensitivity of gastric cancer to paclitaxel. Cancer Chemother Pharmacol. 2013;71:1159–1171. doi: 10.1007/s00280-013-2108-y. [DOI] [PubMed] [Google Scholar]
- 142.Tian L, Zhao Z, Xie L, Zhu J. MiR-361-5p suppresses chemoresistance of gastric cancer cells by targeting FOXM1 via the PI3K/Akt/mTOR pathway. Oncotarget. 2017;9:4886–4896. doi: 10.18632/oncotarget.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kang YK, Ryoo BY, Yoon S, Shen L, Lee J, Wei C, Zhou Y, Ryu MH. A Phase I study of cabazitaxel in patients with advanced gastric cancer who have failed prior chemotherapy (GASTANA) Cancer Chemother Pharmacol. 2015;75:309–318. doi: 10.1007/s00280-014-2638-y. [DOI] [PubMed] [Google Scholar]
- 144.Ju C, Wen Y, Zhang L, Wang Q, Xue L, Shen J, Zhang C. Neoadjuvant chemotherapy based on abraxane/human neutrophils cytopharmaceuticals with radiotherapy for gastric cancer. Small. 2018;15:e1804191. doi: 10.1002/smll.201804191. [DOI] [PubMed] [Google Scholar]
- 145.Simón-Gracia L, Hunt H, Scodeller PD, Gaitzsch J, Braun GB, Willmore AM, Ruoslahti E, Battaglia G, Teesalu T. Paclitaxel-loaded polymersomes for enhanced intraperitoneal chemotherapy. Mol Cancer Ther. 2016;15:670–679. doi: 10.1158/1535-7163.MCT-15-0713-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Harada K, Mizrak Kaya D, Shimodaira Y, Ajani JA. Global chemotherapy development for gastric cancer. Gastric Cancer. 2017;20(Suppl 1):92–101. doi: 10.1007/s10120-016-0655-8. [DOI] [PubMed] [Google Scholar]
- 147.Venturutti L, Cordo Russo RI, Rivas MA, Mercogliano MF, Izzo F, Oakley RH, Pereyra MG, De Martino M, Proietti CJ, Yankilevich P, et al. MiR-16 mediates trastuzumab and lapatinib response in ErbB-2-positive breast and gastric cancer via its novel targets CCNJ and FUBP1. Oncogene. 2016;35:6189–6202. doi: 10.1038/onc.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 149.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 152.Abril-Rodriguez G, Ribas A. SnapShot: Immune checkpoint inhibitors. Cancer Cell. 2017;31:848–848.e841. doi: 10.1016/j.ccell.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 153.Kamath SD, Kalyan A, Benson AB., III Pembrolizumab for the treatment of gastric cancer. Expert Rev Anticancer Ther. 2018;18:1177–1187. doi: 10.1080/14737140.2018.1526084. [DOI] [PubMed] [Google Scholar]
- 154.Smolle MA, Calin HN, Pichler M, Calin GA. Noncoding RNAs and immune checkpoints-clinical implications as cancer therapeutics. FEBS J. 2017;284:1952–1966. doi: 10.1111/febs.14030. [DOI] [PubMed] [Google Scholar]
- 155.Wei J, Nduom EK, Kong LY, Hashimoto Y, Xu S, Gabrusiewicz K, Ling X, Huang N, Qiao W, Zhou S, et al. MiR-138 exerts anti-glioma efficacy by targeting immune checkpoints. Neuro Oncol. 2016;18:639–648. doi: 10.1093/neuonc/nov292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fujita Y, Yagishita S, Hagiwara K, Yoshioka Y, Kosaka N, Takeshita F, Fujiwara T, Tsuta K, Nokihara H, Tamura T, et al. The clinical relevance of the miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant non-small-cell lung cancer. Mol Ther. 2015;23:717–727. doi: 10.1038/mt.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bullock MD, Silva AM, Kanlikilicer-Unaldi P, Filant J, Rashed MH, Sood AK, Lopez-Berestein G, Calin GA. Exosomal non-coding RNAs: Diagnostic, prognostic and therapeutic applications in cancer. Noncoding RNA. 2015;1:53–68. doi: 10.3390/ncrna1010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bullrich F, Fujii H, Calin G, Mabuchi H, Negrini M, Pekarsky Y, Rassenti L, Alder H, Reed JC, Keating MJ, et al. Characterization of the 13q14 tumor suppressor locus in CLL: Identification of ALT1, an alternative splice variant of the LEU2 gene. Cancer Res. 2001;61:6640–6648. [PubMed] [Google Scholar]
- 159.Bader AG, Brown D, Winkler M. The promise of microRNA replacement therapy. Cancer Res. 2010;70:7027–7030. doi: 10.1158/0008-5472.CAN-10-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gandellini P, Profumo V, Folini M, Zaffaroni N. MicroRNAs as new therapeutic targets and tools in cancer. Expert Opin Ther Targets. 2011;15:265–279. doi: 10.1517/14728222.2011.550878. [DOI] [PubMed] [Google Scholar]
- 161.van der Ree MH, van der Meer AJ, van Nuenen AC, de Bruijne J, Ottosen S, Janssen HL, Kootstra NA, Reesink HW. Miravirsen dosing in chronic hepatitis C patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. Aliment Pharmacol Ther. 2016;43:102–113. doi: 10.1111/apt.13432. [DOI] [PubMed] [Google Scholar]
- 162.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 163.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang H, Wang L, Wu Z, Sun R, Jin H, Ma J, Liu L, Ling R, Yi J, Wang L, et al. Three dysregulated microRNAs in serum as novel biomarkers for gastric cancer screening. Med Oncol. 2014;31:298. doi: 10.1007/s12032-014-0298-8. [DOI] [PubMed] [Google Scholar]
- 165.Kim CH, Kim HK, Rettig RL, Kim J, Lee ET, Aprelikova O, Choi IJ, Munroe DJ, Green JE. miRNA signature associated with outcome of gastric cancer patients following chemotherapy. BMC Med Genomics. 2011;4:79. doi: 10.1186/1755-8794-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dehghanzadeh R, Jadidi-Niaragh F, Gharibi T, Yousefi M. MicroRNA-induced drug resistance in gastric cancer. Biomed Pharmacother. 2015;74:191–199. doi: 10.1016/j.biopha.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 167.Baumann V, Winkler J. miRNA-based therapies: Strategies and delivery platforms for oligonucleotide and non-oligonu-cleotide agents. Future Med Chem. 2014;6:1967–1984. doi: 10.4155/fmc.14.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Boudreau RL, Martins I, Davidson BL. Artificial microRNAs as siRNA shuttles: Improved safety as compared to shRNAs in vitro and in vivo. Mol Ther. 2009;17:169–175. doi: 10.1038/mt.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, Howell SB. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4:1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 170.Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, Morelli D, Villa A, Della Mina P, Menard S, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227:658–667. doi: 10.1002/jcp.22773. [DOI] [PubMed] [Google Scholar]
- 171.Zhang HD, Jiang LH, Hou JC, Zhong SL, Zhu LP, Wang DD, Zhou SY, Yang SJ, Wang JY, Zhang Q, et al. Exosome: A novel mediator in drug resistance of cancer cells. Epigenomics. 2018;10:1499–1509. doi: 10.2217/epi-2017-0151. [DOI] [PubMed] [Google Scholar]
- 172.Shuwen H, Qing Z, Yan Z, Xi Y. Competitive endogenous RNA in colorectal cancer: A systematic review. Gene. 2018;645:157–162. doi: 10.1016/j.gene.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 173.Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y, Chen W, Liu F, Sun W, Li XF, et al. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29:653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 174.Wang D, Yang S, Wang H, Wang J, Zhang Q, Zhou S, He Y, Zhang H, Deng F, Xu H, et al. The progress of circular RNAs in various tumors. Am J Transl Res. 2018;10:1571–1582. [PMC free article] [PubMed] [Google Scholar]
- 175.Kun-Peng Z, Xiao-Long M, Chun-Lin Z. Overexpressed circPVT1, a potential new circular RNA biomarker, contributes to doxorubicin and cisplatin resistance of osteosarcoma cells by regulating ABCB1. Int J Biol Sci. 2018;14:321–330. doi: 10.7150/ijbs.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.